Abstract
Worldwide increase incidences of allergic diseases have heightened the interest of clinicians and researchers to understand the role of neuroendocrine cells in the recruitment and activation of inflammatory cells. Several pieces of evidence revealed the association of neuropeptides in the pathogenesis of allergic diseases. Importantly, one such peptide that is secreted by neuronal cells and immune cells exerts a wide spectrum of immunological functions as cytokine/chemokine is termed as Vasoactive Intestinal Peptide (VIP). VIP mediates immunological function through interaction with specific receptors namely VPAC-1, VPAC-2, CRTH2 and PAC1 that are expressed on several immune cells such as eosinophils, mast cells, neutrophils, and lymphocytes; therefore, provide the basis for the action of VIP on the immune system. Additionally, VIP mediated action varies according to target organ depending upon the presence of specific VIP associated receptor, involved immune cells and the microenvironment of the organ. Herein, we present an integrative review of the current understanding on the role of VIP and associated receptors in allergic diseases, the presence of VIP receptors on various immune cells with particular emphasis on the role of VIP in the pathogenesis of allergic diseases such as asthma, allergic rhinitis, and atopic dermatitis. Being crucial signal molecule of the neuroendocrine-immune network, the development of stable VIP analogue and/or antagonist may provide the future therapeutic drug alternative for the better treatment of these allergic diseases. Taken together, our current review summarizes the current understandings of VIP biology and further explore the significance of neuroendocrine cells derived VIP in the recruitment of inflammatory cells in allergic diseases that may be helpful to the investigators for planning the experiments and accordingly predicting new therapeutic strategies for combating allergic diseases. Summarized graphical abstract will help the readers to understand the significance of VIP in allergic diseases.
Keywords: Allergy, Asthma, EoE, Eosinophils, Mast cells, VIP
Graphical Abstract
1. Introduction
In the last few decades, the allergic diseases have become more prevalent affecting up to 20–30% of the world population that results in lower life quality [1, 2]. The condition becomes worse as the occurrence of one allergic disease leads to predisposition for another [3]. Importantly, the recent advances to understand the pathogenesis of allergic diseases have highlighted the neuroimmune interaction as a crucial player in the development of allergic diseases; therefore, the understanding of neuroimmune communication is particularly required for gaining the better knowledge in the context of allergic diseases [4]. The link between the neuroendocrine system and the immune system depends upon the several key factors such as the secretion of neuropeptides by immune cells and cytokines by neuroendocrine cells, the presence of shared receptors on the cells for both the systems, the effect of neuropeptides and cytokines on immunological and neurological functions respectively. All these regulatory processes provide the basis for neuropeptides to function as cytokine or chemokine in the immune system thereby modulating the provoked immune responses [5]. Interestingly, the large number of neuropeptides such as Calcitonin Gene-Related Peptide (CGRP), Substance P (SP), Protein gene product 9.5, Neuropeptide Y, Nerve growth factor, and Vasoactive Intestinal Peptide (VIP) have been implicated in the pathogenesis of the allergic diseases [6, 7]. However, in the last decade, VIP has emerged as one such potent neuropeptide that exerts a wide spectrum of immunological functions controlling both innate and adaptive immunity. VIP exerts its biological function by regulating the production of both anti- and pro-inflammatory mediators. In terms of innate immunity, VIP inhibits the production of inflammatory cytokines and chemokines from immune cells as well as reduces the expression of co-stimulatory molecules on antigen-presenting cells that reduces the stimulation of antigen-specific CD4+T cells. Additionally, in adaptive immunity, VIP promotes T-helper (Th)2 type immune responses and reduces inflammatory Th1 type responses [8].
VIP is 28-amino acid peptide hormone that was originally isolated as a vasodilator peptide from the intestine [9, 10]. VIP is reported as one of the most abundant neuropeptides of the human body and secreted in the significant amount in the central and peripheral nervous systems as well as in various peripheral tissues and organs [11]. Although VIP is primarily secreted by neuronal tissue, it is also produced by several immune cells such as eosinophils, mast cells and lymphocytes [12–14]. The secreted VIP like cytokine or chemokine has the ability to regulate the homeostasis of the immune system. The expression of VIP is noticed in the brain, heart, lungs, kidney, urinary bladder, pancreas and gastrointestinal tract [15]. Importantly, the sequence of VIP peptide is well conserved among the different species and is identical in human, mice, cow, pig and dog species suggesting the significance of this neuropeptide in immunomodulation [16]. Further, the presence of VIP-containing nerves near the elements of the immune system and the presence of VIP receptors on immune cells indicate the interaction of VIP with immune system [11]. The immunological responses of VIP are mediated through interaction with specific receptors namely Vasoactive Intestinal Peptide Receptor Type 1 (VPAC-1), Vasoactive Intestinal Peptide Receptor Type 2 (VPAC-2), Chemoattractant Receptor-Homologous Molecule Expressed on Th2 Cells (CRTH2) and Pituitary adenylate cyclase-activating polypeptide (PACAP) receptors (PAC1) that are present on different types of immune cells [17, 18]. In addition, the emerging pieces of evidence have indicated that VIP and its receptors mediated signaling plays the decisive role in the pathogenesis of allergic diseases such as asthma, allergic rhinitis, dermatitis and food allergy [18–20]. Therefore, it will be of great significance to explore the mechanistic pathways involved in VIP mediated biological effect on the immune system that may open the novel avenues for therapeutic intervention in allergic diseases. The review provides most current knowledge and understanding of VIP mediated diverse biological functions on the immune system, the interaction of VIP with specific receptors that are expressed on various immune cells, further to understand the VIP induced signaling pathways with special attention on the impact of VIP in the pathogenesis of allergic diseases. Furthermore, exploring the pathways involved in cross-talk between the nervous and immune system may be useful for understanding the neuropeptide mediated signaling events in the pathophysiology of allergic diseases. The summarized information will be helpful to propose VIP and its agonists/antagonists as promising alternative candidates for the treatment of the allergic diseases.
2. General characteristics of Vasoactive Intestinal Peptide and its receptors
VIP was first isolated from swine duodenum and named so because of its vasodilating action that modifies the intestinal blood flow [10]. VIP works as ‘cytokine-like peptide’ and exerts a wide spectrum of biological functions through specific receptors present on immune effector cells, thus triggering a signal transduction cascade, and regulating the immunological response by the production of both anti and pro-inflammatory mediators [21, 22]. Basically, human VIP gene encodes 7 exons and is localized to chromosome 6q25.2. VIP is synthesized as a precursor molecule where VIP is encoded by the fifth exon and signal peptide of 22 amino acids by the second exon. The active VIP molecule of 28 amino acids is produced after the processing of precursor molecule [23, 24]. VIP is reported to have the helical conformation with one α-helix (residues11–26) and two β-bends (residues 2–5 and 7–10) at the N-terminus [25]. As VIP has high similarity in primary and secondary structures with glucagon/secretin, thus VIP classified with these peptides in glucagon/secretin superfamily, the ligand of class II G protein–coupled receptors. This family also includes peptides such as glucagon-like peptides (GLPs), gastric inhibitory polypeptide (GIP), corticotropin-releasing factor (CRF), growth hormone-releasing factor (GRF), parathyroid hormone (PTH), calcitonin and pituitary adenylate cyclase-activating peptide (PACAP) [26]. VIP expressed in numerous organs, and tissues at various concentrations thereby exert an array of different biological effects in the body. The biological functions of VIP are mediated via specific receptors namely VPAC-1, VPAC-2, CRTH2 and PAC1 that are expressed on immune cells. VIP having low affinity for PAC1 while for remaining receptors affinity is comparatively high. The general characteristics of VIP receptors are summarized in Table 1.
Table 1.
Characterization of VIP associated receptors
| Receptors | VPAC-1 | VPAC-2 | PAC1 | CRTH2 |
|---|---|---|---|---|
| Gene name | VIPR1 | VIPR2 | ADCYAP1R1 | PTGDR2 |
| Human chromosome location | 3p22 | 7q36.3 | 7p14 | 11q12.2 |
| Receptor type | G-protein coupled receptor | G-protein coupled receptor | G-protein coupled receptor | G-protein coupled receptor |
| Tissue expression | CNS and peripheral tissues including liver, lung and intestine | CNS and Peripheral tissues, including smooth muscles in cardiovascular, gastrointestinal and reproductive systems | CNS, anterior pituitary, pancreatic acini, uterus, and adrenal medulla | CNS, thymus, digestive tract, heart, spleen, spinal cord, skeletal muscle |
| mRNA expression on immune cells | T lymphocytes, Neutrophils, Monocytes, Macrophages, Thymocyte, Keratinocyte THP-1 (monocytic) | Mast cells, Thymocyte, Keratinocyte. Lymphocytes, Macrophages (Upon activation) |
Macrophages THP-1 (monocytic) |
Eosinophils, Basophils, Th2 cells, Mast cells, monocytes |
| Selective agonists | [Ala11,22,28]VIP [K15, R16, L27]VIP(1– 7)/GRF(8–27)-NH2 |
Ro 25–1392; Ro 25–1553 |
Maxadilan | Delta12-prostaglandin D2; Δ12-Prostaglandin D2; L-888,607 |
| Selective antagonist | PG97–269 | PG99–465 | Max.d.4; M65; PACAP (6–38). |
Ramatroban; OC000459; Fevipiprant; Setipiprant. |
| VIP mediated Signaling mechanism | Primarily coupled to adenylate cyclase | Primarily coupled to adenylate cyclase | Adenylate cyclase/protein kinase A (PKA) and phospholipase C (PLC)/protein kinase C (PKC) pathways | VIP-CRTH2 ligation involved Ca2+ independent PKC and PKA cytosol-to-membrane translocation |
2.1. VPAC receptors (VPAC-1 & VPAC-2)
The VPAC receptors (VPAC-1 & VPAC-2) are widely distributed in the body suggesting the crucial role in the diverse immunological processes [27]. These VPAC receptors belong to the subfamily of G protein-coupled receptor family (GPCR) known as class B GPCR. This subfamily is also known as ‘secretin-like’ receptors family because the secretin receptor was the first member of a new GPCR family. These receptors consist of one polypeptide chain with seven transmembrane segments having N-terminal extracellular domain and C-terminal cytoplasmic end [28]. VPAC-1 receptors are mainly expressed in the central nervous system (CNS), liver, lung, breast, kidney, prostate, spleen, mucosa of stomach and small intestine [29] while VPAC-2 receptors have been localized in the CNS, pancreas, lung, heart, kidney, stomach skeletal muscle, smooth muscle, adipose tissue, and thyroid follicular cells [30]. Structurally, VPAC-1 receptor consists of 457 amino acids with a long extracellular N-terminal having Mw of 52 kDa. VPAC-1 receptor gene (VIPR1) is composed of 13 exons ranging in size from 42 to 1400 bp and located on short arm of human chromosome 3 (3p22) [31, 32]. However, VPAC-2 receptor consists of 438 amino acids with Mw of 49 kDa. The human VPAC-2 receptor gene (VIPR2) is reported to be present on chromosome 7q36.3 and encoded by 13 exons [33, 34]. Both VPAC-1 and VPAC-2 receptors possess highly conserved amino acid sequence suggesting that these receptors may be evolved from a common ancestral gene thereby transduce the signal in the same way from the extracellular surface to the underlying involved molecules upon ligand binding [35]. Importantly, VPAC-1 receptors are expressed constitutively on T cells, monocytes, and macrophages whereas the expression of VPAC-2 receptors are induced on T cells and macrophages only after bacterial/or viral infections [36].
These VPAC receptors also interact with few accessory proteins that modulate the signaling events. These proteins are commonly known as accessory proteins’ or ‘GPCR interacting proteins which provided a new concept in GPCR signaling to transduce the signals independently of the G-proteins [37]. GPCR mediated signaling is also affected by Calmodulin (CaM) i.e. a calcium-binding messenger protein expressed in all eukaryotic cells. Upon binding to Ca2+, Calmodulin acts as part of calcium signal transduction pathway by promoting the different modes of association with many target protein such as kinases or phosphatases as well as GPCR signaling involved enzymes such as adenylyl cyclase, phospholipases etc [38]. Another study by Gee et al., (2009) suggested that S-SCAM (synaptic scaffolding molecule), also named membrane-associated guanylate kinase inverted-2 (MAGI-2), interacts and regulates VPAC-1 intracellular localization in epithelial cells and also inhibits VPAC-1 agonist-induced activation and internalization. The physical interaction between VPAC-1 and S-SCAM was confirmed by immunoprecipitation in HEK 293 mammalian cells and human pancreatic and colonic tissues [39].
2.2. Chemoattractant receptor-homologous molecule expressed on Th2 cells (CRTH2) receptor
In the recent years, the involvement of CRTH2 receptor in the allergic diseases has gained much attention due to its presence on the inflammatory cells such as eosinophils, basophils, and Th2 lymphocytes [40, 41]. CRTH2 receptor is G protein-coupled receptor (GPCR) containing seven putative transmembrane domains. CRTH2 also designated as Prostaglandin D2 receptor 2 (DP2), and cluster of differentiation 294 (CD294) is encoded by the PTGDR2 gene in human. Several pieces of evidence indicate that CRTH2 receptor may play an important role in the allergic diseases, since the blockade of this receptor reduces the allergic airway inflammation [42, 43]. Further, the previous findings have highlighted the role of Prostaglandin D2 (PGD2) and CRTH2 receptor interaction in the pathogenesis of allergic diseases [44]. Generally, PGD2 is a major arachidonic acid metabolite released from immune cells such as mast cell [45], and Th2 cells [46]. In addition, several other studies implicate PGD2-induced inflammatory cells recruitment in allergic diseases that are mediated via CRTH2 receptor [40, 47] and blockage of this novel CRTH2 receptor attenuates the allergic manifestation in the patients [48, 49]. However, recently, the novel association between VIP and CRTH2 receptor in recruiting eosinophils in allergic rhinitis patients have been reported. Interestingly, the activation of CRTH2 receptor expressed on human eosinophils by VIP was noticed and further, the ability of VIP to induce protein synthesis of CRTH2 and its surface expression was reported [18]. This study points the possible role of VIP and CRTH2 receptor interaction in modulating the other allergic diseases and there is still need more efforts in this direction.
2.3. Pituitary adenylate cyclase-activating polypeptide type I receptor (PAC1)
VIP also interacts with another member of GPCRs known as Pituitary adenylate cyclase-activating polypeptide type I receptor (PAC1). PAC1 receptor was first identified in a rat pancreatic acinar carcinoma cell line [50]. With respect to the tissue, the expression of PAC1 receptor is particularly observed in CNS, the anterior pituitary, pancreatic acini, uterus, and predominantly in the adrenal medulla [51]. Notably, VPAC receptors respond to both VIP and PACAP with high affinity whereas PACAP has more than >100-fold affinity as compared to VIP for PAC1 receptor suggesting the minimal role of this receptor in VIP mediated allergic diseases [52]. However, the study performed by Lauenstein et al., (2011) suggested that PAC1 receptor mediates anti-inflammatory effects in allergic airway inflammation. Thus, PAC1 receptor agonists may be a promising candidate in airway allergic diseases such as bronchial asthma [53].
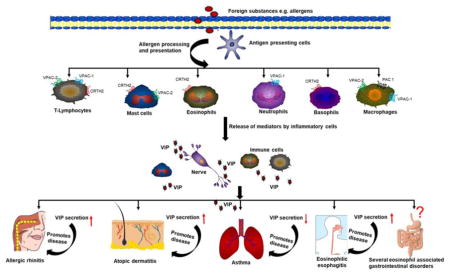
3. Vasoactive Intestinal Peptide receptors expression on the immune cells
The significance of VIP in the immune system is further enhanced due to its ability to interact with multiple immune cells such as mast cells, eosinophils, neutrophils, lymphocytes, dendritic cells, NK cells, and macrophages through VIP associated receptors VPAC-1, VPAC-2, PAC1 and CRTH2 [36, 54].
3.1. Mast cells, Basophils, and VIP receptors expression
Mast cells have an important regulatory role in innate as well as adaptive immunity and act as effector cells that manifest the allergic disorders via releasing allergic mediators such as histamine, leukotrienes, prostaglandins, cytokines, proteases, and heparin. Generally, mast cells perform the biological functions via FcεRI mediated degranulation; however, these mast cells also respond to neuropeptides during inflammation in FcεRI independent manner. Further, FcεRI-independent activation of human mast cells and their presence in the close proximity of neurons suggest the mast cell and neuropeptides interaction in the pathophysiology of allergic diseases [4, 55]. The late phase response in asthma is mediated by activation of human mast cells by neuropeptides that may indirectly provoke inflammation via cellular recruitment [56]. Furthermore, human mast cells degranulation and production of chemokines mainly MCP-1, inducible protein-10, RANTES and IL-8 occurs in response to neuropeptides including VIP. These secreted chemokines, in turn, recruit the other immune cells such as eosinophils, monocytes, and neutrophils [57]. Moreover, Groneberg et al (2003) reported VPAC-2 mRNA expression in human skin mast cells, as well as human mast cell line (HMC-1) and down-regulation of this VPAC-2 receptor in human mast cells in acute lesions of atopic dermatitis was noticed highlighting the significance of VPAC-2 receptor and mast cell interaction in allergic disease pathophysiology [58]. Consistent with the earlier study, VIP mediated activation and degranulation of both human cultured mast cells LAD2 and primary cultured human mast cells were observed. In addition, real-time PCR analysis showed that LAD2 express mRNA for VPAC-2 but not VPAC-1 receptor. The expression of VIP and its receptors is enhanced particularly in mast cell-mediated inflammatory diseases suggesting that VIP may cause in vivo mast cell activation [57]. The tryptase serum levels were also correlated significantly with neuropeptide levels demonstrating the involvement of neuropeptides in the pathomechanism of mastocytosis [59]. In addition, VIP also governs intestinal barrier function and inflammation as stresses disturb follicle-associated epithelium by VIP and VPAC receptors mediated mechanism on mucosal mast cells [60]. Furthermore, when murine mast cells were cultured in the presence of IgE that subsequently lead to stimulation and release of truncated VIP together with histamine [13]. Importantly, the expression of CRTH2 receptor was also reported on mast cells and basophils. In human nasal polyps, 34% of mast cell express CRTH2 receptor. Additionally, 87% of LAD2 human mast cell line and 98% of primary cultured human mast cell showed expression of intracellular CRTH2 [61]. The expression of CRTH2 receptor has also been noticed on basophils that may be responsible for chemotaxis of these cells at the inflammatory site [41, 62]. Together, current evidence suggests that the expression of VIP specific receptors on mast cells and basophils provide an opportunity for neuropeptide VIP to act on these cells and modulate the immunological reactions. We recently reported the expression of CRTH2 receptor on tissue mast cells of eosinophilic esophagitis (EoE) patients as well as in the experimental murine model of EoE [63].
3.2. Eosinophils and VIP receptors expression
Eosinophils are responsible for combating multicellular parasites in vertebrates. Along with mast cells, the molecular and cellular mechanisms related to eosinophils in allergic diseases such as asthma, rhinitis, eosinophilic gastrointestinal disorders, and eosinophilic pancreatitis is well studied [64–68]. Despite considerable advances in understanding eosinophil-mediated allergic manifestations, the impact of neuropeptides on eosinophils in allergic disease is vastly under-studied. Only a few earlier reports have pointed out the immuno-regulatory effect of neuropeptides on eosinophils [69, 70]. A recent report showed the role of VIP and CRTH2 receptor interaction in recruiting eosinophils in allergic rhinitis (AR) patients [18]. The relatively high expression of CRTH2 receptor in various regions of the brain was observed indicating the association between neuropeptide and CRTH2 receptor. These evidence point out that VIP/CRTH2 receptor interaction may play a crucial role in allergic manifestations through its stimulatory effects on Th2 cells, eosinophils, and basophils [71]. The secretion of VIP by eosinophils is also reported [12]. All these functional studies have pointed out a strong association between VIP and eosinophils in the pathophysiology of allergic diseases. However, the role of PGD2-CRTH2 interaction in chemotaxis of eosinophils in allergic condition has also been reported [40, 47]. Additionally, in nasal tissue of AR patients, ligation of PGD2 to CRTH2 receptor appears to be selectively involved in eosinophils recruitment. However, the amount of CRTH2 but not PGD2 was highly and significantly correlated with the number of eosinophils infiltrating into nasal mucosa suggesting the involvement of another factor in eosinophils trafficking [72]. Recently, the involvement of CRTH2 receptor in modulating the biochemical events in VIP-induced eosinophil chemotaxis in AR patients was reported [18]. Our study also reported CRTH2 receptor expression on blood and tissue eosinophils in EoE patients [63].
The significant decrease in inflammatory biomarkers such as eosinophil peroxidase (EPO), myeloperoxidase and lactate dehydrogenase were observed in the lung of OVA-respirable powder challenged murine model when intra-tracheal administration of IK312532-respirable powder (Long-acting analogue of VIP) was done. These results reveal the VIP mediated attenuation of existing eosinophilia in the lung [73]. Taken together, it seems reasonable that the association exists between eosinophils and VIP in the allergic diseases. This interaction needs further investigation to explore the VIP biology with respect to eosinophils in allergic diseases.
3.3. Neutrophils and VIP receptors expression
O’Dorisio et al (1980) detected VIP in neutrophils isolated from leukaemic patients that may provoke either inhibitory or/excitatory response upon neutrophils and this action is mediated through VPAC-1 receptor. The expression of VPAC-1 receptor on human resting neutrophils was first detected by RT-PCR analysis [74]. Palermo et al (1996) demonstrated that VIP has the ability to affect the expression of receptors for Fc portion of IgG (Fcγ R) in human neutrophils. In the experiment, antibody dependent cellular cytotoxicity (ADCC) activity was evaluated in basal conditions and following in vitro stimulation with interferon gamma (IFNγ). The incubation with VIP reduced the cytotoxicity of both stimulated and non-stimulated neutrophils. The inhibitory effect of VIP was more pronounced on neutrophils stimulated with IFNγ. Thus, by regulating the neutrophil cytotoxic response, VIP may limit tissue injury during inflammation [75]. The priming of neutrophils by VIP has also been reported [76, 77]. Additionally, VIP peptide analogue could inhibit antigen-or cytokine-induced neutrophil recruitment in vivo in airways [78].
3.4. Lymphocytes and VIP receptors expression
Both T and B lymphocytes express VIP associated receptors [79] and the expression of VPAC-1 receptor on murine CD4+ and CD8+ T-cells point out the effect of VIP on cytokine production and proliferation of T-lymphocytes [80]. However, another study has demonstrated the presence of both VPAC-1 and VPAC-2 receptors on CD4+ and CD8+ T cells [81]. VIP as immunomodulatory neuropeptide has the ability to promote or inhibit individually the differentiation or function of some T-helper subsets [82]. Both T and B lymphocytes produce VIP suggesting the lymphocytes may modulate the immune response through secretion of VIP [14, 83, 84].
The expression of CRTH2 receptor has also been reported on T lymphocytes and it is preferentially expressed on Th2 cells [85]. The role of CRTH2 receptor in mediating chemotaxis of CD4+ Th2 lymphocytes in response to mast cell released substances has been observed [86]. Further, the migration of Th2 cells through a CRTH2 dependent mechanism via mast cells released mediators in nasal polyp tissue has also been reported [87]. More studies needed in this direction to explore the effect of lymphocyte and VIP interaction on the immune system.
3.5. Monocytes, macrophages, dendritic cells and VIP receptors expression
Monocytes are the part of the innate immune system and also have the prominent effect on adaptive immunity. The inhibitory or stimulatory effect of VIP on monocytes or macrophages depends upon the expression of specific receptors at the differentiated or activated cells that lead to activation of different transduction pathways and subsequently distinct immune response [88]. Importantly, in resting human monocytes, the expression of VPAC-1 was reported but not VPAC-2 receptor [89]. Murine peritoneal macrophages, and the human monocytic cell line THP-1 express VPAC-1 and PAC1 mRNA constitutively, and VPAC-2 following LPS stimulation [90, 91]. Interestingly, VIP shows the inhibitory effect on LPS-induced inflammatory responses by murine monocytes and macrophages that is mediated via VPAC-1 receptor, even though both VPAC-1 and VPAC-2 receptors are expressed [92]. Additionally, VIP exerts anti-inflammatory action by inhibition of pro-inflammatory cytokine production (such as TNFα, IL-6, IL-12) by activated macrophages; and by up-regulation of anti-inflammatory cytokine IL-10 production. VIP has an excitatory effect on monocytes and macrophages but inhibits LPS-induced or IFN-γ-induced inflammatory pathways in these cells. VIP arrest LPS-induced inflammation in monocytes and macrophages via cAMP-dependent or independent mechanisms [54, 93]. The presence of VPAC-1 and VPAC-2 receptors on dendritic cells has also been reported [94, 95].
4. Mechanistic events involved in VIP mediated signaling
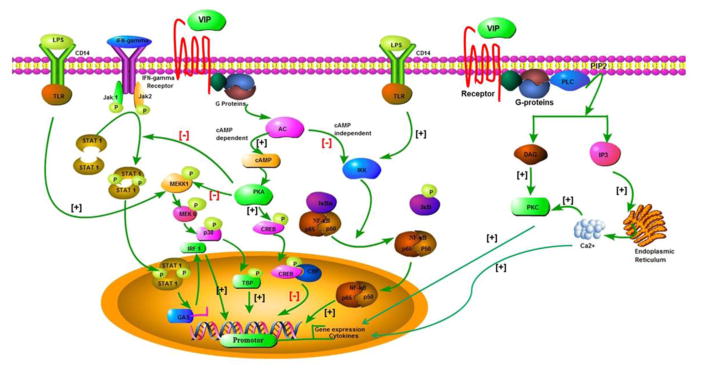
VIP exert their immunomodulatory effects through VPAC-1, VPAC-2, PAC1 and CRTH2 receptors via the stimulation of various protein kinases such as the phospholipase C/PKC and the mitogen-activated protein kinase (MAPK) pathways, as well as the adenylate cyclase/PKA pathway [96]. Further, PAC1 receptor mainly mediates effects via linking with phospholipase C, whereas VPAC-1 and VPAC-2 receptors generally couple to adenylate cyclase. The mechanistic pathways involved in VIP mediated induction of immune responses are depicted in Fig. 1. Two pathways are operational in VPAC mediated response:-cAMP-dependent pathway and cAMP-independent pathway. In cAMP-dependent pathway, the binding of VIP with VPAC receptors increase the intracellular cAMP by stimulating adenylate cyclase that leads to activation of protein kinase A (PKA). The activated PKA further mediates its action via two different mechanisms- through promoting phosphorylation of cAMP response element binding protein (CREB), which finally leads to inhibition of NF-κB. CREB binds to co factor known as CRBP binding protein (CBP), therefore preventing the interaction of CREB with nuclear factor kB (NF-KB). The another downstream effect of PKA activation causes inhibition of phosphorylation of downstream MAP/ERK kinase(MEK) KINASE 1 (MEKK1) that leads to inhibitor of MEKK3/6/P38 pathway that subsequently prevents the another NF-κB co factor known as TATA box binding protein (TBP) minimizing the affinity for DNA and inhibit NFkB pathway [54]. In addition, this pathway also blocks the IFN-γ induced inflammatory response by inhibiting phosphorylation of the Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway [23].
Fig. 1.
The summarized mechanistic events operational in VIP mediated signalling pathways. PKA =Protein kinase A; MAP kinase= Mitogen-activated protein kinase; CREB= cAMP response element binding protein; CBP= CRBP binding protein; NF-KB= Nuclear factor KB; TBP= TATA box binding protein; JAK/STAT= Janus kinase/signal transducer and activator of transcription; IκK=inhibitory κB kinase; LPS= Lipopolysaccharides; MEKK1= MAP/ERK kinase(MEK) KINASE 1; TLR= Toll-like receptor; STAT 1= Signal transducer and activator of transcription 1; JAK 1=Janus kinase 1;IRF 1=Interferon regulatory factor 1; PKC= Protein kinase C; DAG=Diacylglycerol; PIP2: Phosphatidylinositol 4,5-bisphosphate; PLC= Phospholipase C; IP3= Inositol 1,4,5 –triphosphate.
Apart from above discussed signaling mechanism, there is another cAMP-independent pathway operational in VIP mediated responses that prevent the nuclear entry of NF-κB via inhibiting IκB phosphorylation. The cAMP-independent pathway prevents inhibitory κB kinase (IκK) activity that inhibits the phosphorylation of the IκB and enhances the stabilization of p65/p50/IκB complex. Finally, all these events prevent the nuclear translocation of NFκB subunits. VIP mediated signaling also inhibits the inflammatory response induced by LPS [36, 54]. Additionally, the stimulation of other intracellular messenger systems including calcium and phospholipase D have also been reported [24]. Recently, the possible mechanism of association between VIP and CRTH2 receptor has been explored. The study demonstrated that VIP-induced eosinophil chemotaxis in allergic rhinitis patients is mediated through the CRTH2 receptor involving Ca2++ independent protein kinase C (PKC) and protein kinase A (PKA) activity. Moreover, VIP-CRTH2 binding involves novel PKC δ, PKCε, PKAα, PKAα IIreg, and PKAγ cytosol-to-membrane translocation without the change in the total contents of these proteins [18]. Importantly, VIP mediated activation of the particular signal transduction pathway depends on expression of the predominant receptor subtype, cellular level of activation, and use of cell lines [97].
5. Significance of VIP in the allergic diseases
VIP has been proposed to play a key role in many physiological processes occurring in the gastrointestinal tract, airways, cardiovascular system, reproductive system, and endocrine system. The immunomodulatory effect of VIP is indicated by the study in which the peripheral blood mononuclear leukocytes from allergic rhinitis and asthma subjects were incubated with a number of neuropeptides such as VIP, Substance P, morphine, and ACTH and in both normal and allergic subjects, only VIP showed stimulatory effects [98].
5.1. Significance of VIP in asthma pathogenesis
Asthma is a common chronic inflammatory disease of the airways characterized by reversible airflow obstruction, airway hyperreactivity, and mucus hypersecretion. The allergic manifestations involve the release of allergic substances from mast cells, eosinophils, and excessive production of Th2 cytokines such as IL-4, IL-5, IL-13, and IgE antibody [99]. It is estimated that asthma is affecting >7.0 million children in the United States and the prevalence of the disease is still increasing [100]. A rich supply of VIPergic fibers were observed in the airway along with vascular smooth muscles of trachea and bronchi in the respiratory tract pointing out the role of VIP in the immunopathogenesis of respiratory diseases [101]. As VIP exerts a variety of biological actions, reducing vascular- and bronchial-constriction, and works as a potent anti-inflammatory factor, thus may be used for the treatment of cardiopulmonary disorders such as pulmonary arterial hypertension, asthma and chronic obstructive pulmonary disease (COPD). VIP has the ability to improve blood circulation to lungs and also balancing airway secretions [102]. Furthermore, VIP released from inhibitory nonadrenergic noncholinergic (i-NANC) nerves works as an airway smooth muscle dilator but during allergic condition, VIP mediated effect is attenuated by released inflammatory mediators that may cause airway hyperresponsiveness. The mechanistic role of VIP in asthma pathogenesis is shown by the study in which intraperitoneal administration of VIP eliminated the airway hyperresponsiveness and reduces the inflammation in VIP gene-deficient (−/−) mice [19]. Consistent with this study, VIP gene deficient (−/−) mice showed peribronchiolar airway inflammation along with the production of pro-inflammatory cytokines and airway hyper-responsiveness to inhaled cholinergic agonist methacholine [103]. Further, the significance of VIP was analyzed in VIP gene deficient (−/−) mouse model of sulfite-sensitive asthma in which VIP modulate the oxidant/antioxidant balance in asthma. The wild-type mice with VIP gene have functional lung carbonyl reductase without any symptoms of asthma, whereas VIP−/− mice have abnormal carbonyl reductase and have spontaneous asthma suggesting that deficiency of VIP gene may cause a predisposition to asthma development [104]. The decreased VIP concentration in the bronchoalveolar lavage fluid in rat model is negatively correlated with c-fos protein expression and more prone to asthma attacks [105]. Moreover, the decrease in VIP content in murine lungs, especially in the columnar epithelia of the airways in Aspergillus-treated mice, was also observed [106]. The positive correlation between VIP staining and mast cell infiltration in the smooth muscle layer was also noticed [107]. It is well established that tryptase, a serine proteinase, released after mast cell activation is capable of causing bronchial hyperresponsiveness and recruitment of inflammatory cells in asthma. This effect is probably mediated by activation of other allergic mediators and cleavage of the bronchodilating peptides VIP and peptide histidine-methionine (PHM) [108]. Purified tryptase obtained from human lung extract has the ability to rapidly hydrolyze VIP at multiple sites at Arg12, Arg14, Lys20, and Lys21 and PHM at a single site Lys20 indicating tryptase-mediated degradation of the bronchodilators including VIP may be responsible for bronchial responsiveness in asthma [109]. The effect of VIP administration on neutrophil trafficking in the lungs were also observed demonstrating the systemic or/local administration of VIP analogue decreases cytokine-induced neutrophil recruitment in airways in Sprague-Dawley rats that were treated intratracheally with recombinant interleukin (IL)-1 β [78]. Stimulation of airway smooth muscle (ASM) cell with endogenous mitogens may lead to increased airway resistance/ASM hyperplasia in bronchial asthma. However, VIP has the ability to inhibit ASM cell growth and inhibit the mitogenic effect of histamine by a PKA-mediated mechanism [110]. Further, targeting the VIP receptors can also be promising approach for reducing the asthma severity. Several lines of evidence suggest that there was no correlation exist between severity of asthma and the number of VIP-nerves in the biopsies [111, 112]. A study was performed in 25 adult asthmatic patients in which VIP plasma levels were found lower in patients than normal individuals indicating the relevance of VIP in asthma pathogenesis [113]. Collectively, it can be mentioned that VIP acts as an anti-asthma factor as decreased VIP concentration in asthmatic patients leads to increased airway inflammation and hyperresponsiveness. The diagrammatic representation of VIP mediated immune responses in asthma is summarized in Fig. 2.
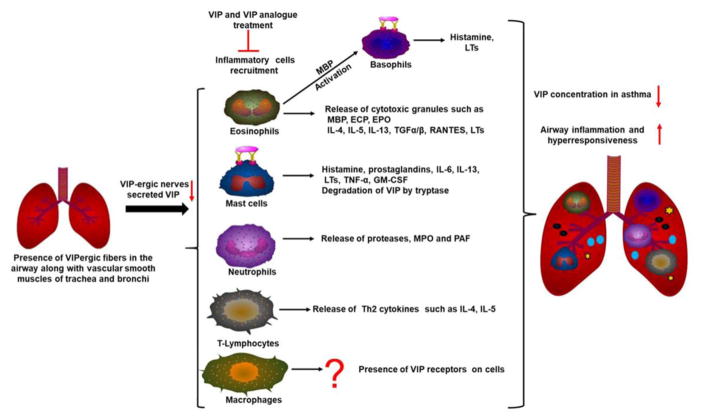
Fig. 2.
The significance of VIP in asthma pathogenesis. Decreased VIP level in lungs leads to recruitment of inflammatory cells such as eosinophils, mast cells, neutrophils, lymphocytes in the lungs. These cells release the allergic mediators that promote airway inflammation and hyperresponsiveness. MBP=Major basic protein; ECP= Eosinophil cationic protein; EPO=Eosinophil peroxidase; LTs=Leukotrienes; TNF= Tumor necrosis factor; RANTES= regulated on activation, normal T cell expressed and secreted; TGF= Transforming growth factor; MPO=Myeloperoxidase; PAF=Platelet activating factor; GM-CSF=Granulocyte macrophage-colony stimulating factor.
5.2. Significance of VIP in promoting allergic rhinitis pathogenesis
Allergic rhinitis (AR) is an IgE-mediated immediate allergic reaction with the main symptoms of nasal obstruction, pruritus, nasal itching, sneezing and rhinorrhea [65]. Several allergic mediators such as histamine, leukotrienes, prostaglandins, interleukins, and platelet-activating factor playing role in AR pathogenesis [65]. Nasal biopsy examinations have shown an accumulation of inflammatory cells such as mast cells, eosinophils, and basophils in AR patients [114] and Th2 mediated response is reported [115].
The human nasal mucosa is abundantly innervated by nerve fibers, secretes various neuropeptides that have the impact on the disease pathogenesis. Notably, the enhanced expression of VIP and its receptors (VPAC-1 and VPAC-2) in the nasal mucosa of AR patients were observed suggesting an increased expression level of VIP receptors as one of the possible explanation for nasal hyperresponsiveness in allergic rhinitis patients [116]. Similarly, the significant increase in neuropeptide-containing nerve fibers especially VIPergic fibers were observed emphasized the involvement of innervation in these allergic rhinitis patients [117].
Mucociliary clearance (MCC) is the physical defense mechanism that protects the airway against inhaled pathogens, toxins and allergens [118]. The expression of VIP is observed in the sinonasal epithelium that was found up-regulated in allergic state suggesting the possibility that an increased VIP level in histamine-driven allergic rhinitis may enhance the sinonasal fluid secretion causing allergic rhinorrhea [119]. In the study, allergic rhinitis patients were challenged nasally with histamine or allergen and after histamine challenge, only VIP level was found enhanced in nasal lavages. However, SP, CGRP, and VIP were significantly enhanced immediately after allergen challenge. This data suggest that histamine-induced cholinergic reflexes induce the release of VIP and its crucial role in nasal allergy [120]. Additionally, parasympathetic nerves are involved in the pathophysiology of rhinosinusitis and estimation of secreted VIP in human saliva may be used as markers for parasympathetic nerve activity in these patients. The baseline salivary levels of VIP was significantly elevated between attacks in allergic rhinosinusitis subjects and returned to baseline values following treatment with pseudoephedrine demonstrating that VIP level in saliva may indicate the neuronal mechanisms involved in rhinosinusitis [121].
Posterior nasal neurectomy (cholinergic nerve) has been shown to remarkably improve subjective nasal symptoms in patients with severe allergic rhinitis as it decreases the IL-5 and mast cells in severe AR [122]. Posterior nasal neurectomy (PNN) is a common surgical treatment for allergic rhinitis in Asia and it is based on the concept that posterior nasal neurectomy may induce denervation of the nasal mucosa and relieve the nasal symptoms of allergic rhinitis. The posterior nasal nerve is considered the main source of the sympathetic, parasympathetic and sensory fibers that innervate the nasal mucosa. However, recently rat model of PNN was developed and examined the effects of PNN on allergic rhinitis in ovalbumin-sensitized rats. Although these rats showed reduced nasal secretion, PNN did not affect eosinophils and mast cells infiltration, and other allergic symptoms (i.e. sneezing and nasal scratching). The study points out that PNN may be a therapeutic option for management of hyperrhinorrhea, but not allergic rhinitis hypersensitivity as nerves and secreted neuropeptides regulate only nasal secretion, but not hypersensitivity in AR [123]. Recent literature on allergic rhinitis patients provided the first evidence of the association between VIP and CRTH2 receptor in eosinophil chemotaxis in these patients. The CRTH2 receptor expression by eosinophils in AR patients was found to be up-regulated following VIP treatment. Further, VIP induces PGD2 secretion by eosinophils enhancing the allergic manifestation. This study suggests that VIP-induced eosinophil chemotaxis in AR patients is mediated through CRTH2 receptor [18]. Additionally, tyrosine kinase inhibitors induce the expression of CRTH2 receptor in both human lymphocytes and eosinophils thereby augmenting the VIP/PGD2 induced eosinophils recruitment in allergic rhinitis patients [124]. Taken together, these findings provide preliminary supportive evidence that VIP may be playing a crucial role in AR pathogenesis and there is still need to explore the further significance of VIP in AR immunopathogenesis. The possible mechanistic aspects and cell types involved in VIP mediated immunological responses in allergic diseases such as allergic rhinitis have been summarized in Fig. 3.
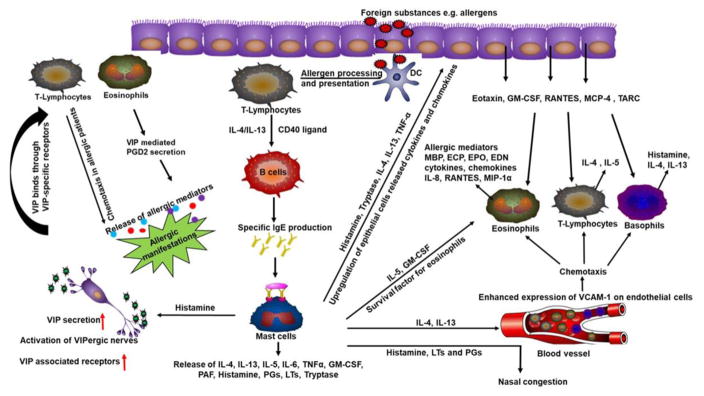
Fig. 3.
The significance of VIP in the pathogenesis of allergic rhinitis. Allergic mediators activate VIP-ergic nerves to induce the secretion of VIP which in turn promotes the trafficking of inflammatory cells in the tissue and manifest allergic responses. VCAM-1= Vascular cell adhesion molecule 1; TARC= Thymus-and activation Regulated chemokine; PGs=Prostaglandins; PGD2=Prostaglandin D2; DC=Dendritic cells; MCP-4=Monocyte chemotactic protein-4; EDN=Eosinophil-derived neurotoxin; MIP-1α=Macrophage Inflammatory Proteins -1α.
5.3. Significance of VIP in promoting atopic dermatitis pathogenesis
Atopic dermatitis (AD) is a type of skin inflammation and it affects 10%–20% of the population [125, 126]. Nervous system modulates the immunologic responses in the skin of AD patients. Altered expression of cutaneous innervation and released neuropeptides in AD patients provoke the immunological responses in the skin by modulating the functions of langerhans cells, keratinocytes, mast cells, and other immune cells [127, 128]. Neuropeptides are released by the skin innervating nerves that affect the skin immunity and cell function. Therefore, the better understanding of neuropeptides interaction with skin immune cells/system is necessary for developing new approaches for skin disease treatment. The skin inflammation may be Th1/Th2-mediated and APC cells involved in the Th1 and Th2 process are distinct. Thus, the effect of neuropeptides in cutaneous immunity occurs through a complex mechanism and interaction of neuropeptides with particular immune cells changes the immune response [6]. The existence of VIP- immunoreactive nerve fibers in the deeper part of the dermis close to sweat glands and hair follicles as well as the presence of VIP receptors on keratinocytes in the basal layer of the epidermis have been reported indicating the possible role of VIP in skin physiology [129]. Diminished SP levels and increased VIP levels in lesional skin from AD patients were observed as compared to control skin [130]. VIP concentration is elevated in skin biopsies from patients with eczema, psoriasis and VIP may increase local blood flow in these patients [131].
An increase in the numbers of VIP-positive nerve fibers in lesional psoriatic skin were observed as compared to normal skin [132]. The study by Fisher et al., (2002) demonstrated the marked staining for VPAC-2 mRNA in epidermal cells with most pronounced hybridization signals observed in keratinocytes of the basal layer and in glandular cells surrounded by VIP-immunoreactive nerve fibers. Hair follicle cells next to VIP-positive nerve fibers also showed hybridization signals [133]. The elevated plasma levels of neuropeptides including VIP was reported to be enhanced in AD patients as comparison to normal non-atopic controls [134]. Further, enhanced plasma levels of neuropeptides including VIP was found correlated with the intensity of pruritus in the intrinsic type of AD and the higher risk of IgE-mediated sensitization to moulds in the extrinsic type of AD suggesting neuropeptides may be used as better alternative biomarkers of AD [135]. The enhanced VIP levels were reported in AD patients not only in the skin but also in the serum [20]. Interestingly, computer-induced stress enhanced the allergen-specific skin wheal responses with the concomitant increase in plasma levels of SP and VIP in AD patients aggravating the severity of AD [136]. However, a study performed by Kang et al (2000) where the action of neuropeptides VIP and SP to affect cytokine release (IFN-gamma & IL-4) in the peripheral blood mononuclear cells (PBMCs) of AD patients were analyzed. The ratios of IFN-gamma: IL-4 production was significantly elevated in the SP treated AD group, although VIP had no specific noticeable modulatory effects on these cytokine production suggesting the no VIP mediated modulatory effects on the cytokine production in AD patients [137]. Only type I VIP receptor (VPAC-1) mRNA was reported to express in normal human keratinocytes while DJM-1 cells (human epidermal keratinocyte cell line) expressed both VPAC-1 and VPAC-2 receptor. VIP also induced the production of inflammatory cytokines (IL-6, IL-8, and RANTES) and these effects were nullified by VPAC-1 selective antagonist indicating that these effects are mediated by type I VIP receptor [138]. It is proposed that in the dermis, inflammatory cells secrete different cytokines which in turn upregulates the expression of VIP receptor thereby enhancing response to nerve fibers released VIP. This nerve endings derived VIP ultimately enhances the proliferation as well as cytokine production by keratinocytes. This cytokine network around keratinocytes may be playing a crucial role in the pathogenesis of inflammatory dermatoses [138]. However, in another study, the role of mast cell in allergic skin disease has been observed. The enhanced VPAC-2 mRNA expression in mast cells was noticed that was increased compared to other receptors such as VPAC-1 or VIP in the human mast cell line HMC-1. Stimulation of HMC-1 cells led to a downregulation of VPAC-2. Similarly, significantly decreased VPAC-2 immunoreactivity in mast cell was noticed in acute atopic dermatitis lesions implicating the role of VPAC-2 receptor in the pathophysiology of atopic dermatitis [58, 139]. Taken together, the current pieces of evidence have clearly shown the participation of VIP in the pathophysiologic background of skin inflammatory disorders.
5.4. Significance of VIP in pathogenesis of esophageal disorders
Gastroesophageal reflux disease (GERD) is the most common gastrointestinal disorder that is characterized by heartburn, difficulty in swallowing (dysphagia), and regurgitation of food or sour liquid (acid reflux) [140]. GERD patients who have delayed gastric emptying, have the abnormalities in the peptide-immunoreactive fibers that innervate the gastric external muscle [141]. Interestingly, abnormally high serum VIP level may have a role in GERD pathogenesis. VIP mediated relaxant effect on lower esophageal sphincter causes exposure of esophageal mucosa to harmful acid refluxed that subsequently increases the nitric oxide (NO) levels responsible for low lower esophageal sphincter (LES) pressure [142]. Additionally, the study by Rossiter et al., (1991) demonstrated that the decreased LES resting pressure noticed in patients with Barrett’s esophagus and severe gastroesophageal reflux may be due to impairment of the VIPergic innervation that subsequently enhances local release of VIP with possible overflow to peripheral plasma [143]. The symptoms include vomiting, diarrhoea, itching, low blood pressure, and respiratory problems, and sometimes anaphylaxis. In addition, GERD like symptoms is also observed in another esophageal chronic allergic disease termed as EoE. EoE is characterized by esophageal dysfunction, accumulation of ≥15 eosinophils/high-powered field, induced mast cell accumulation, basal cell hyperplasia and a lack of response to 8-week proton pump inhibitor treatment. Patients with primary EoE commonly report symptoms that include difficulty feeding, vomiting, chest pain, dysphagia, and food impaction [144, 145]. Most recently, we showed that neuroendocrine cells released VIP in the esophageal mucosa promotes eosinophils and mast cells trafficking and accumulation in all segments of the esophagus, indicating an important role of VIP in the pathogenesis of EoE. Furthermore, we showed that inhibition of VIP-CRTH2 axis ameliorates eosinophils and mast cells inflammation in the esophagus. Therefore, anti-VIP or anti CRTH2 receptor therapy may be beneficial to treat the dysphagia, stricture and motility dysfunction of chronic EoE [63].
VIP have modulatory effects on immune system activity especially on Th1/Th2 balance and may lead to allergic sensitization in children. Notably, VIP showed a positive relationship for allergic sensitization with food allergens [146]. Collectively, it can be clearly mentioned that allergic diseases become the rapidly growing public health problem worldwide and there is still the gap in the understanding of the significance of VIP in other allergic diseases such as Eosinophilic Gastrointestinal Disorders. Therefore exploring the relationship between VIP and allergic diseases may provide the basis for future research to develop therapeutic approaches for the management of these allergic diseases.
6. Therapeutic approaches
Our increasing understanding regarding the impact of VIP in the pathogenesis of allergic diseases proposes the VIP and stable VIP-derived agents as an efficient therapeutical option to cure inflammatory allergic disorders such as asthma, rhinitis, atopic dermatitis, and several eosinophil associated gastrointestinal disorders [22]. The synthesis of stable peptidase resistant VIP agonist can give promising results to treat bronchial asthma. The recent finding revealed the novel stabilized inhaled VIP agonists for respiratory therapeutics may be used with minimum side effects [147]. The use of VIP may also be a fruitful approach to protect asthmatic patients against histamine-induced bronchoconstriction [148]. Inhalation of VIP analogue Ro 25–1553 (a selective VPAC-2 receptor agonist) leads to a rapid bronchodilatory effect in asthmatic patients without any side effects [149]. Additionally, conjugated alpha-alumina nanoparticle with VIP has been proposed as an effective nano-drug for the treatment of asthma [150]. Additionally, induced expression of VIP and VIP associated receptors were reported in patients with atopic dermatitis [20, 129], allergic rhinitis [116] and EoE [63], suggesting the use of stable VIP antagonists or specific VIP receptors antagonist may be the logical approach for improving these inflammatory diseases.
7. Conclusions and future perspectives
VIP is a neuropeptide with a broad distribution in the body that performs pleiotropic functions in several systems and serves as the modulator of immune response. To date, several studies have demonstrated the role of VIP in regulating the recruitment of inflammatory cells in the allergic diseases. Owing to the ability of VIP to activate immune cells of both the innate and adaptive immune system, it may be a decisive neuropeptide in modulating the pathophysiology of allergic diseases. The main concern in the usage of VIP as a therapeutic option is the very short half-life as rapid enzymatic degradation occurs. However, in the light of recent advancement, better stable VIP agonists/antagonists have been developed providing the convincing support to be used as a therapeutic alternative for the treatment of allergic diseases. However, there is still need to conduct more studies to investigate the interaction and cross talk of VIP with the immune cells to get the better idea regarding the pathogenesis of the allergic disease. A better way to see VIP as both anti-inflammatory and pro-inflammatory agent, therefore, the better understanding of VIP immune biology will be helpful to develop efficient future therapeutic strategies to treat allergic diseases.
Highlights.
Neuroendocrine cells are the source of Vasoactive Intestinal Peptide (VIP) that exerts a wide spectrum of immunological functions as cytokine/chemokine.
VIP mediates immunological function via VPAC-1, VPAC-2, CRTH2 and PAC1 receptors that are expressed on immune cells.
VIP has the ability to modulate the immune response induced by inflammatory cells such as mast cells, eosinophils, neutrophils, lymphocytes, and macrophages.
The current review provides an updated understanding on the significance of VIP in the pathogenesis of allergic diseases such as asthma, allergic rhinitis, and atopic dermatitis.
Acknowledgments
Dr. Mishra is the Endowed Schlieder Chair; therefore, authors thank Edward G. Schlieder Educational Foundation for the support. Additionally, Dr. Mishra’s NIH R01 AI080581 grant funding supports the authors.
Abbreviations
- VIP
Vasoactive Intestinal Peptide
- VPAC-1
Vasoactive Intestinal Peptide Receptor Type 1
- VPAC-2
Vasoactive Intestinal Peptide Receptor Type 2
- CRTH2
Chemoattractant Receptor-Homologous Molecule Expressed on Th2 Cells
- PAC1
Pituitary adenylate cyclase-activating polypeptide (PACAP) receptor
- GPCR
G protein-coupled receptor
- PGs
Prostaglandins
- DC
Dendritic cell
- MCP-1
Monocyte chemotactic protein 1
- CNS
Central nervous system
- PGD2
Prostaglandin D2
- AR
Allergic rhinitis
- AD
Atopic dermatitis
- CGRP
Calcitonin Gene-Related Peptide
- SP
Substance P
- ELISA
Enzyme-linked immunosorbent assay
- IL
Interleukin
- Mw
Molecular weight
- EoE
Eosinophilic esophagitis
Biographies
 Dr. Alok K. Verma, PhD, is postdoctoral research fellow at Department of Medicine, Section of Pulmonary Diseases, Tulane University, New Orleans, LA, USA. Dr. Verma’s long-term research interest involves to understand the underlying immune mechanism of various cells such as T cells, B cells, mast cells, macrophages and eosinophils in modulating the immune response. Dr. Verma has received his master’s degree from University of Lucknow, India and PhD degree in Biological Sciences from the Academy of Scientific and Innovative research (AcSIR), CSIR-IITR, India. He has completed his initial PhD training at CSIR-Indian Institute of Toxicological Research, a Government of India sponsored research organization involved in understanding the mechanism and regulation of toxic agents and their effects on different human organs. His doctoral research work was mainly focused to understand the mechanism involved in the allergenicity of food allergens using in vitro and in vivo model systems. Dr. Verma has identified seven IgE binding proteins which are responsible for chickpea induced allergy in sensitized individuals. He is currently working in the area of eosinophil biology and exploring the role of different involved molecules in the pathogenesis of Eosinophilic esophagitis. Dr Verma is also member of several prestigious scientific societies such as The American Association of Immunologists (AAI), American Gastroenterological Association (AGA), European academy of allergy and clinical immunology (EAACI), and Indian Immunology Society.
Dr. Alok K. Verma, PhD, is postdoctoral research fellow at Department of Medicine, Section of Pulmonary Diseases, Tulane University, New Orleans, LA, USA. Dr. Verma’s long-term research interest involves to understand the underlying immune mechanism of various cells such as T cells, B cells, mast cells, macrophages and eosinophils in modulating the immune response. Dr. Verma has received his master’s degree from University of Lucknow, India and PhD degree in Biological Sciences from the Academy of Scientific and Innovative research (AcSIR), CSIR-IITR, India. He has completed his initial PhD training at CSIR-Indian Institute of Toxicological Research, a Government of India sponsored research organization involved in understanding the mechanism and regulation of toxic agents and their effects on different human organs. His doctoral research work was mainly focused to understand the mechanism involved in the allergenicity of food allergens using in vitro and in vivo model systems. Dr. Verma has identified seven IgE binding proteins which are responsible for chickpea induced allergy in sensitized individuals. He is currently working in the area of eosinophil biology and exploring the role of different involved molecules in the pathogenesis of Eosinophilic esophagitis. Dr Verma is also member of several prestigious scientific societies such as The American Association of Immunologists (AAI), American Gastroenterological Association (AGA), European academy of allergy and clinical immunology (EAACI), and Indian Immunology Society.
 Dr. Murli Manohar, PhD, is a post-doctoral research fellow at Tulane University, New Orleans, USA. Dr. Manohar’s research interest includes immunological regulation of gastrointestinal disorders such as eosinophilic esophagitis, eosinophilic pancreatitis, fibrosis, and pancreatic malignancy. Currently, he is working on the various the role of eosinophils, IL-5 and IL-18 in the pathophysiology of acute pancreatitis, chronic pancreatitis, eosinophilic pancreatitis, fibrosis as well as pancreatic cancer. Dr. Manohar obtained his Ph.D in Biochemistry from the CSIR-Central Drug Research Institute/Jamia Hamdrd, INDIA where he studied the pathophysiology and treatment options for endocrine related cancers and explored the anti-tumor effect as well as molecular mechanism of polyphenolic phyto-compound “Epigallocatechin-3-gallate” on endometrial cancer. Dr. Manohar has gained his B.Sc. and M.Sc. form University of Lucknow, India. He serves on the reviewer board of the Cancer Letter, Life Sciences, RBM online, Mediators of Inflammation, and SOJ Journal of Gastroenterology, Pancreatology & Liver Disorders. Dr. Manohar is the member of various scientific societies such as American Association of Cancer Research, American Gastroenterological Association, and American Association of Immunologist.
Dr. Murli Manohar, PhD, is a post-doctoral research fellow at Tulane University, New Orleans, USA. Dr. Manohar’s research interest includes immunological regulation of gastrointestinal disorders such as eosinophilic esophagitis, eosinophilic pancreatitis, fibrosis, and pancreatic malignancy. Currently, he is working on the various the role of eosinophils, IL-5 and IL-18 in the pathophysiology of acute pancreatitis, chronic pancreatitis, eosinophilic pancreatitis, fibrosis as well as pancreatic cancer. Dr. Manohar obtained his Ph.D in Biochemistry from the CSIR-Central Drug Research Institute/Jamia Hamdrd, INDIA where he studied the pathophysiology and treatment options for endocrine related cancers and explored the anti-tumor effect as well as molecular mechanism of polyphenolic phyto-compound “Epigallocatechin-3-gallate” on endometrial cancer. Dr. Manohar has gained his B.Sc. and M.Sc. form University of Lucknow, India. He serves on the reviewer board of the Cancer Letter, Life Sciences, RBM online, Mediators of Inflammation, and SOJ Journal of Gastroenterology, Pancreatology & Liver Disorders. Dr. Manohar is the member of various scientific societies such as American Association of Cancer Research, American Gastroenterological Association, and American Association of Immunologist.
 Dr. Sathisha Upparahalli Venkateshaiah, PhD is Postdoctoral Research Fellow in the Section of Pulmonary Diseases at Tulane School of Medicine, New Orleans, LA. Dr. Sathisha UV had an exceptional background, foundation with his renowned education at University of Mysore, Mysore, India where he graduated with Bachelor of Science. Having excelled in science education, he then undertook PhD training at University of Mysore, Mysore, India. Dr. Sathisha UV skilled in Cancer Biology and Molecular Biology training from an esteemed Central food Technological Research Institute (CSIR), Mysore, India. Dr. Sathisha UV completed his postdoctoral training at Myeloma Institute for Research and Therapy, Winthrop P. Rockefeller Cancer Institute, UAMS, Little Rock, AR, USA, Tulane School of Medicine, New Orleans, LA. Dr. Sathisha UV most important contribution was role of bone marrow microenvironment in Myeloma tumorigenesis. Dr. Sathisha UV’s most important contribution was IL-15’s protective role in the pathogenesis of asthma (SATHISHA U V., et al Journal of Allergy and Clinical Immunology (JACI) 2017, In press).
Dr. Sathisha Upparahalli Venkateshaiah, PhD is Postdoctoral Research Fellow in the Section of Pulmonary Diseases at Tulane School of Medicine, New Orleans, LA. Dr. Sathisha UV had an exceptional background, foundation with his renowned education at University of Mysore, Mysore, India where he graduated with Bachelor of Science. Having excelled in science education, he then undertook PhD training at University of Mysore, Mysore, India. Dr. Sathisha UV skilled in Cancer Biology and Molecular Biology training from an esteemed Central food Technological Research Institute (CSIR), Mysore, India. Dr. Sathisha UV completed his postdoctoral training at Myeloma Institute for Research and Therapy, Winthrop P. Rockefeller Cancer Institute, UAMS, Little Rock, AR, USA, Tulane School of Medicine, New Orleans, LA. Dr. Sathisha UV most important contribution was role of bone marrow microenvironment in Myeloma tumorigenesis. Dr. Sathisha UV’s most important contribution was IL-15’s protective role in the pathogenesis of asthma (SATHISHA U V., et al Journal of Allergy and Clinical Immunology (JACI) 2017, In press).
 Dr. Anil Mishra, PhD is Edward G. Schlieder Educational Foundation Endowed Chair and Professor of Medicine. He is also the Director of Tulane Eosinophilic Disorder Center in the Section of Pulmonary Diseases at Tulane University School of Medicine. Dr. Mishra’s research established that eosinophils are the resident cells of the gastrointestinal tract that home prenatally (Mishra et al., J. Clin. Invest. 1999). He showed that eosinophil active chemokine eotaxin-1 constitutively expressed and has significant role for eosinophils homing into the gastrointestinal tract. He developed the first murine model of eosinophilic esophagitis (EoE) (Mishra et al., J. Clin. Invest. 2001). His findings implicated aeroallergen in the etiology of EoE and suggested that esophageal eosinophilic inflammation is mechanistically regulated by IL-5, IL-13 and eotaxin (J. Immunology 2002, and Gastroenterology, 2003). Furthermore, we also indicated that T cell subset iNKT cells are critical and the source of eosinophil active cytokines in human EoE, and targeting the cell surface receptors of iNKT cells improve EoE in experimental model of EoE (Clinical and Translational Immunology, 2014). Most recently, the laboratory of Dr. Mishra has reported that rIL-15 is a therapeutic molecule for the allergen-induced airway hyperactivity and fibrosis for chronic asthma and other pulmonary functional impairment (J. Allergy Clin. Immunol. 2017). Dr. Mishra is an elected fellow of the American Academy of Allergy Asthma Immunology (FAAAAI) and the American Gastrointestinal Association (FAGA). He has published over 85 articles, book chapters and reviews on molecular mechanisms of pulmonary and gastrointestinal allergic responses in high impact factor journals. Dr. Mishra’ research is supported by The National Institutes of Health via NIDDK and NIAID institutes Dr Mishra is also a member of several NIH study sections and serving Editor and Editorial Board member in a number of international journals.
Dr. Anil Mishra, PhD is Edward G. Schlieder Educational Foundation Endowed Chair and Professor of Medicine. He is also the Director of Tulane Eosinophilic Disorder Center in the Section of Pulmonary Diseases at Tulane University School of Medicine. Dr. Mishra’s research established that eosinophils are the resident cells of the gastrointestinal tract that home prenatally (Mishra et al., J. Clin. Invest. 1999). He showed that eosinophil active chemokine eotaxin-1 constitutively expressed and has significant role for eosinophils homing into the gastrointestinal tract. He developed the first murine model of eosinophilic esophagitis (EoE) (Mishra et al., J. Clin. Invest. 2001). His findings implicated aeroallergen in the etiology of EoE and suggested that esophageal eosinophilic inflammation is mechanistically regulated by IL-5, IL-13 and eotaxin (J. Immunology 2002, and Gastroenterology, 2003). Furthermore, we also indicated that T cell subset iNKT cells are critical and the source of eosinophil active cytokines in human EoE, and targeting the cell surface receptors of iNKT cells improve EoE in experimental model of EoE (Clinical and Translational Immunology, 2014). Most recently, the laboratory of Dr. Mishra has reported that rIL-15 is a therapeutic molecule for the allergen-induced airway hyperactivity and fibrosis for chronic asthma and other pulmonary functional impairment (J. Allergy Clin. Immunol. 2017). Dr. Mishra is an elected fellow of the American Academy of Allergy Asthma Immunology (FAAAAI) and the American Gastrointestinal Association (FAGA). He has published over 85 articles, book chapters and reviews on molecular mechanisms of pulmonary and gastrointestinal allergic responses in high impact factor journals. Dr. Mishra’ research is supported by The National Institutes of Health via NIDDK and NIAID institutes Dr Mishra is also a member of several NIH study sections and serving Editor and Editorial Board member in a number of international journals.
Footnotes
Conflict of interest
All authors have declared no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Verma AK, Kumar S, Das M, Dwivedi PD. A comprehensive review of legume allergy. Clin Rev Allergy Immunol. 2013;45(1):30–46. doi: 10.1007/s12016-012-8310-6. [DOI] [PubMed] [Google Scholar]
- 2.Devis DL, Davies JM, Zhang D. Molecular features of grass allergens and development of biotechnological approaches for allergy prevention. Biotechnol Adv. 2017;35(5):545–556. doi: 10.1016/j.biotechadv.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Shaker M. New insights into the allergic march. Curr Opin Pediatr. 2014;26(4):516–20. doi: 10.1097/MOP.0000000000000120. [DOI] [PubMed] [Google Scholar]
- 4.Groneberg DA, Quarcoo D, Frossard N, Fischer A. Neurogenic mechanisms in bronchial inflammatory diseases. Allergy. 2004;59(11):1139–52. doi: 10.1111/j.1398-9995.2004.00665.x. [DOI] [PubMed] [Google Scholar]
- 5.Chorny A, Gonzalez-Rey E, Varela N, Robledo G, Delgado M. Signaling mechanisms of vasoactive intestinal peptide in inflammatory conditions. Regul Pept. 2006;137(1–2):67–74. doi: 10.1016/j.regpep.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 6.Mikami N, Fukada S, Yamamoto H, Tsujikawa K. Neuronal derivative mediators that regulate cutaneous inflammations. Crit Rev Immunol. 2012;32(4):307–20. doi: 10.1615/critrevimmunol.v32.i4.20. [DOI] [PubMed] [Google Scholar]
- 7.Smolyannikova VA, Kubanova AA, Karamova AE, Nefedova MA, Chikin VV. Role of the skin expression of neuropeptides, neurotrophins and their receptors in the pathogenesis of dermatoses. Arkh Patol. 2015;77(4):33–39. doi: 10.17116/patol201577433-39. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez-Rey E, Delgado M. Role of vasoactive intestinal peptide in inflammation and autoimmunity. Curr Opin Investig Drugs. 2005;6(11):1116–23. [PubMed] [Google Scholar]
- 9.Said SI, Mutt V. Potent peripheral and splanchnic vasodilator peptide from normal gut. Nature. 1970;225(5235):863–4. doi: 10.1038/225863a0. [DOI] [PubMed] [Google Scholar]
- 10.Said SI, Mutt V. Polypeptide with broad biological activity: isolation from small intestine. Science. 1970;169(3951):1217–8. doi: 10.1126/science.169.3951.1217. [DOI] [PubMed] [Google Scholar]
- 11.Bellinger DL, Lorton D, Brouxhon S, Felten S, Felten DL. The significance of vasoactive intestinal polypeptide (VIP) in immunomodulation. Adv Neuroimmunol. 1996;6(1):5–27. doi: 10.1016/s0960-5428(96)00008-3. [DOI] [PubMed] [Google Scholar]
- 12.Metwali A, Blum AM, Ferraris L, Klein JS, Fiocchi C, Weinstock JV. Eosinophils within the healthy or inflamed human intestine produce substance P and vasoactive intestinal peptide. J Neuroimmunol. 1994;52(1):69–78. doi: 10.1016/0165-5728(94)90164-3. [DOI] [PubMed] [Google Scholar]
- 13.Wershil BK, Turck CW, Sreedharan SP, Yang J, An S, Galli SJ, Goetzl EJ. Variants of vasoactive intestinal peptide in mouse mast cells and rat basophilic leukemia cells. Cell Immunol. 1993;151(2):369–78. doi: 10.1006/cimm.1993.1246. [DOI] [PubMed] [Google Scholar]
- 14.Leceta J, Martinez C, Delgado M, Garrido E, Gomariz RP. Expression of vasoactive intestinal peptide in lymphocytes: a possible endogenous role in the regulation of the immune system. Adv Neuroimmunol. 1996;6(1):29–36. doi: 10.1016/s0960-5428(96)00001-0. [DOI] [PubMed] [Google Scholar]
- 15.Sundler F, Ekblad E, Grunditz T, Hakanson R, Uddman R. Vasoactive intestinal peptide in the peripheral nervous system. Ann N Y Acad Sci. 1988;527:143–67. doi: 10.1111/j.1749-6632.1988.tb26979.x. [DOI] [PubMed] [Google Scholar]
- 16.Foster N. Editorial: vasoactive intestinal peptide (vip): historic perspective and future potential. Endocr Metab Immune Disord Drug Targets. 2012;12(4):303–7. doi: 10.2174/187153012803832521. [DOI] [PubMed] [Google Scholar]
- 17.Laburthe M, Couvineau A, Tan V. Class II G protein-coupled receptors for VIP and PACAP: structure, models of activation and pharmacology. Peptides. 2007;28(9):1631–9. doi: 10.1016/j.peptides.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 18.El-Shazly AE, Begon DY, Kustermans G, Arafa M, Dortu E, Henket M, Lefebvre PP, Louis R, Delvenne P. Novel association between vasoactive intestinal peptide and CRTH2 receptor in recruiting eosinophils: a possible biochemical mechanism for allergic eosinophilic inflammation of the airways. J Biol Chem. 2013;288(2):1374–84. doi: 10.1074/jbc.M112.422675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szema AM, Hamidi SA, Lyubsky S, Dickman KG, Mathew S, Abdel-Razek T, Chen JJ, Waschek JA, Said SI. Mice lacking the VIP gene show airway hyperresponsiveness and airway inflammation, partially reversible by VIP. Am J Physiol Lung Cell Mol Physiol. 2006;291(5):L880–6. doi: 10.1152/ajplung.00499.2005. [DOI] [PubMed] [Google Scholar]
- 20.Umemoto N, Kakurai M, Okazaki H, Kiyosawa T, Demitsu T, Nakagawa H. Serum levels of vasoactive intestinal peptide are elevated in patients with atopic dermatitis. J Dermatol Sci. 2003;31(2):161–4. doi: 10.1016/s0923-1811(03)00004-5. [DOI] [PubMed] [Google Scholar]
- 21.Delgado M, Abad C, Martinez C, Leceta J, Gomariz RP. Vasoactive intestinal peptide prevents experimental arthritis by downregulating both autoimmune and inflammatory components of the disease. Nat Med. 2001;7(5):563–8. doi: 10.1038/87887. [DOI] [PubMed] [Google Scholar]
- 22.Gomariz RP, Martinez C, Abad C, Leceta J, Delgado M. Immunology of VIP: a review and therapeutical perspectives. Curr Pharm Des. 2001;7(2):89–111. doi: 10.2174/1381612013398374. [DOI] [PubMed] [Google Scholar]
- 23.Igarashi NFH, Ito T, Nakamura T, Oono T, Nakamura K, Suzuki K, Jensen R, Takayanagi R. Abstractive Intestinal Peptide (VIP) and VIP Receptors-Elucidation of Structure and Function for Therapeutic Applications. International Journal of Clinical Medicine. 2011;2(4):500–508. [Google Scholar]
- 24.Dickson L, Finlayson K. VPAC and PAC receptors: From ligands to function. Pharmacol Ther. 2009;121(3):294–316. doi: 10.1016/j.pharmthera.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Fry DC, Madison VS, Bolin DR, Greeley DN, Toome V, Wegrzynski BB. Solution structure of an analogue of vasoactive intestinal peptide as determined by two-dimensional NMR and circular dichroism spectroscopies and constrained molecular dynamics. Biochemistry. 1989;28(6):2399–409. doi: 10.1021/bi00432a010. [DOI] [PubMed] [Google Scholar]
- 26.Laburthe M, Couvineau A, Gaudin P, Maoret JJ, Rouyer-Fessard C, Nicole P. Receptors for VIP, PACAP, secretin, GRF, glucagon, GLP-1, and other members of their new family of G protein-linked receptors: structure-function relationship with special reference to the human VIP-1 receptor. Ann N Y Acad Sci. 1996;805:94–109. doi: 10.1111/j.1749-6632.1996.tb17476.x. discussion 110–1. [DOI] [PubMed] [Google Scholar]
- 27.Langer I. Mechanisms involved in VPAC receptors activation and regulation: lessons from pharmacological and mutagenesis studies. Front Endocrinol (Lausanne) 2012;3:129. doi: 10.3389/fendo.2012.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laburthe M, Couvineau A. Molecular pharmacology and structure of VPAC Receptors for VIP and PACAP. Regul Pept. 2002;108(2–3):165–73. doi: 10.1016/s0167-0115(02)00099-x. [DOI] [PubMed] [Google Scholar]
- 29.Reubi JC, Laderach U, Waser B, Gebbers JO, Robberecht P, Laissue JA. Vasoactive intestinal peptide/pituitary adenylate cyclase-activating peptide receptor subtypes in human tumors and their tissues of origin. Cancer Res. 2000;60(11):3105–12. [PubMed] [Google Scholar]
- 30.Harmar AJ, Sheward WJ, Morrison CF, Waser B, Gugger M, Reubi JC. Distribution of the VPAC2 receptor in peripheral tissues of the mouse. Endocrinology. 2004;145(3):1203–10. doi: 10.1210/en.2003-1058. [DOI] [PubMed] [Google Scholar]
- 31.Goetzl EJ, Xia M, Ingram DA, Kishiyama JL, Kaltreider HB, Byrd PK, Ichikawa S, Sreedharan SP. Neuropeptide signaling of lymphocytes in immunological responses. Int Arch Allergy Immunol. 1995;107(1–3):202–4. doi: 10.1159/000236977. [DOI] [PubMed] [Google Scholar]
- 32.Sreedharan SP, Huang JX, Cheung MC, Goetzl EJ. Structure, expression, and chromosomal localization of the type I human vasoactive intestinal peptide receptor gene. Proc Natl Acad Sci U S A. 1995;92(7):2939–43. doi: 10.1073/pnas.92.7.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mackay M, Fantes J, Scherer S, Boyle S, West K, Tsui LC, Belloni E, Lutz E, Van Heyningen V, Harmar AJ. Chromosomal localization in mouse and human of the vasoactive intestinal peptide receptor type 2 gene: a possible contributor to the holoprosencephaly 3 phenotype. Genomics. 1996;37(3):345–53. doi: 10.1006/geno.1996.0569. [DOI] [PubMed] [Google Scholar]
- 34.Lutz EM, Sheward WJ, West KM, Morrow JA, Fink G, Harmar AJ. The VIP2 receptor: molecular characterisation of a cDNA encoding a novel receptor for vasoactive intestinal peptide. FEBS Lett. 1993;334(1):3–8. doi: 10.1016/0014-5793(93)81668-p. [DOI] [PubMed] [Google Scholar]
- 35.Segre GV, Goldring SR. Receptors for secretin, calcitonin, parathyroid hormone (PTH)/PTH-related peptide, vasoactive intestinal peptide, glucagonlike peptide 1, growth hormone-releasing hormone, and glucagon belong to a newly discovered G-protein-linked receptor family. Trends Endocrinol Metab. 1993;4(10):309–14. doi: 10.1016/1043-2760(93)90071-l. [DOI] [PubMed] [Google Scholar]
- 36.Delgado M, Pozo D, Ganea D. The significance of vasoactive intestinal peptide in immunomodulation. Pharmacol Rev. 2004;56(2):249–90. doi: 10.1124/pr.56.2.7. [DOI] [PubMed] [Google Scholar]
- 37.Bockaert J, Roussignol G, Becamel C, Gavarini S, Joubert L, Dumuis A, Fagni L, Marin P. GPCR-interacting proteins (GIPs): nature and functions. Biochem Soc Trans. 2004;32(Pt 5):851–5. doi: 10.1042/BST0320851. [DOI] [PubMed] [Google Scholar]
- 38.Chin D, Means AR. Calmodulin: a prototypical calcium sensor. Trends Cell Biol. 2000;10(8):322–8. doi: 10.1016/s0962-8924(00)01800-6. [DOI] [PubMed] [Google Scholar]
- 39.Gee HY, Kim YW, Jo MJ, Namkung W, Kim JY, Park HW, Kim KS, Kim H, Baba A, Yang J, Kim E, Kim KH, Lee MG. Synaptic scaffolding molecule binds to and regulates vasoactive intestinal polypeptide type-1 receptor in epithelial cells. Gastroenterology. 2009;137607–17(2):617e1–4. doi: 10.1053/j.gastro.2009.01.065. [DOI] [PubMed] [Google Scholar]
- 40.Hirai H, Tanaka K, Yoshie O, Ogawa K, Kenmotsu K, Takamori Y, Ichimasa M, Sugamura K, Nakamura M, Takano S, Nagata K. Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven-transmembrane receptor CRTH2. J Exp Med. 2001;193(2):255–61. doi: 10.1084/jem.193.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagata K, Hirai H, Tanaka K, Ogawa K, Aso T, Sugamura K, Nakamura M, Takano S. CRTH2, an orphan receptor of T-helper-2-cells, is expressed on basophils and eosinophils and responds to mast cell-derived factor(s) FEBS Lett. 1999;459(2):195–9. doi: 10.1016/s0014-5793(99)01251-x. [DOI] [PubMed] [Google Scholar]
- 42.Diamant Z, Sidharta PN, Singh D, O’Connor BJ, Zuiker R, Leaker BR, Silkey M, Dingemanse J. Setipiprant, a selective CRTH2 antagonist, reduces allergen-induced airway responses in allergic asthmatics. Clin Exp Allergy. 2014;44(8):1044–52. doi: 10.1111/cea.12357. [DOI] [PubMed] [Google Scholar]
- 43.Horak F, Zieglmayer P, Zieglmayer R, Lemell P, Collins LP, Hunter MG, Steiner J, Lewis T, Payton MA, Perkins CM, Pettipher R. The CRTH2 antagonist OC000459 reduces nasal and ocular symptoms in allergic subjects exposed to grass pollen, a randomised, placebo-controlled, double-blind trial. Allergy. 2012;67(12):1572–9. doi: 10.1111/all.12042. [DOI] [PubMed] [Google Scholar]
- 44.Monneret G, Gravel S, Diamond M, Rokach J, Powell WS. Prostaglandin D2 is a potent chemoattractant for human eosinophils that acts via a novel DP receptor. Blood. 2001;98(6):1942–8. doi: 10.1182/blood.v98.6.1942. [DOI] [PubMed] [Google Scholar]
- 45.O’Sullivan S. On the role of PGD2 metabolites as markers of mast cell activation in asthma. Acta Physiol Scand Suppl. 1999;644:1–74. [PubMed] [Google Scholar]
- 46.Tanaka K, Ogawa K, Sugamura K, Nakamura M, Takano S, Nagata K. Cutting edge: differential production of prostaglandin D2 by human helper T cell subsets. J Immunol. 2000;164(5):2277–80. doi: 10.4049/jimmunol.164.5.2277. [DOI] [PubMed] [Google Scholar]
- 47.Shiraishi Y, Asano K, Nakajima T, Oguma T, Suzuki Y, Shiomi T, Sayama K, Niimi K, Wakaki M, Kagyo J, Ikeda E, Hirai H, Yamaguchi K, Ishizaka A. Prostaglandin D2-induced eosinophilic airway inflammation is mediated by CRTH2 receptor. J Pharmacol Exp Ther. 2005;312(3):954–60. doi: 10.1124/jpet.104.078212. [DOI] [PubMed] [Google Scholar]
- 48.Kuna P, Bjermer L, Tornling G. Two Phase II randomized trials on the CRTh2 antagonist AZD1981 in adults with asthma. Drug Des Devel Ther. 2016;10:2759–70. doi: 10.2147/DDDT.S105142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Erpenbeck VJ, Popov TA, Miller D, Weinstein SF, Spector S, Magnusson B, Osuntokun W, Goldsmith P, Weiss M, Beier J. The oral CRTh2 antagonist QAW039 (fevipiprant): A phase II study in uncontrolled allergic asthma. Pulm Pharmacol Ther. 2016;39:54–63. doi: 10.1016/j.pupt.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 50.Pisegna JR, Wank SA. Molecular cloning and functional expression of the pituitary adenylate cyclase-activating polypeptide type I receptor. Proc Natl Acad Sci U S A. 1993;90(13):6345–9. doi: 10.1073/pnas.90.13.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vaudry D, Falluel-Morel A, Bourgault S, Basille M, Burel D, Wurtz O, Fournier A, Chow BK, Hashimoto H, Galas L, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev. 2009;61(3):283–357. doi: 10.1124/pr.109.001370. [DOI] [PubMed] [Google Scholar]
- 52.Harmar AJ, Fahrenkrug J, Gozes I, Laburthe M, May V, Pisegna JR, Vaudry D, Vaudry H, Waschek JA, Said SI. Pharmacology and functions of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide: IUPHAR review 1. Br J Pharmacol. 2012;166(1):4–17. doi: 10.1111/j.1476-5381.2012.01871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lauenstein HD, Quarcoo D, Plappert L, Schleh C, Nassimi M, Pilzner C, Rochlitzer S, Brabet P, Welte T, Hoymann HG, Krug N, Muller M, Lerner EA, Braun A, Groneberg DA. Pituitary adenylate cyclase-activating peptide receptor 1 mediates anti-inflammatory effects in allergic airway inflammation in mice. Clin Exp Allergy. 2011;41(4):592–601. doi: 10.1111/j.1365-2222.2010.03636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smalley SG, Barrow PA, Foster N. Immunomodulation of innate immune responses by vasoactive intestinal peptide (VIP): its therapeutic potential in inflammatory disease. Clin Exp Immunol. 2009;157(2):225–34. doi: 10.1111/j.1365-2249.2009.03956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bienenstock J, MacQueen G, Sestini P, Marshall JS, Stead RH, Perdue MH. Mast cell/nerve interactions in vitro and in vivo. Am Rev Respir Dis. 1991;143(3 Pt 2):S55–8. doi: 10.1164/ajrccm/143.3_Pt_2.S55. [DOI] [PubMed] [Google Scholar]
- 56.Munitz A, Piliponsky AM, Levi-Schaffer F. IgE-Independent Activation of Human Mast Cells Indicates their Role in the Late Phase Reaction of Allergic Inflammation. Cell Tissue Bank. 2003;4(1):25–8. doi: 10.1023/A:1026307812980. [DOI] [PubMed] [Google Scholar]
- 57.Kulka M, Sheen CH, Tancowny BP, Grammer LC, Schleimer RP. Neuropeptides activate human mast cell degranulation and chemokine production. Immunology. 2008;123(3):398–410. doi: 10.1111/j.1365-2567.2007.02705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Groneberg DA, Welker P, Fischer TC, Dinh QT, Grutzkau A, Peiser C, Wahn U, Henz BM, Fischer A. Down-regulation of vasoactive intestinal polypeptide receptor expression in atopic dermatitis. J Allergy Clin Immunol. 2003;111(5):1099–105. doi: 10.1067/mai.2003.1477. [DOI] [PubMed] [Google Scholar]
- 59.Maintz L, Wardelmann E, Walgenbach K, Fimmers R, Bieber T, Raap U, Novak N. Neuropeptide blood levels correlate with mast cell load in patients with mastocytosis. Allergy. 2011;66(7):862–9. doi: 10.1111/j.1398-9995.2011.02550.x. [DOI] [PubMed] [Google Scholar]
- 60.Keita AV, Carlsson AH, Cigehn M, Ericson AC, McKay DM, Soderholm JD. Vasoactive intestinal polypeptide regulates barrier function via mast cells in human intestinal follicle-associated epithelium and during stress in rats. Neurogastroenterol Motil. 2013;25(6):e406–17. doi: 10.1111/nmo.12127. [DOI] [PubMed] [Google Scholar]
- 61.Moon TC, Campos-Alberto E, Yoshimura T, Bredo G, Rieger AM, Puttagunta L, Barreda DR, Befus AD, Cameron L. Expression of DP2 (CRTh2), a prostaglandin D(2) receptor, in human mast cells. PLoS One. 2014;9(9):e108595. doi: 10.1371/journal.pone.0108595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yoshimura-Uchiyama C, Iikura M, Yamaguchi M, Nagase H, Ishii A, Matsushima K, Yamamoto K, Shichijo M, Bacon KB, Hirai K. Differential modulation of human basophil functions through prostaglandin D2 receptors DP and chemoattractant receptor-homologous molecule expressed on Th2 cells/DP2. Clin Exp Allergy. 2004;34(8):1283–90. doi: 10.1111/j.1365-2222.2004.02027.x. [DOI] [PubMed] [Google Scholar]
- 63.Verma AK, Manohar M, Sathisha UV, Blecker U, Collins MH, Mishra A. Role of vasoactive intestinal peptide in promoting the pathogenesis of eosinophilic esophagitis (EoE) Cell Mol Gastroenterol Hepatol. 2017 Sep; doi: 10.1016/j.jcmgh.2017.09.006. Accepted, Article in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Venkateshaiah SU, Zhu X, Rajavelu P, Niranjan R, Manohar M, Verma AK, Lasky JA, Mishra A. Regulatory effects of IL-15 on allergen-induced airway obstruction. J Allergy Clin Immunol. 2017 doi: 10.1016/j.jaci.2017.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pawankar R, Mori S, Ozu C, Kimura S. Overview on the pathomechanisms of allergic rhinitis. Asia Pac Allergy. 2011;1(3):157–67. doi: 10.5415/apallergy.2011.1.3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shukla A, Mishra A, Venkateshaiah SU, Manohar M, Mahadevappa CP, Mishra A. Elements Involved In Promoting Eosinophilic Gastrointestinal Disorders. J Genet Syndr Gene Ther. 2015;6(2) doi: 10.4172/2157-7412.1000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Manohar M, Verma AK, Venkateshaiah SU, Sanders NL, Mishra A. Pathogenic mechanisms of pancreatitis. World J Gastrointest Pharmacol Ther. 2017;8(1):10–25. doi: 10.4292/wjgpt.v8.i1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Manohar M, Verma AK, Venkateshaiah SU, Mishra A. Role of eosinophils in the initiation and progression of pancreatitis pathogenesis. Am J Physiol Gastrointest Liver Physiol. 2017 Sep 19; doi: 10.1152/ajpgi.00210.2017. Accepted, Article in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dunzendorfer S, Meierhofer C, Wiedermann CJ. Signaling in neuropeptide-induced migration of human eosinophils. J Leukoc Biol. 1998;64(6):828–34. doi: 10.1002/jlb.64.6.828. [DOI] [PubMed] [Google Scholar]
- 70.Numao T, Agrawal DK. Neuropeptides modulate human eosinophil chemotaxis. J Immunol. 1992;149(10):3309–15. [PubMed] [Google Scholar]
- 71.Nagata K, Hirai H. The second PGD(2) receptor CRTH2: structure, properties, and functions in leukocytes. Prostaglandins Leukot Essent Fatty Acids. 2003;69(2–3):169–77. doi: 10.1016/s0952-3278(03)00078-4. [DOI] [PubMed] [Google Scholar]
- 72.Okano M, Fujiwara T, Sugata Y, Gotoh D, Masaoka Y, Sogo M, Tanimoto W, Yamamoto M, Matsumoto R, Eguchi N, Kiniwa M, Isik AU, Urade Y, Nishizaki K. Presence and characterization of prostaglandin D2-related molecules in nasal mucosa of patients with allergic rhinitis. Am J Rhinol. 2006;20(3):342–8. doi: 10.2500/ajr.2006.20.2865. [DOI] [PubMed] [Google Scholar]
- 73.Misaka S, Aoki Y, Karaki S, Kuwahara A, Mizumoto T, Onoue S, Yamada S. Inhalable powder formulation of a stabilized vasoactive intestinal peptide (VIP) derivative: anti-inflammatory effect in experimental asthmatic rats. Peptides. 2010;31(1):72–8. doi: 10.1016/j.peptides.2009.09.032. [DOI] [PubMed] [Google Scholar]
- 74.Harfi I, D’Hondt S, Corazza F, Sariban E. Regulation of human polymorphonuclear leukocytes functions by the neuropeptide pituitary adenylate cyclase-activating polypeptide after activation of MAPKs. J Immunol. 2004;173(6):4154–63. doi: 10.4049/jimmunol.173.6.4154. [DOI] [PubMed] [Google Scholar]
- 75.Palermo MS, Vermeulen ME, Giordano MN. Human antibody-dependent cellular cytotoxicity mediated by interferon gamma-activated neutrophils is impaired by vasoactive intestinal peptide. J Neuroimmunol. 1996;69(1–2):123–8. doi: 10.1016/0165-5728(96)00078-1. [DOI] [PubMed] [Google Scholar]
- 76.Pedrera C, Lucas M, Bellido L, Lopez-Gonzalez MA. Receptor-independent mechanisms are involved in the priming of neutrophil’s oxidase by vasoactive intestinal peptide. Regul Pept. 1994;54(2–3):505–11. doi: 10.1016/0167-0115(94)90548-7. [DOI] [PubMed] [Google Scholar]
- 77.Lopez-Gonzalez MA, Lucas M. Priming effect of vasoactive intestinal peptide on the respiratory burst of neutrophils non-mediated by plasma membrane receptors. Experientia. 1994;50(5):486–8. doi: 10.1007/BF01920753. [DOI] [PubMed] [Google Scholar]
- 78.Sergejeva S, Hoshino H, Yoshihara S, Kashimoto K, Lotvall J, Linden A. A synthetic VIP peptide analogue inhibits neutrophil recruitment in rat airways in vivo. Regul Pept. 2004;117(2):149–54. doi: 10.1016/j.regpep.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 79.O’Dorisio MS, Hermina NS, O’Dorisio TM, Balcerzak SP. Vasoactive intestinal polypeptide modulation of lymphocyte adenylate cyclase. J Immunol. 1981;127(6):2551–4. [PubMed] [Google Scholar]
- 80.Johnson MC, McCormack RJ, Delgado M, Martinez C, Ganea D. Murine T-lymphocytes express vasoactive intestinal peptide receptor 1 (VIP-R1) mRNA. J Neuroimmunol. 1996;68(1–2):109–19. doi: 10.1016/0165-5728(96)00085-9. [DOI] [PubMed] [Google Scholar]
- 81.Delgado M, Martinez C, Johnson MC, Gomariz RP, Ganea D. Differential expression of vasoactive intestinal peptide receptors 1 and 2 (VIP-R1 and VIP-R2) mRNA in murine lymphocytes. J Neuroimmunol. 1996;68(1–2):27–38. doi: 10.1016/0165-5728(96)00063-x. [DOI] [PubMed] [Google Scholar]
- 82.Jimeno R, Leceta J, Martinez C, Gutierrez-Canas I, Perez-Garcia S, Carrion M, Gomariz RP, Juarranz Y. Effect of VIP on the balance between cytokines and master regulators of activated helper T cells. Immunol Cell Biol. 2012;90(2):178–86. doi: 10.1038/icb.2011.23. [DOI] [PubMed] [Google Scholar]
- 83.Gomariz RP, Leceta J, Garrido E, Garrido T, Delgado M. Vasoactive intestinal peptide (VIP) mRNA expression in rat T and B lymphocytes. Regul Pept. 1994;50(2):177–84. doi: 10.1016/0167-0115(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 84.Delgado M. VIP: a very important peptide in T helper differentiation. Trends Immunol. 2003;24(5):221–4. doi: 10.1016/s1471-4906(03)00069-3. [DOI] [PubMed] [Google Scholar]
- 85.Mutalithas K, Guillen C, Day C, Brightling CE, Pavord ID, Wardlaw AJ. CRTH2 expression on T cells in asthma. Clin Exp Immunol. 2010;161(1):34–40. doi: 10.1111/j.1365-2249.2010.04161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gyles SL, Xue L, Townsend ER, Wettey F, Pettipher R. A dominant role for chemoattractant receptor-homologous molecule expressed on T helper type 2 (Th2) cells (CRTH2) in mediating chemotaxis of CRTH2+ CD4+ Th2 lymphocytes in response to mast cell supernatants. Immunology. 2006;119(3):362–8. doi: 10.1111/j.1365-2567.2006.02440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Perez-Novo CA, Holtappels G, Vinall SL, Xue L, Zhang N, Bachert C, Pettipher R. CRTH2 mediates the activation of human Th2 cells in response to PGD(2) released from IgE/anti-IgE treated nasal polyp tissue. Allergy. 2010;65(3):304–10. doi: 10.1111/j.1398-9995.2009.02204.x. [DOI] [PubMed] [Google Scholar]
- 88.Ganea D, Delgado M. Neuropeptides as modulators of macrophage functions. Regulation of cytokine production and antigen presentation by VIP and PACAP. Arch Immunol Ther Exp (Warsz) 2001;49(2):101–10. [PubMed] [Google Scholar]
- 89.Lara-Marquez M, O’Dorisio M, O’Dorisio T, Shah M, Karacay B. Selective gene expression and activation-dependent regulation of vasoactive intestinal peptide receptor type 1 and type 2 in human T cells. J Immunol. 2001;166(4):2522–30. doi: 10.4049/jimmunol.166.4.2522. [DOI] [PubMed] [Google Scholar]
- 90.Delgado M, Munoz-Elias EJ, Gomariz RP, Ganea D. VIP and PACAP inhibit IL-12 production in LPS-stimulated macrophages. Subsequent effect on IFNgamma synthesis by T cells. J Neuroimmunol. 1999;96(2):167–81. doi: 10.1016/s0165-5728(99)00023-5. [DOI] [PubMed] [Google Scholar]
- 91.Delgado M, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit expression of Fas ligand in activated T lymphocytes by regulating c-Myc, NF-kappa B, NF-AT, and early growth factors 2/3. J Immunol. 2001;166(2):1028–40. doi: 10.4049/jimmunol.166.2.1028. [DOI] [PubMed] [Google Scholar]
- 92.Ganea D, Delgado M. Vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase-activating polypeptide (PACAP) as modulators of both innate and adaptive immunity. Crit Rev Oral Biol Med. 2002;13(3):229–37. doi: 10.1177/154411130201300303. [DOI] [PubMed] [Google Scholar]
- 93.Delgado M, Munoz-Elias EJ, Gomariz RP, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide prevent inducible nitric oxide synthase transcription in macrophages by inhibiting NF-kappa B and IFN regulatory factor 1 activation. J Immunol. 1999;162(8):4685–96. [PubMed] [Google Scholar]
- 94.Delneste Y, Herbault N, Galea B, Magistrelli G, Bazin I, Bonnefoy JY, Jeannin P. Vasoactive intestinal peptide synergizes with TNF-alpha in inducing human dendritic cell maturation. J Immunol. 1999;163(6):3071–5. [PubMed] [Google Scholar]
- 95.Delgado M, Reduta A, Sharma V, Ganea D. VIP/PACAP oppositely affects immature and mature dendritic cell expression of CD80/CD86 and the stimulatory activity for CD4(+) T cells. J Leukoc Biol. 2004;75(6):1122–30. doi: 10.1189/jlb.1203626. [DOI] [PubMed] [Google Scholar]
- 96.Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol Rev. 2000;52(2):269–324. [PubMed] [Google Scholar]
- 97.Onoue S, Yamada S, Yajima T. Bioactive analogues and drug delivery systems of vasoactive intestinal peptide (VIP) for the treatment of asthma/COPD. Peptides. 2007;28(9):1640–50. doi: 10.1016/j.peptides.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 98.Guarnaccia S, Russo Mancuso G, Peters SM, Scanlon RT, Bellanti JA. In vitro studies of the modulatory effects of sensory neuropeptides on peripheral blood mononuclear leukocytes of normal and allergic subjects. Ann Allergy. 1990;64(1):58–61. [PubMed] [Google Scholar]
- 99.Harper RW, Zeki AA. Immunobiology of the critical asthma syndrome. Clin Rev Allergy Immunol. 2015;48(1):54–65. doi: 10.1007/s12016-013-8407-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vercelli D. Does epigenetics play a role in human asthma? Allergol Int. 2016;65(2):123–6. doi: 10.1016/j.alit.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 101.Luts A, Uddman R, Alm P, Basterra J, Sundler F. Peptide-containing nerve fibers in human airways: distribution and coexistence pattern. Int Arch Allergy Immunol. 1993;101(1):52–60. doi: 10.1159/000236498. [DOI] [PubMed] [Google Scholar]
- 102.Wu D, Lee D, Sung YK. Prospect of vasoactive intestinal peptide therapy for COPD/PAH and asthma: a review. Respir Res. 2011;12:45. doi: 10.1186/1465-9921-12-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hamidi SA, Szema AM, Lyubsky S, Dickman KG, Degene A, Mathew SM, Waschek JA, Said SI. Clues to VIP function from knockout mice. Ann N Y Acad Sci. 2006;1070:5–9. doi: 10.1196/annals.1317.035. [DOI] [PubMed] [Google Scholar]
- 104.Szema AM, Hamidi SA, Koller A, Martin DW. Vasoactive Intestinal Peptide Knockout (VIP KO) mouse model of sulfite-sensitive asthma: up-regulation of novel lung carbonyl reductase. BMC Immunol. 2011;12:66. doi: 10.1186/1471-2172-12-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu H, Yang X, Hou W. Correlation of c-fos protein expression with neuropeptide content in the lung of bronchial asthmatic rat. Int J Clin Exp Pathol. 2014;7(12):8657–65. [PMC free article] [PubMed] [Google Scholar]
- 106.Samarasinghe AE, Hoselton SA, Schuh JM. Spatio-temporal localization of vasoactive intestinal peptide and neutral endopeptidase in allergic murine lungs. Regul Pept. 2010;164(2–3):151–7. doi: 10.1016/j.regpep.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.El-Shazly A, Berger P, Girodet PO, Ousova O, Fayon M, Vernejoux JM, Marthan R, Tunon-de-Lara JM. Fraktalkine produced by airway smooth muscle cells contributes to mast cell recruitment in asthma. J Immunol. 2006;176(3):1860–8. doi: 10.4049/jimmunol.176.3.1860. [DOI] [PubMed] [Google Scholar]
- 108.Zhang MQ, Timmerman H. Mast cell tryptase and asthma. Mediators Inflamm. 1997;6(5–6):311–7. doi: 10.1080/09629359791433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tam EK, Caughey GH. Degradation of airway neuropeptides by human lung tryptase. Am J Respir Cell Mol Biol. 1990;3(1):27–32. doi: 10.1165/ajrcmb/3.1.27. [DOI] [PubMed] [Google Scholar]
- 110.Maruno K, Absood A, Said SI. VIP inhibits basal and histamine-stimulated proliferation of human airway smooth muscle cells. Am J Physiol. 1995;268(6 Pt 1):L1047–51. doi: 10.1152/ajplung.1995.268.6.L1047. [DOI] [PubMed] [Google Scholar]
- 111.Chanez P, Springall D, Vignola AM, Moradoghi-Hattvani A, Polak JM, Godard P, Bousquet J. Bronchial mucosal immunoreactivity of sensory neuropeptides in severe airway diseases. Am J Respir Crit Care Med. 1998;158(3):985–90. doi: 10.1164/ajrccm.158.3.9608104. [DOI] [PubMed] [Google Scholar]
- 112.Howarth PH, Springall DR, Redington AE, Djukanovic R, Holgate ST, Polak JM. Neuropeptide-containing nerves in endobronchial biopsies from asthmatic and nonasthmatic subjects. Am J Respir Cell Mol Biol. 1995;13(3):288–96. doi: 10.1165/ajrcmb.13.3.7654385. [DOI] [PubMed] [Google Scholar]
- 113.Cardell LO, Uddman R, Edvinsson L. Low plasma concentrations of VIP and elevated levels of other neuropeptides during exacerbations of asthma. Eur Respir J. 1994;7(12):2169–73. doi: 10.1183/09031936.94.07122169. [DOI] [PubMed] [Google Scholar]
- 114.Bradding P, Feather IH, Wilson S, Holgate ST, Howarth PH. Cytokine immunoreactivity in seasonal rhinitis: regulation by a topical corticosteroid. Am J Respir Crit Care Med. 1995;151(6):1900–6. doi: 10.1164/ajrccm.151.6.7767538. [DOI] [PubMed] [Google Scholar]
- 115.Ricci M, Romagnani S. Pathogenetic aspects of allergic respiratory syndromes. Ann Ital Med Int. 1991;6(1 Pt 2):183–91. [PubMed] [Google Scholar]
- 116.Kim DH, Park IH, Cho JS, Lee YM, Choi H, Lee HM. Alterations of vasoactive intestinal polypeptide receptors in allergic rhinitis. Am J Rhinol Allergy. 2011;25(1):e44–7. doi: 10.2500/ajra.2011.25.3568. [DOI] [PubMed] [Google Scholar]
- 117.Fischer A, Wussow A, Cryer A, Schmeck B, Noga O, Zweng M, Peiser C, Dinh QT, Heppt W, Groneberg DA. Neuronal plasticity in persistent perennial allergic rhinitis. J Occup Environ Med. 2005;47(1):20–5. doi: 10.1097/01.jom.0000150238.77663.49. [DOI] [PubMed] [Google Scholar]
- 118.Antunes MB, Gudis DA, Cohen NA. Epithelium, cilia, and mucus: their importance in chronic rhinosinusitis. Immunol Allergy Clin North Am. 2009;29(4):631–43. doi: 10.1016/j.iac.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 119.Lee RJ, Chen B, Doghramji L, Adappa ND, Palmer JN, Kennedy DW, Cohen NA. Vasoactive intestinal peptide regulates sinonasal mucociliary clearance and synergizes with histamine in stimulating sinonasal fluid secretion. FASEB J. 2013;27(12):5094–103. doi: 10.1096/fj.13-234476. [DOI] [PubMed] [Google Scholar]
- 120.Mosimann BL, White MV, Hohman RJ, Goldrich MS, Kaulbach HC, Kaliner MA. Substance P, calcitonin gene-related peptide, and vasoactive intestinal peptide increase in nasal secretions after allergen challenge in atopic patients. J Allergy Clin Immunol. 1993;92(1 Pt 1):95–104. doi: 10.1016/0091-6749(93)90043-f. [DOI] [PubMed] [Google Scholar]
- 121.Bellamy JL, Cady RK, Durham PL. Salivary levels of CGRP and VIP in rhinosinusitis and migraine patients. Headache. 2006;46(1):24–33. doi: 10.1111/j.1526-4610.2006.00294.x. [DOI] [PubMed] [Google Scholar]
- 122.Ogawa T, Takeno S, Ishino T, Hirakawa K. Submucous turbinectomy combined with posterior nasal neurectomy in the management of severe allergic rhinitis: clinical outcomes and local cytokine changes. Auris Nasus Larynx. 2007;34(3):319–26. doi: 10.1016/j.anl.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 123.Nishijima H, Kondo K, Toma-Hirano M, Iwasaki S, Kikuta S, Fujimoto C, Ueha R, Kagoya R, Yamasoba T. Denervation of nasal mucosa induced by posterior nasal neurectomy suppresses nasal secretion, not hypersensitivity, in an allergic rhinitis rat model. Lab Invest. 2016;96(9):981–93. doi: 10.1038/labinvest.2016.72. [DOI] [PubMed] [Google Scholar]
- 124.El-Shazly AE, Roncarati P, Lejeune M, Lefebvre PP, Delvenne P. Tyrosine kinase inhibition is an important factor for gene expression of CRTH2 in human eosinophils and lymphocytes: A novel mechanism for explaining eosinophils recruitment by the neuro-immune axis in allergic rhinitis. Int Immunopharmacol. 2017;45:180–186. doi: 10.1016/j.intimp.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 125.Kim KH. Overview of atopic dermatitis. Asia Pac Allergy. 2013;3(2):79–87. doi: 10.5415/apallergy.2013.3.2.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mu Z, Zhao Y, Liu X, Chang C, Zhang J. Molecular biology of atopic dermatitis. Clin Rev Allergy Immunol. 2014;47(2):193–218. doi: 10.1007/s12016-014-8415-1. [DOI] [PubMed] [Google Scholar]
- 127.Tobin D, Nabarro G, Baart de la Faille H, van Vloten WA, van der Putte SC, Schuurman HJ. Increased number of immunoreactive nerve fibers in atopic dermatitis. J Allergy Clin Immunol. 1992;90(4 Pt 1):613–22. doi: 10.1016/0091-6749(92)90134-n. [DOI] [PubMed] [Google Scholar]
- 128.Salomon J, Baran E. The role of selected neuropeptides in pathogenesis of atopic dermatitis. J Eur Acad Dermatol Venereol. 2008;22(2):223–8. doi: 10.1111/j.1468-3083.2007.02399.x. [DOI] [PubMed] [Google Scholar]
- 129.Lundeberg L, Nordlind K. Vasoactive intestinal polypeptide in allergic contact dermatitis: an immunohistochemical and radioimmunoassay study. Arch Dermatol Res. 1999;291(4):201–6. doi: 10.1007/s004030050394. [DOI] [PubMed] [Google Scholar]
- 130.Giannetti A, Fantini F, Cimitan A, Pincelli C. Vasoactive intestinal polypeptide and substance P in the pathogenesis of atopic dermatitis. Acta Derm Venereol Suppl (Stockh) 1992;176:90–2. [PubMed] [Google Scholar]
- 131.Anand P, Springall DR, Blank MA, Sellu D, Polak JM, Bloom SR. Neuropeptides in skin disease: increased VIP in eczema and psoriasis but not axillary hyperhidrosis. Br J Dermatol. 1991;124(6):547–9. doi: 10.1111/j.1365-2133.1991.tb04948.x. [DOI] [PubMed] [Google Scholar]
- 132.Al’Abadie MS, Senior HJ, Bleehen SS, Gawkrodger DJ. Neuropeptides and general neuronal marker in psoriasis--an immunohistochemical study. Clin Exp Dermatol. 1995;20(5):384–9. doi: 10.1111/j.1365-2230.1995.tb01354.x. [DOI] [PubMed] [Google Scholar]
- 133.Fischer TC, Dinh QT, Peiser C, Loser C, Fischer A, Groneberg DA. Simultaneous detection of receptor mRNA and ligand protein in human skin tissues. J Cutan Pathol. 2002;29(2):65–71. doi: 10.1034/j.1600-0560.2002.290201.x. [DOI] [PubMed] [Google Scholar]
- 134.Hodeib A, El-Samad ZA, Hanafy H, El-Latief AA, El-Bendary A, Abu-Raya A. Nerve growth factor, neuropeptides and cutaneous nerves in atopic dermatitis. Indian J Dermatol. 2010;55(2):135–9. doi: 10.4103/0019-5154.62735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Teresiak-Mikolajczak E, Czarnecka-Operacz M, Jenerowicz D, Silny W. Neurogenic markers of the inflammatory process in atopic dermatitis: relation to the severity and pruritus. Postepy Dermatol Alergol. 2013;30(5):286–92. doi: 10.5114/pdia.2013.38357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kimata H. Enhancement of allergic skin wheal responses and in vitro allergen-specific IgE production by computer-induced stress in patients with atopic dermatitis. Brain Behav Immun. 2003;17(2):134–8. doi: 10.1016/s0889-1591(03)00025-4. [DOI] [PubMed] [Google Scholar]
- 137.Kang H, Byun DG, Kim JW. Effects of substance P and vasoactive intestinal peptide on interferon-gamma and interleukin-4 production in severe atopic dermatitis. Ann Allergy Asthma Immunol. 2000;85(3):227–32. doi: 10.1016/S1081-1206(10)62471-4. [DOI] [PubMed] [Google Scholar]
- 138.Kakurai M, Fujita N, Murata S, Furukawa Y, Demitsu T, Nakagawa H. Vasoactive intestinal peptide regulates its receptor expression and functions of human keratinocytes via type I vasoactive intestinal peptide receptors. J Invest Dermatol. 2001;116(5):743–9. doi: 10.1046/j.1523-1747.2001.01306.x. [DOI] [PubMed] [Google Scholar]
- 139.Jarvikallio A, Harvima IT, Naukkarinen A. Mast cells, nerves and neuropeptides in atopic dermatitis and nummular eczema. Arch Dermatol Res. 2003;295(1):2–7. doi: 10.1007/s00403-002-0378-z. [DOI] [PubMed] [Google Scholar]
- 140.Sandhu DS, Fass R. Current Trends in the Management of Gastroesophageal Reflux Disease. Gut Liver. 2017 doi: 10.5009/gnl16615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wattchow DA, Jamieson GG, Maddern GJ, Furness JB, Costa M. Distribution of peptide-containing nerve fibers in the gastric musculature of patients undergoing surgery for gastroesophageal reflux. Ann Surg. 1992;216(2):153–60. doi: 10.1097/00000658-199208000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kassim SK, El Touny M, El Guinaidy M, El Moghni MA, El Mohsen AA. Serum nitrates and vasoactive intestinal peptide in patients with gastroesophageal reflux disease. Clin Biochem. 2002;35(8):641–6. doi: 10.1016/s0009-9120(02)00399-5. [DOI] [PubMed] [Google Scholar]
- 143.Rossiter A, Guelrud M, Souney PF, Mendoza S, Rossiter G, Gelrud D. High vasoactive intestinal polypeptide plasma levels in patients with Barrett’s esophagus. Scand J Gastroenterol. 1991;26(5):572–6. doi: 10.3109/00365529108998582. [DOI] [PubMed] [Google Scholar]
- 144.Zaidi AK, Mussarat A, Mishra A. Diagnostic and therapeutic strategies for eosinophilic esophagitis. Clin Pract (Lond) 2014;11(3):351–367. doi: 10.2217/cpr.14.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Niranjan R, Mavi P, Rayapudi M, Dynda S, Mishra A. Pathogenic role of mast cells in experimental eosinophilic esophagitis. Am J Physiol Gastrointest Liver Physiol. 2013;304(12):G1087–94. doi: 10.1152/ajpgi.00070.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Herberth G, Daegelmann C, Weber A, Roder S, Giese T, Kramer U, Schins RP, Behrendt H, Borte M, Lehmann I, Group LIS. Association of neuropeptides with Th1/Th2 balance and allergic sensitization in children. Clin Exp Allergy. 2006;36(11):1408–16. doi: 10.1111/j.1365-2222.2006.02576.x. [DOI] [PubMed] [Google Scholar]
- 147.Mathioudakis A, Chatzimavridou-Grigoriadou V, Evangelopoulou E, Mathioudakis G. Vasoactive intestinal Peptide inhaled agonists: potential role in respiratory therapeutics. Hippokratia. 2013;17(1):12–6. [PMC free article] [PubMed] [Google Scholar]
- 148.Morice A, Unwin RJ, Sever PS. Vasoactive intestinal peptide causes bronchodilatation and protects against histamine-induced bronchoconstriction in asthmatic subjects. Lancet. 1983;2(8361):1225–7. doi: 10.1016/s0140-6736(83)91272-2. [DOI] [PubMed] [Google Scholar]
- 149.Linden A, Hansson L, Andersson A, Palmqvist M, Arvidsson P, Lofdahl CG, Larsson P, Lotvall J. Bronchodilation by an inhaled VPAC(2) receptor agonist in patients with stable asthma. Thorax. 2003;58(3):217–21. doi: 10.1136/thorax.58.3.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Athari SS, Pourpak Z, Folkerts G, Garssen J, Moin M, Adcock IM, Movassaghi M, Ardestani MS, Moazzeni SM, Mortaz E. Conjugated Alpha-Alumina nanoparticle with vasoactive intestinal peptide as a Nano-drug in treatment of allergic asthma in mice. Eur J Pharmacol. 2016;791:811–820. doi: 10.1016/j.ejphar.2016.10.014. [DOI] [PubMed] [Google Scholar]