Abstract
Gap junctions provide the basis for electrical synapses between neurons. Early studies in well-defined circuits in lower vertebrates laid the foundation for understanding various properties conferred by electrical synaptic transmission. Knowledge surrounding electrical synapses in mammalian systems unfolded first with evidence indicating the presence of gap junctions between neurons in various brain regions, but with little appreciation of their functional roles. Beginning at about the turn of this century, new approaches were applied to scrutinize electrical synapses, revealing the prevalence of neuronal gap junctions, the connexin protein composition of many of those junctions, and the myriad diverse neural systems in which they occur in the mammalian CNS. Subsequent progress indicated that electrical synapses constitute key elements in synaptic circuitry, govern the collective activity of ensembles of electrically coupled neurons, and in part orchestrate the synchronized neuronal network activity and rhythmic oscillations that underlie fundamental integrative processes.
Keywords: Neuronal gap junctions, Mixed chemical/electrical synapses, Connexins, Cell localization, Ultrastructural diversity, Protein composition
1. Introduction
Electrical synapses, formed by gap junctions between neurons, have been studied extensively in the CNS of non-mammalian vertebrates beginning in the mid-20th century, and more recently have received intensive scrutiny in the mammalian CNS following the development and application of an arsenal of genetic and molecular biological tools. To many established scientists in the neuroscience community who are uninitiated with respect to the many decades of literature on electrical synaptic transmission, the field of electrical synapses in mammalian systems may appear to have emerged suddenly at the beginning of this century, with the subsequent pace of progress in the area being nothing short of astonishing. Consequently, because electrical synapses are now unambiguously recognized to constitute a vital component in mammalian brain circuitry, it would be appropriate for those outside our field to ask why recognition of their importance and widespread distribution did not occur much earlier, and what factors contributed to this apparently abrupt transformation in knowledge. This is perhaps best answered by our initial consideration of two points in this review. First is the evolution in understanding of electrical synapses in invertebrates and non-mammalian vertebrates beginning mid-way through the 20th century, as contrasted with the more convoluted history surrounding investigations of those synapses in mammals, beginning shortly thereafter. Second is the confluence of multiple discoveries and technical advances that enabled major leaps in understanding and ultimately general acceptance of the critical role of electrical synapses in the mammalian CNS.
Also covered in this review are overlapping studies that implicate roles for electrical synapses in the high-speed neuronal network oscillatory activities that underlie the intricate processes of human consciousness, learning and memory, fine motor control, and complex information processing. Scores of investigations not only repeatedly confirmed earlier observations of neuronal gap junctions in various CNS areas and at different developmental stages, but they also revealed numerous previously unsuspected regions where electrical synapses occur. In particular, studies have for the first time exposed the high density that neuronal gap junctions occur in many of those locations, as visualized by immunofluorescence imaging of identified neuronal connexins. In addition, these investigations established the remarkable diversity of electrical synapses in the mammalian CNS, including the disparate neuronal types that are coupled by these synapses, the distinct subcellular sites at which these synapses are deployed, the connexin proteins that form neuronal gap junctions, and the wide variety of ultrastructural configurations that these junctions exhibit. And in the two final components, we focus on a few key unresolved issues, including those recently appearing on the horizon. One of these concerns the many brain regions where electrical synapses are almost certain to occur based on the presence of neuronally expressed connexin proteins, but where these synapses have not yet been studied by electrophysiological or other approaches. Another is the increasing evidence for the presence of gap junctions at mammalian axon terminals in the CNS, forming morphologically-mixed (i.e., chemical plus electrical) synapses but where the functional contribution of the gap junction component of these abundant synapses remains an enigma.
2. Electrical synapses in non-mammalian vertebrates
Following on the heels of the debate during the first half of the last century regarding whether neuronal communication was mediated electrically vs. by neuronal release of diverse chemical mediators, and shortly after the mid-century conclusion of that debate in favor of neurotransmission by chemical neurotransmitters, reports began to reveal the occurrence of direct intercellular electrical transmission, at least between neurons in non-mammalian systems. The first evidence for the presence of electrical transmission was obtained at contacts between the giant axon and the giant motor fiber of the crayfish [1], and it was soon followed by evidence in fish [2–8] and birds [9], demonstrating the presence of electrical synaptic communication in vertebrates. Moreover, the ability to combine electrophysiological analysis with ultrastructural imaging led to the identification of areas of close apposition between neurons and the discovery of gap junctions, the intercellular structures that underlie electrical transmission. Thus, studies in fish were seminal in establishing the basic properties and structural basis of electrical transmission in vertebrates, including the role of electrical synapses in promoting synchronized activity, and later providing evidence for the plasticity and diverse molecular composition of the constituent gap junctions (reviewed in [10]).
Many of the examples in which electrical transmission was observed in fish occurred at contacts in which gap junctions were associated with chemical synapses. The most studied of this synaptic configuration was the large myelinated club endings on the goldfish Mauthner cell [11]. At these large synapses, transmission is simultaneously supported by both chemical and electrical means [12,13], the coexistence of which greatly contributed to the identification of the presence and structural bases of electrical transmission in vertebrates [4,5]. These contacts later contributed to the description of novel plastic properties and exposed close interactions between electrical and chemical synapses (see below). Besides goldfish and electric fish, functional evidence for the presence of mixed synapses was also observed in lamprey ([14–16] and other cold-blooded vertebrates [17–22]; reviewed in [23]). Interestingly, coincident with the description of mixed synapses in the goldfish Mauthner cell, Martin and Pilar [9] reported the presence of mixed synaptic transmission in the chick ciliary ganglion, an anatomically peculiar synaptic contact between a parasympathetic preganglionic cholinergic fiber and the cell body of a postganglionic cell. These findings suggested that such mixed synaptic configurations in warm-blooded animals could occur elsewhere, including in mammals. A decade later, mixed synaptic transmission was reported to occur between primary vestibular afferents in the rat [24] and other species, including primates and humans (reviewed in [25]), and increasing anatomical evidence suggested their widespread distribution in the mammalian brain (see below).
3. Mammalian neuronal gap junctions; pre-millennial perspectives
Early on, direct cell-to-cell electrical and ionic communication and, in particular, proposals for direct intercellular propagation of electrical action potentials [26] were correlated with the existence of newly-recognized intercellular junctions called “gap junctions” (aka “nexuses”) in diverse neuronal and non-neuronal tissues [26–29]. The role of gap junctions in intercellular electrical communications was further confirmed in vitro by the de novo formation of gap junction-like close membrane appositions between pre-fusion skeletal myoblasts within seconds of the onset of electrical coupling [30] and by the subsequent freeze-fracture confirmation of abundant gap junctions between adjacent pre-fusion myoblasts in vivo [31]. Now, with electrical synapses as secure participants in the process of mammalian neuronal communication, it is perhaps less appreciated that the relatively recent work documenting the existence, abundance, functionality, and ultrastructural diversity of these synapses was preceded by a considerable body of literature suggesting the presence, if not the abundance, of these synapses in mammalian systems. This included more than 50 early ultrastructural, dye-coupling, and electrophysiological reports of neuronal gap junctions and electrical synapses in various areas of adult mammalian CNS published in the 1960’s [32], 1970’s [24,25,33–50], 1980’s [51–68], and 1990’s [69–75]; reviewed in 2000 [76]).
As discussed in reviews contemporaneous with the early reports [77–80], a number of factors contributed to the early general ambivalence surrounding the functional relevance of electrical synapses in mammals:
1. Ultrastructural factors
Neuronal gap junctions in mammalian CNS were difficult to find by thin-section electron microscopy (EM), which required optimal tissue fixation and staining, and much higher magnifications than used to find chemical synapses. Many more hours of microscope time than usually used for analyses of the much wider variety of excitatory, inhibitory and modulatory chemical synapses was necessary, and time-consuming goniometric tilting) analyses was required to obtain the requisite optimum viewing angles that allowed convincing visualization of the tell-tale close heptalaminar structures of gap junctions. The appearance of these structures (e.g., pentalaminar or septalaminar) depended on the chemical fixatives and stains used – potassium permanganate, osmium tetroxide alone, glutaraldehyde/osmium tetroxide fixation [81], with or without aqueous uranyl acetate post-stain [82]) to define gap junctions in thin sections [41,46,54,83]. The few neuronal gap junctions that had been found were relatively large (often >0.5 μm), leading to the idea that gap junctions/electrical synapses in mammals were equally large as those in lower vertebrates; and where these large gap junctions were found in mammalian brain, they were proposed to represent an evolutionary vestige remaining from “when we were fish”. Consequently, the much more abundant but much smaller gap junctions subsequently found in mammalian CNS [73,84] did not conform with earlier functional perspectives, in part, because the intercellular transmission capabilities of very small gap junctions were not consistent with the then-current ideas that regarded gap junctions as devices that serve primarily for rapid and efficient intercellular propagation of action potentials [27–29,85].
2. Synapse complexity and flexibility
Because electrical synapses were already known to occur widely in non-mammalian vertebrates, they initially were considered evolutionarily primitive, and perhaps not suited for the more sophisticated computations afforded by the diversity and flexibility of chemical synapses. Voicing a then common concept, Shepherd [86] proposed that the apparent absence of gap junctions in most areas of the CNS of higher vertebrates “could reflect a mechanism to increase the metabolic and functional independence of neurons, in order to permit more complex information processing” (emphasis added). This concept of gap junctions as sites for high-efficiency electrical coupling for direct relay of action potentials appeared to account for the reported restriction of gap junctions to “primitive” areas of the mammalian brain [24,32–35] and spinal cord [87,88], wherein propagated electrical activity was proposed to exist for synchronization of nerve activity in networks responsible for stereotyped motor behaviors as occurs for example, in direct conduction of sensory action potentials into the Mauthner cells that then initiate the “tail-flip” escape response, and in motoneurons controlling ocular movements in fish [8]. Thus, in those early days, propagation and synchronization of action potentials was the only electrical function that was widely recognized for neuronal gap junctions.
3. Technical challenges to addressing function
Except in ideal test systems, such as the locations where large gap junctions were found in lower vertebrates [3–6,89], early electrophysiological approaches to studies of electrical synapses in more complex systems were technically challenging; and only a few sites in mammalian brain lent themselves easily to even indirect detection of electrical coupling [24,35,40,41,44]. Even in mammalian CNS regions having strong neuronal coupling, this technical challenge was compounded by the absence of specific and robust pharmacological agents that could be used to manipulate or perturb functions of electrical synapses. Further, studies that did provide evidence for neuronal gap junctions by dye-transfer between neurons following injection of single neurons in tissue slices suffered criticisms that transfer of dye might have been artefactual, resulting from unrecognized impalement of multiple cells (the “shish kabob effect” [90]). Thus, technical limitations led to a paucity in functional correlates of electrical synapses in mammalian systems, which diminished enthusiasm for studies of these synapses.
4. Speed advantage of electrical transmission
A more subtle point related to the comparative speed advantage of electrical vs. chemical synaptic transmission is that the speed of chemical transmission was known to be temperature dependent, whereas transmission speed of electrical synapses was thought to be essentially independent of temperature. In cold blooded species, electrical synapses therefore were thought to provide an advantage, especially in sensory and motor systems, where speed of synaptic transmission is essential, as in the well-documented escape response in fish (reviewed in [91]). Indeed, in non-mammalian vertebrates, it was widely presumed that likely sites within neuronal networks in which to find electrical synapses were those where speed of transmission confers obvious survival advantages [92], but it should be noted that equivalent examination of other CNS areas in the same species were not conducted to determine whether they, too, contained gap junctions -- of any size. Instead, recent studies revealed abundant electrical synapses throughout goldfish brain [93]. Nevertheless, in early studies, such sites in mammals that were presumed to require rapid propagation of action potentials were less obvious, so much so that arguments were made that warm-blooded animals had abandoned or greatly reduced their reliance on fast electrical synapses. Even though all of these considerations fueled doubt about the importance of electrical transmission in mammals, it was repeatedly emphasized by Bennett that speed difference in electrical vs. chemical transmission can be minimal in warm blooded animals [78–80,94]. Thus, at mammalian body temperature, synaptic delay times in the two modes can be very nearly the same. Gap junctions, particularly the small gap junctions now documented to occur abundantly between mammalian neurons [73,84,95,96], may subserve as-yet-unidentified electrical, ionic, or metabolic functions [97] other than propagation of action potentials.
5. Electrical vs. chemical transmission
Perhaps because electrical transmission had been debated since early in the last century as a serious contender for mediation of neuronal communication, initial demonstrations of its occurrence in lower vertebrates and invertebrates were met with relatively little resistance and quickly accepted. This was in contrast to mammalian systems, where concepts regarding electrical synapses had a more prolonged emergence, beginning at a time when their potential roles needed to be considered against a slew of already-well-entrenched ideas and concepts surrounding chemical transmission.
6. Neuronal gap junctions during CNS development in mammals
A wealth of evidence indicating that electrical synapses are widely expressed during early stages of mammalian brain development became available in the early-mid nineties, and those observations included structures such as the neo-cortex [98–100], retina [101], and spinal cord [102]. Early gap junction connections were believed to create functional compartments and determine early network connectivity [98–100]; and coupling, often at the limit of detectability [102], was extrapolated to disappear (but not explicitly shown to disappear) as brain and spinal cord development progressed [102,103]. These observations provided unambiguous evidence for the presence and functional relevance of electrical synapses in mammalian brain development, with emphasis placed on relevance in immature systems and less so in adult CNS, where tools did not then exist that allowed equivalent analyses in adult brain and spinal cord slices (cf. [51]). By the turn of the new millennium, however, studies of electrical synapses in developing neural systems helped pave the way to the recognition that these synapses persist in vast areas of adult brain, particularly those gap junctions between inhibitory interneurons [58,104,105], which are widely distributed throughout the central nervous system.
For some of us, it was difficult to imagine at that time that the above cited bulk of early data on neuronal gap junctions in mammals did not have more profound implications; hence the impetus and dogged determination to study them with the then-available approaches. Perhaps better said is a quote from an early review by Bennett [77], where in considering favorably the value of electrical synapses vs. the then-current bias regarding the primacy of chemical transmission, in biasing his comparisons of electrical vs. chemical transmission to include only what were at that time physiologically demonstrated cases, he stated “This bias is introduced in order to confront the prejudices of those who regard electrical mediation as beneath the lofty mental processes of higher animals”. We conclude from the foregoing that, prior to about 1998, there was already sufficient evidence to seriously consider potential roles for electrical synapses in the mammalian CNS, with clear examples in the retina and inferior olive; but cautious interpretation, mixed with healthy skepticism and a modicum of bias against these synapses, prevailed in the absence of knowledge surrounding the biochemical underpinnings, abundance, and functional/electrophysiological manifestations of mammalian neuronal gap junctions, all of which came to light in close temporal succession with the technical and conceptual advances described below.
4. Transformative surge in understanding
By the last decade of the last century, a number of developments occurring in multiple disciplines led to a surge of reports that revitalized efforts to delve broadly and deeply into what would eventually become understanding of the occurrence, diverse types, wide-spread distribution, and functional roles of electrical synapses in mammalian brain and spinal cord. The evolution of events leading up to and beyond the renaissance that occurred at the turn of the century is illustrated in Figure 1. These developments included:
Fig. 1. Time line of significant technical advances and discoveries regarding electrical synapses in vertebrate species.
The blue overlay is a graphical representation of the previous 2632 publications that have investigated electrical synapses by a diversity of approaches in a variety of invertebrate and vertebrate species, with average number of publications per year for each decade indicated by numbered blue dots on the left margin. The center column indicates years from the point electrical synapses were discovered to the present. The left side lists the discovery dates for all mammalian connexins, with the first neuronal connexin identified towards the end of the 1990’s. The right side indicates landmark discoveries regarding the structure, function, and connexin composition of neuronal gap junctions and the development of methods used to study electrical synapses. Note the dense concentration of significant event around the turn of the century.
1. Discovery of Cx36
Among the family of 20 connexin protein members in mammals, connexin36 (Cx36), the 14th connexin to be discovered in 1998 [106,107] (1998) (Fig. 1), was the first found unequivocally to be expressed in multiple types of neurons in retina, brain, and spinal cord [84,108], strongly suggesting the presence of electrical synapses in widespread regions of the CNS. Cx36 protein was then quickly detected in ultrastructurally-defined gap junctions in retinal and spinal cord neurons [109–111]. Of note is that mRNA for Cx35, the fish ortholog of mammalian Cx36, was identified two years earlier in skate neurons [112], but Cx35 and Cx34.7 proteins were not immunocytochemically localized to neurons until 2003–2004 [113,114]. The relatively late discovery of Cx36 was much to the consternation of those of us seeking to study connexins localized at neuronal gap junctions, having examined its thirteen predecessors for such localization. Further, many other connexins, either based on detection of their mRNAs or their proteins, had (erroneously) been ascribed to neurons, including Cx26 [115], Cx32 [115–121], Cx47 [122], or a combination of Cx26, Cx37, Cx40, Cx43 [123–125]; but none of those connexin proteins were confirmed to be present by their detection in ultrastructurally-defined gap junctions [109–111] that simultaneously were found to contain Cx36 (with or without Cx45). Detection of connexin mRNAs without detection of the corresponding protein was later proposed, but not as yet demonstrated, to result from active suppression of translation of specific mRNAs into protein by newly-discovered micro interfering RNA (miRNA) mechanisms present in mammalian CNS neurons vs. glia ([126,127], as discussed in [128].
Subsequently, mRNAs for at least two more connexins, Cx45 and Cx57, were found to be expressed in neurons [129–135], and Cx45 was quickly documented to occur in ultrastructurally-defined gap junctions [136]. More recently, Cx57 and Cx50 immunofluorescence was localized to discrete puncta in the outer plexiform layer of rabbit and mouse retina [137–140] and localized to putative gap junctions in thin sections examined by TEM [138,140]. To date, neither of these connexin proteins has been documented in other brain regions. We have also found Cx57 in a few ultrastructurally-defined gap junctions in the outer plexiform layer of rat retina (Rash and Nagy, unpublished observations.) Likewise, lacZ reporter expression systems indicate that Cx30.2 may also be expressed in neurons throughout the brain and spinal cord [141], including in retinal ganglion cells [142], but remains to be confirmed by demonstration of Cx30.2 protein expression. Nevertheless, to date, Cx36 remains the most abundantly detected neuronal connexin protein in mammals.
The discovery of Cx36 allowed the generation of antibodies against this protein, use of which provided all of the powerful approaches that antibodies afford. In particular, visualization of Cx36 localization by immunofluorescence is well-correlated with its localization in gap junctions at the ultrastructural level [84,114] (Fig. 2), at least in vivo [95,96,128,137]. This is a fortuitous feature arising from immunofluorescence labeling for Cx36 exclusively at gap junctions, with its other potential subcellular and intracellular sites (i.e., rough endoplasmic reticulum, Golgi apparatus, or cytoplasmic transport vesicles) either remaining masked or below the limit of fluorescence detectability by all anti-Cx36 antibodies that we have tested [138,143,144]. Such sites of gap junctions are illustrated by punctate immunofluorescence labeling of Cx36 in the olfactory bulb (Fig. 3A–C). Where visualization of marker proteins in neurons that express Cx36 is possible, labeling of Cx36 appears as Cx36-puncta distributed on neuronal somata and dendrites, as shown in cerebral cortex (Fig. 3D, striatum (Fig 3E), arcuate nucleus (Fig. 3F) and sexually dimorphic motor nuclei in spinal cord (Fig. 3G,H). Thus, visualization of Cx36 immunofluorescent puncta along neuronal surfaces provides a powerful tool for light microscopic identification of Cx36-containing neuronal gap junctions above a certain minimal size, represented roughly by >30–50 connexons, which corresponds to >70% of gap junctions in both retina [84] and spinal cord [73], thereby revealing their quantitative cellular and subcellular localization over large areas of the CNS [84,95,96,128,136–138,143–148]. It is highly likely that other sites with Cx36 puncta contain bona fide gap junctions, and will reveal functional correlates of both electrical and tracer coupling.
Fig. 2. Correlation of immunofluorescence and FRIL methods used to identify, quantify, map, and immunocytochemically characterize the wide variety of Cx36-containing gap junctions in adult rat retina.
A: Confocal image showing Cx36-puncta (green) vs. Nissl stained (red) neurons in a full-thickness section of rat retina. B: Correlative FRIL image showing exact locations of photomapped immunogold-labeled gap junctions. Numbered red triangles = locations of string gap junctions (all in sublaminae S2 and S1). Numbered blue circles = plaque gap junctions (in S2–S5). C: Higher magnification of boxed area in B. This crystalline plaque gap junction, mapped to sublamina S4, contains ca. 300 connexons that were immunogold double-labeled for Cx36 by 6-nm and 18-nm gold beads. D: Small string gap junction, mapped to S2, contains ca. 30 connexons labeled by 18-nm gold beads. E: Tiny crystalline plaque gap junction mapped to S2; 11 connexons are labeled by a single 12-nm gold bead. Based on quantitative FRIL analysis [84] showing that virtually all string gap junctions (mostly <100 connexons) and many small plaque gap junctions were abundant in S2 of both rats and mice (where others had found few or no gap junctions), we calculated that conventional immunofluorescence imaging had not revealed gap junctions smaller than ca. 100 connexons, which comprise the majority of gap junctions in retina, particularly in S1 and S2. F–G: For correlation, we compared immunofluorescence imaging protocols in which photomultiplier gain was reduced to avoid autofluorescence “noise” in tissue sections (F), with images in which photomultiplier gain was set at increased (optimum) gain in the same 8-μm section of retina (G). In F, relatively few puncta were detected in sublamina S2, where FRIL had revealed a high density of string and miniature plaque gap junctions (<100 connexons). With photomultiplier gain increased, many additional puncta were detected in the same area of S2 (G; circles), but without significantly increasing background autofluorescence. In contrast, the number of puncta visible in ON sublamina S4 (F; square) (where small gap junctions were rare by FRIL) was only slightly increased (G; square box), thereby allowing the first semi-quantitative immunofluorescence detection of this new class of abundant gap junctions containing 30–100 connexons. (Those smaller than 30 connexons remain undetectable by immunofluorescence in tissue slices.) F–G may be viewed as a stereo pair to visualize the 3-D distribution of small to large puncta in the various sublaminae. Offset arrows at C reveal gap junctions at top and bottom of section. RPE = retinal pigment epithelium; OS and IS = outer and inner segments of rods and cones; ONL = outer nuclear layer; OPL = outer plexiform layer; INL = inner nuclear layer; IPL = inner plexiform layer; GCL = ganglion cell layer; Ax = axons on the anterior surface of the retina. Numbers at left margins indicate approximate locations of sublaminae S1–S5. All images modified from [84], with permission. Calibration bars in all FRIL images are 0.1 μm unless otherwise indicated.
Fig. 3. Immunofluorescence labeling of Cx36 in various CNS regions of adult mouse and rat.
A: Main olfactory bulb of mouse, showing overview of a fluorescence Nissl-stained whole transverse section of the olfactory bulb. B,C: Higher magnification images showing labeling of Cx36 in an area (B) of the mitral cell layer (MCL) indicated by the boxed region to the right in A, and labeling of Cx36 in an area (C) of the glomerular cell layer (GCL) shown by the boxed region to the left in A. Detection of Cx36 appears exclusively punctate in both the mitral cell layer and glomeruli, which correspond to sites of heavily-concentrated neuronal gap junctions. D–F: Double immunofluorescence labeling for Cx36 and parvalbumin (PV) in the cerebral cortex (D) and the striatum (E), and double labelling for Cx36 and tyrosine hydroxylase (TH) in the hypothalamic arcuate nucleus (F), showing PV-positive and TH-positive neurons (arrowheads) with Cx36-puncta distributed on their somata and/or processes (arrows). G,H: Double immunofluorescence labeling for Cx36 and the motoneuron marker peripherin, showing closely packed peripherin-positive motoneurons in the sexually dimorphic dorsomedial nucleus (DMN) (G, arrows) in a horizontal section of the lumbosacral region of rat spinal cord, with Cx36-puncta surrounding the motoneuronal somata and localized along motoneuron dendrites. Higher magnification shows peripherin-positive motoneurons (H, arrowheads) displaying Cx36-puncta at their somatic appositions (H, arrows).
2. Development of Cx36 null mice
The identification of Cx36 as a major neuronal connexin led to the generation of Cx36 null mice and the revelation of functional deficits in various neural systems of these mice, including visual, motor and memory impairments [149–156], helping considerably to expose the functional contributions of electrical synapses in mammalian neuronal networks. Some of the deficits found in these mice are further indicated in section 5.1 below.
3. Development and application of FRIL
Anatomically, difficulties imposed in the identification, documentation, and quantification of neuronal gap junctions by thin-section EM and by conventional freeze fracture were substantially surmounted by the advent of novel approaches to ultrastructural detection and characterization of neuronal gap junctions and their connexin constituents. These included the development of freeze-fracture replica immunogold labeling (FRIL), first introduced as SDS-FRL [157,158], and latter improved for use in complex CNS neuropil by combining SDS-FRL with “confocal grid-mapped freeze fracture” as “FRIL” [159]. The term FRIL was coined by Gruijters a decade earlier [160], and has precedence. More recent developments have included “matched-double-replica FRIL” (MDR-FRIL) [93,136], specific applications of which are described below. This approach allowed high-resolution, semi-quantitative analyses of symmetric vs. asymmetric connexin labeling in matching apposed hemiplaques of individual ultrastructurally-visualized gap junctions throughout the CNS. In contrast to thin-section EM, searches for neuronal gap junctions was facilitated by traditional freeze fracture because the fracture plane exposed much broader areas of tissue and revealed defining features of gap junctions in readily identifiable cell types [38,73,161]. Additional to the advantages of conventional freeze fracture, FRIL allows immunogold labeling and examination of connexins at ultrastructurally-identified gap junctions in equally broad expanses of tissue (Fig. 4). Moreover, the larger immunogold beads (20- and 30-nm) served as highly-visible “flags” that were readily detected at much lower magnifications than used when searching for gap junctions by conventional freeze fracture, and hence allowed rapid examination of greater tissue areas. Thus, the rapid advancement that FRIL provided for studies of electrical synapses is that many-fold more neuronal gap junctions were found by FRIL (now >4000) than by all previous ultrastructural studies in mammals combined (op cit), with the additional advantage that their constituent connexins were identified [84,109,128], simultaneously revealing the presence of Cx36 [110,111,162] and/or Cx45 [136] and absence of Cx26, Cx30, Cx32, and Cx43 [110,111].
Fig. 4. FRIL images of a Cx36 immunogold-labeled reticular gap junction on neuronal soma in adult rat suprachiasmatic nucleus.
A: Neuron cell body, revealing its nucleus (N, with abundant nuclear pores), cross-fractured cytoplasm (*), and its somatic plasma membrane containing an immunogold-labeled gap junction (inscribed box). Note the complete absence of “background” labeling. Double-ended arrow traces continuity of cytoplasm, as maintained by the carbon support film, even in the absence of an electron-dense platinum coating. B: High magnification of the boxed area in A, showing a distinctive reticular gap junction, characterized by the presence of connexon-free voids within the junction (modified from Fig. 3 in [96] with permission). Both images originally published as stereo pairs to facilitate 3-D analysis.)
4. Dual cell recording under direct visualization
A greater sophistication of electrophysiological analyses was achieved in the late nineties with the advent of dual cell recording [163–165] (Fig. 5), which was enabled by the development of patch-clamp electrodes (reviewed in [166]) that allowed simultaneous recordings of neighboring cells, thereby increasing the probability of detecting electrical coupling. Dual whole-cell recording under visualized conditions, permitted recording from neighboring neurons, at which the incidence of coupling is much higher [164], thus overcoming difficulties arising from the pattern of connectivity that is unknown to the experimenters when doing blind simultaneous recording from two cells in morphologically complex tissue [167]. Moreover, recordings from electrically-coupled pairs was faciitated by the generation of transgenic mice at which cell-specific proteins are labeled with fluorescent proteins (i.e., parvalbunim) and transgenic mice with Bacterial Artificial Chromosome (BAC) expression of EGFP linked to Cx36 promoter (Cx36-BC-EGFP). This rapid expansion of electrophysiological technology provided strong support for functional electrical coupling of CNS neurons at a time when morphological evidence was mounting for wider and wider occurrence of neuronal gap junctions and for widespread expression of Cx36 in their gap junctions.
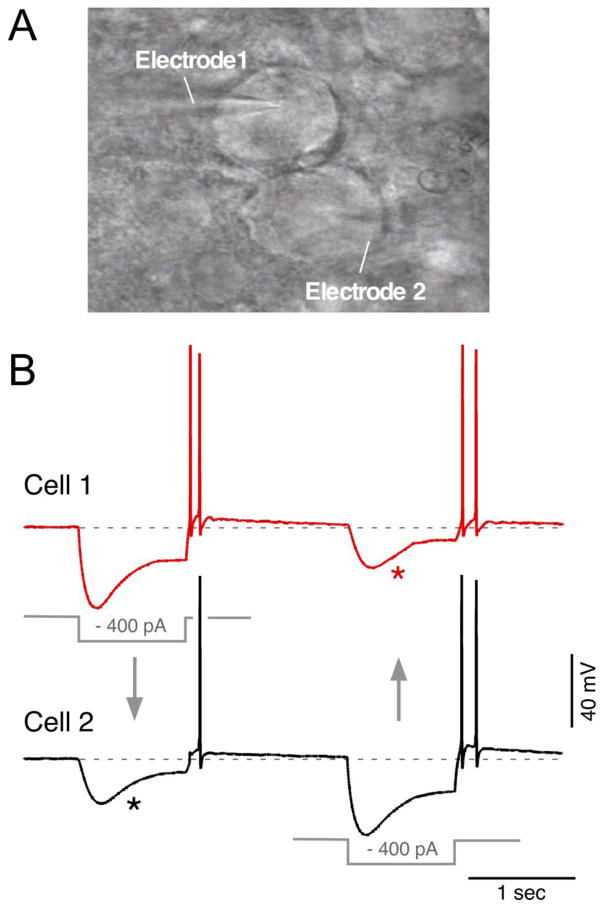
Fig. 5. Visualized paired recordings of electrically coupled cells in the mesencephalic trigeminal nucleus (MesV).
A: Infrared-differential interference contrast (IR-DIC) image of two contiguous MesV neurons during a simultaneous whole-cell recording (electrode 1 and electrode 2). B: Simultaneous recordings from a pair of strongly coupled MesV neurons. Voltage responses to 200 ms hyperpolarizing ( 400 pA) current pulses injected either in cell 1 (red trace) or cell 2 (black trace) evoke corresponding coupling potentials (asterisks). Rebound depolarization after hyperpolarizing pulses causes cell firing. Unpublished image and recordings kindly provided by S. Curti (UdelaR, Montevideo, Uruguay).
5. New tracer molecules
While dual cell recording may still remain challenging, an alternative means for analysis of neuronal gap junction coupling is through intracellular dye- or tracer-injection, and subsequent visualization of tracer transferred between cells via gap junctions. Such transfer between neurons in mammalian CNS first utilized the dye Lucifer Yellow (LY; mw = 444) [55,56,69]. However, its use and use of other higher molecular weight dyes was soon contraindicated with emerging data showing that most mammalian electrical synapses are composed of Cx36, and by findings that LY is poorly permeable through Cx36-containing gap junction channels [152,168]). The development and use of smaller tracers, including Neurobiotin (mw = 287) and biocytin (mw = 372) [72], for visualization of neuronal coupling and, in particular, demonstration of large networks of coupled neurons (Fig. 6), represented another major improvement and addition to the arsenal of approaches available to tackle questions surrounding mammalian electrical synapses. It is noteworthy, nevertheless, that early demonstrations of LY transfer between neurons suggested the possible presence of neuronal gap junctions composed of a connexin (other than Cx36) that forms channels that are at least weakly permeable to this dye. It should also be noted that among some neurons known to be electrically coupled by Cx36-containing neuronal gap junctions, such as preganglionic sympathetic neurons in the spinal cord [169,170] and neocortical interneurons [164], tracer transfer, even with the above lower molecular weight tracers, was not detectable or was barely detectable above fluorescence noise. This was possibly due to the very small size and low rate of flux through the otherwise abundant gap junctions associated with these neurons, as determined by their display of a high concentration of albeit very small Cx36-puncta (Nagy unpublished observations).
Fig. 6. Tracer coupling reveals the organization of functional compartments formed by electrical synapses.
A: Cluster of neurons in the thalamic reticular nucleus following intracellular injection of Neurobiotin (0.5% in the internal solution) during a whole cell recording. While the injected cells appear completely filled and labeled by Neurobiotin, only the cell bodies and proximal process are Neurobiotin-positive in the coupled cells. B: Dye coupling reveals that clusters can be spherical or elongated (compare the extension and spread of clusters), suggesting differences in coupling architecture. These clusters project to different regions of the ventrobasal (VB) and posterior medial (POm) nuclei of the thalamus. Panels A and B are modified from Lee et al, 2014 [253], with permission.
6. More effective gap junction blockers
A wider range of drugs with gap junction actions became available, and their specificity and off-targets effects were better characterized. These included the quinine derivative, mefloquine [171–173], which more specifically blocked Cx36-containing gap junctions. Although less specific, an additional gap junction blocker, meclofenamic acid, also has been useful in the study of electrical synapses [174,175].
7. Spikelets correlated with gap junctions
Transmission of spikes (action potentials) through electrical synapses evokes corresponding coupling potentials in a postsynaptic cell, roughly resembling the time course of the presynaptic action potential (see below). These so called “spikelets” (or “fast pre-potentials”) can be spontaneously observed in electrically-coupled networks, indicating the occurrence of action potentials (spikes) in cells that are presynaptic to the recorded cell. However, spikelets were initially proposed to represent dendritic spikes [176] (i.e., active dendritic signals), casting doubts on whether spikelets were indicative of the presence of electrical synapses, as had been proposed [52]. More recently, paired recordings [163–165,177–179] confirmed initial observations [52] indicating that indeed spikelets often represent the intercellular propagation of presynaptic action potentials. Moreover, the detection of spikelets was found to be correlated with dye coupling [179] and with immunolabeling for neuronal connexins [180]. Spikelets have also been observed during in-vivo intracellular recordings [181,182]. The detection of spikelets as indicative of the presence of electrical synapses should, however, be taken with caution, as dendritic spikes were also reported to occur in many cell types [183–185], and the presence of gap junctions should be independently confirmed using additional approaches such as freeze fracture, dye coupling, immunolabeling, and/or paired simultaneous recordings.
5. Mammalian electrical synapses; post-millennial perspectives
5.1. Functional impact of electrical synapses
Application of the newly-derived knowledge and innovations described above, together with an expanded effort of neuroscientists venturing into the field of electrical synaptic transmission, led to a surge of reports indicating both the prevalence and functional importance of electrical synapses in many diverse regions of the mammalian CNS, especially in inhibitory interneurons [104,186], which are widely distributed in many CNS structures. Most enlightening were revelations that electrical synapses participated in mediating neuronal electrical activities and physiological processes that were already well-established to be fundamental properties of neural circuitry, including synchronization of neuronal network activity [150–152]. A short but non-exhaustive list of structures in the mammalian CNS where evidence has been gathered for the functional importance of electrical synapses includes: 1. Retina is the CNS structure with the highest diversity of neuronal connexins, and where electrical synapses and their regulation are known to underlie a variety of functional processes [187]. For example, electrical synapses, often at glutamatergic mixed synapses, are essential in the transmission and modulation of rod and cone signals through multiple pathways, ultimately to retinal ganglion cells, and where transmission of these signals is impaired in Cx36 null mice [188,189]; 2. Olfactory bulb, where electrical synapses formed primarily by Cx36 [128] are necessary for rapid spike synchronization of mitral cell activity [190]; 3. Cerebral cortex, where electrical coupling between GABAergic interneurons is proposed to generate synchronous activity in interneuronal networks and in neocortical pyramidal cells [163,164,186,191–194]; 4. Hippocampus, where electrical synapses promote synchronous activity among inhibitory interneurons, enabling synchronous high-frequency γ-oscillations in pyramidal cells, and these γ-oscillations are disrupted in Cx36 null mice [105,195–200]; 5. Reticular thalamic nucleus, where Cx36-containing gap junctions promote synchronization of burst-firing [201] and are capable of supporting spindle frequency rhythms among small clusters of neurons [201,202]; 6. Suprachiasmatic nucleus, where Cx36-containing neuronal gap junctions [96] are reportedly required for normal circadian behavior and where deficits in circadian rhythms may arise from loss of these gap junctions in the suprachiasmatic nucleus in Cx36 null mice [203,204]; 7. Hypothalamus, where electrical synapses linking magnocellular neurons are thought to enable synchronization of burst firing for pulsatile oxytocin release [67,205–207]; 8. Inferior olive, where electrical synapses [41] containing Cx36 [208–211] provide for subthreshold network oscillations and for synchronous and rhythmic activation of Purkinje cells by climbing fibers, providing for temporal precision of movement, which is impaired in Cx36 null mice and is also impaired after manipulations of junctional coupling between inferior olivary neurons [209–216]. 9. Brainstem, where electrical synapses in respiratory nuclei contribute to inspiratory-phase synchronous activity and modulation of respiratory frequency of coupled neurons [217–220]; 10. Spinal cord motor systems, where electrical synapses between interneurons provide synchronization of subthreshold potentials and rhythmic firing that is in phase with ventral root motor output, suggesting a contribution of these synapses to operation of the locomotor central pattern generator [221]. 11. Spinal cord sensory systems, where the well-known phenomenon of primary afferent presynaptic inhibition was found to be impaired in Cx36 null mice [146], indicating that electrical synapses play an essential role somewhere in the circuitry governing this inhibition. 12. Gastrointestinal system, where enteric neurons of the myenteric plexus were found to be linked by Cx36-containing gap junctions, and where Cx36 null mice displayed physiological deficits in regulation of gut smooth-muscle contractility [222].
In the above examples, promotion of synchronous activity is one recurring feature attributed to the activity of electrical synapses. Transmission at electrical synapses is usually bidirectional, and therefore changes in the membrane potential of cells within an electrically-coupled compartment are presumably shared with all the partners of the network [151,223]. This includes not only action potentials but also subthreshold responses, such as synaptic potentials [224] and spontaneous oscillations [210]. Such arrangement allows electrical synapses to serve as coincidence detectors [179,225,226], because temporally-correlated changes in the membrane potential of some of the cells will be detected by the others. This occurs because electrical synapses allow electrical currents to leak to coupled cells, which markedly reduce cellular excitability. However, this effect can be transiently cancelled if both cells are simultaneously depolarized. This “coincidence detection” property enormously impacts the integrative properties of circuits and can lead to coordinated cellular activity.
Another salient property of electrical synapses in mammals is that they behave as low pass filters [151]. This is not a property of the gap junction per se, but results from the interaction of the passive properties of the coupled cells. The strength of electrical transmission is determined by the conductance of the gap junction and the resistance and time constant of the coupled cells [227]. Brief, high frequency-containing signals such as spikes are greatly attenuated, whereas longer lasting, low frequency-containing signals are less attenuated by the time constant of the postsynaptic cell. One of the functional consequences of low-pass filtering is that the coupling evoked by presynaptic spikes containing a long-lasting pronounced after-hyperpolarization will be predominantly hyperpolarizing and thus inhibitory, as the after-hyperpolarization will be substantially less attenuated than the spike [151,180,228]. Interestingly, this property was shown to lead to the desynchronization of the cerebellar Golgi cell network [180], indicating that electrical synapses can endow networks with more complex properties.
Finally, electrical synapses have been reported to be highly plastic and capable of modifying their coupling strength under various physiological conditions [229–233], and these changes could quickly reconfigure networks, such as occurs in the retina [187]. Thus, altogether, these properties provide sophisticated computational capabilities to neuronal networks containing electrical synapses.
The efficiency with which electrical synapses promote synchronization of neuronal activity has led prominent investigators to suggest that “the presence of electrical synapses among a population of neurons should prompt us to ask when and why they need to synchronize their activity” [234]. In this context, an emerging principle is that synchronous neuronal activity enables synchronous oscillations (e.g., theta 4–12 Hz, gamma 30–80 Hz, and high-frequency 200 Hz oscillations) among ensembles of neurons, where these oscillations temporally correlate firing patterns in disparate networks. It has been stated [235–238] that “brain oscillations are generated in almost every part of the brain”, that “network oscillations may take part in representing information, regulate the flow of information in neural circuits, and help store and retrieve information in synapses distributed throughout cortical networks”, and that “in primate brain there is growing evidence that oscillations are linked to behavior and cognitive tasks”. Further, “even the Holy Grail of cognitive neuroscience – consciousness – has been proposed to involve oscillations of coordinated neural activity in multiple brain areas” [239], as elaborated in several reviews [240,241]). Thus, electrical synapses, with their propensity to support synchronous neuronal activity, have emerged from relative obscurity in mammalian brain to now a place at center stage in contributing to the generation of network oscillations that are considered to be fundamental for information processing in the CNS. Indeed, it has been considered that “as more is learned, we may soon witness the emergence of the electrical synapse as an important element in textbook wiring diagrams of the vertebrate brain” [239]. And finally, it has been noted that “if one considers the experimental evidence indicating that connexins are important for neurophysiology, it is also likely that they may be involved in pathology” [152]. For example, mutation in the non-coding (regulatory) region of the gene for Cx36 has been linked to debilitating juvenile myoclonic epilepsy [242,243].
5.2. Anatomical distribution of electrical synapses
Although it is common to read in the literature that neuronal gap junctions are localized primarily to inhibitory GABAergic interneurons [68,104,105,186], this generalization belies the true diversity of neuronal systems in which these synapses occur. In fact, studies involving analyses of either electrical coupling, dye-coupling, localization of ultrastructurally-identified neuronal gap junctions, and/or immunofluorescence imaging of Cx36 have provided evidence for abundant electrical synapses (including morphologically-mixed synapses) interlinking excitatory neurons, including those with long-axon projections [25]. The best examples of these latter categories were the first systems in which mammalian electrical synapses were identified, namely between: 1. Excitatory glutamatergic inferior olivary cells [41], which project as climbing fibers to the cerebellum; and 2. Glutamatergic excitatory trigeminal mesencephalic primary afferent neurons [34,35], which innervate masseter muscles and periodontal ligaments. Other examples include: 3. Excitatory glutamatergic retinal ganglion cells [188,244], which have long centrally-projecting axons to several brain areas; 4. Excitatory glutamatergic olfactory mitral cells [245,246], which also have long centrally-projecting axons to multiple brain regions; 5. Excitatory glutamatergic cortical and hippocampal pyramidal cells [177,247], whose projections are numerous and extend into diverse regions of the brain; 6. Noradrenergic locus coeruleus neurons [248], which have widespread projections throughout the brain; 7. Vestibular and cochlear primary afferents, which innervate the auditory complex and terminate in the central vestibular nucleus (see [143] and references therein) and cochlear nuclei ([249] and references therein); 8. Excitatory glutamatergic fusiform and inhibitory stellate interneurons in the dorsal cochlear nucleus, where fusiform neurons are electrically coupled to stellate cells, and stellate cells are coupled amongst themselves in this nucleus [250]; 9. Cholinergic spinal preganglionic sympathetic neurons [169,170], which have long projections to diverse peripheral tissues; 10. Glutamatergic spinal myelinated primary sensory afferent neurons [144], whose cell bodies located in dorsal root ganglia have large-diameter fibers that innervate muscle spindles and terminate centrally on motoneurons in the spinal cord ventral horn; 11. Cholinergic sexually dimorphic motoneurons [87,120,147], which innervate sexually dimorphic perineal musculature; 12. Spinal unmyelinated primary afferent neurons [64,251,252], which are likely peptidergic, innervate skin, and terminate in the dorsal spinal cord.
Based on these diverse examples, it is almost certain that electrical coupling will be found to be a feature of additional non-GABAergic neuronal populations. Detailed studies of coupled GABAergic systems, however, have also reached a considerable level of sophistication [253,254]. Moreover, a novel combination of sequential confocal immunofluorescence mapping and TEM examination of the same contact areas revealed Cx36-containing gap junctions linking GABAergic interneurons in striatum [255] and cortex [256], with those studies providing novel quantitative insights into neuronal network connectivity.
5.3. Subcellular locations of diverse types of electrical synapses
Based on cumulative immunocytochemical, ultrastructural, and physiological data from diverse disciplines, it is now clear that in mammalian CNS: a) there are at least five anatomical and functional types of electrical synapses, b) each type of electrical synapse contains from one to several gap junctions, all of which are composed predominately of a single distinguishing type of connexin (i.e., Cx36, Cx45, or Cx57), c) each type of electrical synapse occurs at a specific subcellular location that is related to its primary function, and d) that there are specific targeting mechanisms for these neuronal connexins at each type of subcellular location, as well as for co-targeting of their accessory synaptic proteins (e.g., cell adhesion molecules, neurotransmitter receptors, synaptic vesicle proteins). These broadly-defined electrical synapse types include:
1. Purely electrical dendro-dendritic synapses [58,59,62,68,105,186,245,257–260]
So far, Cx36 is the only connexin localized at these purely electrical synapses [178,180,261] in various brain regions other than retina. In retina, purely electrical synapses formed by connexins Cx57 occur at dendro-dendritic contacts between horizontal cells in rabbit [139], whereas axo-axonic and axo-dendritic contacts between horizontal cells in mice are reportedly formed primarily by Cx50 [140]. Note, however, that either or both of these types of appositions have not been determined physiologically or ultrastructurally to be purely electrical vs. morphologically-mixed synapses.
2. Purely electrical somato-somatic synapses
As originally described in fish (relay cells in electric fish), electrical synapses have been shown to occur between the cell bodies of neurons, such as in the case of the mesencephalic trigeminal nucleus [32,34,35,179].
3. Axo-axonic electrical synapses
Although these were originally reported in fish [6–8], there is substantial literature on the role of hypothesized axo-axonal gap junctions in high-frequency electrical network activity in rodents. Sharp-wave ripples (SWRs) occurring at a frequency of 100–250 Hz in the hippocampus are of particular importance because these oscillations are reported to be generated in axons [262], have high temporal and spatial coherence, and occur during experience-specific reactivation (“replay”) of sequences of neurons [263–265]. The mechanisms responsible for production of SWRs are not known, but experimental and modeling data predict their mediation by electrical synapses that have been proposed (but not yet documented) to link the axon initial segments (AIS) of principal cells in the hippocampus and in the cerebral cortex [266–270]. The AIS of hippocampal pyramidal cells are devoid of labeling for Cx36 (Nagy, unpublished observations), and SWRs apparently persist in Cx36 ko mice [195,197,271], yet the inference of gap junctions in the AIS presumably composed of some neuronal connexin other than Cx36 is supported by observations of dye-coupling between hippocampal pyramidal cells [266], elimination of SWRs by gap junction blockers [272], and observations that SWRs can occur in the absence of chemical synaptic transmission [266,270].
4. Excitatory “mixed” synapses
These are combined chemical plus electrical synapses [25,37,40,41] that are established, at the minimum, to link axon terminals with somata or dendrites (axo-somatic and axo-dendritic), where the gap junction component contains Cx36, as we have shown in rodent spinal cord [144,147,148], trigeminal motor nucleus [144,148], and lateral vestibular nucleus [143], and which so far are exclusively glutamatergic [137,143,273]. However, in the retina [136] and olfactory bulb [128], a few axo-somatic and axo-dendritic gap junctions also contain Cx45 [136] or a mixture of Cx36 and Cx45, the latter forming “bi-homotypic” gap junctions [136]. In the latter, MDR-FRIL (Fig. 7) revealed domains of Cx36 gold beads (10-nm) opposite domains of gold beads for Cx36, and domains of gold beads for Cx45 (5-nm) opposite domains of gold beads for Cx45, the combination not forming heterotypic (i.e., Cx36:Cx45 gap junctions) but instead, forming bi-homotypic (Cx36:Cx36 plus Cx45:Cx45) gap junctions in retina. Such MDR-FRIL mapping has not yet been conducted in olfactory bulb or in other locations co-expressing Cx36 and Cx45.
Fig. 7. Matched double-replica FRIL (MDR-FRIL) showing separate gap junctional domains labeled solely for Cx36 adjacent to domains labeled solely for Cx45.
The image shows a single gap junction viewed after capture of both its hemiplaques in apposing membranes, with one hemiplaque showing areas of particles (A, arrow) and pits (A, arrowhead) and the other in corresponding areas showing instead pits (B, arrowhead) and particles (B, arrow), separating the regions where the membrane fracture skips from one apposed membrane to the other (B, double arrowhead). This and six other matched gap junctions displayed separate domains of Cx36 localization in one hemiplaque opposite Cx36 localization in the apposed hemiplaque, and similarly, Cx45 localization in the same hemiplaque opposite labeling for Cx45 in the apposed hemiplaque. Combined with data from 60 other unmatched hemiplaques containing both Cx45 and Cx36, we concluded that most/all retinal neurons expressing Cx45 also express Cx36, with both connexins co-existing in both apposed hemiplaques. Thus in retina, these two connexins form bi-homotypic (Cx36:Cx36 + Cx45:Cx45) gap junctions [136], rather than forming heterotypic Cx36:Cx45 gap junctions, as previously proposed (references cited in [136]). (Modified from Fig. 6 in [136], with permission.)
5. “Reciprocal” (mirror) dendro-dendritic mixed synapses [83,128,159,190,274,275]
These have been proposed to form high-speed “reciprocal” (positive feedback or self-amplifying) oscillations, and so far have been found only at these glutamatergic appositions, which apparently contain only Cx36 [128].
Among the above configurations of gap junctions where Cx36 has been identified, it is noteworthy that, in at least mammalian systems, this connexin has been found so far to be engaged exclusively in homotypic gap junction channels, meaning that Cx36 on one side of a hemiplaque always couples with Cx36 in the apposing hemiplaque, and never to another connexin. Indeed, it was reported that Cx36 was non-permissive for coupling with nearly half of the other connexin family members that were tested for coupling permissiveness [276]. This extraordinary selectivity of Cx36 coupling only with itself distinguishes Cx36 and Cx36-containing neuronal gap junctions from other connexins that are found in a variety of other cell types. Astrocytes and oligodendrocytes, for example, each express three connexins, five of which engage in various combinations of heterotypic coupling, in both homologous (i.e., same cell type) and heterologous (i.e., different cell type) configurations. Notwithstanding assorted cellular factors that may determine different cell types that have capability to form gap junctions with each other, about which little is known, the lack of coupling permissiveness of Cx36 with other connexins may be required in part to prevent its coupling with, for example, the various glial connexins.
The above five synaptic configurations established so far may provide evidence for multiple mechanisms of regulation of electrical synapses in neuronal synaptic circuitry, but if so, most configurations are not yet characterized physiologically. It also should be noted that inhibitory mixed synapses formed by GABAergic axon terminals on neuronal somata and dendrites (axo-somatic and axo-dendritic) have been suggested, but evidence for the existence of inhibitory mixed synapses is considered tenuous because the single report [277] showing a gap junction at a putative inhibitory mixed synapse ostensibly labeled for GABAa receptors has not been confirmed by others, and the clump of gold beads denoting GABA receptors was present in an area of membrane E-face that did not exhibit clusters of intramembrane particles or pits, which are widely acknowledged as the freeze-fracture correlates of all transmembrane proteins.
5.4. Ultrastructural diversity of Cx36-containing gap junctions
Gap junctions have been almost universally described as “plaques” of tightly clustered intramembrane particles (“connexons”), either in regular hexagonal array (“crystalline”) or in irregular distributions (“non-crystalline”) (Fig. 8a). Early reports attempted to determine whether gap junctions with regular crystalline order were either the conductive or the non-conductive form, or whether one or the other of those two forms resulted from artifacts of chemical fixation ([278,279] and references therein). However, reports of unusual but rare “string” gap junctions in retina [38], and descriptions of gap junctions with dispersed connexons or with internal voids [38,280] (“lacey” or “reticular” gap junctions [84,96,281]) hinted at more diverse forms. With the advent of FRIL, with its capacity for finding large numbers of immunogold-labeled neuronal gap junctions (now >4000 FRIL-labeled mammalian neuronal gap junctions individually photographed by us), at least seven distinct gap junction morphologies based on connexon distributions are now recognized [84]: 1. crystalline plaques (Fig. 8A), ranging from a few connexons to a few thousand connexons, all in crystalline hexagonal array; 2. non-crystalline plaques (Fig. 8B), ranging from a few connexons to several thousand connexons in irregular, sometimes slightly dispersed array; 3. reticular gap junctions (Fig. 8C; also Fig. 4B), having from a few dozen to several hundred connexons, with one or more internal voids larger than 30 nm; 4. ribbon gap junctions (Fig. 8D), composed of one or more extended but centrally-connected or circular strips 2–4 connexons wide and up to 100 connexons long; 5. string gap junctions (Fig. 8E), consisting of a few to several hundred connexons arranged as rows of individual connexons (like beads on a string); 6. “meandering” gap junctions (Fig. 8F), consisting of a few to several dozen loosely-organized, non-crystalline connexons that sinuously wander over short distances; and 7. “cluster” gap junctions, consisting of several small, closely-spaced groups of connexons, with no obvious linkages between clusters. In addition, plaque gap junctions with greatly increased spacing between connexons have been reported in gap junctions of the outer plexiform layer in rabbits [280]. Whether these represent an eighth type of gap junction formed from a different connexin having more dispersed connexins (e.g., Cx50 or Cx57 [282]), species-specific differences, or other factor, remains unexplored. The functional relevance of these distinctive ultrastructural configurations are unknown, but the abundant string gap junctions in the inner plexiform layer normally occur only in the functionally-defined OFF lamina, primarily in sublamina S2 [84,161], as well as occasionally in the outer plexiform layer [280]. Although quantitative analysis of gap junction morphologies have been conducted primarily in retina [84], reticular gap junctions also have been reported linking hippocampal neurons [283] and neurons of the suprachiasmatic nucleus [96] (Fig. 4). Moreover, preliminary data suggest the inter-convertibility of plaque, ribbon, and string gap junctions, based on patterns and frequency of neuronal activity and on state of connexin phosphorylation [284], but such studies are in their infancy [285–287].
Fig. 8. Seven basic morphologies of neuronal gap junctions in retina and hippocampus immunogold labeled for Cx36.
A: Large-diameter crystalline plaque gap junction from ON lamina of adult rat retina (ca. 900 connexons); B: E-face image of small-diameter non-crystalline plaque gap junction in ON sublamina of rat retina (ca. 90 connexons immunogold labeled with two sizes of gold beads. C: Reticular gap junction from adult rat hippocampus. D: Large multi-strand ribbon gap junction (ca. 490 connexons; pink overlay) in the OFF sublamina of adult rat retina (S2) labeled for Cx36 with two sizes of gold beads. E. Portion of E-face image of large compound string gap junction in the OFF sublamina S2 (previously unpublished image). F: “Meandering” gap junction in rat hippocampus, consisting of 25 connexons labeled for Cx36 beneath their E-face pits. G: Dispersed clusters of connexons in adult rat hippocampus (unpublished image). (A and B, modified form Fig. 3 in [84]; C and F, modified from Fig. 11 in [273]), D, modified from Fig. 5A in [84]; all with permission.
6. Mixed synapses in mammalian brain
Shortly after or concomitant with demonstrations of neuronal gap junctions in the inferior olive and the mesencephalic trigeminal nucleus of rat [32,35,40], ultrastructural investigations of mammalian brain convincingly revealed mixed chemical/electrical synapses [25] in regions corresponding anatomically to those in non-mammalian vertebrates, where mixed synapses were first found [4–7,22]. These and several more recently-identified areas at which immunolabeling for Cx36, in combination with the nerve terminal synaptic vesicle marker vesicular glutamate transporter-1 (vglut1), has been found at axon terminals in rat and/or mouse, presumably forming mixed synapses, include: 1. Lateral vestibular nucleus [24,25,33,44,54,143]; 2. Cochlear nucleus [54,249]; 3. Medial nucleus of the trapazoid body [249]; 4. Lateral superior olivary complex. [249]; 5. Hippocampus, specifically in the CA3 region of rat ventral hippocampus [138,273]; 6. Brainstem and spinal cord motoneurons [73,144,148], and 7. Retina [38,84,161,280].
In many of the above mammalian systems, there is a paucity of electrophysiological evidence that the gap junction component of those mixed synapses serves to mediate electrical transmission. Such evidence, albeit obtained by indirect means, has been reported in primary vestibular afferent fibers contacting vestibular neurons [24,39], and in mixed synapses formed by hippocampal mossy fibers [288,289]. This is in contrast to the extensive studies on electrical transmission by mixed synapses formed by, for example, eighth nerve afferents in goldfish (discussed above), as well as in other species, including toadfish [22], frog [19,290], lizard [291], and pigeon [292]. In contrast to studies in lower vertebrates, analysis of electrical transmission at mixed synapses is more challenging in mammalian systems, where synaptic delay of chemical vs. electrical mediation is less distinguishable due to the slower time constant of mammalian neurons that attenuate brief changes in membrane potential (low pass filtering effect). More detailed considerations of mixed synapses in mammalian CNS and their possible functional roles are discussed elsewhere [293].
7. Issues on the horizon
Early on, it was considered by some that the presence of neuronal gap junctions, as identified ultrastructurally or by immunofluorescence visualization of their connexin constituents, did not necessarily require that these junctions ever serve as functionally-active electrical synapses. The field has moved beyond such views, which should be held as no longer tenable because electrical coupling and/or dye-coupling between specific neurons has been found at many locations where there is evidence for gap junctions between those same neurons. At the current state of knowledge, it might be more prudent to suppose that where neuronal gap junctions occur, they are likely to have and will ultimately be found to have functional roles. Nevertheless, many outstanding issues remain, some of which are listed as follows.
1. Gap junction and connexin characterization required at identified sites of coupling
There are situations where electrical and/or dye-coupling between neurons has been demonstrated, but where there is an absence of evidence for gap junctions linking those coupled neurons. Examples of this, among others, include pyramidal cells in the cerebral cortex [247], pyramidal cells in the hippocampus [138,177], and dopaminergic neurons in the substantia nigra [60,294]. A different version of this scenario occurs where neurons have been found to be electrically-coupled and to display gap junctions, but where the connexin constituent of those junctions have defied identification. Examples of this are the hypothalamic magnocellular nuclei, including neurons that express oxytocin or vasopressin in the supraoptic nucleus and paraventricular nucleus, where we have failed to detect Cx36 (Nagy, unpublished observations) or any other putative neuronal connexin.
2. Neuronal connexin compensation
A related problem concerns examination of electrical coupling in Cx36-null mice. In at least three brain areas where neurons are extensively coupled by gap junctions, the primary known constituent of which is Cx36, Cx36 null mice still exhibit residual electrical coupling. These include the reticular thalamic nucleus [253,295], inferior olivary nucleus [296], and the mesencephalic trigeminal nucleus [179]. While compensation for the absence of Cx36 by another connexin in these areas is possible, we have failed to detect at least a dozen candidate connexins in the two latter regions of Cx36 null mice, including Cx45 and Cx57 (Nagy, unpublished observations). However, connexins may be present in fewer than 30–50 connexons, and therefore be functional but not detectable by immunofluorescence (Fig. 2F,G; modified from Fig. 10 in [84]). The nature and extent of the residual coupling and the identity of the connexin involved remains an important issue because the full scope of functional deficits examined in the Cx36 null mice might not be manifest if some degree of coupling compensation occurs. Moreover, it is not known whether the connexin that underlies the observed residual coupling in the Cx36 null mice is normally present in gap junctions between neurons in the above three systems and, if so, whether it contributes to coupling in wild-type mice.
3. Morphologically-mixed vs. functionally-mixed synapses
Morphologically-mixed synapses in mammals were first identified and characterized by Sotelo, Llinas, and coworkers at axon terminals on somata and dendrites [25,33]. Within the axon terminal, as identified by its content of abundant uniform-diameter synaptic vesicles, pre-synaptic “active zones”, and distinctive electron-dense postsynaptic densities (PSDs), the gap junction component was found to occur in close proximity to one or more specializations characteristic of chemical synapses, including electron-opaque active zones that in turn were in direct intercellular apposition to an electron-opaque PSD. In this “dyadic” arrangement (the classical morphologically-mixed synapse), depolarization or hyperpolarization of the postsynaptic cell is thought to influence, via the electrical synapse, the amount of neurotransmitter released during each axon terminal depolarization, as recently shown to occur after depolarization or hyperpolarization at other types of axon terminals [297]. Subsequent to the discovery of dyadic mixed synapses, a “‘triadic” arrangement, where an axon terminal forms a chemical synapse to one dendrite, which is separately coupled via a gap junction to a second, immediately adjacent dendrite, has been observed in multiple CNS locations (see Figs. 13–14 in [25]). In this first of several forms of “functionally-mixed” synapse, the gap junction linking to the second dendrite could allow depolarization/calcium influx into the second dendrite to activate “silent” AMPA receptors in the first (postsynaptic) dendrite, providing another potential type of regulatory circuit mechanism, especially during development wherein both electrical and silent glutamatergic synapses coexist [99,298,299]. Conversely, influx of Ca++ through postsynaptic NMDA glutamate receptors in the first dendrite may modulate electrical conductance of the nearby gap junction, potentially providing yet another type of regulatory mechanism [300–302].
Unfortunately, the word “functional” (as opposed to “non-functional”) has also been applied to mixed synapses (“functional mixed synapse”), in analogy to active vs. “silent synapses” [303], further confusing terminology. For the purposes of this review, the term “functionally-mixed” synapses applies only to several diverse triadic arrangements in which a gap junction links two closely successive postsynaptic elements, as described above.
4. Regulation of electrical synapses
It is long established that gap junctional coupling in peripheral tissues is highly regulated [304,305], and it is therefore no surprise that gap junction channels between neurons are equally subject to regulation of their deployment, turnover, and permeability state. Indeed, there recently has been considerable emphasis placed on the plastic nature of electrical synapses [232,301,306]. Ideas surrounding this plasticity have resurrected interest in earlier work showing that neuronal gap junctional coupling in mammalian systems can be altered by a host of neurotransmitter and neuromodulatory substances, including dopamine [307–310], noradrenaline [200], serotonin [311], histamine [312], acetylcholine [313], and nitric oxide [311,314]. It is now accepted that the coupling strength of electrical synapses, involving changes in gap junctional conductance, is regulated by modulatory neurotransmitters, and mounting evidence indicates it is also influenced by the activity of the network in which they participate via activation of glutamatergic synapses. Interactions between glutamatergic transmission and electrical synapses, as well as activity-dependent regulation of these synapses in developing and adult CNS, have gained more recent attention (reviewed in [301,306]). While the presence of neuromodulators seems to be required to maintain the changes in coupling strength, plastic changes involve long-term modifications in coupling strength triggered by brief activation of chemical synapses, which outlast the induction period. This distinction is somehow arbitrary, as neuromodulators were reported to trigger long-term changes in electrical transmission that outlast the exposure period [315,316]. Neuronal burst firing was also shown to trigger long-term changes in coupling strength, adding a second form of activity-dependent plasticity [231,317].
The influence of monoamines on electrical coupling has particularly far-reaching implications. Neuromodulatory transmitters were shown to modify electrical coupling in the retina, leading to profound reorganization of neural circuits [187], suggesting that electrical synapses in other areas of the CNS could be similarly regulated to modify function. Widespread axonal projections to many areas of the CNS arise from dopaminergic neurons in the substantia nigra and ventral tegmental area, noradrenergic neurons in the locus coeruleus, serotonergic neurons in brainstem nuclei, and histaminergic neurons in the tuberomammillary nucleus. Each of these systems has a broad influence on a variety of physiological processes, motor and sensory functions, and cognition. Further, the first three of these systems are acted upon by many drugs used clinically to treat neurological disorders (e.g., Parkinson’s disease, schizophrenia, depression) and by drugs of addiction (e.g., cocaine, amphetamine). It is quite possible that by modifying activities of these transmitters, therapeutic and addictive drugs may exert their beneficial or adverse actions, in part, by altering signal transmission at electrical synapses [318–322]. In the case of the locus coeruleus, which has been intensively studied in relation to roles in learning, memory, and reinforcement behavior [323,324], its diverse projections greatly expand brain regions where monoamines and drugs acting on monoamine systems could modulate neuronal electrical coupling. Further, neurons in the locus coeruleus are themselves coupled by gap junctions [95] and are heavily innervated by dopaminergic fibers [325], providing for potential dopamine and dopaminergic drug actions at electrical synapses between these neurons. Just as understanding of processes involved in chemical synaptic transmission has served as a basis for deciphering sites and mechanisms of drug action at chemical synapses, so too, knowledge of modulators and cellular processes that regulate electrical synapses will be essential for understanding how malfunction of these synapses may contribute to CNS disease and is expected to provide insight into mechanisms for potential actions of drugs on cellular processes that regulate electrical synapses.
5. Regulation of Cx36 by phosphorylation
Cx36 contains three c-terminus serine amino acid residues (ser293, ser306 and ser315) and an additional cytoplasmic loop residue (ser110), all of which are phosphorylated by protein kinase (PKA) in vitro, with ser293 being the primary phosphorylation site [326,327]. Several early studies of the effects of Cx36 phosphorylation state on conductance at electrical synapses in mammalian CNS [326,328,329] suggested that uncoupling of ON cone bipolar cells and AII amacrine cells [326] resulted from phosphorylation of Cx36 following activation of D1 receptors and consequent activation of cyclic AMP (cAMP) and PKA acting on Cx36 [326]. This view has been challenged by Kothmann et al [330], who provided evidence that uncoupling occurs via an alternative pathway to that proposed by Urschel et al [326]. In this model, D1 receptors activate PKA, which in turn activates protein phosphatase-2A (PP2A) localized to Cx36-containing gap junctions, resulting in uncoupling by Cx36 dephosphorylation. Indeed, small ensembles of gap-junctionally-coupled neurons were shown to undergo modulated moment-to-moment changes in their strength of coupling based on state of phosphorylation, as determined by differential labeling using antibodies against phosphorylated vs. non-phosphorylated Cx36 [330].
6. Rectification of electrical transmission in the mammalian CNS
Rectification of electrical transmission has been reported in invertebrates [1,331,332] and fish [286,333], but no clear examples of this property have been observed to occur between mammalian neurons. However, asymmetry of junctional conductance between some pairs of neurons was observed in the inferior olive [334], the striatum [335], and the reticular thalamic nucleus [230]. Moreover, asymmetry of junctional conductance was recently observed between neurons of the thalamic reticular nucleus following coordinated burst firing of these cells that also triggers long-term depression of electrical transmission [230], suggesting that rectification might be achieved by regulation of gap junction channels. Further studies of these properties are likely to indicate the presence of mechanisms underlying asymmetric electrical transmission, and may reveal that rectification also occurs at electrical synapses between mammalian neurons. Finally, not involving asymmetry of junctional conductance, strong asymmetry of electrical transmission based on differences in the input resistance of the coupled cells (which is greatly determined by cell size) was shown to occur between fusiform and stellate cells of the dorsal cochlear nucleus [250].
7. Molecular complexity of electrical synapses
The molecular basis for regulation of electrical synapses by the above list of neurotransmitters has been most studied in the context of phosphorylating/dephosphorylating agents that act through intracellular cAMP signaling and activation of PKA, or alternatively through CAMKII signaling, followed by phosphorylation of Cx36, leading to attendant changes in channel coupling state (reviewed in [301,306]). However, electrical synapses are emerging as multi-molecular composites of proteins [336]. Identification of the macromolecular protein components of Cx36-containing gap junctions provides opportunities to consider novel mechanisms whereby intracellular signaling pathways could influence the formation and structural integrity of electrical synapses and thereby contribute to regulating strength and potentially, the preferential direction of electrical coupling. These components include structural and scaffolding proteins, transcellular adhesion proteins, and a variety of regulatory and signaling proteins. We originally reported that the PDZ-domain-containing protein zonula occludens-1 (ZO-1) interacts with Cx36 and its fish ortholog Cx35, and is localized at electrical synapses [137,162,337,338]. ZO-1 interaction with connexins in other cells is essential for gap junction electrical coupling and for gap junction accretion and disassembly [339,340]. Subsequently, we found Cx36 co-localization and molecular association with the effector protein AF6 (a.k.a. afadin), the scaffolding protein MUPP1 (multi-PDZ domain protein-1), and the cytoskeleton-interacting-protein cingulin at electrical synapses in widespread brain regions, including mixed synapses on neurons in the vestibular nuclei and at mossy fiber terminals in the hippocampus; and we have shown that the carboxy (C)-terminus PDZ interaction motif of Cx36 interacts with a PDZ domain in AF6 and the 10th PDZ domain of MUPP1 [138,341,342]. Association of ZO-1 with neuronal gap junctions via its interaction with Cx36 and via its known direct interaction with AF6 [343,344], together with association of AF6 and ZO-1 with the cytoskeleton via their known interaction with F-actin, could promote assembly and/or maintain connexon aggregation within neuronal gap junction plaques.
Localization of AF6 at electrical synapses implicates a host of additional regulatory and structural mechanisms that may be operative at these synapses and amenable to investigation. For example, AF6 is an effector for the Ras family of GTP-binding proteins, of which Rap1 is a member [345,346]. The Rap1 signaling pathway contributes to cell development, differentiation and adhesion [347,348], and to dendritic spine remodeling in neurons [349]. Rap1 is a master regulator of cell-cell junctions, and generally promotes the formation of adherens junctions [347,348,350]. Rap1 in turn is activated by the effector protein Epac (exchange protein activated by cAMP), which is a guanine nucleotide exchange factor (GEF) that was distinguished from the other major cAMP effector, PKA. Thus, actions of cAMP may also occur by its activation of Epac. Regulation of neuronal gap junctions by the cAMP/Epac signaling pathway has not yet been studied, but it is possible that Epac/Rap1 signaling to AF6 at electrical synapses serves to organize the assembly, maintenance, and spatial deployment of these structures.
Although gap junction plaques may partially disperse into ribbons and strings, they are always present within defined gap junctions and were never observed dispersed into widely separated IMPs. Even during formation and growth, FRIL revealed labeling of individual connexons and small groups of connexons immediately after insertion into the plasma membrane and before migration and incorporation into nearby jap junctions [351]. (Also see [352] for similar data on gap junction formation in vitro.) However, in no case were abundant immunogold labels dispersed over the surface of retinal neurons, as would have been easily detectable by FRIL if hundreds or thousands of connexons in a plaque were to disperse over several square micrometers, or if connexin proteins were present as a high density of unpaired “hemichannels”, as has been proposed [353]. The ultrastructurally-observed feature of gap junction assembly involving absence of dispersed connexons prior to their aggregation is very likely related to mechanisms whereby proposed Epac/Rap1 signaling to AF6 and other structural proteins orchestrate the construction of Cx36-containing neuronal gap junctions.
8. Conclusions
As pervasive as electrical synapses are now known to be in the mammalian CNS, we are still far from understanding the full scope of the prevalence, let alone functional contributions, of these synapses in neuronal circuitry. For example, our immunofluorescence approaches to localization of Cx36-containing gap junctions have revealed brain regions that harbor an impressive density of immunofluorescent Cx36-puncta (Nagy, unpublished observations), but where electrical synapses have neither been detected by other approaches nor even suspected to exist. While there has been rapid progress deciphering the molecular structure, cellular localization, and functional contributions of electrical synapses to mammalian neural systems, it may be that these endeavors will continue for as long as has occurred for studies of chemical synapses.
There are clear demonstrations of the functionally-relevant roles of electrical synapses in a number of mammalian neural systems. However, these are still relatively few compared with the large numbers of reports on the pervasiveness of these synapses in mammalian CNS, where their physiological relevance has only been inferred or remains unexplained. Indeed, the functional roles of electrical synapses in many systems described above, as well as others not listed, are sometimes superficially understood or have not yet been investigated in the context of the physiology governed by the circuits in which these synapses occur. This is perhaps indicative that the subject of electrical synapses in mammalian systems is still an emerging field, possibly providing at least one reason why recognition of the potential importance of these synapses and the ways in which they contribute to shape neuronal activity has not as yet widely permeated the general neuroscience community. In this context, it is pertinent to consider the standing that electrical synapses and electrical neuronal communication have in the area of connectomics and the massive initiatives of such undertakings as the Human Connectome Project, the Human Brain Project, the Open Connectome Project, the Brain Observatory, and efforts at the Allen Institute for Brain Science to comprehensively understand brain circuitry. With the emergence of more concrete examples of the fundamental importance of electrical synapses and their functional contributions to neural integrative processes, it will become inescapable that a better understanding of electrical synapses and their intricate deployment in neural micro-circuitry will be required in any attempt to develop broader theories of how the brain processes and stores information. We anticipate that aficionados and enthusiasts of electrical synapses will eventually be faced with the challenge of bringing electrical synaptic transmission to the attention of those mega projects for inclusion as an essential component of the connectome.
Highlights.
Analyses of electrical synapses in lower vertebrates led to their study in mammals.
Key factors revealed functional roles of electrical synapses in mammalian CNS.
Neuronal gap junctions are structurally diverse and occur between various neuron types.
Morphologically mixed synapses are identified at many sites in the mammalian CNS.
Recent advances point to exciting new research directions.
Acknowledgments
This work was supported by grants from the Canadian Institutes of Health Research and the Natural Sciences and Engineering Research Council to J. I. Nagy; National Institutes of Health (NIH) grants DC03186, DC011099, NS055726, NS085772 and NS0552827 to A. E. Pereda; and NS31027, NS44010, and NS44395 to J. E. Rash. We thank B. McLean, K. Vanderpool and T. Yasumura for excellent technical assistance, and Dr. Sebastian Curti (UdelaR, Montevideo, Uruguay) for kindly providing the image and recordings of Fig. 5.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Furshpan EJ, Potter DD. Transmission at the giant motor synapses of the crayfish. J Physiol (London) 1959;145:289–325. doi: 10.1113/jphysiol.1959.sp006143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennett MVL, Crain SM, Grundfest H. Electrophysiology of Supramedullary Neurons in Spheroides maculatus III. Organization of the supramedulary neurons. J Gen Physiol. 1959;43:221–250. doi: 10.1085/jgp.43.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennett MVL, Aljure E, Nakajima Y, Pappas GD. Electrotonic junctions between teleost spinal neurons: Electrophysiology and ultrastructure. Science. 1963;141:262–264. doi: 10.1126/science.141.3577.262. [DOI] [PubMed] [Google Scholar]
- 4.Robertson JD. The occurrence of a subunit pattern in the unit membranes of club endings in Mauthner cell synapses in goldfish brains. J Cell Biol. 1963;19:201–221. doi: 10.1083/jcb.19.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robertson JD, Bodenheimer TS, Stage DE. The ultrastructure of Mauthner cell synapses and nodes in goldfish brains. J Cell Biol. 1963;19:159–199. doi: 10.1083/jcb.19.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennett MV, Pappas GD, Gimenez M, Nakajima Y. Physiology and ultrastructure of electrotonic junctions. IV. Medullary electromotor nuclei in gymnotid fish. J Neurophysiol. 1967;30:236–300. doi: 10.1152/jn.1967.30.2.236. [DOI] [PubMed] [Google Scholar]
- 7.Bennett MVL, Pappas GD, Aljure E, Nakajima Y. Physiology and ultrastructure of electrotonic junctions. II. Spinal and medullary electromotor nuclei in mormyrid fish. J Neurophysiol. 1967;30:180–208. doi: 10.1152/jn.1967.30.2.180. [DOI] [PubMed] [Google Scholar]
- 8.Kriebel ME, Bennett MVL, Waxman SG, Pappas GD. Oculomotor neurons in fish: electrotonic coupling and multiple sites of impulse initiation. Science. 1969;166:520–524. doi: 10.1126/science.166.3904.520. [DOI] [PubMed] [Google Scholar]
- 9.Martin AR, Pilar G. Transmission through the ciliary ganglion of the chick. J Physiol (London) 1963;168:464–475. doi: 10.1113/jphysiol.1963.sp007203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pereda AE, Bennett MVL. Electrical synapses in fish: relevance to synaptic transmission. In: Jing J, editor. Electrical Coupling and Microcircuits: Network Functions and Plasticity. 2017. in press. [Google Scholar]
- 11.Bartelmez GW, Hoerr NL. The vestibular club endings in Ameiurus. Further evidence on the morphology of the synapse. J Comp Neurol. 1933;57:401–428. [Google Scholar]
- 12.Furshpan EJ. “Electrical transmission” at an excitatory synapse in a vertebrate brain. Science. 1964;144:878–880. doi: 10.1126/science.144.3620.878. [DOI] [PubMed] [Google Scholar]
- 13.Lin JW, Faber DS. Synaptic transmission mediated by single club endings on the goldfish Mauthner cell. I. Characteristics of electrotonic and chemical postsynaptic potentials. J Neurosci. 1988;8:1302–1312. doi: 10.1523/JNEUROSCI.08-04-01302.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rovainen CM. Synaptic interactions of reticulospinal neurons and nerve cells in the spinal cord of the sea lamprey. J Comp Neurol. 1974;154:207–224. doi: 10.1002/cne.901540207. [DOI] [PubMed] [Google Scholar]
- 15.Rovainen CM. Synaptic interactions of identified nerve cells in the spinal cord of the sea lamprey. J Comp Neurol. 1974;154:189–206. doi: 10.1002/cne.901540206. [DOI] [PubMed] [Google Scholar]
- 16.Brodin L, Christenson J, Grillner S. Single sensory neurons activate excitatory amino acid receptors in the lamprey spinal cord. Neurosci Let. 1987;75:75–79. doi: 10.1016/0304-3940(87)90078-4. [DOI] [PubMed] [Google Scholar]
- 17.Shapovalov AI, Shiriaev BI. Electrical coupling between primary afferents and amphibian motoneurons. Exptl Brain Res. 1978;33:299–312. doi: 10.1007/BF00235555. [DOI] [PubMed] [Google Scholar]
- 18.Shapovalov AP. Evolution of the mechanisms of connection between neurons: Electrical, mixed, and chemical synapses. Neurosci Behav Physiol. 1982;12:169–176. doi: 10.1007/BF01189329. [DOI] [PubMed] [Google Scholar]
- 19.Babalian AL, Shapovalov AI. Mode of synaptic transmission between vestibular afferents and neurons of the vestibular nucleus in the frog. Brain Res. 1984;309:163–167. doi: 10.1016/0006-8993(84)91023-0. [DOI] [PubMed] [Google Scholar]
- 20.Shapovalov AI, Shiriaev BI, Velumian AA. A mechanism of post-synaptic excitation in amphibian motoneurons. J Physiol (London) 1978;279:437–455. doi: 10.1113/jphysiol.1978.sp012355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shapovalov AI, Shiriaev BI. Two types of electrotonic EPSP evoked in amphibian motoneurones by ventral root stimulation. Exp Brain Res. 1978;33:313–323. doi: 10.1007/BF00235556. [DOI] [PubMed] [Google Scholar]
- 22.Korn H, Sotelo C, Bennett MVL. The lateral vestibular nucleus of the toadfish Opsanus tau: Ultrastructural and electrophysiological observations with special reference to electrotonic transmission. Neuroscience. 1977;2:851–884. [Google Scholar]
- 23.Grillner S. Biological pattern generation: the cellular and computational logic of networks in motion. Neuron. 2006;52:751–766. doi: 10.1016/j.neuron.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 24.Korn H, Sotelo C, Crepel F. Electrotonic coupling between neurons in the rat lateral vestibular nucleus. Exp Brain Res. 1973;16:255–275. [PubMed] [Google Scholar]
- 25.Sotelo C, Korn H. Morphological correlates of electrical and other interactions through low-resistance pathways between neurons of the vertebrate central nervous system. Internat Rev Cytol. 1978;55:67–107. doi: 10.1016/s0074-7696(08)61887-2. [DOI] [PubMed] [Google Scholar]
- 26.Barr L, Dewey MM, Berger W. Propagation of action potentials and the structure of the nexus in cardiac muscle. J Gen Physiol. 1965;48:797–823. doi: 10.1085/jgp.48.5.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dewey MM, Barr L. Intercellular connections between smooth muscle cells: The nexus. Science. 1962;137:670–672. doi: 10.1126/science.137.3531.670-a. [DOI] [PubMed] [Google Scholar]
- 28.Dewey MM, Barr L. A study of the structure and distribution of the nexus. J Cell Biol. 1964;23:553–585. doi: 10.1083/jcb.23.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loewenstein WR. Permeability of membrane junctions. Ann N Y Acad Sci. 1966:441–472. doi: 10.1111/j.1749-6632.1966.tb50175.x. [DOI] [PubMed] [Google Scholar]
- 30.Rash JE, Fambrough D. Ultrastructural and electrophysiological correlates of cell coupling and cytoplasmic fusion during myogenesis in vitro. Develop Biol. 1973;30:166–186. doi: 10.1016/0012-1606(73)90055-9. [DOI] [PubMed] [Google Scholar]
- 31.Rash JE, Staehelin LA. Freeze-cleave demonstration of gap junctions between skeletal myogenic cells in vivo. Develop Biol. 1974;36:455–461. doi: 10.1016/0012-1606(74)90066-9. [DOI] [PubMed] [Google Scholar]
- 32.Hinrichsen CFL, Larramendi LMH. Synapses and cluster formation of the mouse mesencephalic fifth nucleus. Brain Res. 1968;7:296–299. doi: 10.1016/0006-8993(68)90105-4. [DOI] [PubMed] [Google Scholar]
- 33.Sotelo C, Palay SL. The fine structure of the lateral vestibular nucleus in the rat. II. Synaptic organization. Brain Res. 1970;18:93–115. doi: 10.1016/0006-8993(70)90459-2. [DOI] [PubMed] [Google Scholar]
- 34.Hinrichsen CFL. Coupling between cells of the trigeminal mesencephalic nucleus. J Dental Res. 1970;49:1369–1373. doi: 10.1177/00220345700490063701. [DOI] [PubMed] [Google Scholar]
- 35.Baker R, Llinás R. Electrotonic coupling between neurones in the rat mesencephalic nucleus. J Physiol. 1971;212:45–63. doi: 10.1113/jphysiol.1971.sp009309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pinching AJ, Powell TPS. The neuropil of the glomeruli of the olfactory bulb. J Cell Science. 1971;9:347–377. doi: 10.1242/jcs.9.2.347. [DOI] [PubMed] [Google Scholar]
- 37.Sotelo C, Llinás R. Specialized membrane junctions between neurons in the vertebrate cerebellar cortex. J Cell Biol. 1972;53:271–289. doi: 10.1083/jcb.53.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raviola E, Gilula NB. Gap junctions between photoreceptor cells in the vertebrate retina. Proc Natl Acad Sci (USA) 1973;70:1677–1681. doi: 10.1073/pnas.70.6.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wylie RM. Evidence of electrotonic transmission in the vestibular nuclei of the rat. Brain Res. 1973;50:179–183. doi: 10.1016/0006-8993(73)90605-7. [DOI] [PubMed] [Google Scholar]
- 40.Sotelo C, Llinás R, Baker R. Structural study of inferior olivary nucleus of the cat: Morphological correlates of electrotonic coupling. J Neurophysiol. 1974;37:541–559. doi: 10.1152/jn.1974.37.3.541. [DOI] [PubMed] [Google Scholar]
- 41.Llinás R, Baker R, Sotelo C. Electronic coupling between neurons in cat inferior olive. J Neurophysiol. 1974;37:560–571. doi: 10.1152/jn.1974.37.3.560. [DOI] [PubMed] [Google Scholar]
- 42.Gogan P, Gueritaud JP, Horcholle-Bossavit G, Tyc-Dumont S. Electrotonic coupling between motoneurones in the abducens nucleus of the cat. Exp Brain Res. 1974;21:139–154. doi: 10.1007/BF00234386. [DOI] [PubMed] [Google Scholar]
- 43.Matthews B, Holland GR. Coupling between nerves in teeth. Brain Res. 1975;98:354–358. doi: 10.1016/0006-8993(75)90013-x. [DOI] [PubMed] [Google Scholar]
- 44.Sotelo C. Morphological correlates of electrotonic coupling between neurons in mammalian nervous system. Raven Press; New York: 1975. pp. 355–365. [Google Scholar]
- 45.Matthews B. Proceedings: Coupling between cutaneous nerves. J Physiol. 1976;254:37P–38P. [PubMed] [Google Scholar]
- 46.Sotelo C, Gentschev T, Zamora AJ. Gap junctions in ventral cochlear nucleus of the rat. A possible new example of electrotonic junctions in the mammalian CNS. Neurosci. 1976;1:5-IN2. doi: 10.1016/0306-4522(76)90041-5. [DOI] [PubMed] [Google Scholar]
- 47.Gwyn DG, Nicholson GP, Flumerfelt BA. The inferior olivary nucleus of the rat: a light and electron microscopic study. J Comp Neurol. 1977;174:489–519. doi: 10.1002/cne.901740305. [DOI] [PubMed] [Google Scholar]
- 48.Rutherford JG, Gwyn DG. Gap junctions in the inferior olivary nucleus of the squirrel monkeySaimiri sciureus. Brain Research. 1977;128:374–378. doi: 10.1016/0006-8993(77)91004-6. [DOI] [PubMed] [Google Scholar]
- 49.Sloper JJ, Powell TPS. Gap junctions between dendrites and somata of neurons in the primate sensori-motor cortex. Proc R Soc London,Ser B. 1978;203:39–47. doi: 10.1098/rspb.1978.0089. [DOI] [PubMed] [Google Scholar]
- 50.Korn H, Faber DS. In: Electrical interactions between vertebrate neurons: field effects and electrotonic coupling. Schmitt FO, Worden FG, editors. MIT Press; Cambridge: 1979. pp. 333–358. [Google Scholar]
- 51.MacVicar BA, Dudek FE. Dye coupling between CA3 pyramidal cells in slices of rat hippocampus. Brain Res. 1980;196:494–497. doi: 10.1016/0006-8993(80)90413-8. [DOI] [PubMed] [Google Scholar]
- 52.MacVicar BA, Dudek FE. Electrotonic coupling between pyramidal cells: A direct demonstration in rat hippocampal slices. Science. 1981;213:782–785. doi: 10.1126/science.6266013. [DOI] [PubMed] [Google Scholar]
- 53.Andrew RD, MacVicar BA, Dudek FE, Hatton GI. Dye transfer through gap junctions between neuroendocrine cells of rat hypothalamus. Science. 1981;211:1187–1189. doi: 10.1126/science.7466393. [DOI] [PubMed] [Google Scholar]
- 54.Sotelo C, Triller A. Morphological correlates of electrical, chemical and dual modes of transmission. Chemical neurotransmission. 1981;75:13–28. [Google Scholar]
- 55.Andrew RD, Taylor CP, Snow RW, Dudek FE. Coupling in rat hippocampal slices: dye transfer between CA1 pyramidal cells. Brain Res Bull. 1982;8:211–222. doi: 10.1016/0361-9230(82)90048-x. [DOI] [PubMed] [Google Scholar]
- 56.MacVicar BA, Dudek FE. Electrotonic coupling between granule cells of rat dentate gyrus: Physiological and anatomical evidence. J Neurophysiol. 1982;47:579–592. doi: 10.1152/jn.1982.47.4.579. [DOI] [PubMed] [Google Scholar]
- 57.Brenan A, Matthews B. Coupling between nerve fibers supplying normal and injured skin in the cat. J Physiol (London) 1983;334:70–71. [Google Scholar]
- 58.Kosaka T. Gap junctions between non-pyramidal cell dendrites in the rat hippocampus (CA1 and CA3 regions) Brain Res. 1983;271:157–161. doi: 10.1016/0006-8993(83)91377-x. [DOI] [PubMed] [Google Scholar]
- 59.Kosaka T. Neuronal gap junctions in the polymorph layer of the rat dentate gyrus. Brain Res. 1983;277:347–351. doi: 10.1016/0006-8993(83)90943-5. [DOI] [PubMed] [Google Scholar]
- 60.Grace AA, Bunney BS. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons-1. Identification and characterization. Neuroscience. 1983;10:301–315. doi: 10.1016/0306-4522(83)90135-5. [DOI] [PubMed] [Google Scholar]
- 61.Wouterlood FG, Mugnaini E, Osen KK, Dahl AL. Stellate neurons in rat dorsal cochlear nucleus studied with combined Golgi impregnation and electron microscopy: synaptic connections and mutual coupling by gap junctions. J Neurocytol. 1984;13:639–664. doi: 10.1007/BF01148083. [DOI] [PubMed] [Google Scholar]
- 62.Kosaka T, Hama K. Gap junctions between non-pyramidal cell dendrites in the rat hippocampus (CA1 and CA3 regions): a combined golgi-electron microscopy study. J Comp Neurol. 1985;231:150–161. doi: 10.1002/cne.902310203. [DOI] [PubMed] [Google Scholar]
- 63.Cobbett P, Smithson KG, Hatton GI. Dye-coupled magnocellular peptidergic neurons of the rat para ventricular nucleus show homotypic immunoreactivity. Neuroscience. 1985;16:885–895. doi: 10.1016/0306-4522(85)90103-4. [DOI] [PubMed] [Google Scholar]
- 64.Meyer RA, Raja SN, Campbell JN. Coupling of action potential activity between unmyelinated fibers in the peripheral nerve of monkey. Science. 1985;227:184–187. doi: 10.1126/science.3966152. [DOI] [PubMed] [Google Scholar]
- 65.Sotelo C, Gotow T, Wassef M. Localization of glutamic–acid–decarboxylase–immunoreactive axon terminals in the inferior olive of the rat, with special emphasis on anatomical relations between GABAergic synapses and dendrodendritic gap junctions. J Comp Neurol. 1986;252:32–50. doi: 10.1002/cne.902520103. [DOI] [PubMed] [Google Scholar]
- 66.Meyer RA, Campbell JN. Coupling between unmyelinated peripheral nerve fibers does not involve sympathetic efferent fibers. Brain Res. 1987;437:181–182. doi: 10.1016/0006-8993(87)91542-3. [DOI] [PubMed] [Google Scholar]
- 67.Hatton GI, Yang QZ, Smithson KG. Synaptic inputs and electrical coupling among magnocellular neuroendocrine cells. Brain Res Bull. 1988;20:751–755. doi: 10.1016/0361-9230(88)90087-1. [DOI] [PubMed] [Google Scholar]
- 68.Katsumaru H, Kosaka T, Heizmann CW, Hama K. Gap junctions on GABAergic neurons containing the calcium-binding protein parvalbumin in the rat hippocampus (CA1 region) Exp Brain Res. 1988;72:363–370. doi: 10.1007/BF00250257. [DOI] [PubMed] [Google Scholar]
- 69.Baimbridge KG, Peet MJ, McLennan H, Church J. Bursting response to current-evoked depolarization in rat CA1 pyramidal neurons is correlated with lucifer yellow dye coupling but not with the presence of calbindin–D28k. Synapse. 1991;7:269–277. doi: 10.1002/syn.890070404. [DOI] [PubMed] [Google Scholar]
- 70.Church J, Baimbridge KG. Exposure to high-pH medium increases the incidence and extent of dye coupling between rat hippocampal CA1 pyramidal neurons in vitro. J Neurosci. 1991;11:3289–3295. doi: 10.1523/JNEUROSCI.11-10-03289.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reyher CK, Lubke J, Larsen WJ, Hendrix GM, Shipley MT, Baumgarten HG. Olfactory bulb granule cell aggregates: morphological evidence for interperikaryal electrotonic coupling via gap junctions. J Neurosci. 1991;11:1485–1495. doi: 10.1523/JNEUROSCI.11-06-01485.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vaney DI. Many diverse types of retinal neurons show tracer coupling when injected with biocytin or Neurobiotin. Neurosci Let. 1991;125:187–190. doi: 10.1016/0304-3940(91)90024-n. [DOI] [PubMed] [Google Scholar]
- 73.Rash JE, Dillman RK, Bilhartz BL, Duffy HS, Whalen LR, Yasumura T. Mixed synapses discovered and mapped throughout mammalian spinal cord. Proc Natl Acad Sci (USA) 1996;93:4235–4239. doi: 10.1073/pnas.93.9.4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Van der Want JJL, Gramsbergen A, Ijkema-Paassen J, De Weerd H, Liem RSB. Dendro-dendritic connections between motoneurons in the rat spinal cord: an electron microscopic investigation. Brain Res. 1998;779:342–345. doi: 10.1016/s0006-8993(97)01238-9. [DOI] [PubMed] [Google Scholar]
- 75.Koos T, Tepper JM. Inhibitory control of neostriatal projection neurons by GABAergic interneurons. Nat Neurosci. 1999;2:467–472. doi: 10.1038/8138. [DOI] [PubMed] [Google Scholar]
- 76.Nagy JI, Dermietzel R. In: Gap junctions and connexins in the mammalian central nervous system. Hertzberg EL, editor. JAI Press; Greenwich: 2000. pp. 323–396. [Google Scholar]
- 77.Bennett MVL. A comparison of electrically and chemically mediated transmission. In: Pappas GD, Purpura DP, editors. Structure and Function of Synapses. Raven Press; NY: 1972. pp. 221–256. [Google Scholar]
- 78.Bennett MVL. Flexibility and rigidity in electrotonically coupled systems. In: Bennett MVL, editor. Synaptic Transmission and Neuronal Interaction. Raven Press; NY: 1974. pp. 153–178. [Google Scholar]
- 79.Bennett MVL. Electrical transmission: a functional analysis and comparison with chemical transmission. In: Kandel ER, editor. Handbook of Physiology, Section I, The Nervous System, Vol. 1, Cellular Biology of Neurons, Part 1. American Physiological Society; Bethesda: 1977. pp. 357–416. [Google Scholar]
- 80.Bennett MVL, Goodenough DA. Gap junctions, electrotonic coupling and intercellular communication. Neuroscie Res Prog Bulletin. 1978;16:373–486. [PubMed] [Google Scholar]
- 81.Sabatini DD, Bench K, Barrnett RJ. Cytochemistry and electron microscopy. The preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. J Cell Biol. 1963;17:19–58. doi: 10.1083/jcb.17.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kellenberger E, Ryter A, Sechaud J. Electron microscope study of DNA containing plasma. II. Vegatative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958;4:671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rash JE, Yasumura T, Dudek FE. Ultrastructure, histological distribution, and freeze-fracture immunocytochemistry of gap junctions in rat brain and spinal cord. Cell Biol Internat. 1998;22:731–749. doi: 10.1006/cbir.1998.0392. [DOI] [PubMed] [Google Scholar]
- 84.Kamasawa N, Furman CS, Davidson KGV, Sampson JA, Magnie AR, Gebhardt B, Kamasawa M, Morita M, Yasumura T, Pieper M, Zumbrunnen JR, Pickard GE, Nagy JI, Rash JE. Abundance and ultrastructural diversity of neuronal gap junctions in the OFF and ON sublaminae of the inner plexiform layer of rat and mouse retina. Neuroscience. 2006;142:1093–1117. doi: 10.1016/j.neuroscience.2006.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Loewenstein WR. Junctional intercellular communication: the cell-to-cell membrane channel. Physiol Rev. 1981;61:829–913. doi: 10.1152/physrev.1981.61.4.829. [DOI] [PubMed] [Google Scholar]
- 86.Shepherd GM. Synapse. Vol. 4. Oxford University Press; New York: 1994. pp. 1–784. [Google Scholar]
- 87.Matsumoto A, Arnold AP, Zampighi G, Micevych PE. Androgenic regulation of gap junctions between motoneurons in the rat spinal cord. J Neurosci. 1988;8:4177–4138. doi: 10.1523/JNEUROSCI.08-11-04177.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Matsumoto A, Arnold AP, Micevych PE. Gap junctions between lateral spinal motoneurons in the rat. Brain Res. 1989;495:362–366. doi: 10.1016/0006-8993(89)90229-1. [DOI] [PubMed] [Google Scholar]
- 89.Pappas GD, Bennett MVL. Specialized junctions involved in electrical transmission between neurons. Ann N Y Acad Sci. 1966;166:495–508. doi: 10.1111/j.1749-6632.1966.tb50177.x. [DOI] [PubMed] [Google Scholar]
- 90.Spray DC, Bennett MVL. Physiology and pharmacology of gap junctions. Annu Rev Physiol. 1985;47:281–303. doi: 10.1146/annurev.ph.47.030185.001433. [DOI] [PubMed] [Google Scholar]
- 91.Pereda A, Rash JE, Nagy JI, Bennett MVL. Dynamics of electrical transmission at club endings on the Mauthner cells. Brain Res Brain Res Rev. 2004;47:227–244. doi: 10.1016/j.brainresrev.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 92.Bennett MVL. Electrical synapses, a personal perspective (or history) Brain Res Rev. 2000;32:16–28. doi: 10.1016/s0165-0173(99)00065-x. [DOI] [PubMed] [Google Scholar]
- 93.Rash JE, Kamasawa N, Vanderpool KG, Yasumura T, O'Brien J, Nannapaneni S, Pereda A, Nagy JI. Heterotypic gap junctions at glutamatergic mixed synapses are abundant in goldfish brain. Neuroscience. 2014;285:166–193. doi: 10.1016/j.neuroscience.2014.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bennett MVL. Gap junctions as electrical synapses. J Neurocytol. 1997;26:349–366. doi: 10.1023/a:1018560803261. [DOI] [PubMed] [Google Scholar]
- 95.Rash JE, Olson C, Davidson KGV, Yasumura T, Kamasawa N, Nagy JI. Identification of connexin36 in gap junctions between neurons in rodent locus coeruleus. Neuroscience. 2007;147:938–956. doi: 10.1016/j.neuroscience.2007.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rash JE, Olson CO, Pouliot WA, Davidson KGV, Yasumura T, Furman CS, Royer S, Kamasawa N, Nagy JI, Dudek FE. Connexin36, miniature neuronal gap junctions, and limited electrotonic coupling in rodent suprachiasmatic nucleus. Neuroscience. 2007;149:350–371. doi: 10.1016/j.neuroscience.2007.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gilula NB, Reeves OR, Steinbach A. Metabolic coupling, ionic coupling and cell contacts. Nature. 1972;235:262–265. doi: 10.1038/235262a0. [DOI] [PubMed] [Google Scholar]
- 98.Yuste R, Peinado A, Katz LC. Neuronal domains in developing neocortex. Science. 1992;257:665–669. doi: 10.1126/science.1496379. [DOI] [PubMed] [Google Scholar]
- 99.Peinado A, Yuste R, Katz LC. Extensive dye coupling between rat neocortical neurons during the period of circuit formation. Neuron. 1993;10:103–114. doi: 10.1016/0896-6273(93)90246-n. [DOI] [PubMed] [Google Scholar]
- 100.Kandler K, Katz LC. Coordination of neuronal activity in developing visual cortex by gap junction- mediated biochemical communication. J Neurosci. 1998;18:1419–1427. doi: 10.1523/JNEUROSCI.18-04-01419.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Penn AA, Wong RO, Shatz CJ. Neuronal coupling in the developing mammalian retina. J Neurosci. 1994;14:3805–3815. doi: 10.1523/JNEUROSCI.14-06-03805.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Walton KD, Navarette R. Postnatal changes in motoneurone electronic coupling studied in the in vitro rat lumbar spinal cord. J Physiol. 1991;433:283–305. doi: 10.1113/jphysiol.1991.sp018426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Peinado A, Yuste R, Katz LC. Gap junctional communication and the development of local circuits in neocortex. Cereb Cortex. 1993;3:488–498. doi: 10.1093/cercor/3.5.488. [DOI] [PubMed] [Google Scholar]
- 104.Fukuda T, Kosaka T. The dual network of GABAergic interneurons linked by both chemical and electrical synapses: a possible infrastructure of the cerebral cortex. Neurosci Res. 2000;38:123–130. doi: 10.1016/s0168-0102(00)00163-2. [DOI] [PubMed] [Google Scholar]
- 105.Fukuda T, Kosaka T. Gap junctions linking the dendritic network of GABAergic interneurons in the hippocampus. J Neurosci. 2000;20:1519–1528. doi: 10.1523/JNEUROSCI.20-04-01519.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Söhl G, Degen J, Teubner B, Willecke K. The murine gap junction gene connexin36 is highly expressed in mouse retina and regulated during brain development. FEBS Letters. 1998;428:27–31. doi: 10.1016/s0014-5793(98)00479-7. [DOI] [PubMed] [Google Scholar]
- 107.Condorelli DF, Parenti R, Spinella F, Salinaro AT, Belluardo N, Cardile V, Cicirata F. Cloning of a new gap junction gene (Cx36) highly expressed in mammalian brain neurons. Eur J Neurosci. 1998;10:1202–1208. doi: 10.1046/j.1460-9568.1998.00163.x. [DOI] [PubMed] [Google Scholar]
- 108.Condorelli DF, Belluardo N, Trovato-Salinaro A, Mudo G. Expression of Cx36 in mammalian neurons. Brain Res Brain Res Rev. 2000;32:72–85. doi: 10.1016/s0165-0173(99)00068-5. [DOI] [PubMed] [Google Scholar]
- 109.Rash JE, Yasumura T, Dudek FE, Nagy JI. Cell-specific expression of connexins, and evidence for restricted gap junctional coupling between glial cells and between neurons. J Neurosci. 2001;21:1983–2001. doi: 10.1523/JNEUROSCI.21-06-01983.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rash JE, Yasumura T, Davidson K, Furman CS, Dudek FE, Nagy JI. Identification of cells expressing Cx43, Cx30, Cx26, Cx32 and Cx36 in gap junctions of rat brain and spinal cord. Cell Commun Adhes. 2001;8:315–320. doi: 10.3109/15419060109080745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rash JE, Staines WA, Yasumura T, Patel D, Hudson CS, Stelmack GL, Nagy JI. Immunogold evidence that neuronal gap junctions in adult rat brain and spinal cord contain connexin36 (Cx36) but not Cx32 or Cx43. Proc Natl Acad Sci (USA) 2000;97:7573–7578. doi: 10.1073/pnas.97.13.7573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.O'Brien J, Al-Ubaidi MR, Ripps H. Connexin35: a gap junctional protein expressed preferentially in the skate retina. Mol Biol Cell. 1996;7:233–243. doi: 10.1091/mbc.7.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.O'Brien J, Nguyen HB, Mills SL. Cone photoreceptors in bass retina use two connexins to mediate electrical coupling. J Neurosci. 2004;24:5632–5642. doi: 10.1523/JNEUROSCI.1248-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pereda A, O'Brien J, Nagy JI, Bukauskas F, Davidson KGV, Kamasawa N, Yasumura T, Rash JE. Connexin35 mediates electrical transmission at mixed synapses on Mauthner cells. J Neurosci. 2003;23:7489–7503. doi: 10.1523/JNEUROSCI.23-20-07489.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Alvarez-Maubecin V, Garcia-Hernandez F, Williams JT, Van Bockstaele EJ. Functional coupling between neurons and glia. J Neurosci. 2000;20:4091–4098. doi: 10.1523/JNEUROSCI.20-11-04091.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Colwell CS. Rhythmic coupling among cells in the suprachiasmatic nucleus. J Neurobiol. 2000;43:379–388. doi: 10.1002/1097-4695(20000615)43:4<379::aid-neu6>3.0.co;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dermietzel R, Traub O, Hwang TK, Beyer E, Bennett MVL, Spray DC, Willecke K. Differential expression of three gap junction proteins in developing and mature brain tissues. Proc Natl Acad Sci (USA) 1989;86:10148–10152. doi: 10.1073/pnas.86.24.10148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dermietzel R, Spray DC. Gap junctions in the brain: Where, what type, how many and why? Trends Neurosci. 1993;16:186–192. doi: 10.1016/0166-2236(93)90151-b. [DOI] [PubMed] [Google Scholar]
- 119.Micevych PE, Abelson L. Distribution of mRNAs coding for liver and heart gap junction proteins in the rat central nervous system. J Comp Neurol. 1991;305:96–118. doi: 10.1002/cne.903050110. [DOI] [PubMed] [Google Scholar]
- 120.Matsumoto A, Arai Y, Urano A, Hyodo S. Effect of androgen on the expresssion of gap junction and beta-actin mRNAs in adult rat motoneurons. Neurosci Res. 1992;14:133–144. doi: 10.1016/0168-0102(92)90089-u. [DOI] [PubMed] [Google Scholar]
- 121.Micevych PE, Popper P, Hatton GI. Connexin 32 mRNA levels in the rat supraoptic nucleus; up- regulation prior to parturition and during lactation. Neuroendocrinology. 1996;63:39–45. doi: 10.1159/000126933. [DOI] [PubMed] [Google Scholar]
- 122.Teubner B, Odermatt B, Guldenagel M, Sohl G, Degen J, Bukauskas FF, Kronengold J, Veselis VK, Jung YT, Kosak CA, Schilling K, Willecke K. Functional expression of the new gap junction gene connexin47 transcribed in mouse brain and spinal cord neurons. J Neurosci. 2001;21:1117–1126. doi: 10.1523/JNEUROSCI.21-04-01117.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chang Q, Gonzalez M, Pinter MJ, Balice-Gordon RJ. Gap junctional coupling and patterns of connexin expression among neonatal rat lumbar spinal neurons. J Neurosci. 1999;19:10813–10828. doi: 10.1523/JNEUROSCI.19-24-10813.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chang Q, Balice-Gordon RJ. Gap junctional communication among developing and injured motor neurons. Brain Res Rev. 2000;32:242–249. doi: 10.1016/s0165-0173(99)00085-5. [DOI] [PubMed] [Google Scholar]
- 125.Honma S, De S, Li D, Shuler CF, Turman JE. Developmental regulation of connexins 26, 32, 36, and 43 in trigeminal neurons. Synapse. 2004;52:258–271. doi: 10.1002/syn.20022. [DOI] [PubMed] [Google Scholar]
- 126.Kloosterman WP, Wienholds E, de Bruijn E, Kauppinen S, Plasterk RHA. In situ detection of miRNAs in animal embryos using LNA-modified oligonucleotide probes. Nat Methods. 2006;3:12. doi: 10.1038/nmeth843. [DOI] [PubMed] [Google Scholar]
- 127.Smirnova L, Grafe A, Seiler A, Schumacher S, Nitsch R, Wulczyn FG. Regulation of miRNA expression during neural cell specification. Eur J Neurosci. 2005;21:1469–1477. doi: 10.1111/j.1460-9568.2005.03978.x. [DOI] [PubMed] [Google Scholar]
- 128.Rash JE, Davidson KGV, Kamasawa N, Yasumura T, Kamasawa M, Zhang C, Michaels R, Restrepo D, Ottersen OP, Olson C, Nagy JI. Ultrastructural localization of connexins (Cx36, Cx43, Cx45), glutamate receptors and aquaporin-4 in rodent olfactory mucosa, olfactory nerve and olfactory bulb. J Neurocytol. 2005;34:307–341. doi: 10.1007/s11068-005-8360-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dedek K, Schultz K, Pieper M, Dirks P, Maxeiner S, Willecke K, Weiler R, Janssen-Bienhold U. Localization of heterotypic gap junctions composed of connexin45 and connexin36 in the rod pathway of the mouse retina. Eur J Neurosci. 2006;24:1675–1686. doi: 10.1111/j.1460-9568.2006.05052.x. [DOI] [PubMed] [Google Scholar]
- 130.Maxeiner S, Dedek K, Janssen-Bienhold U, Ammermuller J, Brune H, Kirsch T, Pieper M, Degen J, Kruger O, Willecke K, Weiler R. Deletion of connexin45 in mouse retinal neurons disrupts the rod/cone signaling pathway between AII amacrine and ON cone bipolar cells and leads to impaired visual transmission. J Neurosci. 2005;25:566–576. doi: 10.1523/JNEUROSCI.3232-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schubert T, Maxeiner S, Kruger O, Willecke K, Weiler R. Connexin45 mediates gap junctional coupling of bistratified ganglion cells in the mouse retina. J Comp Neurol. 2005;490:29–39. doi: 10.1002/cne.20621. [DOI] [PubMed] [Google Scholar]
- 132.Van Der Giessen RS, Maxeiner S, French PJ, Willecke K, De Zeeuw CI. Spatiotemporal distribution of Connexin45 in the olivocerebellar system. J Comp Neurol. 2006;495:173–184. doi: 10.1002/cne.20873. [DOI] [PubMed] [Google Scholar]
- 133.Hombach S, Janssen-Bienhold U, Sohl G, Schubert T, Bussow H, Ott T, Weiler R, Willecke K. Functional expression of connexin57 in horizontal cells of the mouse retina. Eur J Neurosci. 2004;19:2633–2640. doi: 10.1111/j.0953-816X.2004.03360.x. [DOI] [PubMed] [Google Scholar]
- 134.Palacios-Prado N, Sonntag S, Skeberdis VA, Willecke K, Bukauskas FF. Gating, permselectivity and pH-dependent modulation of channels formed by connexin57, a major connexin of horizontal cells in the mouse retina. J Physiol. 2009;587:3251–3269. doi: 10.1113/jphysiol.2009.171496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ciolofan C, Lynn BD, Wellershaus K, Willecke K, Nagy JI. Spatial relationships of connexin36, connexin57 and zonula occludens-1 (ZO-1) in the outer plexiform layer of mouse retina. Neuroscience. 2007;148:473–488. doi: 10.1016/j.neuroscience.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 136.Li X, Kamasawa N, Ciolofan C, Olson CO, Lu S, Davidson KGV, Yasumura T, Shigemoto R, Rash JE, Nagy JI. Connexin45-containing neuronal gap junctions in rodent retina also contain connexin36 in both apposing hemiplaques, forming bi-homotypic gap junctions, with scaffolding contributed by zonula occludens-1. J Neurosci. 2008;28:9769–9789. doi: 10.1523/JNEUROSCI.2137-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rash JE, Pereda A, Kamasawa N, Furman CS, Yasumura T, Davidson KGV, Dudek FE, Olson C, Nagy JI. High-resolution proteomic mapping in the vertebrate central nervous system: Close proximity of connexin35 to NMDA glutamate receptor clusters and co-localization of connexin36 with immunoreactivity for zonula occludens protein-1 (ZO-1) J Neurocytol. 2004;33:131–152. doi: 10.1023/B:NEUR.0000029653.34094.0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nagy JI. Evidence for connexin36 localization at hippocampal mossy fiber terminals suggesting mixed chemical/electrical transmission by granule cells. Brain Res. 2012;1487:107–122. doi: 10.1016/j.brainres.2012.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.O'Brien JJ, Li W, Pan F, Keung J, O'Brien J, Massey SC. Coupling between A-type horizontal cells is mediated by connexin 50 gap junctions in the rabbit retina. J Neurosci. 2006;26:11624–11636. doi: 10.1523/JNEUROSCI.2296-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dorgau B, Herrling R, Schultz K, Greb H, Segelken J, Ströh S, Bolte P, Weiler R, Dedek K, Janssen-Bienhold U. Connexin50 couples axon terminals of mouse horizontal cells by homotypic gap junctions. J Comp Neurol. 2015;523:2062–2081. doi: 10.1002/cne.23779. [DOI] [PubMed] [Google Scholar]
- 141.Kreuzberg MM, Deuchars J, Weiss E, Schober A, Sonntag S, Wellershaus K, Draguhn A, Willecke K. Expression of connexin30. 2 in interneurons of the central nervous system in the mouse. Mol Cell Neurosci. 2008;37:119–134. doi: 10.1016/j.mcn.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 142.de Sevilla Müller LP, Dedek K, Janssen-Bienhold U, Meyer A, Kreuzberg M, Lorenz S, Willecke K, Weiler R. Expression and modulation of connexin30. 2, a novel gap junction protein in the mouse retina. Vis Neurosci. 2010;27:91–101. doi: 10.1017/S0952523810000131. [DOI] [PubMed] [Google Scholar]
- 143.Nagy JI, Bautista W, Blakley B, Rash JE. Morphologically mixed chemical-electrical synapses formed by primary afferents in rodent vestibular nuclei as revealed by immunofluorescence detection of connexin36 and vesicular glutamate transporter-1. Neuroscience. 2013;252:468–488. doi: 10.1016/j.neuroscience.2013.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bautista W, McCrea DA, Nagy JI. Connexin36 identified at morphologically mixed chemical/electrical synapses on trigeminal motoneurons and at primary afferent terminals on spinal cord neurons in adult mouse and rat. Neuroscience. 2014;263:159–180. doi: 10.1016/j.neuroscience.2013.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nagy JI, Dudek FE, Rash JE. Update on connexins and gap junctions in neurons and glia in the mammalian central nervous system. Brain Res Brain Res Rev. 2004;47:191–215. doi: 10.1016/j.brainresrev.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 146.Bautista W, Nagy JI, Dai Y, McCrea DA. Requirement of neuronal connexin36 in pathways mediating presynaptic inhibition of primary afferents in functionally mature mouse spinal cord. J Physiol. 2012;590:3821–3839. doi: 10.1113/jphysiol.2011.225987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bautista W, Nagy JI. Connexin36 in gap junctions forming electrical synapses between motoneurons in sexually dimorphic motor nuclei in spinal cord of rat and mouse. Eur J Neurosci. 2013;39:771–787. doi: 10.1111/ejn.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bautista W, Rash JE, Vanderpool KG, Yasumura T, Nagy JI. Re-evaluation of connexins associated with motoneurons in rodent spinal cord, sexually dimorphic motor nuclei and trigeminal motor nucleus. Eur J Neurosci. 2013;39:757–770. doi: 10.1111/ejn.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bennett MVL. Seeing is relieving: electrical synapses between visualized neurons. Nat Neurosci. 2000;3:7–9. doi: 10.1038/71082. [DOI] [PubMed] [Google Scholar]
- 150.Bennett MVL, Zukin RS. Electrical coupling and neuronal synchronization in the mammalian brain. Neuron. 2004;41:495–511. doi: 10.1016/s0896-6273(04)00043-1. [DOI] [PubMed] [Google Scholar]
- 151.Connors BW, Long MA. Electrical synapses in the mammalian brain. Ann Rev Neurosci. 2004;27:393–418. doi: 10.1146/annurev.neuro.26.041002.131128. [DOI] [PubMed] [Google Scholar]
- 152.Hormuzdi SG, Filippov MA, Mitropoulou G, Monyer H, Bruzzone R. Electrical synapses: a dynamic signaling system that shapes the activity of neuronal networks. Biochim Biophys Acta (BBA) - Biomembranes. 2004;1662:113–137. doi: 10.1016/j.bbamem.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 153.LeBeau FEN, Traub RD, Monyer H, Whittington MA, Buhl EH. The role of electrical signaling via gap junctions in the generation of fast network oscillations. Brain Res Bull. 2003;62:3–13. doi: 10.1016/j.brainresbull.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 154.Söhl G, Odermatt B, Maxeiner S, Degen J, Willecke K. New insights into the expression and function of neural connexins with transgenic mouse mutants. Brain Res Rev. 2004;47:245–259. doi: 10.1016/j.brainresrev.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 155.Söhl G, Maxeiner S, Willecke K. Expression and functions of neuronal gap junctions. Nat Rev Neurosci. 2005;6:191–200. doi: 10.1038/nrn1627. [DOI] [PubMed] [Google Scholar]
- 156.Frisch C, De Souza-Silva MA, Sohl G, Guldenagel M, Willecke K, Huston JP, Dere E. Stimulus complexity dependent memory impairment and changes in motor performance after deletion of the neuronal gap junction protein connexin36 in mice. Behav Brain Res. 2005;157:177–185. doi: 10.1016/j.bbr.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 157.Fujimoto K. Freeze-fracture replica electron microscopy combined with SDS digestion for cytochemical labeling of integral membrane proteins. Application to the immunogold labeling of intercellular junctional complexes. J Cell Sci. 1995;108:3443–3449. doi: 10.1242/jcs.108.11.3443. [DOI] [PubMed] [Google Scholar]
- 158.Fujimoto K. SDS-digested freeze-fracture replica labeling electron microscopy to study the two-dimensional distribution of integral membrane proteins and phospholipids in biomembranes: practical procedure, interpretation and application. Histochem Cell Biol. 1997;107:87–96. doi: 10.1007/s004180050092. [DOI] [PubMed] [Google Scholar]
- 159.Rash JE, Yasumura T. Direct immunogold labeling of connexins and aquaporin4 in freeze-fracture replicas of liver, brain and spinal cord: factors limiting quantitative analysis. Cell Tissue Res. 1999;296:307–321. doi: 10.1007/s004410051291. [DOI] [PubMed] [Google Scholar]
- 160.Gruijters WTM, Kistler J, Bullivant S, Goodenough DA. Immunolocalization of MP70 in lens fiber 16–17-nm intercellular junctions. J Cell Biol. 1987;104:565–572. doi: 10.1083/jcb.104.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Raviola E, Raviola G. Structure of the synaptic membranes in the inner plexiform layer of the retina: A freeze-fracture study in monkeys and rabbits. J Comp Neurol. 1982;209:233–248. doi: 10.1002/cne.902090303. [DOI] [PubMed] [Google Scholar]
- 162.Li X, Olson C, Lu S, Kamasawa N, Yasumura T, Rash JE, Nagy JI. Neuronal connexin36 association with zonula occludens-1 protein (ZO-1) in mouse brain and interaction with the first PDZ domain of ZO-1. Eur J Neurosci. 2004;19:2132–2146. doi: 10.1111/j.l460-9568.2004.03283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Galarreta M, Hestrin S. A network of fast-spiking cells in the neocortex connected by electrical synapses. Nature. 1999;402:72–75. doi: 10.1038/47029. [DOI] [PubMed] [Google Scholar]
- 164.Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402:75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- 165.Mann-Metzer P, Yarom Y. Electrotonic coupling interacts with intrinsic properties to generate synchronized activity in cerebellar networks of inhibitory interneurons. J Neurosci. 1999;19:3298–3306. doi: 10.1523/JNEUROSCI.19-09-03298.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sontheimer H, Ransom BR. Whole-cell patch-clamp recordings. In: Walz W, Boulton AA, Baker GB, editors. Patch-clamp analysis: advanced techniques. Springer Science & Business Media; 2002. pp. 35–67. [Google Scholar]
- 167.Dudek FE, Snow RW, Taylor CP. Role of electrical interactions in synchronization of epileptiform bursts. Adv Neurol. 1986;44:593–617. [PubMed] [Google Scholar]
- 168.Bukauskas FF. Neurons and β-cells of the pancreas express connexin36, forming gap junction channels that exhibit strong cationic selectivity. J Membr Biol. 2012;245:243–253. doi: 10.1007/s00232-012-9445-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Logan SD, Pickering AE, Gibson IC, Nolan MF, Spanswick D. Electrotonic coupling between rat sympathetic preganglionic neurones in vitro. J Physiol. 1996;495:491–502. doi: 10.1113/jphysiol.1996.sp021609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Logan SD, Spanswick D, Pickering AE, Gibson IC, Nolan MF, Cammack C. Gap junction coupling and the regulation of excitability in sympathetic preganglionic neurons. J Autonom Nerv Syst. 1996;58:202–206. [Google Scholar]
- 171.Van Essen TA, Van Der Giessen RS, Koekkoek SKE, VanderWerf F, De Zeeuw CI, Van Genderen PJJ, Overbosch D, De Jeu MTG. Anti-malaria drug mefloquine induces motor learning deficits in humans. Frontiers Neurosci. 2010;191:1–8. doi: 10.3389/fnins.2010.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Margineanu DG, Klitgaard H. The connexin 36 blockers quinine, quinidine and mefloquine inhibit cortical spreading depression in a rat neocortical slice model in vitro. Brain Res Bull. 2006;71:23–28. doi: 10.1016/j.brainresbull.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 173.Cruikshank SJ, Hopperstad M, Younger M, Connors BW, Spray DC, Srinivas M. Potent block of Cx36 and Cx50 gap junction channels by mefloquine. Proc Natl Acad Sci (USA) 2004;101:12364–12369. doi: 10.1073/pnas.0402044101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Pan F, Mills SL, Massey SC. Screening of gap junction antagonists on dye coupling in the rabbit retina. Vis Neurosci. 2007;24:609–618. doi: 10.1017/S0952523807070472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Veruki ML, Hartveit E. Meclofenamic acid blocks electrical synapses of retinal AII amacrine and on-cone bipolar cells. J Neurophysiol. 2009;101:2339–2347. doi: 10.1152/jn.00112.2009. [DOI] [PubMed] [Google Scholar]
- 176.Spencer WA, Kandel ER. Electrophysiology of hippocampal neurons: IV. Fast prepotentials. J Neurophysiol. 1961;24:272–285. doi: 10.1152/jn.1961.24.3.272. [DOI] [PubMed] [Google Scholar]
- 177.Mercer A, Bannister AP, Thomson AM. Electrical coupling between pyramidal cells in adult cortical regions. Brain Cell Biol. 2006;35:13–27. doi: 10.1007/s11068-006-9005-9. [DOI] [PubMed] [Google Scholar]
- 178.Vervaeke K, Lörincz A, Nusser Z, Silver RA. Gap junctions compensate for sublinear dendritic integration in an inhibitory network. Science. 2012;335:1624–1628. doi: 10.1126/science.1215101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Curti S, Hoge G, Nagy JI, Pereda AE. Synergy between electrical coupling and membrane properties promotes strong synchronization of neurons of the mesencephalic trigeminal nucleus. J Neurosci. 2012;32:4341–4359. doi: 10.1523/JNEUROSCI.6216-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Vervaeke K, Lörincz A, Gleeson P, Farinella M, Nusser Z, Silver RA. Rapid desynchronization of an electrically coupled interneuron network with sparse excitatory synaptic input. Neuron. 2010;67:435–451. doi: 10.1016/j.neuron.2010.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Chorev E, Brecht M. In vivo dual intra-and extracellular recordings suggest bidirectional coupling between CA1 pyramidal neurons. J Neurophysiol. 2012;108:1584–1593. doi: 10.1152/jn.01115.2011. [DOI] [PubMed] [Google Scholar]
- 182.Scholl B, Andoni S, Priebe NJ. Functional characterization of spikelet activity in the primary visual cortex. J Physiol. 2015;593:4979–4994. doi: 10.1113/JP270876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Llinás R, Sugimori M. Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices. J Physiol. 1980;305:171. doi: 10.1113/jphysiol.1980.sp013357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Golding NL, Spruston N. Dendritic sodium spikes are variable triggers of axonal action potentials in hippocampal CA1 pyramidal neurons. Neuron. 1998;21:1189–1200. doi: 10.1016/s0896-6273(00)80635-2. [DOI] [PubMed] [Google Scholar]
- 185.Kampa BM, Letzkus JJ, Stuart GJ. Requirement of dendritic calcium spikes for induction of spike–timing–dependent synaptic plasticity. J Physiol. 2006;574:283–290. doi: 10.1113/jphysiol.2006.111062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Fukuda T, Kosaka T, Singer W, Galuske RAW. Gap junctions among dendrites of cortical GABAergic neurons establish a dense and widespread intercolumnar network. J Neurosci. 2006;26:3434–3443. doi: 10.1523/JNEUROSCI.4076-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Bloomfield SA, Volgyi B. The diverse functional roles and regulation of neuronal gap junctions in the retina. Nature Rev Neurosci. 2009;10:495–506. doi: 10.1038/nrn2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Deans MR, Volgyi B, Goodenough DA, Bloomfield SA, Paul DL. Connexin36 is essential for transmission of rod-mediated visual signals in the mammalian retina. Neuron. 2002;36:703–712. doi: 10.1016/s0896-6273(02)01046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Guldenagel M, Ammermuller J, Feigenspan A, Teubner B, Degen Ji, Sohl G, Willecke K, Weiler R. Visual transmission deficits in mice with targeted disruption of the gap junction gene Connexin36. J Neurosci. 2001;21:6036–6044. doi: 10.1523/JNEUROSCI.21-16-06036.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Christie JM, Bark C, Hormuzdi SG, Helbig I, Monyer H, Westbrook GL. Connexin36 mediates spike synchrony in olfactory bulb glomeruli. Neuron. 2005;46:761–772. doi: 10.1016/j.neuron.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 191.Blatow M, Rozov A, Katona I, Hormuzdi SG, Meyer AH, Whittington MA, Caputi A, Monyer H. A novel network of multipolar bursting interneurons generates theta frequency oscillations in neocortex. Neuron. 2003;38:805–817. doi: 10.1016/s0896-6273(03)00300-3. [DOI] [PubMed] [Google Scholar]
- 192.Deans MR, Gibson JR, Sellitto C, Connors BW, Paul DL. Synchronous activity of inhibitory networks in neocortex requires electrical synapses containing connexin36. Neuron. 2001;31:477–485. doi: 10.1016/s0896-6273(01)00373-7. [DOI] [PubMed] [Google Scholar]
- 193.Diesmann M, Gewaltig MO, Aertsen A. Stable propagation of synchronous spiking in cortical neural networks. Nature. 1999;402:529–533. doi: 10.1038/990101. [DOI] [PubMed] [Google Scholar]
- 194.Hestrin S, Galarreta M. Electrical synapses define networks of neocortical GABAergic neurons. Trends Neurosci (TINS) 2005;28:304–309. doi: 10.1016/j.tins.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 195.Buhl DL, Harris KD, Hormuzdi SG, Monyer H, Buzsáki G. Selective impairment of hippocampal gamma oscillations in connexin-36 knock-out mouse in vivo. J Neurosci. 2003;23:1013–1018. doi: 10.1523/JNEUROSCI.23-03-01013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hormuzdi SG, Pais I, LeBeau FEN, Towers SK, Rozov A, Buhl EH, Whittington MA, Monyer H. Impaired electrical signaling disrupts gamma frequency oscillations in connexin 36-deficient mice. Neuron. 2001;31:487–495. doi: 10.1016/s0896-6273(01)00387-7. [DOI] [PubMed] [Google Scholar]
- 197.Maier N, Guldenagel M, Sohl G, Siegmund H, Willecke K, Draguhn A. Reduction of high-frequency network oscillations (ripples) and pathological network discharges in hippocampal slices from connexin 36-deficient mice. J Physiol. 2002;541:521–528. doi: 10.1113/jphysiol.2002.017624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Skinner FK, Zhang L, Perez-Velasquez JL, Carlen PL. Bursting in inhibitory interneuronal networks: a role for gap-junctional coupling. J Neurophysiol. 1999;81:1274–1283. doi: 10.1152/jn.1999.81.3.1274. [DOI] [PubMed] [Google Scholar]
- 199.Traub RD, Kopell N, Bibbig A, Buhl EH, LeBeau FEN, Whittington MA. Gap junctions between interneuron dendrites can enhance synchrony of gamma oscillations in distributed networks. J Neurosci. 2001;21:9478–9486. doi: 10.1523/JNEUROSCI.21-23-09478.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zsiros V, Maccaferri G. Electrical coupling between interneurons with different excitable properties in the stratum lacunosum-moleculare of the juvenile CA1 rat hippocampus. J Neurosci. 2005;25:8686–8695. doi: 10.1523/JNEUROSCI.2810-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Landisman CE, Long MA, Beierlein M, Deans MR, Paul D, Connors BW. Electrical synapses in the thalamic reticular nucleus. J Neurosci. 2002;22:1002–1009. doi: 10.1523/JNEUROSCI.22-03-01002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Long MA, Landisman CE, Connors BW. Small clusters of electrically coupled neurons generate synchronous rhythms in the thalamic reticular nucleus. J Neuroscience. 2004;24:341–349. doi: 10.1523/JNEUROSCI.3358-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Jiang ZG, Yang YQ, Allen CN. Tracer and electrical coupling of rat suprachiasmatic nucleus. Neurosci. 1997;77:1059–1066. doi: 10.1016/s0306-4522(96)00539-8. [DOI] [PubMed] [Google Scholar]
- 204.Long MA, Jutras MJ, Connors BW, Burwell RD. Electrical synapses coordinate activity in the suprachiasmatic nucleus. Nat Neurosci. 2005;8:61–66. doi: 10.1038/nn1361. [DOI] [PubMed] [Google Scholar]
- 205.Hatton GI. Function-related plasticity in hypothalamus. Ann Rev Neurosci. 1997;20:375–397. doi: 10.1146/annurev.neuro.20.1.375. [DOI] [PubMed] [Google Scholar]
- 206.Hatton GI, Zhao Yang Q. Peripartum interneuronal coupling in the supraoptic nucleus. Brain Res. 2002;932:120–123. doi: 10.1016/s0006-8993(02)02279-5. [DOI] [PubMed] [Google Scholar]
- 207.Yang QZ, Hatton GI. Direct evidence for electrical coupling among rat supraoptic nucleus neurons. Brain Res. 1988;463:47–56. doi: 10.1016/0006-8993(88)90525-2. [DOI] [PubMed] [Google Scholar]
- 208.Hoge G, Davidson KG, Yasumura T, Castillo PE, Rash JE, Pereda AE. The extent and strength of electrical coupling between inferior olivary neurons is heterogeneous. J Neurophysiol. 2011;105:1089–1101. doi: 10.1152/jn.00789.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Placantonakis DG, Bukovsky AA, Zeng XH, Kiem HP, Welsh JP. Fundamental role of inferior olive connexin 36 in muscle coherence during tremor. Pro Natl Acad Sci (USA) 2004;101:7164–7169. doi: 10.1073/pnas.0400322101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Placantonakis DG, Bukovsky AA, Aicher SA, Kiem HP, Welsh JP. Continuous electrical oscillations emerge from a coupled network: a study of the inferior olive using lentiviral knockdown of connexin36. J Neurosci. 2006;26:5008–5016. doi: 10.1523/JNEUROSCI.0146-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Leznik E, Linas RL. Role of gap junctions in synchronized neuronal oscillations in the inferior olive. J Neurophysiol. 2005;94:2447–2456. doi: 10.1152/jn.00353.2005. [DOI] [PubMed] [Google Scholar]
- 212.Blenkinsop TA, Lang EJ. Block of inferior olive gap junctional coupling decreases Purkinje cell complex spike synchrony and rhythmicity. J Neurosci. 2006;26:1739–1748. doi: 10.1523/JNEUROSCI.3677-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Sasaki K, Bower JM, Llinás R. Multiple Purkinje cell recording in rodent cerebellar cortex. Europ J Neurosci. 1989;1:572–586. doi: 10.1111/j.1460-9568.1989.tb00364.x. [DOI] [PubMed] [Google Scholar]
- 214.Van Der Giessen RS, Koekkoek SK, van Dorp S, De Gruijl JR, Cupido A, Khosrovani S, Dortland B, Wellershaus K, Degen J, Deuchars J, Fuchs EC, Monyer H, Willecke K, De Jeu MTG, De Zeeuw CI. Role of olivary electrical coupling in cerebellar motor learning. Neuron. 2008;58:599–612. doi: 10.1016/j.neuron.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 215.Marshall SP, Van Der Giessen RS, De Zeeuw CI, Lang J. Altered olivocerebellar activity patterns in the connexin36 knockout mouse. Cerebellum. 2007;6:287–299. doi: 10.1080/14734220601100801. [DOI] [PubMed] [Google Scholar]
- 216.De Gruijl JR, Hoogland TM, De Zeeuw CI. Behavioral correlates of complex spike synchrony in cerebellar microzones. J Neurosci. 2014;34:8937–8947. doi: 10.1523/JNEUROSCI.5064-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Bou-Flores C, Berger AJ. Gap junctions and inhibitory synapses modulate inspiratory motoneuron synchronization. J Neurophysiol. 2001;85:1543–1551. doi: 10.1152/jn.2001.85.4.1543. [DOI] [PubMed] [Google Scholar]
- 218.Rekling JC, Shao XM, Feldman JL. Electrical coupling and excitatory synaptic transmission between rhythmogenic respiratory neurons in the preBötzinger complex. J Neurosci. 2000;20:RC113. doi: 10.1523/JNEUROSCI.20-23-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Solomon IC. Connexin36 distribution in putative CO2-chemosensitive brainstem regions in rat. Resp Physiol Neurobiol. 2003;139:1–20. doi: 10.1016/j.resp.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 220.Solomon IC, Chon KH, Rodriguez MN. Blockade of brain stem gap junctions increases phrenic burst frequency and reduces phrenic burst synchronization in adult rat. J Neurophysiol. 2003;89:135–149. doi: 10.1152/jn.00697.2002. [DOI] [PubMed] [Google Scholar]
- 221.Hinckley CA, Ziskind-Conhaim L. Electrical coupling between locomotor-related excitatory interneurons in the mammalian spinal cord. J Neurosci. 2006;26:8477–8483. doi: 10.1523/JNEUROSCI.0395-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Nagy JI, Urena-Ramirez V, Ghia J-E. Functional alterations in gut contractility after connexin36 ablation and evidence for gap junctions forming electrical synapses between nitrergic enteric neurons. FEBS Letts. 2014;588:1480–1490. doi: 10.1016/j.febslet.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Wheal HV, Thomson AM. The electrical properties of neurones of the rat suprachiasmatic nucleus recorded intracellularly in vitro. Neuroscience. 1984;13:97–104. doi: 10.1016/0306-4522(84)90262-8. [DOI] [PubMed] [Google Scholar]
- 224.Zsiros V, Aradi I, Maccaferri G. Propagation of postsynaptic currents and potentials via gap junctions in GABAergic networks of the rat hippocampus. J Physiol. 2007;578:527–544. doi: 10.1113/jphysiol.2006.123463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Galarreta M, Hestrin S. Spike Transmission and Synchrony Detection in Networks of GABAergic Interneurons. Science. 2001;292:2295–2299. doi: 10.1126/science.1061395. [DOI] [PubMed] [Google Scholar]
- 226.Veruki ML, Hartveit E. AII (Rod) amacrine cells form a network of electrically coupled interneurons in the mammalian retina. Neuron. 2002;33:935–946. doi: 10.1016/s0896-6273(02)00609-8. [DOI] [PubMed] [Google Scholar]
- 227.Bennett MVL. Physiology of electrotonic junctions. Ann NY Acad Sci. 1966;137:509–539. doi: 10.1111/j.1749-6632.1966.tb50178.x. [DOI] [PubMed] [Google Scholar]
- 228.Galarreta M, Hestrin S. Electrical synapses between GABA-releasing interneurons. Nature Rev Neurosci. 2001;2:425–433. doi: 10.1038/35077566. [DOI] [PubMed] [Google Scholar]
- 229.Landisman CE, Connors BW. Long-term modulation of electrical synapses in the mammalian thalamus. Science. 2005;310:1809–1813. doi: 10.1126/science.1114655. [DOI] [PubMed] [Google Scholar]
- 230.Haas JS, Zavala B, Landisman CE. Activity-dependent long-term depression of electrical synapses. Science. 2011;334:389–393. doi: 10.1126/science.1207502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Haas JS, Greenwald CM, Pereda AE. Activity-dependent plasticity of electrical synapses: increasing evidence for its presence and functional roles in the mammalian brain. BMC cell biology. 2016;17:51. doi: 10.1186/s12860-016-0090-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.O'Brien J. The ever-changing electrical synapse. Current opinion in neurobiology. 2014;29:64–72. doi: 10.1016/j.conb.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Wang Z, Neely R, Landisman CE. Activation of group I and group II metabotropic glutamate receptors causes LTD and LTP of electrical synapses in the rat thalamic reticular nucleus. J Neurosci. 2015;35:7616–7625. doi: 10.1523/JNEUROSCI.3688-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Connors BW. Electrical signaling with neuronal gap junctions. In: Harris A, Locke D, editors. Connexins: A Guide. Humana Press/Springer; 2009. pp. 143–164. [Google Scholar]
- 235.Gelperin A. Olfactory computations and network oscillation. J Neurosci. 2006;26:1663–1668. doi: 10.1523/JNEUROSCI.3737-05b.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Kahana MJ. The cognitive correlates of human brain oscillations. J Neurosci. 2006;26:1669–1672. doi: 10.1523/JNEUROSCI.3737-05c.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Paulsen O, Sejnowski TJ. From invertebrate olfaction to human cognition: emerging computational functions of synchronized oscillatory activity. J Neurosci. 2006;26:1661–1662. doi: 10.1523/JNEUROSCI.3737-05a.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Sejnowski TJ, Paulsen O. Network oscillations: emerging computational principles. J Neurosci. 2006;26:1673–1676. doi: 10.1523/JNEUROSCI.3737-05d.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Spruston N. Axonal gap junctions send ripples through the hippocampus (Previews) Neuron. 2001;31:669–671. doi: 10.1016/s0896-6273(01)00426-3. [DOI] [PubMed] [Google Scholar]
- 240.Singer W, Gray CM. Visual feature integration and the temporal correlation hypothesis. Ann Rev Neurosci. 1995;18:555–586. doi: 10.1146/annurev.ne.18.030195.003011. [DOI] [PubMed] [Google Scholar]
- 241.Singer W. Neuronal synchrony: a versatile code for the definition of relations? Neuron. 1999;24:49–65. doi: 10.1016/s0896-6273(00)80821-1. [DOI] [PubMed] [Google Scholar]
- 242.Hempelmann A, Heils A, Sander T. Confirmatory evidence for an association of the connexin-36 gene with juvenile myoclonic epilepsy. Epilepsy Res. 2006;71:223–228. doi: 10.1016/j.eplepsyres.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 243.Mas C, Taske N, Deutsch S, Guipponi M, Thomas P, Covanis A, Friis M, Kjeldsen MJ, Pizzolato GP, Villemure JG, Buresi C, Rees M, Malafosse A, Gardiner M, Antonarakis SE, Meda P. Association of the connexin36 gene with juvenile myoclonic epilepsy. J Med Genet. 2004;41:e93. doi: 10.1136/jmg.2003.017954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Pan F, Paul DL, Bloomfield SA, Volgyi B. Connexin36 is required for gap junctional coupling of most ganglion cell subtypes in the mouse retina. J Comp Neurol. 2010;518:911–927. doi: 10.1002/cne.22254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Kosaka T, Kosaka K. Intraglomerular dendritic link connected by gap junctions and chemical synapses in the mouse main olfactory bulb: Electron microscopic serial section analyses. Neuroscience. 2005;131:611–625. doi: 10.1016/j.neuroscience.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 246.Kosaka T, Kosaka K. Neuronal gap junctions in the rat main olfactory bulb, with special reference to intraglomerular gap junctions. Neurosci Res. 2003;45:189–209. doi: 10.1016/s0168-0102(02)00222-5. [DOI] [PubMed] [Google Scholar]
- 247.Wang Y, Barakat A, Zhou H. Electrotonic coupling between pyramidal neurons in the neocortex. PLoS One. 2010;5:e10253. doi: 10.1371/journal.pone.0010253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Travagli RA, Dunwiddie TV, Williams JT. Opioid inhibition in locus coeruleus. J Neurophysiol. 1995;74:518–528. doi: 10.1152/jn.1995.74.2.519. [DOI] [PubMed] [Google Scholar]
- 249.Rubio ME, Nagy JI. Connexin36 expression in major centers of the auditory system in the CNS of mouse and rat: Evidence for neurons forming purely electrical synapses and morphologically mixed synapses. Neuroscience. 2015;303:604–629. doi: 10.1016/j.neuroscience.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Apostolides PF, Trussell LO. Regulation of interneuron excitability by gap junction coupling with principal cells. Nature Neurosci. 2013;16:1764–1772. doi: 10.1038/nn.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Wall PD, Gutnick MJ. Properties of afferent nerve impulses originating from a neuroma. Nature. 1974;248:740–743. doi: 10.1038/248740a0. [DOI] [PubMed] [Google Scholar]
- 252.Lisney SJW, Devor M. Afterdischarge and interactions among fibers in damaged peripheral nerve in the rat. Brain Res. 1987;415:122–136. doi: 10.1016/0006-8993(87)90275-7. [DOI] [PubMed] [Google Scholar]
- 253.Lee S-C, Patrick SL, Richardson KA, Connors BW. Two functionally distinct networks of gap junction-coupled inhibitory neurons in the thalamic reticular nucleus. J Neurosci. 2014;34:13182. doi: 10.1523/JNEUROSCI.0562-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Lee S-C, Cruikshank SJ, Connors BW. Electrical and chemical synapses between relay neurons in developing thalamus. J Physiol. 2010;588:2403–2415. doi: 10.1113/jphysiol.2010.187096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Fukuda T. Network architecture of gap junction-coupled neuronal linkage in the striatum. J Neurosci. 2009;29:1235–1243. doi: 10.1523/JNEUROSCI.4418-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Fukuda T. Structural organization of the gap junction network in the cerebral cortex. Neuroscientist. 2007;13:199–207. doi: 10.1177/1073858406296760. [DOI] [PubMed] [Google Scholar]
- 257.Kosaka T, Kosaka K. Neuronal gap junctions between intraglomerular mitral/tufted cell dendrites in the mouse main olfactory bulb. Neurosci Res. 2004;49:373–378. doi: 10.1016/j.neures.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 258.Kosaka T, Deans MR, Paul DL, Kosaka K. Neuronal gap junctions in the mouse main olfactory bulb: Morphological analyses on transgenic mice. Neuroscience. 2005;134:757–769. doi: 10.1016/j.neuroscience.2005.04.057. [DOI] [PubMed] [Google Scholar]
- 259.Landis DMD, Weinstein LA, Skordeles CJ. Serum influences the differentiation of membrane structure in cultured astrocytes. Glia. 1990;3:212–221. doi: 10.1002/glia.440030308. [DOI] [PubMed] [Google Scholar]
- 260.Tamás G, Buhl EH, Lörincz A, Somogyi P. Proximally targeted GABAergic synapses and gap junctions synchronize cortical interneurons. Nat Neurosci. 2000;3:366–371. doi: 10.1038/73936. [DOI] [PubMed] [Google Scholar]
- 261.Szoboszlay M, Lörincz A, Lanore F, Vervaeke K, Silver RA, Nusser Z. Functional properties of dendritic gap junctions in cerebellar Golgi cells. Neuron. 2016;90:1043–1056. doi: 10.1016/j.neuron.2016.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Bähner F, Weiss EK, Birke G, Maier N, Schmitz D, Rudolph U, Frotscher M, Traub RD, Both M, Draguhn A. Cellular correlate of assembly formation in oscillating hippocampal networks in vitro. Proc Natl Acad Sci (USA) 2011;108:E607–E616. doi: 10.1073/pnas.1103546108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Bendor D, Wilson MA. Biasing the content of hippocampal replay during sleep. Nature Neurosci. 2012;15:1439–1444. doi: 10.1038/nn.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Jadhav SP, Kemere C, German PW, Frank LM. Awake hippocampal sharp-wave ripples support spatial memory. Science. 2012;336:1454–1458. doi: 10.1126/science.1217230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Nádasdy Z, Hirase H, Czurkó A, Csicsvari J, Buzsaki G. Replay and time compression of recurring spike sequences in the hippocampus. J Neurosci. 1999;19:9497–9507. doi: 10.1523/JNEUROSCI.19-21-09497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Schmitz D, Schuchmann S, Fisahan A, Draguhn A, Buhl EH, Petrasch-Parwez E, Dermietzel R, Heinemann U, Traub RD. Axo-axonal coupling: A novel mechanism for ultrafast neuronal communication. Neuron. 2001;31:831–840. doi: 10.1016/s0896-6273(01)00410-x. [DOI] [PubMed] [Google Scholar]
- 267.Roopun AK, Middleton SJ, Cunningham MO, LeBeau FEN, Bibbig A, Whittington MA, Traub RD. A beta2-frequency (20–30 Hz) oscillation in nonsynaptic networks of somatosensory cortex. Proc Natl Acad Sci (USA) 2006;103:15646–15650. doi: 10.1073/pnas.0607443103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Wang Y, Barakat A, Zhou H. Electrotonic coupling between pyramidal neurons in the neocortex. PLoS One. 2010;5:e10253. doi: 10.1371/journal.pone.0010253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Vladimirov N, Tu Y, Traub RD. Synaptic gating at axonal branches, and sharp-wave ripples with replay: a simulation study. Eur J Neurosci. 2013;38:3435–3447. doi: 10.1111/ejn.12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Traub RD, Duncan R, Russell AJC, Baldeweg T, Tu Y, Cunningham MO, Whittington MA. Spatiotemporal patterns of electrocorticographic very fast oscillations (> 80 Hz) consistent with a network model based on electrical coupling between principal neurons. Epilepsia. 2010;51:1587–1597. doi: 10.1111/j.1528-1167.2009.02420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Pais I, Hormuzdi SG, Monyer H, Traub RD, Wood IC, Buhl EH, Whittington MA, LeBeau FEN. Sharp wave-like activity in the hippocampus in vitro in mice lacking the gap junction protein connexin 36. J Neurophysiol. 2003;89:2046–2054. doi: 10.1152/jn.00549.2002. [DOI] [PubMed] [Google Scholar]
- 272.Sheffield M, Best T, Mensh B, Kath W, Spruston N. Slow integration leads to persistent action potential firing in distal axons of coupled interneurons. BMC Neurosci. 2011;12:O17. doi: 10.1186/1471-2202-12-S1-O17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Hamzei-Sichani F, Davidson KGV, Yasumura T, Janssen WGM, Wearne SL, Hof PR, Traub RD, Gutierrez R, Ottersen OP, Rash JE. Mixed electrical-chemical synapses in adult rat hippocampus are primarily glutamatergic and coupled by connexin-36. Front Neuroanat. 2012;6:1–26. doi: 10.3389/fnana.2012.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Christie JM, Schoppa NE, Westbrook GL. Tufted cell dendrodendritic inhibition in the olfactory bulb is dependent on NMDA receptor activity. J Neurophysiol. 2001;85:169–173. doi: 10.1152/jn.2001.85.1.169. [DOI] [PubMed] [Google Scholar]
- 275.Schoppa NE, Westbrook GL. AMPA autoreceptors drive correlated spiking in olfactory bulb glomeruli. Nature Neurosci. 2002;5:1194–1202. doi: 10.1038/nn953. [DOI] [PubMed] [Google Scholar]
- 276.Teubner B, Degen J, Sohl G, Guldenagel M, Bukauskas FF, Trexler EB, Verselis VK, DeZeeuw CI, Lee CG, Kozak CA, Petrasch-Parwez E, Dermietzel R, Willecke K. Functional expression of the murine connexin 36 gene coding for a neuron-specific gap junctional protein. J Membr Biol. 2000;176:249–262. doi: 10.1007/s00232001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Masugi-Tokita M, Shigemoto R. High-resolution quantitative visualization of glutamate and GABA receptors at central synapses. Curr Opinion Neurobiol. 2007;17:387–393. doi: 10.1016/j.conb.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 278.Larsen WJ. Biological implications of gap junction structure, distribution and composition: A review. Tissue and Cell. 1983;15:645–671. doi: 10.1016/0040-8166(83)90041-1. [DOI] [PubMed] [Google Scholar]
- 279.Wolburg H, Rohlmann A. Structure-function relationships in gap junctions. Internat Rev Cytol. 1995;157:315–373. doi: 10.1016/s0074-7696(08)62161-0. [DOI] [PubMed] [Google Scholar]
- 280.Raviola E, Gilula NB. Intramembrane organization of specialized contacts in the outer plexiform layer of the retina. J Cell Biol. 1975;65:192–222. doi: 10.1083/jcb.65.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Dudek FE, Shao LR, Rash JE. Possible roles of nonsynaptic mechanisms in synchronization of epileptic seizures: Potential antiepileptic targets? In: Jong MR, Raman S, Stafstrom Carl E, editors. Epilepsy: Mechanisms, Models, and Translational Perspectives. 2010. pp. 209–228. [Google Scholar]
- 282.Massey SC, O'Brien JJ, Trexler EB, Li W, Keung JW, Mills SL, O'Brien J. Multiple neuronal connexins in the mammalian retina. Cell Commun Adhes. 2003;10:425–430. doi: 10.1080/cac.10.4-6.425.430. [DOI] [PubMed] [Google Scholar]
- 283.Dudek FE, Yasumura T, Rash JE. Nonsynaptic mechanisms in seizures and epileptogenesis. Cell Biol Internat. 1998;22:793–805. doi: 10.1006/cbir.1999.0397. [DOI] [PubMed] [Google Scholar]
- 284.Rash JE, Pickard GE, Davidson KGV, O'Brien J, Hartwick ATE, Kamasawa N, Yasumura T, Nagy JI. Exposure to dopamine and its D1 receptor antagonist SCH23390 produces large-scale ultrastructural plasticity and changes in phosphorylation of connexin36 in neuronal gap junctions of adult rat retina. Mol Biol Cell. 2007;18(supplement) Abstract #910. [Google Scholar]
- 285.Rash JE, Kamasawa N, Davidson K, Yasumura T, Nannapaneni S, Flores C, O'Brien J, Nagy J, Pereda AE. Heterotypic Cx35/Cx34. 7 coupling at rectifying gap junctions in goldfish giant club ending / Mauthner cell synapses demonstrated by matched double-replica FRIL. Mol Biol Cell. 2010;21:1466–1467. (Abstract 2225/B622) [Google Scholar]
- 286.Rash JE, Curti S, Vanderpool KG, Kamasawa N, Nannapaneni S, Palacios-Prado N, Flores CE, Yasumura T, O'Brien J, Lynn BD, Bukauskas FF, Nagy JI, Pereda AE. Molecular and functional asymmetry at a vertebrate electrical synapse. Neuron. 2013;79:957–969. doi: 10.1016/j.neuron.2013.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Curti S, Pereda AE. Voltage-dependent enhancement of electrical coupling by a subthreshold sodium current. J Neurosci. 2004;24:3999–4010. doi: 10.1523/JNEUROSCI.0077-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Vivar C, Traub RD, Gutierrez R. Mixed electrical-chemical transmission between hippocampal mossy fibers and pyramidal cells. Eur J Neurosci. 2012;35:76–82. doi: 10.1111/j.1460-9568.2011.07930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Münster-Wandowski A, Gómez-Lira G, Gutiérrez R. Mixed neurotransmission in the hippocampal mossy fibers. Front Cell Neurosci. 2015;7 doi: 10.3389/fncel.2013.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Precht W, Richter A, Ozawa S, Shimazu H. Intracellular study of frog's vestibular neurons in relation to the labyrinth and spinal cord. Exptl Brain Res. 1974;19:377–393. doi: 10.1007/BF00234462. [DOI] [PubMed] [Google Scholar]
- 291.Richter A, Precht W, Ozawa S. Responses of neurons of lizard's, Lacerta viridis, vestibular nuclei to electrical stimulation of the ipsi-and contralateral VIIIth nerves. Pflügers Arch. 1975;355:85–94. doi: 10.1007/BF00584802. [DOI] [PubMed] [Google Scholar]
- 292.Wilson VJ, Wylie RM. A short-latency labyrinthine input to the vestibular nuclei in the pigeon. Science. 1970;168:124–127. doi: 10.1126/science.168.3927.124. [DOI] [PubMed] [Google Scholar]
- 293.Nagy JI, Pereda AE, Rash JE. On the enigma of mixed chemical/electrical synapses in the mammalian CNS. Neurosci Let. 2017 doi: 10.1016/j.neulet.2017.09.021. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Grace AA, Bunney BS. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons-3. Evidence for electrotonic coupling. Neuroscience. 1983;10:333–348. doi: 10.1016/0306-4522(83)90137-9. [DOI] [PubMed] [Google Scholar]
- 295.Zolnik TA, Connors BW. Electrical synapses and the development of inhibitory circuits in the thalamus. J Physiol. 2016 doi: 10.1113/JP271880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Long MA, Deans MR, Paul DL, Connors BW. Rhythmicity without synchrony in the electrically uncoupled inferior olive. J Neurosci. 2002;22:10898–10905. doi: 10.1523/JNEUROSCI.22-24-10898.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Zbili M, Rama S, Debanne D. Dynamic control of neurotransmitter release by presynaptic potential. Front Cell Neurosci. 2016;10:278. doi: 10.3389/fncel.2016.00278. (electronic citation) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Isaac JTR, Crair MC, Nicoll RA, Malenka RC. Silent synapses during development of thalamocortical inputs. Neuron. 1997;18:269–280. doi: 10.1016/s0896-6273(00)80267-6. [DOI] [PubMed] [Google Scholar]
- 299.Poncer JC, Malinow R. Postsynaptic conversion of silent synapses during LTP affects synaptic gain and transmission dynamics. Nature Neurosci. 2001;4:989–996. doi: 10.1038/nn719. [DOI] [PubMed] [Google Scholar]
- 300.Kothmann WW, Trexler EB, Whitaker CM, Li W, Massey SC, O'Brien J. Nonsynaptic NMDA receptors mediate activity-dependent plasticity of gap junctional coupling in the AII amacrine cell network. J Neurosci. 2012;32:6747–6759. doi: 10.1523/JNEUROSCI.5087-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Pereda AE. Electrical synapses and their functional interactions with chemical synapses. Nature Rev Neurosci. 2014;15:250–263. doi: 10.1038/nrn3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Turecek J, Yuen GS, Han VZ, Zeng XH, Bayer KU, Welsh JP. NMDA receptor activation strengthens weak electrical coupling in mammalian brain. Neuron. 2014;81:1375–1388. doi: 10.1016/j.neuron.2014.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Malenka RC, Nicoll RA. Silent synapses speak up. (Minireview) Neuron. 1997;19:473–476. doi: 10.1016/s0896-6273(00)80362-1. [DOI] [PubMed] [Google Scholar]
- 304.Sáez JC, Berthoud VM, Branes MC, Martinez AD, Beyer EC. Plasma membrane channels formed by connexins: their regulation and functions. Physiol Rev. 2003;83:1359–1400. doi: 10.1152/physrev.00007.2003. [DOI] [PubMed] [Google Scholar]
- 305.Nielsen MS, Nygaard AL, Sorgen PL, Verma V, Delmar M, Holstein-Rathlou NH. Gap junctions. Wiley Online Library; 2012. pp. 1981–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Pereda AE, Curti S, Hoge G, Cachope R, Flores CE, Rash JE. Gap junction-mediated electrical transmission: Regulatory mechanisms and plasticity. Biochim Biophys Acta (BBA) - Biomembranes. 2013;1828:134–146. doi: 10.1016/j.bbamem.2012.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Hampson EC, Vaney DI, Weiler R. Dopaminergic modulation of gap junction permeability between amacrine cells in mammalian retina. J Neurosci. 1992;12:4911–4922. doi: 10.1523/JNEUROSCI.12-12-04911.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Onn SP, Grace AA. Dye coupling between rat striatal neurons recorded in vivo: compartmental organization and modulation by dopamine. J Neurophysiol. 1994;71:1917–1934. doi: 10.1152/jn.1994.71.5.1917. [DOI] [PubMed] [Google Scholar]
- 309.He S, Weiler R, Vaney DI. Endogenous dopaminergic regulation of horizontal cell coupling in the mammalian retina. J Comp Neurol. 2000;418:33–40. doi: 10.1002/(sici)1096-9861(20000228)418:1<33::aid-cne3>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 310.Onn SP, West AR, Grace AA. Dopamine-mediated regulation of striatal neuronal and network interactions. Trends Neurosci (TINS) 2000;23:S48–S56. doi: 10.1016/s1471-1931(00)00020-3. [DOI] [PubMed] [Google Scholar]
- 311.Rörig B, Sutor B. Serotonin regulates gap junction coupling in the developing rat somatosensory cortex. Eur J Neurosci. 1996;8:1685–1695. doi: 10.1111/j.1460-9568.1996.tb01312.x. [DOI] [PubMed] [Google Scholar]
- 312.Hatton GI, Yang QZ. Synaptically released histamine increases dye coupling among vasopressinergic neurons of the supraoptic nucleus: mediation by H1 receptors and cyclic nucleotides. J Neurosci. 1996;16:123–129. doi: 10.1523/JNEUROSCI.16-01-00123.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Velazquez JL, Han D, Carlen PL. Neurotransmitter modulation of gap junctional communication in the rat hippocampus. Eur J Neurosci. 1997;9:2522–2531. doi: 10.1111/j.1460-9568.1997.tb01681.x. [DOI] [PubMed] [Google Scholar]
- 314.O'Donnell P, Grace AA. Cortical afferents modulate striatal gap junction permeability via nitric oxide. Neuroscience. 1996;76:1–5. doi: 10.1016/s0306-4522(96)00433-2. [DOI] [PubMed] [Google Scholar]
- 315.Pereda A, Triller A, Korn H, Faber DS. Dopamine enhances both electrotonic coupling and chemical excitatory postsynaptic potentials at mixed synapses. Proc Natl Acad Sci (USA) 1992;89:12088–12092. doi: 10.1073/pnas.89.24.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Cachope R, Mackie K, Triller A, O'Brien J, Pereda AE. Potentiation of electrical and chemical synaptic transmission mediated by endocannabinoids. Neuron. 2007;56:1034–1047. doi: 10.1016/j.neuron.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Sevetson J, Fittro S, Heckman E, Haas JS. A calcium-dependent pathway underlies activity- dependent plasticity of electrical synapses in the thalamic reticular nucleus. J Physiol. 2017 doi: 10.1113/JP274049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Cepeda C, Walsh JP, Hull CD, Howard SG, Buchwald NA, Levine MS. Dye-coupling in the neostriatum of the rat: I. Modulation by dopamine-depleting lesions. Synapse. 1989;4:229–237. doi: 10.1002/syn.890040308. [DOI] [PubMed] [Google Scholar]
- 319.Onn SP, Grace AA. Repeated treatment with haloperidol and clozapine exerts differential effects on dye coupling between neurons in subregions of striatum and nucleus accumbens. J Neurosci. 1995;15:7024–7036. doi: 10.1523/JNEUROSCI.15-11-07024.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Onn S-P, Grace AA. Alterations in electrophysiological activity and dye coupling of striatal spiny and aspiny neurons in dopamine–denervated rat striatum recorded in vivo. Synapse. 1999;33:1–15. doi: 10.1002/(SICI)1098-2396(199907)33:1<1::AID-SYN1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 321.McCracken CB, Patel KM, Vrana KE, Paul DL, Roberts DC. Amphetamine withdrawal produces region-specific and time-dependent changes in connexin36 expression in rat brain. Synapse. 2005;56:39–44. doi: 10.1002/syn.20127. [DOI] [PubMed] [Google Scholar]
- 322.McCracken CB, Hamby SM, Patel KM, Morgan D, Vrana KE, Roberts DC. Extended cocaine self-administration and deprivation produces region-specific and time-dependent changes in connexin36 expression in rat brain. Synapse. 2006;58:141–150. doi: 10.1002/syn.20194. [DOI] [PubMed] [Google Scholar]
- 323.Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: Adaptive gain and optimal performance. Ann Rev Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- 324.Takeuchi T, Duszkiewicz AJ, Sonneborn A, Spooner PA, Yamasaki M, Watanabe M, Smith CC, Fernández G, Deisseroth K, Greene RW. Locus coeruleus and dopaminergic consolidation of everyday memory. Nature. 2016;537:357–362. doi: 10.1038/nature19325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Maeda T. Dopaminergic innervation of the locus ceruleus of the rat with special reference to aminergic interaction. Psych Clin Neuroscie. 1991;45:517–518. doi: 10.1111/j.1440-1819.1991.tb02539.x. [DOI] [PubMed] [Google Scholar]
- 326.Urschel S, Hoher T, Schubert T, Alev C, Sohl G, Worsdorfer P, Asahara T, Dermietzel R, Weiler R, Willecke K. Protein kinase A-mediated phosphorylation of connexin36 in mouse retina results in decreased gap junctional communication between AII amacrine cells. J Biol Chem. 2006;281:33163–33171. doi: 10.1074/jbc.M606396200. [DOI] [PubMed] [Google Scholar]
- 327.Ouyang X, Winbow VM, Patel LS, Burr GS, Mitchell CK, O'Brien J. Protein kinase A mediates regulation of gap junctions containing connexin35 through a complex pathway. Molec Brain Res. 2005;135:1–11. doi: 10.1016/j.molbrainres.2004.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Alev C, Urschel S, Sonntag S, Zoidl G, Fort AG, Hoher T, Matsubara M, Willecke K, Spray DC, Dermietzel R. The neuronal connexin36 interacts with and is phosphorylated by CaMKII in a way similar to CaMKII interaction with glutamate receptors. Pro Natl Acad Sci (USA) 2008;105:20964–20969. doi: 10.1073/pnas.0805408105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Urbano FJ, Leznik E, Llinás R. Modafinil enhances thalamocortical activity by increasing neuronal electrotonic coupling. Proc Natl Acad Sci (USA) 2007;104:12554–12559. doi: 10.1073/pnas.0705087104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Kothmann WW, Massey SC, O'Brien J. Dopamine-stimulated dephosphorylation of Connexin 36 mediates AII amacrine cell uncoupling. J Neurosci. 2009;29:14903–14911. doi: 10.1523/JNEUROSCI.3436-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Rela L, Szczupak L. In situ characterization of a rectifying electrical junction. J Neurophysiol. 2007;97:1405–1412. doi: 10.1152/jn.00973.2006. [DOI] [PubMed] [Google Scholar]
- 332.Phelan P, Goulding LA, Tam JLY, Allen MJ, Dawber RJ, Davies JA, Bacon JP. Molecular mechanism of rectification at identified electrical synapses in the Drosophila giant fiber system. Current Biol. 2008;18:1955–1960. doi: 10.1016/j.cub.2008.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Auerbach AA, Bennett MVL. A rectifying electrotonic synapse in the central nervous system of a vertebrate. J Gen Phys. 1969;53:211–237. doi: 10.1085/jgp.53.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Devor A, Yarom Y. Electrotonic coupling in the inferior olivary nucleus revealed by simultaneous double patch recordings. J Neurophysiol. 2002;87:3048–3058. doi: 10.1152/jn.2002.87.6.3048. [DOI] [PubMed] [Google Scholar]
- 335.Venance L, Glowinski J, Giaume C. Electrical and chemical transmission between striatal GABAergic output neurones in rat brain slices. J Physiol. 2004;559:215–230. doi: 10.1113/jphysiol.2004.065672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Herve JC, Derangeon M, Sarrouilhe D, Giepmans BNG, Bourmeyster N. Gap junctional channels are parts of multiprotein complexes. Biochim Biophys Acta (BBA) - Biomembranes. 2012;1818:1844–1865. doi: 10.1016/j.bbamem.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 337.Flores CE, Li X, Bennett MVL, Nagy JI, Pereda AE. Connexin35 and Zonula Occludens-1 directly interact: implications for the regulation of electrical transmission. Proc Natl Acad Sci USA. 2008;105:12545–12550. doi: 10.1073/pnas.0804793105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Hyman SE. Addiction to cocaine and amphetamine. Neuron. 1996;16:901–904. doi: 10.1016/s0896-6273(00)80111-7. [DOI] [PubMed] [Google Scholar]
- 339.Hunter AW, Barker RJ, Zhu C, Gourdie RG. Zonula occludens-1 alters connexin43 gap junction size and organization by influencing channel accretion. Mol Biol Cell. 2005;16:5686–5698. doi: 10.1091/mbc.E05-08-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Akoyev V, Takemoto DJ. ZO-1 is required for protein kinase C gamma-driven disassembly of connexin 43. Cell Signalling. 2007;19:958–967. doi: 10.1016/j.cellsig.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Lynn BD, Li X, Nagy JI. Under construction: Building the macromolecular superstructure and signaling components of an electrical synapse. J Membr Biol. 2012;245:303–317. doi: 10.1007/s00232-012-9451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Li X, Lynn BD, Nagy JI. The effector and scaffolding proteins AF6 and MUPP1 interact with connexin36 and localize at gap junctions that form electrical synapses in rodent brain. Europ J Neurosci. 2012;35:166–181. doi: 10.1111/j.1460-9568.2011.07947.x. [DOI] [PubMed] [Google Scholar]
- 343.Ooshio T, Kobayashi R, Ikeda W, Miyata M, Fukumoto Y, Matsuzawa N, Ogita H, Takai Y. Involvement of the interaction of afadin with ZO-1 in the formation of tight junctions in Madin-Darby canine kidney cells. J Biol Chem. 2010;285:5003–5012. doi: 10.1074/jbc.M109.043760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Yamamoto H, Maruo T, Majima T, Ishizaki H, Tanaka-Okamoto M, Miyoshi J, Mandai K, Takai Y. Genetic deletion of afadin causes hydrocephalus by destruction of adherens junctions in radial glial and ependymal cells in the midbrain. PLoS One. 2013;8:e80356. doi: 10.1371/journal.pone.0080356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Boettner B, Govek EE, Cross J, Van Aelst L. The junctional multidomain protein AF-6 is a binding partner of the Rap1A GTPase and associates with the actin cytoskeletal regulator profilin. Proc Natl Acad Sci (USA) 2000;97:9064–9069. doi: 10.1073/pnas.97.16.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Boettner B, Herrmann C, Van Aelst L. Ras and rap 1 interaction with AF-6 effector target. Meth Enzymol. 2001;332:151–168. doi: 10.1016/s0076-6879(01)32199-7. [DOI] [PubMed] [Google Scholar]
- 347.Caron E. Cellular functions of the Rap1 GTP-binding protein: a pattern emerges. J Cell Sci. 2003;116:435–440. doi: 10.1242/jcs.00238. [DOI] [PubMed] [Google Scholar]
- 348.Kooistra MRH, Dube N, Bos JL. Rap1: a key regulator in cell-cell junction formation. J Cell Sci. 2007;120:17–22. doi: 10.1242/jcs.03306. [DOI] [PubMed] [Google Scholar]
- 349.Xie Z, Huganir RL, Penzes P. Activity-dependent dendritic spine structural plasticity is regulated by small GTPase Rap1 and its target AF-6. Neuron. 2005;48:605–618. doi: 10.1016/j.neuron.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 350.Watanabe T, Sato K, Kaibuchi K. Cadherin-mediated intercellular adhesion and signaling cascades involving small GTPases. Cold Spring Harbor Perspect Biol. 2009;1:a003020. doi: 10.1101/cshperspect.a003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Flores CE, Nannapaneni S, Davidson KGV, Yasumura T, Bennett MVL, Rash JE, Pereda AE. Trafficking of gap junction channels at a vertebrate electrical synapse in vivo. Proc Natl Acad Sci (USA) 2012;109:E573–E582. doi: 10.1073/pnas.1121557109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Johnson RG, Reynhout J, TenBroek E, Quade BJ, Yasumura T, Davidson KGV, Sheridan JD, Rash JE. Gap junction assembly: Roles for the Formation Plaque and regulation by the C-terminus of connexin43. Mol Biol Cell. 2012;23:71–86. doi: 10.1091/mbc.E11-02-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Janssen-Bienhold U, Schultz K, Gellhaus A, Schmidt P, Ammermüller J, Weiler R. Identification and localization of connexin26 within the photoreceptor-horizontal cell synaptic complex. Vis Neurosci. 2001;18:169–178. doi: 10.1017/s0952523801182015. [DOI] [PubMed] [Google Scholar]