Abstract
Background and aims
Atherosclerosis is a systemic disease. We examined whether the cumulative burden of thoracic extra-coronary calcification (ECC) improves prediction of stroke, transient ischemic attack (TIA), and stroke mortality beyond traditional risk factors and coronary artery calcium (CAC).
Methods
We followed a total of 6,805 participants (mean age 62.1±10.2 years, 47.2% male) from the Multi-Ethnic Study of Atherosclerosis (MESA) over a median of 12.1 years. The presence or absence of calcification at 4 thoracic ECC sites (mitral valve annulus, aortic valve, aortic root, and thoracic aorta) was determined from baseline cardiac-gated non-contrast CT scans. A multisite thoracic ECC score, ranging 0–4, was calculated by summing the 4 individual sites, which were treated as binary variables. Multivariable Cox proportional hazards regression models, controlled for traditional risk factors and CAC, were used to estimate hazard ratios for ischemic (primary endpoint) and hemorrhagic stroke, total stroke, TIA, and stroke mortality with increasing thoracic ECC.
Results
With an increasing number of thoracic ECC sites, there was a significant (p<0.05) multivariable adjusted step-wise increase in the risk for ischemic stroke (n=184), total stroke (n=235), and TIA (n=85), but not hemorrhagic stroke (n=32) and stroke mortality (n=42). Thoracic ECC increased the c-statistic and net reclassification index beyond traditional risk factors and CAC, but the results were not significant (p>0.10).
Conclusions
Although multisite thoracic ECC is independently associated with ischemic stroke, total stroke, and TIA, the incremental predictive value of thoracic ECC beyond traditional risk factors and CAC appears to be minimal.
1. Introduction
Atherosclerosis is a systemic process.1 Detection of subclinical atherosclerosis using imaging, particularly coronary artery calcium (CAC) from computed tomography (CT) scans, has proven to be superior to traditional risk factors for prediction of coronary heart disease (CHD).2 The 2013 American College of Cardiology/American Heart Association (ACC/AHA) guidelines placed an emphasis on the prediction of both CHD and stroke events.3 However, CAC is a stronger predictor of CHD2 than stroke4, 5. Therefore, further risk information, in particular other imaging data, may be needed beyond CAC to enhance prediction of stroke.
Extra-coronary calcification (ECC) can be visualized on a variety of imaging modalities, including routine non-gated and cardiac-gated non-contrast chest CT scans, plain radiography, and echocardiography6–8 and, as such, its identification does not require additional cost or radiation exposure. Of these modalities, CT scanning has a superior sensitivity for identifying vascular calcification and allows for a more quantitative assessment of ECC. Previous studies have demonstrated the association between individual sites of thoracic ECC, including aortic or mitral valve6, ascending aorta7, thoracic aorta8, abdominal aorta10, and carotid arteries9, and the risk for CVD. In the Multi-Ethnic Study of Atherosclerosis (MESA), thoracic aortic11 and aortic valve12 calcification have been shown to predict CHD and CVD events beyond CAC.
ECC may reflect the manifestation of CVD risk factors, such as hypertension and diabetes, on the systemic vasculature.13 Previous studies have shown that individual thoracic ECC sites herald the presence or risk of atherosclerotic calcification at other vascular sites.6, 10 As such, thoracic ECC sites may be suitable for prediction of cerebrovascular events, such as ischemic stroke and TIA, as these sites are more proximal to the cerebrovascular circulation, compared with CAC and ECC sites outside the thoracic region.13 In MESA, Tison et al. suggested a strong association of an ordinal multisite thoracic ECC score with total CVD and mortality rather than with CHD.14 No further studies have evaluated the utility of a multisite thoracic ECC score for predicting the risk for total and individual stroke types.
In this study, we sought to evaluate the discrimination and reclassification ability of thoracic ECC, including mitral valve calcification (MVC), aortic valve calcification (AVC), aortic root calcification (ARC), and thoracic aorta calcification (TAC), for the prediction of ischemic and hemorrhagic stroke, total stroke, TIA, and stroke mortality.
2. Patients and methods
2.1. Study population
MESA is a longitudinal study of 6,814 White, Black, Hispanic, or Chinese Americans aged 45 to 84 years old free of known CVD at baseline, enrolled from 6 US centers between 2000–2002. Participants were excluded if they had a confirmed diagnosis of myocardial infarction, stroke, TIA, heart failure, angina, atrial fibrillation, or any history of cardiovascular procedures, weight >300 pounds, pregnancy, or any medical conditions that could prevent long-term participation. Exam 2 was held from Fall 2002 to Winter 2004, Exam 3 from Spring 2004 to Fall 2005, Exam 4 from Fall 2005 to Spring 2007, and the fifth exam between Spring 2010 and Winter 2012. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Institutional Review Board at each site in accordance with the Health Insurance Portability and Accountability Act. Details regarding protocol and design of MESA were reported elsewhere.15
2.2. Data collection
Baseline data were collected using self-administered questionnaires and obtained linical and laboratory data. Total cholesterol, high-density lipoprotein cholesterol, triglyceride and blood glucose measurements were performed after a 12-hour fast. Diabetes was defined as fasting blood glucose ≥7.0 mmol/l (126 mg/dl), self-reported diabetes, or use of hypoglycemic drugs. The definition of hypertension was untreated systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or use of antihypertensive medication. Metabolic syndrome was defined according to the modified National Cholesterol Education Program Adult Treatment Panel III definition.16 Smoking status was considered as never, former, and current use of cigarettes. Self-reported education and income level were assessed as parameters of socioeconomic status.
2.3. Measuring coronary and extra-coronary calcium
The details of the MESA cardiac CT protocol have been published elsewhere.17 Briefly, CAC and ECC were measured by Agatston method by two consecutive baseline non-contrast cardiac CT scans, which were EKG-gated to the R-R interval. To assess CAC, all participants underwent two consecutive noncontrast cardiac-gated CT scans. A minimum of 35 images was obtained starting above the left main coronary artery to the bottom of both ventricles during a single breath hold. To reconstruct raw image data, a slice thickness of 3 mm, field of view of 35 cm, and a matrix of 512 × 512 was used. Three sites used an electron beam CT scanner (GE–Imatron C–150XL, San Francisco, CA), and three sites used a 4-slice multidetector CT scanner. The nominal section thicknesses were 3.0 for electron beam scanner and 2.5 mm for multidetector scanner. Spatial resolution was described as 1.38 mm3 for electron beam scanner (0.68 × 0.68×3.00 mm) and 1.15 mm3 for multidetector scanner (0.68×0.68 ×2.50 mm).
Axial datasets of CT scans were also reviewed for the presence of calcification at 4 non-coronary sites: 1) the level of mitral annulus (MVC), 2) from aortic valve to just before aortic root (AVC), 3) the level of aortic root (ARC), 4) and ascending or descending thoracic aorta (TAC). Ascending aortic calcification was measure from the aortic annulus to the lower edge of pulmonary artery. Descending aortic calcification was measured from the lower edge of pulmonary artery to the cardiac apex. Therefore, aortic arch was not visualized on scans. We excluded participants with at least one thoracic ECC site information missing.
Due to the heterogeneity in Agatston scores across different extra-coronary vascular sites18, and to increase the generalizability of results, we used a binary variable for each of 4 individual thoracic ECC sites based on presence (1) or absence (0) of any calcifications. The presence of CAC or ECC was defined as any measured Agatston scores higher than zero. The integrated multisite thoracic ECC score was the sum of individual ordinal binary scores at each calcification site.
2.4. Follow-up and ascertainment of events
During a median follow-up of 12.1 years, new cases of stroke, TIA, and stroke mortality were recorded using interviews to document interim hospital admissions, outpatient diagnoses, and deaths. Two neurologists adjudicated independently all events. Stroke endpoints were classified by their type comprising ischemic and hemorrhagic (subarachnoid and intra-parenchymal hemorrhage). TIA was defined as having symptoms that lasted less than 24 hours and negative imaging. Primary endpoint was ischemic stroke. Detailed description of follow-up of MESA participants is available online (www.mesa-nhlbi.org).
2.5. Statistical analyses
For comparison of discrete or normally distributed continuous variables across the four thoracic ECC groups, Pearson’s Chi-square test or ANOVA were performed, respectively. For skewed variables, medians and interquartile ranges were reported and Kruskal-Wallis tests performed for comparison. We ran logistic regression models to calculate odds ratios (ORs) with 95% confidence intervals (CIs) for CAC>0 based on thoracic ECC score.
Kaplan-Meier curves were generated to estimate rates of stroke, TIA, and stroke mortality events. Three Cox proportional hazard models were built to estimate hazard ratios (HRs) with 95% CIs with increasing thoracic ECC sites. The first model was unadjusted, and the second model also included traditional CVD risk factors and other potential confounders including age, gender, race/ethnicity, education, estimated glomerular filtration rate, LDL-C, HDL-C, total cholesterol, diabetes mellitus, hypertension, cigarette smoking status (never, former, current), any lipid-lowering medications, anti-hypertensive medications, aspirin use, and family history of heart attack or stroke. In addition, third model also included the continuous CAC score, which was calculated by natural logarithmic transformation of CAC+1 (LogeCAC). For each model, trend analysis was conducted to test whether stroke or mortality increased linearly with increasing number of thoracic ECC sites. Two-sample log-rank test was used to compare the survival experiences between the four thoracic ECC groups. The proportional hazards assumption was assessed by Schoenfeld residuals and plotting log-log survival.
We assessed discrimination by calculating and comparing area under the Receiver Operating Characteristic (ROC) curves for models with and without thoracic ECC. The base model included traditional CVD risk factors and CAC, and the second model also included multisite thoracic ECC. We compared the two models using the likelihood ratio test. Integrated discrimination improvement (IDI) was also calculated.19
Thresholds of Framingham risk score for stroke were calculated using sex-specific calibration factors for individual CVD as reported previously.20 The ability of thoracic ECC to reclassify risk beyond traditional risk factors with or without CAC was assessed by calculating the net reclassification improvement (NRI). Information is insufficient on how to justify categories of NRI for stroke. Therefore, we calculated the category-less, continuous NRI that is independent of risk thresholds and has more statistical power.21
We performed secondary analysis for individual thoracic ECC sites can significantly predict stroke events and stroke mortality. Thus, we added MVC, AVC, ARC, and TAC as binary (presence or absence of calcification) or continuous (Agatston score) covariates into models. Subgroup analyses were performed by restricting the analysis to participants with no CAC (CAC=0), CAC>100, and CAC>75th percentile in the population. We used multivariable adjusted competing risk regression considering competing risk from total mortality and stroke mortality. Moreover, we adjusted for atrial fibrillation, all interim non-stroke CVD events, and change in lipid-lowering and aspirin use prior to incident stroke in Cox regression models. We tested for interaction between thoracic ECC score and age, sex, gender, and estimated glomerular filtration rate as well as hypertension and diabetes status, as they are different mechanistic roles in ischemic and hemorrhagic strokes.22 A p of <0.05 was considered significant. All analyses were performed using Stata (version 13.0, College Station, TX, USA).
3. Results
3.1. Baseline characteristics
A total of 6,805 participants were included in the final analyses. The mean age was 62.1±10.2 years and 47% were male. Characteristics of the participants by number of ECC sites are provided in Table 1. Participants with higher number of thoracic ECC sites were older and more likely to be White, hypertensive, diabetic, and have higher waist circumference, pack-years of smoking, systolic blood pressure, triglyceride, and total cholesterol levels. Use of lipid-lowering and anti-hypertensive medications at baseline was higher among participants with higher thoracic ECC scores. ARC had the highest prevalence within each thoracic ECC score group, followed by TAC, AVC, and MVC. (Fig. 1A)
Table 1.
Baseline characteristics of asymptomatic participants without a history of cardiovascular disease in Multi-Ethnic Study of Atherosclerosis by the number of thoracic extra-coronary calcification sites.
| Variable | Entire cohort | Thoracic extra-coronary calcification sites | p value | |||
|---|---|---|---|---|---|---|
|
| ||||||
| (N=6805) | 0, N= 3617 | 1, N=1474 | 2, N=1052 (%) | 3–4, N=662 | ||
| Age | 62.1±10.2 | 56.6±8.3 | 65.0±8.4 | 69.9±7.4 | 72.5±7.0 | <0.001 |
| Male, n (%) | 3,209 (47.2) | 1,685 (46.6) | 713 (48.4) | 491 (46.7) | 320 (48.3) | 0.61 |
| Race, n (%) | <0.001 | |||||
| White | 2,615 (38.4) | 1277 (35.3) | 559 (37.9) | 451 (42.9) | 328 (49.6) | |
| African American | 1,892 (27.8) | 1065 (29.4) | 424 (28.8) | 266 (25.3) | 137 (20.7) | |
| Hispanics | 1,495 (22.0) | 802 (22.2) | 330 (22.4) | 215 (20.4) | 148 (22.4) | |
| Chinese American | 803 (11.8) | 473 (13.1) | 161 (10.9) | 120 (11.4) | 49 (7.4) | |
| High school education, n (%) | 4,323 (63.7) | 2,503 (69.5) | 900 (61.3) | 572 (54.4) | 348 (52.9) | <0.001 |
| Income ≥ $40,000 | 3,503 (51.5) | 2,083 (57.6) | 712 (48.3) | 442 (42.0) | 266 (40.2) | <0.001 |
| Body mass index, kg/m2 | 28.3±5.5 | 28.4±5.7 | 28.4±5.3 | 28.1±5.3 | 28.4±4.9 | 0.34 |
| Waist circumference, cm | 98.1±14.4 | 96.9±14.8 | 99.2±14.2 | 98.9±13.8 | 101.3±13.1 | <0.001 |
| Metabolic syndrome, n (%) | 2,444 (36.0) | 1,080 (29.9) | 581 (39.6) | 458 (43.5) | 325 (49.4) | <0.001 |
| Triglycerides (mg/dL) | 111 (78, 161) | 107 (74, 157) | 113 (81, 163) | 118 (80, 163) | 120 (86, 169) | <0.001 |
| Total cholesterol, mg/dL | 194.1±35.7 | 192.2±34.7 | 197.0±36.3 | 195.3±36.2 | 196.5±38.3 | <0.001 |
| HDL cholesterol, mg/dL | 51.0±14.8 | 51.2±15.0 | 50.9±14.2 | 50.9±15.4 | 50.2±14.1 | 0.48 |
| Systolic blood pressure, mmHg | 126.6±21.5 | 120.7±19.0 | 129.8±21.1 | 134.9±21.7 | 138.4±23.9 | <0.001 |
| Estimated GFR, mL/min/1.73 m2 | 81.2±18.5 | 83.7±16.6 | 81.1±12.4 | 77.5±18.3 | 73.6±18.3 | <0.001 |
| Hypertension, n (%) | 3,055 (44.9) | 1,188 (32.8) | 759 (51.5) | 651 (61.9) | 457 (69.0) | <0.001 |
| Diabetes, n (%) | 858 (12.6) | 334 (9.2) | 212 (14.4) | 180 (17.1) | 132 (19.9) | <0.001 |
| Hypertension medication, n (%) | 2,532 (37.2) | 987 (27.3) | 628 (42.6) | 533 (50.7) | 384 (58.0) | <0.001 |
| Lipid-lowering medication, n (%) | 1,096 (16.1) | 380 (10.5) | 278 (18.9) | 237 (22.6) | 201 (30.3) | <0.001 |
| Smoking status, n (%) | <0.001 | |||||
| Never | 3,415 (50.4) | 1,889 (52.4) | 734 (50.0) | 492 (46.8) | 300 (45.5) | |
| Former | 2,482 (36.6) | 1,212 (33.6) | 551 (37.5) | 425 (40.4) | 294 (44.6) | |
| Current | 886 (13.1) | 503 (14.0) | 184 (12.5) | 134 (12.8) | 65 (9.9) | |
| Pack-year | 11.3±20.9 | 8.7±16.8 | 12.7±23.6 | 14.6±24.0 | 16.9±26.5 | <0.001 |
| CAC score, n (%) | <0.001 | |||||
| 0 | 3,414 (50.2) | 2,526 (69.8) | 591 (40.1) | 216 (20.5) | 81 (12.2) | |
| >0–100> | 1,793 (26.4) | 785 (21.7) | 498 (33.7) | 349 (33.2) | 161 (24.3) | |
| ≥100–400 | 926 (13.6) | 233 (6.4) | 231 (15.7) | 279 (26.5) | 183 (27.6) | |
| ≥400 | 672 (9.9) | 73 (2.0) | 154 (10.5) | 208 (19.8) | 237 (35.8) | |
| ASCVD risk for MESA | <0.001 | |||||
| <7.5% | 2,919 (42.9) | 2,317 (64.1) | 440 (29.9) | 136 (12.9) | 26 (3.9) | |
| ≥7.5% and <15.0% | 1,539 (22.6) | 774 (21.4) | 412 (28.0) | 250 (23.8) | 103 (15.6) | |
| ≥15.0% | 2,295 (33.7) | 499 (13.8) | 607 (41.2) | 664 (63.1) | 525 (79.3) | |
ASCVD, atherosclerotic cardiovascular disease; CAC, coronary artery calcium; ECC, extra-coronary calcification; HDL-C, high-density lipoprotein cholesterol; IQR, interquartile range; MESA, Multi-Ethnic Study of Atherosclerosis; SD, standard deviation.
Figure 1.
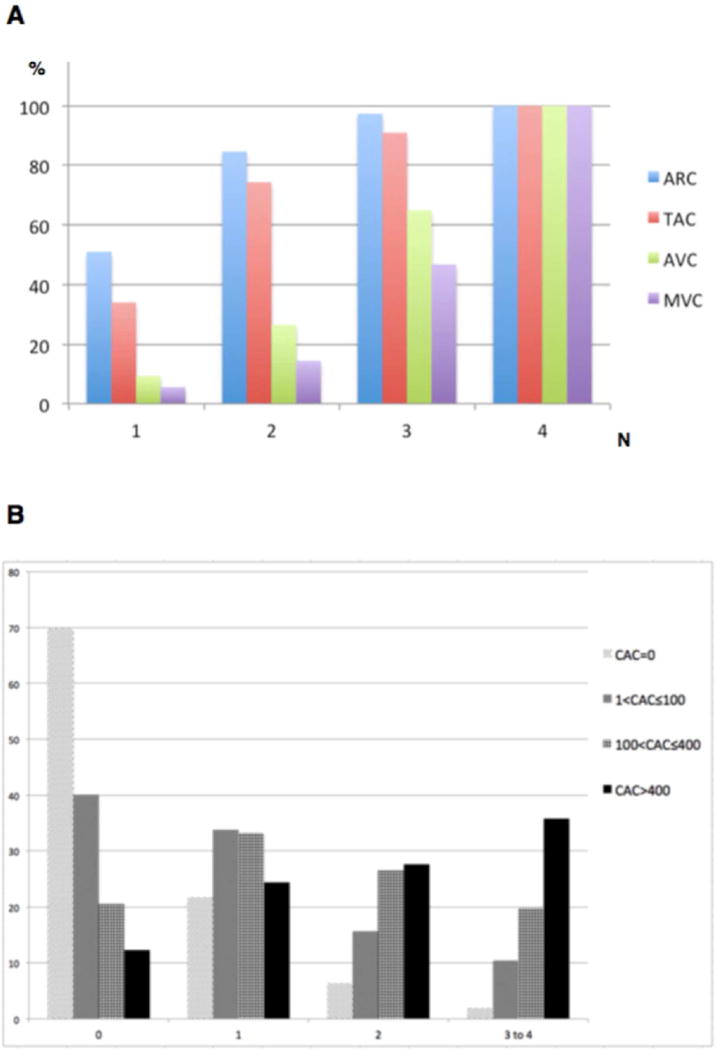
Individual thoracic calcification sites and coronary artery calcium (CAC) by multisite thoracic extra-coronary calcification (ECC) in MESA.
(A) Prevalence of aortic root calcification (ARC), thoracic aorta calcification (TAC), aortic valve calcification (AVC), mitral valve calcification (MVC) by number of multisite thoracic ECC; All 4 thoracic extra-coronary calcification sites were involved in 183 participants (not shown). (B) Prevalence of CAC, stratified into 4 categories, by the number of multisite thoracic ECC. Scale, 1.5:1
An increase in thoracic ECC sites was associated with step-wise increase in baseline logeCAC. Prevalence of CAC was 30% (1,091 participants) among 3,617 participants without thoracic ECC, and 88% (581 participants) among 662 participants with 3–4 thoracic ECC sites. (Fig. 1B) At baseline, compared with participants without thoracic ECC, there were higher odds of CAC>0 for participants with 1 (OR=2.27 [1.96, 2.64]), 2 (OR=4.57 [3.75, 5.58]), and 3–4 (OR=6.1 [4.60, 8.08]) thoracic ECC sites, after adjusting for potential confounders. Among 3,414 participants with CAC=0, about 26% had calcifications in at least one thoracic extra-coronary site.
3.2. Survival analyses
Over a median of 12.1 years of follow-up, there were 184 ischemic strokes, 32 hemorrhagic strokes, 235 total strokes, 85 TIAs, and 42 deaths due to stroke. (Table 2) Incidence rates per 1,000 person-years for stroke outcomes are shown on Fig. 2.
Table 2.
Hazard ratios by multisite thoracic extra-coronary calcification for stroke endpoints, transient ischemic attack, and stroke mortality
|
|
||||||
|---|---|---|---|---|---|---|
| Thoracic ECC | ||||||
|
| ||||||
| n = 0 (reference) |
n = 1 | n = 2 | n = 3–4 |
p value (trend) |
||
|
|
||||||
| Ischemic stroke | Events/total at risk (%) | 52/3,617 (1.44) | 37/1,474 (2.51) | 50/1,052 (4.75) | 45/662 (6.80) | |
| Model 1 | 1.00 | 1.87 (1.23–2.86) | 3.72 (2.52–5.49) | 5.92 (3.97–8.83) | <0.001 | |
| Model 2 | 1.00 | 1.22 (0.76–1.96) | 1.97 (1.22–3.17) | 2.35 (1.37–4.02) | <0.001 | |
| Model 3 | 1.00 | 1.15 (0.70–1.76) | 1.76 (1.09–2.92) | 2.02 (1.15–3.58) | 0.001 | |
| Hemorrhagic stroke | Events/total at risk (%) | 14/3,617 (0.39) | 10/1,474 (0.68) | 5/1,052 (0.47) | 3/662 (0.45) | |
| Model 1 | 1.00 | 1.86 (0.82–4.18) | 1.35 (0.49–3.75) | 1.43 (0.41–4.98) | 0.62 | |
| Model 2 | 1.00 | 1.13 (0.45–2.83) | 0.68 (0.21–2.14) | 0.64 (0.15–2.72) | 0.33 | |
| Model 3 | 1.00 | 1.12 (0.44–2.86) | 0.66 (0.20–2.22) | 0.64 (0.14–2.85) | 0.36 | |
| Total stroke | Events/total at risk (%) | 70/3,617 (1.94) | 50/1,474 (3.40) | 60/1,052 (5.70) | 55/662 (8.31) | |
| Model 1 | 1.00 | 1.88 (1.31–2.70) | 3.32 (2.35–4.69) | 5.39 (3.78–7.68) | <0.001 | |
| Model 2 | 1.00 | 1.18 (0.79–1.78) | 1.65 (1.08–2.52) | 2.00 (1.25–3.22) | <0.001 | |
| Model 3 | 1.00 | 1.11 (0.73–1.68) | 1.47 (0.95–2.27) | 1.71 (1.04–2.81) | 0.01 | |
| TIA | Events/total at risk (%) | 21/3,617 (0.58) | 26/1,474 (1.76) | 26/1,052 (2.47) | 12/662 (1.81) | |
| Model 1 | 1.00 | 3.27 (1.84–5.81) | 4.82 (2.71–8.57) | 3.86 (1.89–7.84) | <0.001 | |
| Model 2 | 1.00 | 2.82 (1.46–5.44) | 3.67 (1.77–7.57) | 3.00 (1.24–7.25) | <0.01 | |
| Model 3 | 1.00 | 2.63 (1.35–5.11) | 3.21 (1.52–6.80) | 2.54 (1.02–6.33) | 0.02 | |
| Stroke mortality | Events/total at risk (%) | 10/3,617 (0.28) | 5/1,474 (0.34) | 14/1,052 (1.33) | 13/662 (1.96) | |
| Model 1 | 1.00 | 1.29 (0.44–3.77) | 5.35 (2.38–12.06) | 8.59 (3.76–19.63) | <0.001 | |
| Model 2 | 1.00 | 0.36 (0.11–1.23) | 1.00 (0.48–2.63) | 1.02 (0.35–2.98) | 0.24 | |
| Model 3 | 1.00 | 0.34 (0.10–1.18) | 0.90 (0.32–2.48) | 0.89 (0.29–2.79) | 0.36 | |
Model 1 includes multisite thoracic ECC.
Model 2 includes thoracic ECC and traditional cardiovascular risk factors and other potential confounders including age, gender, race/ethnicity, estimated glomerular filtration rate, LDL-C, HDL-C, total cholesterol, diabetes mellitus, hypertension, cigarette smoking status (never, former, current), any lipid-lowering medications, anti-hypertensive medications, aspirin use, family history of heart attack or stroke, and education.
Model 3 includes log (CAC+1) and all factors in Model 2.
CAC, coronary artery calcification; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; ECC, extra-coronary calcification; TIA, transient ischemic attack.
Figure 2.
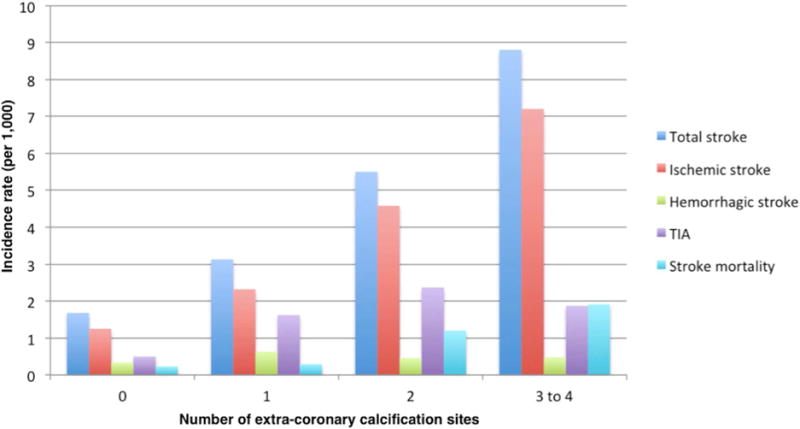
Incidence rate per 1,000 person-years for stroke outcomes by number of extra-coronary calcification sites. Scale, 2:1
TIA, transient ischemic attack.
The results of the Cox models are shown in Table 2. An increase in the number of thoracic ECC sites was associated with a graded increase in HRs for ischemic and total stroke in all three models. HRs were attenuated in Models 2 and 3 but results remained significant for 3–4 thoracic ECC score. Hemorrhagic stroke events were not associated with thoracic ECC in any of three models. For TIA, the HRs were attenuated in models 2 and 3, but results remained significant in all models and across all thoracic ECC score groups. An increase in the number of thoracic ECC sites was associated with a step-wise increase in the rate of stroke mortality only in the unadjusted models. Supplementary Fig. 1 shows cumulative incidence of stroke events by thoracic ECC score.
3.3. Discrimination and reclassification
Adding thoracic ECC to traditional cardiovascular risk factors, other potential confounders, and CAC increased the area under the ROC curve for all endpoints, but results were not statistically significant (Supplementary Table 1). IDI was only statistically significant for ischemic stroke (0.0023; p= 0.005), but not hemorrhagic stroke (−0.0000; p= 0.927), total stroke (0.0012; p= 0.058), TIA (0.0013; p= 0.101), and stroke mortality (0.0000; p=0.990). Thoracic ECC improved continuous NRI but results were not statistically significant. (Tables 3)
Table 3.
Net reclassification improvement (NRI) analyses for stroke endpoints after adding thoracic extra-coronary calcium (ECC) score to models adjusted for traditional risk factors (TRF) and coronary artery calcium (CAC) score.
| Endpoint | TRF/confounders vs. TRF /confounders + thoracic ECC | TRF/confounders + CAC vs. TRF/confounders + CAC + thoracic ECC | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Continuous event NRI | Continuous non-event NRI | Continuous NRI | Continuous event NRI | Continuous non-event NRI | Continuous NRI | |
| Ischemic stroke | 0.060 (−0.074, 0.179) | 0.079 (0.002, 0.152) | 0.139 (−0.047, 0.305) | 0.048 (−0.113, 0.176) | 0.047 (−0.026, 0.125) | 0.095 (−0.118, 0.292) |
| Hemorrhagic stroke | 0.267 (−0.473, 0.483) | 0.053 (−0.015, 0.174) | 0.320 (−0.460, 0.607) | 0.133 (−0.448, 0.429) | 0.065 (−0.018, 0.178) | 0.198 (−0.427, 0.555) |
| Total stroke | 0.023 (−0.085, 0.135) | 0.061 (−0.000, 0.123) | 0.085 (−0.067, 0.239) | −0.014 (−0.157, 0.115) | 0.024 (−0.028, 0.090) | 0.010 (−0.168, 0.191) |
| TIA | 0.038 (−0.169, 0.218) | 0.115 (0.014, 0.204) | 0.153 (−0.127, 0.390) | 0.013 (−0.212, 0.208) | 0.074 (−0.015, 0.187) | 0.087 (−0.181, 0.348) |
| Stroke mortality | −0.243 (−0.488, 0.406) | −0.006 (−0.111, 0.281) | −0.249 (−0.447, 0.488) | −0.189 (−0.485, 0.355) | −0.021 (−0.107, 0.279) | −0.210 (−0.466, 0.461) |
CAC, coronary artery calcium; ECC, extra-coronary calcium; NRI, net reclassification improvement; TRF, traditional.
3.4. Secondary, subgroup, and sensitivity analyses
Compared with other thoracic ECC sites, MVC was a better predictor for ischemic stroke and total stroke in all 3 models, while TAC could better predict TIA in models 1 and 2 (Supplementary Table 2). The results were largely unchanged after restricting the analyses to participants with CAC=0, CAC>100, and CAC>75th percentile in the population (Supplementary Tables 3–5). The results were unchanged after considering competing risk from total mortality and stroke mortality, as well as further adjustment for atrial fibrillation, all CVD events, and change in lipid-lowering and aspirin use in Cox regression models. There was no interaction between age, sex, race, body mass index, body weight, estimated glomerular filtration rate, and hypertensive status and thoracic ECC score for any outcomes. The interaction was borderline significant between diabetes and thoracic ECC for TIA (p=0.060), such that all estimates were significant among participants without diabetes and no endpoints were significant for participants with diabetes. (Supplementary Tables 6 and 7).
4. Discussion
In this study, we demonstrated that with increasing number of thoracic ECC sites there is a step-wise increase in the risk for ischemic stroke, total stroke, and TIA, but not hemorrhagic stroke, and stroke mortality. In addition, we showed that multisite thoracic ECC minimally improves global risk prediction for ischemic stroke over traditional risk factors and CAC. However, the increase in risk discrimination and reclassification was not significant for other stroke-related outcomes.
Although, a quantitative assessment of ECC can be more robust from methodological perspective, it cannot be replicated clinically in general chest CT imaging. Our study is amongst the few that devised and utilized a novel multisite thoracic ECC score for more pragmatic and comprehensive clinically relevant risk stratification. The clinical significance of our findings is rooted in the widespread use of various imaging methods on which thoracic ECC data can be easily obtained and is often reported. Calcification outside the coronary beds can be found incidentally on several imaging modalities, such as gated CT, non-gated chest CT scans, plain radiography, ultrasonography, echocardiography, magnetic resonance imaging, and dual energy X-ray absorptiometry (DEXA). ECC may appear earlier or in the absence of CAC, and therefore, may be a more sensitive marker for CHD and cardiovascular disease (CVD) risk assessment.6, 8 As such, the benefits of multisite ECC scoring regarding improving current risk assessment strategies, altering treatment decisions, and improving clinical outcomes has been hypothesized to outweigh healthcare expenses in the absence of additive radiation exposure.23
A few previous studies have shown the role of extra-coronary calcifications for predicting CHD risk. Van der Meer et al. combined carotid plaque and carotid intima-media thickness as early markers of atherosclerosis with abdominal aortic calcification to create a score for predicting myocardial infarction in Rotterdam Study population. In this study, the HRs for moderate and severe atherosclerosis according to composite atherosclerosis score were 1.71 (1.06–2.76) and 2.77 (1.70–4.52), respectively, as compared with no atherosclerosis.24 Munter et al. combined MVC, AVC, and abdominal aortic calcification along with age and history of dialysis to create a “cardiovascular calcification index”, which was significantly predictive of CAC.25 Tison et al. reported that a multisite thoracic ECC score, consisting of TAC, MVC, MVC, ARC, can improve risk prediction for CHD events, CHD mortality, and all-cause mortality when combined with traditional risk factors. However, when CAC was added to the model, thoracic ECC significantly predicted all-cause mortality, but not CHD events or CHD mortality.13 Our results support the ability of thoracic ECC, regardless of the presence of CAC, for prediction of stroke, TIA, and stroke mortality, in addition to CHD and other mortality endpoints. However, thoracic ECC does not improve measured of global discrimination above that provided by CAC.
New guidelines have recommendations for the risk assessment of atherosclerotic CVD, which includes fatal and nonfatal stroke events in addition to CHD.3 CAC has already been shown to be a stronger predictor of CHD than stroke.4,5 The fact that more patients have CT scans with thoracic ECC than CAC, make thoracic ECC scoring a valuable method to be studied and utilized for patient risk assessment. Although CAC=0 is deemed to predict lower CVD risk26, Wong et al. showed that more than 55% of MESA participants had extra-coronary calcification that may put them at high risk for CVD events.27 In this regard, our results demonstrated that thoracic ECC is predictive of stroke beyond traditional risk factors when CAC was not in the model (Model 2). Therefore, thoracic ECC can stratify patients for stroke, and consequently, may help physicians make decisions about starting appropriate lifestyle or pharmacological primary prevention measures when CAC is not available.
This study has limitations. The Agatston score was developed in early 1990s in order to quantify calcification in coronary vessels. Due to the heterogeneity of calcification in extra-coronary vascular beds, the utility of thoracic ECC as a continuous score for the prediction of CVD events is questionable. To address this problem, we used an ordinal multisite thoracic ECC score that only considered presence of calcification at locations outside coronary beds, in order to refrain from the bias that results from outliers and difference in Agatston score across thoracic ECC sites. Volume and density of calcification were also not measured in this study. Moreover, although individual thoracic ECC, such as TAC and MVC, were treated the same in the calculation of thoracic ECC score, they may be associated with different clinical outcomes.6, 9 However, the rationale to create a multisite score in this study was to reduce reliance on individuals ECC sites, increase the generalizability of results, and simplify stroke risk prediction for clinicians. Another limitation of our study might be the low number of events for stroke endpoints, as compared with the total population. Small numbers resulted in wide confidence intervals for sensitivity analyses that included evaluation of individual stroke types, individual thoracic ECC sites, and race-based risk. Finally, the MESA cohort lacks CT measurement for other calcification sites, such as carotid arteries, aortic arch, and iliac arteries, which may potentially provide more complete CVD risk prediction.8
In conclusion, our study shows that multisite thoracic ECC is associated with increased risk of ischemic stroke, total stroke, and TIA and minimally improves global risk prediction for all stroke-related outcomes independent of traditional risk factors and CAC. Although significant associations between thoracic ECC and stroke events may be useful for predictive purposes, this study was not designed to show their causal relationships. However, while incidentally found calcifications on various imaging modalities may provide some prognostic information without further cost or radiation exposure, their value to global risk prediction is limited in comprehensive models. More studies with larger number of stroke events are needed to show if particular subgroups defined by age, sex, race, or comorbid conditions might benefit from thoracic ECC-based stroke risk prediction.
Supplementary Material
Highlights.
Extra-coronary calcification (ECC) present in an increasing number of sites was associated with a graded increase in the risk for ischemic stroke, total stroke, and TIA.
There was no significant increase in hemorrhagic stroke or stroke mortality, although these outcomes were rate.
ECC site involvement increased the c-statistic and net reclassification index beyond traditional risk factors and coronary artery calcification (CAC), but the results were not significant (p>0.10).
Therefore, the incremental risk predictive value of thoracic ECC beyond traditional risk factors and CAC appears to be minimal.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Financial support
This research was supported by R01 HL071739 and contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165 and N01 HC 95169 from the National Heart, Lung, and Blood Institute. This funding covered costs of participation in MESA for all volunteers. NIH covered all costs, and there was a nominal dollar amount payed to individuals for their time.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declared they do not have anything to disclose regarding conflict of interest with respect to this manuscript.
Author contributions
Sina Kianoush, Mahmoud Al Rifai, and Michael J. Blaha contributed to the literature search, study design, data interpretation, and writing and editing of the manuscript. Sina Kianoush contributed to the statistical analyses. All other co-authors contributed to the study design, data interpretation, and editing of the manuscript.
References
- 1.Demer LL, Tintut Y. Vascular calcification: pathobiology of a multifaceted disease. Circulation. 2008;117(22):2938–48. doi: 10.1161/CIRCULATIONAHA.107.743161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yeboah J, McClelland RL, Polonsky TS, Burke GL, Sibley CT, O’Leary D, et al. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA. 2012;308(8):788–95. doi: 10.1001/jama.2012.9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goff DC, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63 doi: 10.1016/j.jacc.2013.11.005. (25_PA) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gepner AD, Young R, Delaney JA, Budoff MJ, Polak JF, Blaha MJ, Post WS, Michos ED, Kaufman J, Stein JH. Comparison of Carotid Plaque Score and Coronary Artery Calcium Score for Predicting Cardiovascular Disease Events: The Multi‐Ethnic Study of Atherosclerosis. JAHA. 2017;6(2):e005179. doi: 10.1161/JAHA.116.005179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Folsom AR, Kronmal RA, Detrano RC, O’Leary DH, Bild DE, Bluemke DA, Budoff MJ, Liu K, Shea S, Szklo M, Tracy RP. Coronary artery calcification compared with carotid intima-media thickness in the prediction of cardiovascular disease incidence: the Multi-Ethnic Study of Atherosclerosis (MESA) Arch Intern Med. 2008 Jun 23;168(12):1333–39. doi: 10.1001/archinte.168.12.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barasch E, Gottdiener JS, Larsen EKM, Chaves PH, Newman AB. Cardiovascular morbidity and mortality in community-dwelling elderly individuals with calcification of the fibrous skeleton of the base of the heart and aortosclerosis (The Cardiovascular Health Study) Am J Cardiol. 2006;97(9):1281–6. doi: 10.1016/j.amjcard.2005.11.065. [DOI] [PubMed] [Google Scholar]
- 7.Gondrie MJ, van der Graaf Y, Jacobs PC, Oen AL, Willem PTM, Group PS The association of incidentally detected heart valve calcification with future cardiovascular events. Eur Radiol. 2011;21(5):963–73. doi: 10.1007/s00330-010-1995-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iribarren C, Sidney S, Sternfeld B, Browner WS. Calcification of the aortic arch: risk factors and association with coronary heart disease, stroke, and peripheral vascular disease. JAMA. 2000;283(21):2810–5. doi: 10.1001/jama.283.21.2810. [DOI] [PubMed] [Google Scholar]
- 9.Allison MA, Hsi S, Wassel CL, Morgan C, Ix JH, Wright CM, et al. Calcified atherosclerosis in different vascular beds and the risk of mortality. Arterioscler Thromb Vasc Biol. 2012;32(1):140–6. doi: 10.1161/ATVBAHA.111.235234. [DOI] [PubMed] [Google Scholar]
- 10.Wilson PW, Kauppila LI, O’Donnell CJ, Kiel DP, Hannan M, Polak JM, et al. Abdominal aortic calcific deposits are an important predictor of vascular morbidity and mortality. Circulation. 2001;103(11):1529–34. doi: 10.1161/01.cir.103.11.1529. [DOI] [PubMed] [Google Scholar]
- 11.Budoff MJ, Nasir K, Katz R, Takasu J, Carr JJ, Wong ND, et al. Thoracic aortic calcification and coronary heart disease events: the multi-ethnic study of atherosclerosis (MESA) Atherosclerosis. 2011;215(1):196–202. doi: 10.1016/j.atherosclerosis.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Owens DS, Budoff MJ, Katz R, Takasu J, Shavelle DM, Carr JJ, et al. Aortic valve calcium independently predicts coronary and cardiovascular events in a primary prevention population. J Am Coll Cardiol Img. 2012;5(6):619–25. doi: 10.1016/j.jcmg.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lavy S, Melamed E, Cahane E, CARMON A. Hypertension and diabetes as risk factors in stroke patients. Stroke. 1973;4(5):751–9. doi: 10.1161/01.str.4.5.751. [DOI] [PubMed] [Google Scholar]
- 14.Tison GH, Guo M, Blaha MJ, McClelland RL, Allison MA, Szklo M, et al. Multisite extracoronary calcification indicates increased risk of coronary heart disease and all-cause mortality: The Multi-Ethnic Study of Atherosclerosis. J Cardiovasc Comput. 2015;9(5):406–14. doi: 10.1016/j.jcct.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bild DE, Bluemke DA, Burke GL, Detrano R, Roux AVD, Folsom AR, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 16.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation. 2005;112(17):2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 17.Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, Jacobs DR, Jr, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of multi-ethnic study of atherosclerosis (MESA) and coronary artery risk development in young adults (CARDIA) study 1. Radiology. 2005;234(1):35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 18.Allison MA, Criqui MH, Wright CM. Patterns and risk factors for systemic calcified atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24(2):331–6. doi: 10.1161/01.ATV.0000110786.02097.0c. [DOI] [PubMed] [Google Scholar]
- 19.Pencina MJ, D’Agostino RB, D’Agostino RB, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27(2):157. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 20.D’Agostino RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care the Framingham Heart Study. Circulation. 2008;117(6):743–53. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 21.Pencina MJ, D’Agostino RB, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30(1):11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Air EL, Kissela BM. Diabetes, the metabolic syndrome, and ischemic stroke epidemiology and possible mechanisms. Diabetes care. 2007;30(12):3131–40. doi: 10.2337/dc06-1537. [DOI] [PubMed] [Google Scholar]
- 23.Waugh N, Black C, Walker S, McIntyre L, Cummins E, Hillis G. The effectiveness and cost-effectiveness of computed tomography screening for coronary artery disease: systematic review. Health Technol Assess. 2006;10(39) doi: 10.3310/hta10390. [DOI] [PubMed] [Google Scholar]
- 24.van der Meer IM, Bots ML, Hofman A, del Sol AI, van der Kuip DA, Witteman JC. Predictive Value of Noninvasive Measures of Atherosclerosis for Incident Myocardial Infarction The Rotterdam Study. Circulation. 2004;109(9):1089–94. doi: 10.1161/01.CIR.0000120708.59903.1B. [DOI] [PubMed] [Google Scholar]
- 25.Muntner P, Ferramosca E, Bellasi A, Block GA, Raggi P. Development of a cardiovascular calcification index using simple imaging tools in haemodialysis patients. Nephrol Dial Transplant. 2007;22(2):508–14. doi: 10.1093/ndt/gfl609. [DOI] [PubMed] [Google Scholar]
- 26.Sarwar A, Shaw LJ, Shapiro MD, Blankstein R, Hoffman U, Cury RC, et al. Diagnostic and prognostic value of absence of coronary artery calcification. J Am Coll Cardiol Img. 2009;2(6):675–88. doi: 10.1016/j.jcmg.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 27.Wong ND, Lopez VA, Allison M, Detrano RC, Blumenthal RS, Folsom AR, et al. Abdominal aortic calcium and multi-site atherosclerosis: the Multiethnic Study of Atherosclerosis. Atherosclerosis. 2011;214(2):436–41. doi: 10.1016/j.atherosclerosis.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


