Summary
Depressive mood in youth has been associated with distinct sleep dimensions, such as timing, duration, and quality. To identify discrete sleep phenotypes we applied person-centered analysis (latent class mixture models) based on self-reported sleep patterns and quality, and examined associations between phenotypes and mood in high school seniors. Students (n=1451; mean age=18.4±0.3; 648 M) completed a survey near the end of high school. Indicators used for classification included school night bed and rise-times, differences between non-school night and school night bed and rise-times, sleep onset latency, number of awakenings, naps, and sleep quality and disturbance. Mood was measured using the total score on the Center for Epidemiologic Studies-Depression Scale. One-way ANOVA tested differences between phenotype for mood. Fit indexes were split between 3-, 4- and 5-phenotype solutions. For all solutions, between phenotype differences were shown for all indicators: Bedtime showed the largest difference, thus, classes were labeled from earliest to latest bedtime as “A” (n=751), “B” (n=428), and “C” (n=272) in the 3-class solution. Class B showed the lowest sleep disturbances and remained stable, whereas classes C & A each split in the 4 & 5-class solutions, respectively. Associations with mood were consistent, albeit small, with class B showing the lowest scores. Person centered analysis identified sleep phenotypes that differed in mood, such that those with the fewest depressive symptoms had moderate sleep timing, shorter sleep onset latencies, and fewer arousals. Sleep characteristics in these groups may add to our understanding of how sleep and depressed mood associate in teens.
Keywords: sleep patterns, depression, adolescence, mixture models
The functional correlates of inadequate sleep in adolescence have received growing attention in recent years (for reviews see Owens 2014; Shochat et al., 2014). Poor and insufficient sleep has been associated with mental (Blank et al., 2015; Coulombe et al., 2011; Fredriksen et al., 2004; Johnson et al., 2006; Roberts and Duong, 2014; Sivertsen et al, 2014; Short et al., 2013; Tzischinsky & Shochat, 2011), physical (Blank et al., 2015; Roberts et al., 2009), behavioral (McKnight-Eily et al., 2011; Umlauf et al., 2011), and academic (Carskadon et al., 1998; Dewald et al., 2010; Mak et al., 2012; Short et al., 2013; Wolfson & Carskadon, 1998) impairments. Depressed mood is an important outcome that has also often been linked with inadequate sleep. Previous studies have reported independent associations with various features of adolescent sleep, such as short sleep duration (Fredriksen et al., 2004; Roberts et al., 2009; Roberts and Duong, 2014; Ojio et al., 2016), late sleep timing (Díaz-Morales et al, 2015; Tzischinsky & Shochat, 2011), sleep problems, sleep disturbances, and insomnia (Coulombe et al., 2011; Johnson et al., 2006; Kaneita et al., 2009; Sivertsen et al., 2014; Short et al., 2013).
While it is informative to examine distinct features of sleep (e.g., duration, timing, quality) in relation to depressive mood (Short et al., 2013), in an ecological context these features are not independent. For example, early school start times require adolescents to maintain early sleep schedules, which often conflict with their developmental tendency towards later bedtimes (Carskadon et al., 1998; Hagenauer et al., 2009; Short et al., 2013), resulting in shorter sleep duration during the school week and delayed sleep timing and longer sleep duration on non-school nights (Carskadon et al., 1998; Short et al., 2013; Yang et al., 2005). Moreover, an evening “night owl” tendency and irregular weekly sleep schedules have been associated with poor sleep quality (Giannotti et al., 2002; Short et al., 2013; Tzischinsky & Shochat, 2011). Accounting for this interrelatedness among sleep features by articulating distinct sleep phenotypes in an ecological setting may provide additional insight into the relation between depressed mood and sleep. For example, do early vs. late sleep schedules associate with distinct features of sleep, e.g., sleep onset latency, number of awakenings, delayed sleep timing on non-school nights, or daytime naps; and if so, do these distinct phenotypes associate with variations in depressed mood?
Person-centered analytic approaches such as latent class, latent profile, or mixture models (Berlin et al, 2014) are attractive approaches because they utilize the relationships among features to classify participants into groups, with each group showing similar patterns. Using sleep measures derived by self-report from a large cohort of high-school seniors, we applied a person-centered analysis to classify individuals based on their self-reported habitual sleep patterns and perceived sleep quality. Our aims were to identify distinct sleep patterns and to explore their associations with depressed mood. Despite the exploratory, data driven nature of the study, based on the extant literature, we hypothesized that features indicating later sleep timing, disturbed sleep patterns and poorer sleep quality would comprise a distinct sleep phenotype that would be associated with depressed mood.
Methods
Participants
One thousand, four hundred and fifty-one high school seniors who accepted admission to Brown University in 2010–2014 (mean age = 18.4±0.3; 648 M) participated in the study with no exclusion criteria.
Procedure
Participants completed a paper and pencil survey that was sent to their home address by mail near the end of high school. The survey included demographic information, the Sleep Habits Survey (SHS, Wolfson & Carskadon, 1998), the Pittsburgh Sleep Quality Index (PSQI, Buysse et al., 1989) and the Center for Epidemiologic Studies-Depression Scale (CES-D, Radloff, 1977). The study was approved by the Rhode-Island Hospital/Lifespan Institutional Review Board for the Protection of Human Subjects. After reading information about the study, participants provided informed consent by checking a box at the beginning of the survey. The mailings included $5 in years 2010–2013 and $2 in 2014; participants were asked to complete the survey and return it in a prepaid envelope. The average response rate was 31%.
Materials
The SHS (Wolfson & Carskadon, 1998) asks participants to report bedtimes, rise-times, and other sleep-related behaviors for school nights and non-school nights over the previous two weeks. It includes the Bradley Sleepiness Scale (BSS, Carskadon et al., 1991), a 17-item, 3-point scale (0–2) summed to obtain a total score for the assessment of subjective sleepiness in different daily situations (e.g., during a test, doing homework), with higher scores indicating increased sleepiness; and the Horne Östberg Questionnaire (HÖQ, Horne & Östberg, 1976), a 19-item scale with 14 items ranging from 1–4 and 5 items ranging from 1–5, summed to assess morning-evening preference (e.g., preferred time of day for performing mental or physical activity), with higher scores indicating a morning preference. Internal consistency (Chronbach’s alpha) for the present sample was 0.77 and 0.80 for the BSS and the HÖQ, respectively. The PSQI (Buysse et al., 1989) is a short, retrospective self-rated questionnaire that measures subjective sleep quality over the past month and has been validated for use in college students (Lund et al., 2010). It is composed of 19 items assessing seven domains of sleep difficulty (e.g., sleep duration, sleep efficiency). Each domain is converted to a weighted score ranging from 0 to 3, and the sum of these scores represents global sleep quality, with higher scores indicating lower sleep quality. In the present study, only two of the seven domains were used (see statistical analysis below). The CES-D (Radloff, 1977) is a commonly used 20-item depression screener, validated for use in adolescents (Roberts et al., 1990). Symptoms of depression are rated for their frequency on a 4-point scale, from “rarely or none of the time” to “most or all of the time.” Items are summed to obtain a total score, with higher scores indicating a higher frequency of symptoms. A cutoff of ≥16 is an indicator of clinically elevated depression (Roberts et al., 1990). Internal consistency in the present sample was 0.88.
Statistical analysis
A person-centered approach, i.e., mixture modeling, was used to group individuals who reported similar sleep patterns (Berlin et al., 2014). The mixture model included four continuous and five categorical indicators. Continuous indicators derived from the SHS included reported school night bedtime (BT) and rise-time (RT), as well as the difference between non-school night and school night BT (dBT) and RT (dRT) (computed by subtracting school night from non-school night BT and RT, so that positive values indicated later, and negative values indicated earlier BT and RT on non-school nights, respectively). Time in Bed (TIB) was not included in the model because it is defined by variables already in the model (BT & RT). Three more indicators from the SHS included reported minutes to fall asleep (sleep onset latency, SOL), number of arousals (NOA) on school nights, and number of naps (NAPS) per school week. All three of these items were highly skewed toward zero and were converted from continuous to ordered categorical indicators each with three categories (SOL: ≤15 m, 16–30m, >30m; NOA: 0, 1–2, > 2; NAPS: 0, 1–2, > 2). The final two indicators were perceived sleep quality (PSQ) and sleep disturbances (SD) based on PSQI categorical subscales 1 and 5, respectively. Due to low endorsement of the higher scores (2 and 3), these were collapsed on both PSQI subscales resulting in a range of three scores (0–2) for each scale.
Mixture models were fit using Mplus version 7.2 (Muthén & Muthén, 1998–2012) using a maximum likelihood estimator with robust standard errors, with 500 initial-stage random starts and 10 final stage optimizations (Hipp & Bauer, 2006). The number of classes was determined through an iterative process (Berlin et al., 2014). Ten models were fit where the number of classes increased from 1–10. Model fit was compared among the 10 models using the negative log likelihood (lower values indicating better fit), Consistent Akaike’s Information Criteria (CAIC; nadir indicates best fit), Bayesian Information Criteria (BIC; nadir indicates best fit); and Vuong-Lo-Mendel-Rubin likelihood ratio test (VLMR-LRT; significant p-value indicates significant improvement in fit (k vs. k-1)). Class differentiation was evaluated using a measure of relative entropy, with 0 indicating no differentiation and 1 indicating perfect differentiation. Typically, entropy should be greater than .8 in order to reliably apply a classification model to other samples in future research. (Muthén & Muthén, 1998–2012).
Classes were labeled and characterized according to visual inspection of profiles across the indicators. For the visual inspection, indicators were placed on a comparable metric by z-scoring the estimated means for each class. For ordered categorical indicators, probit thresholds were mapped onto a standard normal distribution so that each of the ordered-categorical indicators could be represented by single values as opposed to multiple thresholds.
To evaluate classification solution(s), classes were compared across all of the sleep indicators used as part of the mixture model, as well as across sleep-related measures not entered in the mixture model (i.e., BSS, HÖQ, and school night and non-school-night TIB). Class differences were also compared for depression scores. For continuous variables, the comparisons were tested using one-way analyses of variance (ANOVA) and eta-squared was used to estimate effect size (~.01 small, ~.06 medium, ~.14 large, Cohen, 1988). For categorical variables, chi-square tests of independence were used and Cramér’s V was used to estimate effect size (~.1 small, ~.3 medium, ~.5 large, Cohen, 1988).
Results
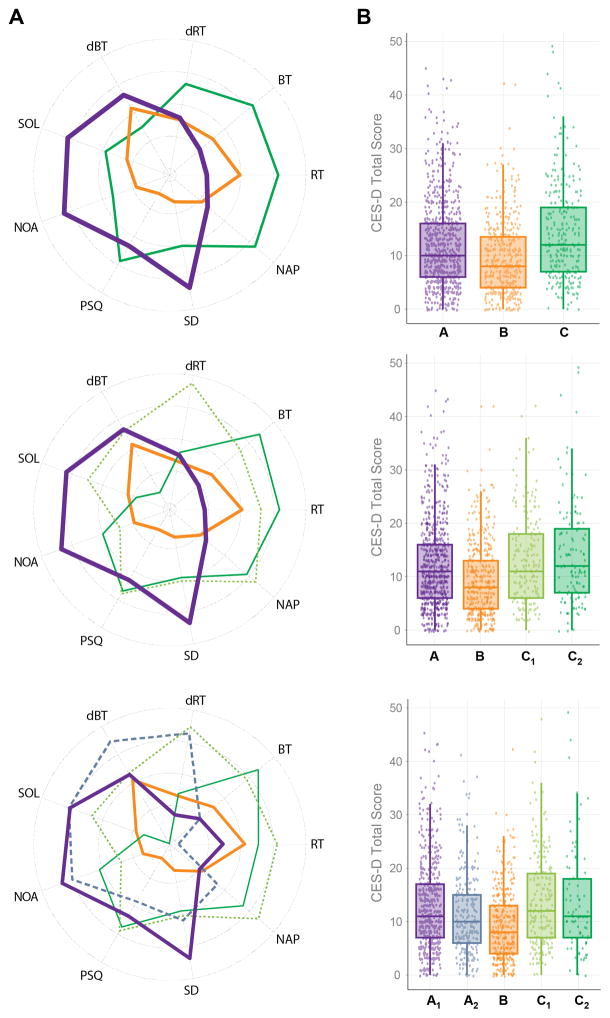
Fit indexes were split among three solutions (Table 1). The BIC suggested a 5-class solution, the CAIC a 4-class solution, and the VLMR-LRT suggested a 3-class solution. Entropy was also similar across the three solutions. Thus, all three classification solutions were evaluated and plotted (Figure 1A). Because the 3-class solution was the most parsimonious, and the associations with mood scores were similar across solutions, we have chosen to present results for this solution. Results for the 4-class and 5-class solution are included as an appendix.
Table 1.
Fit indexes and tests for class solutions, 1–10.
| Class solution (#) | Negative log likelihood | Number of Estimated Parameters | CAICa | BICa | VLMR-LRT p-valueb | Relative entropyc |
|---|---|---|---|---|---|---|
| 1 | −13867.25 | 18 | 27883.54 | 27865.54 | NA | NA |
| 2 | −13577.52 | 33 | 27428.27 | 27395.27 | <.01 | 0.713 |
| 3 | −13462.27 | 48 | 27321.97 | 27273.97 | <.01 | 0.615 |
| 4 | −13388.7 | 63 | 27299.05 | 27236.05 | 0.32 | 0.646 |
| 5 | −13332.27 | 78 | 27310.38 | 27232.38 | 0.24 | 0.622 |
| 6 | −13279.57 | 93 | 27329.17 | 27236.17 | 0.14 | 0.646 |
| 7 | −13234.76 | 108 | 27363.77 | 27255.77 | 0.25 | 0.659 |
| 8 | −13200.98 | 123 | 27420.39 | 27297.39 | 0.86 | 0.667 |
| 9 | −13167.7 | 138 | 27478.05 | 27340.04 | 0.80 | 0.692 |
| 10 | −13142.07 | 153 | 27550.98 | 27397.98 | 0.37 | 0.694 |
CAIC: Consistent Akaike’s Information Criteria, BIC: Bayesian Information Criteria, LRT: Likelihood ratio test, VLMR: Vuong-Lo-Mendel-Rubin.
BIC and CAIC: lowest value indicates best fitting model.
VLMR-LRT: significant p-value indicates significant improvement in fit (k vs. k-1).
measure of class differentiation ranges from no differentiation (0) to perfect differentiation (1).
Fig. 1.
(A) Radar plots of the three, four and five class solutions based on continuous (BT: bedtime, RT: rise time, dBT: difference in BT between non-school night and school night, dRT: difference in RT between nonschool night and school night) and categorical (SOL: sleep onset latency (school nights), NOA: number of awakenings (school-nights), NAP: weekly naps PSQ: perceived sleep quality, SD: sleep disturbance) indicators. (B) Box (interquartile range) and scatter plots of the Center for Epidemiologic Studies-Depression Scale (CES-D) scores by the three, four and five class solutions.
The 3-class solution showed differences on all of the sleep indicators entered into the mixture model and all but one of the variables not entered into the model (time in bed (TIB) on non-school nights; Table 2). Effect sizes indicated that the largest difference among classes was for reported bedtime. Classes were labeled from earliest to latest bedtime (BT) as “A” (n=751), “B” (n=428), and “C” (n=272). There was a main effect for age (F(2,1444)=6.08, p=0.002). Mean age in class C (18.46±0.43) was significantly higher than in classes B (18.38±0.29) and A (18.39±0.33). There were no sex differences across classes.
Table 2.
Three class solution class comparisons (means (SD) / percentages) for continuous and categorical sleep indicators, sleep measures not in the mixture model, and CES-D.
| Class A (n=751) | Class B (n=428) | Class C (n=272) | partial η2/Cramér’s V | |
|---|---|---|---|---|
| Continuous indicators (means (SD)) | ||||
| BT | 22.95 (0.6) | 23.47 (0.6) | 24.84 (0.7) | 0.54* |
| RT | 6.50 (0.7) | 6.68 (0.6) | 6.84 (0.7) | 0.04* |
| dBT | 1.46 (0.9) | 0.98 (0.9) | 0.44 (1.2) | 0.14* |
| dRT | 2.70 (1.3) | 2.60 (1.2) | 3.39 (1.4) | 0.05* |
| Categorical indicators (percentages) | ||||
| SOL (0,1,2) | 43,43,14 | 95,4,1 | 78,17,5 | 0.34* |
| NOA (0,1,2) | 46,5,50 | 96,1,3 | 84,2,15 | 0.35* |
| NAPS (0,1,2) | 57,27,16 | 59,31,10 | 11,35,54 | 0.31* |
| PSQ (0,1,2) | 10,69,21 | 40,57,3 | 7,60,34 | 0.30* |
| SD (0,1,2) | 0,89,11 | 30,70,0 | 10,83,7 | 0.31* |
| Sleep variables not entered in the mixture model (means (SD)) | ||||
| BSS | 5.30 (4.00) | 4.72 (3.62) | 7.68 (4.94) | 0.06* |
| HÖQ | 50.21 (8.02) | 50.29 (8.24) | 43.68 (7.95) | 0.09* |
| TIB (school-nights) | 7.58 (0.83) | 7.22 (0.78) | 5.96 (01.99) | 0.34* |
| TIB (non-school-nights) | 8.79 (1.26) | 8.84 (1.11) | 8.95 (1.46) | 0.002 |
| Depression (means (SD)) | ||||
| CES-D | 12.23 (8.19) | 9.48 (7.00) | 13.87 (9.04) | 0.04* |
p<0.001;
BT: bedtime, RT: rise time, dBT: difference in BT between non-school night and school night, dRT: difference in RT between non-school night and school night, SOL: sleep onset latency (school nights), NOA: number of awakenings (school-nights), PSQ: perceived sleep quality, SD: sleep disturbance, BSS: Bradley Sleepiness Scale, HÖQ: Horne-Östeberg Questionnaire, TIB: time in bed, CES-D: Center for Epidemiologic Studies - Depression Scale, η2 : eta squared.
There was a significant but relatively small association between class membership and depressed mood scores (Table 2). Class B showed the lowest and class C showed the highest CES-D score (Figure 1B).
Discussion
Person-centered analysis from our large sample of over fourteen hundred college-bound high school seniors suggested three classification solutions, each representing possible sleep “phenotypes” characterized by profiles of reported sleep timing and disturbance. In all three of the solutions we evaluated, three relatively stable profiles emerged, with class B demonstrating the highest stability. Class B was characterized by moderate sleep timing and low sleep disturbance indexes, and included nearly 30% of the study sample. The other two classes (A & C) were characterized by different constellations of early or late (i.e., averages near 2300 or near 0100 hours, respectively) sleep timing and indexes of elevated sleep disturbance. Class A was the largest group, and included about 50% of the sample.
Classification solutions were evaluated using additional sleep-related variables not included in the models, i.e., daytime sleepiness, phase preference, and time in bed (TIB). These associations supported class B as a moderate sleep profile, demonstrating intermediate phase preference and weekday TIB and lowest sleepiness levels. Class C stood out as the least “moderate,” with a clear evening preference, shortest reported TIB, and highest sleepiness levels. Beyond being the most consistent group, class B showed the lowest depressive mood symptomatology across classification models.
High school students in class A reported the earliest BT (near 2300 hours on average), possibly indicating an attempt to increase their weekday sleep quotas and adhere to early sleep schedules during the school week due to early school start times. This strategy, however, is not without cost. Discrepancy between intrinsic sleep timing and environmentally imposed sleep practices in adolescence has been described as a conflict between psychosocial requirements and bioregulatory mechanisms favoring delayed sleep patterns; insufficient sleep arises due to inappropriately timed forced arousals in adherence with early school start times (Carskadon et al., 1998; Carskadon et al., 2004). In the present study, this discrepancy assumed a different form: the data indicate that early BT and long TIB were associated with increased sleep disruption. Similar findings were reported in a cross-ethnic comparison study of sleep patterns in Israeli teens, where sleep in Arab adolescents was characterized by earlier BT and longer SOL compared to their Jewish counterparts (Shochat, 2013). Our findings here suggest that advanced BT may coincide with a circadian phase that is not permissive of sleep, also known as the “forbidden zone” for sleep (Lavie, 1986). We surmise that for adolescents with late intrinsic sleep timing, early BT that adhere to cultural or societal norms are detrimental to sleep onset and continuity.
Furthermore, a tendency favoring increasingly later BT in the course of adolescent development is well established (Carskadon et al., 1998; Hagenauer et al., 2009; Short et al., 2013); and indeed, elements of the sleep profile in class C, including late BT, inadequate sleep quotas, and irregular sleep patterns have previously been observed in adolescent population based studies (Hysing et al., 2013; Yang et al., 2005). Findings from our person centered approach, however, show that late sleep timing (near 0100) is not habitual for most high school seniors in this mostly North American sample, suggesting that despite their delayed tendency, a sizable proportion of students opt for BTs near 2300 on average. Taken together, our findings suggest that both later and earlier sleep patterns are associated with disturbed and irregular sleep patterns.
These findings show that sleep profiles differed in association with depressive mood symptoms, with higher depressed mood scores for classes A and C compared with class B, suggesting that the sleep features in class B comprise a constellation that may be protective not only for sleep disturbance but also for depressed mood. It is important to note that mean scores in all classes did not reach the CES-D clinical cutoff of 16 (Radloff, 1977). Nevertheless, scrutinizing the distributions of the individual scores shows that approximately 15%, 25%, and 33% exceeded the cutoff in classes B, A, and C, respectively.
As person-centered approaches have not been used in the study of sleep and depression, we struggle to draw comparisons with studies that typically correlate depression with single dimensions of sleep (e.g., sleep length or quality). For example, in an adolescent population based study from Norway, later BT and shorter TIB were associated with depression (Sivertsen et al., 2014). These linear relationships stand in contrast to our findings that represent a U-shaped relationship, whereby classes characterized by both earlier and later BT and both shorter and longer TIB, were associated with elevated levels of depressed mood. A population based study of adolescents aged 12–18 in Japan provides evidence for a U-shaped relationship between sleep duration and risk for anxiety and depression (Ojio et al., 2016). Rates of anxiety and depression were lowest for males reporting 8.5–9.5 hours of sleep, and for females reporting 7.5–8.5 hours of sleep. Sleeping <7.5 hours but also between 8.5–9.5 hours significantly increased the risk for anxiety and depression in females, compared to 7.5–8.5 hours. Taken together with our findings, we hypothesize that sleep impairments associated with more extreme sleep patterns, in terms of both timing and duration, may mediate their relationship with depressed mood. These findings challenge current sleep recommendations that generally acknowledge linear associations suggesting that more sleep is associated with optimal health outcomes (Beebe, 2016).
Study limitations
There are a number of limitations to this study. First, the data are cross-sectional and relied exclusively on self-report. Second, the data came from a non-representative sample of college-bound high school seniors. Nevertheless, self-reported measures of sleep timing and duration are fairly consistent with those reported by the National Sleep Foundation Sleep in America Poll (2006). Third, within each solution, significant variability between participants was indexed by entropy scores that were less than 0.8, the suggested cut-off for broader application. This variability suggests that there may be additional classes that were too small to reliably detect given our sample or that additional measures or features of sleep may need to be considered before this classification model can be applied to other samples. It may help to examine non-survey data, such as diary data or actigraphy, to improve the differentiation between the models. Finally, as is true with any empirically-based classification method, the sleep classifications generated by the mixture model need to be replicated in additional samples and linked with additional outcomes before being considered as reified sleep classifications.
Conclusions
This person-centered approach provides a comprehensive picture of how diverse reported sleep variables aggregated in our sample of high school seniors. The sleep patterns and disturbances that characterize these groups may add to our understanding of how sleep and mood associate in older teens in the context of high school. Our findings suggest that more extreme (early/late) sleep timing is characterized by more sleep and mood disturbances than moderate sleep timing. Early rise times characterized the largest group, likely due to early school start times rather than to intrinsic sleep preference. These findings carry important clinical implications suggesting that the promotion of moderate rather than early sleep patterns may be protective for sleep and depressed mood in adolescents.
Acknowledgments
Funding: NIMH MH079179
The authors wish to thank their data coordinator Caroline Gredvig-Ardito at the E.P. Bradley Sleep and Chronobiology Research Lab for her meticulous work and tireless efforts to support this project.
Appendix A
Similar to the 3-class solution, the 4- and 5-class solutions also showed significant differences on reported sleep variables, both those included and not included in the mixture models (Tables A.1 and A.2). Based on visual inspection of class profiles for the 3-, 4-, and 5-class solutions (Figure 1A), class B remained highly stable across all classifications, whereas classes C and A each split into two subgroups (labeled C1, C2 and A1, A2) in the 4- and 5-class solutions, respectively (Figure 1A).
Table A.1.
Four class solution class comparisons (means (SD) / percentages) for continuous and categorical sleep indicators, sleep measures not in the mixture model, and CES-D.
| Class A (n=704) | Class B (n=423) | Class C1 (n=197) | Class C2 (n=127) | partial η2/Cramér’s V | |
|---|---|---|---|---|---|
| Continuous indicators (means (SD)) | |||||
| BT | 22.91 (0.62) | 23.43 (0.63) | 24.42 (0.64) | 01.10 (0.65) | 0.57* |
| RT | 6.49 (0.67) | 6.69 (0.60) | 6.75 (0.66) | 6.85 (0.65) | 0.04* |
| dBT | 1.45 (0.90) | 0.93 (0.82) | 1.45 (0.78) | −0.51 (1.04) | 0.29* |
| dRT | 2.64 (1.21) | 2.45 (1.03) | 4.22 (1.20) | 2.67 (1.28) | 0.19* |
| Categorical indicators (percentages) | |||||
| SOL (0,1,2) | 42,43,15 | 93,6,1 | 65,27,8 | 93,5,2 | 0.35* |
| NOA (0,1,2) | 43,5,52 | 96,1,3 | 88,1,11 | 75,3,22 | 0.36* |
| NAPS (0,1,2) | 58,27,15 | 62,27,11 | 7,44,49 | 19,33,48 | 0.32* |
| PSQ (0,1,2) | 10,70,20 | 40,56,4 | 7,64,29 | 10,56,34 | 0.29* |
| SD (0,1,2) | 0,89,11 | 28,72,0 | 9,85,6 | 13,81,6 | 0.30* |
| Sleep variables not entered in the mixture model (means (SD)) | |||||
| BSS | 5.29 (4.06) | 4.63(3.58) | 7.42(4.78) | 7.43(4.69) | 0.06* |
| HÖQ | 50.59(7.76) | 50.87(8.14) | 41.26(7.26) | 46.03(7.52) | 0.16* |
| TIB (school-nights) | 7.62(0.81) | 7.26(0.77) | 6.33(0.86) | 5.66(1.03) | 0.37* |
| TIB (non-school-nights) | 9.10(1.35) | 8.93(1.55) | 8.78(1.26) | 8.77(1.09) | 0.01** |
| Depression (means (SD)) | |||||
| CES-D | 12.33(8.25) | 9.48(7.00) | 12.95(8.29) | 13.93(9.66) | 0.03* |
p<0.001;
p<0.01;
BT: bedtime, RT: rise time, dBT: difference in BT between non-school night and school night, dRT: difference in RT between non-school night and school night, SOL: sleep onset latency (school nights), NOA: number of awakenings (school-nights), PSQ: perceived sleep quality, SD: sleep disturbance, BSS: Bradley Sleepiness Scale, HÖQ: Horne-Östeberg Questionnaire, TIB: time in bed, CES-D: Center for Epidemiologic Studies - Depression Scale, η2 : eta squared.
Table A.2.
Five class solution class comparisons (means (SD) / percentages) for continuous and categorical sleep indicators, sleep measures not in the mixture model, and CES-D.
| Class A1 (n=516) | Class A2 (n=245) | Class B (n=385) | Class C1 (n=221) | Class C2 (n=84) | p, partial η2/Cramér’s V | |
|---|---|---|---|---|---|---|
| Continuous indicators (means (SD)) | ||||||
| BT | 22.94(0.62) | 22.97(0.63) | 23.47(0.66) | 24.60(0.70) | 01.01(0.66) | 0.53* |
| RT | 6.57(0.65) | 6.35(0.65) | 6.68(0.62) | 6.85(0.67) | 6.74(0.62) | 0.53* |
| dBT | 1.07(0.72) | 2.28(0.74) | 0.91(0.76) | 1.17(0.74) | −1.06(0.86) | 0.48* |
| dRT | 2.06(0.95) | 3.93(1.04) | 2.48(0.95) | 3.92(1.17) | 2.56(1.32) | 0.38* |
| Categorical indicators (percentages) | ||||||
| SOL (0,1,2) | 46,40,14 | 46,41,13 | 94,6,0 | 67,24,9 | 95,4,1 | 0.32* |
| NOA (0,1,2) | 42,5,53 | 53,5,42 | 98,0,2 | 87,1,12 | 73,5,22 | 0.36* |
| NAPS (0,1,2) | 66,24,10 | 45,35,20 | 59,28,13 | 7,39,54 | 21,36,43 | 0.33* |
| PSQ (0,1,2) | 10,65,25 | 13,77,10 | 41,58,1 | 6,58,35 | 10,63,27 | 0.31* |
| SD (0,1,2) | 0,87,13 | 1,95,3 | 30,70,0 | 9,84,7 | 16,79,5 | 0.32* |
| Sleep variables not entered in the mixture model (means (SD)) | ||||||
| BSS | 4.89(3.68) | 5.99(4.49) | 4.63(3.56) | 7.55(4.81) | 7.68(5.01) | 0.07* |
| HÖQ | 52.44(7.70) | 46.47(7.16) | 50.48(7.99) | 41.98(7.40) | 47.08(7.51) | 0.19* |
| TIB (school-nights) | 7.68(0.82) | 7.41(0.79) | 7.21(0.79) | 6.23(0.93) | 5.61(1.08) | 0.35* |
| TIB (non-school-nights) | 8.63(1.20) | 9.02(1.33) | 8.78(1.09) | 8.99(1.36) | 9.35(1.5) | 0.03* |
| Depression (means (SD)) | ||||||
| CES-D | 12.70(8.45) | 11.27(7.71) | 9.15(6.62) | 13.74(8.64) | 13.56(9.93) | 0.05* |
p<0.001;
BT: bedtime, RT: rise time, dBT: difference in BT between non-school night and school night, dRT: difference in RT between non-school night and school night, SOL: sleep onset latency (school nights), NOA: number of awakenings (school-nights), PSQ: perceived sleep quality, SD: sleep disturbance, BSS: Bradley Sleepiness Scale, HÖQ: Horne-Östeberg Questionnaire, TIB: time in bed, CES-D: Center for Epidemiologic Studies - Depression Scale, η2: eta squared.
Footnotes
Conflict of Interest: Authors have none to declare.
Author contributorship: TS: statistical analysis, data interpretation, manuscript writing and editing; DHB: analysis conception and design, statistical analysis, data interpretation, manuscript editing; KMS, EVR, BMR, and MAC: study design, data interpretation, manuscript editing.
References
- Beebe DW. WEIRD considerations when studying adolescent sleep need. SLEEP. 2016;39(8):1491–1492. doi: 10.5665/sleep.6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin KS, Williams NA, Parra GR. An introduction to latent variable mixture modeling (part 1): Overview and cross-sectional latent class and latent profile analyses. J Pediatr Psychol. 2014;39:174–187. doi: 10.1093/jpepsy/jst084. [DOI] [PubMed] [Google Scholar]
- Blank M, Zhang J, Lamers F, Taylor AD, Hickie IB, Merikangas KR. Health correlates of insomnia symptoms and comorbid mental disorders in a nationally representative sample of US adolescents. Sleep. 2015;38:197–204. doi: 10.5665/sleep.4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Wolfson AR, Acebo C, Tzischinsky O, Seifer R. Adolescent sleep patterns, circadian timing, and sleepiness at a transition to early school days. Sleep. 1998;21:871–881. doi: 10.1093/sleep/21.8.871. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Acebo C, Jenni OG. Regulation of adolescent sleep: Implications for behavior. Ann N Y Acad Sci. 2004;1021:276–291. doi: 10.1196/annals.1308.032. [DOI] [PubMed] [Google Scholar]
- Carskadon M, Seifer R, Acebo C. Reliability of six scales in a sleep questionnaire for adolescents. J Sleep Res. 1991;20:421. [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2 Hillsdale, New Jersey: 1988. [Google Scholar]
- Coulombe JA, Reid GJ, Boyle MH, Racine Y. Sleep problems, tiredness, and psychological symptoms among healthy adolescents. J Pediatr Psychol. 2011;36:25–35. doi: 10.1093/jpepsy/jsq028. [DOI] [PubMed] [Google Scholar]
- Dewald JF, Meijer AM, Oort FJ, Kerkhof GA, Bögels SM. The influence of sleep quality, sleep duration and sleepiness on school performance in children and adolescents: A meta-analytic review. Sleep Med Rev. 2010;14:179–189. doi: 10.1016/j.smrv.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Díaz-Morales JF, Escribano C, Jankowski KS. Chronotype and time-of-day effects on mood during school day. Chronobiol Int. 2015;32:37–42. doi: 10.3109/07420528.2014.949736. [DOI] [PubMed] [Google Scholar]
- Fredriksen K, Rhodes J, Reddy R, Way N. Sleepless in Chicago: Tracking the effects of adolescent sleep loss during the middle school years. Child Dev. 2004;75:84–95. doi: 10.1111/j.1467-8624.2004.00655.x. [DOI] [PubMed] [Google Scholar]
- Giannotti F, Cortesi F, Sebastiani T, Ottaviano S. Circadian preference, sleep and daytime behaviour in adolescence. J Sleep Res. 2002;11:191–199. doi: 10.1046/j.1365-2869.2002.00302.x. [DOI] [PubMed] [Google Scholar]
- Hagenauer MH, Perryman JI, Lee TM, Carskadon MA. Adolescent changes in the homeostatic and circadian regulation of sleep. Dev Neurosci. 2009;31:276–284. doi: 10.1159/000216538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipp JR, Bauer DJ. Local solutions in the estimation of growth mixture models. Psychol Methods. 2006;11:36. doi: 10.1037/1082-989X.11.1.36. [DOI] [PubMed] [Google Scholar]
- Horne JA, Östberg OA. self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- Hysing M, Pallesen S, Stormark KM, Lundervold AJ, Sivertsen B. Sleep patterns and insomnia among adolescents: a population-based study. J Sleep Res. 2013;22:549–56. doi: 10.1111/jsr.12055. [DOI] [PubMed] [Google Scholar]
- Johnson EO, Roth T, Breslau N. The association of insomnia with anxiety disorders and depression: Exploration of the direction of risk. J Psychiatr Res. 2006;40:700–708. doi: 10.1016/j.jpsychires.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Kaneita Y, Yokoyama E, Harano S, et al. Associations between sleep disturbance and mental health status: A longitudinal study of Japanese junior high school students. Sleep Med. 2009;10:780–786. doi: 10.1016/j.sleep.2008.06.014. [DOI] [PubMed] [Google Scholar]
- Lavie P. Ultrashort sleep-waking schedule. III.’Gates’ and ‘forbidden zones’ for sleep. Electroencephalogr Clin Neurophysiol. 1986;63:414–425. doi: 10.1016/0013-4694(86)90123-9. [DOI] [PubMed] [Google Scholar]
- Lund HG, Reider BD, Whiting AB, Prichard JR. Sleep patterns and predictors of disturbed sleep in a large population of college students. J Adolesc Health. 2010;46:124–132. doi: 10.1016/j.jadohealth.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Mak KK, Lee SL, Ho SY, Lo WS, Lam TH. Sleep and academic performance in Hong Kong adolescents. J Sch Health. 2012;82:522–527. doi: 10.1111/j.1746-1561.2012.00732.x. [DOI] [PubMed] [Google Scholar]
- McKnight-Eily LR, Eaton DK, Lowry R, Croft JB, Presley-Cantrell L, Perry GS. Relationships between hours of sleep and health-risk behaviors in US adolescent students. Prev Med. 2011;53:271–273. doi: 10.1016/j.ypmed.2011.06.020. [DOI] [PubMed] [Google Scholar]
- Muthén B, Muthén L. User’s guide. Muthén & Muthén; Los Angeles, CA: 2012. Mplus version 7. [Google Scholar]
- National Sleep Foundation. [Accessed January 13, 2016];Sleep in America Poll. 2006 [Online]. Available at: https://sleepfoundation.org/sleep-polls-data/sleep-in-america-poll/2006-teens-and-sleep.
- Ojio Y, Nishida A, Shimodera S, Togo F, Sasaki T. Sleep Duration Associated with the Lowest Risk of Depression/Anxiety in Adolescents. Sleep. 2016;39(8):1555–62. doi: 10.5665/sleep.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens J. Insufficient Sleep in Adolescents and Young Adults: An Update on Causes and Consequences. Pediatrics. 2014;134:e921–e932. doi: 10.1542/peds.2014-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff L. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- Roberts RE, Andrews JA, Lewinsohn PM, Hops H. Assessment of depression in adolescents using the Center for Epidemiologic Studies Depression Scale. Psychol Assess. 1990;2:122–8. [Google Scholar]
- Roberts RE, Roberts CR, Duong HT. Sleepless in adolescence: Prospective data on sleep deprivation, health and functioning. J Adolesc. 2009;32:1045–1057. doi: 10.1016/j.adolescence.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RE, Duong HT. The prospective association between sleep deprivation and depression among adolescents. Sleep. 2014;37:239–244. doi: 10.5665/sleep.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shochat T. Sleep patterns and daytime sleep-related behaviors in male and female Arab and Jewish adolescents in Israel. Sleep Biol Rhythms. 2013;11:82–89. [Google Scholar]
- Shochat T, Cohen-Zion M, Tzischinsky O. Functional consequences of inadequate sleep in adolescents: A systematic review. Sleep Med Rev. 2014;18:75–87. doi: 10.1016/j.smrv.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Short MA, Gradisar M, Lack LC, Wright HR. The impact of sleep on adolescent depressed mood, alertness and academic performance. J Adolesc. 2013;36:1025–1033. doi: 10.1016/j.adolescence.2013.08.007. [DOI] [PubMed] [Google Scholar]
- Sivertsen B, Harvey AG, Lundervold AJ, Hysing M. Sleep problems and depression in adolescence: Results from a large population-based study of Norwegian adolescents aged 16–18 years. Eur Child Adolesc Psychiatry. 2014;23:681–689. doi: 10.1007/s00787-013-0502-y. [DOI] [PubMed] [Google Scholar]
- Tzischinsky O, Shochat T. Eveningness, sleep patterns, daytime functioning, and quality of life in Israeli adolescents. Chronobiol Int. 2011;28:338–343. doi: 10.3109/07420528.2011.560698. [DOI] [PubMed] [Google Scholar]
- Umlauf MG, Bolland JM, Lian BE. Sleep disturbance and risk behaviors among inner-city African American adolescents. J Urban Health. 2011;88:1130–1142. doi: 10.1007/s11524-011-9591-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson AR, Carskadon MA. Sleep schedules and daytime functioning in adolescents. Child Dev. 1998;69:875–887. [PubMed] [Google Scholar]
- Yang CK, Kim JK, Patel SR, Lee JH. Age-related changes in sleep/wake patterns among Korean teenagers. Pediatrics. 2005;115:250–256. doi: 10.1542/peds.2004-0815G. [DOI] [PubMed] [Google Scholar]