Abstract
BACKGROUND
Since plasma oxalate (POx) concentrations increase at lower glomerular filtration rate (GFR) levels, even among those without enteric (EH) or primary hyperoxaluria (PH), the appropriate thresholds for considering a disorder of oxalate metabolism are poorly defined. The current study was completed to establish relationships between POx, GFR, and urine oxalate excretion (UOx) among patients with PH, EH, and routine urinary stone disease (USD).
METHODS
The most recent POx measurement on all Mayo Clinic patients between 2005–2015 were electronically pulled from the Lab Information System together with the closest serum creatinine within 14 days and 24 hr urine study within 60 days. After exclusion of patients not in steady state at the time of blood draw, 270 patients were available for study. Records were reviewed for clinical diagnoses to categorize patients as PH, EH, or USD. Waste plasma for Pox was also obtained from controls without USD undergoing clinical GFR testing.
RESULTS
In all 3 groups POx increased as eGFR fell. For any given eGFR, POx was highest in the PH group and lowest in the USD and control groups (p<0.0001). POx was also influenced by UOx excretion (reflecting total body oxalate burden, absorption from diet and endogenous production). Generalized estimating equations of POx vs eGFR revealed higher average POx levels in PH compared to EH,USD or control, and for EH compared to USD or control. GEE prediction models were created that use POx, UOx, age, and serum creatinine to estimate the probability of a PH diagnosis.
CONCLUSIONS
New models were developed to help interpret POx when considering PH in clinical practice even when it was not previously suspected and/or eGFR is reduced.
Keywords: calcium oxalate, enteric hyperoxaluria, glomerular filtration rate, plasma oxalate, primary hyperoxaluria
INTRODUCTION
Primary hyperoxaluria (PH) is a rare group of genetic diseases caused by defects in specific enzymes that lead to overproduction of oxalate, and can be caused by mutations in one of three genes (1). PH1 is the most severe form and has the highest urinary oxalate (UOx) levels and most severe symptoms, accounting for up to 80% of cases (2, 3). Enteric hyperoxaluria (EH) is associated with gastrointestinal disorders that cause fat malabsorption, which in turn leads to over-absorption of oxalate from food (4). Potential causes of EH include Roux-en-Y gastric bypass for obesity, inflammatory bowel disease, and chronic pancreatitis (3). In all forms of PH and EH, the excess oxalate load is primarily excreted by the kidney. Thus, a common complication is calcium oxalate kidney stones. Patients can also develop chronic kidney damage from the renal response to calcium oxalate crystals and/or oxalate ion. Once glomerular filtration rate (GFR) falls below critical levels, oxalate elimination no longer equals its production and absorption and deposition in organs such as bone and heart can ensue, a condition termed oxalosis (5).
Among patients with normal kidney function, a measurement of 24-hour urinary oxalate excretion is usually considered the most helpful screen for PH and/or EH (1). However, among patients with stage 4 or especially stage 5 CKD the diagnosis of PH can become challenging. (1). However, since POx concentrations increase at lower GFR levels, even in persons without EH or PH (6), (7), the question often arises: “Is this POx value high enough to make the diagnosis of PH a possibility?”. Thus, the aim of the current study was to establish relationships between POx, GFR, and oxalate load (as reflected by UOx) in cohorts of patients with PH, EH, routine urinary stone disease (USD), and controls with a range of GFR but no known USD or oxalate disorder.
METHODS
This study was approved by the Mayo Clinic Institutional Review Board.
Data was obtained from the Rare Kidney Stone Consortium (RKSC) PH registry (8) and Mayo Clinic lab information system (LIS). Urine and plasma oxalate were both measured in the Mayo Clinic Renal Testing Laboratory by an oxalate oxidase based assay (9, 10). The upper (95%) reference range for POx was 1.8 μml/L (9) while for urine oxalate it was 0.46 mM/24 hr (10). For POx measurement all samples were handled as per the standard Mayo Renal Laboratory protocol (9). Blood was drawn in a sodium heparin tube and centrifuged for 10 minutes at 3,500 rpm, at 4°C within 1 hour of draw. The plasma specimen was then adjusted to a pH of 1.0–4.0 with approximately 10 μL concentrated (12N) HCl per 1.0 mL plasma. Samples were then stable for storage at 4°C until further processing as previously published (9).
All POx measurements on Mayo Clinic patients between 2005–2015 were electronically pulled from the LIS (407 patients); for each individual only the most recent POx prior to 2/2015 was used and charts were reviewed for clinical diagnoses. Patients without PH were categorized as those with known or suspected EH, patients with urinary stone disease (USD) without evidence for PH or EH, and other. Patients were excluded (137 patients) if they were hospitalized and met clinical criteria for AKI (AKIN stage 1 or greater (11)). The closest serum creatinine within 14 days of POx measurement was recorded, as were the closest 24-hour urine oxalate, creatinine, citrate, calcium, and volume values (within 60 days). Race, age, sex, height, and weight were abstracted from the medical record. For PH patients the same variables were also obtained from the Rare Kidney Stone Consortium PH registry as available. To obtain POx values from a control population without USD across a clinical range of GFR, waste plasma was obtained from patients undergoing clinical GFR testing in the Mayo Renal Testing Laboratory. No 24 hour urine data was available for this last cohort.
Demographics, serum laboratory values, and urine laboratory values between study groups (PH and EH; PH and USD; PH and control) were analyzed. The eGFR was calculated from the serum creatinine using the CKD-EPI equation (12) or the Schwartz equation (age < 18 years old) (13). Some laboratory values were considered in multiple ways, such as standardized by urine creatinine concentration or body surface area (BSA), or after natural log transformation. Univariate comparison of either PH versus EH, PH versus USD, or PH vs control was made using generalized estimating equations (GEE) to account for repeated measures, with a logit link to provide odds ratios.
The relationship between POx and eGFR were compared for the four study groups with a scatterplot (both on the natural log scale to account for skewness), and with a linear GEE model in which ln (POx) was predicted simultaneously by ln (eGFR) and the categorical disease variable with indicator variables for each of EH, USD, and control to differentiate them from PH. Interaction terms were also assessed in this model to allow the relationship between eGFR and POx to vary by disease group. Using the model without the interaction terms, the predicted POx level for each disease group at several values of eGFR was quantified.
Multivariable models were next developed to use in clinical practice to differentiate PH and EH, and PH and control. POx, UOx, eGFR, serum creatinine, and age were all used as potential predictors of PH (versus EH) in GEE models analogous to those used at the univariate level; mixed stepwise models were utilized to identify a parsimonious set of predictors. Four final models were identified with separate statistically significant predictors. All models were reported with the Quasilikelihood under the Independence model Criterion (QIC) to differentiate their relative strength. Within a constant dataset (i.e. PH vs. EH or PH vs. control) a lower QIC is preferred. To describe the results of these models, the predicted probabilities of PH for each of the four study groups for all observations were compared using boxplots.
In order to better understand the relationship between a given POx and UOx, a linear GEE model was developed to predict UOx from POx using all observations. The relationship between UOx and POx was shown on a scatterplot to assess differences by study group, with separate predicted linear association lines identified for different levels of eGFR. Univariate odds ratios and generalized estimating equations (GEE) were used to compare POx, eGFR, and other factors between groups.
RESULTS
A total of 39 PH (with 175 observations), and 151 EH and 80 USD patients (one observation each) were identified (Table 1). For the control population without USD, data was obtained for 77 patients. There were 2 patients with ethylene glycol ingestion and one with unexplained oxalate nephropathy in a renal allograft (not studied further). In the USD group stones were composed of majority calcium oxalate (47), majority calcium phosphate (8), cystine (1), or uric acid (2), while in 22 cases composition was unknown. Other demographics are listed in Table 1. Clinical diagnoses for the EH group included bariatric surgical procedures for weight loss (n=82), inflammatory bowel disease (n=25), short bowel syndrome (n=25), chronic diarrhea syndrome (n=15), chronic pancreatitis (n=1), chronic tube feeds (n=1), carcinoid (n=1) and oxalate nephropathy after kidney transplant (n=1). The PH group was younger (25±19 yrs versus EH 57±14 yrs and USD 48±21 yrs and controls 57±13 yrs; mean±SD), while the EH group was majority female (61%).
Table 1.
Demographics and Patient Characteristics
| PH (N=39) | EH (N=151) | USD (N=80) | Controls (N=77) | OR (PH vs EH) | OR (PH vs USD) | OR (PH vs Control) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD)@ | (95% CI) | p-value1 | (95% CI) | p-value2 | (95% CI) | p-value3 | |
| Age, years | 24.9 (18.7) | 56.6 (14.2) | 48.1 (20.7) | 57.4 (13.3) | 0.90(0.86, 0.93) | <0.0001 | 0.93 (0.90, 0.96) | <0.0001 | 0.89 (0.86, 0.93) | <0.0001 |
| Male | 20 (51%) | 59 (39%) | 43 (54%) | 47 (61%) | 1.58 (0.70, 3.58) | 0.28 | 0.87 (0.36, 2.08) | 0.75 | 0.65 (0.27, 1.56) | 0.33 |
| Weight, kg | 66.4 (33.6) | 84.3 (23.6) | 80.2 (28.8) | 0.97 (0.95, 0.99) | 0.002 | 0.98 (0.96, 0.995) | 0.01 | |||
| BMI, kg/m2 | 25 (7.6) | 29.9 (7.7) | 28.3 (7.5) | 0.89 (0.83, 0.95) | 0.0008 | 0.91 (0.85, 0.98) | 0.009 | |||
| eGFR, mL/min/1.73 m2 | 87 (43) | 49 (30) | 74 (33) | 58 (27) | 1.04 (1.02, 1.05) | <0.0001 | 1.01 (0.996, 1.02) | 0.18 | 1.03 (1.01, 1.05) | 0.0004 |
| Serum creatinine, mg/dL | 1.08 (0.76) | 1.94 (1.38) | 1.31 (1.11) | 1.5 (0.7) | 0.23 (0.11, 0.49) | 0.0001 | 0.61 (0.37, 0.98) | 0.04 | 0.27 (0.10, 0.72) | 0.0089 |
| Plasma oxalate, mcmol/L | 6.71 (8.66) | 5.52 (6.44) | 2.15 (2.71) | 2.0 (1.5) | 1.02 (0.96, 1.07) | 0.53 | 1.53 (1.19, 1.95) | 0.0007 | 1.94 (1.47, 2.57) | <0.0001 |
| Urine Creatinine, mg/day | 1451 (623) | 1241 (500) | 1471 (588) | 1.00 (0.999, 1.001) | 0.37 | 1.00 (0.999, 1.0004) | 0.39 | |||
| Urine Calcium, mg/day | 119.9 (98) | 123 (105) | 215 (127) | 1.00 (0.99, 1.00) | 0.33 | 0.99 (0.986, 0.995) | < 0.0001 | |||
| Urine Citrate, mg/day | 456 (329) | 281 (329) | 569 (375) | 1.002 (1.00, 1,003) | 0.05 | 0.999 (0.997, 1.00) | 0.01 | |||
| Urine Calcium/Urine Creatinine | 0.10 (0.07) | 0.10 (0.07) | 0.16 (0.10) | 0.17 (0.002, 18.48) | 0.46 | 0.0001 (0, 0.14) | 0.01 | |||
| Urine Citrate/Urine Creatinine | 0.39 (0.32) | 0.10 (0.07) | 0.43 (0.31) | 8.97 (1.39, 57.88) | 0.02 | 0.9547 (0.35, 2.58) | 0.93 | |||
| Urine oxalate, mmol/day | 1.22 (0.89) | 0.56 (0.38) | 0.36 (0.18) | 12.98 (4.50, 37.42) | <0.0001 | 665(56.3, 7850.8) | <0.0001 | |||
| Urine oxalate adjusted for BSA, mmol/(day*m2) | 1.18 (0.76) | 0.50 (0.33) | 0.33 (0.16) | 24.61 (7.49, 80.90) | <0.0001 | 4009 (114.4, 111316) | <0.0001 |
p-values for OR of the covariate from the GEE model predicting PH vs EH
p-values for OR of the covariate from the GEE model predicting PH vs USD
p-values for OR of the covariate from the GEE model predicting PH vs controls
39 In PH Group (34 had urine collected), 151 in EH (76 had urine collected), and 80 in USD (58 had urine collected)
No urine data for the control (no stone) group
Mean eGFR was 87±43 (PH), 49±30 (EH), 74±33 (USD), and 58±27 (control) ml/min/1.73m2. PH patients had a higher eGFR (OR=1.04, p<0.0001), urine citrate (OR=1.002, p=0.05), urine citrate/urine creatinine ratio (OR=8.97, p=0.02), UOx (OR=12.98, p<0.0001), and UOx after adjustment for BSA (OR=24.61, p<0.0001), compared to EH. PH patients also had lower age (OR=0.90, p<0.0001), weight (OR=0.97, p=0.002), BMI (OR=0.89, p=0.0008), and serum creatinine (OR=0.23, p=0.0001) than EH. Compared to USD patients, PH patients had a higher POx (OR=1.53, p=0.0007), UOx (OR=665, p<0.0001), and UOx after adjustment for BSA (OR=4009, p<0.0001). PH patients were of lower age (OR=0.93, p<0.0001), weight (OR=0.98, p=0.01), BMI (OR=0.91, p=0.009), serum creatinine (OR=0.61, p=0.04), urine calcium (OR=0.99, p<0.0001), urine citrate (OR=0.999, p=0.01), and urine calcium/urine creatinine ratio (OR=0.0001, p=0.01). Compared to controls, PH patients had lower age (OR=0.89, p<0.0001), lower serum creatinine (OR=0.27, p=0.0089), higher eGFR (OR=1.03, p=0.0004) and higher POx (OR=1.94, p<0.0001).
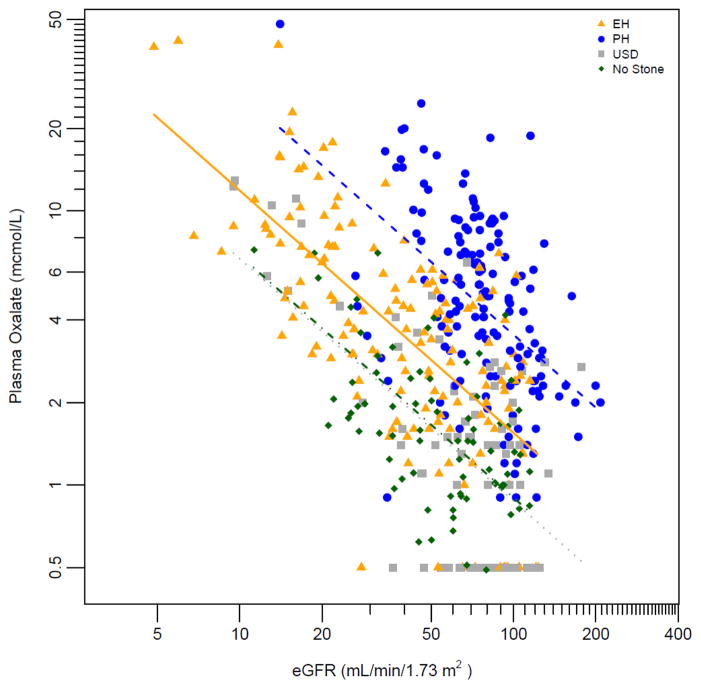
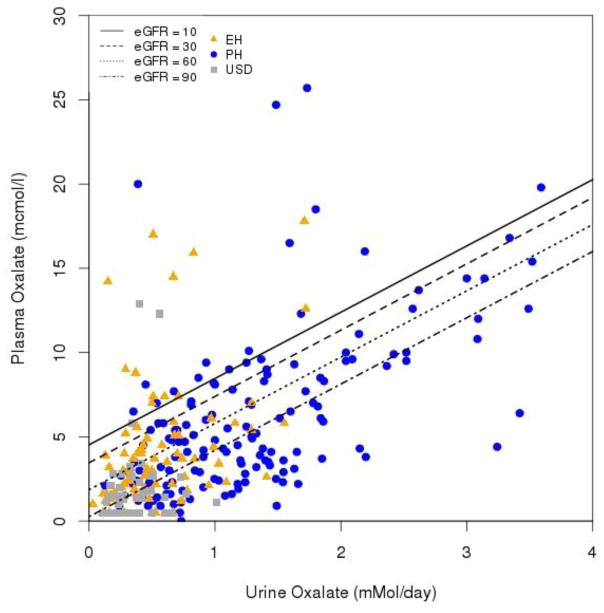
In all 4 groups POx increased as eGFR fell (Figure 1; p<0.0001). In addition, for any given eGFR, POx was highest in the PH group, lowest in the USD and control groups, and intermediate in the EH group (p<0.0001). POx was also influenced by UOx excretion (Figure 2). GEE models of POx vs eGFR revealed higher average POx levels in PH compared to either EH, USD or control, and for EH compared to USD or control (Figure 1; p<0.0001). Figure 2 further illustrates the relationship between POx and UOx with lines indicating eGFR levels of 10, 30, 60, and 90 ml/min/1.73 m2. In general, for a given UOx the POx increased as GFR fell, regardless of disease group. Table 2 contains the predicted mean POx by representative eGFR levels for the three disease groups based upon the model.
Figure 1. Relationship between plasma oxalate and eGFR in PH, EH, USD and non-stone former patients.
ln(Plasma Ox.) = 5.2531 – 0.8734 * ln(eGFR) – 0.7814 * (Group = EH) – 1.3604 * (Group = USD) – 1.3295 * (Group = Non Stone Former) (p<0.0001 for all beta).
Figure 2. Relationship between plasma oxalate and urine oxalate at different eGFR.
UOx=0.0809+0.1022*Pox+0.0044*eGFR; p≤0.002. One outlier left out of the plot had a POx of 48.1 and UOx of 3.54.
Table 2.
POx (μmol/L) for PH, EH and urinary stone patients at different levels of eGFR.
| eGFR (mL/min/1.73 m2) | log(eGFR) | POx PH group (μmol/L) | POx EH group (μmol/L) | POx Stone group (μmol/L) | POx Control group (μmol/L) |
|---|---|---|---|---|---|
| 20 | 3 | 14.6 | 6.5 | 3.7 | 3.7 |
| 33 | 3.5 | 9.3 | 4.1 | 2.4 | 2.4 |
| 54 | 4 | 5.9 | 2.6 | 1.5 | 1.5 |
| 90 | 4.5 | 3.7 | 1.7 | 0.9 | 1.0 |
Given the interrelationships between POx, UOx, and kidney function, 4 GEE prediction models were created to determine how POx or UOx (corrected for BSA) might be used to suspect PH; the first two models comparing PH and EH and the second two models comparing PH and controls (Table 3). Since both POx and UOx are highly correlated, if both were included together in the model only one remained significant. In model 1, the significant covariates based upon QIC were POx (OR=1.2 per μmol/l of POx, p=0.02), age (OR=0.9 per year of age, p<0.0001), and serum creatinine (OR=0.35 per unit (mg/dl) of serum creatinine, p=0.08); the QIC for this model was 200. In model 2, the covariates were UOx corrected for BSA (OR=10.54 per mmol/(day*m2), p=0.003) and age*eGFR (OR=1.06 if < 30 yrs old, p<0.0001); the QIC for this model was 111. In model 3 for predicting PH vs control, the significant covariates were POx (OR=3.0 per μmol/l of POx, p=0.003), age (OR=0.89 per year of age, p<0.0001), and serum creatinine (OR=0.14 per unit (mg/dl) of serum creatinine, p=0.053); the QIC for this model was 99. In model 4 covariates were POx (OR=2.86 per μmol/L, p=0.002), age > 30 yrs old (p<0.001) and age*serum creatinine (OR=0.17 if > 30 yrs old, p=0.10); the QIC for this model was 90. Equations for calculating the OR for PH vs EH, PH vs controls and probability of PH (P(PH)) are given in Table 4. An equation for calculating predicted UOx based on POx and eGFR is given in the legend for Figure 2. In the supplemental data (Figure 3–5) Figures 3 and 4 show the predicted probability that a subject has PH using each model and for each of the study groups. Figure 5 shows the same predicted probabilities broken down for eGFR < 45 versus > 45 ml/min/1.73m2. In general, both models become more specific for PH at lower eGFR, but also less sensitive.
Table 3.
Prediction of PH as compared to EH or Control
| Parameter | Beta Estimate | OR | 95% CI | p-value | Model QIC* |
|---|---|---|---|---|---|
| PH vs EH | |||||
| Model 1 | 200 | ||||
| Plasma Oxalate, μmol/L | 0.1627 | 1.2 | (1.0, 1.3) | 0.02 | |
| Age, yrs | −0.1022 | 0.90 | (0.86, 0.94) | <.0001 | |
| Serum Creatinine**, mg/dL | −1.0597 | 0.35 | (0.11, 1.12) | 0.08 | |
| Model 2 | 111 | ||||
| eGFR* Age, < 30 | 0.0628 | 1.06 | (1.04, 1.09) | <.0001 | |
| BSA Corrected UOx, mmol/(day* m2) | 2.3551 | 10.54 | (2.26, 49.21) | 0.003 | |
| PH vs Controls | |||||
| Model 3 | 99 | ||||
| Plasma Oxalate, μmol/L | 1.1046 | 3.02 | (1.45, 6.27) | 0.003 | |
| Age, yrs | −0.1223 | 0.89 | (0.84, 0.94) | <.0001 | |
| Serum Creatinine**, mg/dL | −1.9938 | 0.14 | (0.02, 1.03) | 0.053 | |
| Model 4 | 90 | ||||
| Plasma Oxalate, μmol/L | 1.0496 | 2.86 | (1.46, 5.59) | 0.002 | |
| Age > 30 yrs | −17.5304 | <0.001 | (0, 0.0004) | <.0001 | |
| Serum Creatinine* Age>30 | −1.7905 | 0.17 | (0.02, 1.44) | 0.1034 | |
| Serum Creatinine* Age ≤30 | −11.5826 | <0.001 | (0, 0.01) | 0.0004 | |
Within a comparison, lower QIC indicates a stronger model.
Despite p-values greater than 0.05 for serum creatinine in these models, the QIC was lower for these models than for those from which serum creatinine had been removed, indicating that these models were superior and more parsimonious.
Table 4.
Equations for predicting risk and odds of PH versus EH and PH versus Controls
| PH vs EH based on POx, age, and serum creatinine | |
|
| |
|
| |
| PH vs EH based on UOx (BSA corrected), and the interaction eGFR*I(Age < 30) | |
|
| |
|
| |
| PH vs Controls based on POx, age, and serum creatinine | |
|
| |
|
| |
| PH vs Controls based on Pox, I(Age ≥ 30), and the interaction serum creatinine*I(Age ≥ 30) | |
|
| |
|
|
DISCUSSION
UOx excretion is a key parameter for the diagnosis of PH and EH, as well as for the evaluation and treatment of diverse forms of USD. In the event of advanced CKD, UOx declines and may no longer reflect daily oxalate loads (7). In these circumstances POx can potentially be useful to indicate that PH is a possibility. However, the effect of CKD on POx can be an important confounder. Thus in the current study we quantified the relationship of both GFR and UOx with POx in four different patient groups: PH, EH, USD, and control patients. These data were used to define mean levels of POx by disease group and level of GFR (Table 2). These expected POx values can thus be used to interpret a given POx result, and in particular when to suspect PH or EH, especially in relationship to eGFR. In addition, models were developed to estimate the probability of PH that use POx or UOx plus serum creatinine and age. This information can be clinically helpful to decide whether a PH diagnosis might be reasonably pursued, for example using genetic testing for the implicated genes.
In the current study an equation was also developed to predict UOx based upon POx and eGFR (Figure 2). This data helps interpret POx to infer likely disease process since in general UOx is > 1.0 mM/day in PH, 0.5–1.0 mM/day in EH, and lower in routine USD (14). In a patient with CKD and history of calcium oxalate stones, these expected POx (or UOx) values can be helpful when considering PH, since the presence of conditions that predispose to EH are usually clinically apparent. The interrelationship between POx and eGFR differs by disease group as visualized in Figure 1. In general the relationship between POx and GFR was very similar between USD and controls without stones, likely because disorders of oxalate metabolism and handling, when present, are quite subtle in the vast majority of USD patients as compared to PH and EH. Another intuitive and useful way to think about these data is the interrelationship between POx and UOx, since UOx reflects oxalate burden that must be eliminated by the kidney (Figure 2). In particular, POx increases at a given level of UOx as the eGFR declines.
Other laboratory and clinical features differed between the three patient groups. As anticipated EH patients were older than PH patients and had lower eGFR (49 vs 87 ml/min/1.73m2). Although eGFR declines with age, other comorbidities and factors likely explain the lower eGFR in the EH group. Also as expected, the PH group had a higher UOx (1.22±0.89mmol/day) than EH (0.56±0.38 mmol/day)(OR=12.98, p<0.0001) and USD (0.36±0.18 mmol/day)(OR=665, p<0.0001), with stronger odds after adjustment for BSA in both EH (OR=24.61, p<0.0001), and USD (OR=4009, p<0.0001). A published algorithm (15) suggests suspicion for PH when the UOx excretion is greater than 0.7 mmol/1.73m2/day in the presence of an eGFR > 50 ml/min/1.73m2. In our study 56.4 % of PH, 5.3 % of EH, and 1.3 % of USD patients exceeded this threshold. Our study also suggests values of POx that might be used to differentiate PH when the eGFR is < 50 ml/min/1.73m2 (Figure 1).
Since UOx (and POx) overlap between PH and EH, two models were created to predict PH versus EH (Table 3) based on age, serum creatinine, and either BSA corrected UOx or POx. The prediction model that included UOx corrected for BSA was stronger (lower QIC) than the model using POx. This is illustrated in Figure 3–5 (Supplemental data), which also compare PH vs USD and PH vs controls and among those with an eGFR less than versus more than 45 ml/min/1.73m2. One factor possibly contributing to this outcome might be that oxalate can be secreted from the proximal tubule, and this secretion is markedly increased among PH patients (16). It is currently thought that high POx concentrations might be driving oxalate secretion, but further studies are needed to verify this. Thus POx may be falsely “low” in PH, explaining the poorer positive predictive value of POx for PH at lower eGFR (Figure 5A). Conversely, the negative predictive value of POx for PH was better in the low eGFR group (Figure 5A), perhaps for the same reason.
Since EH is often clinically apparent due to an underlying clinical condition associated with fat malabsorption, models were also constructed to differentiate PH versus controls based upon the POx values. In order to construct robust models we obtained new data on a cohort of patients undergoing clinical GFR testing and enriched for moderate chronic kidney disease to mirror the GFR in the PH cohort (Figure 1). Together with clinical information these figures and models can be used to help determine if an elevated POx can be attributed to CKD alone, or if PH is a possibility.
In general, POx increased as eGFR decreased (Figure 1), or at higher levels of UOx (Figure 2). In addition, UOx was highest in the PH and lowest in the USD group. Thus POx by eGFR differed by disease group (Table 2). These data provide potential insight regarding the risk for oxalosis. In general, the risk of systemic oxalate deposition increases dramatically as the POx exceeds 35–40 μmol/L (14). As Figure 1 demonstrates, on average this POx threshold is almost universally exceeded in PH when eGFR dips below 10 ml/min/1.73m2. On the other hand, in the EH group POx only approaches 20 μmol/L as the eGFR declines to <5 ml/min/1.73m2. In USD, POx values are even lower and most patients never exceed a POx of 20 μmol/L. These observations likely explain why oxalosis is nearly universal in PH, variable (but relatively rare) in EH, and not observed in routine ESRD.
Our study has certain limitations. The population was largely Americans of European descent, and thus results will need to be confirmed in other racial and ethnic groups. Further studies will be needed to verify the prediction equations in other cohorts, preferably including a wider range of eGFR values and ages (although the observed age distributions likely reflect the average age of presentation of the 3 conditions). We also did not have sufficient power to break down analyses by PH type. It is likely the relationships between POx and eGFR vary by PH type since UOx is highest in PH1, lowest in PH3, an intermediate in PH2. Nevertheless, to our knowledge, this is the first study to quantify the relationship between POx and eGFR in PH, EH, and USD patients. This will aid clinicians in catching undiagnosed PH patients, particularly in patients with stage 4–5 CKD and serve as a reference range for POx at different eGFR.
In conclusion, we propose new models for use in clinical practice to help interpret POx when considering PH at reduced eGFR even when PH was not previously suspected.
Highlights.
Relationships between plasma oxalate, GFR and urine oxalate are established among patients with primary hyperoxaluria, enteric hyperoxaluria, and routine urinary stone disease.
New models were developed to help interpret plasma oxalate when considering PH in clinical practice
The models also help interpret plasma oxalate even when Primary Hyperoxaluria was not previously suspected and/or when eGFR is reduced
Acknowledgments
GRANT FUNDING
This study was supported by the Mayo Foundation, the Mayo Clinic O‘Brien Urology Research Center (U54DK U54DK100227), and the Rare Kidney Stone Consortium (U54KD083908), a part of the Rare Diseases Clinical Research Network (RDCRN), an initiative of the Office of Rare Diseases Research (ORDR), National Center for Advancing Translational Sciences‘ (NCATS). This consortium is funded through a collaboration between NCATS, and National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The study sponsor had no role in study design; collection, analysis, and interpretation of data; writing the report; and the decision to submit the report for publication.
Footnotes
DISCLOSURES
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Edvardsson VO, Goldfarb DS, Lieske JC, Beara-Lasic L, Anglani F, Milliner DS, et al. Hereditary causes of kidney stones and chronic kidney disease. Pediatric nephrology. 2013 doi: 10.1007/s00467-012-2329-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoppe B, Langman CB. A United States survey on diagnosis, treatment, and outcome of primary hyperoxaluria. Pediatr Nephrol. 2003;18(10):986–91. doi: 10.1007/s00467-003-1234-x. [DOI] [PubMed] [Google Scholar]
- 3.Lorenzo V, Torres A, Salido E. Primary hyperoxaluria. Nefrologia. 2014;34(3):398–412. doi: 10.3265/Nefrologia.pre2014.Jan.12335. [DOI] [PubMed] [Google Scholar]
- 4.Sinha MK, Collazo-Clavell ML, Rule A, Milliner DS, Nelson W, Sarr MG, et al. Hyperoxaluric nephrolithiasis is a complication of Roux-en-Y gastric bypass surgery. Kidney Int. 2007;72(1):100–7. doi: 10.1038/sj.ki.5002194. [DOI] [PubMed] [Google Scholar]
- 5.Lorenz EC, Michet CJ, Milliner DS, Lieske JC. Update on oxalate crystal disease. Curr Rheumatol Rep. 2013;15(7):340. doi: 10.1007/s11926-013-0340-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Worcester EM, Nakagawa Y, Bushinsky DA, Coe FL. Evidence that serum calcium oxalate supersaturation is a consequence of oxalate retention in patients with chronic renal failure. JClinInvest. 1986;77:1888–96. doi: 10.1172/JCI112516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elgstoen KB, Johnsen LF, Woldseth B, Morkrid L, Hartmann A. Plasma oxalate following kidney transplantation in patients without primary hyperoxaluria. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2010;25(7):2341–5. doi: 10.1093/ndt/gfq065. [DOI] [PubMed] [Google Scholar]
- 8.Hopp K, Cogal AG, Bergstralh EJ, Seide BM, Olson JB, Meek AM, et al. Phenotype-Genotype Correlations and Estimated Carrier Frequencies of Primary Hyperoxaluria. Journal of the American Society of Nephrology: JASN. 2015 doi: 10.1681/ASN.2014070698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ladwig PM, Liedtke RR, Larson TS, Lieske JC. Sensitive spectrophotometric assay for plasma oxalate. Clinical chemistry. 2005;51(12):2377–80. doi: 10.1373/clinchem.2005.054353. [DOI] [PubMed] [Google Scholar]
- 10.Wilson DM, Liedtke RR. Modified enzyme-based colorimetric assay of urinary and plasma oxalate with improved sensitivity and no ascorbate interference: reference values and sample handling procedures. Clinical chemistry. 1991;37(7):1229–35. [PubMed] [Google Scholar]
- 11.Ricci Z, Cruz DN, Ronco C. Classification and staging of acute kidney injury: beyond the RIFLE and AKIN criteria. Nature reviews Nephrology. 2011;7(4):201–8. doi: 10.1038/nrneph.2011.14. [DOI] [PubMed] [Google Scholar]
- 12.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. The New England journal of medicine. 2012;367(1):20–9. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, et al. New equations to estimate GFR in children with CKD. Journal of the American Society of Nephrology: JASN. 2009;20(3):629–37. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoppe B, Beck BB, Milliner DS. The primary hyperoxalurias. Kidney international. 2009;75(12):1264–71. doi: 10.1038/ki.2009.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edvardsson VO, Goldfarb DS, Lieske JC, Beara-Lasic L, Anglani F, Milliner DS, et al. Hereditary causes of kidney stones and chronic kidney disease. Pediatric nephrology. 2013;28(10):1923–42. doi: 10.1007/s00467-012-2329-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Worcester EM, Evan AP, Coe FL, Lingeman JE, Krambeck A, Sommers A, et al. A test of the hypothesis that oxalate secretion produces proximal tubule crystallization in primary hyperoxaluria type I. American journal of physiology Renal physiology. 2013;305(11):F1574–84. doi: 10.1152/ajprenal.00382.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]