Abstract
HIV-positive young Black MSM (YBMSM) experience poor outcomes along the HIV Care Continuum, yet few interventions have been developed expressly for YBMSM retention in care. Project nGage was a randomized controlled trial conducted across five Chicago clinics with 98 HIV-positive YBMSM aged 16–29 between 2012 and 2015. The intervention used a social network elicitation approach with index YBMSM (n=45) to identify and recruit a support confidant (SC) to the study. Each index-SC dyad met with a social worker to improve HIV-care knowledge, activate dyadic social support, and develop a retention in care plan. Each index and SC also received four mini-booster sessions. Control participants (n=53) received treatment as usual. Surveys and medical records at baseline, 3-, and 12-months post-intervention assessed visit history (3 or more visits over 12 months; primary outcome), and sociodemographic, network, social-psychological, and behavioral factors. At baseline, there were no differences in age (M=23.8 years), time since diagnosis (M≤2 years), clinic visits in the previous 12 months (M=4.1), and medication adherence (68.6%≥90% adherence). In multivariate logistic regression analysis, intervention participants were 3.01 times more likely to have had at least 3 provider visits (95% CI: 1.0–7.3) than were control participants over 12 months. Project nGage demonstrates preliminary efficacy in improving retention in care among YBMSM. Results suggest that engaging supportive network members may improve key HIV Care Continuum outcomes.
Keywords: Young Black men who have sex with men, randomized controlled trial, retention in HIV care, social support, social networks
INTRODUCTION
The HIV Care Continuum includes a range of important activities necessary to limit onward transmission and promote longevity among people living with HIV, i.e., HIV testing and diagnosis, linkage to care, retention in care, antiretroviral [ARV] treatment and adherence, and virologic suppression (1, 2). In the United States, younger Black men who have sex with men (YBMSM) experience poorer outcomes than their peers along the entire HIV Care Continuum (3–6). Retention in care, typically defined as seeing an HIV healthcare provider every 3 to 4 months, is an especially important target for improvement, as optimal retention in care is associated with better ARV adherence, virologic suppression, and reduced mortality (7). Furthermore, maximizing retention in care can have ancillary benefits by connecting people to other services, such as mental health care, substance use treatment, housing support, and sexual risk reduction, all of which may limit onward transmission and support people living with HIV to lead healthy and fulfilling lives (8–10). Despite this, few retention in care interventions have been developed expressly for YBMSM aged 16–29 years old, the group most impacted by HIV (11).
The current study presents efficacy data from a pilot randomized controlled trial (RCT) of Project nGage, a social network support intervention designed to improve retention in care among YBMSM who have been successfully linked to HIV care (12). The intervention is novel relative to extant approaches in that it identifies, activates, and harnesses a naturally existing supportive relationship in the lives of HIV-positive YBMSM as a means to improve retention in care. In contrast, current approaches often connect people living with HIV to an exogenous source of social support, such as intensive case management (13–15), peer support groups (16), peer health navigators (17), and multimodal peer outreach (18, 19). Although effective, these programs require significant human resource costs and may not be sustainable in all funding climates. For Project nGage, we used a flexible approach that selects supportive network members based on their supportive role (20, 21), e.g., emotional support, as opposed to their relationship status, e.g., mother, partner, or case manager. This approach is particularly important when working with people living with HIV and with lesbian, gay, bisexual, transgender, and queer communities, as the support available within their social networks is affected by knowledge of and responses to one’s sexual orientation and serostatus. As such, people who serve a supportive function are likely important not only for retention in care, but also for sustained health, risk reduction behavior maintenance, and ARV adherence (22–24).
The philosophical underpinnings for Project nGage are that social networks and social support are critical for improving and maintaining people’s health and well-being. Social network oriented interventions have been effective for preventing HIV-related risk behaviors (25, 26), and for increasing HIV counseling and testing and the likelihood of identifying newly infected cases (27). In addition, studies indicate that social support is associated with better outcomes in the HIV Continuum of Care (28, 29). Indeed, in a diverse sample of people living with HIV, Catz et al. found that social support was the only psychosocial factor associated with non-attendance in HIV care, and was a stronger correlate of retention in care than were medical and clinical factors, such as physician consistency and CD-4 count (30). Despite robust research on the important role of social networks and social support for people living with HIV, few social network support interventions have been developed to improve retention in HIV care for YBMSM living with HIV.
Consequently, the primary aim of this pilot RCT was to test the efficacy of a brief, theory-based intervention designed to improve retention in care (primary outcome) and ARV adherence and viral load (secondary outcomes) among YBMSM living with HIV. Based on prior research on barriers to engaging in the HIV Care Continuum, we controlled for social network size, family social support, self-efficacy, HIV stigma, and criminal justice involvement when examining intervention effects (31–33).
METHODS
Recruitment
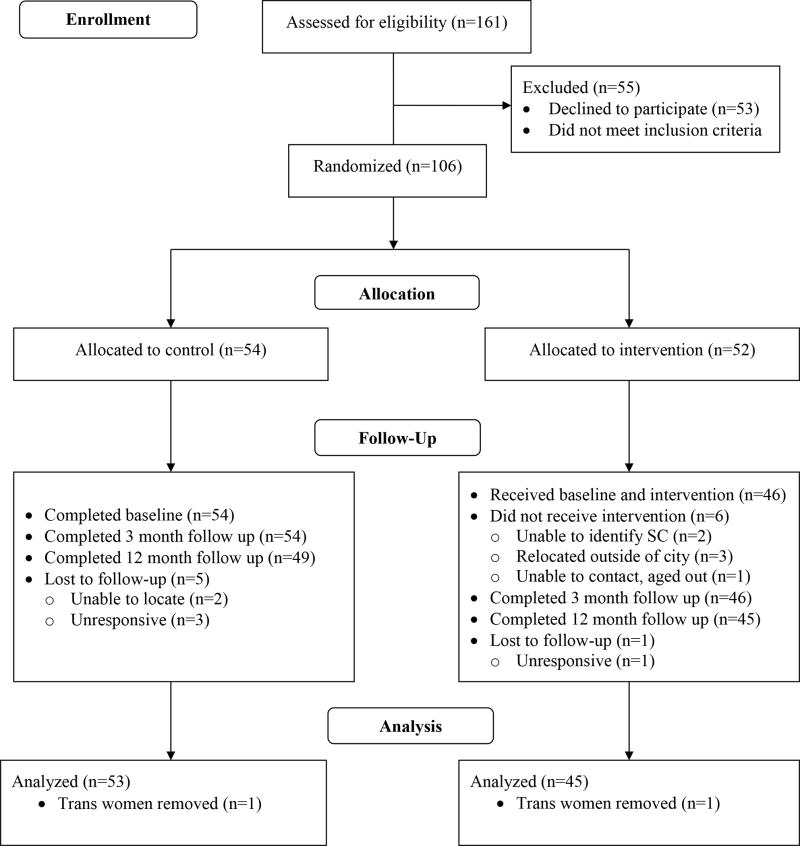
Participants were recruited from five federally qualified health centers (FQHCs) using flyers and face-to-face recruitment. Index participants were eligible if they: (1) identified as Black/African American, (2) were aged 16–29 years old, inclusive, (3) were assigned male sex at birth, (4) self-reported anal or oral sex with a cisgender male in the past 2 years; (4) spoke English; and (5) had been diagnosed with HIV for more than 3 months. In addition, participants had to own a cell phone not shared with other persons, agree to receive text messages, and have at least one person in their social network who knew of their HIV diagnosis. YBMSM were excluded if they were unable to provide assent/consent or planned to move out of the study area in the next 12 months. In total, 161 prospective participants were screened for eligibility. Of these, 55 were not eligible or declined to participate (see Figure 1 for Consort Diagram).
Figure 1.
Project nGage Consort Diagram
Eligible adults provided written consent and eligible minors provided written assent. In total, 106 HIV-positive young Black MSM and transgender women age 16–29 were consented into the study and randomized to an experimental (n=52) or a control arm (n=54) consisting of treatment as usual (TAU), e.g., standard HIV primary care in each clinical site. The present efficacy analysis includes data on N=98 participants, as 6 participants withdrew from the study and too few transwomen were enrolled (n=2) to conduct meaningful subgroup analysis (see Figure 1).
Index participants were scheduled for a baseline visit within 30 days of being consented into the study. At the baseline visit, participants randomized to the experimental arm met with a social work interventionist (SWI), who conducted a social network diagram (sociogram) activity using pen and paper—a technique recommended for engaging participants, generating rich data, and being a feasible strategy in low resource settings (34, 35). The SWI started by using a confidant name generator, asking each participant to “Please identify people in your life with whom you share personal information with and can count on in a time of need. When we say personal information, we mean private things about yourself that you might not tell everyone.” Following this, the SWI elicited the names, nicknames, or initials of up to 10 network members and wrote each identified member in one of 10 empty cells of a table. The first five of these individuals were then populated into a sociogram. Once the network was populated and visualized, the SWI asked a series of questions about each network member to systematically assess demographics (e.g., age, gender identity, sexual orientation), relationship to index (e.g., family, friend, romantic partner), knowledge of sexual orientation and HIV serostatus, emotional characteristics of the index-alter relationship (e.g., had the identified network member betrayed the index’s trust in the previous 6 months; did the index anticipate that the person would be available and supportive of them in the next 12 months), and indicators of stability and availability (e.g., frequency of moving, substance abuse problems, changing telephone numbers, and incarceration). Based on these measures, the SWI used an algorithm to determine the optimal network member to be an SC. Finally, the SWI utilized a network matrix to elicit information on how each alter (network member) was connected to other network members, which enabled the SWI to determine how knowledge of sexual orientation of HIV serostatus was distributed across network members.
Using the information gathered from these tools, the SWI presented the index with the populated tables of their social network and the algorithm selection and discussed which alter would be the best SC. This conversation was guided by shared decision-making around the ideal SC as a network member who knew the index’s sexual orientation and serostatus, had not betrayed the index, and would not disclose the index’s serostatus and sexual orientation to others. In instances where there was disagreement on which SC should be selected, the SWI explained the rationale for their decision for SC selection but let the YBMSM select their SC of choice. Prior to finalizing the SC selection, the SWI utilized an abuse screener to ensure there was no evidence of physical, emotional, or sexual abuse between the Index and SC. The data on network members and the SC selection process was later entered into RedCap, a secure web-based application used for data collection (secure web-based application (Harris, Taylor et al., 2009).
Once identified, the index contacted the SC to invite them to join the study and a dyadic appointment was made to screen and consent the SC and to complete the baseline survey and first intervention session. SCs were eligible to participate if they: (1) were aged 18 or over; (2) spoke English; (3) owned a cell phone not shared with other persons, (4) were identified by the index as a candidate SC; and (5) were willing to attend a face-to-face intervention and to receive telephonic booster sessions. SC participants were excluded if they were unable to provide informed consent. In addition, study consent/assent forms stated that screening for dyadic abuse would occur at all study visits and that a positive screen for either person would result in study withdrawal, psychosocial evaluation, referral to supportive services, or other action as was legally and ethically appropriate.
Project nGage Intervention
Project nGage is a client-centered intervention consisting of a 90-minute session that included an individual SC and a dyadic SC-index component. During this time, the SC and SC-index dyad met with the SWI to discuss the importance of HIV primary care and the critical role that social support plays for people living with HIV, and to problem-solve barriers to social support and engaging in HIV primary care. The intervention, which was based on Information-Motivation-Behavioral Skills theory(36, 37) and incorporated strategies from motivational interviewing (MI) (38) and Cognitive Behavioral Therapy (CBT) (39, 40), has been described in detail elsewhere (12). We briefly review key components here.
At the beginning of the session, the SWI met for approximately 20 minutes with the SC-index dyad to discuss the importance of HIV primary care and social support for people living with HIV. After delivering this content, the SWI led a client-centered conversation on the nature of social support in the context of each SC-index relationship. Following this component, the index YBMSM left to receive TAU at the clinic while the SWI met with the SC alone for approximately 40 minutes. This component included content on HIV/AIDS, the importance of HIV primary care for people living with HIV, common barriers to retention in HIV care and ARV adherence (41), and a client-centered conversation where the SWI and SC worked together to identify and problem-solve potential barriers to providing social support to the index YBMSM.
After the individual session, the SWI then met with the SC-index dyad for a final 20 minutes to develop a tailored “Care and Support Plan” (39, 40) that first elicited barriers to retention in care and ARV adherence for each index YBMSM and then identified and selected the best solutions for each barrier. As part of this process, the SWI engaged each dyad in a collaborative problem-solving process designed to activate social support and develop their individual and collective self-efficacy and behavioral skills.
Following the face-to-face intervention, the SWI delivered four telephonic booster sessions to the index, scheduled at two months, five months, eight months, and eleven months after baseline. The boosters focused on implementing the “Care and Support Plan” and the emotional quality of the SC-index relationship. Selected SCs received the same boosters, along with a supplemental refresher on potential means of support following the intervention.
If a member of the dyad was struggling with any aspect of the Care and Support plan, the SWI problem-solved with them to identify and enact appropriate corrective strategies. In completing the booster calls, the study team attempted calls from two weeks prior to the scheduled booster date and until two weeks after the scheduled booster date. When phone contact was not possible, project staff utilized text messaging, email, and Facebook messages (with index consent).
In addition, project staff also assessed each member of the dyad for relationship strain or abuse during the booster calls. Prior to this assessment, each index and SC was asked to generally describe how their relationship was going, including how often they were in contact and the types of contact they had with each other (e.g., in person via telephone or text), what types of social activities they had engaged in together, etc. This information was then entered into a project database.
Overall, there was a 73.7% success rate for booster calls with index YBMSM and a 57.6% success rate with selected SCs. Frequent changes in cell phone numbers or discontinuation were a major factor in missed boosters for both index and SC clients. For more than half of the sample, cell phones were either disconnected or phone numbers were changed over the year—up to six times for some participants. Despite this, most lines of communication were eventually reestablished.
Data Collection
All YBMSM completed the sociogram activity, as described above, which elicited the relational and functional characteristics of their social network. In addition, all index YBMSM completed an interviewer-administered close-ended survey at baseline, 3, and 12 months post-intervention. All survey data was collected via RedCap, a secure online survey software (42). All data collection staff were trained on survey administration from the University of Chicago Survey Lab, and each survey took approximately 40 minutes to complete. In addition, all index YBMSM completed a release of information form that gave the study team permission to access clinical information on HIV clinic appointment attendance, viral load, and other clinical care variables.
Measures
Retention in Care
Retention in HIV care was our primary outcome measure. Based on routine care where clients are seen by an HIV prescriber every three months, we defined retention in care as having at least three HIV primary care visits in the previous 12 months. Many retention in care measures exist (43). The 12-month study period precluded use of other commonly used definitions, including those used by the Health Resources and Services Administration, which require 24 months of follow-up. Clinical visit history was abstracted from medical records.
Other HIV Care measures
We also measured ARV medication adherence and HIV viral load as secondary outcome measures. Medication adherence was measured based on a visual analog scale which assessed adherence for medications taken in the previous 30 days from 0–100% (44). Self-reported measures of adherence have been found to correlate with viral load (45), and we used 90% adherence as the cut-off for optimal adherence (46). Viral load was abstracted from medical records, measured in RNA copies per milliliter, with ≤500 copies per mL defined as viral suppression.
Intervention Condition
Randomization to experimental and TAU arms were coded as one and zero, respectively.
Social Network
Average network size (degree), and proportion of network members with characteristics likely to impact retention were included from the sociogram (47, 48). These factors included disclosure of HIV status to network members, incarceration of network members, substance use by network members, and betrayal of trust by network members.
Social-Psychological Factors
Index participants answered additional questions on satisfaction with family social support, self-efficacy to engage in care (49), HIV stigma (50), criminal justice involvement (0=no, 1=yes) and mental health (e.g., Brief Symptom Inventory (51)).
Behavioral Factors
Index participants reported if they had ever used marijuana (52) and engaged in condomless anal sex with a male partner of unknown HIV status in the past 6 months (53).
Socio-demographic Factors
Index participants reported their age, highest level of educational attainment, and employment.
Statistical Analyses
The study evaluated intervention effects in retention of HIV care over 12 months using an intention to treat approach. Chi-square or Fisher’s exact test and independent sample T-test or Mann- Whitney test were used for group comparisons in demographic, clinical, network, and social-psychological variables. Univariate logistic regressions examined potential factors associated with the primary outcome of retention in HIV care, i.e., having at least three HIV primary care visits in the prior 12 months. The final multivariable model included all variables that were significant at p<.10 in the univariate analysis. Univariate modeling and multivariable analysis (MVA) for repeated measurement of medication adherence and viral load (secondary outcomes) was performed using generalized estimation equations (GEE) with an AR (1) correlation structure to identify demographic and variables of interest associated with changes in medication adherence over time and to compare between different time points within each group and different groups at each time point. The GEE models incorporated baseline (pre-adherence rate) as a covariate. Statistical analyses were performed using STATA/SE software version 13 (StataCorp LP, Texas), and statistical significance was set at p<.05 for all multivariate analyses.
RESULTS
Baseline demographic, clinical, network, and behavioral characteristics of index participants as a function of intervention condition are presented in Table 1. At baseline, there were no differences in mean age (M=23.8 years), time since HIV diagnosis (M≤2 years), the number of HIV primary care in the previous 12 months (M=4.1), and self-reported ARV adherence (68.6%≥90% adherence). However, a significantly greater percentage of intervention condition participants reported substance abuse by one or more social network members than did participants in the control condition (22.6% vs. 44.2%, p=.05). Of the 98 index participants who completed the baseline, 94% were successfully interviewed at the 12-month follow-up. There were no significant differences in attrition by study condition and attrition was not associated with any baseline characteristics. Table 2 presents descriptive characteristics of all enrolled SCs (n=45). The majority were friends (n=22), followed by mothers (n=6) and female relatives (n=7), with a mean age of 30.8 years. Overall, 55.6% of SCs identified as gay or bisexual, and reported low levels of substance use (n=4) and criminal justice involvement (n=2).
Table 1.
Baseline Sample Characteristics, Project nGage, Chicago, 2012 – 2015 (N = 98)
| Characteristics | Control (n=53) | Intervention (n=45) | p-value |
|---|---|---|---|
| Sociodemographic | |||
| Mean Age (year) (SD) | 23.9 (2.8) | 23.7 (3.0) | 0.79 |
| Education, N (%) | |||
| < High School | 9 (17.0) | 11 (24.4) | 0.83 |
| High School/GED | 15 (28.3) | 13 (28.9) | |
| Some College | 23 (43.4) | 17 (37.8) | |
| College or other school | 6 (11.3) | 4 (8.9) | |
| Employed, N (%) | 31 (58.5) | 23 (51.1) | 0.54 |
| Network | |||
| Network degree, Med (IQR) | 4 (3–5) | 5 (3–5) | 0.34 |
| 1–3, N (%) | 21 (39.6) | 12 (26.7) | 0.20 |
| 4–5, N (%) | 32 (60.4) | 33 (73.3) | |
| HIV status network disclosure,1 N (%) | 48 (90.6) | 44 (97.8) | 0.21 |
| Network criminal justice involvement,1 N (%) | 13 (24.5) | 7 (15.6) | 0.32 |
| Network betrayal of trust,1 N (%) | 16 (30.2) | 20 (44.4) | 0.21 |
| Network substance abuse,1 N (%) | 12 (22.6) | 19 (42.2) | 0.05 |
| Clinical (Baseline) | |||
| Time since diagnosis, Med (IQR) | 1.8 (0.7–4.2) | 1.6 (0.4–3.7) | 0.61 |
| Viral load, N (%) | |||
| 0–500 copies/mL | 29 (54.7) | 29 (65.9) | 0.30 |
| >500 copies/mL | 24 (45.3) | 15 (34.1) | |
| Medication adherence ≥ 90%, N (%) | 34 (69.4) | 25 (67.6) | 1.0 |
| Retained in care, previous 12 mo., Med (IQR) | |||
| Total visits | 4 (3–5) | 4 (3–5) | 0.63 |
| Missed visits | 2 (0–3) | 1 (0–2) | 0.14 |
| Appointment Adherence | 0.7 (0.5–1) | 0.8 (0.63–1) | 0.22 |
| Social-Psychological (Baseline) | |||
| Mental health, BSI ≥ 62, N (%) | 4 (7.6) | 6 (13.3) | 0.51 |
| Family social support, N (%) | |||
| Very dissatisfied | 8 (15.1) | 4 (8.9) | 0.41 |
| Somewhat dissatisfied | 5 (9.4) | 3 (6.7) | |
| Somewhat satisfied | 15 (28.3) | 20 (44.4) | |
| Very satisfied | 25 (47.2) | 18 (40.0) | |
| Overall self-efficacy, Med (IQR) | 15 (13–15) | 14 (12–15) | 0.25 |
| Overall stigma, Mean (SD) | 23.2 (4.6) | 23.6 (5.6) | 0.70 |
| Criminal justice involvement, N (%) | 4 (8.2) | 2 (4.9) | 0.69 |
| Behavioral (Baseline) | |||
| Ever used marijuana, N (%) | 35 (83.3) | 32 (80.0) | 0.78 |
| Condomless anal sex, N (%) | 8 (15.1) | 15 (33.3) | 0.05 |
Numerical superscript indicates at least one network member
Table 2.
Characteristics for Support Confidants in Project nGage Experimental Arm (N = 45)
| Characteristics | Intervention (n=45) |
|---|---|
| Relationship Category, N (%) | |
| Friend | 21 (46.7) |
| Mother | 7 (15.6) |
| Sister | 7 (15.6) |
| Partner | 4 (8.9) |
| Other* | 6 (13.3) |
| Mean Age (SD) | 30.8 (11.0) |
| Ethnicity, N (%) | |
| Black/African-American | 40 (88.9) |
| Hispanic/Latino | 2 (4.4) |
| Mixed race or ethnicity | 3 (6.7) |
| Gender Identity, N (%) | |
| Female | 25 (55.6) |
| Male | 20 (44.4) |
| Sexual Orientation, N (%) | |
| Gay | 17 (37.8) |
| Bisexual | 8 (17.8) |
| Straight | 17 (37.8) |
| Don’t know | 3 (6.7) |
| Education, N (%) | |
| < High school | 4 (8.9) |
| High school/GED | 15 (33.3) |
| Some college | 18 (40.0) |
| College or other school | 6 (13.3) |
| Don’t know | 2 (4.4) |
| Substance use, N (%)† | 4 (8.9) |
| Criminal justice involvement, N (%)†† | 2 (4.4) |
Other relationships included a neighbor, cousins and older mentors
Substance use negatively impacted SC’s life at least once in the six months prior to baseline
SC was detained by police at least one in the twelve months prior to baseline
SC engagement by YBMSM included a range of different network members in Project nGage. Six of the participants chose their mother to be their SC and about half of these index YBMSM also lived with their mothers. SC mothers tended to be the most responsive to booster calls, with a successful completion rate of 74.3%. In booster sessions, SC mothers described feeling very busy as they worked full-time, took care of grandchildren and aging parents, and managed their own health issues. Conflict within each dyad remained minimal, although a few index YBMSM portrayed their mothers as too involved or “over supportive.” Though index YBMSM seemed to rely heavily on SC mothers for both emotional and instrumental support, many of the SC mothers described feeling grateful for the opportunity to be involved with the project and to work on their relationship with their child.
Other index YBMSM chose their sisters (n=7) or female cousin (n=1) to be their SC. There was a 55% completion rate for boosters with these female relatives and the study team experienced several challenges with engaging this group. Five of these SC relatives had their phones disconnected or their numbers changed at least once, which was difficult when attempting to complete subsequent booster calls. Two SC relatives moved to another state. In addition, five of the SC relatives had young children or became pregnant during the study, which may have limited their time to be involved with the project. Despite these difficulties in reaching SCs, the index YBMSM and SC relatives described their relationships as strong and as a constant source of emotional support.
About half of the index YBMSM chose a friend as their study SC (n=22). SC friends had a 57.1% booster completion rate and it was common for phone numbers to change, for cell phones to be disconnected, and for SCs to move. In fact, seven of the SC friends moved out of Chicago or out of state, though these moves did not always negatively affect the dyadic relationship. SC friends tended to belong to a similar peer group, including many young Black women or YBMSM living on the South Side of Chicago. Many of the index YBMSM chose SC friends who were also living with HIV. Both index YBMSM and SC friends living with HIV spoke about the importance of mutual understanding, informational support, and being able to remind one another about taking medications or attending appointments.
Of these 22 SC friends, seven were dropped from the study by the index YBMSM. In two cases, boundaries between friendship and romance were blurred and consequently, index YBMSM ended the SC’s involvement in the study. In another instance, the SC decided they just did not want to be in the study anymore, though the dyad’s relationship remained supportive. In the remaining four cases, the index YBMSM and the SC had a falling out and the index YBMSM requested that the SC friend no longer be a part of the study.
Four of the index YBMSM included their romantic partners as SCs in the study. Booster calls to the SC partners had a 60% completion rate. Though these relationships were initially characterized by stability and closeness, three of these dyads later noted concerns around trust and monogamy. Still, index YBMSM and SC continued to reference their relationship as a source of emotional support and none of the index YBMSM chose to remove their partner SC from the study. At the 12-month follow-up, when asked about his relationship with his SC romantic partner, one participant reported that he had broken up with this partner.
Among the other SCs selected (n=3), there was one social service staff member, one pastor, and one father figure. These relationships were supportive and stable, though as community leaders, these SCs were often busy supporting others. These SCs may have been difficult to engage for both the index YBMSM and for nGage study staff, as evidenced by the low booster completion rate of 33.3%.
Table 3 presents the univariate and multivariate results for the primary outcome of retention in care. Multivariate analyses examining differences in retention in care indicated that index YBMSM randomly assigned to the intervention condition had significantly better outcomes. Specifically, in multivariate logistic regression, index YBMSM who received the intervention were 3.01 times more likely to have had at least 3 HIV primary care visits in the previous 12 months (95% CI: 1.05–8.69, p=0.04). GEE analyses examining self-reported ARV adherence also indicated that intervention participants were 2.91 times more likely to report ≥90% medication adherence (95% CI: 1.10–7.71; p=0.031) than were control participants who received TAU (data not shown). There were no differences in VL between groups at 12 months (AOR=.49, 95% CI: .16–1.55; p=0.23; data not shown).
Table 3.
Univariate and Multivariable Models for Retention in Care (3 or more HIV Provider Visits) in the Past 12 Months
| Characteristics | Univariate | Multivariate | ||
|---|---|---|---|---|
|
|
||||
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Experimental Condition | ||||
| Control: TAU | 1 (-) | … | ||
| Intervention: Project nGage | 2.39 (0.92–6.17) | 0.08+ | 3.01 (1.05–8.69) | 0.04* |
| Sociodemographic | ||||
| Age (year) | 0.81 (0.69 -0.96) | 0.02* | 1.46 (0.54–3.94) | 0.06+ |
| Education | ||||
| < High School | 1 (-) | … | ||
| High School/GED | 3.23 (0.80–13.12) | 0.10 | ||
| Some College | 1.85 (0.57–6.04) | 0.31 | ||
| College or other school | 0.36 (0.08–1.71) | 0.20 | ||
| Employed | 1.63 (0.66–4.02) | 0.29 | ||
| Network | ||||
| Network degree | 1.28 (0.88–1.86) | 0.20 | ||
| Network degree ≥ 4 | 1.67 (0.66–4.21) | 0.28 | ||
| HIV status network disclosure1 | 0.99 (0.32–3.07) | 0.98 | ||
| Network criminal justice involvement1 | 2.37 (0.63–8.87) | 0.20 | ||
| Network betrayal of trust1 | 2.38 (0.85–6.63) | 0.10+ | 2.26 (0.53–9.63) | 0.27 |
| Network substance abuse1 | 1.77 (0.63–4.98) | 0.28 | ||
| Clinical | ||||
| Time since diagnosis (year) | 0.88 (0.73–1.07) | 0.21 | ||
| Viral load >500 copies/mL (at | 0.52 (0.20–1.31) | 0.17 | ||
| CD4 > 200 (at baseline) | 1.13 (0.20–0.62) | 0.89 | ||
| Adherence ≥ 90% (at baseline) | 1.89 (0.71–5.06) | 0.21 | ||
| Retained in care in prior 12 months | 1.45 (1.10–1.92) | 0.01** | 1.44 (1.07–1.93) | 0.01* |
| Social-Psychological | ||||
| Brief Symptom Inventory ≥ 62 | 3.57 (0.43–29.67) | 0.24 | ||
| Family social support | ||||
| Very / Somewhat dissatisfied | 1 (-) | … | ||
| Somewhat satisfied | 2.07 (0.57–7.59) | 0.27 | ||
| Very satisfied | 0.89 (0.28–2.80) | 0.84 | ||
| Overall self-efficacy | 0.85 (0.65–1.11) | 0.24 | ||
| Overall stigma | 1.04 (0.95–1.14) | 0.39 | ||
| Criminal justice involvement | 0.33 (0.06–1.78) | 0.20 | ||
| Behavioral | ||||
| Marijuana use | 0.39 (0.08–1.89) | 0.24 | ||
| Condomless anal sex | 2.95 (0.80–10.92) | 0.11 | ||
p < .10,
p < .05,
p < .01,
Numerical superscript indicates at least one network member
DISCUSSION
YBMSM living with HIV report poorer retention in HIV care and poorer ARV adherence than do their White MSM peers (54, 55). Adherence to primary HIV care and ARVs is critical for supporting the health and wellbeing of YBMSM living with HIV and for limiting the likelihood of onward transmission. Interventions to improve these two outcomes in the HIV Care Continuum for YBMSM are limited and have tended to rely on providing YBMSM with external resources and social support via case managers and peers (13–19). In contrast, we developed a social support intervention that identifies and leverages naturally existing supportive relationships in the lives of YBMSM living with HIV as a mechanism to improve adherence to HIV primary care and ARVs among YBMSM (12). Results from our pilot efficacy study suggest that such an approach is not only feasible, but is associated with significantly better retention in HIV primary care and ARV adherence relative to YBMSM who received TAU.
There were several other notable findings from this work. First, both treatment and control conditions had relatively high rates of retention, ARV adherence, and viral suppression at baseline. This was not by design, and likely reflects a combination of the excellent care already being provided at the partnering FQHCs, as well as what a clinic-based sample looks like compared to a community-based sample. High baseline retention in care was the other main variable predictive of successful retention in care across the entire sample over time, and was adjusted for in the final analysis. Notably, the nGage intervention was potent enough to result in even higher rates of retention despite high baseline retention in care. In addition, age was a predictor of retention in univariate analysis with younger BMSM having worse retention. This finding is consistent with other work and has been well-described in the literature (56, 57). Although age was marginally significant in the multivariate analysis, retaining younger MSM in care remains an important priority. Indeed, studies have emphasized the need for youth-friendly structures for retaining younger MSM in clinical care (58).
An early concern in the development of Project nGage was whether participants would have enough SCs in their networks and if SCs could be sufficiently engaged in care. Both of these concerns proved to be moot. For example, concerns over whether SCs could be identified were unfounded, as less than 2% of participants were unable to identify an SC. We attribute the success of the SC selection procedure, in part, to the process of network visualization and the value of reflection in bolstering beneficial support networks (59, 60). By starting with a blank table, the Index was given opportunity to conceive of all possible SC options. Following this, the systematic evaluation of network members allowed the Index to compare potential SCs and think critically about the characteristics of an effective support system. Additionally, concerns over the potential for abuse, disclosure, or conflict impacting the intervention were not observed or associated with retention in care in the sample. However, we did find that the majority of intervention participants whose SC dropped from the study had included a friend or roommate, as opposed to a family member. While research has documented that shifts in friendship and sex partner statuses are frequent among YBMSM (61), it was unknown if these relationships were also romantic or sexual. Future research is needed to better understand the potential role of partners in impacting continuums of care, including the PrEP Continuum, particularly as they may have a vested interest in the success of their partner.
Network interventions, such as Project nGage, are increasingly recognized as effective approaches to improving behavior change at the individual, group, or larger network levels (62). To date, five network intervention typologies have been described in the extant literature (63). The first and most widely used type of network intervention identifies individuals in key network positions who can wield influence on other network members (64, 65). In such interventions, peer change agents are identified and can work at larger community levels (66), or in a personalized intervention approach, as in Project nGage. The strength of this approach is that network members can assist in behavior change of individuals, and may thus undergo change themselves (67). Multiple individuals changing at the same time can create an environment where social norms begin to change, allowing for diffusion of innovation to be most successful and thus making interventions more impactful at the group or network level (68). Although we did not examine behavior change among SCs, future research should examine the extent to which Project nGage and similar interventions may impact the health and well-being of SCs connected to YBMSM living with HIV.
CONCLUSIONS
Although the results of the Project nGage intervention are promising, the findings should be interpreted in the context of the study limitations. First, the clinic-based sample of YBMSM was recruited via convenience methods from FQHCs and study findings may not generalize to other settings or populations living with HIV. In addition, retention was based on 12 months of follow-up; as such, longer-term durability and impact on newly infected persons is unclear. The present study also did not assess which components of the Project nGage intervention were responsible for improved outcomes among YBMSM randomized to the experimental arm. Future research with a larger sample size is needed to identify the key mediators of intervention effectiveness, which will be important for implementing the intervention in other real world settings. Despite these limitations, the present study is among the first to examine a social network support intervention designed to improve retention in care and ARV adherence among YBMSM living with HIV, who continue to have a higher incidence of HIV and poorer outcomes along the HIV Care Continuum relative to their peers from other ethno-racial groups. The dearth of interventions specifically designed to retain YBMSM living with HIV in care thus represents a significant gap in current public health efforts to stem the epidemic. This study addresses this gap by focusing exclusively on YBMSM and documenting that identifying, engaging, and bringing supportive network members into care may improve HIV Continuum Care outcomes among YBMSM, a vulnerable and underserved population.
Acknowledgments
This work was supported by the National Institute of Mental Health [R34MH097622, R01DA039934 and R01DA033875]. ClinicalTrials.gov Identifier: NCT01726712. This manuscript was also made possible with help from the Third Coast Center for AIDS Research (P30 AI 117943). We would like to thank the study participants for their participation and Milton “Mickey” Eder, Molly Pilloton, Natasha Flatt, Tim Walsh, Tiffany Washington, Keisha Hampton and Montre Washington for their valuable contributions to the project.
Footnotes
Conflicts of Interest The authors have no conflicts of interest to declare.
Ethical Approval All protocols and policies for this study were approved by the Institutional Review Board at the University of Chicago. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.”
Informed Consent Informed consent was obtained from all individual participants included in the study.
References
- 1.Gardner EM, McLees MP, Steiner JF, del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clinical infectious diseases. 2011;52(6):793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hull MW, Wu Z, Montaner JS. Optimizing the engagement of care cascade: a critical step to maximize the impact of HIV treatment as prevention. Current Opinion in HIV and AIDS. 2012;7(6):579–86. doi: 10.1097/COH.0b013e3283590617. [DOI] [PubMed] [Google Scholar]
- 3.Whiteside YO, Cohen SM, Bradley H, Skarbinski J, Hall HI, Lansky A. Progress along the continuum of HIV care among blacks with diagnosed HIV-United States, 2010. MMWR Morb Mortal Wkly Rep. 2014;63(5):85–9. [PMC free article] [PubMed] [Google Scholar]
- 4.Singh S, Bradley H, Hu X, Skarbinski J, Hall HI, Lansky A. Men living with diagnosed HIV who have sex with men: progress along the continuum of HIV care—United States, 2010. MMWR Morb Mortal Wkly Rep. 2014;63(38):829–33. [PMC free article] [PubMed] [Google Scholar]
- 5.Campsmith ML, Rhodes PH, Hall HI, TA G. Undiagnosed HIV prevalence among adults and adolescents in the United States at the end of 2006. J Acquir Immune Defic Syndr. 2010;53:619–24. doi: 10.1097/QAI.0b013e3181bf1c45. [DOI] [PubMed] [Google Scholar]
- 6.Traeger L, O'Cleirigh C, Skeer MR, Mayer KH, Safren SA. Risk factors for missed HIV primary care visits among men who have sex with men. J Behav Med. 2011 doi: 10.1007/s10865-011-9383-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giordano TP, Gifford AL, White AC, Jr, Suarez-Almazor ME, Rabeneck L, Hartman C, et al. Retention in care: a challenge to survival with HIV infection. Clin Infect Dis. 2007;44(11):1493–9. doi: 10.1086/516778. [DOI] [PubMed] [Google Scholar]
- 8.Ford MA, Spicer CM. Monitoring HIV care in the United States: indicators and data systems: National Academies Press. 2012 [PubMed] [Google Scholar]
- 9.Duncan KC, Salters K, Forrest JI, Palmer AK, Wang H, O’Brien N, et al. Cohort Profile: Longitudinal investigations into supportive and ancillary health services. International journal of epidemiology. 2013;42(4):947–55. doi: 10.1093/ije/dys035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Neil CR, Palmer AK, Coulter S, O'Brien N, Shen A, Zhang W, et al. Factors associated with antiretroviral medication adherence among HIV-positive adults accessing highly active antiretroviral therapy (HAART) in British Columbia, Canada. Journal of the International Association of Physicians in AIDS Care (JIAPAC) 2012 doi: 10.1177/1545109711423976. 1545109711423976. [DOI] [PubMed] [Google Scholar]
- 11.Prejean J, Song R, Hernandez A, Ziebell R, Green T, Walker F HIV Incidence Surveillance Group. Estimated HIV incidence in the United States, 2006–2009. PloS one. 2011;6(8):e17502. doi: 10.1371/journal.pone.0017502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bouris A, Voisin D, Pilloton M, Flatt N, Eavou R, Hampton K, et al. Project nGage: network supported HIV care engagement for younger black men who have sex with men and transgender persons. Journal of AIDS & clinical research. 2013;4 doi: 10.4172/2155-6113.1000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Millett GA, Flores SA, Peterson JL, Bakeman R. Explaining disparities in HIV infection among black and white men who have sex with men: A meta-analysis of HIV risk behaviors. Aids. 2007;21:2083–91. doi: 10.1097/QAD.0b013e3282e9a64b. [DOI] [PubMed] [Google Scholar]
- 14.Voisin DR, DiClemente RJ, Salazar LF, Crosby RA, Yarber WL. Ecological factors associated with STD risk behaviors among detained female adolescents. Soc Work. 2006;51(1):71–9. doi: 10.1093/sw/51.1.71. [DOI] [PubMed] [Google Scholar]
- 15.Voisin DR, Salazar LF, Crosby R, Diclemente RJ, Yarber WL, Staples-Horne M. Teacher connectedness and health-related outcomes among detained adolescents. J Adolesc Health. 2005;37(4):337. doi: 10.1016/j.jadohealth.2004.11.137. [DOI] [PubMed] [Google Scholar]
- 16.Mo PKH, Coulson NS. Exploring the communication of social support within virtual communities: A content analysis of messages posted to an online HIV/AIDS support group. CyberPsychology & Behavior. 2008;11(3):371–4. doi: 10.1089/cpb.2007.0118. [DOI] [PubMed] [Google Scholar]
- 17.Berg KM, Wilson IB, Li X, Arnsten JH. Comparison of Antiretroviral Adherence Questions. Aids Behav. 2010 doi: 10.1007/s10461-010-9864-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magnus M, Jones K, Phillips G, 2nd, Binson D, Hightow-Weidman LB, Richards-Clarke C, et al. Characteristics associated with retention among African American and Latino adolescent HIV-positive men: results from the outreach, care, and prevention to engage HIV-seropositive young MSM of color special project of national significance initiative. J Acquir Immune Defic Syndr. 2010;53(4):529–36. doi: 10.1097/QAI.0b013e3181b56404. [DOI] [PubMed] [Google Scholar]
- 19.Hightow-Weidman LB, Jones K, Wohl AR, Futterman D, Outlaw A, Phillips G, et al. Early linkage and retention in care: findings from the outreach, linkage, and retention in care initiative among young men of color who have sex with men. AIDS Patient Care STDS. 2011;25(Suppl 1):S31–8. doi: 10.1089/apc.2011.9878. [DOI] [PubMed] [Google Scholar]
- 20.Schwarzer R, Dunkel-Schetter C, Kemeny M. The multidimensional nature of received social support in gay men at risk of HIV infection and AIDS. American journal of community psychology. 1994;22(3):319–39. doi: 10.1007/BF02506869. [DOI] [PubMed] [Google Scholar]
- 21.Detrie PM, Lease SH. The relation of social support, connectedness, and collective self-esteem to the psychological well-being of lesbian, gay, and bisexual youth. Journal of Homosexuality. 2007;53(4):173–99. doi: 10.1080/00918360802103449. [DOI] [PubMed] [Google Scholar]
- 22.Simoni JM, Huh D, Frick PA, Pearson CR, Andrasik MP, Dunbar PJ, et al. Peer support and pager messaging to promote antiretroviral modifying therapy in Seattle: a randomized controlled trial. Journal of acquired immune deficiency syndromes (1999) 2009;52(4):465–73. doi: 10.1097/qai.0b013e3181b9300c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Remien RH, Stirratt MJ, Dolezal C, Dognin JS, Wagner GJ, Carballo-Dieguez A, et al. Couple-focused support to improve HIV medication adherence: a randomized controlled trial. Aids. 2005;19(8):807–14. doi: 10.1097/01.aids.0000168975.44219.45. [DOI] [PubMed] [Google Scholar]
- 24.Simoni JM, Pantalone DW, Plummer MD, Huang B. A randomized controlled trial of a peer support intervention targeting antiretroviral medication adherence and depressive symptomatology in HIV-positive men and women. Health Psychology. 2007;26(4):488. doi: 10.1037/0278-6133.26.4.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelly JA, St Lawrence JS, Diaz YE, Stevenson LY, Hauth AC, Brasfield TL, et al. HIV risk behavior reduction following intervention with key opinion leaders of population: an experimental analysis. American Journal of Public Health. 1991;81(2):168–71. doi: 10.2105/ajph.81.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kegeles SM, Hays RB, Coates TJ. The Mpowerment Project: a community-level HIV prevention intervention for young gay men. American journal of public health. 1996;86(8_Pt_1):1129–36. doi: 10.2105/ajph.86.8_pt_1.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kimbrough LW, Fisher HE, Jones KT, Johnson W, Thadiparthi S, Dooley S. Accessing social networks with high rates of undiagnosed HIV infection: the social networks demonstration project. American journal of public health. 2009;99(6):1093–9. doi: 10.2105/AJPH.2008.139329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelly JD, Hartman C, Graham J, Kallen MA, Giordano TP. Social support as a predictor of early diagnosis, linkage, retention, and adherence to HIV care: results from the steps study. Journal of the Association of Nurses in AIDS Care. 2014;25(5):405–13. doi: 10.1016/j.jana.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wohl AR, Galvan FH, Myers HF, Garland W, George S, Witt M, et al. Do social support, stress, disclosure and stigma influence retention in HIV care for Latino and African American men who have sex with men and women? Aids Behav. 2011;15(6):1098–110. doi: 10.1007/s10461-010-9833-6. [DOI] [PubMed] [Google Scholar]
- 30.Catz SL, McClure JB, Jones GN, Brantley PJ. Predictors of outpatient medical appointment attendance among persons with HIV. AIDS Care. 1999;11(3):361–73. doi: 10.1080/09540129947983. [DOI] [PubMed] [Google Scholar]
- 31.Mannheimer S, Hirsch-Moverman Y. What We Know and What We Do Not Know About Factors Associated with and Interventions to Promote Antiretroviral Adherence. Current Infectious Disease Reports. 2015;17(4):1–8. doi: 10.1007/s11908-015-0466-9. [DOI] [PubMed] [Google Scholar]
- 32.Iroh PA, Mayo H, Nijhawan AE. The HIV care cascade before, during, and after incarceration: a systematic review and data synthesis. American journal of public health. 2015;105(7):e5–e16. doi: 10.2105/AJPH.2015.302635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bekker L-G, Johnson L, Wallace M, Hosek S. Building our youth for the future. J Int Aids Soc. 2015;18:1Á7. doi: 10.7448/IAS.18.2.20027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hogan B, Carrasco JA, Wellman B. Visualizing personal networks: Working with participant-aided sociograms. Field Method. 2007;19(2):116–44. [Google Scholar]
- 35.McFadden RB, Bouris AM, Voisin DR, Glick NR, Schneider JA. Dynamic social support networks of younger black men who have sex with men with new HIV infection. Aids Care-Psychological and Socio-Medical Aspects of Aids/Hiv. 2014;26(10):1275–82. doi: 10.1080/09540121.2014.911807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fisher JD, Fisher WA, Amico KR, Harman JJ. An information-motivation-behavioral skills model of adherence to antiretroviral therapy. Health Psychology. 2006;25(4):462. doi: 10.1037/0278-6133.25.4.462. [DOI] [PubMed] [Google Scholar]
- 37.Starace F, Massa A, Amico KR, Fisher JD. Adherence to antiretroviral therapy: an empirical test of the information-motivation-behavioral skills model. Health Psychology. 2006;25(2):153. doi: 10.1037/0278-6133.25.2.153. [DOI] [PubMed] [Google Scholar]
- 38.Miller WRRS. Motivational Interviewing: Helping People Change. Third. The Guilford Press; 2012. [Google Scholar]
- 39.Safren SA, O'Cleirigh C, Tan JY, Raminani SR, Reilly LC, Otto MW, et al. A randomized controlled trial of cognitive behavioral therapy for adherence and depression (CBT-AD) in HIV-infected individuals. Health Psychol. 2009;28(1):1–10. doi: 10.1037/a0012715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Safren SGJ, Soroudi N. Coping with chronic illness: A Cognitive-Behavioral approach for adherence and depression: therapist guide. New York: Oxford University Press; 2007. [Google Scholar]
- 41.Christopoulos KA, Das M, Colfax GN. Linkage and retention in HIV care among men who have sex with men in the United States. Clinical infectious diseases. 2011;52(suppl 2):S214–S22. doi: 10.1093/cid/ciq045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics. 2009;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mugavero MJ, Westfall AO, Zinski A, Davila J, Drainoni M-L, Gardner LI, et al. Measuring retention in HIV care: the elusive gold standard. Journal of acquired immune deficiency syndromes (1999) 2012;61(5):574. doi: 10.1097/QAI.0b013e318273762f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuhns LM, Hotton AL, Garofalo R, Muldoon AL, Jaffe K, Bouris A, et al. An Index of Multiple Psychosocial, Syndemic Conditions Is Associated with Antiretroviral Medication Adherence Among HIV-Positive Youth. Aids Patient Care St. 2016;30(4):185–92. doi: 10.1089/apc.2015.0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murphy DA, Sarr M, Durako SJ, Moscicki A-B, Wilson CM, Muenz LR. Barriers to HAART adherence among human immunodeficiency virus–infected adolescents. Arch Pediat Adol Med. 2003;157(3):249–55. doi: 10.1001/archpedi.157.3.249. [DOI] [PubMed] [Google Scholar]
- 46.Paterson DL, Swindells S, Mohr J, Brester M, Vergis EN, Squier C, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Annals of internal medicine. 2000;133(1):21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 47.Schneider JA, Cornwell B, Ostrow D, Michaels S, Schumm P, Laumann EO, et al. Network mixing and network influences most linked to HIV infection and risk behavior in the HIV epidemic among black men who have sex with men. American journal of public health. 2013;103(1):e28–e36. doi: 10.2105/AJPH.2012.301003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schneider J, Michaels S, Bouris A. Family network proportion and HIV risk among black men who have sex with men. Journal of Acquired Immune Deficiency Syndromes. 2012;61(5):627. doi: 10.1097/QAI.0b013e318270d3cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Outlaw A, Naar-King S, Green-Jones M, Wright K, Condon K, Sherry L, et al. Brief report: Predictors of optimal HIV appointment adherence in minority youth: A prospective study. Journal of pediatric psychology. 2010;35(9):1011–5. doi: 10.1093/jpepsy/jsq002. [DOI] [PubMed] [Google Scholar]
- 50.Wright K, Naar-King S, Lam P, Templin T, Frey M. Stigma scale revised: reliability and validity of a brief measure of stigma for HIV+ youth. J Adolescent Health. 2007;40(1):96–8. doi: 10.1016/j.jadohealth.2006.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Derogatis L. Brief symptom inventory: Administration, scoring, and procedures manual. Minneapolis, MN: National Computer Systems, Inc.; 1993. [Google Scholar]
- 52.Group W. The alcohol, smoking and substance involvement screening test (ASSIST): development, reliability and feasibility. Addiction. 2002;97(9):1183–94. doi: 10.1046/j.1360-0443.2002.00185.x. [DOI] [PubMed] [Google Scholar]
- 53.Bouris A, Hill BJ, Fisher K, Erickson G, Schneider JA. Mother–son communication about sex and routine human immunodeficiency virus testing among younger men of color who have sex with men. J Adolescent Health. 2015;57(5):515–22. doi: 10.1016/j.jadohealth.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park W, Choe P, Kim SH, Jo J, Bang J, Kim H, et al. One - year adherence to clinic visits after highly active antiretroviral therapy: a predictor of clinical progress in HIV patients. J Intern Med. 2007;261(3):268–75. doi: 10.1111/j.1365-2796.2006.01762.x. [DOI] [PubMed] [Google Scholar]
- 55.Mugavero MJ, Lin H-Y, Willig JH, Westfall AO, Ulett KB, Routman JS, et al. Missed visits and mortality among patients establishing initial outpatient HIV treatment. Clinical Infectious Diseases. 2009;48(2):248–56. doi: 10.1086/595705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Magnus M, Jones K, Phillips G, Binson D, Hightow-Weidman LB, Richards-Clarke C, et al. Characteristics associated with retention among African American and Latino adolescent HIV-positive men: results from the outreach, care, and prevention to engage HIV-seropositive young MSM of color special project of national significance initiative. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2010;53(4):529–36. doi: 10.1097/QAI.0b013e3181b56404. [DOI] [PubMed] [Google Scholar]
- 57.Woods ER. Overview of the special projects of national significance program’s 10 models of adolescent HIV care. J Adolescent Health. 1998;23(2):5–10. doi: 10.1016/s1054-139x(98)00049-4. [DOI] [PubMed] [Google Scholar]
- 58.Lee L, Yehia BR, Gaur AH, Rutstein R, Gebo K, Keruly JC, et al. The Impact of Youth-Friendly Structures of Care on Retention Among HIV-Infected Youth. Aids Patient Care St. 2016;30(4):170–7. doi: 10.1089/apc.2015.0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kennedy DP, Hunter SB, Osilla KC, Maksabedian E, Golinelli D, Tucker JS. A computer-assisted motivational social network intervention to reduce alcohol, drug and HIV risk behaviors among Housing First residents. Addict Sci Clin Prac. 2016;11 doi: 10.1186/s13722-016-0052-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Osilla KC, Kennedy DP, Hunter SB, Maksabedian E. Feasibility of a computer-assisted social network motivational interviewing intervention for substance use and HIV risk behaviors for housing first residents. Addict Sci Clin Prac. 2016;11 doi: 10.1186/s13722-016-0061-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schneider JCB, Jonas A, Behler R, Lancki N, Skaathun B, Michaels S, Khanna AS, Young LE, Morgan E, Duvoisin R, Friedman S, Schumm P, Laumann E for the uConnect Study Team. Network dynamics and HIV risk and prevention in a population-based cohort of young Black men who have sex with men. Network Science. in press. [Google Scholar]
- 62.Valente TW. Social networks and health: Models, methods, and applications: Oxford University Press. 2010 [Google Scholar]
- 63.Valente TW. Network interventions. Science. 2012;337(6090):49–53. doi: 10.1126/science.1217330. [DOI] [PubMed] [Google Scholar]
- 64.Schneider JA, Zhou AN, Laumann EO. A new HIV prevention network approach: sociometric peer change agent selection. Social Science & Medicine. 2015;125:192–202. doi: 10.1016/j.socscimed.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Group NCHSPT. Results of the NIMH collaborative HIV/STD prevention trial of a community popular opinion leader intervention. Journal of acquired immune deficiency syndromes (1999) 2010;54(2):204. doi: 10.1097/QAI.0b013e3181d61def. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schneider JA, McFadden RB, Laumann EO, Kumar SP, Gandham SR, Oruganti G. Candidate change agent identification among men at risk for HIV infection. Soc Sci Med. 2012;75(7):1192–201. doi: 10.1016/j.socscimed.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Latkin C, Donnell D, Liu TY, Davey - Rothwell M, Celentano D, Metzger D. The dynamic relationship between social norms and behaviors: the results of an HIV prevention network intervention for injection drug users. Addiction. 2013;108(5):934–43. doi: 10.1111/add.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Valente TW. Network models of the diffusion of innovations. Computational & Mathematical Organization Theory. 1996;2(2):163–4. [Google Scholar]