Abstract
Purpose
To determine the extent to which trajectories of maternal preconception leisure time physical activity (LTPA) and sedentary behavior (LTSB) during adolescence and young adulthood are associated with offspring birthweight (BW), and to test if these associations differ by offspring sex or maternal pre-pregnancy overweight/obese status.
Methods
Participants with ≥1 birth (N=1,408) were identified from the National Longitudinal Study of Adolescent to Adult Health. Group-based trajectory modeling was used to characterize trajectories of LTPA (frequency/week) and LTSB (hours/week) which were measured, on average, over 7 years between age 15–22. Weighted regression and Wald tests were used to estimate and test mean differences and ORs for BW, small-, and large-for-gestational age (SGA, LGA).
Results
Three trajectories were identified for LTPA and five for LTSB. Associations differed by offspring sex for continuous BW and LGA (interaction P=0.10 and 0.008, respectively). Among female offspring, participants with high followed by decreasing LTPA delivered offspring with 90 g greater BW (95% CI: −4, 184) and 72% greater risk of LGA (95% CI: 0.94–3.14), compared to participants with low LTPA. Among male offspring, LTPA patterns were not associated with BW. A pattern of high then decreasing LTPA among normal weight, but not overweight/obese women, was associated with 2.03 times greater risk of LGA (95% CI: 1.06, 3.88). LTSB trajectories were not associated with BW.
Conclusion
Associations of preconception trajectories of LTPA with offspring BW may differ by offspring sex and maternal pre-pregnancy overweight/obese status.
Keywords: Physical activity, sedentary behavior, life course, birthweight, adolescence, trajectory
INTRODUCTION
Maternal preconception lifestyle behaviors influence maternal health and can affect pregnancy outcomes including offspring birthweight (1, 2). Leisure time physical activity (LTPA) in adolescence and adulthood is associated with cardiovascular and metabolic health benefits, including lower body mass index (BMI) and body fat (3–5), greater insulin sensitivity (6, 7), lower blood pressure (8, 9), and lower inflammation (10). Prolonged sedentary behavior, independent of LTPA, is associated with increased risk of overweight (5), higher glucose and insulin levels, greater waist circumference, systolic blood pressure, and triglycerides (11, 12). These health consequences of LTPA and leisure time sedentary behavior (LTSB) in adolescence and young adulthood affect disease processes (body fat accumulation (3, 4), insulin sensitivity (6, 7, 12, 13), vascular function (14), and inflammation (10)) throughout adulthood and impact future maternal health. Maternal health status and preparedness for pregnancy is directly related to pregnancy outcomes.
A life course approach to healthy pregnancy and fetal development focuses not just on the pregnancy itself but also on preconception lifestyle and behavior (1). Prevention of chronic diseases by targeting upstream factors in adolescence and young adulthood may be less costly, easier, and more effective than prevention or treatment efforts focused only on adulthood (15). However, most previous research on determinants of healthy pregnancy has focused on behaviors during pregnancy or the short time period before the pregnancy. To our knowledge, the only previous study of patterns of maternal LTPA during adolescence and adulthood reported associations of long-term preconception LTPA with reduced risk of preterm birth but not low birthweight (16). This study did not consider macrosomia or preconception LTSB. Further, despite potential differences in response to maternal health status and behavior between male and female fetuses (2, 17, 18) and between fetuses born to women who are of normal weight and women who are obese (19, 20), roles of offspring sex and maternal pre-pregnancy BMI in these associations have not been studied.
Birthweight is an important measure of fetal growth and development (21). Extremes of the birthweight distribution have been associated with adverse short- and long-term health outcomes. Low birthweight and small-for-gestational age are associated with perinatal morbidity and mortality (22), as well as risk of chronic diseases in adulthood (23–27). Macrosomia and large-for-gestational age are associated with development of obesity and type 2 diabetes in later life (28, 29).
The objective of this study was to determine the extent to which trajectories of maternal preconception LTPA or LTSB during adolescence and young adulthood were associated with offspring birthweight, and to test if these associations differed by offspring sex or maternal pre-pregnancy overweight/obese status.
METHODS
Study setting and study population
This study was conducted using data collected from 1994–2008 as part of the National Longitudinal Study of Adolescent to Adult Health (Add Health), a prospective cohort study designed to identify social, behavioral, and biological determinants of health across the life course (30). Add Health participants were identified from 132 “feeder” schools (schools that send graduates to a specific high school) of a stratified, random sample of high schools in the United States based on region, rural/urban setting, school size/type, and racial composition of the student body. At each school, a stratified, random sample of students based on grade and sex were selected for participation in the study. A total of 20,745 adolescents in grades 7–12 (age 11–21 years) participated in Wave I (1994–1995). Follow up of participants occurred in 1996 (Wave II), 2001–2002 (Wave III), and 2007–2009 (Wave IV) when participants were 12–22 years old, 18–26 years old, and 24–32 years old, respectively. Between 71% and 76% of the baseline cohort completed study interviews at each follow-up assessment. Among 2,983 participants with at least one live birth after Wave III, 78%, 100%, and 99.9% of participants completed study interviews at Wave II, III, and IV, respectively. Add Health was approved by the Institutional Review Board of the University of North Carolina at Chapel Hill. This secondary analysis was conducted using deidentified data obtained under an Add Health Restricted-Use Data Contract at the University of Washington.
Participants with ≥1 live, singleton birth between Wave III and IV were included in this study (N=2,983). For participants who reported ≥1 live, singleton birth between Wave III and IV, only the first birth after Wave III study interview was included in this study. Participants with missing data for survey weight (N=182), birthweight (N=23), LTPA or LTSB trajectory (N=649), parental income and pre-pregnancy BMI (N=711), or other covariates (N=10) were excluded. A total of 1,408 participants with follow-up, on average, over 7 years between age 15–22, remained for analyses. Sociodemographic and pregnancy characteristics were similar between all eligible participants, participants included in this study, and participants excluded due to missing data (Supplementary Table 1).
Data collection
During in-person study interviews, data on sociodemographic characteristics (age, race, income of participant’s parents), pregnancy history and outcomes (live births, smoking and alcohol use during pregnancies), anthropometric measurements (height and weight), LTPA and LTSB were collected. Pre-pregnancy BMI was calculated using reported height and weight at Wave III and categorized according to standard cutoffs (underweight: <18.5 kg/m2, normal: 18.5–24.9 kg/m2, overweight: 25–29.9 kg/m2, obese: ≥30 kg/m2) (31).
Physical activity
In Waves I and II, participants reported frequency of the following LTPAs in the past week: jogging, walking, karate, jumping rope, gymnastics, dancing, roller-blading, roller-skating, skate-boarding, bicycling, baseball, softball, basketball, soccer, swimming, and football. At Wave III, participants reported frequency of the following LTPAs in the past week: bicycling, skate-boarding, dancing, hiking, hunting, yard work, gymnastics, weight lifting, strength training, running, wrestling, swimming, cross-country skiing, cycle racing, martial arts, football, soccer, basketball, lacrosse, rugby, field hockey, ice hockey, gold, fishing, bowling, softball, baseball, roller-blading, roller-skating, downhill skiing, snowboarding, racquet sports, aerobics, and walking. Because more LTPAs were assessed at Wave III (34 activities) than at Wave I or II (16 activities), total reported frequency of LTPA at Wave III was scaled by (16/34) to avoid overestimating LTPA at Wave III, as has previously been done in Add Health (32). The LTPA questionnaire used in Add Health has not undergone validity testing. However, validity compared to heart rate monitoring (r=0.45 for 7 day Physical Activity Recall) and test-retest reliabilities (r=0.49 for 7 day Physical Activity Recall, r=0.76 for Godin-Shephard Survey) of self-reported LTPA was reported to be reasonable among female children and adolescents (33).
Sedentary behavior
Participants reported frequency and duration (hours) of the following LTSBs in the past week at each study visit: watching a movie, playing video or computer games, and using a computer for something other than school work.
Birthweight
At Wave IV, participants reported current pregnancy status and pregnancy history, including birth date, birthweight, sex, and gestational age of live births. Offspring birthweight was categorized as small-for-gestational age (SGA, <10th percentile) or large-for-gestational age (LGA, >90th percentile) using INTERGROWTH-21st birthweight standards, which are considered to be valid for multiethnic populations (34).
Statistical analyses
Maternal and offspring characteristics were summarized for the analytic population. Weighted mean and standard deviation using survey weights was used to describe continuous variables. Frequency and weighted percentage was used to describe categorical variables.
Group-based trajectory analysis using a censored normal model for continuous measures, implemented through the TRAJ procedure in SAS, was used to identify trajectories of LTPA and LTSB from Wave I to Wave III (35, 36). This approach assumes the population is composed of a mixture of distinct groups defined by their trajectories. Sets of probability distributions for the specified number of LTPA and LTSB trajectory groups were calculated using maximum likelihood with age as the time scale. The optimal number and form (shape of the pattern of change) of trajectories were identified by comparing Bayesian Information Criteria (BIC) for models with different numbers of trajectory groups. Model comparisons with logged Bayes factor (2*ΔBIC)>10 were considered strong evidence for the better model (36). Additionally, even if logged Bayes factor comparison suggested a model with <20 participants in one trajectory group was a better model, this model was not considered better because of the challenges of using such small numbers in adjusted regression models (37). First, models with different numbers of trajectory groups with all groups of quadratic form were compared. After the optimal numbers of trajectory groups were determined, the form of all trajectory groups was changed to cubic form and the model was compared to one with all groups in quadratic form to identify the optimal form of each trajectory group. For the final models with the optimal number of trajectory groups and optimal form for each group, the probability of group membership in each of the identified trajectory groups was calculated for each participant. Each participant was assigned to the trajectory group for which she had the highest posterior predictive probability on an age time scale. Trajectory groups were identified in the total analytic study population and in groups defined by pre-pregnancy BMI category (normal weight or overweight/obese). Final forms for trajectory groups are presented in Supplementary Table 2. Final models had good discrimination for LTPA (average posterior predictive probability=0.67) and LTSB (average posterior predictive probability=0.77). Identified trajectories were labeled based on the relative distribution of the trajectories.
Weighted linear regression using survey weights was used to estimate mean differences and 95% confidence intervals (CI) in offspring birthweight associated with LTPA and LTSB trajectories. Weighted logistic regression was used to estimate odds ratios and 95% CIs for SGA and LGA associated with trajectories. Wald tests were used to test joint significance of all LTPA or LTSB trajectories. Model I was adjusted for maternal age at delivery (years), race (non-Hispanic white/non-Hispanic black/Hispanic/other), parental income category (<$20K/$20-60K/>$60K), nulliparity (Y/N), smoking during pregnancy (Y/N), alcohol use during pregnancy (Y/N), gestational age at delivery (weeks), and offspring sex. Model II was additionally adjusted for pre-pregnancy BMI category (underweight/normal weight/overweight/obese).
Regression models were also fit stratified by offspring sex and by pre-pregnancy overweight/obese status. Two-way multiplicative interaction terms and corresponding P values were used to assess interactions of LTPA or LTSB trajectories with offspring sex. Because trajectories were different for normal weight and overweight/obese women, there was no test of interaction for these groups. We used another longitudinal data approach, generalized estimating equations with repeated exposure and exchangeable correlation structure, to conduct sensitivity analyses.
A two-sided alpha level of 0.05 was used for statistical significance in all analyses. Analyses were performed using SAS 9.4 (SAS Institute Inc., Cary NC) and Stata 12.1 (StataCorp, College Station TX).
RESULTS
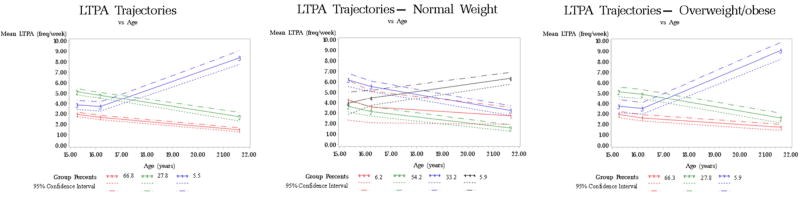
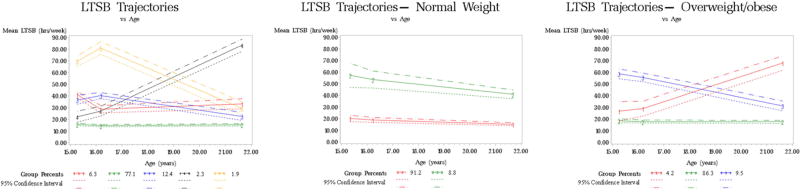
Participants were 24 years old at delivery and delivered newborns that weighted 3,281g, on average (Table 1). Sixty-seven percent of women were white, 14% were black, and 11% were Hispanic. About half of the participants were nulliparous and normal weight. Twenty-four percent smoked during pregnancy. We identified three LTPA trajectories in adolescence and early adulthood among all participants, four LTPA trajectories among women with normal pre-pregnancy BMI, and three LTPA trajectories among women with overweight/obese pre-pregnancy BMI (Figure 1, Supplementary Table 3). We identified five LTSB trajectories among all participants, two LTSB trajectories among women with a normal pre-pregnancy BMI, and three LTSB trajectories among women with overweight/obese pre-pregnancy BMI (Figure 2, Supplementary Table 4).
Table 1.
Maternal and offspring characteristics of Add Health study participants, 1994–2008
| Total | |
|---|---|
| (N=1,408) | |
| Maternal characteristics | |
| Age at delivery (years), weighted mean (SE) | 24 (0.2) |
| Race, n (weighted %) | |
| Non-Hispanic white | 813 (67) |
| Non-Hispanic black | 274 (14) |
| Hispanic | 213 (11) |
| Other | 108 (7) |
| Married at time of pregnancy, (n, weighted %) | 770 (81)a |
| Parental incomeb ($K), weighted mean (SE) | 40 (1.4) |
| Parental incomeb category (n, weighted %) | |
| <$20,000 | 335 (25) |
| $20,000–60,000 | 776 (55) |
| >$60,000 | 297 (20) |
| Nulliparous, n (weighted %) | 813 (56) |
| Pre-pregnancy BMI (kg/m2), weighted mean (SE) | 26 (0.3) |
| Pre-pregnancy BMI category, n (weighted %) | |
| Underweight | 51 (4) |
| Normal weight | 662 (47) |
| Overweight | 336 (24) |
| Obese | 359 (25) |
| Smoking during pregnancy, n (weighted %) | 284 (24) |
| Alcohol use during pregnancy, n (weighted %) | 86 (7) |
| Offspring characteristics | |
| Birthweight (grams), weighted mean (SE) | 3281 (23.0) |
| Low birthweight (<2,500g), n (weighted %) | 113 (8) |
| SGA, n (weighted %) | 110 (8) |
| LGA, n (weighted %) | 216 (15) |
| Gestational age at delivery (weeks), weighted mean (SE) | 39 (0.1) |
| Male, n (weighted %) | 717 (52) |
N=937
Income of participant’s parents at age 11–21 years.
Figure 1.
Leisure time physical activity trajectory groups for study participants overall (N=1,408) and by pre-pregnancy overweight/obesity status (N=662 for normal weight, N=695 for overweight/obese)
Figure 2.
Leisure time sedentary behavior trajectory groups for study participants (N=1,408) and by pre-pregnancy overweight/obesity status (N=662 for normal weight, N=695 for overweight/obese)
LTPA trajectories and birthweight
LTPA trajectories were not associated with offspring birthweight in the overall population or by pre-pregnancy BMI category (Supplementary Table 5), but there was a suggestion that associations differed by offspring sex (P value for interaction=0.10) (Table 2). There were no statistically significant associations between LTPA trajectories and offspring birthweight in sex-stratified analyses. However, there was a suggested association among participants who delivered female offspring. Among participants who delivered female offspring, participants with high LTPA at 15–16 years old followed by decreasing LTPA at 22 years old delivered offspring with 90g greater birthweight (95% CI: −4, 184; P=0.06) compared to participants with low then decreasing LTPA. Among participants delivering male offspring, the same LTPA trajectory was not associated with birthweight (mean difference=−22; 95% CI: −110, 67; P=0.63). Results were similar after adjusting for pre-pregnancy BMI category.
Table 2.
Associations of leisure time physical activity trajectory groups with birthweight
| N | Mean birthweight (g) |
Mean difference (95% CI) | ||
|---|---|---|---|---|
| Model Ia | Model IIb | |||
| Leisure time physical activity trajectory | ||||
| OVERALL | 1408 | |||
| 1 (decreasing from low activity) | 940 | 3293 | Reference | Reference |
| 2 (decreasing from high activity) | 391 | 3273 | 26 (−44, 96) | 25 (−44, 94) |
| 3 (increasing activity) | 77 | 3171 | −35 (−191, 120) | −48 (−202, 106) |
| FEMALESc | 691 | |||
| 1 (decreasing from low activity) | 468 | 3208 | Reference | Reference |
| 2 (decreasing from high activity) | 181 | 3178 | 90 (−4, 184) | 92 (−1, 186) |
| 3 (increasing activity) | 42 | 3067 | −73 (−258, 112) | −70 (−261, 121) |
| MALESc | 717 | |||
| 1 (decreasing from low activity) | 472 | 3375 | Reference | Reference |
| 2 (decreasing from high activity) | 210 | 3341 | −22 (−110, 67) | −27 (−113, 59) |
| 3 (increasing activity) | 35 | 3307 | 15 (−217, 246) | −10 (−230, 209) |
Model is adjusted for maternal age at delivery (years), race (non-Hispanic white/non-Hispanic black/Hispanic/other), parental income category (<$20K/$20-60K/>$60K), nulliparity (Y/N), smoking during pregnancy (Y/N), alcohol use during pregnancy (Y/N), gestational age at delivery (weeks), and offspring sex.
Model II is adjusted for all covariates in Model I and pre-pregnancy BMI category (underweight/normal weight/overweight/obese).
P value for interaction of LTPA trajectories with offspring sex= Model I: 0.10, Model II: 0.08
LTPA trajectories and birthweight categories
LTPA trajectories were not associated with SGA or LGA in the overall population (Supplementary Table 6), but associations with LGA differed by offspring sex (P value for interaction=0.008) (Table 3). There were no statistically significant associations between LTPA trajectories and LGA in sex-stratified analyses. However, there was a suggested association among participants who delivered female offspring. Among participants who delivered female offspring, participants with high LTPA at 15–16 years old followed by decreasing LTPA at 22 years old delivered offspring with 72% greater odds of LGA (95% CI: 0.94, 3.14; P=0.08) than participants with low then decreasing LTPA. Among participants delivering male offspring, LTPA trajectories were not associated with LGA (P=0.15). LTPA trajectories were associated with LGA among normal weight women, but not overweight/obese women. Among normal weight women, participants with high LTPA at 15–16 years old followed by decreasing LTPA at 22 years old delivered offspring with 2.03-fold greater odds of LGA (95% CI: 1.06, 3.88) than participants with consistently moderate LTPA (Table 2). There were no associations of LTPA trajectories with SGA in female offspring, male offspring, normal weight women, or overweight/obese women. Results were similar after adjusting for pre-pregnancy BMI category.
Table 3.
Associations of leisure time physical activity trajectory groups with categorical birthweight
| N | SGA | N | LGA | |||
|---|---|---|---|---|---|---|
| Odds Ratio (95% CI) | Odds Ratio (95% CI) | |||||
| Model Ia | Model IIb | Model Ia | Model IIb | |||
| Leisure time physical activity trajectory | ||||||
| OVERALL | ||||||
| 1 (decreasing from low activity) | 76/922 | Reference | Reference | 139/922 | Reference | Reference |
| 2 (decreasing from high activity) | 24/376 | 0.85 (0.39, 1.87) | 0.86 (0.39, 1.89) | 64/376 | 1.07 (0.98, 1.69) | 1.08 (0.69, 1.70) |
| 3 (increasing activity) | 10/74 | 1.71 (0.70, 4.20) | 1.89 (0.74, 4.84) | 13/74 | 1.07 (0.46, 2.46) | 1.03 (0.46, 2.33) |
| FEMALES | ||||||
| 1 (decreasing from low activity) | 37/462 | Reference | Reference | 64/462 | Reference | Reference |
| 2 (decreasing from high activity) | 9/172 | 0.81 (0.26, 2.52) | 0.75 (0.23, 2.41) | 33/172 | 1.72 (0.94, 3.14) | 1.83 (0.98, 3.41) |
| 3 (increasing activity) | 6/41 | 1.28 (0.35, 4.70) | 1.54 (0.35, 6.86) | 5/41 | 0.38 (0.11, 1.33) | 0.40 (0.12, 1.33) |
| MALES | ||||||
| 1 (decreasing from low activity) | 39/460 | Reference | Reference | 75/460 | Reference | Reference |
| 2 (decreasing from high activity) | 15/204 | 0.75 (0.32, 1.78) | 0.74 (0.31, 1.77) | 31/204 | 0.69 (0.40, 1.21) | 0.68 (0.39, 1.18) |
| 3 (increasing activity) | 4/33 | 2.51 (0.61, 10.3) | 2.49 (0.62, 10.0) | 8/33 | 1.84 (0.74, 4.62) | 1.66 (0.65, 4.21) |
| NORMAL WEIGHT | ||||||
| 1 (consistently moderate activity) | 3/40 | 1.10 (0.18, 6.76) | Reference | 8/40 | 2.33 (0.71, 7.67) | Reference |
| 2 (decreasing from low activity) | 33/355 | Reference | - | 44/355 | Reference | - |
| 3 (decreasing from high activity) | 17/218 | 1.11 (0.51, 2.42) | - | 35/218 | 2.03 (1.06, 3.88) | - |
| 4 (increasing from high activity)c | - | - | - | 6/41 | 0.52 (0.15, 1.87) | - |
| OVERWEIGHT/OBESE | ||||||
| 1 (consistently low activity) | 35/450 | Reference | Reference | 76/450 | Reference | Reference |
| 2 (decreasing activity) | 8/179 | 0.54 (0.21, 1.37) | - | 37/179 | 1.11 (0.58, 2.14) | - |
| 3 (increasing activity) | 6/39 | 2.23 (0.42, 11.9) | - | 8/39 | 1.51 (0.62, 3.68) | - |
P value for interaction with of LTPA trajectory with offspring sex: SGA Model I=0.89, SGA Model II=0.84; LGA Model I=0.008, LGA Model II=0.007
Model I is adjusted for maternal age at delivery (years), race (non-Hispanic white/non-Hispanic black/Hispanic/other), parental income category (<$20K/$20-60K/>$60K), nulliparity (Y/N), smoking during pregnancy (Y/N), alcohol use during pregnancy (Y/N), gestational age at delivery (weeks), and offspring sex.
Model II is adjusted for all covariates in Model I and pre-pregnancy BMI category (underweight/normal weight/overweight/obese).
Number of SGA in this category is too small (<3), so cannot show estimate according to Add Health rules.
LTSB trajectories and birthweight
LTSB trajectories were not associated with offspring birthweight (continuous or categorical) in the overall population, by offspring sex, or by pre-pregnancy BMI category. Results were similar with additional adjustment for pre-pregnancy BMI category. Preconception LTPA or LTSB was not associated with offspring birthweight in sensitivity analyses (Supplementary Table 7).
DISCUSSION
Our study found that maternal preconception LTPA and LTSB trajectories were not significantly associated with offspring birthweight overall. However, we observed several suggested associations that differed by offspring sex and maternal pre-pregnancy BMI that, while not statistically significant, are of interest. Among participants who delivered female offspring, there was a suggested association of high LTPA in adolescence followed by decreasing LTPA in early adulthood and 90g greater offspring birthweight and 72% greater odds for offspring LGA compared to participants with low then decreasing LTPA. This association was not observed among participants who delivered male offspring. Among women with normal pre-pregnancy BMI, participants with high LTPA in adolescence (15–16 years) followed by decreasing LTPA in early adulthood (22 years) had 2.0-fold greater odds for having LGA offspring compared with participants with consistently moderate LTPA throughout adolescence and young adulthood. This association was not observed among overweight/obese women.
A previous study of patterns of maternal preconception LTPA and pregnancy outcomes using Add Health data by Vamos et al found that maintaining high LTPA (≥5 times per week, based on meeting physical activity recommendations through 30 minutes of physical activity 5 days per week) in adolescence and adulthood was not associated with low birthweight (OR=0.79; 95% CI: 0.45, 1.39) (16). Vamos et al identified patterns of LTPA a priori, which may not capture observed patterns in the data. In order to better capture observed LTPA patterns, we used a data-driven approach to identify trajectories in our analysis. We did not find associations of any identified LTPA trajectory with SGA. Vamos et al did not examine LTSB or LGA in their analysis or stratify by offspring sex or maternal pre-pregnancy BMI. No other study, to our knowledge, has examined these associations.
The sex-specific and pre-pregnancy BMI-specific findings we report are consistent with sex-specific and pre-pregnancy BMI-specific associations of LTPA in the year before pregnancy with offspring birthweight we reported previously (2). In a cohort based in western Washington State, we found that pre-pregnancy LTPA was associated with lower offspring birthweight among female offspring and among offspring of women with normal pre-pregnancy BMI. LTPA in adolescence and young adulthood is correlated with LTPA later in adulthood, which influences adult health (38). Participants with high LTPA in adolescence followed by decreasing LTPA in early adulthood likely continued to have a low LTPA level in adulthood, including during the time period immediately preceding the pregnancy. Low levels of LTPA in the year before pregnancy and during early pregnancy may have sex-specific direct effects on early placental development (39), resulting in greater offspring birthweight among females, but not males, as we observed previously. Female fetuses may respond to changes in the intrauterine environment by changing growth rate, while male fetuses may respond by adapting placental function to continue normal growth (17). Additionally, low LTPA in adolescence and adulthood is associated with greater risk of abnormal glucose metabolism (6, 7, 13), which is a strong pre-pregnancy risk factor for gestational diabetes and large offspring birth size (40). Decreasing LTPA across adolescence to adulthood may be a stronger risk factor for abnormal glucose metabolism before pregnancy in normal weight women than in overweight/obese women, which may contribute to the observed increased risk of LGA in normal weight women.
The data driven approach we used in our study identified similar distributions of longitudinal LTPA and LTSB patterns as a previous study among the female Add Health population using a priori specified patterns (32). However, we did not identify a pattern of consistently high LTPA, as reported previously (16, 32). We also identified an additional LTSB trajectory with consistently moderate LTSB.
Strengths of our study include characterization of long-term, longitudinal patterns of preconception LTPA and LTSB, use of continuous and categorical measures of birthweight to better characterize associations with birth size, and generalizability of results to the United States female population, as this was a nationally representative sample. However, results should be interpreted with several limitations in mind. LTPA and LTSB were recalled and self-reported, and LTPA duration was not collected, which may have introduced measurement error. Although the questionnaires used in Add Health have not undergone validity or reliability testing, similar LTPA recalls had reasonable validity (r=0.45 for 7 day Physical Activity Recall) compared to heart rate monitors and reasonable test-retest reliabilities (r=0.49 for 7 day Physical Activity Recall, r=0.76 for Godin-Shephard Survey) among female respondents (33). LTSB self-report among adolescent girls tends to underestimate sedentary behavior by about 3 hours per week compared to accelerometry (41) and has moderate reliability (κ=0.42–0.55) (42). Measurement error is likely non-differential as LTPA and LTSB data were assessed before participants became pregnant. This may have biased our results toward the null. Offspring birthweight and gestational age at delivery were also recalled and self-reported. Maternal recall of birthweight has excellent validity (r=0.90) compared to birthweight in the medical record (43). Maternal recall of gestational age is also good (ICC=0.68) compared with the medical record (44). A large portion of eligible participants were excluded due to missing data; however, sociodemographic and pregnancy characteristics were similar between eligible participants, participants included in this analysis, and participants excluded due to missing data, reducing concerns about selection bias. Low P values for associations of LTSB trajectories with LGA in males and females were driven by one trajectory with no cases of LGA and are not reliable estimates (Supplementary Table 8). Three time points is the minimum number of time points necessary in order to apply trajectory modeling, which may have affected the estimation of trajectories. In sensitivity analyses using generalized estimating equations, we did not observe associations of preconception LTPA or LTSB with offspring birthweight. Finally, we may have had low power to identify statistically significant associations by offspring sex.
In conclusion, we found potential associations of a pattern of high LTPA in adolescence followed by decreasing LTPA in young adulthood with larger offspring birth size among female offspring and participants with normal pre-pregnancy BMI. Our results may help identify women at risk for LGA offspring at the beginning of pregnancy based on history of long-term LTPA prior to conception. Our results also contribute to a growing body of literature on sex-specific determinants of fetal growth and development. Future studies confirming our observed associations should objectively measure LTPA and LTSB at more than three time points to better characterize trajectories of these behaviors.
Supplementary Material
Acknowledgments
Funding
This work was supported by grants from the National Institutes of Health (T32 HD052462, R01HD-32562, and K01HL103174).
LIST OF ABBREVIATIONS
- BMI
body mass index
- CI
confidence interval
- LGA
large-for-gestational age
- LTPA
leisure time physical activity
- LTSB
leisure time sedentary behavior
- SGA
small-for-gestational age
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Misra DP, Guyer B, Allston A. Integrated perinatal health framework. A multiple determinants model with a life span approach. American journal of preventive medicine. 2003;25(1):65–75. doi: 10.1016/s0749-3797(03)00090-4. [DOI] [PubMed] [Google Scholar]
- 2.Badon SE, Wander PL, Qiu C, Miller RS, Williams MA, Enquobahrie DA. Maternal Leisure Time Physical Activity and Infant Birth Size. Epidemiology (Cambridge, Mass) 2016;27(1):74–81. doi: 10.1097/EDE.0000000000000399. [DOI] [PubMed] [Google Scholar]
- 3.Moore LL, Gao D, Bradlee ML, Cupples LA, Sundarajan-Ramamurti A, Proctor MH, et al. Does early physical activity predict body fat change throughout childhood? Prev Med. 2003;37(1):10–7. doi: 10.1016/s0091-7435(03)00048-3. [DOI] [PubMed] [Google Scholar]
- 4.Stevens J, Suchindran C, Ring K, Baggett CD, Jobe JB, Story M, et al. Physical activity as a predictor of body composition in American Indian children. Obes Res. 2004;12(12):1974–80. doi: 10.1038/oby.2004.248. [DOI] [PubMed] [Google Scholar]
- 5.O’Brien M, Nader PR, Houts RM, Bradley R, Friedman SL, Belsky J, et al. The ecology of childhood overweight: a 12-year longitudinal analysis. International journal of obesity (2005) 2007;31(9):1469–78. doi: 10.1038/sj.ijo.0803611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmitz KH, Jacobs DR, Jr, Hong CP, Steinberger J, Moran A, Sinaiko AR. Association of physical activity with insulin sensitivity in children. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2002;26(10):1310–6. doi: 10.1038/sj.ijo.0802137. [DOI] [PubMed] [Google Scholar]
- 7.Imperatore G, Cheng YJ, Williams DE, Fulton J, Gregg EW. Physical activity, cardiovascular fitness, and insulin sensitivity among U.S. adolescents: the National Health and Nutrition Examination Survey, 1999–2002. Diabetes care. 2006;29(7):1567–72. doi: 10.2337/dc06-0426. [DOI] [PubMed] [Google Scholar]
- 8.Gidding SS, Barton BA, Dorgan JA, Kimm SY, Kwiterovich PO, Lasser NL, et al. Higher self-reported physical activity is associated with lower systolic blood pressure: the Dietary Intervention Study in Childhood (DISC) Pediatrics. 2006;118(6):2388–93. doi: 10.1542/peds.2006-1785. [DOI] [PubMed] [Google Scholar]
- 9.Parker ED, Schmitz KH, Jacobs DR, Jr, Dengel DR, Schreiner PJ. Physical activity in young adults and incident hypertension over 15 years of follow-up: the CARDIA study. American journal of public health. 2007;97(4):703–9. doi: 10.2105/AJPH.2004.055889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Platat C, Wagner A, Klumpp T, Schweitzer B, Simon C. Relationships of physical activity with metabolic syndrome features and low-grade inflammation in adolescents. Diabetologia. 2006;49(9):2078–85. doi: 10.1007/s00125-006-0320-6. [DOI] [PubMed] [Google Scholar]
- 11.Healy GN, Dunstan DW, Salmon J, Shaw JE, Zimmet PZ, Owen N. Television time and continuous metabolic risk in physically active adults. Medicine and science in sports and exercise. 2008;40(4):639–45. doi: 10.1249/MSS.0b013e3181607421. [DOI] [PubMed] [Google Scholar]
- 12.Dunstan DW, Salmon J, Healy GN, Shaw JE, Jolley D, Zimmet PZ, et al. Association of television viewing with fasting and 2-h postchallenge plasma glucose levels in adults without diagnosed diabetes. Diabetes care. 2007;30:516–22. doi: 10.2337/dc06-1996. United States. [DOI] [PubMed] [Google Scholar]
- 13.Cornelissen VA, Fagard RH. Effects of endurance training on blood pressure, blood pressure-regulating mechanisms, and cardiovascular risk factors. Hypertension. 2005;46(4):667–75. doi: 10.1161/01.HYP.0000184225.05629.51. [DOI] [PubMed] [Google Scholar]
- 14.Dias KA, Green DJ, Ingul CB, Pavey TG, Coombes JS. Exercise and Vascular Function in Child Obesity: A Meta-Analysis. Pediatrics. 2015;136(3):e648–59. doi: 10.1542/peds.2015-0616. [DOI] [PubMed] [Google Scholar]
- 15.Cheng TL, Solomon BS. Translating Life Course Theory to clinical practice to address health disparities. Maternal and child health journal. 2014;18(2):389–95. doi: 10.1007/s10995-013-1279-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vamos CA, Flory S, Sun H, DeBate R, Bleck J, Thompson E, et al. Do Physical Activity Patterns Across the Lifecourse Impact Birth Outcomes? Maternal and child health journal. 2015;19(8):1775–82. doi: 10.1007/s10995-015-1691-4. [DOI] [PubMed] [Google Scholar]
- 17.Clifton VL. Review: Sex and the human placenta: mediating differential strategies of fetal growth and survival. Placenta. 2010;(31 Suppl):S33–9. doi: 10.1016/j.placenta.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 18.Di Renzo GC, Rosati A, Sarti RD, Cruciani L, Cutuli AM. Does fetal sex affect pregnancy outcome? Gender medicine. 2007;4(1):19–30. doi: 10.1016/s1550-8579(07)80004-0. [DOI] [PubMed] [Google Scholar]
- 19.Sewell MF, Huston-Presley L, Super DM, Catalano P. Increased neonatal fat mass, not lean body mass, is associated with maternal obesity. American journal of obstetrics and gynecology. 2006;195(4):1100–3. doi: 10.1016/j.ajog.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 20.Sebire NJ, Jolly M, Harris JP, Wadsworth J, Joffe M, Beard RW, et al. Maternal obesity and pregnancy outcome: a study of 287,213 pregnancies in London. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2001;25(8):1175–82. doi: 10.1038/sj.ijo.0801670. [DOI] [PubMed] [Google Scholar]
- 21.Hediger ML, Joseph KS. Fetal Growth: Measurement and Evaluation. In: Buck Lewis G, Platt R, editors. Reproductive and Perinatal Epidemiology. New York: Oxford University Press; 2011. pp. 168–85. [Google Scholar]
- 22.Clausson B, Cnattingius S, Axelsson O. Outcomes of post-term births: the role of fetal growth restriction and malformations. Obstetrics and gynecology. 1999;94(5 Pt 1):758–62. doi: 10.1016/s0029-7844(99)00387-7. [DOI] [PubMed] [Google Scholar]
- 23.Santos MS, Joles JA. Early determinants of cardiovascular disease. Best practice & research Clinical endocrinology & metabolism. 2012;26(5):581–97. doi: 10.1016/j.beem.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 24.Osmond C, Barker DJ, Winter PD, Fall CH, Simmonds SJ. Early growth and death from cardiovascular disease in women. Bmj. 1993;307(6918):1519–24. doi: 10.1136/bmj.307.6918.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berends LM, Ozanne SE. Early determinants of type-2 diabetes. Best practice & research Clinical endocrinology & metabolism. 2012;26(5):569–80. doi: 10.1016/j.beem.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992;35(7):595–601. doi: 10.1007/BF00400248. [DOI] [PubMed] [Google Scholar]
- 27.Botting KJ, Wang KC, Padhee M, McMillen IC, Summers-Pearce B, Rattanatray L, et al. Early origins of heart disease: low birth weight and determinants of cardiomyocyte endowment. Clinical and experimental pharmacology & physiology. 2012;39(9):814–23. doi: 10.1111/j.1440-1681.2011.05649.x. [DOI] [PubMed] [Google Scholar]
- 28.Eriksson JG, Forsen T, Tuomilehto J, Jaddoe VW, Osmond C, Barker DJ. Effects of size at birth and childhood growth on the insulin resistance syndrome in elderly individuals. Diabetologia. 2002;45(3):342–8. doi: 10.1007/s00125-001-0757-6. [DOI] [PubMed] [Google Scholar]
- 29.Eriksson J, Forsen T, Tuomilehto J, Osmond C, Barker D. Size at birth, childhood growth and obesity in adult life. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2001;25(5):735–40. doi: 10.1038/sj.ijo.0801602. [DOI] [PubMed] [Google Scholar]
- 30.Harris KM, Halper CT, Whitsel E, Hussey J, Tabor J, Entzel P, et al. The National Longitudinal Study of Adolescent to Adult Health: Research Design. 2009 Available from: http://www.cpc.unc.edu/projects/addhealth/design.
- 31.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organization technical report series. 2000;894:1–253. i-xii. [PubMed] [Google Scholar]
- 32.Gordon-Larsen P, Nelson MC, Popkin BM. Longitudinal physical activity and sedentary behavior trends: adolescence to adulthood. American journal of preventive medicine. 2004;27(4):277–83. doi: 10.1016/j.amepre.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 33.Sallis JF, Buono MJ, Roby JJ, Micale FG, Nelson JA. Seven-day recall and other physical activity self-reports in children and adolescents. Medicine and science in sports and exercise. 1993;25(1):99–108. doi: 10.1249/00005768-199301000-00014. [DOI] [PubMed] [Google Scholar]
- 34.Villar J, Cheikh Ismail L, Victora CG, Ohuma EO, Bertino E, Altman DG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet. 2014;384(9946):857–68. doi: 10.1016/S0140-6736(14)60932-6. [DOI] [PubMed] [Google Scholar]
- 35.Jones B, Nagin D, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociological Methods & Research. 2001;29(3):374–93. [Google Scholar]
- 36.Jones B, Nagin D. Advances in group-based trajectory modeling and an SAS procedure for estimating them. Sociological Methods & Research. 2007;35(4):542–71. [Google Scholar]
- 37.Nagin DS. Group-Based Modeling of Development. Cambridge MA: Harvard University Press; 2005. [Google Scholar]
- 38.Hallal PC, Victora CG, Azevedo MR, Wells JC. Adolescent physical activity and health: a systematic review. Sports medicine (Auckland, NZ) 2006;36(12):1019–30. doi: 10.2165/00007256-200636120-00003. [DOI] [PubMed] [Google Scholar]
- 39.Clapp JF, 3rd, Kim H, Burciu B, Lopez B. Beginning regular exercise in early pregnancy: effect on fetoplacental growth. American journal of obstetrics and gynecology. 2000;183(6):1484–8. doi: 10.1067/mob.2000.107096. [DOI] [PubMed] [Google Scholar]
- 40.Ehrenberg HM, Mercer BM, Catalano PM. The influence of obesity and diabetes on the prevalence of macrosomia. American journal of obstetrics and gynecology. 2004;191(3):964–8. doi: 10.1016/j.ajog.2004.05.052. [DOI] [PubMed] [Google Scholar]
- 41.Hardy LL, Bass SL, Booth ML. Changes in sedentary behavior among adolescent girls: a 2.5-year prospective cohort study. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2007;40(2):158–65. doi: 10.1016/j.jadohealth.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 42.Schmitz KH, Harnack L, Fulton JE, Jacobs DR, Jr, Gao S, Lytle LA, et al. Reliability and validity of a brief questionnaire to assess television viewing and computer use by middle school children. The Journal of school health. 2004;74(9):370–7. doi: 10.1111/j.1746-1561.2004.tb06632.x. [DOI] [PubMed] [Google Scholar]
- 43.Shenkin SD, Zhang MG, Der G, Mathur S, Mina TH, Reynolds RM. Validity of recalled v. recorded birth weight: a systematic review and meta-analysis. Journal of developmental origins of health and disease. 2017;8(2):137–48. doi: 10.1017/S2040174416000581. [DOI] [PubMed] [Google Scholar]
- 44.Keenan K, Hipwell A, McAloon R, Hoffmann A, Mohanty A, Magee K. Concordance between maternal recall of birth complications and data from obstetrical records. Early human development. 2017;105:11–5. doi: 10.1016/j.earlhumdev.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.