Abstract
Purpose
We previously reported that fibroblasts migrating within 3-D collagen matrices move independently, whereas fibroblasts within 3-D fibrin matrices form an interconnected network. Similar networks have been identified previously during in vivo corneal wound healing. In this study, we investigate the role of fibronectin in mediating this mechanism of collective cell spreading, migration and patterning.
Methods
To assess cell spreading, corneal fibroblasts were plated within fibrillar collagen or fibrin matrices. To assess migration, compacted cell-populated collagen matrices were nested inside cell-free fibrin matrices. Constructs were cultured in serum-free media containing PDGF, with or without RGD peptide, anti-α5 or anti-fibronectin blocking antibodies. In some experiments, LifeAct and fluorescent fibronectin were used to allow dynamic assessment of cell-induced fibronectin reorganization. 3-D and 4-D imaging were used to assess cell mechanical behavior, connectivity, F-actin, α5 integrin and fibronectin organization.
Results
Corneal fibroblasts within 3-D fibrin matrices formed an interconnected network that was lined with cell-secreted fibronectin. Live cell imaging demonstrated that fibronectin tracks were formed at the leading edge of spreading and migrating cells. Furthermore, fibroblasts preferentially migrated through fibronectin tracks laid down by other cells. Interfering with cell-fibronectin binding with RGD, anti α5 integrin or anti fibronectin antibodies inhibited cell spreading and migration through fibrin, but did not affect cell behavior in collagen.
Conclusions
In this study, a novel mode of cell patterning was identified in which corneal fibroblasts secrete and attach to fibronectin via α5β1 integrin to facilitate spreading and migration within 3-D fibrin matrices, resulting in the formation of localized fibronectin tracks. Other cells use these fibronectin tracks as conduits, resulting in an interconnected cell-fibronectin network.
Keywords: Extracellular Matrix, Corneal Keratocytes, Cell Mechanics, Fibrin, Fibronectin
INTRODUCTION
The corneal stroma is a highly ordered structure consisting of approximately 200 collagen lamellae [1]. Corneal stromal cells (keratocytes) reside between the collagen lamellae, and are responsible for secreting extracellular matrix (ECM) components required to develop and maintain normal corneal structure and function [2–4]. Cell-matrix mechanical interactions within the corneal stroma play a central role in fundamental biological processes such as developmental morphogenesis and wound healing. In adult corneal tissue, resting keratocytes are mechanically quiescent; they do not organize cellular actin into stress fibers or generate substantial contractile forces [5, 6]. Following injury, surgery or other insults, corneal keratocytes can become activated by growth factors and other cytokines present in the wound environment, and transform into a fibroblastic repair phenotype [7, 8]. Corneal fibroblasts proliferate, develop intracellular stress fibers, migrate into the wound and reorganize the ECM through the application of mechanical forces. In certain wound types, the presence of transforming growth factor beta (TGFp) in the wound can induce transformation of corneal fibroblasts to myofibroblasts, which generate even stronger forces on the matrix and synthesize a disorganized fibrotic ECM [9, 10]. Together these processes can impact visual acuity by altering corneal shape and reducing transparency due to increased light scattering by both cells and the newly synthesized ECM [11–16].
In addition to growth factors, the extracellular matrix also provides both biophysical and chemical signals that can regulate cell differentiation and mechanical behavior during wound healing [17–20] and other biological processes including development, morphogenesis and cancer [21–23]. In recent studies, we demonstrated that ECM composition has a profound impact on the pattern of corneal fibroblast spreading and migration in 3-D culture. Specifically, cells interacting with collagen matrices develop dendritic processes and move independently, whereas cells interacting with fibrin matrices develop stress fibers and form an interconnected meshwork [24, 25]. Interestingly, corneal fibroblasts form an interconnected mesh as they migrate into the wound space following incisional surgery, and these interconnections are hypothesized to mediate force transduction during wound contraction [26, 27]. Furthermore, following a transcorneal freeze or keratectomy injury in the rabbit, highly aligned streams of interconnected fibroblasts migrate into the damaged stromal tissue [19, 28]. Overall, the mode of cell migration (individual versus collective) can influence cell patterning, force generation and tissue organization in other systems, and also plays a pivotal role in cancer invasion [29–31]. Thus a better understanding of the factors that can mediate collective cell migration and patterning could have broad biological significance.
One possible candidate for mediating corneal fibroblast network formation within 3-D fibrin matrices is fibronectin. Fibronectin is an adhesive protein secreted by cells that facilitates binding to other ECM proteins (including fibrin) [32–35], and it is expressed by corneal fibroblasts during clustering on top of fibrin ECM [25]. Fibronectin synthesis has been shown to contribute to vascular endothelial cell network formation and tubulogenesis in vitro [36, 37]. Furthermore, blocking fibronectin secretion or interfering with the associated α5β1 integrin protein prevents contraction-induced cluster formation by dermal fibroblasts on compliant collagen matrices [38]. Importantly, in vivo studies of corneal injuries have shown an accumulation of fibronectin during corneal fibrosis [28, 39, 40], and TGFβ-induced myofibroblast transformation requires fibronectin fibril formation [9, 41]. In this study, we directly investigate the role of fibronectin and α5β1 integrin in mediating cellular patterning and network formation during fibroblast spreading and migration within fibrin ECM.
RESULTS
Migrating corneal fibroblasts form fibronectin tracks in nested fibrin matrices
We previously demonstrated that cells interacting with collagen matrices develop dendritic processes and move independently, whereas cells interacting with fibrin matrices develop stress fibers and form an interconnected meshwork [24, 25]. In constructs in which cell-seeded compressed collagen matrices are nested within acellular fibrin matrixes, interconnected streams of cells are formed during migration into fibrin (Supplemental Movie 1). Although cells within these streams can become temporarily separated, cells behind the region of separation continue to follow the same path or trajectory that the leader cells followed. Leader cells can also move backwards and/or extend rear protrusions over the same path to reattach to the cells behind them. This pattern of movement suggests the formation of “tracks” by cells at the leading edge during migration that facilitate preferential attachment, spreading and migration of trailing cells.
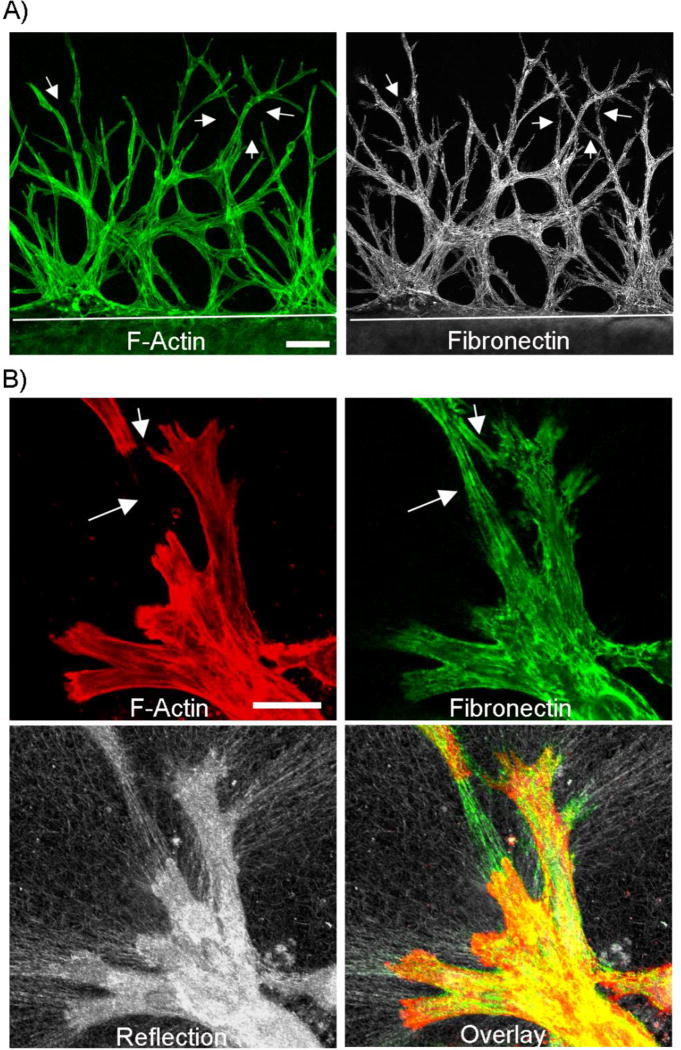
Fibronectin is a cell-secreted protein that can serve as a bridge between integrins and the extracellular substrate [42]. To investigate whether fibronectin plays a role in mediating collective cell migration, we first determined the pattern of fibronectin organization in our nested fibrin matrix model using anti-fibronectin labeling. As shown in Figure 1 A, an extensive network of fibronectin is present around the migrating cells after 48 hours. Both the cells and the fibronectin form an interconnected network. Interestingly, fibronectin “tracks” were also observed between cells in some areas (arrows). To verify that the fibronectin was extracellular, fibronectin labeling was also performed prior to permeabilizing the cells (Figure 1B). A fibrillar pattern of fibronectin labeling was present both around and between cells (arrows). Confocal reflection microscopy showed that the ECM was also compacted and aligned by migrating cells (figure 1B, lower panel). These findings suggest that cells secrete and organize fibronectin into tracks when migrating into fibrin ECM. These tracks may then serve as paths or conduits for other cells.
Figure 1.
Fibroblasts migrating through fibrin create fibronectin tracks. Nested matrix constructs were cultured for 48h in PDGF-containing media to stimulate cell migration. A) Corneal fibroblasts form an interconnected network as they migrate into the outer fibrin matrix (top part of image, white line delineates edge of inner matrix). This network contained significant fibronectin. As shown by arrows, fibronectin “tracks” were sometimes present in between cells. Scale bar is 100 µm. B) Higher magnification images from a sample that was not permeabilized prior to fibronectin labeling. Extracellular fibronectin tracks were observed around cells. Fibronectin tracks were also found in spaces between cells in some areas (arrows). As shown by reflection microscopy, localized ECM compaction was produced by migrating fibroblasts. Scale bar is 100 µm.
α5 integrin colocalizes with fibronectin during cell spreading in 3-D fibrin matrices
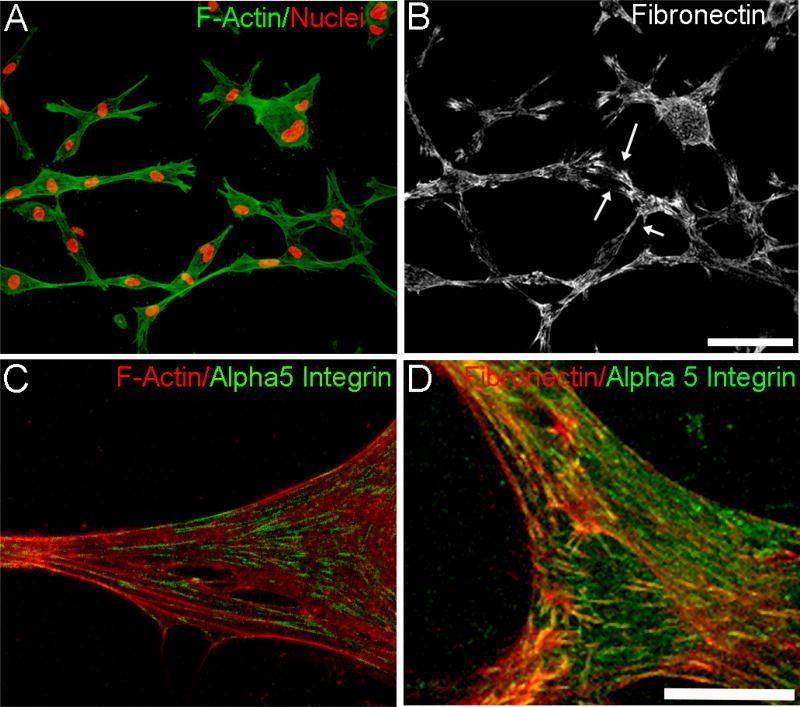
To investigate fibronectin organization during cell spreading, corneal fibroblasts were embedded in standard (non-nested) fibrin matrices and incubated overnight. Similar patterns of F-actin (Fig. 2A) and fibronectin (Fib. 2B) labeling were observed. As in the nested model, fibronectin “tracks” were sometimes present in between cells (arrows). A similar pattern of cell interconnectivity and fibronectin network formation was observed when using primary cultures of rabbit corneal keratocytes (supplemental figure 1). Previous studies have established that α5β1 integrin is used for corneal fibroblast attachment to fibronectin [43]. In order to study the expression and localization of α5β1 integrin in fibrin and collagen matrices, we used immunolabeling with an α5 integrin antibody. Overlays of F-actin/α5 integrin (Fig. 2C) and fibronectin/α5 integrin (Fig. 2D) after overnight culture in fibrin matrices show punctate α5 labeling that is colocalized with F-actin and fibronectin fibers. This labeling pattern suggests that cells in 3-D fibrin use α5 integrin for attachment to the fibronectin network. In contrast, α5 integrin labeling was not detected during cell spreading in collagen matrices (not shown).
Figure 2.
Cell-fibronectin networks and α5 integrin organization during 3-D cell spreading of human corneal fibroblasts cultured in 3-D fibrin matrices. A) Maximum intensity projection of F-actin and nuclei showing interconnected cells. B) Corresponding maximum intensity projection of fibronectin network formed by cells. Arrows show fibronectin tracks in spaces between some cells. Scale bar is 100 µm. C and D) Higher magnification maximum intensity projection of overlays of F-actin/α5 integrin (C) and fibronectin/α5 integrin (D). Scale bar is 25 µm.
Dynamic correlation of cell spreading and migration with fibronectin organization
Fluorescent fibronectin has been previously used by other labs to visualize dynamic changes in cell-induced fibronectin organization [38]. In order to validate that exogenous fluorescent fibronectin would integrate with cell secreted fibronectin in our model, we used two different processes for staining. 1) After overnight incubation to allow cell spreading, fibrin matrices were fixed and labeled with an anti-fibronectin antibody. At the end of the staining process, samples were incubated for an additional 60 min with exogenous fluorescent fibronectin. 2) After overnight culture, fluorescent fibronectin was added to the media and cells were incubated for an additional 60 min. Media was then removed and samples were fixed and stained using the anti-fibronectin antibody. In both cases, the samples were not permeabilized. For both staining protocols, fluorescent fibronectin colocalized with the anti-fibronectin antibody (supplemental figure 2), confirming fluorescent fibronectin can be used to map cell-secreted fibronectin organization.
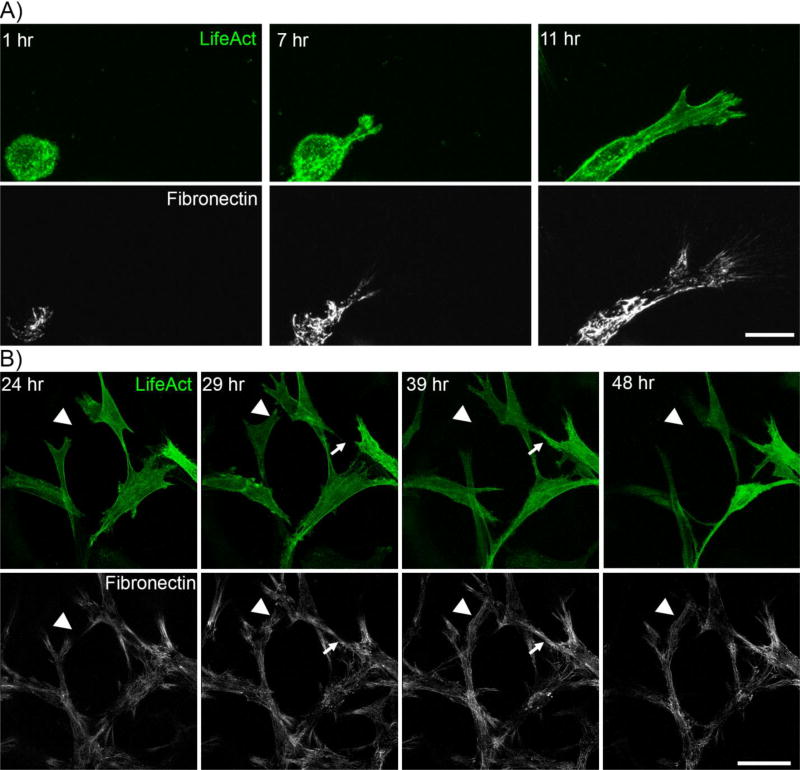
In order to visualize changes in F-actin and fibronectin organization simultaneously, cells were transfected with the LifeAct adenoviral vector, which has a TagGFP2 marker that labels filamentous actin. For these experiments DIC imaging was first used to verify that all cells in the area being imaged were labeled with LifeAct. To assess the dynamics of cell spreading, media containing PDGF and Rhodamine fibronectin were added immediately after fibrin matrix polymerization. After one hour of incubation, matrices were transferred to the microscope for time-lapse imaging. Figure 3A shows maximum intensity projection images of cell spreading at 1h, 7h, and 11h after seeding (see also supplemental movie 2). In all experiments, formation of fibronectin tracks occurred in concert with cell spreading. .
Figure 3.
Temporal assessment of fibronectin track formation. Cells expressing LifeAct (green) in fibrin matrices were monitored with media containing fluorescent fibronectin (white). A) Initial cell spreading. After one hour of incubation, the matrix was transferred to the microscope for time-lapse imaging. Z-stacks of images were collected at 20 minute intervals. Images show maximum intensity projections. Note that fibronectin is present at the leading edge during cell spreading. Scale bar is 25 µm. B) Cell movement within fibronectin network. Cell/fibrin matrix was incubated for 23 hours in PDGF. After one hour of incubation with Rhodamine fibronectin, the matrix was transferred to the microscope for time-lapse imaging. Z-stacks of images were collected at 20 minute intervals. Images show maximum intensity projections. Arrowheads show areas where new fibronectin tracks were created during migration of the cells. Arrows show a cell spreading into a pre-existing fibronectin track. Scale bar is 50 µm
We also studied cell-fibronectin interactions after 24 hours of culture, when the cell-secreted fibronectin network had already been established. Figure 3B shows maximum intensity projections at the beginning of the recording (24 h) and subsequently at 29h, 39 h and 48h (see also supplemental movie 3). In all experiments, cell spreading and migration was constrained to areas of fibronectin labeling. Arrowheads show areas where new fibronectin tracks were created during cell migration; whereas arrows show cell migration through pre-existing fibronectin tracks.
Cell spreading and migration in fibrin requires attachment to fibronectin
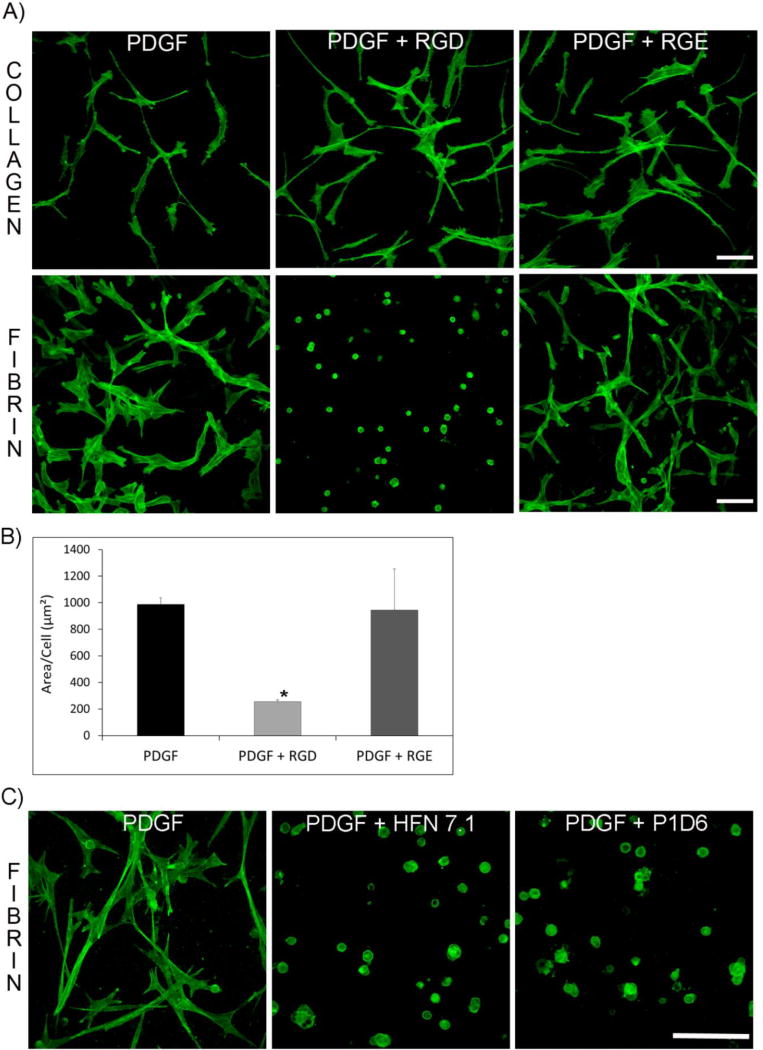
The results above suggest that fibronectin is used to facilitate corneal fibroblast attachment and spreading within fibrin matrices. In order to determine if cell binding to fibronectin is required for cell spreading and network formation within fibrin, we used an RGD peptide which directly interferes with integrin attachment to fibronectin [44]. Figure 4A shows representative pictures of cells in fibrin or in collagen matrices after overnight incubation in media containing RGD peptide, RGE control peptide, or PDGF alone. When cells were incubated in the presence of RGD in fibrin matrices, they were not able to spread and remained rounded. In contrast, no significant effect was observed in collagen matrices. Cells incubated with RGE control peptide or PDGF alone spread normally in both fibrin and collagen matrices. Statistical analysis demonstrated a significant decrease in cell area in media containing RGD (Figure 4B). Time-lapse imaging demonstrated that removing RGD from the media after 24 hours resulted in spreading of rounded cells within 2–4 hours, indicating that the effect was reversible. To further confirm that cells require fibronectin attachment in order to spread, we carried out additional experiments using HFN 7.1, an antibody that binds to fibronectin and blocks cell attachment, and P1D6, an antibody that interferes with binding of α5β1 to fibronectin. Cells cultured overnight in fibrin matrices in the presence of these antibodies did not spread, providing further evidence that fibronectin mediates attachment and spreading within fibrin ECM.
Figure 4.
Blocking cell attachment to fibronectin inhibits cell spreading in fibrin ECM. A) RGD peptide blocks cell spreading in fibrin matrices but not in collagen matrices. Representative pictures of collagen and fibrin matrices cultured overnight with RGD and RGE (control) peptide, and fixed and labeled for F-actin. For collagen, no difference in the pattern and amount of cell spreading was found between the three conditions. For fibrin, cells cultured with RGD peptide were not able to spread and remained rounded. Images are maximal intensity projections. Scale bar is 50 µm. B) Statistical analysis of cell spreading. Cell area was reduced in the presence of RGD. Data are mean ± standard deviation of 3 separate experiments. (*P<0.05, ANOVA on Ranks). C) Representative pictures of cells in fibrin matrices cultured with PDGF media containing antibodies that block fibronectin attachment (HFN 7.1) or α5β1 integrin attachment (P1D6). After overnight incubation, samples were fixed and stained for F-actin. Cells cultured with blocking antibodies were not able to spread and remained rounded. Images show maximum intensity projections. Scale bar is 50 µm.
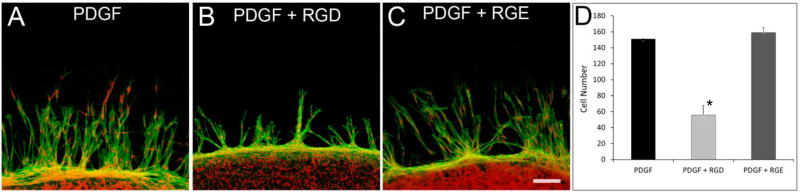
We also investigated whether cell migration in our nested matrix constructs was dependent on fibronectin binding. After 48 hours, cell migration in nested fibrin matrices incubated with PDGF or RGE occurred at a similar rate (figure 5A and 5C). However, cell migration into fibrin was significantly reduced in constructs incubated with the RGD peptide (Figure 5B and 5D). Interestingly, even though incubation of samples with RGD peptide reduced the amount of cell migration, the interconnected pattern of cell migration was still observed.
Figure 5.
RGD peptide decreases cell migration in nested fibrin matrices. F-actin (green) and nuclei (red) staining of migrating cells in fibrin nested matrices. Cells were cultured with PDGF, PDGF plus RGD, or PDGF plus RGE for 48 hours. RGD peptide reduced the cell migration rate but did not change the interconnected pattern of migration. All data are mean ± standard deviation of 3 separate experiments (*P<0.05, ANOVA on Ranks). Scale bar is 100 µm.
DISCUSSION
We previously demonstrated that ECM composition can modulate the mechanism of corneal fibroblast spreading and migration in 3-D culture. Specifically, whereas corneal fibroblasts generally move independently within 3-D collagen matrices, fibrin induces a switch to an interconnected, collective mode of cell spreading and migration which is independent of differences in ECM stiffness [24, 25]. Fibronectin is an adhesive protein secreted by cells that facilitates binding to other ECM proteins [32]. Most cells require fibronectin in order to bind to fibrinogen and fibrin, and fibronectin is required for dermal fibroblast invasion of fibrin matrices [33, 34]. In the current study, we used high resolution 3-D and 4D imaging to investigate the role of fibronectin on corneal fibroblast patterning. We demonstrated for the first time that during migration into 3-D fibrin matrices, leading edge cells secrete and organize fibronectin into tracks. These fibronectin tracks are used as conduits by the cells behind them, which leads to the development of an interconnected cell/fibronectin network.
Integrins are a family of transmembrane heterodimers consisting of alpha and beta subtypes that serve to attach cells to the ECM. The primary integrin for fibronectin attachment is α5 αβ1 integrin [32, 45]. We observed α5 integrin labeling along fibronectin fibrils. When cells apply contractile force through integrins at focal adhesion sites, fibronectin is reorganized into fibrils which generally align with α5β1 integrin and actin filaments [46]. Co-alignment of α5 integrin and fibronectin was also observed in this study. One of the sites where α5β1 integrin binds fibronectin is the RGD motif of fibronectin [46–48]. Experiments using a synthetic RGD peptide to block integrin binding to fibronectin prevented cell spreading in fibrin matrices. The cell migration rate into 3-D fibrin was also significantly decreased when cells were exposed to RGD peptide. In contrast, no significant effect was observed when cells interacted with collagen matrices, as cells were able to spread normally. This is consistent with the fact that corneal fibroblast binding to collagen is mediated by α2β1 integrin [49].
It should be noted that α5β1 also binds to the synergic region of fibronectin, and it is reported that both the RGD motif and the synergic region of fibronectin are required to initiate fibronectin fibril formation [32, 50]. Other integrins can also bind fibronectin at different regions [32, 46]. In the current study, antibody blockade of the α5 integrin subunit using a specific anti α5 integrin antibody had the same effect as RGD; cells remained rounded. Using an anti-fibronectin antibody to interfere with cell attachment to fibronectin also prevented fibroblast spreading. These results further suggest that cell-fibronectin binding via α5β1 integrin is required for corneal fibroblast spreading in 3-D fibrin ECM. However we cannot exclude a role for other integrins in fibronectin attachment.
Fibronectin staining revealed a fibrillar pattern of organization within the tracks. Fibroblasts are known to assemble fibronectin into fibrils which can bind to fibrin ECM [33, 51]. In our dynamic study, fibronectin track formation directly coincided with cell spreading. Fibronectin fibrillogenesis often coincides with morphological changes and cell movement during embryonic development, and this can play an important role in patterning during morphogenesis [32, 52, 53]. We often observed more intense fibronectin fluorescence behind the leading edge of extending cell processes, which is where soluble fibronectin would likely be assembled and reorganized by tractional forces applied at the focal adhesion sites [54]. Importantly, our time-lapse observations demonstrated that while fibroblasts could secrete and organize new fibronectin tracks during spreading and migration, they generally remained within the preexisting fibronectin network. This more “confined” form of cell migration resulted in extensive cell-cell interactions and a reduction in the number of branching cell processes as compared to that observed during individual cell migration in 3-D collagen matrices [55, 56].
It should be noted that in addition to the fibronectin tracks, cell-induced reorganization of the fibrin matrix could also contribute to corneal fibroblast guidance and patterning. In 3-D matrices pre-fabricated with directional gradients in collagen density, fibroblasts migrate towards the stiffer region [57], a phenomenum called “durotaxis” [58]. Cell induced compaction of collagen into aligned tracks can play a role in the transition from individual to collective cell migration in cancer cell invasion [59]. In the current study, compaction of the fibrin ECM was observed between cells in correlation with the fibronectin tracks, thus it is possible that the increased mechanical stiffness of the compacted matrix helped guide cell migration. However, it should be noted that corneal fibroblasts in collagen matrices do not migrate collectively, even under contractile conditions when significant matrix compaction and alignment is observed. Furthermore, we previously demonstrated that collective migration and clustering occur in fibrin matrices even when contractility is inhibited by blocking Rho kinase [24]. Finally, corneal keratocytes maintained in serum-free media, which do not generate significant contractile forces, still formed an interconnected network that was correlated with fibronectin patterning.
The results of this study demonstrate that fibronectin plays a key role in corneal fibroblast migration and patterning in fibrin ECM, and that this mechanism is dependent on α5β1 integrin binding. Interestingly, in vivo studies of corneal injuries have shown an accumulation of fibronectin in the stroma during healing [39], and corneal myofibroblasts express and organize fibronectin [9, 41, 60]. Following full thickness incisional surgery in the rabbit, in which a fibrin plug is formed, corneal fibroblasts form an interconnected mesh as they migrate into the wound space, similar to the pattern of organization observed in the current study [27]. An interconnected mesh is also observed during wound healing following partial thickness incisions [61]. These interconnections between cells are hypothesized to mediate force transduction during wound contraction [26, 27].
In conclusion, our results suggest that corneal fibroblasts secrete, bind and organize fibronectin to facilitate spreading and migration within fibrin matrices. Other cells use these fibronectin tracks as conduits, which leads to the development of in an interconnected network of cells and fibronectin. These findings could explain, in part, the presence of organized cellular networks during incisional wound healing in the cornea, and the pattern of collective corneal fibroblast migration observed during intrastromal cell migration.
EXPERIMENTAL PROCEDURES
Materials
Dulbecco’s modified Eagle medium (DMEM), nonessential amino acids, and 0.25% trypsin/EDTA solution were purchased from Invitrogen (Gaithersburg, MD). Platelet-derived growth factor BB isotype (PDGF) was obtained from Upstate Biotechnology, Inc. (Lake Placid, NY). Fetal bovine serum (FBS), fatty acid-free fraction V bovine serum albumin (BSA), RPMI 1640 vitamin solution, HEPES, Sodium bicarbonate, and thrombin from human plasma were obtained from Sigma-Aldrich (St. Louis, MO). Penicillin, streptomycin, and amphotericin B were obtained from lonza inc. (Walkersville, MD). Type I rat tail collagen and plasma fibronectin were purchased from BD Biosciences (Bedford, MA). Alexa Fluor Phalloidin 488 and Propidium Iodide (PI) were obtained from Molecular Probes, Inc. (Eugene, OR). RNase (DNase free) was purchased from Roche (Indianapolis, IN). For immunostaining, a rabbit anti-human fibronectin polyclonal antibody (sc-9068), a rabbit anti- α5 integrin antibody (EPR7854) and a goat anti-human fibronectin polyclonal antibody (sc-6952) were used (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). FITC conjugated goat anti-rabbit and donkey anti-goat secondary antibodies were obtained from Jackson ImmunoResearch (West Grove, PA). DAPI was obtained from Molecular Probes (Eugene, OR). Cyclo-RGD and -RGE peptides were obtained from AnaSpec Inc (Fremont CA). FITC and Rhodamine labeled fibronectin were obtained from Cytoskeleton, Inc. (Denver, CO). Mouse anti-human α5 integrin blocking antibody (P1D6) and rabbit anti-human fibronectin blocking antibody (HFN 7.10) were obtained from Abcam (Cambridge, MA). LifeAct adenoviral vector (rAVCMV-LifeAct-TagGFP2) was purchased from ibidi USA (Madison, WI).
Cell Culture
A previously published human corneal fibroblast cell line (HTK cells) [62, 63] as well as primary cultures of corneal keratocytes were used. HTK fibroblasts were maintained in tissue culture flasks with DMEM containing 10% FBS, supplemented with 1% penicillin, 1% streptomycin and 1% amphotericin B. Corneal keratocytes (NRK cells) were isolated from rabbit eyes obtained from a slaughterhouse (Pel Freez, Rogers, AR, USA) as previously described [5, 64]. NRK cells were maintained in serum-free media composed of DMEM containing pyruvate, HEPES, 1% RPMI vitamin mix, 100µM non-essential amino acids, 100 µg/mL ascorbic acid and 1% penicillin/streptomycin/amphotericin B to maintain the keratocyte phenotype [5, 65].
Standard 3-D Matrix Model
Cell spreading and patterning was assessed in fibrillar collagen and fibrin matrices. In this model, 150 µl of neutralized solutions of collagen (2 mg/ml) or fibrin (1 mg/ml) containing 5×105 cells were poured onto glass bottom dishes (MatTek, model P35GC-1.5–14-C, Ashland, MA). The collagen solution was prepared by mixing high concentration rat tail type I collagen with 0.1 N NaOH, 10X DMEM and H2O to achieve a final concentration of 2 mg/ml. For fibrin matrices, fibrinogen was warmed for 20 minutes and mixed with DMEM to achieve a final concentration of 1 mg/ml, and the solution was mixed with 0.5 U/ml thrombin to initiate polymerization. Samples were then placed for 30 minutes in a humidified incubator (37°C, 5% CO2) to polymerize. Matrices were then gently rinsed twice with DMEM to remove excess thrombin, and 2 ml of media was then added to each sample. Experiments were carried out using serum-free media supplemented with 5 mg/ml BSA and 50 ng/ml PDGF, PDGF plus RGD (20 mM), PDGF plus RGE (20 mM ), PDGF plus mouse anti-human α5 integrin blocking antibody (P1D6, 1:100), or PDGF plus rabbit anti-human fibronectin blocking antibody (HFN 7.10, 1:100).
Nested Matrix Model
In order to study the pattern and amount of 3-D cell migration, cell-populated compressed collagen constructs were nested within acellular uncompressed fibrin matrices as previously described [24, 25, 55, 66]. In this model, 2ml of a neutralized collagen solution (4 mg/ml) is poured into a rectangular metal mold (3 cm length, 2 cm width, 1 cm height) and placed in a humidified incubator (37°C, 5% CO2) for 30 minutes for polymerization. Cells (6×106) are mixed with a second 2ml collagen solution, which is added on top of the first collagen layer. After 30 minutes to allow collagen polymerization, the construct is compressed as previously described [55, 66–68]. This produces a ~200 µm thick construct with an acellular ECM on the bottom and a cell-populated ECM on top. The first collagen layer serves as a spacer that prevents cells from contacting the glass substrate as they migrate out of the matrix, thus ensuring that they interact with the outer fibrin ECM.
To prepare the nested constructs, 6 mm diameter buttons from the compressed sandwiched matrices were cut with a trephine blade and gently placed on glass bottom dishes. Buttons were then covered with a 100 µl solution of fibrin (1 mg/ml). To prepare fibrin, fibrinogen was warmed for 20 minutes and mixed with DMEM to achieve a final concentration of 1 mg/ml, and the solution was mixed with 0.5 U/ml thrombin to initiate polymerization. Samples were then placed for 30 minutes in a humidified incubator (37°C, 5% CO2) to polymerize. After polymerization, constructs were rinsed twice with DMEM to remove excess thrombin, and 2 ml of media was then added to each sample. Constructs were cultured for 48 hours using serum-free media supplemented with 5 mg/ml BSA and 50 ng/ml PDGF, PDGF plus RGD (20 mM) or PDGF plus RGE (20 mM).
Quantitative analysis of cell migration was carried out as previously described [25, 56]. After 48 hours of culture, f-actin and nuclei were fluorescently labeled as detailed below. Maximum intensity projection images of cell nuclei were generated from image stacks using Metamorph (Molecular Devices Inc., version 7.7), and overlaid with reflection images. An index of cell migration was then determined by counting the number of cells (based on nuclear staining) that migrated from the inner matrix into the outer matrix of the nested constructs. Counts were collected from up to four 750µm wide strips in each construct, at approximately 90° intervals. Each strip included the border of the button and the furthest moving cell.
Fluorescent Labeling and Imaging of 3-D Constructs
For fluorescent labeling, both standard and nested constructs were fixed with 3% paraformadehyde in PBS for 10 min. For double labeling of F-actin and nuclei, cells were permeabilized with 0.5% Triton X-100 in PBS for 15 min, washed for 30 minutes, and incubated with Alexa Fluor 488 Phalloidin (1:150 ratio) for 60 minutes and then washed again for 60 minutes. Nuclear staining was carried out after f-actin labeling, by incubating samples for 30 minutes with Propidium Iodide (1:100) in PBS containing 1:100 RNase-DNase free. For double labeling of α5 integrin and F-actin in HTK cells, samples were blocked with 1% BSA fraction V in PBS for 1 hour, incubated for 120 minutes with a rabbit anti α5 integrin antibody at a ratio of 1:100, washed for 60 minutes with PBS, and then incubated for 60 minutes with FITC conjugated goat anti-rabbit secondary antibody. Samples were washed for 30 minutes, incubated with Alexa Fluor 546 Phalloidin (1:150 ratio) for 60 minutes and then washed for an additional 30 minutes. For double labeling of α5 integrin and fibronectin, samples were pre-incubated with 5 mg/ml Rhodamine fibronectin for 1 hour prior to fixation and immunolabeling for α5 integrin.
For triple labeling of fibronectin, F-actin and nuclei, some samples were permeabilized in Triton X-100 immediately after fixation, whereas others were permeabilized after fibronectin immunolabeling. Samples were blocked with 1% BSA fraction V in PBS for 1 hour. For HTK cells, samples were incubated for 120 minutes with a rabbit anti-human fibronectin polyclonal antibody at a ratio of 1:100, washed for 60 minutes with PBS, and then incubated for 60 minutes with FITC conjugated goat anti-rabbit secondary antibody. For α5 integrin labeling, the primary antibody was replaced with a rabbit monoclonal anti α5 integrin antibody. For NRK cells, samples were incubated for 120 minutes with a goat anti-human fibronectin polyclonal antibody at a ratio of 1:100, washed for 60 minutes with PBS, and then incubated for 60 minutes with FITC conjugated donkey anti-goat secondary antibody. After immunolabeling, samples were permeabilized and/or washed for 30 minutes, incubated with Alexa Fluor 546 Phalloidin (1:150 ratio) for 60 minutes and then washed for an additional 30 minutes. Nuclear staining was carried out after f-actin labeling, by incubating samples for 30 minutes with DAPI (1:100). All staining procedures were performed in the original culture plates to avoid cell or matrix distortion.
Fluorescently labeled samples were imaged using laser scanning confocal microscopy (Leica SP8, Heidelberg, Germany). Appropriate combinations of UV (405 nm), Argon (488 nm) and GreNe (543) lasers were used for fluorescent imaging, and a HeNe laser (633nm) was used for confocal reflection imaging of collagen and fibrin fibrils. Stacks of optical sections (z-series) were acquired using a 10x dry objective, a 25x water immersion objective and/or a 63x oil immersion objective. Sequential scanning was used to image double- and triple-labeled samples to prevent cross-talk between fluorophores.
Quantitative analysis of the area occupied by cells was carried out using Methamorph software. Z-stacks of images from 3-D matrices were combined into a single picture using the maximum intensity projection function. Subsequently the image was thresholded to separate cells from the background and converted to a binary image. The area occupied by cells in each image was then calculated and divided by the cell number to get the average area/cell.
Live Cell Imaging
For dynamic assessment of cell spreading and migration, additional samples were assessed using time-lapse imaging. To assess the general pattern of cell migration, a Nikon Eclipse inverted microscope (Nikon, Tokyo, Japan) equipped with an environmental chamber (In Vivo Scientific, MO) was used, as previously described [69, 70]. Z-series of images were taken at the border between the inner and outer matrices of nested constructs at 10 minute intervals using a 10X dry phase contrast objective. Imaging was carried out for up to 72 hours. To create movies of cell movements, a single plane from the z-series was selected at each time point, so that the same cells were in focus for the entire sequence. MetaMorph software was used to generate movies of cell movements.
For dynamic imaging of cytoskeletal dynamics and cell-induced fibronectin organization and patterning, cells were prepared for live-cell imaging as follows. For visualization of f-actin, cells were incubated for 48 h with the LifeAct adenoviral vector (1 µL/mL). LifeAct is a 17-amino acid peptide that stains filamentous actin (F-actin) structures in living or fixed eukaryotic cells and tissues. It has a TagGFP2 marker to allow visualization of the labeled f-actin using FITC optics. For visualization of fibronectin, 5 mg/ml Rhodamine fibronectin (rhodamine molecules covalently linked to pure fibronectin) was added to the media 60 minutes prior to the start of time-lapse imaging. For imaging of these fluorescently labeled samples, a Leica SP8 confocal microscope with an environmental chamber (Life Imaging Services, Basel, Switzerland) was used. An argon laser (488nm) was used for imaging F-actin and a GreNe (543nm) laser was used for Rhodamine fibronectin. 3-D stacks of 10 – 15 images (1–2 micron steps) were taken at 20 minutes intervals using a 63X oil immersion objective. To generate time lapse movies, maximum intensity projections were generated at each time point and combined into a single sequence using Metamorph. Experiments were carried out at least 3 times for each condition studied.
Statistical Analysis
Statistical analysis of cell migration and spreading area was performed using the analysis module within Sigmaplot (version 12.5, Systat Software, Inc., San Jose, CA). Analysis of variance (ANOVA) was used to compare group means. Post-hoc multiple comparisons between groups were performed using the Holm–Sidak method.
Supplementary Material
Supplemental Movie 1. Collective cell migration of corneal fibroblasts. Time-lapse phase contrast imaging (10 minute intervals) of migrating cells in a nested fibrin matrix model. Cells were cultured in PDGF. Recording started after 24h of incubation once cells appeared in the outside matrix. Arrow shows region where a line of cells temporarily separated, then reconnected along the same “track”. Total time of recording is 24 hours. Horizontal field width = 155 um.
Supplemental Movie 2. Corneal fibroblast spreading in a 3-D fibrin matrix. Time-lapse fluorescent imaging (20 minute intervals) of fibroblasts expressing LifeAct (green, left panel) cultured in PDGF containing Rhodamine fibronectin (white, middle panel). Right panel shows overlay of LifeAct (green) and Fibronectin (red). Note that accumulation and organization of fluorescent fibronectin occurs in parallel with cell spreading. Recording started after 1 hour of incubation. Total time of recording is 11 hours. Horizontal field width for each panel = 74 um.
Supplemental Movie 3. Corneal Fibroblast motility in a 3-D fibrin matrix. Time-lapse fluorescence imaging (20 minute intervals) of fibroblasts expressing LifeAct (green, left panel) cultured in PDGF containing Rhodamine fibronectin (white, right panel). Note that fibroblasts move in and out of the fibronectin network. Recording started after 16 hours of incubation. Total time of recording is 24 hours. Horizontal field width = 185 um.
Highlights.
Corneal fibroblasts form an interconnected network in 3-D fibrin matrix
The cellular network is confined within with extracellular fibronectin tracks
Fibroblast-fibronectin network formation is dependent on α5 β1 integrin
Similar patterns have been observed during in vivo wound healing
Acknowledgments
This study was supported in part by NIH R01 EY 013322, NIH P30 EY020799, and an unrestricted grant from Research to Prevent Blindness, Inc., NY, NY.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None
References
- 1.Pepose JS, Ubels JL. The Cornea. In: Hart WM, editor. Adler's Physiology of the Eye. Mosby Year Book; St. Louis: 1992. pp. 29–70. [Google Scholar]
- 2.Hassell JR, Birk DE. The molecular basis of corneal transparency. Exp Eye Res. 2010;91(3):326–35. doi: 10.1016/j.exer.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chakravarti S, Petroll WM, Hassell J, Jester JV, Lass JH, Paul J, Birk DE. Corneal opacity in lumican-null mice: defects in collagen fibril structure and packing in the posterior stroma. Invest Ophthalmol Vis Sci. 2000;41:3365–3373. [PMC free article] [PubMed] [Google Scholar]
- 4.Funderburgh JL, Mann MM, Funderburgh ML. Keratocyte phenotype mediates proteoglycan structure: a role for fibroblasts in corneal fibrosis. J Biol Chem. 2003;278:45629–45637. doi: 10.1074/jbc.M303292200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jester JV, Barry PA, Lind GJ, Petroll WM, Garana R, Cavanagh HD. Corneal keratocytes: In situ and in vitro organization of cytoskeletal contractile proteins. Invest Ophthalmol Vis Sci. 1994;35:730–743. [PubMed] [Google Scholar]
- 6.Lakshman N, Kim A, Petroll WM. Characterization of corneal keratocyte morphology and mechanical activity within 3-D collagen matrices. Exp Eye Res. 2010;90(2):350–9. doi: 10.1016/j.exer.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stramer BM, Zieske JD, Jung J-C, Austin JS, Fini ME. Molecular mechanisms controlling the fibrotic repair phenotype in cornea: implications for surgical outcomes. Invest Ophthalmol Vis Sci. 2003;44:4237–4246. doi: 10.1167/iovs.02-1188. [DOI] [PubMed] [Google Scholar]
- 8.Garana RM, Petroll WM, Chen WT, Herman IM, Barry P, Andrews HD, Cavanagh HD, Jester JV. Radial keratotomy II: The role of the myofibroblast in corneal wound contraction. Invest Ophthalmol Vis Sci. 1992;33:3271–3282. [PubMed] [Google Scholar]
- 9.Jester JV, Huang J, Barry-Lane PA, Kao WW, Petroll WM, Cavanagh HD. Transforming growth factor(beta)-mediated corneal myofibroblast differentiation requires actin and fibronectin assembly. Invest Ophthalmol Vis Sci. 1999;40:1959–1967. [PubMed] [Google Scholar]
- 10.Blalock TD, Duncan MR, Varela JC, Goldstein MH, Tuli MH, Grotensdorst GR, Schultz GS. Connective tissue growth factor expression and action in human corneal fibroblast cultures and rat corneas after photorefractive keratectomy. Invest Ophthalmol Vis Sci. 2003;44:1879–1887. doi: 10.1167/iovs.02-0860. [DOI] [PubMed] [Google Scholar]
- 11.Jester JV, Brown D, Pappa A, Vasiliou V. Myofibroblast differentiation modulates keratocyte crystallin protein expression concentration and cellular light scattering. Invest Ophthalmol Vis Sci. 2012;53(2):770–8. doi: 10.1167/iovs.11-9092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moller-Pedersen T, Cavanagh HD, Petroll WM, Jester JV. Corneal haze development after PRK is regulated by volume of stromal tissue removal. Cornea. 1998;17:627–639. doi: 10.1097/00003226-199811000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Boote C, Du Y, Morgan S, Harris J, Kamma-Lorger C, Hayes S, Lathrop K, Rob D, Burrow M, Hiller J, Terrill N, Funderburgh J, Meek K. Quantitative assessment of ultrastructure and light scatter in mouse corneal debridement wounds. Invest Ophthalmol Vis Sci. 2012;53(6):2786–2795. doi: 10.1167/iovs.11-9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moller-Pedersen T, Cavanagh HD, Petroll WM, Jester JV. Stromal wound healing explains refractive instability and haze development after photorefractive keratectomy: A 1-year confocal microscopic study. Ophthalmology. 2000;107:1235–1245. doi: 10.1016/s0161-6420(00)00142-1. [DOI] [PubMed] [Google Scholar]
- 15.Dupps WJ, Wilson SE. Biomechanics and wound healing in the cornea. Exp Eye Res. 2006;83:709–720. doi: 10.1016/j.exer.2006.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruberti JW, Roy AS, Roberts CJ. Corneal biomechanics and biomaterials. Annu Rev Biomed Eng. 2011;13:269–95. doi: 10.1146/annurev-bioeng-070909-105243. [DOI] [PubMed] [Google Scholar]
- 17.Schultz GS, Davidson JM, Kirsner RS, Bornstein P, Herman IM. Dynamic reciprocity in the wound microenvironment. Wound Repair Regen. 2011;19(2):134–48. doi: 10.1111/j.1524-475X.2011.00673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaw TJ, Martin P. Wound repair at a glance. J Cell Sci. 2009;122(Pt 18):3209–13. doi: 10.1242/jcs.031187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petroll WM, Kivanany PB, Hagenasr D, Graham EK. Corneal fibroblast migration patterns during intrastromal wound healing correlate with ECM structure and alignment. Invest Ophthalmol Vis Sci. 2015;56:7352–7361. doi: 10.1167/iovs.15-17978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petroll WM, Miron-Mendoza M. Mechanical interactions and crosstalk between corneal keratocytes and the extracellular matrix. Exp Eye Res. 2015;133:49–57. doi: 10.1016/j.exer.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nelson CM, Bissell MJ. Of extracellular matrix scaffolds, signaling: tissue architecture regulates development homeostasis and cancer. Annu Rev Cell Dev Biol. 2006;22:287–309. doi: 10.1146/annurev.cellbio.22.010305.104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghosh K, Ingber DE. Micromechanical control of cell and tissue development: implications for tissue engineering. Adv Drug Deliv Rev. 2007;59(13):1306–18. doi: 10.1016/j.addr.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 23.Cox TR, Erler JT. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis Model Mech. 2011;4(2):165–78. doi: 10.1242/dmm.004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miron-Mendoza M, Graham E, Kivanany P, Quiring J, Petroll WM. The role of thrombin and cell contractility in regulating clustering and collective migration of corneal fibroblasts in different ECM environments. Invest Ophthalmol Vis Sci. 2015;56(3):2079–90. doi: 10.1167/iovs.15-16388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miron-Mendoza M, Lin X, Ma L, Ririe P, Petroll WM. Individual versus collective fibroblast spreading and migration: regulation by matrix composition in 3D culture. Exp Eye Res. 2012;99:36. doi: 10.1016/j.exer.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petroll WM, Cavanagh HD, Barry P, Andrews P, Jester JV. Quantitative analysis of stress fiber orientation during corneal wound contraction. J Cell Sci. 1993;104:353–363. doi: 10.1242/jcs.104.2.353. [DOI] [PubMed] [Google Scholar]
- 27.Jester JV, Petroll WM, Barry PA, Cavanagh HD, Temporal 3-dimensional. cellular anatomy of corneal wound tissue. J Anat. 1995;186:301–311. [PMC free article] [PubMed] [Google Scholar]
- 28.Kivanany PB, Grose KC, Petroll WM. Temporal and spatial analysis of stromal cell and extracellular matrix patterning following lamellar keratectomy. Exp Eye Res. 2016;153:56–64. doi: 10.1016/j.exer.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hegerfeldt Y, Tusch M, Brocker E-B, Friedl P. Collective cell movement in primary melanoma explants: plasticity of cell-cell interaction b1-integrin function and migration strategies. Cancer Res. 2002;62:2125–2130. [PubMed] [Google Scholar]
- 30.Ilina O, Friedl P. Mechanisms of collective cell migration at a glance. J Cell Sci. 2009;122(Pt 18):3203–8. doi: 10.1242/jcs.036525. [DOI] [PubMed] [Google Scholar]
- 31.Friedl P, Zallen JA. Dynamics of cell-cell and cell-matrix interactions in morphogenesis, regeneration and cancer. Curr Opin Cell Biol. 2010;22(5):557–9. doi: 10.1016/j.ceb.2010.08.024. [DOI] [PubMed] [Google Scholar]
- 32.Singh P, Carraher C, Schwarzbauer JE. Assembly of fibronectin extracellular matrix. Annu Rev Cell Dev Biol. 2010;26:397–419. doi: 10.1146/annurev-cellbio-100109-104020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grinnell F, Feld M, Minter D. Fibroblast adhesion to fibronectin and fibrin substrata: requirement for cold-insoluble globulin (plasma fibronectin) Cell. 1980;19:517–525. doi: 10.1016/0092-8674(80)90526-7. [DOI] [PubMed] [Google Scholar]
- 34.Greiling D, Clark RA. Fibronectin provides a conduit for fibroblast transmigration from collagenous stroma into fibrin clot provisional matrix. J Cell Sci. 1997;100:861–870. doi: 10.1242/jcs.110.7.861. [DOI] [PubMed] [Google Scholar]
- 35.Zollinger AJ, Smith ML. Fibronectin the extracellular glue. Matrix biology : journal of the International Society for Matrix Biology. 2017;60–61:27–37. doi: 10.1016/j.matbio.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 36.Reinhart-King CA. How matrix properties control the self-assembly and maintenance of tissues. Ann Biomed Eng. 2011;39(7):1849–56. doi: 10.1007/s10439-011-0310-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou X, Rowe RG, Hiraoke N, George JP, Wirts D, Mosher DF, Virtanen I, Chernousov MA, Weiss SJ. Fibronectin fibrillogenesis regulates three-dimensional neovessel formation. Gene Dev. 2008;22:1231–1243. doi: 10.1101/gad.1643308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.da Rocha-Azevedo B, Ho C-H, Grinnell F. Fibroblast cluster formation on 3D collagen matrices requires cell contraction dependent fibronectin matrix organization. Exp Cell Res. 2013;319(4):546–555. doi: 10.1016/j.yexcr.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jester JV, Petroll WM, Cavanagh HD. Corneal stromal wound healing in refractive surgery: the role of the myofibroblast. Prog. Retinal. Eye. Res. 1999;18:311–356. doi: 10.1016/s1350-9462(98)00021-4. [DOI] [PubMed] [Google Scholar]
- 40.Jester JV, Barry-Lane PA, Petroll WM, Olsen DR, Cavanagh HD. Inhibition of cornea fibrosis by topical application of blocking antibodies to TGFbeta in the rabbit. Cornea. 1999 [PubMed] [Google Scholar]
- 41.Sandbo N, Dulin N. Actin cytoskeleton in myofibroblast differentiation: ultrastructure defining form and driving function. Transl Res. 2011;158(4):181–96. doi: 10.1016/j.trsl.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pankov R, Yamada KM. Fibronectin at a glance. J Cell Sci. 2002;115(Pt 20):3861–3. doi: 10.1242/jcs.00059. [DOI] [PubMed] [Google Scholar]
- 43.Robinson EE, Foty RA, Corbett SA. Fibronectin matrix assembly regulates alpha5beta1-mediated cell cohesion. Mol Biol Cell. 2004;15(3):973–81. doi: 10.1091/mbc.E03-07-0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruoslahti E. RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- 45.Humphries MJ, Travis MA, Clark K, Mould AP. Mechanisms of integration of cells and extracellular matrices by integrins. Biochem Soc Trans. 2004;32(Pt 5):822–5. doi: 10.1042/BST0320822. [DOI] [PubMed] [Google Scholar]
- 46.Leiss M, Beckmann K, Giros A, Costell M, Fassler R. The role of integrin binding sites in fibronectin matrix assembly in vivo. Curr Opin Cell Biol. 2008;20(5):502–7. doi: 10.1016/j.ceb.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 47.Pytela R, Pierschbacher MD, Ruoslahti E. Identification and isolation of a 140 kd cell surface glycoprotein with properties expected of a fibronectin receptor. Cell. 1985;40(1):191–8. doi: 10.1016/0092-8674(85)90322-8. [DOI] [PubMed] [Google Scholar]
- 48.Gouveia RM, Castelletto V, Alcock SG, Hamley IW, Connon CJ. Bioactive films produced from self-assembling peptide amphiphiles as versatile substrates for tuning cell adhesion and tissue architecture in serum-free conditions. J Mater Chem B. 2013;1(44):6157–6169. doi: 10.1039/c3tb21031f. [DOI] [PubMed] [Google Scholar]
- 49.Andresen JL, Ledet T, Hager H, Josephsen K, Ehlers N. The influence of corneal stromal matrix proteins on the migration of human corneal fibroblasts. Exp Eye Res. 2000;71:33–43. doi: 10.1006/exer.2000.0850. [DOI] [PubMed] [Google Scholar]
- 50.Sechler JL, Corbett SA, Schwarzbauer JE. Modulatory roles for integrin activation and the synergy site of fibronectin during matrix assembly. Mol Biol Cell. 1997;8(12):2563–73. doi: 10.1091/mbc.8.12.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wierzbicka-Patynowski I, Schwarzbauer JE. Regulatory role for SRC and phosphatidylinositol 3-kinase in initiation of fibronectin matrix assembly. J Biol Chem. 2002;277(22):19703–8. doi: 10.1074/jbc.M200270200. [DOI] [PubMed] [Google Scholar]
- 52.Schwarzbauer JE, DeSimone DW. Fibronectins, their fibrillogenesis, and in vivo functions. Cold Spring Harb Perspect Biol. 2011;3(7) doi: 10.1101/cshperspect.a005041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Davidson LA, Keller R, DeSimone DW. Assembly and remodeling of the fibrillar fibronectin extracellular matrix during gastrulation and neurulation in Xenopus laevis. Dev Dyn. 2004;231(4):888–95. doi: 10.1002/dvdy.20217. [DOI] [PubMed] [Google Scholar]
- 54.Gudzenko T, Franz CM. Studying early stages of fibronectin fibrillogenesis in living cells by atomic force microscopy. Mol Biol Cell. 2015;26(18):3190–204. doi: 10.1091/mbc.E15-06-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim A, Lakshman N, Karamichos D, Petroll WM. Growth factor regulation of corneal keratocyte differentiation and migration in compressed collagen matrices. Invest Ophthalmol Vis Sci. 2010;51(2):864–75. doi: 10.1167/iovs.09-4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim A, Zhou C, Lakshman N, Petroll WM. Corneal stromal cells use both high- and low-contractility migration mechanisms in 3-D collagen matrices. Exp Cell Res. 2012;318:741. doi: 10.1016/j.yexcr.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hadjipanayi E, Mudera V, Brown RA. Guding cell migration in 3D: A collagen matrix with graded directional stiffness. Cell Motil Cytoskel. 2009;66:121–129. doi: 10.1002/cm.20331. [DOI] [PubMed] [Google Scholar]
- 58.Lo CM, Wang HB, Dembo M, Wang YL. Cell movement is guided by the rigidity of the substrate. Biophys J. 2000;79(1):144–52. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wolf K, Wu YI, Liu Y, Geiger J, Tam E, Overall C, Stack MS, Friedl P. Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat Cell Biol. 2007;9:893–904. doi: 10.1038/ncb1616. [DOI] [PubMed] [Google Scholar]
- 60.Jester JV, Huang J, Petroll WM, Cavanagh HD. TGFbeta induced myofibroblast differentiation of rabbit keratocytes requires synergistic TGFbeta PDGF and integrin signalling. Exp Eye Res. 2002;75:645–657. doi: 10.1006/exer.2002.2066. [DOI] [PubMed] [Google Scholar]
- 61.Petroll WM, Cavanagh HD, Jester JV. Assessment of stress fiber orientation during healing of radial keratotomy wounds using confocal microscopy. Scanning. 1998;20:74–82. doi: 10.1002/sca.1998.4950200202. [DOI] [PubMed] [Google Scholar]
- 62.Jester JV, Huang J, Fisher S, Spiekerman J, Chang JH, Wright WE, Shay JW. Myofibroblast differentiation of normal human keratocytes hTERT extended-life human corneal fibroblasts. Invest Ophthalmol Vis Sci. 2003;44:1850–1858. doi: 10.1167/iovs.02-0973. [DOI] [PubMed] [Google Scholar]
- 63.Petroll WM, Ma L, Kim A, Ly L, Vishwanath M. Dynamic assessment of fibroblast mechanical activity during Rac-induced cell spreading in 3-D culture. J Cell Physiol. 2008;217:162–171. doi: 10.1002/jcp.21487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lakshman N, Petroll WM. Growth factor regulation of corneal keratocyte mechanical phenotypes in 3-D collagen matrices. Invest Ophthalmol Vis Sci. 2012;53:1077–1086. doi: 10.1167/iovs.11-8609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jester JV, Chang J-H. Modulation of cultured corneal keratocyte phenotype by growth factors/cytokines control in vitro contractility and extracellular matrix contraction. Exp Eye Res. 2003;77:581–592. doi: 10.1016/s0014-4835(03)00188-x. [DOI] [PubMed] [Google Scholar]
- 66.Karamichos D, Lakshman N, Petroll WM. An experimental model for assessing fibroblast migration in 3-D collagen matrices. Cell Motil Cytoskel. 2009;66(1):1–9. doi: 10.1002/cm.20326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brown RA, Wiseman M, Chuo C-B, Cheema U, Nazhat SN. Ultrarapid engineering of biomimetic materials and tissues: fabrication of nano- and microstructures by plastic compression. Adv. Funct. Mater. 2005;15:1762–1770. [Google Scholar]
- 68.Neel EAA, Cheema U, Knowles JC, Brown RA, Nazhat SN. Use of multiple unconfined compression for control of collagen gel scaffold density and mechanical properties. Soft. Matter. 2006;2:986–992. doi: 10.1039/b609784g. [DOI] [PubMed] [Google Scholar]
- 69.Zhou C, Petroll WM. Rho Kinase Regulation of Fibroblast Migratory Mechanics in Fibrillar Collagen Matrices. Cell Mol Bioeng. 2010;3(1):76–83. doi: 10.1007/s12195-010-0106-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhou C, Petroll WM. MMP regulation of corneal keratocyte motility and mechanics in 3-D collagen matrices. Exp Eye Res. 2014;121:147–60. doi: 10.1016/j.exer.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Movie 1. Collective cell migration of corneal fibroblasts. Time-lapse phase contrast imaging (10 minute intervals) of migrating cells in a nested fibrin matrix model. Cells were cultured in PDGF. Recording started after 24h of incubation once cells appeared in the outside matrix. Arrow shows region where a line of cells temporarily separated, then reconnected along the same “track”. Total time of recording is 24 hours. Horizontal field width = 155 um.
Supplemental Movie 2. Corneal fibroblast spreading in a 3-D fibrin matrix. Time-lapse fluorescent imaging (20 minute intervals) of fibroblasts expressing LifeAct (green, left panel) cultured in PDGF containing Rhodamine fibronectin (white, middle panel). Right panel shows overlay of LifeAct (green) and Fibronectin (red). Note that accumulation and organization of fluorescent fibronectin occurs in parallel with cell spreading. Recording started after 1 hour of incubation. Total time of recording is 11 hours. Horizontal field width for each panel = 74 um.
Supplemental Movie 3. Corneal Fibroblast motility in a 3-D fibrin matrix. Time-lapse fluorescence imaging (20 minute intervals) of fibroblasts expressing LifeAct (green, left panel) cultured in PDGF containing Rhodamine fibronectin (white, right panel). Note that fibroblasts move in and out of the fibronectin network. Recording started after 16 hours of incubation. Total time of recording is 24 hours. Horizontal field width = 185 um.