Abstract
Objective
Weight loss interventions have begun to receive increased attention in primary care. Motivational interviewing (MI) is compatible with primary care because it requires relatively limited time and resources. Few studies, however, have examined the long-term impact of MI for weight loss in primary care and none have used attention-control comparisons. This study was the first randomized controlled trial with a 12-month follow-up of two web-supported interventions: motivational interviewing (MIC) and nutrition psychoeducation (NPC).
Methods
59 patients with overweight/obesity, with and without binge-eating disorder (BED), were randomized to treatments and assessed at 12-month follow-up after completing 3-month treatments in primary care (15 months total).
Results
Mixed-models examining weight loss at 12-months revealed a group and time interaction effect trend (p=0.054,d=0.57). Secondary endpoint analysis showed a decrease (−1.7%) versus an increase (1.3%) in weight at 12-months among NPC and MIC patients, respectively (p=0.056,d=0.57). Overall, 5 of 44 (11.4%) participants lost/maintained 5% weight losses, differences between treatments were not significant. BED status did not impact weight loss.
Conclusions
Two brief and scalable weight loss interventions resulted in small effect sizes for weight loss 12-months following treatment conclusion. Given MIC required significantly more resources for adequate implementation, NPC may be more cost effective.
Keywords: clinical trial, binge eating, primary care, motivation, weight loss
Introduction
Excess weight is a prevalent, major, and costly health problem (1). Obesity is related to increased risk of early death, cardiovascular disease, hypertension, stroke, and metabolic syndrome (2, 3, 4). Individuals with excess weight also have higher rates of cancer and dementia (5, 6). In 2013 alone, obesity-related healthcare costs were estimated at $116 billion dollars (7). This insidious public health issue impacts men and women of all races and ages (1). With 81.5 million Americans estimated to have obesity (7), the potential harmful consequences of the obesity epidemic cannot be overstated.
To better address the widespread clinical problem that obesity poses, it is important to focus on scalable weight loss treatments to increase dissemination (8). For example, the potential impact of weight loss interventions within primary care offices is being examined extensively (9). Due to barriers of providing weight loss treatment in primary care, including limited resources, time, and training (10, 11), researchers began incorporating motivational interviewing (MI). MI, an evidence-based, time limited, person-centered counseling approach for strengthening a person’s motivation and commitment to behavior change (12), can be effectively implemented by general medical practitioners, without prior psychotherapy experience, to treat health-related behavioral concerns (13).
A recent review of the literature noted that MI may aid in weight loss in primary care (14). Of the 24 randomized controlled trials (RCTs) examined, however, only two included long-term follow-up data (15, 16). Twelve months following the 6-month interventions (i.e., 18 months total), there were no significant differences in weight loss between participants randomized to receive MI compared to usual care or standard of care (i.e., receiving health-related pamphlets at baseline).
With the increasing use of MI for weight loss in primary care, it is important for research to clarify potential long-term effects. The current study aimed to examine weight loss 12-months following a 3-month MI intervention in primary care (i.e., 15 months after treatment commencement). The initial 3-month RCT compared MI to nutrition psychoeducation (attention-control) and usual care at post-treatment and 3-month follow-up (17). The current analyses add to existing literature in several ways. First, this study compared the MI intervention to an attention-control condition at 12-month follow-up; the usual care condition was not included in current analyses because those participants were offered compassionate care following the 3-month follow-up. To our knowledge, this is the first study to test MI for weight loss in primary care against a non-MI intervention with matched time for attention (14). Second, all participants were rigorously assessed for binge-eating disorder (BED), a psychiatric disorder characterized by the presence of weekly binge eating (i.e., consuming large quantities of food in relatively brief periods of time) without regular compensatory behaviors. BED is common within primary care, associated strongly with excess weight and poor health-related outcomes, and thought to negatively impact weight loss treatment outcomes (17, 18, 19). Third, the RCT included online resources, which may be related to greater weight loss (14). The current RCT also incorporated strengths of the previous two studies by including both men and women, including overweight in addition to individuals with obesity, using medical assistants who are readily available in primary care settings, and thorough assessment of treatment fidelity. Results through 3-month follow-up assessment were published previously (17) and showed overall that the attention-control nutrition psychoeducation condition resulted in significant weight loss compared to usual care at post-treatment and 3-months following treatment conclusion. The motivational interviewing condition, however, did not differ statistically from the attention-control or usual care conditions at post-treatment or 3-months following treatment. The present paper reports data from 12-month follow-up assessment (i.e., 15 months from treatment commencement) to assess for long-term intervention effects.
Methods
Participants
Participants were 59 adult patients with overweight or obesity (body mass index (BMI) between 25–55 kg/m2) receiving primary care services at a large urban university-based medical healthcare center. They were recruited through primary care provider referrals and flyers placed in waiting/patient rooms. Recruitment was intended to enhance generalizability by utilizing relatively few exclusionary criteria. Exclusion criteria included over 65 years old, severe psychiatric (e.g., schizophrenia) or medical (e.g., cardiac disease) problems, pregnancy/breastfeeding, and uncontrolled liver or thyroid disease, hypertension, or diabetes. The Physical Activity Readiness Questionnaire (PAR-Q; 20) was used to exclude individuals with cardiovascular problems, chest pains, and unexplained/frequent dizziness. Participants endorsing high blood pressure, physical conditions that may prohibit physical activity, or explainable/infrequent dizziness on the PAR-Q were able to participate with primary care provider consent (20). Participants were required to have regular internet and telephone access. The study was approved by the Yale IRB.
Measures
The Autonomous Motivation (AM) (21) subscale of the Treatment Self-Regulation Questionnaire measures internal/personal reasons for losing weight with satisfactory reliability. Higher scores reflect higher levels of motivation.
The Beck Depression Inventory (BDI) (22) assesses current depression level with higher scores reflecting increased depression; the BDI has excellent reliability and validity (23).
The Eating Disorder Examination-Questionnaire (EDE-Q), (24) self-report version of the EDE interview (25), has received psychometric support, including good test-retest reliability (26) and good convergence with the EDE interview in studies of BED performed in primary care (27). The present study used the EDE-Q version with instructions; this version includes added written definitions and examples of binge eating which has been found to improve the performance of the self-report questionnaire in individuals with BED performed in specialty clinics (28). The EDE-Q Global score provides an index of eating disorder symptomatology, with higher scores reflecting greater severity.
Physical Measurements
Height was measured at baseline only using a wall measure, weight was measured at all assessment points using a large capacity digital scale (Brand: Med-Weigh; Model: MS4600). Blood pressure and pulse were measured using Mabis brand electric sphygmomanometers and used an average of two measurements. Medical assistants followed standardized instructions for obtaining the blood pressure measurements, for example, how to place/adjust armband, asking that participants keep their feet flat on the floor and not speak during measurement, and ensuring participants’ arm is resting on the table.
Procedures
The study had IRB approval and all participants provided written informed consent (See Barnes and colleagues (17) for previously published detailed procedures). Patients completed the battery of self-report measures and were screened by masters- or doctoral-level psychology clinicians trained in eating and weight disorders who were blinded to the patients’ treatment condition. The EDE (interview) (25), administered by masters- or doctoral-level psychology research clinicians, was used to diagnose BED.
Treatment was provided by medical assistants to increase generalizability to generalist primary-care settings. Participants were randomly assigned, stratified by BED diagnosis, to one of three conditions. First, the Motivational Interviewing and Internet Condition (MIC) (n = 30) included five manualized sessions over 12-weeks with guidelines to help medical assistants flexibly apply motivational interviewing strategies to motivate patients for behavior changes that support weight loss. The guidelines allowed focus on BED as needed. The first session included an initial 60-minute in-person individual session. Following this first appointment, patients received up to four additional 20-minute MI sessions. Second, the Nutrition Psychoeducation and Internet Condition (NPC) (n = 29) was designed as an attention-control to provide patients the same frequency and length of sessions. The sessions provided basic nutritional information (e.g., recommended daily fruit/vegetable intake). At their first session, participants randomized to MIC and NPC also received a LEARN manual (29) and orientation to a free website for tracking food intake, setting weight/intake goals, and physical activity (Livestrong.com). Third, the Usual Care (n=30) participants were encouraged to continue working with their primary care providers and did not receive additional weight loss intervention (i.e., they did not receive a LEARN manual or guidance for the free website); as such, they were offered compassionate care (MIC) after completing the 3-month follow-up assessment and were not included in the currently presented 12-month follow-up assessment. Participants were reimbursed $50 for completing the 12-month follow-up assessment.
Statistical Analyses
Analyses designed to compare treatments were performed for all randomized patients (intent-to-treat). Weight and BMI outcomes were log-transformed to minimize skew. Linear mixed models were used to compare anthropometric, physiological, and psychological measures between groups over time. These models included treatment group (MIC vs. NPC) as a between-subjects factor and time (baseline vs. 12-month follow-up) as a within-subjects factor. The group by time interaction was modeled and the best-fitting variance-covariance structure was based on Schwartz Bayesian criterion (BIC). Least-square means were compared to interpret significant effects. Percentage weight change from baseline at 12-month follow-up was compared between groups using a t-test. Fisher’s exact test was used to compare those achieving/maintaining at least 5% reduction in body weight at 12-month follow-up. Both main and interactive effects of BED status were considered in all analyses. All analyses were perform using SAS, version 9.4 (Cary, NC).
Results
Participants had a mean age of 48.0 years (SD=10.7, range 22–65) and a mean BMI of 34.9 kg/m2 (SD=7.2). Most of the participants were female (74.6%, n=44), and 25.4% (n=15) of the participants met DSM-5 criteria for BED. The sample was relatively diverse, with 66.1% (n=39) of participants identifying as White, not Hispanic. There were no significant baseline differences between the conditions (17). Patient retention for the 12-month follow-up assessment was 23/30 (76.7%) in MIC, 26/29 (89.7%) in NPC, and 49/59 (83.1%) overall. Retention rates did not differ significantly between the conditions (p = 0.12).
Weight
Mixed-models examining BMI changes from baseline to 12-month follow-up assessment revealed a nonsignificant interaction effect trend between group and time (F(1, 42) = 3.95, p = 0.053, d=0.56); see Table 1 for means, standard deviations, effect sizes, and confidence intervals. The interaction was explained by decreases in BMI among participants in the NPC group versus BMI increases among MIC subjects. Simple group effects, however, were not statistically significant. Following the same pattern, a similar interaction was observed for weight change (in pounds) measured over time (F(1, 42) = 3.94, p = 0.054, d = 0.57). A secondary endpoint analysis showed a decrease (−1.7%) versus an increase (1.3%) in percentage weight change at 12-month follow-up for NPC and MIC participants, respectively (t(42) = 1.96, p = 0.056, d = 0.57; see Figure 1). On average, the percentage weight change translates to a 3.3 (SD = 12.9) pound weight gain for MIC participants and a −3.1 (SD = 9.2) pound weight loss for NPC participants between baseline and 12-month follow-up assessment.
Table 1.
Variable Descriptive Data.
| Motivational Interviewing + Internet | Nutrition Psychoeducation + Internet | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||
| Baseline | 12-month minus Baseline | Baseline | 12-month minus Baseline | Effect (group difference) | ||||||||||||
|
| ||||||||||||||||
| N | Mean | Std Dev | N* | Mean | Std Dev | N | Mean | Std Dev | N* | Mean | Std Dev | Mean | Lower 95% CI |
Upper 95% CI |
Interaction: Cohen’s d |
|
| Body Mass Index | 30 | 34.65 | 7.06 | 21 | 0.474 | 2.025 | 29 | 35.07 | 7.52 | 23 | −0.521 | 1.544 | 0.996 | −0.094 | 2.085 | 0.56 |
| Weight | 30 | 216.04 | 54.27 | 21 | 3.314 | 12.851 | 29 | 219.36 | 53.88 | 23 | −3.052 | 9.240 | 6.367 | −0.399 | 13.132 | 0.57 |
| Systolic blood pressure | 30 | 120.30 | 11.13 | 21 | 1.500 | 12.915 | 29 | 127.66 | 13.65 | 23 | −3.348 | 10.271 | 4.848 | −2.221 | 11.917 | 0.42 |
| Diastolic blood pressure | 30 | 76.78 | 9.56 | 21 | −0.643 | 8.281 | 29 | 77.52 | 10.76 | 23 | −0.304 | 8.628 | −0.339 | −5.495 | 4.818 | 0.04 |
| Heart rate | 29 | 73.53 | 11.05 | 20 | 1.375 | 9.045 | 29 | 75.40 | 12.76 | 23 | −1.304 | 10.048 | 2.679 | −3.246 | 8.605 | 0.28 |
| BDI | 30 | 8.03 | 7.15 | 23 | −1.130 | 4.693 | 29 | 7.90 | 5.67 | 26 | −1.192 | 5.706 | 0.062 | −2.965 | 3.089 | 0.01 |
| AMQ | 30 | 6.61 | 0.79 | 23 | −0.348 | 1.435 | 29 | 6.62 | 0.80 | 26 | −0.846 | 1.703 | 0.498 | −0.413 | 1.410 | 0.31 |
| Total EDE-Q (avg) | 30 | 2.39 | 0.99 | 23 | −0.343 | 0.836 | 29 | 2.12 | 1.06 | 26 | −0.550 | 0.727 | 0.207 | −0.242 | 0.656 | 0.27 |
Note. BDI=Beck Depression Inventory. AMQ=Autonomous Motivation Questionnaire. EDE-Q=Eating Disorder Examination-Questionnaire.
Participant numbers at 12-month follow-up (15 months after treatment start) differ as some participants completed online self-report questionnaires but did not attend in-person assessments for measurements.
Figure 1.
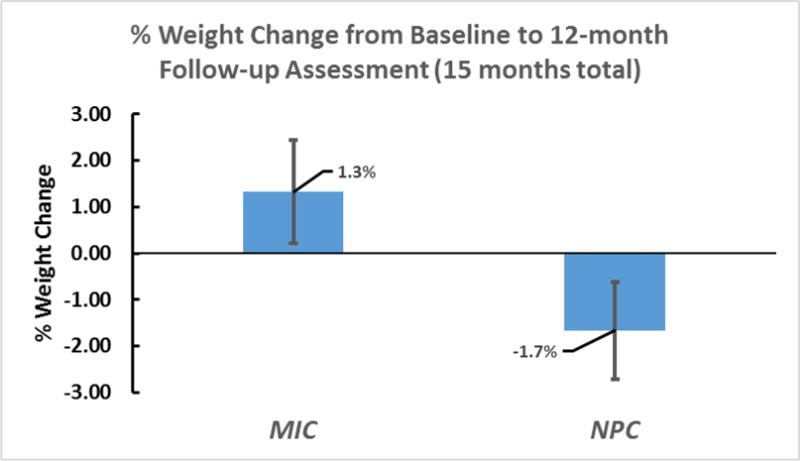
Percent Weight Change from Baseline to 12-months Following Treatment End (15 months total).
Note. MIC = Motivational Interviewing and Internet Condition. NPC = Nutrition Psychoeducation and Internet Condition.
The likelihood of participants reaching/maintaining at least 5% loss of initial body weight at 12-month follow-up assessment did not differ significantly between treatment conditions (p = 0.171); 17.4% (n = 4 of 23) of NPC participants lost/maintained at least 5% weight losses compared to only 4.8% (n = 1 of 21) of MIC participants.
Physical and metabolic assessments
Mixed-models revealed no statistically significant effects when examining the impact of group, time, and group by time interactions for blood pressure and pulse.
Psychological and motivational assessments
The change in depression (BDI) over time was not statistically significant (F(1, 47) = 3.80, p = 0.057) and the effects for group and the group-by-time interaction (d = 0.01) were also not statistically significant. Mixed-models revealed a significant decrease in motivation (AMQ) over time for participants across conditions (F(1, 57) = 7.52, p = 0.008); effects for group and the group-by-time interaction (d=0.31) were not statistically significant. Mixed-models showed a statistically significant decrease in self-reported disordered eating symptomatology (EDE-Q Global) over time (F(1, 47) = 19.78, p < 0.0005); effects for group and the group-by-time interaction (d=0.27) were not significant.
Role of binge-eating disorder
BED did not significantly predict or moderate any treatment outcomes.
Discussion
The 12-month follow-up assessment results indicate that motivational interviewing (MIC) did not result in significant weight losses following a 3-month intervention in primary care. The condition designed as an attention-control (NPC) also did not differ significantly from the motivational interviewing condition. BED diagnosis did not negatively impact weight loss outcomes. There were improvements over time in disordered eating for participants in both conditions and motivation decreased for all participants. Depression, blood pressure, and pulse did not change significantly.
Our findings are consistent with those reported by Hardcastle and colleagues (16) and Martin and colleagues (15); although both studies tested interventions that were 6 months in length, neither reported significant weight losses for individuals receiving motivational interviewing in primary care one year following treatment. In our study, participants randomized to the NPC (attention-control condition) maintained, on average, almost a 2% initial body weight loss after receiving a total of only 2 hours and 20 minutes of nutrition psychoeducation. Similarly, one year after receiving treatment, 4 of 23 (17.4%) participants receiving NPC maintained 5% or more weight loss, a goal associated with attenuating weight-related health consequences (30, 31); only 1 of 21 (4.8%) individuals receiving MI maintained a 5% weight loss. The latter finding is similar to that reported by Martin and colleagues (15), who reported 7% of participants maintained/achieved 5% or more weight loss 12-months following motivational interviewing treatment.
Our previously published data found that participants receiving NPC had greater weight losses at post-treatment and three months following treatment cessation than those receiving MIC (17); however, by 12-month follow-up, the two conditions did not significantly differ in weight loss. Importantly, the current results are despite extensive MI training, supervision, and treatment fidelity assurance. The MI was delivered with expected skill by the carefully trained and monitored medical assistants. Due to the basic nature of the NPC, the attention-control condition required significantly fewer resources to ensure treatment fidelity compared to the MIC. Replication of our findings observed for NPC and MI through 12-months following the interventions warrants further examination as they are the first to compare MI for weight loss in primary care to a non-MI attention matched intervention.
When comparing these weight changes to more intensive behavioral interventions such as the Diabetes Prevention Program, they appear quite minor (32). From a public health standpoint, however, if 1 in approximately every 6 Americans with overweight or obesity could maintain a 5% or more weight loss from a widely disseminated and implemented brief (2 hour and 20-minute) scalable intervention like NPC, the intervention could help large numbers of people lose small, but significant, amounts of weight. Similarly, participants receiving NPC on average maintained a 3-pound weight loss from baseline to 12-month follow-up, whereas participants receiving MIC gained approximately 3 pounds during the same period. Preventing further weight gain may be an important focus for preventing associated health-related consequences.
Similar to the previously published post-treatment and three-month follow-up outcomes (17), there was no significant impact of BED diagnosis on weight loss outcomes. Overall the literature is mixed on whether BED impacts weight loss trials (18, 19). Perhaps individuals with BED recruited from primary care for the current trial differ from those recruited through specialty clinics for weight loss treatment (31).
Overall, disordered eating symptoms improved over time, while motivation decreased, mirroring post- and three-month follow-up assessments (19). The two previous assessments of motivational interviewing for weight loss in primary care did not assess these variables (15, 16). It is not surprising that motivation continued to decrease given the high motivation at treatment onset (17). In terms of physiological assessments, Hardcastle and colleagues (16) also found no significant improvements in blood pressure one year after treatment. Neither of the longer-term follow-up assessments of MI for weight loss in primary care reported data on depression or resting heart rate.
It is important to consider the limitations of the current study. The sample size was small, and while the inclusion/exclusion criteria were meant to mimic typical primary care patients, results may not generalize to non-treatment seeking populations or to patients with significant psychological and physical comorbidities. There was no usual care comparison condition for this longer-term outcome time-point. Based on other similar trials and the initial published RCT results, it would be reasonable to expect that participants randomized to Usual Care would not weigh significantly less on average by the 12-month follow-up assessment (15, 16, 19). Indeeed, on average, adults with overweight/obesity without BED reported gaining 2 pounds and those with BED reported gaining 18 pounds in the year prior to initiating weight loss treatment (33).
In conclusion, the current longer-term follow-up assessment of two brief and scalable weight loss treatments delivered in primary care by medical assistants revealed that neither treatment resulted in significant weight losses 12-months after finishing treatments. The results do suggest, however, that a brief and straightforward nutrition psychoeducation may help prevent weight gain overtime (compared to motivational interviewing) and may result in a 5% weight loss for approximately 1 in 6 individuals. Findings of the initial RCT, combined with current results, also suggest individuals seek weight loss treatment when already highly motivated. Future research should examine whether motivational interviewing enhances treatment enrollment or participation in nutrition psychoeducation in primary care settings or if it is beneficial for individuals less motivated to lose weight.
What is already known about this subject
Primary care settings may be an opportune place to incorporate weight loss interventions to increase dissemination.
Motivational interviewing may result in weight loss and non-specialists can be trained to provide the treatment.
There is limited information about the long-term impact of motivational interviewing for weight loss in primary care.
What this study adds
The first long-term follow-up assessment of motivational interviewing (MI) for weight loss in primary care to include web-based resources, to include a non-MI attention-control condition, and to thoroughly assess for binge-eating disorder.
Nutrition psychoeducation condition (designed as an attention-control) and motivational interviewing did not differ statistically in weight-loss maintenance at 12-months following treatment.
Binge-eating disorder was unrelated to weight loss outcomes 12 months following treatment.
Acknowledgments
This study was supported by NIH career development awards, K23-DK092279 for RDB and K24-DK070052 for CMG.
Footnotes
Clinical Trial Registration: NCT02578199
Conflicts of Interest: RDB, VI, BPP, and SM have no conflicts of interest. CMG reports no relevant conflicts of interest but notes that he has received consultant fees from Shire and Sunovion, honoraria from American Psychological Association, American Academy of CME, Vindico CME, Global Medical Education, Medscape CME, CME Institute of Physicians Postgraduate Press, and from professional and scientific conferences for lectures and presentations, and book royalties from Guilford Press and Taylor and Francis for academic books.
References
- 1.National Center for Health Statistics. With Special Feature on Emergency Care. Health, United States: Hyattsville, MD: 2013. p. 2012. [PubMed] [Google Scholar]
- 2.Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285 doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 3.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C, American Heart A et al. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 4.Ervin RB. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States. National Health Statistics Reports. 2009;13:1–8. [PubMed] [Google Scholar]
- 5.Arnold M, Leitzmann M, Freisling H, Bray F, Romieu I, Renehan A, et al. Obesity and cancer: an update of the global impact. Cancer Epidemiol. 2016;41:8–15. doi: 10.1016/j.canep.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Pedditizi E, Peters R, Beckett N. The risk of overweight/obesity in mid-life and late life for the development of dementia: a systematic review and meta-analysis of longitudinal studies. Age Ageing. 2016;45:14–21. doi: 10.1093/ageing/afv151. [DOI] [PubMed] [Google Scholar]
- 7.Wang YC, Pamplin J, Long MW, Ward ZJ, Gortmaker SL, Andreyeva T. Severe obesity in adults cost state medicaid programs nearly $8 billion in 2013. Health Aff. 2015;34:1923–1931. doi: 10.1377/hlthaff.2015.0633. [DOI] [PubMed] [Google Scholar]
- 8.Pagoto SL, Appelhans BM. A call for an end to the diet debates. JAMA. 2013;310:687–688. doi: 10.1001/jama.2013.8601. [DOI] [PubMed] [Google Scholar]
- 9.Wadden TA, Butryn ML, Hong PS, Tsai AG. Behavioral treatment of obesity in patients encountered in primary care settings: a systematic review. JAMA. 2014;312:1779–1791. doi: 10.1001/jama.2014.14173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsai AG, Wadden TA. Treatment of obesity in primary care practice in the United States: a systematic review. J Gen Intern Med. 2009;24:1073–1079. doi: 10.1007/s11606-009-1042-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Østbye T, Yarnall KS, Krause KM, Pollak KI, Gradison M, Michener JL. Is there time for management of patients with chronic diseases in primary care? Annals Fam Med. 2005;3:209–214. doi: 10.1370/afm.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller WR, Rollnick S. Motivational interviewing: Helping people change. Guilford press; 2012. [Google Scholar]
- 13.Söderlund LL, Madson MB, Rubak S, Nilsen P. A systematic review of motivational interviewing training for general health care practitioners. Patient Educ Couns. 2011;84:16–26. doi: 10.1016/j.pec.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 14.Barnes R, Ivezaj V. A systematic review of motivational interviewing for weight loss among adults in primary care. Obesity Reviews. 2015;16:304–318. doi: 10.1111/obr.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin PD, Dutton GR, Rhode PC, Horswell RL, Ryan DH, Brantley PJ. Weight loss maintenance following a primary care intervention for low income minority women. Obesity. 2008;16:2462–2467. doi: 10.1038/oby.2008.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hardcastle SJ, Taylor AH, Bailey MP, Harley RA, Hagger MS. Effectiveness of a motivational interviewing intervention on weight loss, physical activity and cardiovascular disease risk factors: a randomised controlled trial with a 12-month post-intervention follow-up. Int J Behav Nut Phys Act. 2013;10:40. doi: 10.1186/1479-5868-10-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barnes RD, White MA, Martino S, Grilo CM. A randomized controlled trial comparing scalable weight loss treatments in primary care. Obesity. 2014;22:2508–2516. doi: 10.1002/oby.20889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blaine B, Rodman J. Responses to weight loss treatment among obese individuals with and without BED: a matched-study meta-analysis. Eat Weight Disord. 2007;12:54–60. doi: 10.1007/BF03327579. [DOI] [PubMed] [Google Scholar]
- 19.Grilo CM, White MA. Orlistat with behavioral weight loss for obesity with versus without binge eating disorder: randomized placebo-controlled trial at a community mental health center serving educationally and economically disadvantaged Latino/as. Behav Res Ther. 2013;51:167–175. doi: 10.1016/j.brat.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shephard RJ. PAR-Q, Canadian Home Fitness Test and exercise screening alternatives. Sports Medi. 1988;5:185–195. doi: 10.2165/00007256-198805030-00005. [DOI] [PubMed] [Google Scholar]
- 21.Levesque CS, Williams GC, Elliot D, Pickering MA, Bodenhamer B, Finley PJ. Validating the theoretical structure of the Treatment Self-Regulation Questionnaire (TSRQ) across three different health behaviors. Health Educ Res. 2007;22:691–702. doi: 10.1093/her/cyl148. [DOI] [PubMed] [Google Scholar]
- 22.Beck AT, Steer R. Manual for the revised Beck depression inventory. San Antonio, TX: Psychological Corporation. 1987 [Google Scholar]
- 23.Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- 24.Fairburn CG, Beglin SJ. Assessment of eating disorders: Interview or self-report questionnaire? Int J Eat Disord. 1994;16:363–370. [PubMed] [Google Scholar]
- 25.Fairburn C, Cooper Z. The Eating Disorders Examination. In: Fairburn CG, Wilson GT, editors. Binge Eating: Nature, Assesment and Treatment. New York: Guilford Press; 1993. [Google Scholar]
- 26.Reas DL, Grilo CM, Masheb RM. Reliability of the Eating Disorder Examination-Questionnaire in patients with binge eating disorder. Behav Res Ther. 2006;44:43–51. doi: 10.1016/j.brat.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Barnes RD, Masheb RM, White MA, Grilo CM. Comparison of methods for identifying and assessing obese patients with binge eating disorder in primary care settings. Int J Eat Disord. 2011;44:157–163. doi: 10.1002/eat.20802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldfein JA, Devlin MJ, Kamenetz C. Eating Disorder Examination-Questionnaire with and without instruction to assess binge eating in patients with binge eating disorder. Int J Eat Disord. 2005;37:107–111. doi: 10.1002/eat.20075. [DOI] [PubMed] [Google Scholar]
- 29.Brownell KD. The LEARN program for weight management: lifestyle, exercise, attitudes, relationships, nutrition. American Health Publishing Company. 2004 [Google Scholar]
- 30.Blackburn G. Effect of degree of weight loss on health benefits. Obesity Res. 1995;3:211s–216s. doi: 10.1002/j.1550-8528.1995.tb00466.x. [DOI] [PubMed] [Google Scholar]
- 31.Barnes RD, Barber JA. Preliminary examination of metabolic syndrome response to motivational interviewing for weight loss as compared to an attentional control and usual care in primary care for individuals with and without binge-eating disorder. Eat Behav. 2017;26:108–113. doi: 10.1016/j.eatbeh.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knowler W, Barrett-Connor E, Fowler S, Hamman R, Lachin J, Walker E, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. Scand J Med Sci Sports. 2003;13:208. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ivezaj V, Kalebjian R, Grilo CM, Barnes RD. Comparing weight gain in the year prior to treatment for overweight and obese patients with and without binge eating disorder in primary care. J Psychosom Res. 2014;77:151–154. doi: 10.1016/j.jpsychores.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


