Abstract
Fibronectin fibrils are a major component of the extracellular matrix (ECM) of the trabecular meshwork (TM). They are a key mediator of the formation of the ECM which controls aqueous humor outflow and contributes to the pathogenesis of glaucoma. The purpose of this work was to determine if a fibronectin-binding peptide called FUD, derived from the Streptococcus pyogenes Functional Upstream Domain of the F1 adhesin protein, could be used to control fibronectin fibrillogenesis and hence ECM formation under conditions where its expression was induced by treatment with the glucocorticoid dexamethasone. FUD was very effective at preventing fibronectin fibrillogenesis in the presence or absence of steroid treatment as well as the removal of existing fibronectin fibrils. Disruption of fibronectin fibrillogenesis by FUD also disrupted the incorporation of type IV collagen, laminin and fibrillin into the ECM. The effect of FUD on these other protein matrices, however, was found to be dependent upon the maturity of the ECM when FUD was added. FUD effectively disrupted the incorporation of these other proteins into matrices when added to newly confluent cells that were forming a nascent ECM. In contrast, FUD had no effect on these other protein matrices if the cell cultures already possessed a preformed, mature ECM. Our studies indicate that FUD can be used to control fibronectin fibrillogenesis and that these fibrils play a role in regulating the assembly of other ECM protein into matrices involving type IV collagen, laminin, and fibrillin within the TM. This suggests that under in vivo conditions, FUD would selectively disrupt fibronectin fibrils and de novo assembly of other proteins into the ECM. Finally, our studies suggest that targeting fibronectin fibril assembly may be a viable treatment for POAG as well as other glaucomas involving excessive or abnormal matrix deposition of the ECM.
1. Introduction
Elevated intraocular pressure (IOP) is the most common risk factor for developing primary open-angle glaucoma (POAG) (Quigley, 2011). The elevation in IOP is generally attributed to a reduction in aqueous humor outflow from the trabecular meshwork as a result of excessive TM extracellular matrix (ECM) production and/or decreased turnover (Braunger et al., 2015; Keller et al., 2009; Pattabiraman and Toris, 2016). One protein that has been suggested as having a potentially significant role in the maintenance of ECM in the TM under both normal and glaucomatous conditions is fibronectin (Babizhayev and Brodskaya, 1989; Floyd et al., 1985; Hann et al., 2001)
Fibronectin fibrils are a major component of the ECM produced by many different cell types including TM cells (Acott and Kelley, 2008; Faralli et al., 2009; Schwarzbauer and DeSimone, 2011). It is one of the earliest ECM fibrils to be assembled, and deletion of the fibronectin gene is embryonic lethal (Hynes, 1990). Fibronectin fibrillogenesis has been shown to be a step-wise, cell surface receptor mediated process that is regulated to a large extent by the contractile properties of a given tissue and integrin signaling (Figure 1) (Gagen et al., 2014; Schwarzbauer and DeSimone, 2011; Singh et al., 2010). In addition, there are a number of specific domains within fibronectin that play key roles in promoting intermolecular interactions during fibril formation (Aguirre et al., 1994; Bultmann et al., 1998; Carnemolla et al., 1996; Hocking et al., 1994; Ingham et al., 1997; Maqueda et al., 2007). The most critical site appears to be a 70 kDa fragment from the amino terminus of fibronectin (McKeown-Longo and Mosher, 1983).
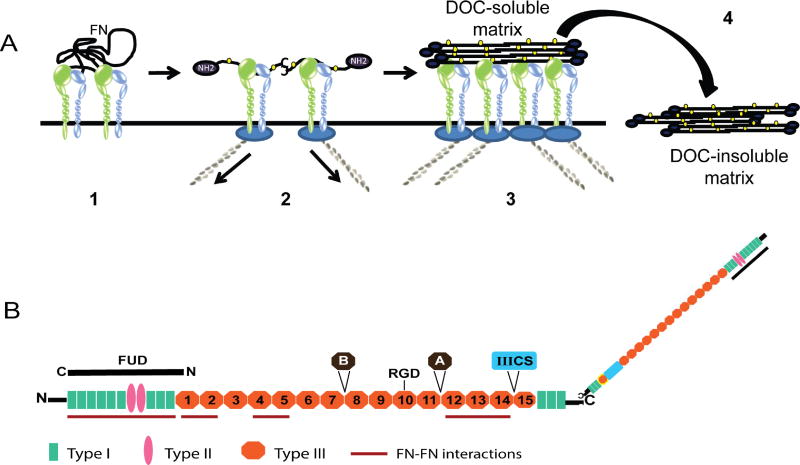
Figure 1. Proposed model for fibronectin matrix assembly.
A) Fibronectin, which is secreted as a globular dimer, binds an integrin on the cell surface (step 1). Contractile forces generated by the actomyosin cytoskeleton (step 2) cause the fibronectin dimer to unfold which exposes fibronectin-fibronectin binding sites (yellow ovals) that mediate fibril formation (step 3). Eventually these fibronectin fibrils are incorporated into the extracellular matrix as a deoxycholic (DOC) acid-insoluble fibril (step 4). B) Fibronectin schematic highlighting the various domains involved in fibril formation. FUD binding to the amino terminus disrupts the intermolecular interactions involved in fibril formation. The domains labeled A, B and IIICS represent the 3 alternatively-spliced domains of fibronectin. The RGD site indicated in the 10th type III repeat is the main integrin-binding site.
Fibronectin fibrils play an important role in mediating the incorporation of other proteins into the ECM. These fibrils can act as a scaffold or organizing nidus upon which additional ECM proteins like collagen types I and III and fibrillin are assembled into the matrix (Dallas et al., 2005; Li et al., 2003; Sabatier et al., 2009; Sottile and Hocking, 2002; Velling et al., 2002). Once incorporated into the matrix, however, it is unclear whether fibronectin fibrils are required for the maintenance of other ECM protein matrices (Dallas et al., 2006). Fibronectin fibrils can also act as a bioreservoir for growth factors such as TGFβ2 along with LTPB TGFβ activating proteins (Dallas et al., 2005) and enzymes like LOX1 (Fogelgren et al., 2005) which contribute to ECM formation and have been implicated in glaucoma (Fuchshofer and Tamm, 2011; Wu et al., 2015). Thus, fibronectin fibrils would be expected to play a major role in maintaining ECM homeostasis in the normal TM and increased deposition of ECM observed in glaucoma.
Given the significance that fibronectin would appear to have with respect to regulating aqueous humor outflow under normal and glaucomatous conditions, peptides and small molecules that are able to affect fibronectin matrix assembly have the potential to be developed into effective therapeutic tools to treat POAG. One such peptide is a fibronectin-binding, 49 amino-acid fragment derived from the functional upstream domain (FUD) of Streptococcus pyogenes adhesin F1 protein (Ozeri et al., 1996). FUD (or pUR4 (Altrock et al., 2015)) has previously been shown to inhibit FN matrix assembly in fibroblasts by binding to the amino-terminal 70-kDa fibrin/gelatin-binding domain of fibronectin (Maurer et al., 2010; Sabatier et al., 2013; Tomasini-Johansson et al., 2001). This 70-kDa domain appears capable of regulating both cell-mediated events in fibrillogenesis as well as the self-association of fibronectin dimers and is critical in promoting fibronectin matrix assembly (McKeown-Longo and Mosher, 1983, 1985; Sottile et al., 1991). Being able to inhibit fibronectin self-association and subsequent fibrillogenesis can ultimately inhibit ECM formation in general, and thus has the potential to have a significant effect on the organization of the ECM within the TM.
In this study, we used recombinant FUD to determine if fibronectin fibril formation could be prevented in dexamethasone-treated cultures of HTM cells where this process is up-regulated. We also examined whether prevention of fibronectin fibril assembly affected the incorporation of collagen type IV, laminin and fibrillin into the ECM of HTM cultures. These studies showed that FUD prevented the de novo synthesis of fibronectin fibrils as well as the incorporation of collagen type IV, laminin and fibrillin into newly developing matrices. The inhibition of fibronectin fibrillogenesis by FUD also appeared to promote the removal of existing fibronectin fibrils, but had no effect on mature matrices of collagen type IV, laminin and fibrillin. This suggests that controlling fibronectin fibril formation may represent a way to control excessive matrix deposition in glaucoma.
2. Materials and Methods
2.1 Materials
Mouse anti-laminin clone LAM-89 (cat. #L8271) against the laminin β1 chain (Geberhiwot et al., 2000) and mouse anti-glial fibrillary acidic protein (GFAP) clone G-A-5 (cat. #3893) were purchased from Sigma (St. Louis, MO). Rabbit polyclonal anti-type IV collagen (cat. #AB748) and mouse monoclonal anti-fibrillin clone 11C1.3 (cat. #MAB1919) were purchased from EMD Millipore (Billerica, MA). Mouse anti-EDA (EIIIA) fibronectin clone IST-9 (cat. #ab6328), and mouse anti-EDB (EIIIB) fibronectin clone BC-1 (cat. ab154210) were purchased from Abcam (Cambridge, MA). Rabbit polyclonal fibronectin antisera was produced in our lab and verified by western blot analysis using purified plasma fibronectin. Normal rabbit serum (R-NS) was purchased from Vector Laboratories (Burlingame, CA).
2.2 Cell Culture
N25TM-8 cells were isolated from the corneal rim of the OS eye from a 25 year old donor. N17TM-2 cells were isolated from the OD eye of a 17 year old donor. Both eyes were non-glaucomatous and did not have any known history of ocular disease. Cells from these eyes were generated as previously described (Filla et al., 2002; Polansky et al., 1981; Polansky et al., 1979) and cultured in low glucose DMEM (Sigma, St. Louis, MO), 15% fetal bovine serum (Atlanta Biologicals, Atlanta, GA), 2 mM L-glutamine (Sigma), 1% amphoteracin B (Mediatech, Herndon, VA), 0.05% gentamicin (Mediatech) and 1 ng/mL FGF-2 (Peprotech, Rocky Hill, NJ).
In some experiments, cell cultures that were maintained at confluence for 7 days prior to treatment where treated for 12–14 days in the presence of 1% FBS and 100nM or 500nM dexamethasone (DEX) or 0.1% ethanol (vehicle). In other experiments cells were treated for 7 days with vehicle or 500 nM DEX once the cells reached confluence. FUD treatment in all experiments began at the same time DEX treatment was initiated. For the duration of whichever treatment protocol was used, cells were re-fed every other day with fresh medium containing vehicle, DEX and/or FUD.
2.3 FUD expression and preparation
Recombinant FUD was expressed as a His-tagged fusion peptide using the pET-ELMER plasmid in cultures of the BL21 (DE3) strain of E. Coli as previously described (Maurer et al., 2010) with the following modifications. The His-tag was removed from the FUD while it was bound to Ni-agarose using 2.4 U/mL thrombin in 20 mM Tris, pH 8.4, 150 mM NaCl and 2.5 mM CaCl2. In order to remove the thrombin post-digestion, the supernatant containing the released peptide was further incubated with p-aminobenzamidine Sepharose 6B beads (Sigma) equilibrated in the same buffer as previously described (Bultmann et al., 1998). After thrombin removal, FUD concentration was determined using a BCA assay (Thermo Fisher Scientific, Waltham, CA). The purity of the peptide was determined by conjugating an aliquot of the peptide with IRDye 680RD NHS ester (LI-COR Biosciences, Lincoln, NE) according to the manufacturer’s instructions and then analyzing it by SDS-PAGE. The gel was then read on a LI-COR Odyssey CLx infrared scanner at a wavelength of 700 nm. In order to conjugate recombinant FUD with AlexaFluor®488, FUD containing an amino terminal cysteine residue (Ma et al., 2015) was purified as described above and then incubated with the fluorochrome AlexaFluor®488-maleimide (Thermo Fisher Scientific) according to the manufacturer’s instructions.
A mutated version of the full length peptide (mFUD) was engineered such that specific amino acids within three of the five C-terminal active domains of the peptide (Ma et al., 2015) were changed in order to eliminate or reduce the binding activity of the peptide to fibronectin (Figure 3A). The mutated FUD was generated using a PCR amplified full length DNA fragment for FUD. A two-step site-directed mutagenesis was then performed to create the mutated FUD. The following primers 5’-TATAACTCTGTTGATCCTGATAAAAAACTTATAAATGAAGGAGGTTTTTCAACA A AT ATGGTTGAGCCAGAAGATACGAAAGCTTAATTAG-’3; and 5’-GCTCAACCATATTTGTTGAAAAACCTCCTTCATTTATAAGTTTTTTATCAGGAT CAACAGAGTTAT ATT GAT TTC CAT AGA CTT CG-’3 were used to generate specific mutations (Q24/Y, P26/S, I29/P, P34/I, T37/G, G41/T and T46/P) into the carboxyl terminus of FUD. The codons in bold are the mutated sequences. This full length DNA fragment of mutated FUD was then amplified using the 5’-ATGGGAGGATC GCATCACCATC-’3 and 5’-GGGCTTTGTTAGCAGCCG GATC-’3 to generate a BamHI PCR product. The resulting PCR product was then digested with the restriction enzymes BamHI and ligated into the pET-Elmer vector in place of the BamHI fragment of the wild type FUD. The plasmid was sequenced to confirm the integrity of the constructed mutant peptide.
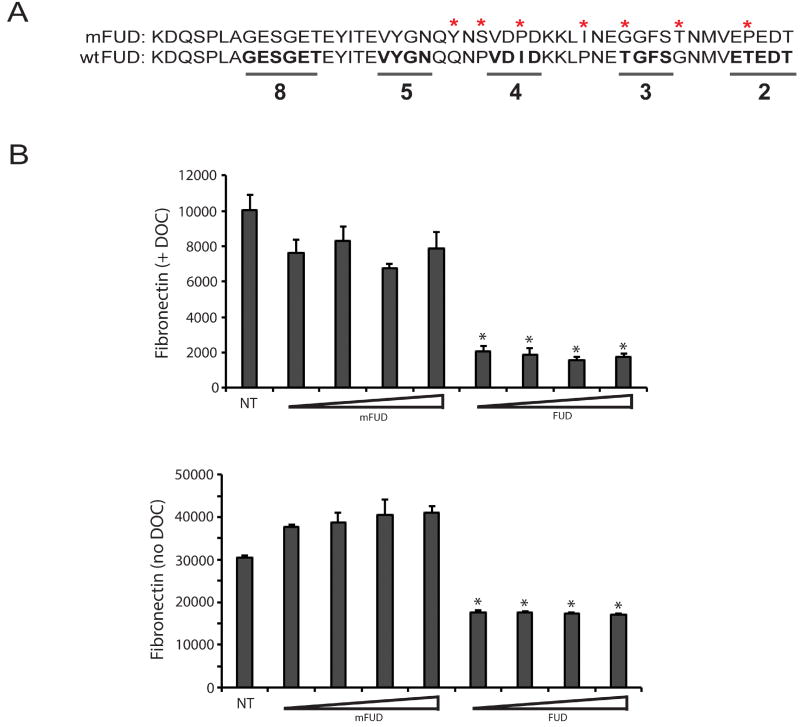
Figure 3. Inhibition of fibronectin fibrillogenesis by FUD is specific.
A) FUD was mutated using site-directed mutagenesis to alter key amino acids found within the C-terminal portion of the peptide in order to abrogate the activity the peptide. The bottom sequence is wild type FUD while the mutant FUD (mFUD) sequence is on top. The mutated amino acids are marked with asterisks. The underlined wild type sequences in bold are critical for FUD binding to fibronectin. The numbers 2, 3, 4, 5 & 8 indicate the fibronectin type I domains that the underlined sequences bind to (N-terminal to C-terminal) (Ma et al., 2015). B) HTM cells were plated at 25,000 cells/well into 96 wells plates and allowed to attach for 3 h. Cells were then left either untreated (NT) or treated with 0.5, 1.0, 2.0 or 4.0 µM mFUD or FUD for 24 h. Cell layers were then extracted with 1% DOC (top graph) or left un-extracted (bottom graph) prior to processing for OCW analysis. For both graphs, all FUD-treated groups are significantly less than untreated cells, p < 0.0001.
2.4 MTT Cell Viability Assay
Cells were plated into 96 well plates and grown to confluence at which point cells were refed every other day with either control medium or medium plus 2 µM FUD for 7 days. During the last 24 h of the assay one group of untreated cells was exposed to 0.1% saponin as a positive control. Viability was determined using a CellQuanti-MTT™ assay kit (BioAssay Systems). The yellow (3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide (MTT) was added for the last 4 h of the assay. After conversion of MTT to formazan, a solubilizing reagent supplemented with 0.5% TX-100 was added and the plates were incubated at room temperature overnight on an orbital shaker. The following day the plates were read at 570 nm on an ELx 800 NB Universal Plate Reader (Bio-Tek Instruments, Inc). The data represent the mean of the data pooled from three experiments ± SE.
2.5 Immunofluorescence Microscopy
At the end of each FUD treatment protocol, cells were fixed with either PBS plus 4% paraformaldehyde at room temperature or −20°C methanol depending upon which primary antibody was to be used. Cells were blocked in PBS plus 1% BSA prior to labeling with the various primary antibodies. Rabbit primary antibodies were detected with AlexaFluor®488 goat anti-rabbit IgG while mouse primary antibodies were detected using AlexaFluor®546 goat anti-mouse IgG (both Thermo Fisher Scientific). Nuclei were labeled using Hoechst 33342 (Thermo Fisher Scientific). Fluorescence was observed with an epifluorescence microscope (Zeiss Axioplan 2) equipped with a digital camera (Axiocam HRm) and image acquisition software (Axiovision ver. 4.8). These experiments were performed two to four times, and the reported results are representative of what was observed.
2.6 On-Cell Western (OCW) Analysis
Seven days after reaching confluence, cells grown in 96-well plates were treated with 0.1% EtOH or 100 nM or 500 nM DEX together with or without 2 µM FUD for an additional 12–14 days. Cells were then processed for OCW analysis. Half the wells were left un-extracted while the other half were extracted a lysis buffer to isolate the insoluble ECM. To isolate the insoluble matrix layer, cell layers were washed twice with PBS, 0.05% BSA and then treated 2 × 5 min with hypotonic lysis buffer (20 mM HEPES, pH 7.4, 1 mM EDTA, 1X HALT protease inhibitors (Thermo Scientific) plus 1% deoxycholate (DOC). The insoluble matrices and un-extracted duplicate cell layers were then fixed for 20 min with PBS plus 4% paraformaldehyde. Post-fixation, cell layers and isolated matrices were labeled with IRDye 680RD NHS ester to allow normalization for protein content. Wells were blocked with PBS plus 1% BSA prior to labeling with rabbit fibronectin polyclonal antisera or normal rabbit sera for 90 min. Bound primary rabbit antibody was detected using IRDye 800CW-conjugated goat-anti-rabbit IgG (LI-COR). The plates were read on a LI-COR Odyssey CLx infrared scanner at wavelengths of 700 and 800 nm. Quantification of the signals at each wavelength was performed using LI-COR Image Studio v. 5.0.21 software. The fibronectin labeled wells were blanked against normal sera labeled wells. For each well, the 800 nm signal was normalized to the corresponding NHS ester signal read at 700 nm. The data represent the mean of data pooled from three experiments ± SE. In experiments comparing the activity of wild type FUD and mutant FUD, 2.5 × 104 cells/well were plated into 96 well plates. Three hours later cells were refed with medium containing 1% FBS alone or plus increasing concentrations (0.5 µM, 1 µM, 2 µM, 4 µM) of either wild type FUD or mutant FUD for an additional 24 h and then processed for OCW analysis as described above. The experiment was performed twice with similar results. The data represent the mean ± SE. In separate experiments, levels of fibronectin, type IV collagen, laminin and fibrillin were assessed in newly confluent cultures of HTM cells in the absence of DEX treatment. Cells were grown to confluence and either immediately processed for OCW analysis as described above or grow for an additional 7 days prior to processing. Fibronectin was detected as described above while type IV collagen, laminin and fibrillin were detected using the same antibodies that were described above for immunofluorescence microscopy. The data represent the mean of data pooled from two experiments ± SE. Triplicate determinations were used in all experiments.
2.7 Statistical Analysis
Statistical analysis comparing the various treatment groups for differences in fibronectin labeling was performed using ANOVA. Where pairs of treatment groups had to be compared, ANOVA analysis was used in conjunction with the Tukey HSD test. For OCW analysis comparing fibronectin, type IV collagen, laminin or fibrillin levels at different time points in confluent HTM cells, a paired Student’s t-test was used.
3. Results
3.1 FUD disrupts fibronectin fibril formation in HTM cultures
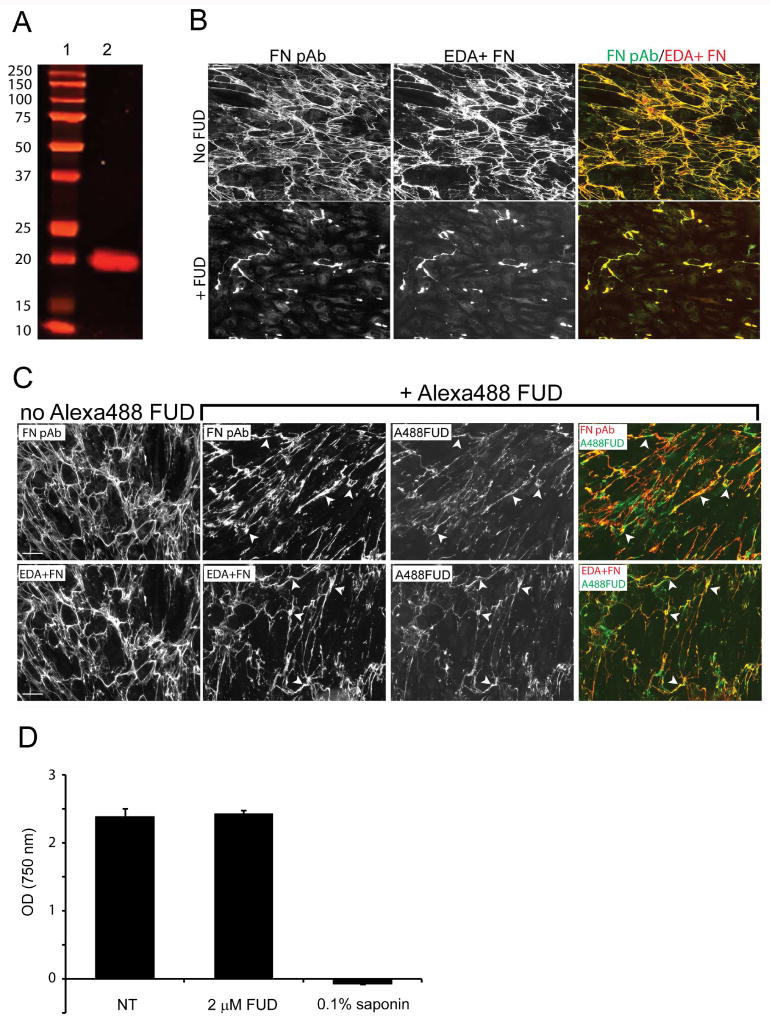
As shown in Figure 1A, fibronectin fibrillogenesis is a cell-mediated process that involves several intermolecular interactions between specific fibronectin-fibronectin binding domains. The amino terminal type I repeats of fibronectin make up one such domain (Figure 1B), and several studies have shown that proteins or peptides that bind to this domain can be used to inhibit fibronectin fibrillogenesis. One such peptide, FUD (pUR4 (Altrock et al., 2015)), was derived from the functional upstream domain of the adhesin F1 protein (Maurer et al., 2010; Ozeri et al., 1996). To determine if FUD could affect fibronectin matrix assembly in HTM cell cultures, a recombinant FUD was produced (Fig 2A). As shown in Figure 2B, the majority of the matrix produced by these cells consisted of the EDA+ fibronectin isoform since there was nearly 100% co-localization between the IST-9 antibody and the anti-fibronectin sera. The few areas where there wasn’t co-localization indicates the presence of fibronectin isoforms that lack the EDA domain in these cultures. Normal rabbit serum and an irrelevant monoclonal antibody against GFAP were used as negative controls and both failed to demonstrate any significant labeling of fixed cells (not shown). When these HTM cultures were treated with FUD for 7 days, there was a significant decrease in fibronectin fibrils (Figure 2B). To show that the decrease in fibril formation was due to FUD interacting with the fibronectin fibrils in culture, AlexaFluor®488-conjugated FUD was added to cultures for only 48 h so that sufficient matrix could still be observed. As shown in Figure 2C, there was a decrease in fibronectin fibrils in the presence of AlexaFluor®488-conjugated FUD compared to untreated cells, and FUD co-localized extensively with the EDA+ fibronectin fibrils still present. The decrease in fibronectin matrices was not due to general cytotoxicity in response to the peptide. Using a MTT-based cell viability assay, no cytotoxic effects of FUD on HTM cells could be detected after a 7 day treatment period (Figure 2D).
Figure 2. Recombinant FUD binds to fibronectin and disrupts fibronectin fibrillogenesis.
A) Recombinant FUD (lane 2) was purified and analyzed by PAGE. Molecular weight markers are in lane 1. The recombinant FUD peptide migrated as a single band corresponding to a molecular weight of ~ 20 kDA which is larger than its theoretical size but consistent with an earlier report of FUD (Ozeri et al., 1996). B) Confluent HTM cells were left untreated (top row) or treated with 2 µM FUD for 7 days prior to fixation and double labeling with the fibronectin polyclonal antisera (FN pAb) and mAb IST-9 against EDA+ FN. C) FUD conjugated to AlexaFluor®488 was added to confluent HTM cultures for forty-eight hours prior to fixation and labeled with either a fibronectin antisera (top row) or mAb IST-9 (bottom row). D) FUD cytotoxicity was assessed using the MTT assay in HTM cells that were plated into 96 well plates and grown to confluence. They were left either untreated (NT) or treated for 7 days with 2 µM FUD. There was no significant difference between untreated and FUD-treated cells. Scale bars = 50 µm.
To further confirm that the effects of FUD on fibronectin matrix assembly were specific, we also compared the ability of FUD to block fibronectin matrix assembly relative to a mutated FUD (mFUD; Figure 3A). The ability of both peptides to block fibronectin fibril formation was assessed using a DOC extraction procedure that removes soluble, cell surface bound and intracellular fibronectin but not fibronectin assembled into a fibril. An On-cell western (OCW) assay was then used to measure the level of fibronectin fibrils in HTM cells treated for 24 h with increasing concentrations of mFUD or FUD. As shown in Figure 3B, all four concentrations of wild type FUD significantly reduced the amount of fibronectin incorporated into the DOC-insoluble matrix by at least 79% compared to untreated cells (p < 0.0001). In contrast, all tested concentrations of mFUD appeared to have only a small blocking effect on the level of fibronectin in the DOC-insoluble matrix relative to untreated cells which was not statistically significant by ANOVA (p = 0.084).
Un-extracted cell layers treated with FUD also showed a very significant decrease in fibronectin levels at all concentrations tested (p < 0.0001) consistent with an earlier report showing that FUD affected binding of soluble fibronectin to cell surfaces in addition to blocking incorporation of fibrils into the insoluble matrix (Tomasini-Johansson et al., 2001). In contrast, unextracted cell layers treated with mFUD showed no decrease in fibronectin levels (Figure 3B) relative to untreated cells. The unextracted cells, however, did show a small increase in the fibronectin levels in response to mFUD compared to untreated cells. However, this increase, was not statistically significant by ANOVA (p = 0.75). Together with the data shown in Figure 2, these studies indicate that FUD inhibits fibronectin fibrillogenesis in HTM cells.
3.2 Dexamethasone-induced increases in DOC-insoluble fibronectin matrix can be inhibited by FUD
Having confirmed the specificity of FUD activity, we then sought to examine its ability to block ECM assembly under conditions where fibronectin expression is upregulated. Since glucocorticoids such as dexamethasone (DEX) are known to increase fibronectin expression and matrix deposition by TM cells (Filla et al., 2002; Steely et al., 1992; Zhou et al., 1998), we used a DEX-induced matrix model to determine the effectiveness of FUD at preventing matrix assembly when fibronectin is upregulated in TM cells. Figure 4A shows immunofluorescence (IF) microscopy images of HTM cells treated with 0.1% ethanol or 500 nM DEX in the presence or absence of 2 µM FUD for 14 days. As expected, an increase in fibronectin levels was detected in cultures treated with DEX relative to control cells treated with ethanol in the absence of FUD. The DEX-induced increase in fibronectin appeared to consist predominantly of the EDA+ isoform as there was nearly 100% co-localization between fibrils detected with mAb IST-9 against the fibronectin EDA domain and fibrils detected with the fibronectin antisera. This suggests that DEX treatment, like treatment with TGFβ2 (Medina-Ortiz et al., 2013), causes an increase in EDA+ fibronectin.
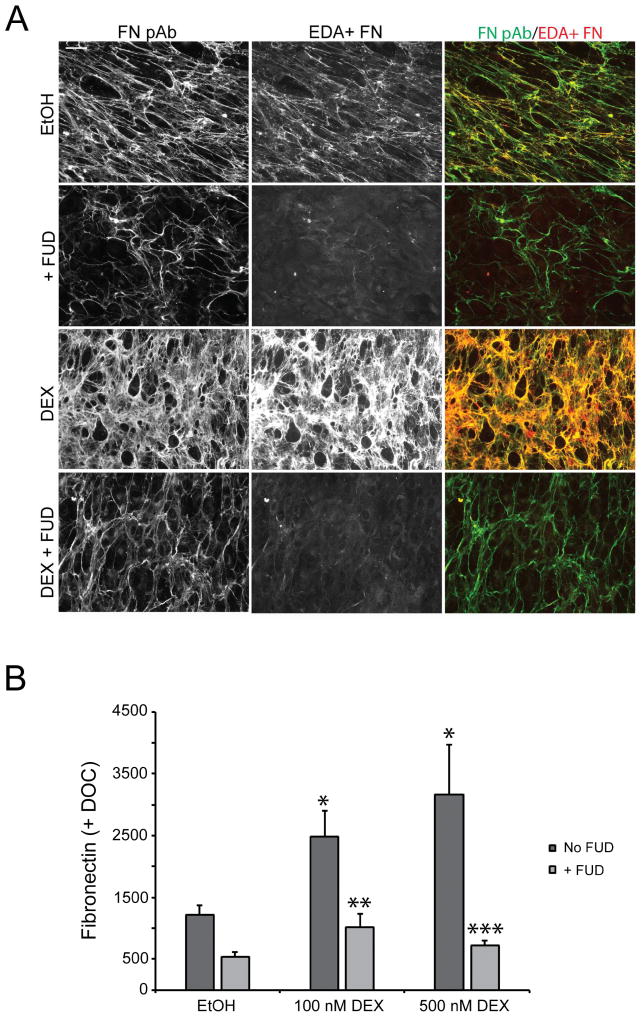
Figure 4. FUD blocks steroid-induced increases in fibronectin fibrillogenesis and matrix assembly.
A) Confluent cell monolayers were treated with vehicle (EtOH) or 500 nM DEX for 12–14 days in the presence or absence of 2 µM FUD prior to fixation and double labeling with fibronectin antisera (FN pAb) and mAb IST-9 against EDA+ FN. B) Cell monolayers in 96 wells plates were treated as in panel A. We used the fibronectin polyclonal antisera in this assay rather than mAb IST-9 as the antisera would detect the multiple isoforms of fibronectin present in our HTM cultures as suggested by the immunofluorescence microscopy results described in panel A. At the end of the treatment period, cell monolayers were extracted with 1% DOC prior to processing for OCW analysis. *, significantly greater than EtOH-treated cells, p < 0.01; **, significantly less than DEX (100nM) treated cells without FUD, p < 0.05; ***, significantly less than DEX (500nM) treated cells without FUD, p < 0.01. Scale bars = 50 µm.
In the presence of FUD, both ethanol- and DEX-treated cells demonstrated visibly decreased fibronectin labeling. The decrease in EDA+ fibronectin in response to FUD was striking as it appears almost undetectable in cells treated with or without DEX. In contrast, some fibronectin is still readily detectable albeit at reduced levels, relative to cells not treated with FUD, with the polyclonal fibronectin antisera in the presence of FUD. This remaining fibronectin could represent either plasma fibronectin present in the tissue culture serum or another cellular fibronectin isoform expressed by HTM cells that lacks the EDA domain such as the EDB+ isoform. Another explanation is that the polyclonal antisera, which recognizes multiple epitopes, is simply more sensitive than the monoclonal antibody which detects a single epitope.
The DOC extraction procedure in conjunction with OCW analysis confirmed the observation that FUD effectively reduced a DEX-induced fibronectin matrix in HTM cultures (Figure 4B). In the absence of FUD, 100 nM and 500 nM DEX significantly increased the level of DOC-insoluble fibronectin over ethanol-treated cells 2.4-fold and 3.6-fold (p < 0.01), respectively. The addition of FUD during the treatment period effectively blocked this increase. FUD caused a 59% decrease in the DOC-insoluble fibronectin induced by 100 nM DEX (p < 0.05) and a 77% decrease in the DOC-insoluble fibronectin induced by 500 nM DEX (p < 0.01). FUD also decreased fibronectin matrix in vehicle-treated cells, however, post-hoc analysis of the ANOVA results found that this decrease was not statistically significant. Taken together, the data from these experiments shows that FUD is effective at blocking fibronectin fibril formation and matrix deposition under conditions when fibronectin expression and matrix deposition are induced.
3.3 FUD conditionally blocks assembly of other proteins into ECM
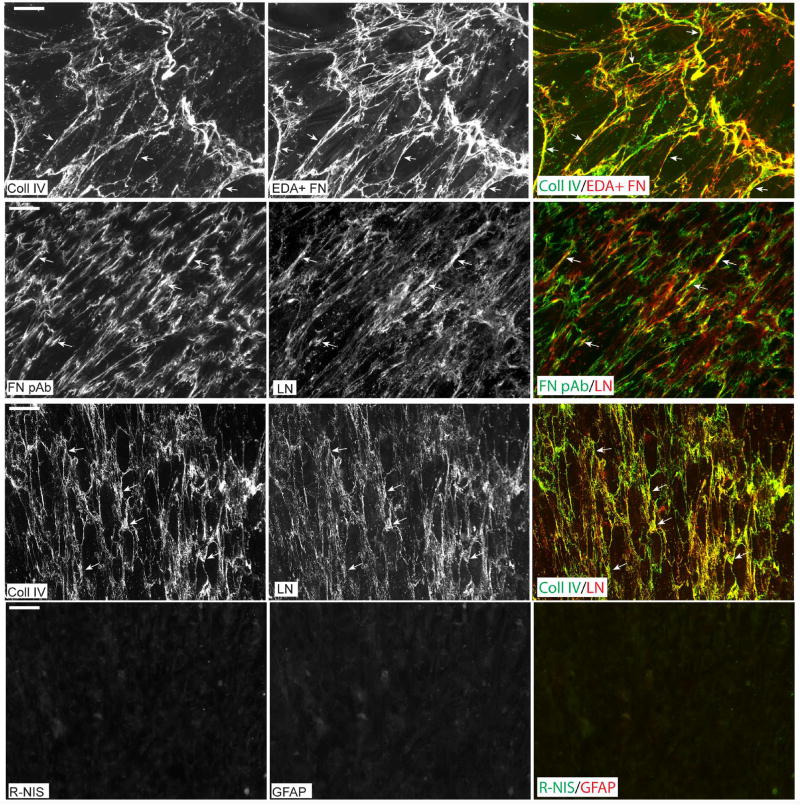
It is well established that fibronectin can interact with other ECM proteins such as fibrillin and types I and III collagen and regulate their incorporation into the ECM (Dallas et al., 2006; Li et al., 2003; Sabatier et al., 2009; Sottile and Hocking, 2002; Velling et al., 2002). In the TM other proteins found in the ECM include type IV collagen, laminin and fibrillin. As shown in Figure 5 (top row), there was extensive, but not complete, co-localization of fibronectin and type IV collagen in the ECM of control HTM cultures. A limited co-localization of fibronectin and laminin was also observed. Additionally, widespread co-localization of type IV collagen and laminin under the same culture conditions was observed which is consistent with reports that these two ECM proteins are associated together within the ECM (Pöschl et al., 2004). We did not look for fibrillin since it has been established that fibrillin and fibronectin co-localize in the ECM (Sabatier et al., 2009; Sabatier et al., 2013).
Figure 5. Co-localization of ECM proteins expressed by HTM cells.
HTM cells that had been maintained at confluence for 7 days were double-labeled for type IV collagen and EDA+ fibronectin (top row), fibronectin and laminin (2nd row) or type IV collagen and laminin (3rd row). Arrows indicate areas of co-localization for the different antibody pairs. Cultures were also double-labeled with rabbit non-immune serum and a mAb against glial fibrillary acidic protein (GFAP) which served as controls for antibody specificity (4th row). Cultures in row 1 were methanol-fixed while the other three rows show labeling in paraformaldehyde-fixed cells. Negative control labeling was also performed in methanol-fixed cells and showed similar results (not shown).
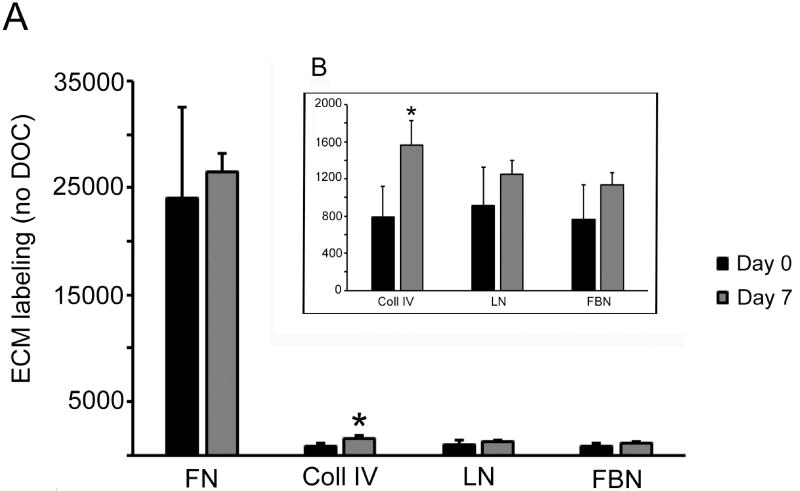
We then examined whether inhibiting fibronectin fibrillogenesis with FUD would also block the incorporation of type IV collagen, laminin, and fibrillin into the ECM. For these studies, we initially used HTM cultures that were undergoing “nascent” matrix formation where it was expected that type IV collagen, laminin and fibrillin levels would be low while fibronectin levels would be relatively high. We confirmed these expectations using OCW analysis to assess levels of fibronectin, type IV collagen, laminin and fibrillin in cultures of HTM cells that were newly confluent (day 0) or 7 days post-confluence (Figure 6). Cell layers were strongly positive for fibronectin at both day 0 and day 7, but the labeling intensity did not change over the seven day culture period. In contrast, the cell layers demonstrated relatively weak labeling for type IV collagen, laminin and fibrillin at both time points. Both the laminin and fibrillin labeling intensities appeared to increase over the seven day period (Figure 6B), however these increases were not statistically significant. In contrast, there was a statistically significant (p < 0.01), twofold increase in type IV collagen labeling at day 7 compared to day 0.
Figure 6. Relative labeling intensities of ECM proteins in newly confluent HTM cultures.
HTM cells were plated into 96 wells plates and allowed to reach confluence at which point they were either fixed with 4% paraformaldehyde and processed for OCW analysis (Day 0) or maintained in culture for 7 days prior to fixation and processing (Day 7). Cell monolayers were not extracted with DOC prior to OCW analysis. A) All four proteins on the same axis. B) Blow up of the type IV collagen, laminin and fibrillin data. *, type IV collagen significantly greater at day 7 relative to day 0, p < 0.01.
Using the same seven day time period, we then examined the effect of FUD on HTM cell cultures treated with or without DEX in the presence or absence of FUD starting on the day they reached confluence. As shown in Figure 7, FUD effectively disrupted the incorporation of all three proteins indicating that fibronectin fibrillogenesis is necessary for the incorporation of other proteins into the ECM of the TM under conditions of nascent matrix formation.
Figure 7. FUD disrupts newly forming matrices of type IV collagen, laminin and fibrillin.
Cells were grown to confluence at which time treatment with vehicle or 500 nM DEX in the presence of absence of 2 µM FUD was initiated. Cells were treated for 7 days prior to fixation and labeling for type IV collagen, laminin or fibrillin. Scale bars = 50 µm.
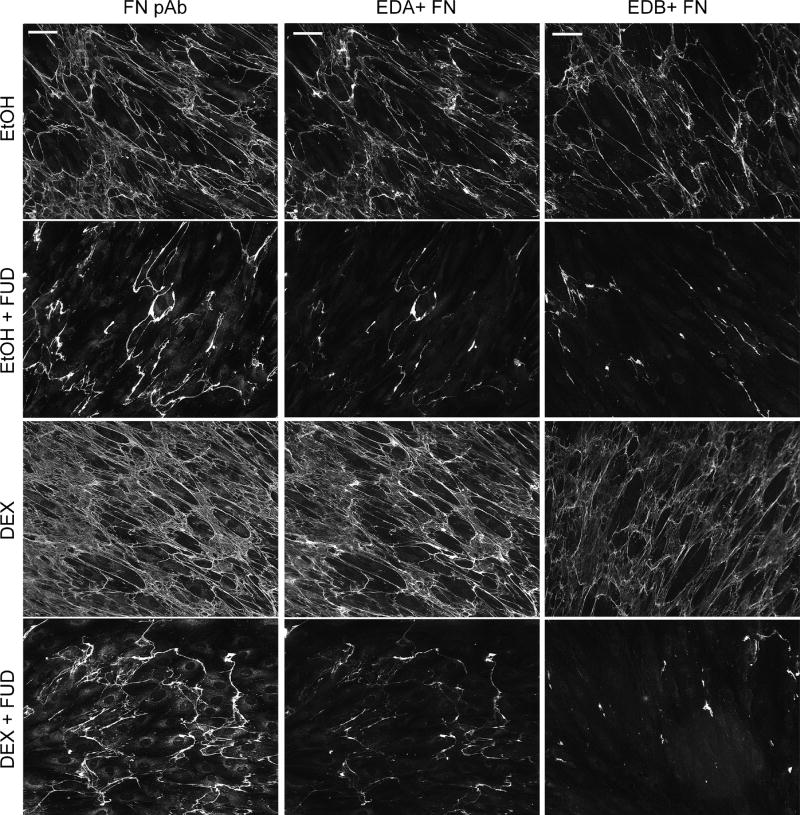
As expected, FUD also disrupted fibronectin fibril formation under these same culture conditions (Figure 8). Since our other studies indicated that other cellular fibronectin isoforms lacking the EDA domain were expressed by HTM cells (Figure 4A), we also looked for the presence of the EDB+ isoform of cellular fibronectin using mAb BC-1 (Carnemolla et al., 1989) in separate experiments. As expected after 7 days in culture, EtOH- and DEX-treated cells assembled a fibronectin matrix that consisted of a mixture of both the EDA+ and EDB+ fibronectin isoforms and both isoforms demonstrated a clear increase in labeling intensity following DEX treatment. In contrast to what we observed for the EDA isoform, however, we observed only partial co-localization of the fibronectin antisera and mAb BC-1 against EDB+ fibronectin suggesting that HTM cells express lower levels of this isoform (Figure 9).
Figure 8. HTM express EDA+ and EDB+ fibronectin isoforms both of which are susceptible to disruption by FUD.
Cells were grown to confluence at which time treatment with vehicle or 500 nM DEX in the presence of absence of 2 µM FUD was initiated. Cells were treated for 7 days prior to fixation and labeling with fibronectin polyclonal antisera (FN pAb), mAb IST-9 against EDA+ fibronectin or mAb BC-1 against EDB+ fibronectin. Scale bars = 50 µm.
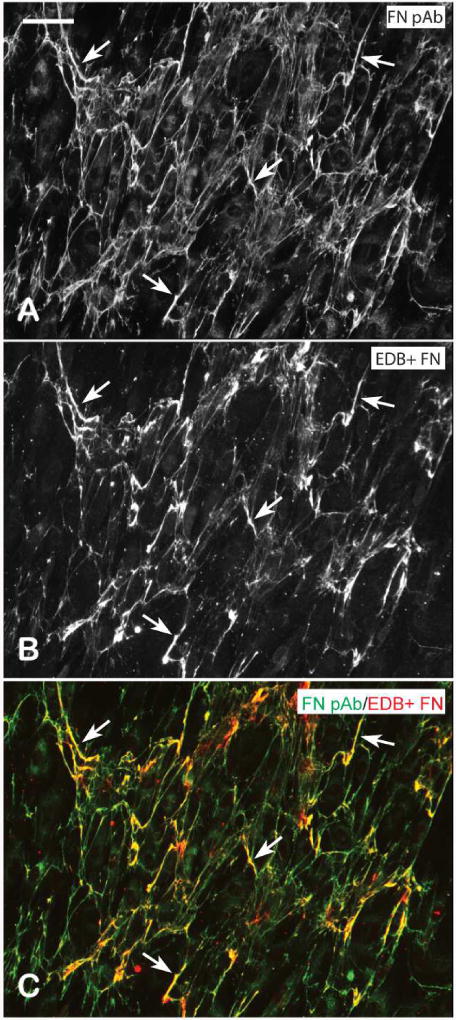
Figure 9. Fibronectin fibrils consist of a mixture of EDB+ and EDB- fibronectin isoforms.
Confluent HTM cells were fixed and labeled with fibronectin polyclonal antisera (A) and mAb BC-1 against EDB+ fibronectin (B). The merged images (C) show select regions of co-localization of the two antibodies (arrowheads). Scale bar = 50 um.
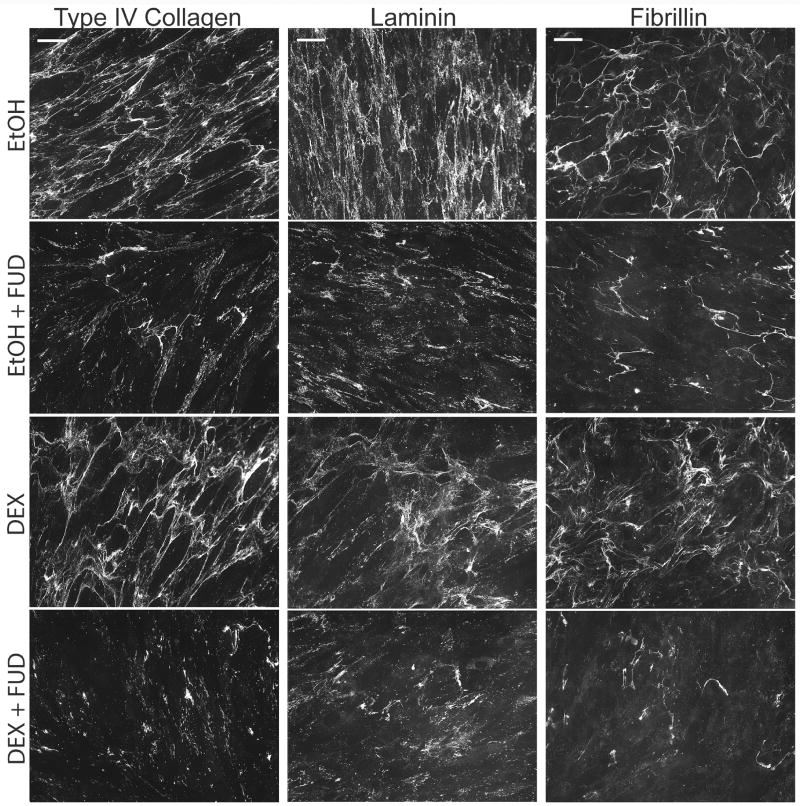
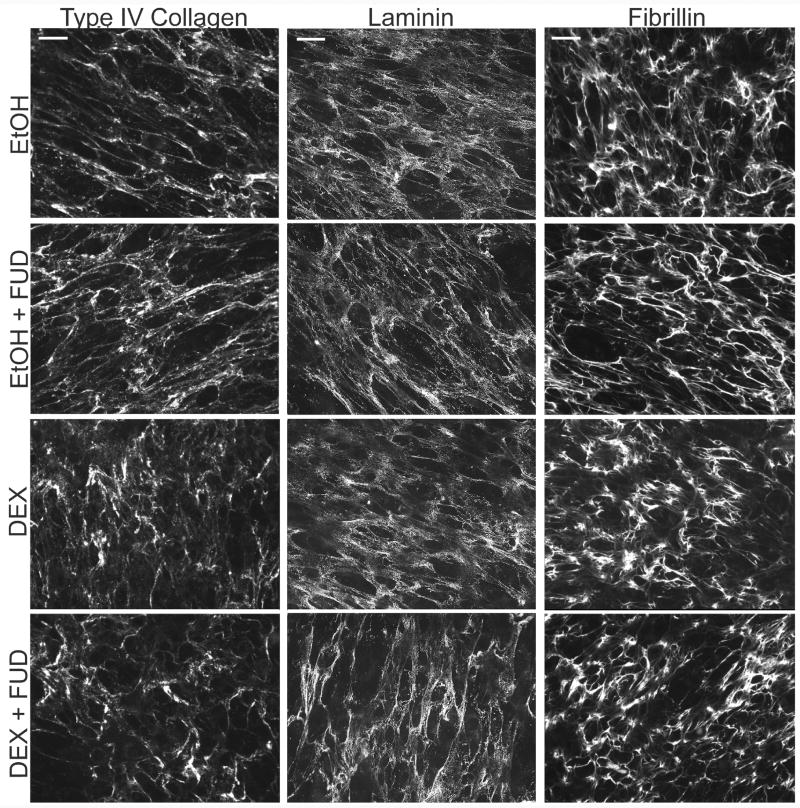
We then repeated the experiments to determine the effects of FUD on these proteins in “mature” matrices (Figure 10). For this study cells were allowed to form their matrix for 1 week before the addition of DEX and/or FUD similar to conditions described for Figure 4. Under these conditions, HTM cells formed an extensive, well developed matrix of fibronectin, type IV collagen, laminin and other ECM proteins as previously reported (Filla et al., 2004; Filla et al., 2002; Kurosawa et al., 1987; Yun et al., 1989). Under these conditions, however, FUD had no effect on the matrices of these three other ECM proteins regardless of the presence or absence of steroid treatment and despite the marked decrease in fibronectin fibrils caused by treatment with FUD (see Figure 4). Together these data suggest that disrupting fibronectin fibril formation only affects the incorporation of other proteins into newly forming matrices but not mature matrices which is consistent with an earlier report by Sabatier, et al. (Sabatier et al., 2013).
Figure 10. FUD has no effect on type IV collagen, laminin or fibrillin matrices when added to cells with a mature pre-formed ECM.
Cells were grown to confluence and then allowed to form a mature ECM for 7 days prior to initiating treatment with vehicle or 500 nM DEX in the presence of absence of 2 µM FUD. Cells were treated for 12 days prior to fixation and labeling for type IV collagen, laminin or fibrillin. Scale bars = 50 µm.
4. Discussion
In this paper we have used a fragment of the S. pyogenes F1 adhesin protein that binds to the amino terminus of fibronectin to show that by blocking fibronectin-fibronectin interactions, fibronectin fibrillogenesis and the deposition of other ECM proteins in HTM cells can be controlled. The decrease in fibronectin matrix in response to FUD appears to be consistent with the mechanism of action previously demonstrated for FUD in fibroblast cultures (Maurer et al., 2010; Tomasini-Johansson et al., 2001) and suggests that the step-wise mechanisms involved in the assembly of fibronectin into the matrix is the same in both fibroblast cultures and trabecular meshwork cultures.
These studies have also shown that fibronectin fibrils can participate in the incorporation of other proteins, such as fibrillin, laminin and type IV collagen, into the ECM provided that matrices of these proteins did not previously exist. FUD was also very effective at disrupting fibronectin fibrillogenesis under conditions of enhanced fibronectin expression and matrix deposition in the presence of steroid treatment which suggests that it may represent an effective approach to control ECM deposition in glaucoma or following steroid treatments.
In addition to blocking nascent fibronectin fibril formation in newly confluent HTM cultures, FUD was very effective at disrupting mature fibronectin matrices in cultures with a pre-existing ECM. With respect to the effects of FUD on mature fibronectin matrices, the presence of FUD in these cultures appeared to remove virtually all cellular fibronectin fibrils from these cultures. Since the mature cultures would have had time to assemble fibronectin fibrils prior to the addition of FUD, this suggests that FUD not only blocked de novo fibronectin fibrillogenesis, but it also promoted the removal of fibronectin fibrils that would have existed prior to the start of FUD treatment. This is consistent with a previous report observed in fibroblasts cultures treated with FUD (Sabatier et al., 2013).
This study also shows that human TM cells in culture normally express both the EDA+ and EDB+ fibronectin isoforms and that both isoforms coexist within the same fibrils which is consistent with an earlier report (Pesciotta Peters et al., 1990). There appeared to be a nearly 100% co-localization of the IST-9 antibody with a polyclonal antiserum that recognizes all forms of fibronectin, including plasma fibronectin, suggesting that most of the detectable fibronectin in these cultures is the EDA+ isoform. In contrast, the EDB+ fibronectin localization suggests that this cellular isoform is not as prevalent in HTM cultures as the EDA+ isoform. This is in contrast to an earlier report (Medina-Ortiz et al., 2013) that EDA+ fibronectin may be induced in response to TGFβ2 expression since very little detectable EDA+ fibronectin was found in the absence of TGFβ2 treatment. The reason for this is unclear, but one possible explanation may be the maturity of the HTM cultures. The Medina-Ortiz study appeared to use sub-confluent, proliferating cultures whereas this study used confluent quiescent cultures similar to that observed in vivo (Polansky et al., 1979; Polansky et al., 1984). Finally, this study showed that both isoforms of fibronectin can also be upregulated by DEX.
The requirement of fibronectin fibrils for the assembly of other proteins into the ECM is not unique to the TM. In fibroblast cultures, type I collagen fibril formation has been found to use fibronectin fibrils since their incorporation into the ECM can be inhibited by blocking interactions between fibronectin-collagen reciprocal binding sites (Dzamba et al., 1993; McDonald et al., 1982). Likewise, fibrillin microfibril formation can be prevented by inhibiting fibrillin binding to the amino terminal domain of fibronectin (Sabatier et al., 2009; Sabatier et al., 2013) or by preventing fibronectin binding to the α5β1 integrin which is a cell surface receptor known to be important in fibronectin fibrillogenesis (Kinsey et al., 2008). Since collagen and fibrillin are capable of self-assembling into a fibril (Davis and Summers, 2012; Mouw et al., 2014) and fibronectin is deposited early into the ECM both in vivo and in vitro before collagen or fibrillin is detected (Hynes, 1990; Papadimitriou et al., 1993; Sabatier et al., 2009; Velling et al., 2002), it has been proposed that fibronectin orchestrates the formation of the ECM by acting as a nidus or organizing site for type I collagen and fibrillin fibril formation, by increasing the local concentration of collagen and fibrillin monomers (Kadler et al., 2008).
Although previous studies have indicated that fibronectin fibrils are needed for collagen types I and III fibrillogenesis and the incorporation of fibrillin into microfibrils, this is the first report finding that fibronectin fibrils may also be involved in the deposition of the type IV collagen/laminin network. The TM is somewhat unique compared to other tissues in that fibronectin, in addition to type IV collagen, laminin, fibrillin and other components (Yurchenco, 2011), is part of the ECM that makes up the TM basement membrane (Hann et al., 2001; Murphy et al., 1987). Since fibronectin is part of this basement ECM, this would suggest that fibronectin could play a role in regulating a type IV collagen/laminin network. The type IV collagen/laminin network is thought to form upon the cell surface starting with laminin sheets that self-assemble and bind to cell surface receptors. The laminin sheets then bind to a scaffold of pre-formed type IV collagen fibrillar networks (Mouw et al., 2014). Thus, it is not clear how fibronectin fibrils would promote this type IV collagen/laminin network formation, since neither laminin nor type IV collagen have been reported to have a fibronectin binding site. However, recent studies support our findings and have shown that fibronectin fibrils may play a role in organizing collagen IV networks, since linearly arranged native type IV collagen co-localized with fibronectin fibrils in endothelial cell (Sylman et al., 2015) or fibroblast (Coelho et al., 2013) cultures. In addition, some co-dependence was observed in Schwann cells where the assembly of fibronectin fibrils was dependent on the presence of native type IV collagen molecules (Chernousov et al., 1998). Thus, one possibility is that by blocking fibronectin fibrillogenesis with FUD, the type IV collagen network fails to form which then indirectly impacts the stability of the laminin sheets bound to the cell surface.
Our studies, however, found that the effect of FUD on ECM proteins other than fibronectin was conditional. Under conditions where HTM cultures were allowed to form a mature ECM, FUD was still effective in disrupting fibronectin matrices (see Figure 4), however, the addition of FUD had no effect on pre-existing matrices of type IV collagen, laminin and fibrillin. Thus, it appears that in HTM cells, fibronectin fibrillogenesis is only needed for the de novo synthesis of fibrillin microfibrils and the type IV collagen/laminin network. This also suggests that, in vivo, FUD would not disrupt these existing matrices and would only prevent the formation of any excess matrix such as might be present under glaucomatous conditions or where a patient was undergoing steroid treatment to prevent ocular inflammation.
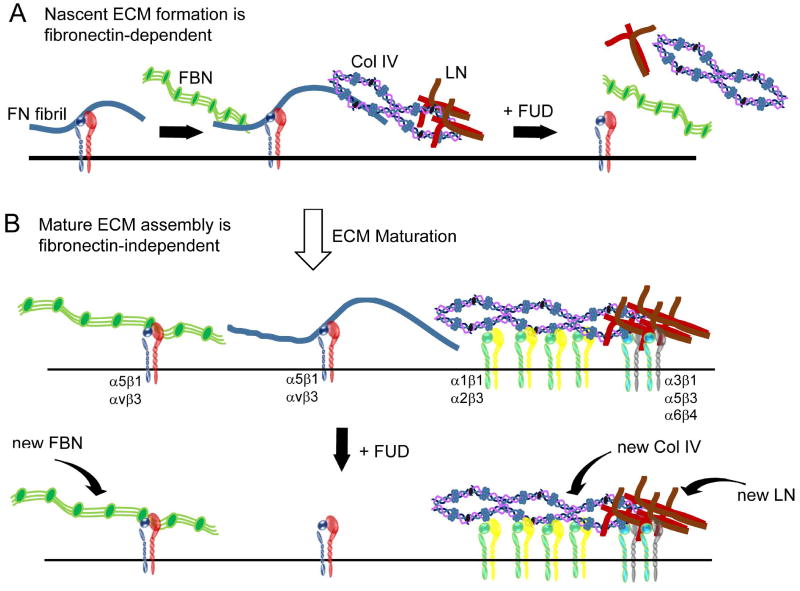
Taken together, these data suggest that there is a step-wise assembly of type IV collagen and laminin proteins into the ECM similar to that previously reported for fibrillin (Sabatier et al., 2009; Sabatier et al., 2013). In this scenario (Figure 11), nascent matrices of laminin, type IV collagen and/or fibrillin are initially deposited in the ECM along pre-existing fibronectin fibrils. Removal of these fibronectin fibrils disrupts their assembly into larger macromolecular structures within the ECM. Once the ECM becomes more established, however, these other proteins do not require pre-existing fibronectin fibrils and can be assembled into large macromolecular structures regardless of the presence or absence of fibronectin. Presumably, this is because a critical concentration of these proteins now exists on the cell surface and the proteins can self-assemble into their unique structures. Hence, any additional laminin, type IV collagen or fibrillin produced would be directly incorporated into those structures without the requirement for pre-existing fibronectin fibrils.
Figure 11. Schematic illustrating step-wise assembly of the ECM in which fibronectin fibrils initially act as organizing centers.
A) In nascent matrices, fibronectin (FN) fibrils act as a scaffold upon which other macromolecular structures are assembled. Disruption of fibronectin fibrillogenesis by FUD blocks the assembly of these other nascent protein structures. B) Addition of FUD to the mature ECM triggers the removal of existing fibronectin matrices and prevents the assembly of any new fibronectin fibrils. Disruption of fibronectin fibrillogenesis by FUD under these conditions, however, does not affect laminin (LN), fibrillin (FBN) and type IV collagen (Col IV). These proteins are in macromolecular structures that now exist independently of fibronectin fibrils and are likely stabilized by their attachment to their own cell surface receptors which can include different integrins. Any newly secreted FBN, LN or Col IV (curved arrows) in these mature cultures presumably would be incorporated directly into each protein’s existing macromolecular structure.
In summary, the data presented here suggests that the fibronectin matrix within the TM can be targeted for disruption by peptides or peptidomimetics. This presents an opportunity for novel therapeutics to be designed that could aid in the treatment of POAG, SIG and possibly other glaucomas where excessive ECM deposition/accumulation is part of the pathogenesis of the disease regardless of the underlying cause. There is precedence for this approach elsewhere. FUD, for example, has been found to be effective in reducing fibrosis in an experimental liver fibrosis model disease (Altrock et al., 2015).
Highlights.
Fibronectin binding peptide, FUD, controls fibronectin fibrillogenesis.
Blocking fibronectin fibrillogenesis causes removal of existing fibronectin fibrils.
Dexamethasone increases the production of EDA+ and EDB+ fibronectin isoforms.
FUD disrupts dexamethasone-induced increases in fibronectin matrix deposition.
Fibronectin controls the deposition of collagen IV, laminin and fibrillin matrices.
Acknowledgments
Funding: This work was supported by NEI grants EY026009, EY017006, a Brightfocus Grant (to D.M.P.) and a Core grant to the Department of Ophthalmology and Visual Sciences (P30 EY016665)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Acott TS, Kelley MJ. Extracellular matrix in the trabecular meshwork. Exp. Eye Res. 2008;86:543–561. doi: 10.1016/j.exer.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre KM, McCormick RJ, Schwarzbaue JE. Fibronectin self-association is mediated by complementary sites within the amino-terminal one-third of the molecule. J. Biol. Chem. 1994;269:27863–27868. [PubMed] [Google Scholar]
- Altrock E, Sens C, Wuerfel C, Vasel M, Kawelke N, Dooley S, Sottile J, Nakchbandi IA. Inhibition of fibronectin deposition improves experimental liver fibrosis. J. Hepatol. 2015;62:625–633. doi: 10.1016/j.jhep.2014.06.010. [DOI] [PubMed] [Google Scholar]
- Babizhayev MA, Brodskaya MW. Fibronectin detection in drainage outflow system of human eyes in ageing and progression of open-angle glaucoma. Mech. Ageing Dev. 1989;47:145–157. doi: 10.1016/0047-6374(89)90017-1. [DOI] [PubMed] [Google Scholar]
- Braunger BM, Fuchshofer R, Tamm ER. The aqueous humor outflow pathways in glaucoma: a unifying concept of disease mechanisms and causative treatment. Eur. J. Pharmaceut. Biopharmaceut. 2015;95:173–181. doi: 10.1016/j.ejpb.2015.04.029. [DOI] [PubMed] [Google Scholar]
- Bultmann H, Santas AJ, Peters DM. Fibronectin fibrillogenesis involves the heparin II binding domain of fibronectin. J. Biol. Chem. 1998;273:2601–2609. doi: 10.1074/jbc.273.5.2601. [DOI] [PubMed] [Google Scholar]
- Carnemolla B, Baiza E, Siri A, Zardi L, Nicotra MR, Bigotti A, Natali PG. A tumor-associated fibronectin isoform generated by alternative splicing of messenger RNA precursors. J. Cell Biol. 1989;108:1139–1148. doi: 10.1083/jcb.108.3.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnemolla B, Leprini A, Querze G, Urbini S, Zardi L. Novel self-association fibronectin sites. Biochem. Cell Biol. 1996;74:745–748. doi: 10.1139/o96-081. [DOI] [PubMed] [Google Scholar]
- Chernousov MA, Stahl RC, Carey DJ. Schwann Cells Use a Novel Collagen-Dependent Mechanism For Fibronectin Fibril Assembly. J. Cell Sci. 1998;111:2763–2777. doi: 10.1242/jcs.111.18.2763. [DOI] [PubMed] [Google Scholar]
- Coelho NM, Salmerón-Sánchez M, Altankov G. Fibroblasts remodeling of type IV collagen at a biomaterials interface. Biomater. Sci. 2013;1:494–502. doi: 10.1039/c3bm00163f. [DOI] [PubMed] [Google Scholar]
- Dallas SL, Chen Q, Sivakumar P. Dynamics of assembly and reorganization of extracellular matrix proteins. Curr. Top. Devel. Biol. 2006;75:1–24. doi: 10.1016/S0070-2153(06)75001-3. [DOI] [PubMed] [Google Scholar]
- Dallas SL, Sivakumar P, Jones CJP, Chen Q, Peters DM, Mosher DF, Humphries MJ, Kielty CM. Fibronectin regulates latent transforming growth factor β (TGFβ) by controlling matrix assembly of latent TGFβ-binding protein-1. J. Biol. Chem. 2005;280:18871–80. doi: 10.1074/jbc.M410762200. [DOI] [PubMed] [Google Scholar]
- Davis MR, Summers KM. Structure and function of the mammalian fbrillin gene family: implications for human connective tissue diseases. Molec. Genetics Metab. 2012;107:635–47. doi: 10.1016/j.ymgme.2012.07.023. [DOI] [PubMed] [Google Scholar]
- Dzamba BJ, Wu H, Jaenisch R, Peters DM. Fibronectin binding site in type I collagen regulates fibronectin fibril formation. J. Cell Biol. 1993;121:1165–1172. doi: 10.1083/jcb.121.5.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faralli JA, Schwinn MK, Gonzalez JJM, Filla MS, Peters DM. Functional properties of fibronectin in the trabecular meshwork. Exp. Eye Res. 2009;88:689–693. doi: 10.1016/j.exer.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filla MS, David G, Weinreb RN, Kaufman PL, Peters DP. Distribution of Syndecans 1–4 Within the Anterior Segment of the Human Eye: Expression of a variant syndecan-3 and matrix associated syndecan-2. Exp. Eye Res. 2004;79:61–74. doi: 10.1016/j.exer.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Filla MS, Lui X, Nguyen TD, Polansky JR, Brandt BR, Kaufman PL, Peters DM. In vitro localization of TIGR/MYOC in trabecular meshwork extracellular matrix and binding to fibronectin. Invest. Ophthalmol. Vis. Sci. 2002;43:151–161. [PubMed] [Google Scholar]
- Floyd BB, Cleveland PH, Worthen DM. Fibronectin in human trabecular drainage channels. Invest. Ophthalmol. Vis. Sci. 1985;26:797–804. [PubMed] [Google Scholar]
- Fogelgren B, Polgár N, Szauter KM, Újfaludi Z, Laczkó R, Fong KSK, Csiszar K. Cellular fibronectin binds to lysyl oxidase with high affinity and is critical for its proteolytic activation. J. Biol. Chem. 2005;280:24690–24697. doi: 10.1074/jbc.M412979200. [DOI] [PubMed] [Google Scholar]
- Fuchshofer R, Tamm ER. The role of TGF-β in the pathogenesis of primary open-angle glaucoma. Cell Tissue Res. 2011;347:279–290. doi: 10.1007/s00441-011-1274-7. [DOI] [PubMed] [Google Scholar]
- Gagen D, Faralli JA, Filla MS, Peters DM. The role of integrins in the trabecular meshwork. J. Ocul. Pharmcol. Ther. 2014;30:110–120. doi: 10.1089/jop.2013.0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geberhiwot T, Wondimu Z, Salob S, Pikkarainenc T, Kortesmaac J, Tryggvasonc K, Virtanend I, Patarroyo M. Chain specificity assignment of monoclonal antibodies to human laminins by using recombinant laminin β1 and γ1 chains. Matrix Biol. 2000;19:163–167. doi: 10.1016/s0945-053x(00)00056-1. [DOI] [PubMed] [Google Scholar]
- Hann CR, Springett MJ, Wang X, Johnson DH. Ultrastructural localization of collagen IV, fibronectin, and laminin in the trabecular meshwork of normal and glaucomatous eyes. Ophthalmic. Res. 2001;33:314–324. doi: 10.1159/000055687. [DOI] [PubMed] [Google Scholar]
- Hocking DC, Sottile J, McKeown-Longo PJ. Fibronectin’s III-1 module contains a conformation-dependent binding site for the amino-terminal region of fibronectin. J Biol Chem. 1994;269:19183–19187. [PubMed] [Google Scholar]
- Hynes RO. Fibronectins. Springer-Verlag; New York: 1990. [Google Scholar]
- Ingham KC, Brew SA, Huff S, Litvinovich SV. Cryptic self-association sites in type III Modules of fibronectin. J. Biol. Chem. 1997;272:1718–1724. doi: 10.1074/jbc.272.3.1718. [DOI] [PubMed] [Google Scholar]
- Kadler KE, Hill A, Canty-Laird EG. Collagen fibrillogenesis: fibronectin, integrins, and minor collagens as organizers and nucleators. Curr. Opin. Cell Biol. 2008;20:495–501. doi: 10.1016/j.ceb.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller KE, Aga M, Bradley JM, Kelley MJ, Acott TS. Extracellular matrix turnover and outflow resistance. Exp. Eye Res. 2009;88:676–682. doi: 10.1016/j.exer.2008.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey R, Williamson MR, Chaudhry S, Mellody KT, McGovern A, Takahashi S, Shuttleworth CA, Kielty CM. Fibrillin-1 microfibril deposition is dependent on fibronectin assembly. J. Cell Sci. 2008;121:2696–2704. doi: 10.1242/jcs.029819. [DOI] [PubMed] [Google Scholar]
- Kurosawa A, Elner VM, Yue BY, Elvart JL, Tso MO. Cultured trabecular-meshwork cells: immunohistochemical and lectin-binding characteristics. Exp. Eye Res. 1987;45:239–251. doi: 10.1016/s0014-4835(87)80147-1. [DOI] [PubMed] [Google Scholar]
- Li S, Van Den Diepstraten C, D’Souza SJ, Chan BMC, Pickering JG. Vascular smooth muscle cells orchestrate the assembly of type I collagen via α2β1 integrin, RhoA, and fibronectin polymerization. Am. J. Pathol. 2003;163:1045–1056. doi: 10.1016/s0002-9440(10)63464-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Ma H, Mosher DF. On-off kinetics of engagement of FNI modules of soluble fibronectin by β-strand addition. PLOS one. 2015;10:1–17. doi: 10.1371/journal.pone.0124941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maqueda A, Moyano JV, Hernández del Cerro M, Peters DM, Garcia-Pardo A. The heparin III-binding domain of fibronectin (III4-5 repeats) binds to fibronectin and inhibits fibronectin matrix assembly. Matrix Biol. 2007;26:642–651. doi: 10.1016/j.matbio.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Maurer LM, Tomasini-Johansson BR, Ma W, Annis DS, Eickstaedt NL, Ensenberger MG, Satyshur KA, Mosher DF. Extended binding site on fibronectin for the functional upstream domain of protein F1 of Streptococcus pyogenes. J. Biol. Chem. 2010;285:41087–41099. doi: 10.1074/jbc.M110.153692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JA, Kelley DG, Broekelmann TJ. Role of fibronectin in collagen deposition : Fab’ to the gelatin-binding domain of fibronectin inhibits both fibronectin and collagen organization in fibroblast extracellular matrix. J. Cell Biol. 1982;92:485–492. doi: 10.1083/jcb.92.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown-Longo PJ, Mosher DF. Binding of plasma fibronectin to cell layers of human skin fibroblasts. J. Cell Biol. 1983;98:22–28. doi: 10.1083/jcb.97.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown-Longo PJ, Mosher DF. Interaction of the 70,000-mol-wt amino-terminal fragment of fibronectin with the matrix-assembly receptor of fibroblasts. J. Cell Biol. 1985;100:364–374. doi: 10.1083/jcb.100.2.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina-Ortiz WE, Belmares R, Neubauer S, Wordinger RJ, Clark AF. Cellular fibronectin expression in human trabecular meshwork and induction by transforming growth factor-β2. Invest. Ophthalmol. Vis. Sci. 2013;54:6779–6788. doi: 10.1167/iovs.13-12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouw JK, Ou G, Weaver VM. Extracellular matrix assembly: a multiscale deconstruction. Nat. Rev. Molec. Cell Biol. 2014;15:771–785. doi: 10.1038/nrm3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CG, Yun AJ, Newsome DA, Alvarado JA. Localization of extracellular proteins of the human trabecular meshwork by indirect immunofluorescence. Am. J. Ophthalmol. 1987;104:33–43. doi: 10.1016/0002-9394(87)90290-x. [DOI] [PubMed] [Google Scholar]
- Ozeri V, Tovi A, Burstein I, Natanson-Yaron S, Caparon MG, Yamada KM, Akiyama SK, Vlodavsky I, Hanskii E. A two-domain mechanism for group A streptococcal adherence through protein F to the extracellular matrix. EMBO J. 1996;15:989–998. [PMC free article] [PubMed] [Google Scholar]
- Papadimitriou ERUB, Maragoudakis ME, Lelke PI. Time course and quantification of extracellular matrix maturation in the chick chorioallantoic membrane and in cultured endothelial cells. Endothelium. 1993;1:207–219. [Google Scholar]
- Pattabiraman PP, Toris CB. The exit strategy: pharmacological modulation of extracellular matrix production and deposition for better aqueous humar drainage. Eur. J. Pharmacol. 2016;787:32–42. doi: 10.1016/j.ejphar.2016.04.048. [DOI] [PubMed] [Google Scholar]
- Pesciotta Peters DM, Portz LM, Fullenwider J, Mosher DF. Co-assembly of plasma and cellular fibronectins into fibrils in human fibroblast cultures. J. Cell Biol. 1990;111:249–256. doi: 10.1083/jcb.111.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polansky JR, Weinreb R, Alvarado JA. Studies on human trabecular cells propagated in vitro. Vis. Res. 1981;21:155–160. doi: 10.1016/0042-6989(81)90151-6. [DOI] [PubMed] [Google Scholar]
- Polansky JR, Weinreb RN, Baxter JD, Alvarado J. Human trabecular cells. I. Establishment in tissue culture and growth characteristics. Invest. Ophthalmol. Vis. Sci. 1979;18:1043–1049. [PubMed] [Google Scholar]
- Polansky JR, Wood IS, Maglio MT, Alvarado JA. Trabecular meshwork cell culture in glaucoma research: evaluation of biological activity and structural properties of human trabecular cells in vitro. Ophthalmol. 1984;91:580–595. doi: 10.1016/s0161-6420(84)34241-5. [DOI] [PubMed] [Google Scholar]
- Pöschl E, Schlötzer-Schrehardt U, Brachvogel B, Saito K, Ninomiya Y, Mayer U. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development. 2004;131:1619–1628. doi: 10.1242/dev.01037. [DOI] [PubMed] [Google Scholar]
- Quigley HA. Glaucoma. Lancet. 2011;377:1367–1377. doi: 10.1016/S0140-6736(10)61423-7. [DOI] [PubMed] [Google Scholar]
- Sabatier L, Chen D, Fagotto-Kaufmann C, Hubmacher D, McKee MD, Annis DS, Mosher DF, Reinhardt DP. Fibrillin assembly requires fibronectin. Molec. Biol. Cell. 2009;20:846–858. doi: 10.1091/mbc.E08-08-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier L, Djokic J, Fagotto-Kaufmann C, Chen M, Annis DS, Mosher DF, Reinhardt DP. Complex contributions of fibronectin to initiation and maturation of microfibrils. Biochem. J. 2013;456:283–295. doi: 10.1042/BJ20130699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzbauer JE, DeSimone DW. Fibronectin, their fibrillogenesis, and in vivo functions. Cold Spring Harb. Perspect. Biol. 2011;3:1–19. doi: 10.1101/cshperspect.a005041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P, Carraher C, Schwarzbauer JE. Assembly of fibronectin extracellular matrix. Annu. Rev. Cell Dev. Biol. 2010;26:397–419. doi: 10.1146/annurev-cellbio-100109-104020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottile J, Hocking DC. Fibronectin polymerization regulates the composition and stability of extracellular matrix fibrils and cell-matrix adhesions. Molec. Biol. Cell. 2002;13:3546–3559. doi: 10.1091/mbc.E02-01-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottile J, Schwarzbauer JE, Selegue J, Mosher DF. Five type I modules of fibronectin form a functional unit that binds to fibroblasts and Staphylococcus aureus. J. Biol. Chem. 1991;266:12840–12843. [PubMed] [Google Scholar]
- Steely HT, Browder SL, Julian MB, Miggans ST, Wilson KL, Clark AF. The effects of dexamethasone on fibronectin expression in cultured human trabecular meshwork cells. Invest. Ophthalmol. Vis. Sci. 1992;33:2242–2250. [PubMed] [Google Scholar]
- Sylman JL, Artzer DT, Ranaa K, Neeves KB. A vascular injury model using focal heat-induced activation of endothelial cells. Integr. Biol. 2015;7:801–814. doi: 10.1039/c5ib00108k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasini-Johansson BR, Kaufman NR, Ensenberger MG, Ozerii V, Hanskii E, Mosher DF. A 49-residue peptide from adhesin F1 of streptococcus pyogenes inhibits fibronectin matrix assembly. J. Biol. Chem. 2001;276:23430–23439. doi: 10.1074/jbc.M103467200. [DOI] [PubMed] [Google Scholar]
- Velling T, Risteli J, Wennerberg K, Mosher DF, Johansson S. Polymerization of type I and III collagens is dependent on fibronectin and enhanced by integrins alpha 11 beta 1 and alpha 2 beta 1. J. Biol. Chem. 2002;277:37377–37381. doi: 10.1074/jbc.M206286200. [DOI] [PubMed] [Google Scholar]
- Wu M, Zhu X-Y, Ye J. Associations of polymorphisms of LOXL1 gene with primary open-angle glaucoma: a meta-analysis based on 5,293 subjects. Molec. Vis. 2015;21:165–172. [PMC free article] [PubMed] [Google Scholar]
- Yun AJ, Murphy CG, Polansky JR. Proteins secreted by human trabecular cells: glucocorticord and other effects. Invest. Ophthalmol. Vis. Sci. 1989;30:2012–2022. [PubMed] [Google Scholar]
- Yurchenco PD. Basement membranes: cell scaffoldings and signaling platforms. Cold Spring Harb. Perspect. Biol. 2011;3:a004911. doi: 10.1101/cshperspect.a004911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou LL, Li YH, Yue B. Glucocorticoid Effects On Extracellular Matrix Proteins and Integrins in Bovine Trabecular Meshwork Cells in Relation to Glaucoma. Int. J. Mol. Med. 1998;1:339–346. [PubMed] [Google Scholar]