Abstract
Factors governing the steady-state IOP have been extensively studied; however, the dynamic aspects of IOP are less understood. Clinical studies have suggested that intraocular pressure (IOP) fluctuation may be associated with glaucoma risk. This study aims to investigate how stiffening of corneoscleral biomechanical properties affects IOP spikes induced by rapid microvolumetric change. Porcine eyes (n = 25 in total) were subjected to volumetric infusions before and after external treatment of a circular area (11 mm diameter) in either the central cornea or posterior sclera. The treated area in the control group was immersed in phosphate-buffered saline (PBS) for 40 min, while the treated area of the chemical crosslinking group was immersed in 4% glutaraldehyde/PBS for 40 min. A subset of the sham-treated eyes was also subjected to volumetric infusions at a raised steady-state IOP. The magnitude of IOP spikes increased after localized chemical crosslinking of either the cornea (27.5% increase, p < 0.001) or the sclera (14.3% increase, p < 0.001) with corneal crosslinking having a stronger effect than scleral crosslinking (p = 0.018). We also observed that raising the steady-state IOP from 15 to 25 mmHg resulted in marked increase in IOP spike magnitudes by 63.9% (p < 0.001). These results suggested that an increased corneoscleral stiffness could significantly increase IOP spike magnitudes at the same volumetric change. Corneal stiffness appeared to have a strong impact on the IOP spike magnitude and may play a major role in regulating rapid volume-pressure dynamics. An increase in steady-state IOP also resulted in larger IOP fluctuations due to the increased “apparent” stiffness of the ocular shell, suggesting a potential interaction between the magnitude of IOP and its fluctuations. Corneoscleral properties may represent additional pathways for understanding and managing glaucoma risk and warrant future investigation.
Keywords: corneoscleral biomechanics, crosslinking, IOP spikes
1. Introduction
Intraocular pressure (IOP) is currently the only known risk factor that can be modified to treat glaucoma; however, IOP alone is not a sufficient predictor of glaucoma development or progression (Gordon et al., 2002; Lee et al., 2014). IOP fluctuation has been suggested as an additional risk factor, as larger fluctuations appeared to correlate with poorer prognosis (Asrani et al., 2000; Musch et al., 2011; Srinivasan et al., 2016). Although its mechanisms and role in glaucoma are still unclear, further understanding of the dynamic aspects of IOP presents an opportunity to gain a more complete understanding of the relationship between glaucoma risk and IOP. (Leidl et al., 2014; Mansouri et al., 2013).
The steady-state IOP is established when there is a balance between aqueous production and outflow, resulting in a constant intraocular volume under a constant pressure. When there is a net change in intraocular volume, caused by blood and/or aqueous flow changes (Friberg et al., 1987; Stamer and Acott, 2012) or displacement of fluid in the eye during blinking or eye rubbing (Coleman and Trokel, 1969), IOP fluctuates. A typical example is the cyclic ocular pulse generated by blood entering and leaving the eye at each heartbeat. Biomechanically, the intraocular volume change underlies the IOP fluctuation, and the mechanical properties of the ocular coat, which encloses the ocular volume, are thus linked to the characteristics of IOP fluctuation. It is reasonable to expect that a compliant shell would experience smaller IOP spikes at the same volumetric change while a stiffer eye may be subject to larger IOP fluctuations.
We have previously presented an infusion model to study the IOP spikes induced by rapid microvolumetric changes. This model was developed based on previous findings in rabbits showing that the postmortem eye has a pressure-volume relationship close to that of the live eye under normal mean arterial pressure (70–100 mmHg) (Kiel, 1995). Infusion in the donor eye thus provides a convenient model to simulate the live eye under normal blood pressure and analyze the corneoscleral effects on dynamic IOP under well-controlled conditions. Using this model, we have shown a dose-dependent effect of corneal stiffening on IOP spikes (Liu and He, 2009) and a correlation between stiffness of the native posterior sclera and IOP spikes (Morris et al., 2013) in porcine eyes.
In this study, we used the same infusion model to evaluate the impact on IOP fluctuation by either stiffening a local area of the corneoscleral shell or increasing steady-state IOP. We first investigated the effect of corneoscleral biomechanical properties on dynamic IOP by comparing the outcome of corneal and scleral stiffening. Corneal stiffening has become a clinical therapy for keratoconus and other corneal conditions (Iseli et al., 2008; Wollensak et al., 2003). Stiffening of the posterior sclera has also been proposed as a potential treatment for glaucoma (Campbell et al., 2017; Coudrillier et al., 2016; Kimball et al., 2014) and myopia (Dotan et al., 2014; Liu et al., 2016; Wollensak and Spoerl, 2004). It would therefore be interesting to evaluate the level of changes in dynamic IOP that can be expected with localized stiffening of the ocular shell. Moreover, we evaluated how an increased steady-state IOP affects IOP spikes, because an increase in IOP effectively increases the “apparent” stiffness of the entire corneoscleral shell due to the nonlinear mechanical properties of the ocular shell (Cruz Perez et al., 2014; Eliaghi et al., 2010; Girard et al., 2009). This allows us to gain insight into the relationship between the clinical mean IOP and its fluctuations from the perspective of corneoscleral biomechanical properties.
2. Methods
2.1 Sample Preparation
Twenty-five porcine globes were obtained within 6 hours of slaughter from a local meatpacking company and stored in phosphate-buffed saline (PBS) at 4° C. Directly before use, the connective tissue and muscles were removed to expose the underlying sclera. To reduce postmortem corneal swelling, the globes were immersed in a 10% dextran solution at 4° C for 1 hour (Terry et al., 1994). The globes were then rinsed in PBS, and a digital caliper was used to measure the nasal-temporal, superior-inferior and anterior-posterior lengths for estimating total ocular volume.
The porcine globes were divided into two categories based on the area of treatment: the cornea group (n = 10 total) and the sclera group (n = 15 total). These groups were then further divided into two treatment groups: the control group (n = 5 for cornea, n = 6 for sclera) and the crosslinked group (n = 6 for cornea, n = 10 for sclera). Four eyes from each control group (n = 8 total) were also used to study the effect of steady-state IOP by performing infusions at both 15 mmHg and 25 mmHg baselines.
2.2 Experimental Setup and Preconditioning
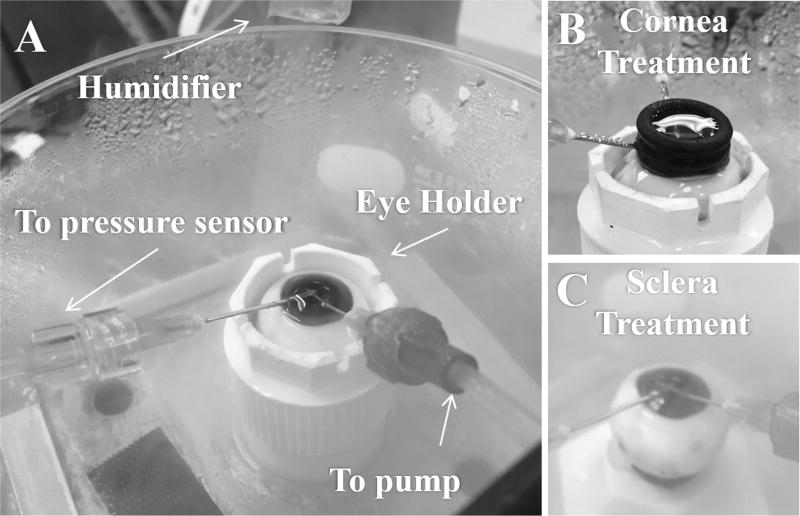
The globes were placed in a custom-made holder that mimics the eye socket (Figure 1A). A 20G needle attached to a programmable syringe pump (UltraPHD, Harvard Apparatus, Boston, MA) was inserted through the nasal side of the peripheral cornea near the limbus and into the posterior chamber of the eye, with the bevel opening oriented towards the lens. Another 20G needle attached to a pressure sensor (P75, Harvard Apparatus) was inserted through the peripheral cornea along the superior-inferior axis into the anterior chamber and allowed for continuous IOP measurements during experimentation. A humidifier was used to keep the globe moist during experimentation. The holder and needle placement is shown in Figure 1A.
Figure 1.
A. Porcine globe in an eye holder. The needle connecting to the syringe pump was inserted into the posterior chamber for infusion and withdrawal, whereas the needle connecting to the pressure sensor was inserted into the anterior chamber for monitoring of IOP. B. The setup for cornea treatment, and C. the setup for sclera treatment, as described in 2.3.
A customized LabVIEW program (National Instruments, Austin, TX) was used to control infusion of PBS via the syringe pump into the globes. Prior to experimentation, 5–8 ml of PBS was flushed through the globe to remove any residual bubbles through the system. The flow rate of the pump was then adjusted until IOP stabilized at 15 mmHg, which was recorded as the outflow rate of the eye. To precondition the globe, the flow rate was temporarily raised by 0.42 µl/s until IOP reached 30 mmHg. The IOP was then held at 30 mmHg for 3 minutes, after which the eye was returned to 15mmHg by adjusting the pump flow rate back to the outflow rate. This procedure was repeated until all residual choroidal blood was expelled from the globe (Morris et al., 2013). Finally, to ensure a stable and repeatable response, a brief test infusion of 7.5 µl of fluid in 0.5 seconds (rate of 15 µl/s) was performed after 5 minutes of equilibration at 15 mmHg. These test runs were repeated until the IOP spikes of two consecutive infusions were within 0.1 mmHg of each other. Prior to all infusion experiments, the globes were equilibrated at baseline IOP for 30 minutes.
2.3 Corneal and scleral stiffening in porcine eyes
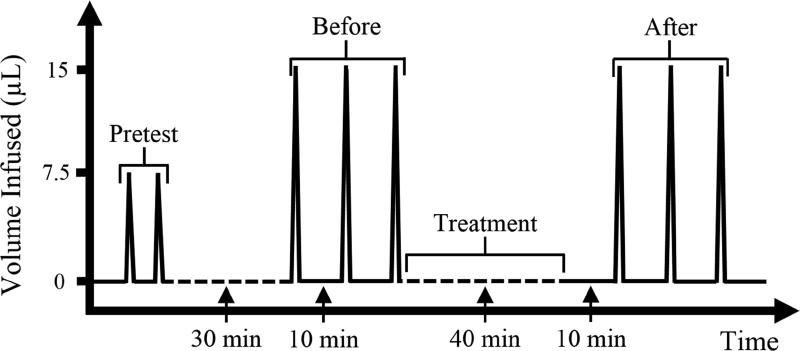
The porcine eyes went through a treatment procedure to evaluate the effects of stiffening either the cornea or the sclera. Before treatment, each globe went through three repeated infusions (the “before” series) at a baseline of 15 mmHg at a rate of 15 µl/s (Figure 2). As our previous study showed high correlation between three different infusion rates in porcine eyes (Morris et al., 2013), only one infusion rate was used in this study. Chemical crosslinking was then performed on either an area of the central cornea or an area of the posterior sclera. During treatment, IOP was maintained at 15 mmHg via a column of fluid. For corneal treatment, a small reservoir of solution was created on top of the central cornea via a rubber ring with an inner diameter of 11 mm (Figure 1B). The solution was 0.5 ml of either PBS (for control group) or 4% glutaraldehyde in PBS (for crosslinking group). For scleral treatment, the posterior sclera was lowered into a prefilled rubber ring of similar fluid volume (Figure 1C). The rubber rings were secured onto the cornea or sclera with ultrasound gel. The tissue was treated for a total of 40 minutes and the solution in all groups was exchanged afresh every 13 minutes. This treatment protocol was designed to induce sufficient tissue fixation/stiffening based on previous experiments (Coudrillier et al., 2016; Liu and He, 2009; Spoerl and Seiler, 1999; Thornton et al., 2009). Given the small volume that can be held by the rubber ring, we replenished the solution to avoid the depletion of effective fixative molecules over time. After treatment, the globes were equilibrated at 15 mmHg for 10 minutes before three repeated infusions were performed (the “after” series). A time series representation of this process is shown in Figure 2. The average of the IOP spikes obtained in each series (before and after) is reported.
Figure 2.
Experimental protocol for studying the effects of corneal or scleral crosslinking. Each spike represents one infusion and one withdrawal at 15 µl/s.
2.4 Comparison between different steady-state IOP’s
In a subset of the control group described above (n = 8, 4 with corneal control treatment and 4 with scleral control treatment), additional infusion experiments were conducted at a raised steady-state IOP (i.e. 25 mmHg). The globes were first equilibrated for 30 minutes at the new steady-state IOP and three repeated infusions were performed as described above. The magnitudes of the IOP spikes obtained at both steady-states from the same eye were compared.
2.5 Statistical Analysis
All statistical analysis was performed using SAS software (version 9.4, SAS Institute, Inc. Cary, NC). The IOP spikes were summarized using mean and standard deviation before and after the treatment for all four groups. Paired t-tests were used to compare changes in IOP spike magnitudes before and after treatment for all four groups and to compare difference in IOP spike magnitudes at 15 mmHg and 25 mmHg steady-state IOPs. Two-sample t-tests were used to compare the percent differences (before and after treatment) between control and crosslinking groups, as well as the corneal crosslinking and scleral crosslinking groups.
3. Results
The average ocular volume of the 25 tested porcine globes was 5.80 ± 0.58 mL (range 4.48–6.71 mL), and the average outflow rate was 0.053 ± 0.029 µL/s (range 0.020–0.133 µL/s).
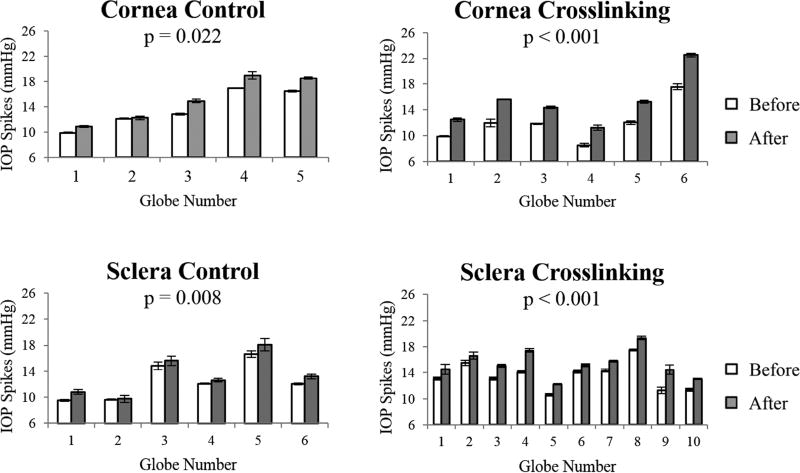
The IOP spikes in porcine globes showed no difference between any groups before treatment (all p’s > 0.05). In all groups including the controls, the IOP spikes increased after treatment (all p’s < 0.05). IOP spikes in each eye before and after corneal or scleral treatment are presented in Figure 3, and the average and standard deviation for each group are reported in Table 1.
Figure 3.
IOP spikes before and after treatment in the control and crosslinking groups (error bars are standard deviation of the three repeated infusions in the same eye), showing a substantial increase in IOP spike magnitudes in the crosslinked eyes and a small increase in control eyes.
Table 1.
IOP spikes (mean ± standard deviation) before and after treatment (sham or glutaraldehyde crosslinking) in porcine eyes.
| Magnitude of IOP Spikes (mmHg) | ||||||
|---|---|---|---|---|---|---|
| Region | Treatment | n | Before | After | Percent Change | P-value |
| Cornea | Control | 5 | 13.6 ± 2.7 | 15.1 ± 3.3 | 10.4% ± 5.2% | 0.022 |
| Crosslinked | 6 | 12.0 ± 2.8 | 15.3 ± 3.6 | 27.5% ± 3.4% | <0.001 | |
| P-value | 0.001 | |||||
| Sclera | Control | 6 | 12.5 ± 2.6 | 13.3 ± 2.8 | 7.2% ± 4.1% | 0.008 |
| Crosslinked | 10 | 13.5 ± 2.0 | 15.4 ± 2.0 | 14.3% ± 6.5% | <0.001 | |
| P-value | 0.025 | |||||
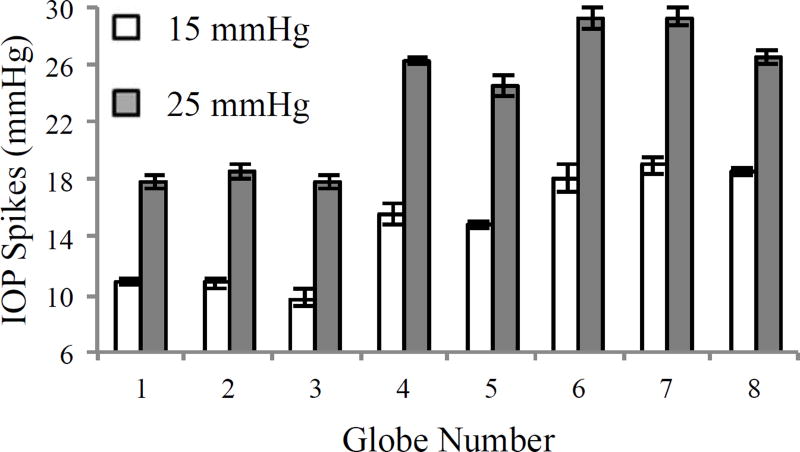
In the 8 control globes tested at different steady-states, a substantial increase in IOP spikes was observed after raising the steady-state IOP from 15 to 25 mmHg (14.70 ± 3.53 mmHg vs. 23.78 ± 4.65 mmHg, p < 0.001, Figure 4). A similar IOP spike increase was observed in globes tested at different steady-states after scleral stiffening treatment (see supplementary material).
Figure 4.
IOP spikes due to fast infusion (15 µl/s) at a steady-state IOP of 15 mmHg or 25 mmHg (error bars are standard deviation of three repeated infusions in the same eye).
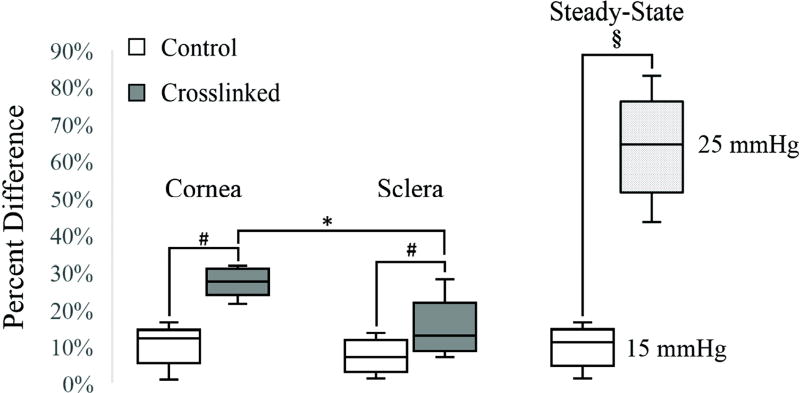
Figure 5 shows the percent change in IOP spikes before and after treatment in the corneal crosslinked and scleral crosslinked groups, as well as the group at a raised steady-state IOP. A rise in steady-state IOP from 15 to 25 mmHg resulted in marked increase in IOP spike magnitudes, much more than what was achieved from localized corneal or scleral stiffening (63.9% vs. 27.5% and 14.3%, respectively). Corneal or scleral crosslinking significantly increased IOP spikes as compared to the respective control group (p < 0.001 and p = 0.025, respectively). Comparing the “after” treatment responses, corneal crosslinking resulted in a larger IOP spikes change than scleral crosslinking (p = 0.018).
Figure 5.
Box-and-whiskers plots for the percent differences in IOP spikes in the control, crosslinked, and steady-state increase groups in porcine globes. While crosslinking had a significantly larger increase in IOP spikes as compared to the control groups (#: p < 0.001 for cornea and p = 0.025 for sclera), corneal crosslinking had a significantly larger effect than scleral crosslinking (*: p = 0.018). Steady-state IOP change from 15 to 25 mmHg in the control globes introduced an even higher increase in IOP spikes due to the overall stiffening of the shell at a higher baseline IOP (§: p < 0.001).
4. Discussion
Rapid IOP spikes are not well understood due to the difficulty in measuring these parameters in vivo. In this study, we applied an ex vivo infusion model in porcine globes to understand how stiffening the corneoscleral shell affects IOP spikes. Our results showed larger IOP spikes occur after localized chemical crosslinking of either the central cornea or the posterior sclera, with corneal crosslinking having a stronger effect than scleral crosslinking. We also observed that increasing the steady-state IOP significantly increased the magnitude of IOP spikes in the same eye. Combined with our previous studies (Cruz Perez et al., 2013; Liu and He, 2009; Morris et al., 2013), these results indicate that dynamic IOP is strongly influenced by the biomechanical properties of the corneoscleral shell, and that the cornea may play a major role in regulating rapid volume-pressure dynamics.
We observed that stiffening either the cornea or the sclera using chemical crosslinking resulted in larger IOP spikes (Figure 3 and Table 1) consistent with the trend reported in our previous study (Liu and He, 2009) using a different infusion rate and volume. Although the glaucoma risk associated with IOP spikes has not been definitively shown in humans, both in vitro and in vivo studies in rats have shown that IOP spikes were more damaging to retinal ganglion cells and their axons than gradually increased IOP to the same magnitude (Resta et al., 2007). In addition, the damage from IOP spikes is accumulative (Resta et al., 2007), suggesting that higher IOP spikes due to permanently altered corneoscleral stiffness may increase glaucoma risk over time. A recent study using a mouse glaucoma model showed increased axonal damage after posterior scleral stiffening (Kimball et al., 2014). Stiffening of the peripapillary sclera has been hypothesized as a protective treatment to halt glaucoma progression by limiting the strains experienced by the lamina cribrosa and other optic nerve head structures (Strouthidis and Girard, 2013). These results suggest that the treatment benefits of corneoscleral stiffening may need to be weighed against the potential risk of heightened IOP spikes.
We also observed that corneal crosslinking had a more significant impact on IOP spikes than scleral crosslinking (Figure 5), which suggests that the cornea may be the primary responsive tissue on the ocular shell to volumetric changes. As the sclera is known to have a larger modulus than the cornea (Cruz Perez et al., 2013; Meek, 2008; Woo et al., 1972), it deforms less in response to volumetric variations. A previous study that subjected corneal buttons to a volume infusion at constant flow rate (Johnson et al., 2007) showed significant corneal expansion using OCT imaging as pressure increased and hypothesized that this expansion may act as a damping mechanism that protects the eye from pressure spikes associated with microvolumetric changes. Johnson et al’s study mounted the corneal buttons on a rigid artificial anterior chamber and thus may not accurately reflect the in vivo corneal inflation under IOP elevations. We have previously observed significant corneal deformation especially through-thickness compression using high-frequency ultrasound speckle tracking (Palko et al., 2014) in eyes mounted at the posterior sclera preserving the corneal boundary conditions. Other corneal inflation studies have reported similar results showing significant corneal deformability during inflation (Boyce et al., 2008; Kling et al., 2010). These observations suggest potential mechanisms linking the cornea to glaucoma risk, as first reported in the Ocular Hypertension Treatment study (Gordon et al., 2002), where a thin cornea was found to be the most potent predictor for glaucoma progression in ocular hypertensives.
It is noted that there was a small but statistically significant increase in IOP spikes after the sham treatment in the controls (Figure 3 and Table 1). This is most likely caused by a slight change in the dehydration status of the ocular shell as both the cornea and sclera dry out quickly in air, resulting in a stiffer response overall. Although we used a humidity chamber to maintain moisture within the outer shell, previous ultrasound imaging data obtained in our lab (not shown) suggests that there was a slight decrease in tissue thickness over the time of experimentation due to the slight dehydration of the outer layer of the ocular coat. Our results (Fig. 5) showed that the cross-linked groups had significantly higher IOP spikes than the control groups after respective treatments in the cornea (p < 0.001) or sclera (p = 0.025), suggesting that the crosslinking response was in addition to the control response.
We also found a significant increase in IOP spike magnitudes after raising the steady-state IOP from 15 mmHg to 25 mmHg (Figures 4 and 5). This response is expected because of the nonlinear mechanical behavior of the corneoscleral shell. Both the cornea and the sclera consist of collagen fibers that have higher resistance to deformation when they are stretched (Meek, 2008; Palko and Liu, 2016; Woo et al., 1972). A higher steady-state IOP results in more pre-stretched collagen fibers and a stiffer response. Previous studies have shown that the naturally occurring ocular pulse has an amplitude that is positively correlated with IOP in healthy human subjects (Kaufmann et al., 2006). Similar results were reported in nonhuman primates based on telemetric data (Downs et al., 2011). This result suggests that an elevated IOP would also result in higher IOP spikes, and it this may be difficult to separate the risk associated with mean IOP from IOP fluctuations. It is noted that the percent increase in IOP spikes was much larger when the steady-state IOP was raised as compared to localized corneal or scleral crosslinking (Figure 5). We also performed an additional explorative study on globes with scleral stiffening. When the steady-state IOP was raised to 25 mmHg in these globes, the increase in IOP spike magnitudes as compared to that at a 15 mmHg steady-state was not significantly different from that found in control globes (see supplementary data). Raising the steady-state IOP effectively increased the stiffness of the entire corneoscleral shell, whereas chemical crosslinking treatment only alters a small region. This suggests that ubiquitous alterations in corneoscleral stiffness due to aging (Coudrillier et al., 2015; Coudrillier et al., 2012; Fazio et al., 2014) or race/genetics (Grytz et al., 2014) could have a much stronger impact on IOP spikes and glaucoma risk than localized treatment such as corneal or scleral crosslinking.
This study may have implications to the interpretation of air puff measurements. Aqueous displacement resultant from a rapid air puff may produce substantial IOP spikes, which in turn may affect the cornea’s response as measured by Scheimpflug or other imaging methods. As our results showed, the cornea’s own stiffness is the dominant factor in determining the magnitude of IOP spikes; however, the rest of the ocular shell also plays a role which will impact the measured cornea deformation (Metzler et al., 2014). Future studies are needed to characterize the IOP spikes during air puff examination and their influences on corneal response.
This study is limited in the following aspects. First, the experiments performed in this study were conducted in porcine globes. Our previous data in human globes (Liu et al., 2014) showed a similar rate-dependent infusion response of slightly higher magnitudes as in porcine eyes, which suggests that the porcine eye provides a good model studying rapid pressure-volume change in the eye. Second, the ex vivo infusion model used in this study does not take into account factors such as choroidal blood flow that could influence the in vivo response to microvolumetric changes. This study presents a first step towards understanding the relationship between corneoscleral biomechanics and dynamic IOP by providing a systematic, simplified model under well-controlled conditions, which provide a necessary basis for developing and interpreting future in vivo studies where multiple interactive factors can be integrated. Lastly, the treated region of the cornea and sclera was of the same size, resulting in a much larger proportion of cornea being altered than the sclera. The comparison between crosslinking of whole cornea or sclera may be of interest in future studies.
In conclusion, this study showed that stiffening of the corneoscleral shell via chemical crosslinking or increasing the “apparent” stiffness by increasing the steady-state IOP resulted in increased magnitudes of IOP spikes during rapid microvolumetric changes. The cornea, generally more compliant than the sclera, may play a major role in damping IOP spikes. These results provide new insights into the relationship between corneoscleral biomechanical properties and the dynamic profile of IOP.
Supplementary Material
Highlights.
Stiffening a local area of either the cornea or the sclera resulted in increased magnitude of IOP spikes.
Corneal stiffening of the same area has a larger impact on IOP spikes than scleral stiffening.
The magnitude of steady-state IOP significantly impacts the magnitude of IOP spikes.
Acknowledgments
This work was partially funded by the National Institute of Health grant RO1EY020929. The authors are grateful for helpful discussions and inputs from Paul Weber, MD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Proprietary Interest: None.
References
- Asrani S, Zeimer R, Wilensky J, Gieser D, Vitale S, Lindenmuth K. Large diurnal fluctuations in intraocular pressure are an independent risk factor in patients with glaucoma. Journal of Glaucoma. 2000;9:134–142. doi: 10.1097/00061198-200004000-00002. [DOI] [PubMed] [Google Scholar]
- Boyce BL, Grazier JM, Jones RE, Nguyen TD. Full-field deformation of bovine cornea under constrained inflation conditions. Biomaterials. 2008;29:3896–3904. doi: 10.1016/j.biomaterials.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Campbell IC, Hannon BG, Read AT, Sherwood JM, Schwaner SA, Ethier CR. Quantification of the efficacy of collagen cross-linking agents to induce stiffening of rat sclera. Journal of the Royal Society Interface. 2017;14:20170014. doi: 10.1098/rsif.2017.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman DJ, Trokel S. Direct-recorded intraocular pressure variations in a human subject. Archives of Ophthalmology. 1969;82:637–640. doi: 10.1001/archopht.1969.00990020633011. [DOI] [PubMed] [Google Scholar]
- Coudrillier B, Campbell IC, Read AT, Geraldes DM, Vo NT, Feola A, Mulvihill J, Albon J, Abel RL, Ethier CR. Effects of peripapillary scleral stiffening on the deformation of the lamina cribrosa. Investigative Ophthalmology and Visual Science. 2016;57:2666–2677. doi: 10.1167/iovs.15-18193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coudrillier B, Pijanka J, Jefferys J, Sorensen T, Quigley HA, Boote C, Nguyen TD. Collagen structure and mechanical properties of the human sclera: analysis for the effects of age. Journal of Biomedical Engineering. 2015;137 doi: 10.1115/1.4029430. [041006]041001-041014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coudrillier B, Tian J, Alexander S, Myers KM, Quigley HA, Nguyen TD. Biomechanics of the human posterior sclera: age- and glaucoma-related changes measured using inflation testing. Investigative Ophthalmology and Visual Science. 2012;53:1714–1728. doi: 10.1167/iovs.11-8009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz Perez B, Morris HJ, Hart RT, Liu J. Finite element modeling of the viscoelastic responses of the eye during microvolumetric changes. Journal of Biomedical Science and Engineering. 2013;6:29–37. doi: 10.4236/jbise.2013.612A005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz Perez B, Tang J, Morris HJ, Palko JR, Pan X, Hart RT, Liu J. Biaxial mechanical testing of posterior sclera using high-resolution ultrasound speckle tracking for strain measurements. Journal of Biomechanics. 2014;47:1151–1156. doi: 10.1016/j.jbiomech.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotan A, Kremer I, Livnat T, Zigler A, Weinberger D, Bourla D. Scleral cross-linking using riboflavin and ultraviolet-A radiation for prevention of progressive myopia in a rabbit model. Experimental Eye Research. 2014;127:190–195. doi: 10.1016/j.exer.2014.07.019. [DOI] [PubMed] [Google Scholar]
- Downs JC, Burgoyne CF, Seigfreid WP, Reynaud JF, Strouthidis NG, Sallee V. 24-hour IOP telemetry in the nonhuman primate: implant system performance and initial characterization of IOP at multiple timescales. Investigative Ophthalmology & Visual Science. 2011;52:7365–7375. doi: 10.1167/iovs.11-7955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliaghi A, Flanagan JG, Tertinegg I, Simmons CA, Brodland GW, Ethier CR. Biaxial mechanical testing of human sclera. Journal of Biomechanics. 2010;43:1696–1701. doi: 10.1016/j.jbiomech.2010.02.031. [DOI] [PubMed] [Google Scholar]
- Fazio MA, Grytz R, Morris JS, Bruno L, Gardiner SK, Girkin C, Downs JC. Age-related changes in human peripapillary scleral strain. Biomechanics and Modeling in Mechanobiology. 2014;13:551–563. doi: 10.1007/s10237-013-0517-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friberg TR, Sanborn G, Weinreb RN. Intraocular and episcleral venous pressure increase during inverted posture. American Journal of Ophthalmology. 1987;103:523–526. doi: 10.1016/s0002-9394(14)74275-8. [DOI] [PubMed] [Google Scholar]
- Girard MJ, Suh J-KF, Bottlang M, Burgoyne CF, Downs JC. Scleral biomechanics in the aging monkey eye. Investigative Ophthalmology & Visual Science. 2009;50:5226–5237. doi: 10.1167/iovs.08-3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon MO, Beiser JA, Brandt JD. The Ocular Hypertension Treatment Study: Baseline factors that predict the onset of primary open-angle glaucoma. Archives of Ophthalmology. 2002;120:714–720. doi: 10.1001/archopht.120.6.714. [DOI] [PubMed] [Google Scholar]
- Grytz R, Fazio MA, Libertiaux V, Bruno L, Gardiner SK, Girkin C, Downs JC. Age- and race-related differences in human scleral material properties. Investigative Ophthalmology and Visual Science. 2014;55:8163–8172. doi: 10.1167/iovs.14-14029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iseli HP, Thiel MA, Hafezi F, Kampmeier J, Seiler T. Ultraviolet A/riboflavin corneal cross-linking for infectious keratitis associated with corneal melts. Cornea. 2008;27:590–594. doi: 10.1097/ICO.0b013e318169d698. [DOI] [PubMed] [Google Scholar]
- Johnson CS, Mian S, Moroi S, Epstein D, Izatt J, Afshari NA. Role of corneal elasticity in damping of intraocular pressure. Investigative Ophthalmology and Visual Science. 2007;48:2540–2544. doi: 10.1167/iovs.06-0719. [DOI] [PubMed] [Google Scholar]
- Kaufmann C, Bachmann LM, Robert YC, Thiel MA. Ocular pulse amplitude in healthy subjects as measured by dynamic contour tonometry. Archives of Ophthalmology. 2006;124:1104–1108. doi: 10.1001/archopht.124.8.1104. [DOI] [PubMed] [Google Scholar]
- Kiel J. The effect of arterial pressure on the ocular pressure-volume relationship in the rabbit. Experimental Eye Research. 1995;60:267–278. doi: 10.1016/s0014-4835(05)80109-5. [DOI] [PubMed] [Google Scholar]
- Kimball EC, Nguyen C, Steinhart MR, Nguyen TD, Pease ME, Oglesby EN, Oveson BC, Quigley HA. Experimental scleral cross-linking increases glaucoma damage in a mouse model. Experimental Eye Research. 2014;128:129–140. doi: 10.1016/j.exer.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kling S, Perez-Escudero A, Merayo-Lloves J, Marcos S. Corneal biomechanical changes after collagen cross-linking from porcine eye inflation experiments. Investigative Ophthalmology and Visual Science. 2010;51:3961–3968. doi: 10.1167/iovs.09-4536. [DOI] [PubMed] [Google Scholar]
- Lee J, Kong M, Kim J, Kee C. Comparison of visual field progression between relatively low and high intraocular pressure groups in normal tension glaucoma patients. Journal of Glaucoma. 2014;23:553–560. doi: 10.1097/IJG.0b013e31829484c6. [DOI] [PubMed] [Google Scholar]
- Leidl MC, Choi CJ, Syed ZA, Melki SA. Intraocular pressure fluctation and glaucoma progression: what do we know? British Journal of Ophthalmology. 2014;98:1315–1319. doi: 10.1136/bjophthalmol-2013-303980. [DOI] [PubMed] [Google Scholar]
- Liu J, He X. Corneal stiffness affects IOP elevation during rapid volume change in the eye. Investigative Ophthalmology and Visual Science. 2009;50:2224–2229. doi: 10.1167/iovs.08-2365. [DOI] [PubMed] [Google Scholar]
- Liu J, Morris HJ, Chen H, Cruz Perez B, Hart RT, Weber PA. Corneoscleral biomechanical properties and whole globe response to microvolumetric changes in human donor eyes. Investigative Ophthalmology and Visual Science. 2014;55:4237. [Google Scholar]
- Liu S, Li S, Wang B, Lin X, Wu Y, Liu H, Qu X, Dai J, Zhou X, Zhou H. Scleral cross-linking using riboflavin UVA irradiation for the prevention of myopia progression in a guinea pig model: blocked axial extension and altered scleral microstructure. PLoS One. 2016;11:e0165792. doi: 10.1371/journal.pone.0165792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri K, Weinreb RN, Medeiros FA. Is 24-hour intraocular pressure monitoring necessary in glaucoma? Seminars in Ophthalmology. 2013;28:157–164. doi: 10.3109/08820538.2013.771201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek KM. The cornea and sclera. In: Fratzl P, editor. Collagen: Structure and Mechanics, an Introduction. Springer Publishing; New York City: 2008. pp. 359–396. [Google Scholar]
- Metzler KM, Mahmoud AM, Liu J, Roberts CJ. Deformation response of paired donor corneas to an air puff: intact whole globe versus mounted corneoscleral rim. Journal of Cataract and Refractive Surgery. 2014;40:888–896. doi: 10.1016/j.jcrs.2014.02.032. [DOI] [PubMed] [Google Scholar]
- Morris HJ, Tang J, Cruz Perez B, Pan X, Hart RT, Weber PA, Liu J. Correlation between biomechanical responses of posterior sclera and IOP elevations during micro intraocular volume change. Investigative Ophthalmology and Visual Science. 2013;54:7215–7222. doi: 10.1167/iovs.13-12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musch DC, Gillespie BW, Niziol LM, Lichter PR, Varma R. Intraocular pressure control and long-term visual field loss in the Collaborative Initial Glaucoma Treatment Study. Ophthalmology. 2011;118:1766–1773. doi: 10.1016/j.ophtha.2011.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palko JR, Liu J. Defintions and concepts. In: Roberts CJ, Liu J, editors. Corneal Biomechanics: From Theory to Practice. Kugler Publications, Amsterdam; The Netherlands: 2016. pp. 1–22. [Google Scholar]
- Palko JR, Tang J, Cruz Perez B, Pan X, Liu J. Spatially heterogeneous corneal mechanical responses before and after riboflavin-ultraviolet-A crosslinking. Journal of Cataract and Refractive Surgery. 2014;40:1021–1031. doi: 10.1016/j.jcrs.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resta V, Novelli E, Vozzi G, Scarpa C, Caleo M, Ahluwalia A, Solini A, Santini E, Parisi V, Di Virgilio F, Galli-Resta L. Acute retinal ganglion cell injury caused by intraocular pressure spikes is mediated by endogenous extracellular ATP. European Journal of Neuroscience. 2007;25:2741–2754. doi: 10.1111/j.1460-9568.2007.05528.x. [DOI] [PubMed] [Google Scholar]
- Spoerl E, Seiler T. Techniques for stiffening the cornea. Journal of Refractive Surgery. 1999;15:711–713. doi: 10.3928/1081-597X-19991101-21. [DOI] [PubMed] [Google Scholar]
- Srinivasan S, Choudhan N, Baskaran M, George R, Shantha B, Vijaya L. Diurnal intracoular pressure fluctuations and its risk factors in angle-closure and open-angle glaucoma. Eye (London) 2016;30:362–368. doi: 10.1038/eye.2015.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamer WD, Acott TS. Current understanding of conventional outflow dysfunction in glaucoma. Current Opinion in Ophthalmology. 2012;23:135–143. doi: 10.1097/ICU.0b013e32834ff23e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strouthidis NG, Girard MJ. Altering the way the optic nerve head responds to intraocular pressure- a potential approach to glaucoma therapy. Current Opinion in Pharmacology. 2013;13:83–89. doi: 10.1016/j.coph.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Terry MA, Ousley PJ, Zjhra ML. Hydration changes in cadaver eyes prepared for practice and experimental surgery. Archives of Ophthalmology. 1994;112:538–543. doi: 10.1001/archopht.1994.01090160118031. [DOI] [PubMed] [Google Scholar]
- Thornton IL, Dupps WJJ, Roy AS, Krueger RR. Biomechanical effects of intraocular pressure elevation on optic nerve/lamina cribrosa before and after peripapllary scleral collagen cross-linking. Investigative Ophthalmology & Visual Science. 2009;50:1227–1233. doi: 10.1167/iovs.08-1960. [DOI] [PubMed] [Google Scholar]
- Wollensak G, Spoerl E. Collagen crosslinking of human and porcine sclera. Journal of Cataract and Refractive Surgery. 2004;30:689–695. doi: 10.1016/j.jcrs.2003.11.032. [DOI] [PubMed] [Google Scholar]
- Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. American Journal of Ophthalmology. 2003;135:620–627. doi: 10.1016/s0002-9394(02)02220-1. [DOI] [PubMed] [Google Scholar]
- Woo SL-Y, Kobayashi A, Schlegel W, Lawrence C. Nonlinear material properties of intact cornea and sclera. Experimental Eye Research. 1972;14:29–39. doi: 10.1016/0014-4835(72)90139-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.