Abstract
Endoplasmic reticulum (ER) stress, a disturbance of the ER function, contributes to cardiac injury. ER and mitochondria are closely connected organelles within cells. ER stress contributes to mitochondrial dysfunction, which is a key factor to increase cardiac injury. Metformin, a traditional anti-diabetic drug, decreases cardiac injury during ischemia-reperfusion. Metformin also inhibits ER stress in cultured cells. We hypothesized that metformin can attenuate the ER stress-induced mitochondrial dysfunction and subsequent cardiac injury. Thapsigargin (THAP, 3 mg/kg) was used to induce ER stress in C57BL/6 mice. Cell injury and mitochondrial function were evaluated in the mouse heart 48 hours after one-time THAP treatment. Metformin was dissolved in drinking water (0.5g/250 ml) and fed to mice for 7 days before THAP injection. Metformin feeding continued after THAP treatment. THAP treatment increased apoptosis in mouse myocardium compared to control. THAP also led to decreased oxidative phosphorylation in heart mitochondria oxidizing complex I substrates. THAP decreased the calcium retention capacity (CRC), indicating that ER stress sensitizes mitochondria to mitochondrial permeability transition pore opening. The cytosolic CHOP content was markedly increased in THAP-treated hearts compared to control, particularly in the nucleus. Metformin prevented the THAP-induced mitochondrial dysfunction and reduced CHOP content in cytosol and nucleus. Thus, metformin reduces cardiac injury during ER stress through the protection of cardiac mitochondria and attenuation of CHOP expression.
Keywords: mitochondria, complex I, thapsigargin, apoptosis
The endoplasmic reticulum (ER) is an intracellular organelle that is responsible for protein folding, lipid synthesis, and calcium homeostasis.1 Accumulation of the unfolded/misfolded proteins in pathological conditions including heart failure results in a disturbance of the ER function that is known as the ER stress.2, 3 While the initial ER stress is an adaptive reaction to restore the ER function through signaling and transcriptional response, severe ER stress ultimately leads to cell injury by activating programmed cell death.4 Induction of CHOP (C/EBP homologous protein) during the ER stress is key pathway to trigger apoptosis.5, 6 CHOP expression increases apoptosis during the ER stress through depletion of bcl-2. The CHOP expression triggers the inflammation cascade that increases cardiac injury in the heart following in vivo ischemia-reperfusion.6
Mitochondrial dysfunction contributes a key role in cell injury in the heart following ischemia-reperfusion.7–11 The ER and mitochondria are closely connected organelles within cells.12, 13 The ER stress is involved in mitochondrial dysfunction in rat hearts.14 Thapsigargin (THAP)-induced ER stress leads to increased ROS (reactive oxygen species) generation through NADPH oxidase and sensitizes mitochondria to permeability transition pore opening in isolated cardiac myocytes.14 Damage to the mitochondrial electron transport chain (ETC) is a key source of ROS production and myocardial injury.15, 16 We first studied if ER stress leads to the ETC damage in mouse heart mitochondria. We next studied if the ER stress increases the ROS generation from the ETC. The sensitivity to permeability transition pore opening was also evaluated in isolated mitochondria following the ER stress.
Metformin, a traditional anti-diabetic drug, decreases cardiac injury during ischemia-reperfusion.17 Based on the UK Prospective Diabetes Study (UKPDS), metformin treatment in diabetic patients substantially improves cardiovascular disease outcomes with a reduction of mortality and myocardial infarction.18 Metformin treatment is protective in experimental models of heart failure19, 20 and is safe when used in diabetic patients with congestive heart. 20, 21 The mechanism of metformin protection is involved in activation of AMP-activated protein kinase (AMPK). AMPK is a cellular energy sensor that regulates mitochondrial metabolism and the redox state of the cell.22–24 Activation of AMPK decreases cell injury during oxidative stress in part through the inhibition of mitochondrial permeability transition pore (MPTP) opening.25 MPTP opening increases cardiac injury during ischemia-reperfusion by reducing bioenergy production, increasing ROS generation, and releasing pro-apoptotic proteins including cytochrome c and AIF from mitochondria into cytosol.26, 27 In addition to its well-known anti-diabetic effect, recent studies show that metformin can decrease ER stress in renal cells.28 Metformin treatment decreases angiotensin II-induced ER stress and hypertension in mice through activation of AMPK.29 Metformin also decreases β-cell lipotoxicity through inhibition of the ER stress.30 Pathologic ER stress leads to calcium release, which is directed toward the mitochondria via domains shared by ER and mitochondria called mitochondria-associated membranes (MAM).31 Therefore, we hypothesized: (1) that metformin will decrease the ER stress-induced MPTP opening measured in isolated heart mitochondria; (2) that metformin will protect the ETC during ER stress; (3) metformin will inhibit ER stress-induced CHOP expression and subsequent cell death.
2. METHODS
2.1. Induction of the ER stress in C57BL/6 mice
The Animal Care and Use Committee of the McGuire VA Medical Center approved the study. Male C57BL/6 mice (2–3 months) were used in the current study. Mice received a normal diet with ad libitum access to food and water during the experiment. Normal diet CHOW including 16% protein and 4% fat. THAP (3 mg/kg) was dissolved in DMSO and diluted with saline to induce ER stress through one-time i.p. injection in mice without fasting (Figure 1A).14 Control mice received vehicle (DMSO) treatment. In metformin treated groups, Metformin (300 mg/kg body weight) was dissolved in drinking water with sucrose (0.2g/100 ml) as sweetener and fed to mice for 7 days before THAP injection.32 The metformin dose was based upon previous studies in the rat.32 Control mice received drinking water with sucrose. Metformin feeding was continued after THAP. Mice were anesthetized with pentobarbital sodium (100 mg/kg, i.p.) 48 hours after one-time THAP treatment (Figure 1A). The mouse heart was quickly excised for mitochondrial isolation or histological examination.
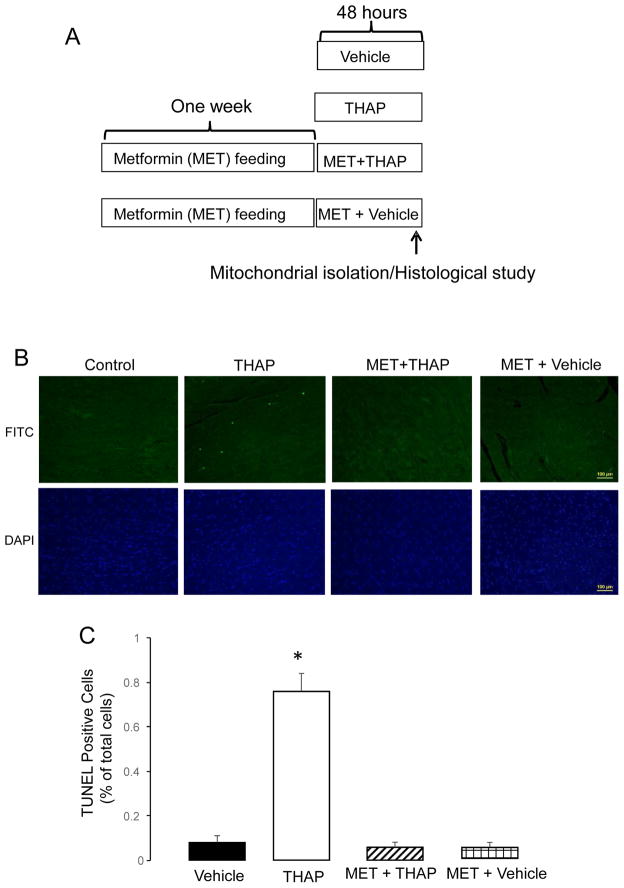
Figure 1. Administration of metformin decreases cardiac injury in THAP-treated mouse hearts.
THAP (3 mg/kg, I.P.) was used to induce the ER stress for 48 hours in C57BL/6 mice. In metformin-treated hearts, mice first received metformin (dissolved in the drinking water) for one week. Then, THAP was administered to mice once and mice followed for 48 hours (Panel A). Apoptotic cell death was assessed using TUNEL staining (green color, Panel B). Total nuclei were quantified using DAPI staining (blue color, Panel B). Arrowhead indicated a typical TUNEL positive nucleus. THAP significantly increased apoptotic cell death compared to vehicle (Panel B&C). Metformin treatment markedly decreased apoptotic cell death in the THAP-treated hearts with no effect in untreated hearts. Mean ± SEM. *p<0.05 vs. other groups. n=3 in each group
2.2 Determination of apoptotic cell death
Apoptotic cell death in myocardium was analyzed by TUNEL staining, using a commercial kit (BD Biosciences, San Jose, CA) that detects nuclear DNA fragmentation via fluorescence assay. In brief, mouse hearts from control, THAP, and MET+THAP groups were excised and stored in a 10% formalin solution. Apoptosis was assessed in transverse paraffin sections with TUNEL staining. The apoptotic index was expressed as the number of apoptotic cells of all cardiomyocytes per field. The apoptotic rate was calculated using 10 random fields per slide. The transverse sections were then counterstained with Vectashield mounting medium with 4,6-diamidino-2-phenylindole (a DNA intercalating dye for visualizing nuclei in fixed cells; catalogue number H-1200, Vector Laboratories). The stained cells were examined under an Olympus IX70 fluorescence microscope.33
2.3. Isolation of cytosol, mitochondria, and nucleus
Heart mitochondria were isolated as previously described.34 The mouse heart was placed in cold buffer A (composition in mM: 100 KCl, 50 MOPS [3-(N-morpholino) propanesulfonic acid], 1 EGTA, 5 MgSO4, and 1 mM ATP). The heart was blotted dry, weighed, and homogenized using a polytron tissue homogenizer at 10,000 rpm for 2.5 seconds with trypsin (5 mg/g tissue). The trypsin was used to generate a combined population of cardiac mitochondria from a single mouse heart. Trypsin treatment also removed potential cytosolic contamination. The homogenate was incubated for 15 min at 4°C, then the same volume of buffer B [buffer A + 0.2% bovine serum albumin (BSA)] was added and the mixture was centrifuged at 500 × g for 10 min. The supernatant was again centrifuged at 3000 × g to pellet mitochondria. The mitochondrial pellet was first washed with buffer B, then re-suspended in KME (100 mM KCl, 50 mM MOPS, 0.5 mM EGTA), and centrifuged at 3000 × g to yield the final mitochondrial pellet. Mitochondria were re-suspended in KME for study.34 In a separate heart, cytosol, mitochondria, and nucleus were isolated using a commercially available kit (#78835) from Thermo Scientific (Waltham, MA).35
Oxygen consumption in mitochondria was measured using a Clark-type oxygen electrode at 30°C as previously described.34 Glutamate (20 mM) + Malate (10 mM) were used as complex I substrate. Succinate (20 mM, complex II substrate) was used as the complex II substrate with the inclusion of 7.5 μM rotenone. ADP (2 mM) was used to determine the maximal rate of ADP-stimulated respiration.26
2.4 Calcium retention capacity (CRC) in isolated mitochondria
The CRC was used to assess the calcium-induced MPTP opening in isolated mitochondria.36 The assay medium included mitochondria (125 μg/ml), 150 mM sucrose, 50 mM KCl, 2 mM KH2PO4, and 5 mM succinate in 20 mM Tris/HCl with pH at 7.4. Sequential exogenous calcium (5 nmol/each pulse) was added into cuvettes until MPTP opening, shown by a burst release of calcium from mitochondria (Figure 3A). Calcium Green-5N (0.5 uM, Thermo Scientific, Waltham, MA) was used to monitor extra-mitochondrial Ca2+ concentration with excitation and emission wavelengths set at 500 and 530 nm, respectively.36
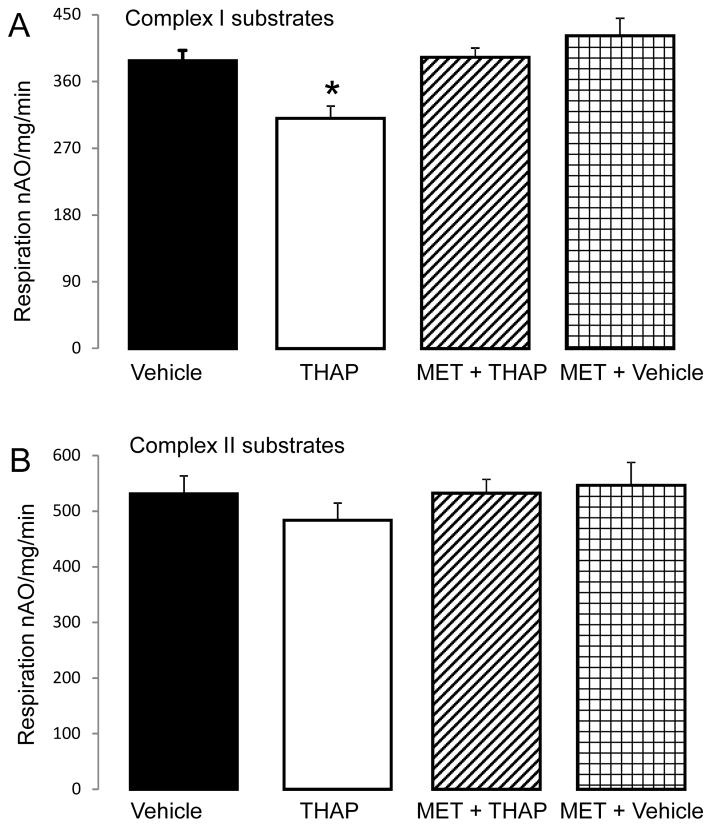
Figure 3. Administration of metformin improves mitochondrial function in THAP-treated mouse hearts.
Compared to vehicle, THAP treatment decreased the rate of oxidative phosphorylation when glutamate + malate was used as complex I substrate (Panel A). Metformin feeding protected oxidative phosphorylation rates in the THAP-treated hearts oxidizing complex I substrates. Metformin feeding alone did not affect the oxidative phosphorylation with complex I substrates (Panel A). There were no differences in the oxidation of succinate (+rotenone) between groups (Panel B). Mean ± SEM; * p <0.05 vs. other groups. N=6 in each group.
2.5 Determination of ROS generation in isolated cardiac mitochondria
H2O2 production from intact mitochondria was measured using the oxidation of the fluorogenic indicator Amplex red in the presence of horseradish peroxidase.37 Glutamate + Malate and succinate (+ rotenone) were used as complex I and complex II substrates, respectively. The concentration of substrates was the same as that used to measure oxidative phosphorylation.
2.6 Western blotting
Proteins were separated using 12% or 4–15% Tris-glycine gels (Bio-Rad, Hercules, CA) and transferred to PVDF membrane (Millipore) using semi-dry transfer (Bio-Rad). The blots were incubated for 1 h at room temperature in 5% (w/v) non-fat dry milk (Bio-Rad) in TBST buffer (10 mM Tris pH 7.5, 150 mM NaCl, 0.1% Tween20) followed by the overnight incubation at 4 °C with primary antibody. After 1h incubation at room temperature with a 1:10,000 dilution of HRP-conjugated anti-mouse or anti-rabbit IgG F(ab)2 (GE Healthcare Life Sciences, Piscataway, NJ), blots were developed using ECL Plus Western Blotting Detection Reagents (GE Healthcare Life Sciences, Piscataway, NJ).
2.7 Statistical analysis
Data are expressed as the mean ± standard error.38 For all analyses, differences between groups in biologic variables and mitochondrial function were compared by two-way ANOVA. For western blotting analyses, differences between groups were compared by one-way ANOVA. When a significant F value was obtained, means were compared using the Student-Newman-Keuls test of multiple comparisons. Statistical significance was defined as a value of p<0.05.
3. Results
3.1 Metformin decreases cell death during ER stress
There were no significant differences in heart and body weight and the ratio of heart/body weight (Table 1) among groups, indicating that THAP treatment did not induce significant cardiomyopathy within 48 hours.
Table 1.
Mouse heart weight and body weight
| Group | Number | Body weight (g) | Heart weight (g) | Ratio of heart/body |
|---|---|---|---|---|
| Control + Vehicle | 6 | 31.9 ± 2.4 | 0.127 ± 0.011 | 0.0040 ± 0.005 |
| THAP | 6 | 31.9 ± 1.2 | 0.137± 0.015 | 0.0043 ± 0.006 |
| MET + Vehicle | 4 | 34.6 ± 7.8 | 0.137± 0.016 | 0.0041 ± 0.008 |
| MET + THAP | 6 | 25.0 ± 3.2 | 0.116± 0.021 | 0.0046 ± 0.005 |
Mean ± SD. p=NS.
TUNEL staining was used to assess apoptosis in mouse hearts with THAP and Metformin treatments. Compared to vehicle-treated control hearts, the frequency of apoptotic nuclei was significantly increased in THAP treated hearts (Figure 1 B&C), indicating that the THAP-induced ER stress increased apoptotic cell death. Metformin treatment markedly decreased the frequency of apoptotic nuclei observed in THAP-treated hearts (Figure 1 B&C), supporting that metformin decreases programmed cell death during ER stress. Metformin treatment alone did not alter the frequency of apoptosis.
3.2 Metformin leads to decreased CHOP expression during ER stress
The induction of ER stress increased CHOP expression that contributed to cell death. The content of CHOP in cytosol was markedly increased in the THAP-treated mice compared to vehicle (Figure 2A). Metformin treatment led to decreased CHOP expression. GAPDH was used as a loading control for the cytosolic proteins. In addition, THAP treatment increased the content of CHOP in the nucleus (Figure 2B) compared to the vehicle only treated group. Metformin decreased the content of CHOP in nucleus following THAP treatment (Figure 2B). Laminin was used as the protein loading control for the nucleus.
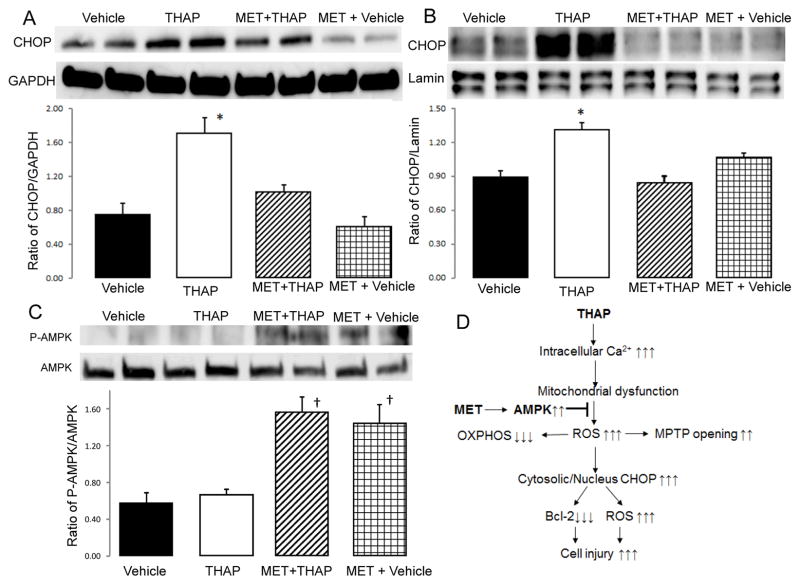
Figure 2. Administration of metformin activates AMPK and decreases CHOP expression in cytosol and nucleus in THAP-treated mouse hearts.
THAP treatment increased the content of CHOP in cytosol (Panel A) and nucleus (Panel B) compared to vehicle. Metformin treatment dramatically decreased the expression of CHOP in cytosol and nucleus in THAP-treated hearts (Panel A&B). In metformin treated hearts, phosphorylated AMPK content was increased compared to non-metformin-treated hearts (Panel C); supporting that metformin feeding activates the AMPK as expected. The potential mechanisms of metformin protection during the ER stress was summarized in the Panel D. Mean ± SEM. *p<0.05 vs. other groups (Panel A&B). †p<0.05 vs. non-metformin treated hearts (Panel C). n=3 in each group.
3.3 Metformin results in phosphorylation of AMPK during ER stress
The phosphorylation of AMPK(Thr172) was dramatically enhanced in metformin treated mice compared to vehicle or THAP treated mice (Figure 2C). There were no differences in total AMPK content between groups. These results support that metformin feeding activates AMPK in mouse hearts as expected.
3.4 Metformin improves oxidative phosphorylation during ER stress
The rate of oxidative phosphorylation was measured using mitochondria isolated from mouse hearts with or without THAP and/or Metformin treatment. Compared to control, treatment of mice with THAP decreased the rate of oxidative phosphorylation when glutamate + malate were used as complex I substrates (Figure 3A). THAP did not alter the oxidative phosphorylation using succinate as the complex II substrate (rotenone was used to block potential reverse electron flow from complex II to complex I). Metformin alone did not alter the oxidative phosphorylation using either complex I or complex II substrates compared to control (Figure 3B). However, metformin treatment improved oxidative phosphorylation in THAP-treated mouse heart mitochondria (Figure 3B).
3.5 Metformin inhibits the MPTP opening during ER stress
Calcium retention capacity (CRC) was used to reflect MPTP opening in isolated heart mitochondria. THAP treatment resulted in a decrease in the CRC compared to control, suggesting that the ER stress sensitizes to MPTP opening (Figure 4A&B). Metformin alone did not affect the sensitivity of the MPTP opening compared to control (Figure 4B). However, metformin decreased the sensitivity to MPTP opening in THAP-treated mouse heart mitochondria (Figure 4B). These results indicate that metformin treatment inhibits the MPTP opening in heart mitochondria observed following the induction of ER stress in vivo.
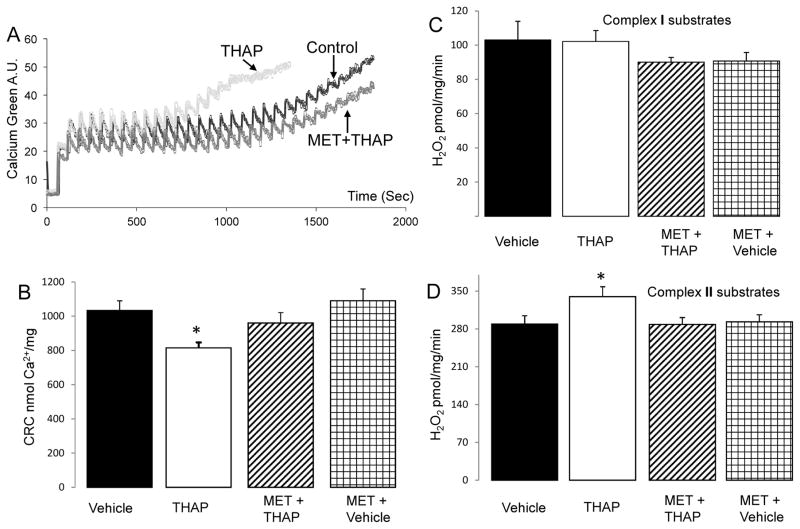
Figure 4. Administration of metformin decreases MPTP opening and ROS generation in THAP-treated mouse hearts.
Calcium retention capacity (CRC) was used to measure the sensitivity to MPTP opening in isolated mitochondria (Panel A). The CRC was decreased in the THAP-treated heart mitochondria compared to vehicle, suggesting that the ER stress sensitizes to MPTP opening in cardiac mitochondria (Panel A&B). Metformin treatment improved the CRC in the THAP-treated mitochondria (Panel A&B), indicating that metformin decreases MPTP opening during the ER stress. THAP treatment also increased the ROS generation in isolated mitochondria oxidizing complex II substrates (Panel D) but not complex I substrates (Panel C) compared to vehicle. Again, metformin decreased the ROS generation in the TAHP-treated mitochondria (Panel D). Mean ± SEM. *p <0.05 vs. other groups. n=6 in each group.
3.6 Metformin decreases the H2O2 generation during ER stress
A net release of H2O2 from the isolated mitochondria was measured using the Amplex red assay. There were no significant differences in H2O2 generation between groups when glutamate + malate were used as substrates (Figure 4C). However, THAP markedly increased H2O2 generation compared to control when succinate was used as substrate in the presence of rotenone. Metformin alone did not alter H2O2 generation compared to control when succinate was oxidized as substrate (Figure 4D). Metformin treatment decreased the THAP-stimulated H2O2 generation compared to THAP alone (Figure 4D). These results indicate that metformin treatment decreases the ER stress-mediated oxidative stress.
4. Discussion
In the present study, we show that THAP-induced ER stress increases cardiac cell death in mouse hearts. THAP leads to decreased oxidative phosphorylation, increased ROS generation, and sensitizes to MPTP opening in cardiac mitochondria. Metformin treatment decreases cardiac injury in the THAP-treated hearts. The ER stress increases the content of CHOP in both cytosol and nucleus, whereas metformin decreases CHOP content in both cytosol and the nucleus. Metformin improves the maximal rate of oxidative phosphorylation with decreased ROS generation in THAP-treated hearts. The sensitivity of the MPTP opening is decreased in metformin + THAP treated hearts. These results suggest that activation of AMPK using metformin decreases the ER stress-induced cell death (Figure 2D). Therefore, chronic metformin treatment may be an alternative strategy to decrease cardiac injury during the ER stress.
Although the initial response to ER stress is to restore ER function by activating specific signaling pathways to increase chaperon protein expression, severe ER stress inevitably increases cell injury and favors cell death.1, 5 In the current study, THAP treatment not only increases cytosolic CHOP expression, but also increases the content of CHOP in the nucleus. An induction of the CHOP expression is a key factor to induce apoptosis during the ER stress. Genetic ablation of CHOP decreases apoptosis and inflammation in hearts following ischemia-reperfusion,39 supporting that CHOP expression plays a key role in cell injury. In cell lines, the overexpression of CHOP increases apoptosis, whereas ablation of the CHOP reduces apoptosis during the ER stress.40 Thus, CHOP plays a key role in the induction of apoptosis during the ER stress. Metformin decreases the expression of CHOP in cytosol and nucleus in the THAP-treated mice, suggesting that metformin protects cells during ER stress through inhibition of CHOP expression. Further study will evaluate the protection from metformin in CHOP-knock out mice.
The increased CHOP content in cytosol leads to reduced bcl-2 content that increases cytosolic BH3-only pro-apoptotic proteins including bax to trigger apoptosis during the ER stress.6 The increased CHOP content in the nucleus leads to a decrease in cytosolic bcl-2 content by retaining bcl-2 at the nucleus through formation of bcl-2 and ASPP2 (Apoptosis-stimulating protein of p53-2) complex.5 Overexpression of CHOP also decreases cellular glutathione content and increases the production of reactive oxygen species in the ER.6 Nuclear factor-κB (NF-κB) is a key transcription factor regulating inflammation.41 An increase in CHOP expression in the nucleus favors NF-κB activation. Knockout of CHOP prevents the cytokine-induced activation of the NF-κB, supporting that the CHOP expression increases inflammation through activation of NF-κB. The increased inflammation contributes to cardiac injury. The THAP-induced ER stress increases cardiac injury through augmentation of the CHOP expression and inflammation.42
Activation of the AMPK using 5-aminoimidazole-4-carboxyamide-1-beta-D-ribofuranoside (AICAR, an AMPK activator) decreases the ER stress and CHOP expression in cardiac myocytes during hypoxia-reoxygenation.43 In the present study, activation of the AMPK in vivo using metformin decreases the THAP-induced CHOP expression. In addition, metformin treatment also decreases CHOP content in the nucleus. Thus, the current study supports that activation of AMPK decreases cardiac injury during ER stress by preventing CHOP expression.
Increased oxidative stress is a key factor to induce CHOP expression during ER stress. In isolated cardiac myocytes, glucose deprivation activates Nox (NADPH oxidase) 4 in the ER that leads to increased ROS generation.1 Genetic knockout of Nox 4 decreases the ROS generation during glucose deprivation, supporting that Nox 4 is a key source of ROS generation in glucose deprivation-induced ER stress. Glucose deprivation does not increase ROS generation from mitochondria.1 In the present study, the THAP-induced ER stress increases ROS generation from mitochondria. THAP-induced ER stress increases ROS generation from NADPH oxidase in H9C2 myoblasts.14 Thus, the THAP-induced ER stress may increase ROS generation from both the ETC and ER.
The generation of ROS is dramatically increased in mitochondria from the THAP-treated hearts compared to control when succinate is used as substrate with rotenone to block reverse electron flow.44 The ROS generation is not significantly altered in the presence of complex I substrates. Metformin treatment decreases the ROS generation in the THAP-treated hearts. These results suggest that activation of AMPK using metformin may decrease CHOP expression by reducing ROS generation from the ETC.
The mechanisms by which metformin treatment decreases ROS generation are not clear. Metformin feeding does not affect antioxidant content in unstressed rats.45 However, metformin improves the antioxidant content in high-fructose-fed rats.45 Thus, metformin feeding may decrease ROS generation through improvement of mitochondrial antioxidants. In addition to ROS generation, THAP treatment impairs the ETC. The decreased oxidative phosphorylation with complex I substrates but not with complex II substrates indicates that THAP leads to damage at the complex I. Metformin treatment improves the oxidative phosphorylation in THAP-treated hearts oxidizing complex I substrates. Thus, metformin feeding may decrease ROS generation in THAP-treated mice by protecting the ETC and improving antioxidant capacity. A recent study found that MPTP opening can increase ROS generation at complex II, especially in the presence of decreased complex III activity.46 In Ca2+ over load-induced PTP opening, H2O2 generation can be increased through activation of glycerophosphate dehydrogenase located in the outer surface of the inner membrane.47 Our study shows that THAP sensitizes MPTP opening in heart mitochondria, whereas metformin treatment inhibit MPTP opening (see discussion below). Thus, we propose that the decreased ROS at complex II in metformin treated mitochondria was likely due to decreased MPTP opening.
MPTP opening is a final downstream step to induce cell death during ischemia-reperfusion.48 THAP treatment has been shown to increase MPTP opening in cultured cells as shown by the depolarized inner mitochondrial membrane potential.14 In the present study, THAP treatment leads to increased sensitivity to MPTP opening in isolated heart mitochondria. Thus, our study provides direct evidence that THAP-induced ER stress sensitizes to MPTP opening. In the current study, administration of metformin improves the CRC in cardiac mitochondria following THAP treatment, supporting that deactivation of AMPK may be involved in MPTP opening. ER stress leads to increased cytosolic calcium that sets the foundation to induce mitochondrial calcium overload. Mitochondrial calcium overload and increased ROS generation are two key factors to induce MPTP opening.48 The decreased ROS generation observed following metformin treatment may lead to decreased MPTP opening observed in mitochondria after in vivo THAP treatment.
Although metformin decreases cardiac injury during ER stress in male mice, metformin treatment may affect female mice differently. There are gender-related differences in antioxidant capacity in mice.49 Also, the phosphorylation of akt is enhanced in female diabetic mice compared to male mice, a potentially cardioprotective increase.50 Therefore, the effect of metformin treatment on ER stress-induced myocardial injury warrants further investigation.
The present study demonstrates that activation of AMPK using metformin decreases cardiac injury during the THAP-induced ER stress. ER stress is increased in many pathological conditions including heart failure, aging, and ischemia-reperfusion. ER stress contributes to cardiomyopathy in the type 2 diabetic rat heart.51 ER stress contributes to a transition from cardiac hypertrophy to heart failure in humans.52 High fat diet induces apoptosis through increased ER stress in mouse hearts.53 Therapeutic interventions to decrease ER stress reduce cardiac hypertrophy and the transition to heart failure in animals following ischemia reperfusion injury or pressure overload.52 The risk of heart failure occurrence is dramatically increased in diabetic patients exposed to ischemia-reperfusion injury.20, 54 Metformin was historically considered to place heart failure patients at increased due to the potential occurrence of lactic acidosis in the setting of reduced renal function.55 However, recent studies show that metformin is a safe treatment in heart failure patients with diabetes.20 Metformin treatment provides cardiac protection in diabetic hearts following experimental ischemia-reperfusion.56 In experimental models, the administration of metformin decreases the incidence of progression of heart failure.19 Metformin also attenuates the transition to heart failure transition in non-diabetic rodents following myocardial infarction and pressure overload.17, 57–59 In the current study, the chronic feeding of metformin decreases the ER stress-induced apoptosis in mouse hearts, indicating that targeting of the ER stress is a potential approach to decrease the occurrence or progression of heart failure. Therefore, chronic metformin treatment may be a promising strategy to decrease cardiac injury, especially in diabetic patients,18 but also possibly in non-diabetic patients as well. This study highlights the potential to “repurpose” metformin, a current FDA-approved drug for a novel therapeutic use in heart failure treatment.
Background.
ER stress contributes to cardiac injury and plays a key role in the development of heart failure. Metformin is a widely used anti-diabetic drug that also activates cytoprotective mechanisms. It is unclear if metformin can decrease ER-stress-mediated cardiac injury.
Translational Significance.
Our studies show that metformin treatment decreases myocardial injury during ER stress through attenuation of mitochondrial damage. The reduction of ER stress and subsequent mitochondrial damage with chronic metformin treatment may be a new potential therapeutic target for treatment of heart failure.
Acknowledgments
Conflict interest: All authors have read the journal’s policy on authorship agreement and disclosure of potential conflicts of interest and have none to declare.
The authors have read the journal’s authorship agreement and the manuscript has been reviewed and approved by all named authors.
This work was supported by a Grant-in-aid (15GRNT24480123) from the American Heart Association (QC), VCU’s CTSA (UL1TR000058 from the National Institutes of Health’s National Center for Advancing Translational Science) and the CCTR Endowment Fund of the Virginia Commonwealth University (QC and AD), the Office of Research and Development, Medical Research Service Merit Review Award (2IO1BX001355-01A2), Department of Veterans Affairs (EJL), the National Institutes of Health RO1HL134366 (AD) and the Pauley Heart Center, Virginia Commonwealth University (QC, AD, EJL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sciarretta S, Zhai P, Shao D, Zablocki D, Nagarajan N, Terada LS, et al. Activation of NADPH oxidase 4 in the endoplasmic reticulum promotes cardiomyocyte autophagy and survival during energy stress through the protein kinase RNA-activated-like endoplasmic reticulum kinase/eukaryotic initiation factor 2alpha/activating transcription factor 4 pathway. Circ Res. 2013;113(11):1253–64. doi: 10.1161/CIRCRESAHA.113.301787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sreedhar R, Arumugam S, Thandavarayan RA, Giridharan VV, Karuppagounder V, Pitchaimani V, et al. Depletion of cardiac 14–3-3eta protein adversely influences pathologic cardiac remodeling during myocardial infarction after coronary artery ligation in mice. Int J Cardiol. 2016;202:146–53. doi: 10.1016/j.ijcard.2015.08.142. [DOI] [PubMed] [Google Scholar]
- 3.Sciarretta S, Volpe M, Sadoshima J. NOX4 regulates autophagy during energy deprivation. Autophagy. 2014;10(4):699–701. doi: 10.4161/auto.27955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdelwahid E, Li H, Wu J, Irioda AC, de Carvalho KA, Luo X. Endoplasmic reticulum (ER) stress triggers Hax1-dependent mitochondrial apoptotic events in cardiac cells. Apoptosis. 2016;21(11):1227–39. doi: 10.1007/s10495-016-1286-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu K, Shi Y, Guo X, Wang S, Ouyang Y, Hao M, et al. CHOP mediates ASPP2-induced autophagic apoptosis in hepatoma cells by releasing Beclin-1 from Bcl-2 and inducing nuclear translocation of Bcl-2. Cell Death Dis. 2014;5:e1323. doi: 10.1038/cddis.2014.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nishitoh H. CHOP is a multifunctional transcription factor in the ER stress response. J Biochem. 2012;151(3):217–9. doi: 10.1093/jb/mvr143. [DOI] [PubMed] [Google Scholar]
- 7.Lesnefsky EJ, Chen Q, Hoppel CL. Mitochondrial Metabolism in Aging Heart. Circ Res. 2016;118(10):1593–611. doi: 10.1161/CIRCRESAHA.116.307505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lesnefsky EJ, Chen Q, Tandler B, Hoppel CL. Mitochondrial Dysfunction and Myocardial Ischemia-Reperfusion: Implications for Novel Therapies. Annu Rev Pharmacol Toxicol. 2017;57:535–65. doi: 10.1146/annurev-pharmtox-010715-103335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang M, Stowe DF, Udoh KB, Heisner JS, Camara AK. Reversible blockade of complex I or inhibition of PKCbeta reduces activation and mitochondria translocation of p66Shc to preserve cardiac function after ischemia. PLoS One. 2014;9(12):e113534. doi: 10.1371/journal.pone.0113534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cabrera JA, Butterick TA, Long EK, Ziemba EA, Anderson LB, Duffy CM, et al. Reduced expression of mitochondrial electron transport chain proteins from hibernating hearts relative to ischemic preconditioned hearts in the second window of protection. J Mol Cell Cardiol. 2013;60:90–6. doi: 10.1016/j.yjmcc.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 11.Agarwal B, Stowe DF, Dash RK, Bosnjak ZJ, Camara AK. Mitochondrial targets for volatile anesthetics against cardiac ischemia-reperfusion injury. Front Physiol. 2014;5:341. doi: 10.3389/fphys.2014.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wieckowski MR, Giorgi C, Lebiedzinska M, Duszynski J, Pinton P. Isolation of mitochondria-associated membranes and mitochondria from animal tissues and cells. Nat Protoc. 2009;4(11):1582–90. doi: 10.1038/nprot.2009.151. [DOI] [PubMed] [Google Scholar]
- 13.Paillard M, Tubbs E, Thiebaut PA, Gomez L, Fauconnier J, Da Silva CC, et al. Depressing mitochondria-reticulum interactions protects cardiomyocytes from lethal hypoxia-reoxygenation injury. Circulation. 2013;128(14):1555–65. doi: 10.1161/CIRCULATIONAHA.113.001225. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Ren J. Thapsigargin triggers cardiac contractile dysfunction via NADPH oxidase-mediated mitochondrial dysfunction: Role of Akt dephosphorylation. Free Radic Biol Med. 2011;51(12):2172–84. doi: 10.1016/j.freeradbiomed.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Ambrosio G, Flaherty JT. Effects of the superoxide radical scavenger superoxide dismutase, and of the hydroxyl radical scavenger mannitol, on reperfusion injury in isolated rabbit hearts. Cardiovasc Drugs Ther. 1992;6(6):623–32. doi: 10.1007/BF00052564. [DOI] [PubMed] [Google Scholar]
- 16.Lesnefsky EJ, Chen Q, Moghaddas S, Hassan MO, Tandler B, Hoppel CL. Blockade of electron transport during Ischemia protects cardiac mitochondria. J Biol Chem. 2004;279(46):47961–7. doi: 10.1074/jbc.M409720200. [DOI] [PubMed] [Google Scholar]
- 17.Gundewar S, Calvert JW, Jha S, Toedt-Pingel I, Ji SY, Nunez D, et al. Activation of AMP-activated protein kinase by metformin improves left ventricular function and survival in heart failure. Circ Res. 2009;104(3):403–11. doi: 10.1161/CIRCRESAHA.108.190918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holman R. Metformin as first choice in oral diabetes treatment: the UKPDS experience. Journ Annu Diabetol Hotel Dieu. 2007:13–20. [PubMed] [Google Scholar]
- 19.Sasaki H, Asanuma H, Fujita M, Takahama H, Wakeno M, Ito S, et al. Metformin prevents progression of heart failure in dogs: role of AMP-activated protein kinase. Circulation. 2009;119(19):2568–77. doi: 10.1161/CIRCULATIONAHA.108.798561. [DOI] [PubMed] [Google Scholar]
- 20.Eurich DT, Weir DL, Majumdar SR, Tsuyuki RT, Johnson JA, Tjosvold L, et al. Comparative safety and effectiveness of metformin in patients with diabetes mellitus and heart failure: systematic review of observational studies involving 34,000 patients. Circ Heart Fail. 2013;6(3):395–402. doi: 10.1161/CIRCHEARTFAILURE.112.000162. [DOI] [PubMed] [Google Scholar]
- 21.Lu DY, Huang CC, Huang PH, Chung CM, Lin SJ, Chen JW, et al. Metformin use in patients with type 2 diabetes mellitus is associated with reduced risk of deep vein thrombosis: a non-randomized, pair-matched cohort study. BMC Cardiovasc Disord. 2014;14:187. doi: 10.1186/1471-2261-14-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daskalopoulos EP, Dufeys C, Bertrand L, Beauloye C, Horman S. AMPK in cardiac fibrosis and repair: Actions beyond metabolic regulation. J Mol Cell Cardiol. 2016;91:188–200. doi: 10.1016/j.yjmcc.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Ma Y, Li J. Metabolic shifts during aging and pathology. Compr Physiol. 2015;5(2):667–86. doi: 10.1002/cphy.c140041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang H, Sun W, Quan N, Wang L, Chu D, Cates C, et al. Cardioprotective actions of Notch1 against myocardial infarction via LKB1-dependent AMPK signaling pathway. Biochem Pharmacol. 2016;108:47–57. doi: 10.1016/j.bcp.2016.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zaha VG, Qi D, Su KN, Palmeri M, Lee HY, Hu X, et al. AMPK is critical for mitochondrial function during reperfusion after myocardial ischemia. J Mol Cell Cardiol. 2016;91:104–13. doi: 10.1016/j.yjmcc.2015.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Q, Paillard M, Gomez L, Ross T, Hu Y, Xu A, et al. Activation of mitochondrial mu-calpain increases AIF cleavage in cardiac mitochondria during ischemia-reperfusion. Biochem Biophys Res Commun. 2011;415(4):533–8. doi: 10.1016/j.bbrc.2011.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson J, Hu Y, Lesnefsky EJ, Chen Q. Activation of mitochondrial calpain and increased cardiac injury: beyond AIF release. Am J Physiol Heart Circ Physiol. 2016;310(3):H376–84. doi: 10.1152/ajpheart.00748.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim H, Moon SY, Kim JS, Baek CH, Kim M, Min JY, et al. Activation of AMP-activated protein kinase inhibits ER stress and renal fibrosis. Am J Physiol Renal Physiol. 2015;308(3):F226–36. doi: 10.1152/ajprenal.00495.2014. [DOI] [PubMed] [Google Scholar]
- 29.Duan Q, Song P, Ding Y, Zou MH. Activation of AMP-activated protein kinase by metformin ablates angiotensin II-induced endoplasmic reticulum stress and hypertension in mice in vivo. Br J Pharmacol. 2017;174(13):2140–51. doi: 10.1111/bph.13833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simon-Szabo L, Kokas M, Mandl J, Keri G, Csala M. Metformin attenuates palmitate-induced endoplasmic reticulum stress, serine phosphorylation of IRS-1 and apoptosis in rat insulinoma cells. PLoS One. 2014;9(6):e97868. doi: 10.1371/journal.pone.0097868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rieusset J, Fauconnier J, Paillard M, Belaidi E, Tubbs E, Chauvin MA, et al. Disruption of calcium transfer from ER to mitochondria links alterations of mitochondria-associated ER membrane integrity to hepatic insulin resistance. Diabetologia. 2016;59(3):614–23. doi: 10.1007/s00125-015-3829-8. [DOI] [PubMed] [Google Scholar]
- 32.Whittington HJ, Hall AR, McLaughlin CP, Hausenloy DJ, Yellon DM, Mocanu MM. Chronic metformin associated cardioprotection against infarction: not just a glucose lowering phenomenon. Cardiovasc Drugs Ther. 2013;27(1):5–16. doi: 10.1007/s10557-012-6425-x. [DOI] [PubMed] [Google Scholar]
- 33.Das A, Durrant D, Koka S, Salloum FN, Xi L, Kukreja RC. Mammalian target of rapamycin (mTOR) inhibition with rapamycin improves cardiac function in type 2 diabetic mice: potential role of attenuated oxidative stress and altered contractile protein expression. J Biol Chem. 2014;289(7):4145–60. doi: 10.1074/jbc.M113.521062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szczepanek K, Chen Q, Derecka M, Salloum FN, Zhang Q, Szelag M, et al. Mitochondrial-targeted Signal transducer and activator of transcription 3 (STAT3) protects against ischemia-induced changes in the electron transport chain and the generation of reactive oxygen species. J Biol Chem. 2011;286(34):29610–20. doi: 10.1074/jbc.M111.226209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu A, Szczepanek K, Hu Y, Lesnefsky EJ, Chen Q. Cardioprotection by modulation of mitochondrial respiration during ischemia-reperfusion: role of apoptosis-inducing factor. Biochem Biophys Res Commun. 2013;435(4):627–33. doi: 10.1016/j.bbrc.2013.05.033. [DOI] [PubMed] [Google Scholar]
- 36.Paillard M, Gomez L, Augeul L, Loufouat J, Lesnefsky EJ, Ovize M. Postconditioning inhibits mPTP opening independent of oxidative phosphorylation and membrane potential. J Mol Cell Cardiol. 2009;46(6):902–9. doi: 10.1016/j.yjmcc.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 37.Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ. Production of reactive oxygen species by mitochondria: Central role of complex III. J Biol Chem. 2003;278:36027–31. doi: 10.1074/jbc.M304854200. [DOI] [PubMed] [Google Scholar]
- 38.Steel R, Torrie J. Principles and procedures of statistics. New York: Mc Graw-Hill; 1960. [Google Scholar]
- 39.Miyazaki Y, Kaikita K, Endo M, Horio E, Miura M, Tsujita K, et al. C/EBP homologous protein deficiency attenuates myocardial reperfusion injury by inhibiting myocardial apoptosis and inflammation. Arterioscler Thromb Vasc Biol. 2011;31(5):1124–32. doi: 10.1161/ATVBAHA.111.224519. [DOI] [PubMed] [Google Scholar]
- 40.Zhang W, Chen L, Shen Y, Xu J. Rifampicin-induced injury in L02 cells is alleviated by 4-PBA via inhibition of the PERK-ATF4-CHOP pathway. Toxicol In Vitro. 2016;36:186–96. doi: 10.1016/j.tiv.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 41.Allagnat F, Fukaya M, Nogueira TC, Delaroche D, Welsh N, Marselli L, et al. C/EBP homologous protein contributes to cytokine-induced pro-inflammatory responses and apoptosis in beta-cells. Cell Death Dis. 2012;19(11):1836–46. doi: 10.1038/cdd.2012.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lebeaupin C, Proics E, de Bieville CH, Rousseau D, Bonnafous S, Patouraux S, et al. ER stress induces NLRP3 inflammasome activation and hepatocyte death. Cell Death Dis. 2015;6:e1879. doi: 10.1038/cddis.2015.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Terai K, Hiramoto Y, Masaki M, Sugiyama S, Kuroda T, Hori M, et al. AMP-activated protein kinase protects cardiomyocytes against hypoxic injury through attenuation of endoplasmic reticulum stress. Mol Cell Biol. 2005;25(21):9554–75. doi: 10.1128/MCB.25.21.9554-9575.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ross T, Szczepanek K, Bowler E, Hu Y, Larner A, Lesnefsky EJ, et al. Reverse electron flow-mediated ROS generation in ischemia-damaged mitochondria: role of complex I inhibition vs. depolarization of inner mitochondrial membrane. Biochim Biophys Acta. 2013;1830(10):4537–42. doi: 10.1016/j.bbagen.2013.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Srividhya S, Anuradha CV. Metformin improves liver antioxidant potential in rats fed a high-fructose diet. Asia Pac J Clin Nutr. 2002;11(4):319–22. doi: 10.1046/j.1440-6047.2002.00306.x. [DOI] [PubMed] [Google Scholar]
- 46.Korge P, John SA, Calmettes G, Weiss JN. Reactive oxygen species production induced by pore opening in cardiac mitochondria: The role of complex II. J Biol Chem. 2017;292(24):9896–905. doi: 10.1074/jbc.M116.768325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tretter L, Adam-Vizi V. High Ca2+ load promotes hydrogen peroxide generation via activation of alpha-glycerophosphate dehydrogenase in brain mitochondria. Free Radic Biol Med. 2012;53(11):2119–30. doi: 10.1016/j.freeradbiomed.2012.09.029. [DOI] [PubMed] [Google Scholar]
- 48.Weiss JN, Korge P, Honda HM, Ping P. Role of the mitochondrial permeability transition in myocardial disease. Circ Res. 2003;93(4):292–301. doi: 10.1161/01.RES.0000087542.26971.D4. [DOI] [PubMed] [Google Scholar]
- 49.Chen Y, Ji LL, Liu TY, Wang ZT. Evaluation of gender-related differences in various oxidative stress enzymes in mice. Chin J Physiol. 2011;54(6):385–90. doi: 10.4077/CJP.2011.AMM080. [DOI] [PubMed] [Google Scholar]
- 50.Ceylan-Isik AF, LaCour KH, Ren J. Sex difference in cardiomyocyte function in normal and metallothionein transgenic mice: the effect of diabetes mellitus. J Appl Physiol. 2006;100(5):1638–46. doi: 10.1152/japplphysiol.01273.2005. [DOI] [PubMed] [Google Scholar]
- 51.Liu X, Xu Q, Wang X, Zhao Z, Zhang L, Zhong L, et al. Irbesartan ameliorates diabetic cardiomyopathy by regulating protein kinase D and ER stress activation in a type 2 diabetes rat model. Pharmacol Res. 2015;93:43–51. doi: 10.1016/j.phrs.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 52.Cominacini L, Mozzini C, Garbin U, Pasini A, Stranieri C, Solani E, et al. Endoplasmic reticulum stress and Nrf2 signaling in cardiovascular diseases. Free Radic Biol Med. 2015;88(Pt B):233–42. doi: 10.1016/j.freeradbiomed.2015.05.027. [DOI] [PubMed] [Google Scholar]
- 53.Hsu HC, Chen CY, Lee BC, Chen MF. High-fat diet induces cardiomyocyte apoptosis via the inhibition of autophagy. Eur J Nutr. 2016;55(7):2245–54. doi: 10.1007/s00394-015-1034-7. [DOI] [PubMed] [Google Scholar]
- 54.Tate M, Grieve DJ, Ritchie RH. Are targeted therapies for diabetic cardiomyopathy on the horizon? Clin Sci (Lond) 2017;131(10):897–915. doi: 10.1042/CS20160491. [DOI] [PubMed] [Google Scholar]
- 55.Bolen S, Feldman L, Vassy J, Wilson L, Yeh HC, Marinopoulos S, et al. Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann Intern Med. 2007;147(6):386–99. doi: 10.7326/0003-4819-147-6-200709180-00178. [DOI] [PubMed] [Google Scholar]
- 56.Calvert JW, Gundewar S, Jha S, Greer JJ, Bestermann WH, Tian R, et al. Acute metformin therapy confers cardioprotection against myocardial infarction via AMPK-eNOS-mediated signaling. Diabetes. 2008;57(3):696–705. doi: 10.2337/db07-1098. [DOI] [PubMed] [Google Scholar]
- 57.Wang Y, Shoemaker R, Powell D, Su W, Thatcher S, Cassis L. Differential effects of Mas receptor deficiency on cardiac function and blood pressure in obese male and female mice. Am J Physiol Heart Circ Physiol. 2017;312(3):H459–h68. doi: 10.1152/ajpheart.00498.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu X, Lu Z, Fassett J, Zhang P, Hu X, Liu X, et al. Metformin protects against systolic overload-induced heart failure independent of AMP-activated protein kinase alpha2. Hypertension. 2014;63(4):723–8. doi: 10.1161/HYPERTENSIONAHA.113.02619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lexis CP, Wieringa WG, Hiemstra B, van Deursen VM, Lipsic E, van der Harst P, et al. Chronic metformin treatment is associated with reduced myocardial infarct size in diabetic patients with ST-segment elevation myocardial infarction. Cardiovasc Drugs Ther. 2014;28(2):163–71. doi: 10.1007/s10557-013-6504-7. [DOI] [PubMed] [Google Scholar]