Abstract
Background and Purpose
To assess associations between radiation dose/volume parameters for cardiac subvolumes and different types of cardiac events in patients treated on radiation dose-escalation trials.
Material and Methods
Patients with Stage III non-small-cell lung cancer received dose-escalated radiation (median 74 Gy) using 3D-conformal radiotherapy on six prospective trials from 1996–2009. Volumes analyzed included whole heart, left ventricle (LV), right atrium (RA), and left atrium (LA). Cardiac events were divided into three categories: pericardial (symptomatic effusion and pericarditis), ischemia (myocardial infarction and unstable angina), and arrhythmia. Univariable competing risks analysis was used.
Results
112 patients were analyzed, with median follow-up 8.8 years for surviving patients. Nine patients had pericardial, seven patients had ischemic, and 12 patients had arrhythmic events. Pericardial events were correlated with whole heart, RA, and LA dose (eg, heart-V30 [p=0.024], RA-V30 [p=0.013], and LA-V30 [p=0.001]), but not LV dose. Ischemic events were correlated with LV and whole heart dose (eg, LV-V30 [p=0.012], heart-V30 [p=0.048]). Arrhythmic events showed borderline significant associations with RA, LA, and whole heart dose (eg, RA-V30 [p=0.082], LA-V30 [p=0.076], heart-V30 [p=0.051]). Cardiac events were associated with decreased survival on univariable analysis (p=0.008, HR 2.09), but only disease progression predicted for decreased survival on multivariable analysis.
Conclusions
Cardiac events were heterogeneous and associated with distinct heart subvolume doses. These data support the hypothesis of distinct etiologies for different types of radiation-associated cardiotoxicity.
Keywords: NSCLC, cardiac toxicity, chemoradiation, dose escalation
Introduction
There is increasing recognition of the significance of radiation (RT)-associated heart toxicity in patients with lung cancer.1–6 To study this issue, we performed a pooled analysis of patients with Stage III non-small-cell lung cancer (NSCLC) treated at the University of North Carolina on several prospective trials using dose-escalated RT between 1996 and 2009.7 Of 112 patients, 26 experienced symptomatic cardiac events at a median of only two years post-RT, and events were associated with heart dose.
Cardiac events in these patients included symptomatic pericardial effusion, pericarditis, myocardial infarction, unstable angina, significant arrhythmia, and new-onset heart failure. The heterogeneity of events begets the question: Do they have different etiologies, and by extension are they dependent on dose to different parts of the heart? There are only limited data addressing cardiac subvolume dosimetry.8–10 We herein assess associations between RT dose to different cardiac subvolumes and different cardiac toxicity endpoints in our previously-studied cohort. We grouped events into three categories: pericardial, ischemic, and arrhythmic. We had three main hypotheses: 1. Pericardial events are most associated with global heart dose due to pericardial anatomy, 2. Ischemic events are most associated with left ventricle dose due to high oxygen demand and contractile function, and 3. Arrhythmic events are most associated with atrial doses given the importance of atrial conduction abnormalities.
Materials and Methods
Study Design
Post hoc analysis of six prospective trials.11–16 Records were retrospectively reviewed to assess three cardiac endpoints: pericardial events, ischemic events, and arrhythmic events. The analysis of the combined cardiotoxicity endpoints, details of the six included trials, and further methodologic details are described in our original publication.7
Patient Population and Treatment
Between 1996 and 2009, 127 patients with stage III NSCLC and ECOG performance status 0–1 were treated on six prospective trials utilizing dose-escalated RT delivering 70–90 Gy. All patients received three-dimensional conformal RT (3D-CRT). Intensity modulated radiation therapy (IMRT) was not used. Heart dose was limited to V40Gy <100% in one trial, left ventricle was limited to V40Gy <100% in one trial, and the remaining four trials did not have cardiac dose limits. Patients received routine protocol-specified clinical and radiographic follow-up every 2–3 months for two years, with reduced frequency of follow-up thereafter. Cardiac evaluations were performed only if clinically indicated. All patients received induction chemotherapy and most received concurrent chemotherapy. After excluding patients who did not complete RT to ≥70 Gy (n=9) and those with inaccessible radiation plans (n=6), there remained 112 patients for the final analysis.
Dosimetric Assessment
3D-CRT dose distributions were reviewed. Heart subvolumes were delineated by the primary investigator per Feng et al.17 The heart and left ventricle (LV) were delineated for our prior publication and independently reviewed for accuracy and consistency by a second investigator (MJE). The right atrium (RA) and left atrium (LA) were delineated for this analysis and reviewed by a third investigator (KAP). The right ventricle (RV), left anterior descending artery (LAD), and pericardium (generated by subtracting the interior of the heart contour [heart-5mm] from the heart) were delineated for supplementary analysis. Dose volume histograms were generated for each volume.
Evaluation of Cardiac Toxicity
Symptomatic cardiac events were defined by an attending cardiologist (BCJ), combined into three distinct categories (see below), and considered separate event endpoints.
-
Pericardial cardiac events:
Symptomatic pericardial effusion: Effusions presenting with shortness of breath, confirmed on echocardiogram as hemodynamically significant and/or requiring procedural intervention (excluding malignant effusions).
Pericarditis: Radiographic, echocardiographic, or electrocardiographic evidence of pericardial inflammation along with shortness of breath or chest pain.
-
Ischemic cardiac events:
Myocardial infarction: Chest pain with increased cardiac biomarkers or as otherwise noted in the medical record.
Unstable angina: Chest pain without biomarker increase but with ischemia on stress test or significant stenosis on cardiac catheterization.
-
Arrhythmic cardiac events:
Significant arrhythmia: New onset tachy- or brady-arrhythmia requiring either medical or procedural intervention.
Radiographic studies and echocardiograms were also reviewed for the development of asymptomatic pericardial effusions. Baseline cardiac risk was assessed by recording pre-treatment diagnosis of coronary artery disease (CAD) and by calculating the WHO / International Society of Hypertension (WHO/ISH) risk score, which estimates 10-year risk of a cardiovascular event.18
Statistical Analysis
Each type of cardiac event was considered as a separate endpoint and the cumulative incidence of each endpoint was estimated. The Fine and Gray competing risks regression model was used to account for the significant competing risk of death.19 Conceptually, patients in this study population are at highest risk for death due to disease progression, and patients who die cannot be at risk for cardiac events.20 Therefore, an unadjusted analysis would overestimate the true incidence of cardiac events by censoring death, leading to a lower “denominator” and higher estimated event incidence. Univariable analysis was used to test the association of cardiac subvolume dose with each cardiac event endpoint, with reporting of subdistribution hazard ratios (HR).21 Consistent with RTOG 0617, mean dose, volume receiving ≥5 Gy (V5Gy), ≥30 Gy (V30Gy), and ≥60 Gy (V60Gy) were chosen for analysis, to represent low, medium, and high dose exposure. As a second method to quantitatively assess dosimetric parameters, area under the curve (AUC) plots were generated for each endpoint. Since 90% of patients had data available through death, we felt it was reasonable to consider the remaining patients as “no event” for this AUC analysis. Multivariable analysis was not performed for toxicity endpoints given the low number of events. Given the expected collinearity of dosimetric data and the anticipated testing of multiple covariates, the main goal of analysis was to assess the strength of correlations. Univariable cox regression was used to analyze the association of covariates with overall survival (OS). Multivariable cox regression was used for covariates found to be significantly associated with OS on univariable analysis. Disease progression and pooled symptomatic cardiac events were considered time-dependent variables for the OS analysis. Two-sided P values <0.05 were considered statistically significant and values <0.1 were considered borderline significant. Analysis was performed using SAS v9.4 (SAS Institute, Cary, NC).
Results
One hundred twelve patients were analyzed. Most patients (72%) received 74 Gy (range, 70–90 Gy). All patients received induction chemotherapy, 90% received concurrent chemotherapy, and 25% received consolidation chemotherapy. Median follow-up for surviving patients was 8.8 years (range, 2.3–17.3 years). Twenty-five patients had symptomatic cardiac events. There were 9 patients with pericardial events, 7 patients with ischemic events, and 12 patients with arrhythmic events. Seven patients had multiple events (three had events of different categories). Further event details for each patient are reported in our prior analysis.7 Patient and dosimetric characteristics are listed in Tables 1 and 2, respectively. Statistical associations between dosimetric parameters and events are shown in Table 3. Supplementary Table 1 contains analyses for additional covariates and subvolumes.
Table 1.
Patient Characteristics
| Characteristic | All patients | Patients with pericardial events | Patients with ischemic events | Patients with arrhythmic events |
|---|---|---|---|---|
| No. | 112 | 9 | 7 | 12 |
| Median age (range, years) | 58 (36–82) | 66 (52–81) | 49 (36–63) | 64 (54–69) |
| Gender | ||||
| Male | 61 (55%) | 3 (33%) | 5 (71%) | 10 (83%) |
| Female | 51 (45%) | 6 (67%) | 2 (29%) | 2 (17%) |
| Tumor laterality | ||||
| Right | 65 (58%) | 8 (89%) | 5 (71%) | 7 (58%) |
| Left | 47 (42%) | 1 (11%) | 2 (29%) | 5 (42%) |
| Stage | ||||
| IIIA | 65 (58%) | 8 (89%) | 5 (71%) | 6 (50%) |
| IIIB | 47 (42%) | 1 (11%) | 2 (29%) | 6 (50%) |
| Histology | ||||
| Adenocarcinoma | 56 (50%) | 4 (44%) | 4 (57%) | 5 (42%) |
| Squamous | 41 (37%) | 5 (56%) | 1 (14%) | 7 (58%) |
| Other | 15 (13%) | 0 (0%) | 2 (29%) | 0 (0%) |
| ECOG Performance status | ||||
| 0 | 73 (65%) | 9 (100%) | 4 (57%) | 10 (83%) |
| 1 | 39 (35%) | 0 (0%) | 3 (43%) | 2 (17%) |
| Baseline WHO/ISH 10-yr risk | ||||
| 0- <10 % | 68 (61%) | 3 (33%) | 5 (71%) | 3 (25%) |
| 10- <20 % | 34 (30%) | 4 (44%) | 2 (29%) | 8 (67%) |
| ≥ 20 % | 10 (9%) | 2 (22%) | 0 (0%) | 1 (8%) |
| Baseline coronary artery disease | ||||
| No | 96 (86%) | 6 (67%) | 5 (71%) | 9 (75%) |
| Yes | 16 (14%) | 3 (33%) | 2 (29%) | 3 (25%) |
| New post-RT pericardial effusion | ||||
| No | 72 (64%) | 0 (0%) | 2 (29%) | 7 (58%) |
| Yes | 40 (36%) | 9 (100%) | 5 (71%) | 5 (42%) |
| Gross tumor volume (median) | 46.6 cc | 48.6 cc | 33.7 cc | 46.4 cc |
| Prescribed RT dose (median) | 74.0 Gy | 74.0 Gy | 74.0 Gy | 74.0 Gy |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; WHO/ISH, World Health Organization / International Society of Hypertension; RT, radiotherapy.
Table 2.
Dosimetric Characteristics
| All patients | Patients with pericardial events | Patients with ischemic events | Patients with arrhythmic events | |||||
|---|---|---|---|---|---|---|---|---|
| No. | 112 | 9 | 7 | 12 | ||||
| Heart | ||||||||
| Mean | 12.3 | Gy | 24.5 | Gy | 17.0 | Gy | 22.5 | Gy |
| V5 | 36.5 | % | 59.9 | % | 52.4 | % | 55.9 | % |
| V30 | 16.8 | % | 34.6 | % | 22.6 | % | 31.3 | % |
| V60 | 3.9 | % | 20.9 | % | 2.9 | % | 16.7 | % |
| Left ventricle | ||||||||
| Mean | 4.0 | Gy | 11.4 | Gy | 11.7 | Gy | 6.4 | Gy |
| V5 | 18.4 | % | 42.0 | % | 44.1 | % | 27.8 | % |
| V30 | 2.2 | % | 13.1 | % | 16.6 | % | 5.2 | % |
| V60 | 0 | % | 0.4 | % | 0 | % | 0 | % |
| Right atrium | ||||||||
| Mean | 11.6 | Gy | 37.8 | Gy | 17.5 | Gy | 31.8 | Gy |
| V5 | 42.7 | % | 83.7 | % | 73.0 | % | 78.4 | % |
| V30 | 13.5 | % | 60.9 | % | 22.1 | % | 45.0 | % |
| V60 | 1.0 | % | 35.8 | % | 0 | % | 13.6 | % |
| Left atrium | ||||||||
| Mean | 24.7 | Gy | 49.4 | Gy | 33.2 | Gy | 38.4 | Gy |
| V5 | 77.9 | % | 99.9 | % | 90.3 | % | 95.8 | % |
| V30 | 37.9 | % | 75.7 | % | 51.2 | % | 57.6 | % |
| V60 | 10.9 | % | 49.2 | % | 10.6 | % | 31.9 | % |
Abbreviations: V5Gy, volume receiving ≥5 Gy; V30Gy, volume receiving ≥30 Gy; V60, volume receiving ≥60 Gy. Note: Data shown are medians.
Table 3.
Univariable Competing Risk-Adjusted Analysis for Dosimetric Parameters and Cardiac Events
| Pericardial events | Ischemic events | Arrhythmic events | |||||||
|---|---|---|---|---|---|---|---|---|---|
| P | HR | (95% CI) | P | HR | (95% CI) | P | HR | (95% CI) | |
| Heart | |||||||||
| Mean | 0.016 | 1.04 | (1.01, 1.07) | 0.079 | 1.04 | (0.996, 1.08) | 0.054 | 1.02 | (1.00, 1.05) |
| V5 | 0.042 | 1.02 | (1.001, 1.04) | 0.014 | 1.03 | (1.01, 1.05) | 0.042 | 1.02 | (1.001, 1.04) |
| V30 | 0.024 | 1.02 | (1.003, 1.04) | 0.048 | 1.03 | (1.00, 1.05) | 0.051 | 1.02 | (1.00, 1.03) |
| V60 | 0.004 | 1.04 | (1.01, 1.07) | 0.48 | 0.97 | (0.90, 1.05) | 0.11 | 1.02 | (0.996, 1.04) |
| Left ventricle | |||||||||
| Mean | 0.38 | 1.01 | (0.98, 1.05) | 0.014 | 1.05 | (1.01, 1.09) | 0.80 | 1.0 | (0.97, 1.04) |
| V5 | 0.14 | 1.01 | (0.996, 1.03) | 0.008 | 1.03 | (1.01, 1.05) | 0.24 | 1.01 | (0.99, 1.02) |
| V30 | 0.66 | 1.0 | (0.99, 1.02) | 0.012 | 1.03 | (1.01, 1.05) | 0.71 | 1.0 | (0.98, 1.03) |
| V60 | 0.66 | 1.01 | (0.97, 1.05) | 0.72 | 1.01 | (0.96, 1.06) | 0.17 | 0.93 | (0.85, 1.03) |
| Right atrium | |||||||||
| Mean | 0.009 | 1.03 | (1.01, 1.06) | 0.95 | 1.0 | (0.97, 1.03) | 0.057 | 1.02 | (0.999, 1.05) |
| V5 | 0.044 | 1.02 | (1.001, 1.04) | 0.54 | 1.01 | (0.98, 1.03) | 0.092 | 1.01 | (0.998, 1.03) |
| V30 | 0.013 | 1.02 | (1.004, 1.04) | 0.59 | 1.01 | (0.99, 1.03) | 0.082 | 1.01 | (0.998, 1.03) |
| V60 | 0.005 | 1.02 | (1.007, 1.04) | 0.28 | 0.98 | (0.93, 1.02) | 0.047 | 1.02 | (1.00, 1.03) |
| Left atrium | |||||||||
| Mean | 0.002 | 1.04 | (1.02, 1.07) | 0.32 | 1.02 | (0.98, 1.05) | 0.12 | 1.01 | (0.996, 1.03) |
| V5 | 0.032 | 1.05 | (1.004, 1.10) | 0.14 | 1.02 | (0.99, 1.06) | 0.072 | 1.03 | (0.998, 1.06) |
| V30 | 0.001 | 1.03 | (1.01, 1.05) | 0.29 | 1.01 | (0.99, 1.04) | 0.076 | 1.01 | (0.999, 1.03) |
| V60 | 0.009 | 1.03 | (1.01, 1.05) | 0.82 | 1.0 | (0.97, 1.04) | 0.43 | 1.02 | (0.99, 1.02) |
Abbreviations: HR, subdistribution hazard ratio; V5, volume receiving ≥5 Gy; V30, volume receiving ≥30 Gy; V60, volume receiving ≥60 Gy. Note: For mean dose, all HR’s are per Gy. For V5, V30, and V60, all HR’s are per %.
Pericardial Cardiac Events
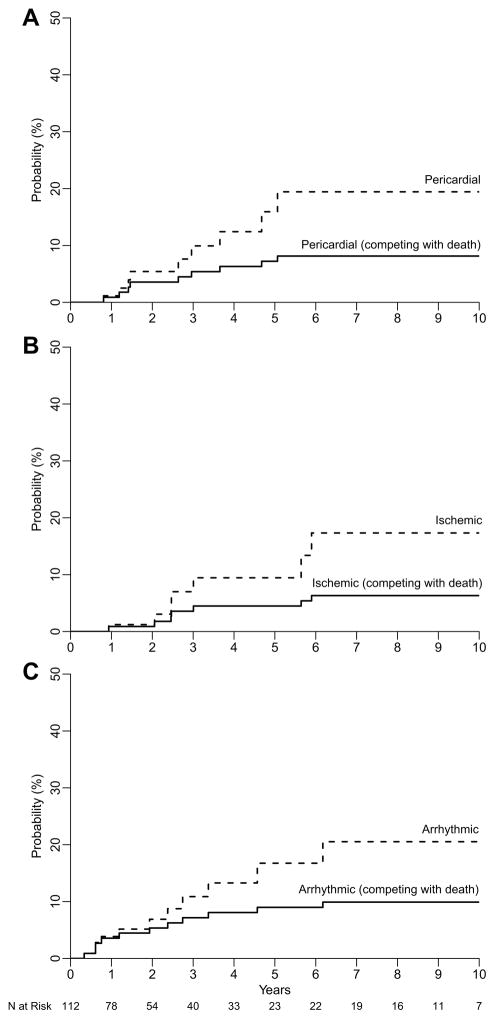
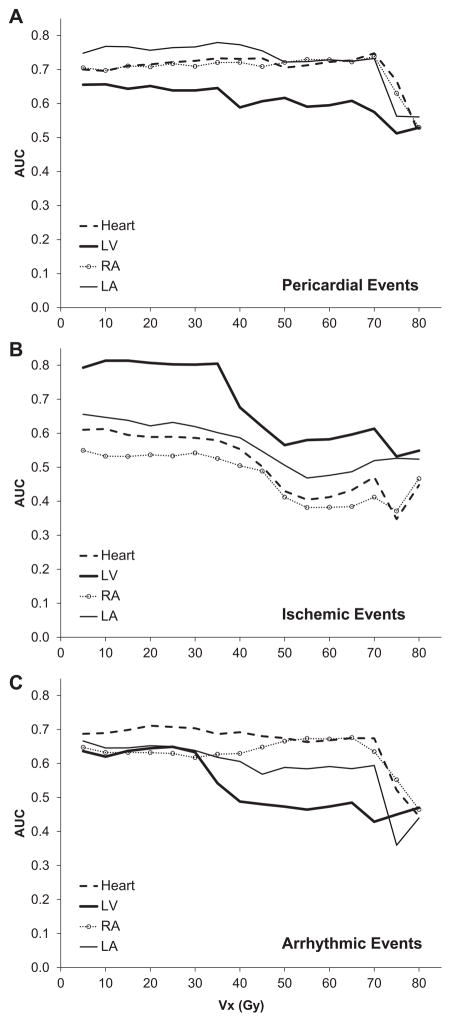
Figure 1A displays the cumulative incidence of pericardial events. Nine patients had pericardial cardiac events at a median of 28 months post-RT (range, 7–58 months). Of these, 2 patients developed severe constrictive pericarditis at 32 and 41 months post-RT, and the remaining 7 had symptomatic effusions at a median of 14 months post-RT. Three patients required pericardiocentesis, 3 underwent a pericardial window procedure, and 3 were managed conservatively. The two and four-year competing risk-adjusted probabilities of pericardial events were 3.6% and 6.3%, respectively. On univariable analysis, pericardial events appeared to be significantly associated with heart, LA, and RA dose, but not LV dose (Table 3). The subvolume dosimetric parameters with the strongest association were LA V30 (p=0.001, HR 1.03/%), LA mean (p=0.002, HR 1.04/Gy), Heart V60 (p=0.004, HR 1.04/%), and RA V60 (p=0.005, HR 1.02/%). The cardiac subvolume AUC plot for pericardial events is shown in Figure 2A. Consistent with the above, the AUC for all subvolume doses except the LV were consistently ≥0.7. Furthermore, 31 patients (27%) had asymptomatic pericardial effusions.
Figure 1.
Cumulative incidence plot of A. pericardial, B. ischemic, and C. arrhythmic events before (dashed lines) and after (solid lines) adjustment for the competing risk of death.
Figure 2.
Area under the curve plots for heart subvolume dosimetric parameters for A. pericardial, B. ischemic, and C. arrhythmic events. Vx (Gy) represents the volume (percentage) of a subvolume receiving “x” Gy.
Ischemic Cardiac Events
Figure 1B displays the cumulative incidence of ischemic events. Seven patients had ischemic events at a median 26 months post-RT (range, 9–68 months). Of these, 4 patients had myocardial infarction at 9, 21, 33, and 68 months post-RT, 2 had unstable angina at 26 months post-RT, and 1 had unstable angina 64 months post-RT, followed by a myocardial infarction 16 years post-RT. One patient had a fatal event, 2 underwent coronary stenting, 2 underwent bypass surgery, and 2 were managed medically. The two and four-year competing risk-adjusted probabilities of ischemic events were 0.9% and 4.5%, respectively. On univariable analysis, ischemic events were associated with LV dose and whole heart dose, but not LA or RA dose. The subvolume dosimetric parameters with the strongest association were LV V5 (p=0.008, HR 1.03/%), LV V30 (p=0.012, HR 1.03/%), LV mean (p=0.014, HR 1.05/Gy), and Heart V5 (p=0.014, HR 1.03/%). The AUC plot for each cardiac subvolume is shown in Figure 2B, with LV doses having the highest AUC values.
Arrhythmic Cardiac Events
Figure 1C displays the cumulative incidence of arrhythmic events. Twelve patients had new onset arrhythmic events (8 atrial fibrillation, 2 atrial flutter, 1 periprocedural complete heart block, 1 sick sinus syndrome), at a median 23 months post-RT (range, 1–190 months). Eight patients were managed with rate-controlling or anti-arrhythmic medications, 1 underwent cardioversion, 1 underwent ablation, and 2 required either temporary or permanent pacing. The two and four-year competing risk-adjusted probabilities of arrhythmic events were 5.4% and 8.1%, respectively. On univariable analysis, arrhythmic events showed borderline significant associations with heart, LA, and RA dose. Arrhythmic events were not associated with LV dose. The subvolume dosimetric parameters with the strongest association were Heart V5 (p=0.042, HR 1.02/%), RA V60 (p=0.047, HR 1.02/%), Heart V30 (p=0.051, HR 1.02/%), and Heart mean (p=0.054, HR 1.02/Gy). The AUC plot for each cardiac subvolume is shown in Figure 2C. Heart and RA doses had the highest AUC values.
Survival
Analyses for OS are presented in Table 4. Performance status, esophageal dose, disease progression, and symptomatic cardiac events were associated with a higher risk of death on univariable analysis. However, only disease progression remained significantly associated with death on multivariable analysis.
Table 4.
Overall Survival Analysis
| Characteristic | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| P | HR (95% CI) | P | HR (95% CI) | |
| Age (years) | 0.95 | 1.00 (0.98, 1.02) | ||
| ECOG PS (1 vs. 0) | 0.044 | 1.52 (1.01, 2.29) | 0.09 | 1.45 (0.94, 2.24) |
| Gross tumor volume (cc) | 0.086 | 1.00 (1.00, 1.004) | ||
| WHO/ISH 10-yr risk (stratum) | 0.73 | 0.96 (0.76, 1.21) | ||
| Baseline CAD | 0.83 | 0.94 (0.54, 1.63) | ||
| Esophagus mean dose (Gy) | <0.001 | 1.04 (1.02, 1.07) | 0.08 | 1.02 (0.997, 1.05) |
| Lung mean dose (Gy) | 0.63 | 1.01 (0.96, 1.06) | ||
| Heart mean dose (Gy) | 0.18 | 1.01 (0.995, 1.03) | ||
| Disease progression* | <0.001 | 7.96 (5.13, 12.35) | <0.001 | 7.48 (4.80, 11.66) |
| Symptomatic cardiac event* | 0.008 | 2.09 (1.21, 3.60) | 0.63 | 1.16 (0.63, 2.13) |
Abbreviations: HR, hazard ratio for death; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; WHO/ISH, World Health Organization / International Society of Hypertension; CAD, coronary artery disease.
Time-dependent covariate
Discussion
In our prior analysis of this population of Stage III NSCLC patients treated on several prospective RT dose-escalation trials, we found a significant association between cardiac dose and combined cardiac events. In this secondary analysis, we describe three distinct types of events – pericardial, ischemic, and arrhythmic, and analyzed their associations with cardiac subvolume dose. Though patients with cardiac events were more likely to have baseline cardiac disease, we did observe three main dosimetric patterns. First, pericardial toxicities had the greatest number and strength of associations with heart subvolume dose. Second, LV dose appeared to be associated only with ischemic events. Third, arrhythmias displayed the weakest associations. Though not all associations were intuitive, these data add to the limited body of available literature on cardiotoxicity after lung cancer RT, and provide reasonable bases for hypotheses regarding the etiologies of these toxicities.
Little is known about the relative significance of different types of cardiac toxicity in patients with lung cancer or their dependence on cardiac subvolume dose. Most early analyses of RT-associated cardiotoxicity have reported only whole heart dose and acute coronary syndromes (myocardial infarction, unstable angina) and death from ischemic heart disease. In patients treated using 2-dimensional planning, heart dosimetry is estimated from simulation radiographs, precluding detailed analyses of cardiac subvolumes. Major reports by Darby et al. and van Nimwegen et al. used this approach to demonstrate a relationship between heart dose and coronary heart disease in patients treated for breast cancer and Hodgkin lymphoma.22,23 However, the heart is complex and may malfunction in ways other than ischemia-induced contractile insufficiency. In almost 2,000 patients treated for Hodgkin lymphoma on nine European trials, ischemic heart disease was the first cardiac event in only 19% of the 703 patients who eventually developed cardiovascular disease. Other initial events included arrhythmia (16%), heart failure (12%), valvular disease (11%), and pericarditis (5%).24 This heterogeneity is further illustrated by the CTCAE grading, spanning three pages and listing 36 distinct cardiac events.25 Similarly, the RTOG mentions angina, arrhythmia, pericarditis, pericardial effusion, and heart failure across different grades of clinical severity.26 In patients with lung cancer, who not only receive substantial heart dose but are older with more comorbidities, all of these cardiac events have the potential to be clinically significant and/or life-threatening.
Pericardial events
Consistent with our hypothesis, pericardial events were strongly associated with global heart dose, in addition to many other dosimetric parameters. RT-associated pericardial effusions and pericarditis have been long recognized. The etiology is thought to be related to acute pericardial inflammation followed by collagen deposition leading to chronic fibrosis.27–30 Prior studies have demonstrated an association between these events and heart and/or pericardial dose.31–33. Though median time to a symptomatic pericardial event was 28 months, 27% of patients also had asymptomatic pericardial effusions at a median of only 11 months post-RT. Compared to patients with other malignancies, patients with lung cancer could have a lower “reserve” and greater likelihood to develop symptoms from effusions.
Ischemic events
Also consistent with our hypothesis, ischemic events showed the greatest associations with LV, and also LAD dose (Supplementary Table 1). Furthermore, ischemic events were not strongly associated with dose to other cardiac subvolumes, and LV dose was not associated with non-ischemic events. RT-associated ischemic heart disease is thought to result from accelerated late atherosclerosis and early microvascular damage manifesting as perfusion deficits,34–37 and there are also reports linking LV dose to troponin and BNP elevation.38,39 In a recent analysis of over 900 patients with breast cancer treated with radiation, van den Bogaard et al. reported that LV dose had the greatest association with ischemic events.10 Thus, the common perception that acute coronary syndrome is related to LV dose may also apply to patients with lung cancer.
Arrhythmic events
Though we hypothesized that arrhythmia would show the greatest association with atrial dose (given that atrial conduction abnormalities are responsible for the most common types of arrhythmia), we only observed weak associations with LA and RA dose. Strength of correlation was low between arrhythmia and most cardiac dosimetric parameters, and there were also unexpected associations (Supplementary Table 1). One explanation is that arrhythmia is relatively common and often due to concurrent acute illnesses, and thus may be the least specific endpoint. We also observed both tachyarrhythmias and bradyarrhythmias (that have different pathophysiologies), underscoring the heterogeneity even within arrhythmic events. Despite this confounding, cardiac dose nonetheless appeared to be associated with arrhythmias to some degree. We are not aware of other studies that have shown a relationship between heart dose and arrhythmia. Strender et al. observed increased electrocardiographic abnormalities in patients previously treated for breast cancer, but most were not clinically significant.40 Rehammar et al. reported that patients receiving breast cancer RT did not have a higher rate of pacemaker or defibrillator placement.41 Another trial evaluated patients receiving ≥20 Gy to the heart and showed that almost half experienced electrocardiographic changes during RT, but again, none were significant.42 More studies are clearly needed on this topic.
The relationship between heart dose, cardiotoxicity, and survival is complex, given heterogeneous patient populations and varying definitions and severities of cardiac events. In the UNC patient cohort, we found an association between pooled symptomatic cardiac events (which ranged from CTCAE grade 2–5) and decreased OS on univariable analysis. However, on multivariable analysis, disease progression predominated as the major predictor of death, and we also did not find an association between heart dose and OS. This is consistent with results from the University of Michigan, where neither heart dose nor grade 2 cardiac events were associated with survival in a similar patient population. However, they did show an association between the more severe grade 3+ cardiac events and survival, though disease progression remained the dominant risk.5 On the other hand, studies with a larger number of patients including RTOG 0617 and analyses by Stam et al. (which analyzed patients with early stage lung cancer) and Speirs et al. were able to find an association between heart dose and decreased survival.4,8,43
Together with prior analyses, the current data improve our understanding of the significance of RT-associated cardiotoxicity in patients with Stage III NSCLC, and may have implications for RT treatment planning. The importance of minimizing cardiac radiation exposure is increasingly recognized and secondary analyses of RTOG 0617 showed reduced toxicities and improved quality of life with the use of IMRT.44,45 Given the importance of tumor control, coverage of gross disease should be given the highest priority. However, guidelines are needed to help clinicians balance the competing priorities of minimizing dose to heart, lung and esophagus. Another question is whether some cardiac subvolumes should take priority over others during the treatment planning process.8–10 Our data provide preliminary information linking cardiac subvolume dose to subsequent toxicity. Given that the three event types showed different patterns of associations with heart subvolumes, it seems advisable to minimize dose to the entirety of the heart if possible. However, this type of information may be applied presently for patients uniquely at risk for certain types of toxicity, where steerage of dose specifically away from a particular heart subvolume may be prudent.
There are several limitations of our study. First, the retrospective nature limits our ability to account for baseline risk and assess toxicity. However, all patients were enrolled on prospective clinical trials and followed closely after treatment. Nonetheless, the lack of protocol-specified cardiac testing may lead to an underestimation of the true frequency of events. Second, there was significant treatment heterogeneity including multiple chemotherapy regimens and one trial which used an alternative fractionation scheme. Nevertheless, all patients were treated at a single institution using uniform treatment planning techniques, and an EQD2 correction was considered but not undertaken for the 11 patients receiving alternative fractionation to ensure consistency in dose reporting between this and our prior published report. Third, patients were treated using induction chemotherapy and high dose radiation, limiting generalizability to patients treated with standard dose chemoradiation alone. However, we would not expect this to affect the observed patterns, though it could affect the magnitude of results. Fourth, the low number of events limits the power of the analysis, but the findings are interesting and can serve as a basis for larger future analyses. Fifth, the expected collinearity of cardiac subvolume data and testing of multiple covariates limits our ability to define what is “significant” and confounds detailed interpretation of results. We therefore examined the strength / frequency of statistical associations by presenting both univariable analyses and AUC plots to convey the overall patterns.
In conclusion, clinically significant cardiac events were heterogeneous with distinct patterns of association between different types of cardiac events and dose to different cardiac subvolumes. The trends observed suggest that there are distinct etiologies for different types of RT-associated cardiotoxicity.
Supplementary Material
Acknowledgments
Funding:
Supported in part by NIH grant CA69579. The study sponsors were not involved in this study’s design or analysis.
Footnotes
Presented at the American Society for Radiation Oncology Annual Meeting, San Diego, CA, September 24-27, 2017.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dautzenberg B, Arriagada R, Chammard AB, et al. A controlled study of postoperative radiotherapy for patients with completely resected nonsmall cell lung carcinoma. Groupe d’Etude et de Traitement des Cancers Bronchiques. Cancer. 1999;86(2):265–273. doi: 10.1002/(sici)1097-0142(19990715)86:2<265::aid-cncr10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 2.Lally BE, Detterbeck FC, Geiger AM, et al. The risk of death from heart disease in patients with nonsmall cell lung cancer who receive postoperative radiotherapy: analysis of the Surveillance, Epidemiology, and End Results database. Cancer. 2007;110(4):911–917. doi: 10.1002/cncr.22845. [DOI] [PubMed] [Google Scholar]
- 3.Douillard JY, Rosell R, De Lena M, et al. Impact of postoperative radiation therapy on survival in patients with complete resection and stage I, II, or IIIA non-small-cell lung cancer treated with adjuvant chemotherapy: the adjuvant Navelbine International Trialist Association (ANITA) Randomized Trial. Int J Radiat Oncol Biol Phys. 2008;72(3):695–701. doi: 10.1016/j.ijrobp.2008.01.044. [DOI] [PubMed] [Google Scholar]
- 4.Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16(2):187–199. doi: 10.1016/S1470-2045(14)71207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dess RT, Sun Y, Matuszak MM, et al. Cardiac Events After Radiation Therapy: Combined Analysis of Prospective Multicenter Trials for Locally Advanced Non-Small-Cell Lung Cancer. J Clin Oncol. 2017;35(13):1395–1402. doi: 10.1200/JCO.2016.71.6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tucker SL, Liu A, Gomez D, et al. Impact of heart and lung dose on early survival in patients with non-small cell lung cancer treated with chemoradiation. Radiother Oncol. 2016;119(3):495–500. doi: 10.1016/j.radonc.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 7.Wang K, Eblan MJ, Deal AM, et al. Cardiac Toxicity After Radiotherapy for Stage III Non-Small-Cell Lung Cancer: Pooled Analysis of Dose-Escalation Trials Delivering 70 to 90 Gy. J Clin Oncol. 2017;35(13):1387–1394. doi: 10.1200/JCO.2016.70.0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stam B, Peulen H, Guckenberger M, et al. Dose to heart substructures is associated with non-cancer death after SBRT in stage I–II NSCLC patients. Radiother Oncol. 2017;123(3):370–375. doi: 10.1016/j.radonc.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 9.McWilliam A, Faivre-Finn C, Kennedy J, Kershaw L, van Herk MB. Data Mining Identifies the Base of the Heart as a Dose-Sensitive Region Affecting Survival in Lung Cancer Patients. Int J Radiat Oncol Biol Phys. 2016;96(2S):S48–S49. [Google Scholar]
- 10.van den Bogaard VA, Ta BD, van der Schaaf A, et al. Validation and Modification of a Prediction Model for Acute Cardiac Events in Patients With Breast Cancer Treated With Radiotherapy Based on Three-Dimensional Dose Distributions to Cardiac Substructures. J Clin Oncol. 2017 doi: 10.1200/JCO.2016.69.8480. JCO2016698480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Socinski MA, Rosenman JG, Halle J, et al. Dose-escalating conformal thoracic radiation therapy with induction and concurrent carboplatin/paclitaxel in unresectable stage IIIA/B nonsmall cell lung carcinoma: a modified phase I/II trial. Cancer. 2001;92(5):1213–1223. doi: 10.1002/1097-0142(20010901)92:5<1213::aid-cncr1440>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 12.Marks LB, Garst J, Socinski MA, et al. Carboplatin/paclitaxel or carboplatin/vinorelbine followed by accelerated hyperfractionated conformal radiation therapy: report of a prospective phase I dose escalation trial from the Carolina Conformal Therapy Consortium. J Clin Oncol. 2004;22(21):4329–4340. doi: 10.1200/JCO.2004.02.165. [DOI] [PubMed] [Google Scholar]
- 13.Socinski MA, Morris DE, Halle JS, et al. Induction and concurrent chemotherapy with high-dose thoracic conformal radiation therapy in unresectable stage IIIA and IIIB non-small-cell lung cancer: a dose-escalation phase I trial. J Clin Oncol. 2004;22(21):4341–4350. doi: 10.1200/JCO.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 14.Socinski MA, Blackstock AW, Bogart JA, et al. Randomized phase II trial of induction chemotherapy followed by concurrent chemotherapy and dose-escalated thoracic conformal radiotherapy (74 Gy) in stage III non-small-cell lung cancer: CALGB 30105. J Clin Oncol. 2008;26(15):2457–2463. doi: 10.1200/JCO.2007.14.7371. [DOI] [PubMed] [Google Scholar]
- 15.Stinchcombe TE, Morris DE, Lee CB, et al. Induction chemotherapy with carboplatin, irinotecan, and paclitaxel followed by high dose three-dimension conformal thoracic radiotherapy (74 Gy) with concurrent carboplatin, paclitaxel, and gefitinib in unresectable stage IIIA and stage IIIB non-small cell lung cancer. J Thorac Oncol. 2008;3(3):250–257. doi: 10.1097/JTO.0b013e3181653cf4. [DOI] [PubMed] [Google Scholar]
- 16.Socinski MA, Stinchcombe TE, Moore DT, et al. Incorporating bevacizumab and erlotinib in the combined-modality treatment of stage III non-small-cell lung cancer: results of a phase I/II trial. J Clin Oncol. 2012;30(32):3953–3959. doi: 10.1200/JCO.2012.41.9820. [DOI] [PubMed] [Google Scholar]
- 17.Feng M, Moran JM, Koelling T, et al. Development and validation of a heart atlas to study cardiac exposure to radiation following treatment for breast cancer. Int J Radiat Oncol Biol Phys. 2011;79(1):10–18. doi: 10.1016/j.ijrobp.2009.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mendis S, Lindholm LH, Mancia G, et al. World Health Organization (WHO) and International Society of Hypertension (ISH) risk prediction charts: assessment of cardiovascular risk for prevention and control of cardiovascular disease in low and middle-income countries. J Hypertens. 2007;25(8):1578–1582. doi: 10.1097/HJH.0b013e3282861fd3. [DOI] [PubMed] [Google Scholar]
- 19.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 20.Kim HT. Cumulative incidence in competing risks data and competing risks regression analysis. Clin Cancer Res. 2007;13(2 Pt 1):559–565. doi: 10.1158/1078-0432.CCR-06-1210. [DOI] [PubMed] [Google Scholar]
- 21.Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med. 2007;26(11):2389–2430. doi: 10.1002/sim.2712. [DOI] [PubMed] [Google Scholar]
- 22.van Nimwegen FA, Schaapveld M, Cutter DJ, et al. Radiation Dose-Response Relationship for Risk of Coronary Heart Disease in Survivors of Hodgkin Lymphoma. J Clin Oncol. 2016;34(3):235–243. doi: 10.1200/JCO.2015.63.4444. [DOI] [PubMed] [Google Scholar]
- 23.Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368(11):987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 24.Maraldo MV, Giusti F, Vogelius IR, et al. Cardiovascular disease after treatment for Hodgkin’s lymphoma: an analysis of nine collaborative EORTC-LYSA trials. Lancet Haematol. 2015;2(11):e492–502. doi: 10.1016/S2352-3026(15)00153-2. [DOI] [PubMed] [Google Scholar]
- 25.NCI. NIH publication # 09–7473. 2009. Common Terminology Criteria for Adverse Events v4.0. [Google Scholar]
- 26.RTOG. [Accessed 1/30/2017];RTOG/EORTC Late Radiation Morbidity Scoring Schema. https://www.rtog.org/ResearchAssociates/AdverseEventReporting/RTOGEORTCLateRadiationMorbidityScoringSchema.aspx.
- 27.Taunk NK, Haffty BG, Kostis JB, Goyal S. Radiation-induced heart disease: pathologic abnormalities and putative mechanisms. Front Oncol. 2015;5:39. doi: 10.3389/fonc.2015.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Veinot JP, Edwards WD. Pathology of radiation-induced heart disease: a surgical and autopsy study of 27 cases. Hum Pathol. 1996;27(8):766–773. doi: 10.1016/s0046-8177(96)90447-5. [DOI] [PubMed] [Google Scholar]
- 29.Chello M, Mastroroberto P, Romano R, Zofrea S, Bevacqua I, Marchese AR. Changes in the proportion of types I and III collagen in the left ventricular wall of patients with post-irradiative pericarditis. Cardiovasc Surg. 1996;4(2):222–226. doi: 10.1016/0967-2109(96)82320-9. [DOI] [PubMed] [Google Scholar]
- 30.Sievert W, Trott KR, Azimzadeh O, Tapio S, Zitzelsberger H, Multhoff G. Late proliferating and inflammatory effects on murine microvascular heart and lung endothelial cells after irradiation. Radiother Oncol. 2015;117(2):376–381. doi: 10.1016/j.radonc.2015.07.029. [DOI] [PubMed] [Google Scholar]
- 31.Carmel RJ, Kaplan HS. Mantle irradiation in Hodgkin’s disease. An analysis of technique, tumor eradication, and complications. Cancer. 1976;37(6):2813–2825. doi: 10.1002/1097-0142(197606)37:6<2813::aid-cncr2820370637>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 32.Martel MK, Sahijdak WM, Ten Haken RK, Kessler ML, Turrisi AT. Fraction size and dose parameters related to the incidence of pericardial effusions. Int J Radiat Oncol Biol Phys. 1998;40(1):155–161. doi: 10.1016/s0360-3016(97)00584-1. [DOI] [PubMed] [Google Scholar]
- 33.Wei X, Liu HH, Tucker SL, et al. Risk factors for pericardial effusion in inoperable esophageal cancer patients treated with definitive chemoradiation therapy. Int J Radiat Oncol Biol Phys. 2008;70(3):707–714. doi: 10.1016/j.ijrobp.2007.10.056. [DOI] [PubMed] [Google Scholar]
- 34.Seddon B, Cook A, Gothard L, et al. Detection of defects in myocardial perfusion imaging in patients with early breast cancer treated with radiotherapy. Radiother Oncol. 2002;64(1):53–63. doi: 10.1016/s0167-8140(02)00133-0. [DOI] [PubMed] [Google Scholar]
- 35.Marks LB, Yu X, Prosnitz RG, et al. The incidence and functional consequences of RT-associated cardiac perfusion defects. Int J Radiat Oncol Biol Phys. 2005;63(1):214–223. doi: 10.1016/j.ijrobp.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 36.Darby SC, Cutter DJ, Boerma M, et al. Radiation-related heart disease: current knowledge and future prospects. Int J Radiat Oncol Biol Phys. 2010;76(3):656–665. doi: 10.1016/j.ijrobp.2009.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang P, Hu X, Yue J, et al. Early detection of radiation-induced heart disease using (99m)Tc-MIBI SPECT gated myocardial perfusion imaging in patients with oesophageal cancer during radiotherapy. Radiother Oncol. 2015;115(2):171–178. doi: 10.1016/j.radonc.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 38.Skytta T, Tuohinen S, Boman E, Virtanen V, Raatikainen P, Kellokumpu-Lehtinen PL. Troponin T-release associates with cardiac radiation doses during adjuvant left-sided breast cancer radiotherapy. Radiat Oncol. 2015;10:141. doi: 10.1186/s13014-015-0436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.D’Errico MP, Petruzzelli MF, Gianicolo EA, et al. Kinetics of B-type natriuretic peptide plasma levels in patients with left-sided breast cancer treated with radiation therapy: Results after one-year follow-up. Int J Radiat Biol. 2015;91(10):804–809. doi: 10.3109/09553002.2015.1027421. [DOI] [PubMed] [Google Scholar]
- 40.Strender LE, Lindahl J, Larsson LE. Incidence of heart disease and functional significance of changes in the electrocardiogram 10 years after radiotherapy for breast cancer. Cancer. 1986;57(5):929–934. doi: 10.1002/1097-0142(19860301)57:5<929::aid-cncr2820570509>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 41.Rehammar JC, Johansen JB, Jensen MB, et al. Risk of pacemaker or implantable cardioverter defibrillator after radiotherapy for early-stage breast cancer in Denmark, 1982–2005. Radiother Oncol. 2017;122(1):60–65. doi: 10.1016/j.radonc.2016.08.024. [DOI] [PubMed] [Google Scholar]
- 42.Gomez DR, Yusuf SW, Munsell MF, et al. Prospective exploratory analysis of cardiac biomarkers and electrocardiogram abnormalities in patients receiving thoracic radiation therapy with high-dose heart exposure. J Thorac Oncol. 2014;9(10):1554–1560. doi: 10.1097/JTO.0000000000000306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Speirs CK, DeWees TA, Rehman S, et al. Heart Dose Is an Independent Dosimetric Predictor of Overall Survival in Locally Advanced Non-Small Cell Lung Cancer. J Thorac Oncol. 2017;12(2):293–301. doi: 10.1016/j.jtho.2016.09.134. [DOI] [PubMed] [Google Scholar]
- 44.Movsas B, Hu C, Sloan J, et al. Quality of Life Analysis of a Radiation Dose-Escalation Study of Patients With Non-Small-Cell Lung Cancer: A Secondary Analysis of the Radiation Therapy Oncology Group 0617 Randomized Clinical Trial. JAMA Oncol. 2016;2(3):359–367. doi: 10.1001/jamaoncol.2015.3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chun SG, Hu C, Choy H, et al. Impact of Intensity-Modulated Radiation Therapy Technique for Locally Advanced Non-Small-Cell Lung Cancer: A Secondary Analysis of the NRG Oncology RTOG 0617 Randomized Clinical Trial. J Clin Oncol. 2017;35(1):56–62. doi: 10.1200/JCO.2016.69.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.