Abstract
Background
Brain-derived neurotrophic factor (BDNF) plays a key role in the pathophysiology and treatment of depression. Recent clinical studies demonstrate that scopolamine, a non-selective muscarinic acetylcholine receptor antagonist, produces rapid antidepressant effects in depressed patients. Rodent studies demonstrate that scopolamine increases glutamate transmission and synaptogenesis in the medial prefrontal cortex (mPFC). Here, we tested the hypothesis that activity-dependent BDNF release within the mPFC is necessary for the antidepressants actions of scopolamine.
Methods
Behavioral effects of scopolamine were assessed in BDNF Val/Met knock-in mice, in which BDNF processing and release are impaired. In addition, intra-mPFC infusion of a BDNF-neutralizing antibody was performed to test the necessity of BDNF release in driving scopolamine-induced behavioral responses. Further in vivo as well as in vitro experiments were performed to delineate BDNF-dependent mechanisms underlying the effects of scopolamine.
Results
We found that BDNF Met/Met mice have attenuated responses to scopolamine, and that anti-BDNF antibody infusions into the mPFC prevented the antidepressant-like behavioral effects of scopolamine. In vitro experiments show that scopolamine rapidly stimulates BDNF release and TrkB-ERK signaling. Moreover, these effects require AMPA receptor activation and are blocked by neuronal silencing. Importantly, pretreatment with verapamil prevented scopolamine-induced behavioral responses and BDNF-TrkB signaling, suggesting that these effects are dependent on activation of voltage-dependent calcium channels.
Conclusion
The results identify an essential role for activity dependent BDNF release in the rapid antidepressant effects of scopolamine. Attenuation of responses in BDNF Met mice indicates that patients with the Met allele may be less responsive to scopolamine.
Keywords: Muscarinic Receptor, Prefrontal Cortex, Depression, TrkB receptor, Voltage Dependent Calcium Channel, mTORC1
Introduction
Depression is a disabling illness, affecting 17 percent of the population in the United States with significant socioeconomic impact (1). While the current therapeutics are effective in some patients, available agents take weeks to months to produce an antidepressant response (2, 3), and about one third of patients fail to respond and are considered treatment resistant. These limitations highlight an urgent need for the development of faster and more effective antidepressant drugs. Recent studies have reported several novel treatments that address these issues, including the NMDA receptor antagonist ketamine and the non-selective muscarinic acetylcholine receptor (mAChR) antagonist scopolamine (4, 5), both of which produce rapid antidepressant actions in depressed patients, even those considered treatment resistant.
We recently reported evidence that scopolamine increases glutamate transmission in the medial prefrontal cortex (mPFC), suggesting a role for neuronal activity in the antidepressant effects of scopolamine (6). This is supported by studies demonstrating that administration of a glutamate AMPA receptor antagonist or infusion of a neuronal silencing agent into the mPFC blocks the behavioral responses to scopolamine (6, 7). The rapid burst of glutamate resulting from scopolamine has been shown to occur via blockade of M1 type AChR on GABA interneurons, resulting in disinhibition of glutamate transmission (8), and a similar disinhibition mechanism has been suggested for ketamine (9, 10).
Brain-derived neurotrophic factor (BDNF) plays a central role in the pathophysiology and treatment of depression (11). BDNF expression is decreased in limbic and cortical regions in rodent chronic stress models and in postmortem brains of depressed subjects; conversely, BDNF expression is increased by chronic administration of typical monoaminergic antidepressants and is required for the behavioral actions of these agents (9, 11). Interestingly, recent studies demonstrate that the behavioral responses to ketamine are blocked in BDNF-deletion mutant mice (12), in BDNF Val/Met knock-in mice (13), which show impaired activity-dependent BDNF release (14, 15), and by infusion of an anti-BDNF antibody into the mPFC (16). However, the role of activity dependent BDNF release in the antidepressant actions of scopolamine has not been investigated.
The current study addresses this question using a combination of mutant mouse (BDNF Val/Met knock-in), anti-BDNF neutralizing antibody, and pharmacological approaches. We also conducted mechanistic studies in cultured primary cortical neurons to further characterize scopolamine-stimulated activity dependent release of BDNF. The results reveal that fast, activity-dependent BDNF release is directly involved in the rapid antidepressant actions of scopolamine and provides a framework for further mechanistic studies. The results could be clinically relevant as the antidepressant responses to ketamine are significantly decreased in depressed patients carrying the Met allele (17).
Methods and Materials
Animals
The BDNF Val/Met knock-in mice were generated and bred as described previously (13, 14). Adult male Sprague-Dawley rats (Charles River Laboratories; 275–300g) were used for all other studies. Pregnant female rats were used as a source of embryonic tissue for primary neuronal cultures. Animals were housed and maintained in standard conditions with a 12-h light/dark cycle and ad libitum food and water unless otherwise noted. All experiments were performed between 8 am and 12 pm during the 12-hour light cycle. Animal use and procedures were in accordance with the National Institutes of Health guidelines and approved by the Yale University Animal Care and Use Committees.
Cannulation studies
mPFC cannulation and intra-mPFC infusions [coordinates from bregma: +3.0 anterior/posterior; +/- 1 medial/lateral; and -4 dorsal/ventral] of IgG or BDNF neutralizing antibody were performed as described previously (16). Rats were bilaterally infused with an anti-BDNF antibody (Chemicon; 0.5 μg/side, 1μg/μl) or IgG (0.25 μl/min).
Drug administration
Scopolamine (Sigma-Aldrich, St. Louis, MO) and verapamil (Sigma-Aldrich, St. Louis, MO) were used for drug administration studies. For in vitro experiments drug [50 μM NBQX, 500 nM K252a, 25 μM ANA-12, or 10 μM muscimol] incubations were conducted as described previously (18, 19) and media was collected 60 min following scopolamine or preferential M1-AChR antagonist VU0255035 (Tocris Bioscience, Minneapolis, MN) incubation for BDNF ELISA.
Behavioral analyses in BDNF Val/Met mice
For the forced swim test (FST) time immobile during the 2- to 6-minute block and novelty suppressed feeding test (NSFT) were performed as described previously (8). Home cage food intake was measured as a control. Locomotor activity was measured using automated activity meters (Omnitech Electronics, Columbus, OH) for a total of 30 minutes.
Behavioral analysis in rats
Behavioral responses in the rat FST and NSFT were conducted as previously described (6, 7). Home cage food intake was measured right after the test as a control. Only rats with correct cannula placement were included for analyses.
Western blot analyses
Western blots of P2 crude synaptosome fractions of dissected mPFC , which included both prelimbic and infralimbic subregions were conducted as previously described (6, 7, 20, 21) using the following antibodies:_pERK, pS6K, pAkt, or p mTORC1 (Cell Signaling, 1:1000) and pTrk B (Santacruz, 1:250). After detection of phospho proteins, membranes were stripped and reprobed with antibodies for total ERK 1/2, S6K, Akt, mTORC1, TrkB (Cell Signaling, 1:1000) overnight. Densitometry was used to quantify protein bands using Image J Software (NIH), and phospho proteins were normalized to their respective total protein.
Primary cortical culture, viral GFP infection, Sholl analysis, and Immunolabeling
Primary cultures were prepared and pharmacological and cell morphology studies conducted as described previously (18, 22). Immunohistochemistical analyses of microtubule-associated protein 2 (MAP2) (Abcam, ab5392, 1:10000), choline acetyl transferase (ChAT) (Millipore, AB144P, 1:1000) and glutamate decarboxylase (GAD67) (Millipore, MAB5406, 1:1000) were performed on DIV13 cells. GAD, ChAT, and MAP2 positive cells were counted.
BDNF ELISA analysis
Measurement of BDNF was performed as previously described (18). Immunoprecipitated BDNF was detected via ELISA ( E-max; Promega, WI).
Statistics
Sample sizes (n) are similar to those reported previously (6-8, 18, 20, 22). Data were subjected to statistical analyses [Students t test, one-way or two-way ANOVA] with Graph-Pad Prism 6 (Graph-pad software, La Jolla, CA). Multiple comparisons with Fisher's least significant difference (LSD) test were applied where main effects and/or interactions were significant, P < .05.
Results
Antidepressant responses to scopolamine are blocked in BDNF Met knock-in mice
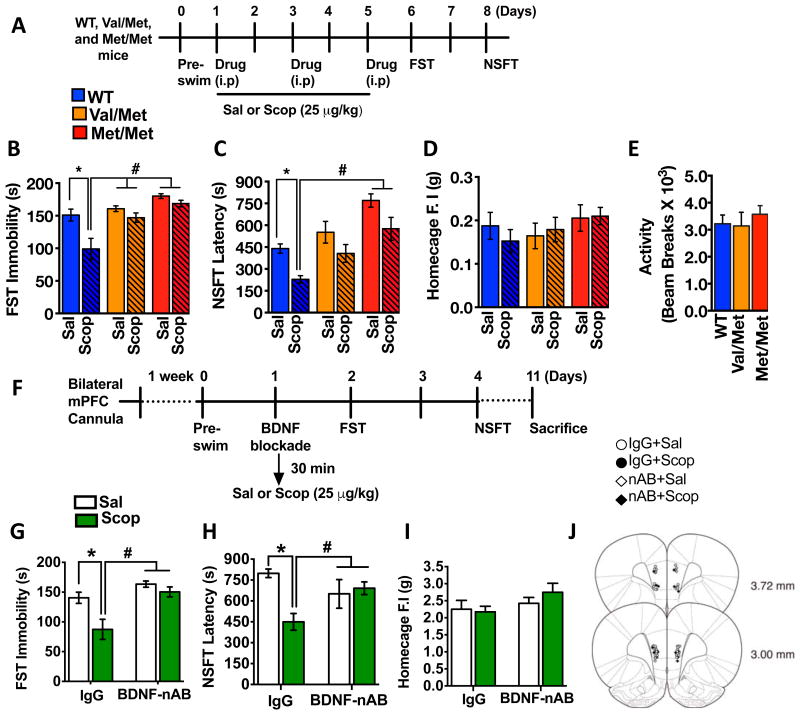
Previously, we showed that 3 doses of scopolamine (25 μg/kg, i.p, every other day), a treatment paradigm based on the clinical dosing schedule (5) produces rapid and long-lasting antidepressant responses in the FST and NSFT when tested 1 to 3 days after the last dose of scopolamine (7, 8). Using the same dosing regimen, we assessed scopolamine-induced behavioral responses in BDNF Met/Met, Val/Met, and wild type (WT, Val/Val) littermates (Figure 1A). In the FST, scopolamine administration significantly reduced immobility time in WT mice (Figure 1B) consistent with an antidepressant effect of scopolamine, but there was no significant difference in immobility time between scopolamine and saline in Val/Met or Met/Met knock-in mice (Figure 1B).
Figure 1. Antidepressant behavioral actions of scopolamine are blocked in BDNF Met knockin mice and by anti-BDNF antibody infusion into mPFC.
(A) Experimental timeline for evaluating the behavioral effects of scopolamine in BDNF Val/Met knockin mice and wild type (WT) littermates. (B, C) Mice (WT, Val/Met, and Met/Met, n=6-8/genotype/group) were injected with saline (sal) or scopolamine (scop) (25 μ/kg) and were tested in the FST and NSFT. The time spent immobile in the FST [main effects of treatment (F1,34 = 12.57, *P < 0.01), genotype (F2,34 = 11.23, *P < 0.01), treatment X genotype interaction (F2,34 = 3.01, P = 0.06)](B) and latency to feed in the NSFT [main effects of treatment (F1,42 = 14.64, *P < 0.01), genotype (F2,42 = 13.47, *P < 0.01), treatment X genotype interaction (F2,42 = 0.017, P < 0.05)]. (C) are shown for each genotype. (D, E) There were no significant differences in home cage feeding or general locomotor activity. (F) Experimental timeline for evaluating the influence of an anti-BDNF antibody on the antidepressant behavioral effects of scopolamine. Infusions of an anti-BDNF neutralizing antibody (n=6-8/group) or IgG control (n=6/group) were made into the mPFC 30 min prior to scopolamine injection (25 μg/kg, i.p.), and behavioral testing was performed at the time points shown. (G, H) In the FST and NSFT, scopolamine significantly reduced the immobility duration [two-way ANOVA, main effects of drug (F1,16 = 11.25 *P < 0.001), antibody (F1,16 = 19.10, *P < 0.01), drug X antibody interaction (F1,16 = 4.13, *P < 0.05)], and decreased the latency to feed [two-way ANOVA, main effects of drug (F1,18 = 4.95 *P < 0.05), and a significant treatment X antibody interaction (F1,18 = 7.91, *P < 0.01)], and these effects were completely blocked by pretreatment with the function-blocking anti-BDNF antibody. (I) There was no effect on home cage feeding regardless of the treatment. (J) Infusion sites of BDNF neutralizing antibody or IgG within the PFC. All data are mean ± SEM. The data included in panels B-D and G-I were analyzed by a two- factor (genotype X Drug) ANOVA with Fisher LSD post hoc test, the data panel E was analyzed by one-way ANOVA. *P < 0.05 vs.WT-Sal in (B-D). #P < 0.05 vs.WT-Scop (B-D). *P < 0.05 vs.IgG-Sal in (F-I). #P < 0.05 vs.IgG-scop in (F-I) respectively.
In the NSFT, scopolamine reduced latency to feed in WT mice (Figure 1C), but again had no effect in Val/Met or Met/Met knock-in mice (Figure 1C). Importantly, there were no differences in home cage feeding (Figure 1D) or locomotor activity (Figure 1E) demonstrating that blockade of scopolamine effects are specific to depression related behaviors. Consistent with a previous report (14), there was a trend for increased latency to feed in the Val/Met and Met/Met saline mice. We also found that scopolamine increased TrkB phosphorylation (measured by the ratio of pTrkB/total TrkB) in the PFC, and this effect was attenuated in the Met/Met mice (Supplementary Figure 1).
Anti-BDNF antibody infusion into the mPFC blocks the antidepressant actions of scopolamine
We have reported that activation of the mPFC is critical for antidepressant-like responses of systemic scopolamine (7), validating mPFC as a key target for infusion studies. To further test the role of BDNF release in the actions of scopolamine a function-blocking anti-BDNF antibody was infused into the mPFC 30 min prior to scopolamine administration, and FST and NSFT were conducted on the subsequent days (Figure 1F). Rats were used because the anti-BDNF antibody conditions and cannula placement have been validated in this species, including blockade of ketamine-induced antidepressant responses (16) , as well as learning-induced mTORC1 signaling (16, 23). Scopolamine administration significantly reduced immobility in the FST in the control IgG pre-treated group (Figure 1G), and infusion of the anti-BDNF antibody completely blocked this effect. Infusions of the antibody alone, in the absence of scopolamine had no effect on immobility when compared to IgG-infused controls.
In the NSFT, scopolamine decreased latency to feed in the control IgG pre-treated group and this effect was blocked by anti-BDNF antibody infusion into the mPFC (Figure 1H). There was no difference in home cage feeding (Figure 1I) between the two treatment groups regardless of the mPFC infusion, indicating that the antidepressant-like responses to scopolamine require BDNF release within the mPFC.
Blockade of L-Type VDCCs inhibits the rapid antidepressant actions of scopolamine
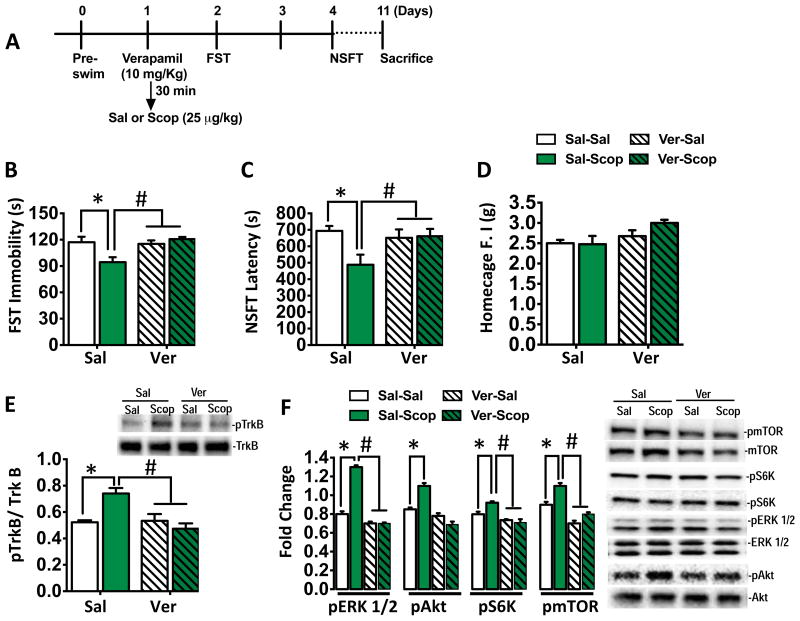
We have reported that the antidepressant actions of scopolamine are blocked by pretreatment with an AMPA receptor antagonist (6), and AMPA-stimulation of mTORC1 requires activation of L-type Voltage-dependent calcium channel (VDCC) (24). To test the role of VDCC's in the actions of scopolamine, we injected rats with verapamil (10 mg/kg) 30 min prior to scopolamine, and examined the behavioral responses in the FST and NSFT (Figure 2A). Scopolamine administration reduced immobility in the FST and verapamil pretreatment completely blocked this effect (Figure 2B); verapamil alone had no effect. Previous studies showed that verapamil has no effect on locomotor activity (16), demonstrating the effects seen in the FST are not due to a generalized decrease in ambulation. Similar effects were observed in the NSFT in that pretreatment with verapamil blocked the reduction in latency to feed in response to scopolamine (Figure 2C), without altering the normal home-cage feeding behavior test (Figure 2D).
Figure 2. L-type VDCC antagonist blocks the antidepressant behavioral effects of scopolamine.
(A) Experimental timeline showing verapamil (10 mg/kg, i.p) and systemic scopolamine (25 μ/kg) dosing, and behavioral and molecular analysis (n=6-10/group). (B) Scopolamine produced a significant decrease in immobility time in the FST and this effect was completely blocked by pretreatment with verapamil (*P < 0.05 vs. saline-treated) [main effects of treatment (F1,20 = 3.27, *P < 0.05), verapamil (F1,20 = 16.46, *P < 0.05), treatment X verapamil interaction (F1,20 = 8.55, *P < 0.01)]. (C) Scopolamine also produced a significant decrease in the latency to feed in the NSFT, which was blocked by verapamil pretreatment (#P < 0.05 vs. scopolamine-treated) [main effects of treatment (F1,36 = 4.12, *P < 0.05), and treatment X verapamil interaction (F1,36 = 4.98, *P < 0.03)]. (D) There were no significant differences in homecage feeding regardless of treatment. (E) Scopolamine (60 mins) significantly increased phosphorylation of TrkB in the rat PFC, and verapamil pretreatment prevented this effect. Total levels of TrKB protein were not different among the four groups. Representative immunoblots for phospho-TrkB (pTrkB) and total TrkB are shown [main effects of treatment (F1,36 = 4.12, *P < 0.05), and treatment X verapamil interaction (F1,36 = 4.98, *P < 0.05)]. (F) Analyses of changes in the mTORC1 signaling pathway within the PFC revealed a significant increase in pERK1/2, pAkt, pS6K and pmTOR in the scopolamine-treated (60 mins) rats; these effects were blocked by verapamil [main effects of treatment *p < 0.05, and treatment X verapamil interaction. p < 0.05]. N=5-9/ group. Representative immunoblots for phosphorylated and total protein levels are shown. All data are mean ± SEM. The data included were analyzed by a two-factor (treatment X Drug) ANOVA with Fisher LSD post hoc test. *p < 0.05 vs.Sal-Sal. #p < 0.05 vs. Sal-Scop.
We next tested the influence of verapamil on BDNF/TrkB activation. We found that scopolamine increased TrkB phosphorylation in the PFC and this effect was completely blocked by pretreatment with verapamil (Figure 2E). We also examined the ability of verapamil pretreatment to block scopolamine-stimulation of mTORC1 signaling. Levels of the phosphorylated and activated forms of mTOR and the downstream target p70S6 kinase 1 (S6K), and two upstream kinases ERK and Akt were analyzed. Scopolamine administration significantly increased levels of pERK 1/2, pAkt, pmTOR, and pS6K (Figure 2F, supplementary Figure 3), and verapamil pretreatment blocked the induction of these phosphoproteins.
Scopolamine increases BDNF release and TrkB-ERK signaling in primary cortical cultures
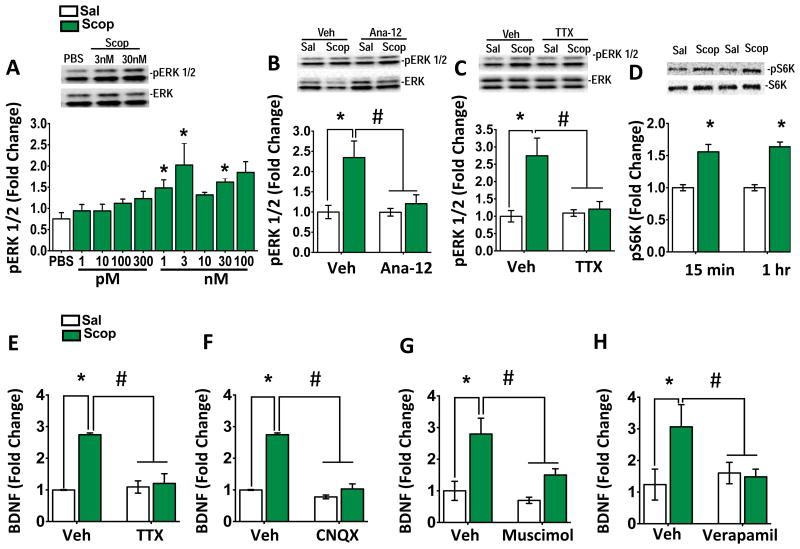
These in vivo studies indicate that the antidepressant actions of scopolamine are dependent on BDNF release, and because BDNF release in vivo is technically difficult to measure, we turned to a primary cortical culture system. We have reported that phosphorylated ERK is a consistent marker of BDNF-TrkB signaling (18), so pERK1/2 levels were used to characterize the concentration response and time course for the actions of scopolamine. Incubation with scopolamine (1 hr) resulted in a concentration-dependent stimulation of pERK1/2 at 1, 3, 30, and 100 nM (Figure 3A). Incubation with the selective BDNF-TrkB inhibitor, ANA-12 (19) (20 min) completely blocked scopolamine-stimulation of pERK1/2 (Figure 3B). Incubation of the cells with TTX, which inhibits glutamate neuronal firing, also blocked scopolamine stimulation of pERK1/2 (Figure 3C). Scopolamine (3 nM, 15, and 60 min) also increased levels of pS6K (Figure 3D).
Figure 3. Incubation with scopolamine increases BDNF release and phospho-ERK signaling in primary neuronal cultures.
(A) Scopolamine incubation (60 mins) produced a concentration dependent increase in phospho-ERK (pERK) (F5, 20 =2.14, *p<0.05). (B) Cultured neurons were incubated with the selective TrkB inhibitor ANA-12 for 20 min prior to addition of scopolamine for 1 hr incubation. Pretreatment with Ana-12 (25 μM) blocked the induction of pERK, and ANA-12 alone had no effect on pERK levels (F2, 18 = 12.65, *p<0.05). (C) Cultured neurons were incubated with TTX (50 μM) for 20 min prior to addition of scopolamine (3 nM). Pretreatment with TTX completely blocked the induction of pERK activation by scopolamine (main effect of scopolamine (F1, 12 = 7.57, *p<0.01); main effect of TTX (F1, 12 = 6.10, *p<0.05); scopolamine X TTX (F1, 12 = 11.81, *p<0.05)). (D) Scopolamine (1 nM) also increased levels of phospho-S6K (pS6K) at 15 and 60 min in primary cultured neurons (15 min (F1, 45 = 6.57, *p<0.01); 60 min (F1, 45 = 2.18, *p<0.001)). (E) Incubation with scopolamine (60 min) increased BDNF release into the media, and pretreatment with TTX completely blocked this effect. TTX on its own did not affect BDNF release (main effect of scopolamine (F1, 19 = 8.57, *p<0.01); scopolamine X TTX (F1, 12 = 10.21, *p<0.05)). (F) The AMPA receptor antagonist CNQX (10 μM) also significantly blocked BDNF release induced by scopolamine (60 min) (F2, 40 = 12.39, *p<0.001). (G) Incubation with the GABAA receptor antagonist muscimol (10 μM) significantly decreased BDNF release and attenuated the scopolamine response (main effect scopolamine (F1,20 = 9.139, *p < 0.01)). (H) Pretreatment with verapamil (10 μM) blocked scopolamine-induced BDNF release in cultures cells (main effect scopolamine (F1,18 = 7.19, *p < 0.05)) . All results are presented as mean ± SEM and fold change. *p < 0.05, compared to vehicle. #p < 0.05, compared to scopolamine. (n=6-10/group).
Next, the influence of scopolamine on BDNF release in to the cortical culture media was determined. Primary cultures were incubated with scopolamine (3 nM) for 1 hr and BDNF release was assessed (18). Scopolamine treatment caused a significant increase (∼2-3 fold) in levels of BDNF into the media (Figure 3E). By contrast, blockade of neuronal activity with TTX prevents scopolamine-induced BDNF release (Figure 3E). We conducted further studies to confirm that BDNF release is glutamate and activity dependent. First, pretreatment (20 min) with an AMPA receptor antagonist, CNQX blocked the effects of scopolamine (Figure 3F). Pre-incubation with the GABAA agonist muscimol, a neuronal silencing agent, also blocked the effects of scopolamine (Figure 3G), these results are consistent with the hypothesis that GABA disinhibition mediates the actions of scopolamine. Complementing our in vitro studies, we found that scopolamine administration (1 hour) significantly increased levels of the mature form of BDNF in the PFC (Supplementary Figure 2). Our in vivo results indicate that the actions of scopolamine require activation of L-type VDCCs (Figure 2), and in primary cortical cultures we found that verapamil (10 μM) completely blocked scopolamine-induced BDNF release, (Figure 3H). The results indicate that scopolamine-stimulated BDNF release is dependent on activation of AMPA receptors and depolarization induced stimulation of VDCCs.
M1-AChR blockade increases BDNF release and TrkB-ERK signaling in primary cortical cultures
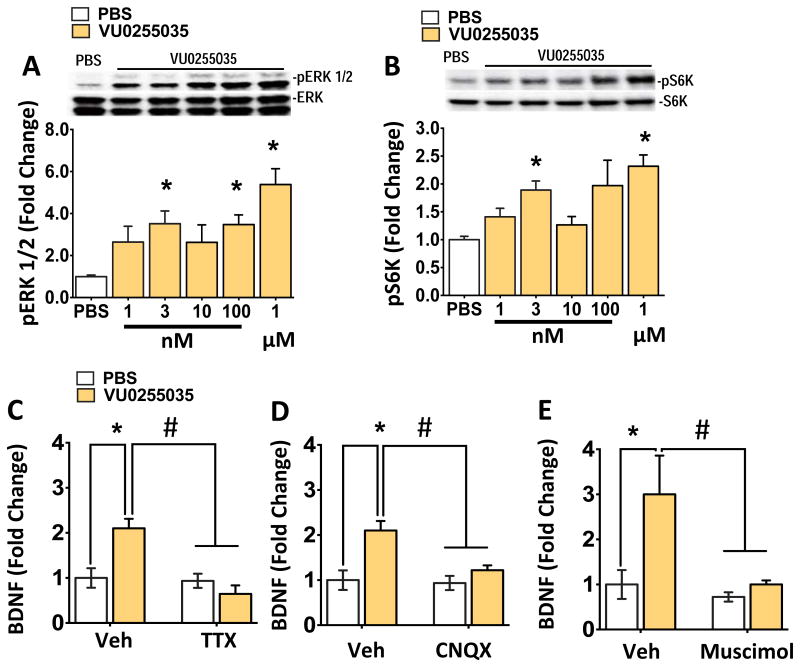
Scopolamine is a nonselective muscarinic receptor antagonist and previous studies indicate that M1-AChR mediates, at least in part the actions of scopolamine (6-8, 25). To determine the role of M1-AChR in BDNF release we tested the selective antagonist, VU0255035 that has approximately 70-fold selectivity for M1-AChR over other AChR muscarinic subtypes (26). Incubation with VU0255035 produced a concentration dependent increase in levels of pERK and pS6K in primary neuronal cultures (Figure 4A, B). Importantly, we found that VU0255035 incubation produced a significant increase in levels of BDNF release into the media (Figure 4C), and this effect was blocked by preincubation with TTX, CNQX or muscimol (Figure 4C-E). Complementing our previous study (7), these findings demonstrate that M1-AChR antagonism produces antidepressant behavioral and molecular signaling responses similar to scopolamine.
Figure 4. VU0255035 stimulates BDNF release and phospho-ERK signaling in primary neuronal cultures.
(A, B) Similar to scopolamine, incubation with a selective M1-AChR antagonist VU0255035 increased in (pERK) and phospho-S6K (pS6K) levels in a concentration dependent manner. (C) VU0255035 incubation (3 nM) also increased BDNF release into the media, and preincubation with TTX (50 μM, 20 min) prior completely blocked this effect. TTX alone had no effect on BDNF release (main effect of scopolamine (F1, 12 = 5.57, *p<0.05); scopolamine X TTX (F1, 12 = 9.11, *p<0.05)). (D, E) Incubation with CNQX (10 μM) or muscimol (10 μM) also significantly blocked BDNF release induced by scopolamine (main effect of scopolamine, *p<0.05); scopolamine X drug (*p<0.05)). Incubation with muscimol alone significantly decreased BDNF release. All results are presented as mean ± SEM and fold change. *p < 0.05, compared to vehicle. #p < 0.05, compared to scopolamine (n=6/group).
Role of GABA and cholinergic neurons in scopolamine-mediated responses
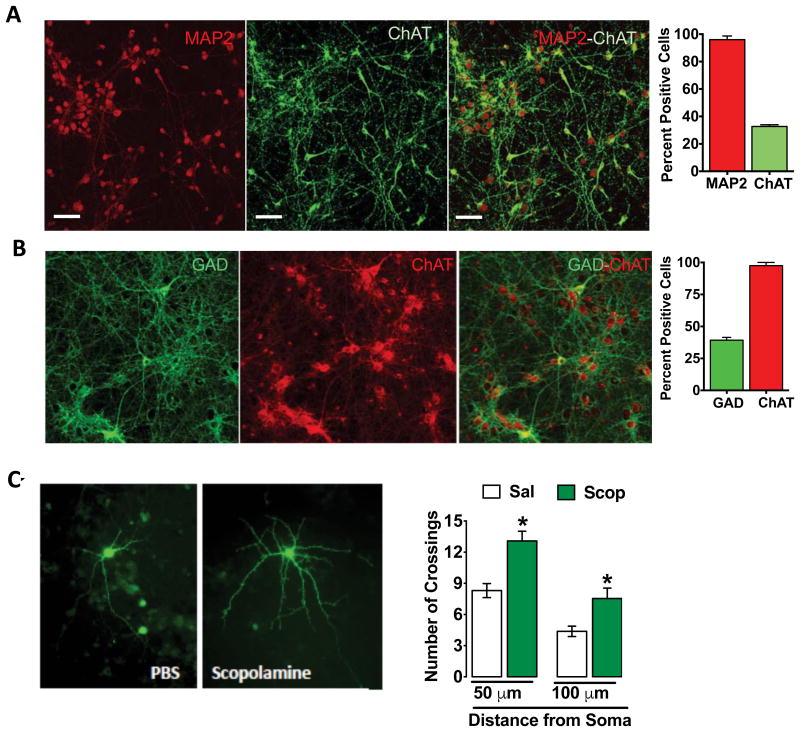
Previously, it has been shown that cortical neuronal cultures, maintained under conditions similar to those used in the current study contain both glutamatergic and GABAergic neurons, as well as a small number of glia (27, 28). Importantly, our prior electrophysiological studies demonstrate that ACh muscarinic receptors stimulate somatostatin (SST)-positive GABA interneurons in the PFC and that these effects are blocked by scopolamine or selective M1-ACh receptors inhibitors (8). The presence of cholinergic and GABAergic neurons was examined using the ACh synthesis marker ChAT and GABA synthesis marker, GAD67, respectively (Figure 5A, B). The results demonstrate that ChAT is expressed by a subpopulation of ∼30-40% of the total MAP2 positive cells (Figure 5A), confirming previous reports (27-29). Double labeling of ChAT and GAD67 demonstrates that many ChAT positive cells also express GAD67 (Figure 5B). These findings demonstrate that our primary neuronal culture system displays the capacity for ACh synthesis that could then regulate the activity of GABA as well as glutamate neurons.
Figure 5. Primary cortical cultures contain ChAT and GABA interneurons: scopolamine incubation increases dendrite complexity.
(A) Double-immunolabeling of cultured cells for the neuronal marker MAP2 and the ACh synthetic enzyme Chat demonstrates a subpopulation of neurons that is co-immunolabled for MAP2 and ChAT. Representative images are shown for each label and for the co-labeled cells as indicated (scale bars in lower right). The total numbers of ChAT immunolabeled cells were determined on 3 separate coverslips and the percentage relative to MAP2+ cells was determined. (B) Double-immunolabeling for the GABA synthetic enzyme GAD67 and ChAT demonstrates a subpopulation of neurons that is co-labeled. Representative images are shown for each label and for the co-labeled cells as indicated. The total numbers of GAD and ChAT immunolabeled cells were determined on 3 separate coverslips and the percentage of GAD positive cells relative to ChAT cells was determined. (C) Cortical cultures were incubated with AAV2-GFP on DIV3 to label neurons for morphological analysis. On DIV 17, cells were incubated with scopolamine (1 nM) for 24 hr. Cells were fixed and imaged for GFP signal. Representative images of labeled cells are shown for each condition. Sholl analysis demonstrates that scopolamine increases dendrite complexity of cultured neurons, including increased number of dendrite crossings at both 50 and 100 μM from the soma (50 μm (t (21) = 8.0, *p<0.05); 50 μm (t (16) = 8.28, *p<0.05)). All results are presented as mean ± SEM and fold change. *p < 0.05, compared to vehicle (n=6-15/group).
Scopolamine increases dendritic complexity in primary neuronal cultures
Scopolamine administration increases spine density and function in layer V pyramidal neurons in the mPFC (6). To test whether scopolamine increases neuronal complexity in vitro, primary cortical neurons were incubated with an AAV2 viral vector that expresses GFP to allow visualization of neuronal processes (Figure 5C). Viral expression and labeling were allowed to proceed for a two-week period and then cells were incubated with vehicle or scopolamine (1 nM) for 24 hr to allow for morphological alterations (17). Scopolamine incubation significantly increased dendrite complexity compared to vehicle treated cells, increasing the number of dendrite branch crossings compared to saline at both 50 and 100 microns from the soma (Figure 5 C).
Discussion
The molecular mechanisms governing the actions of the rapid-acting antidepressants ketamine and scopolamine have been a central focus for identifying novel targets for safer, more efficacious, and rapid-acting agents. In the present study, using mutant mice, pharmacological approaches, and in vitro studies, we show that activity-dependent BDNF release within the mPFC, as well as activation of L-Type VDCCs are required for the antidepressant actions of scopolamine. Interestingly, these mechanisms are similar to the rapid antidepressant effects of ketamine (13, 16), and indicate that activity-dependent stimulation of BDNF release and activation of TrkB-mTORC1 signaling are key convergent targets of rapid acting antidepressants (Figure 6).
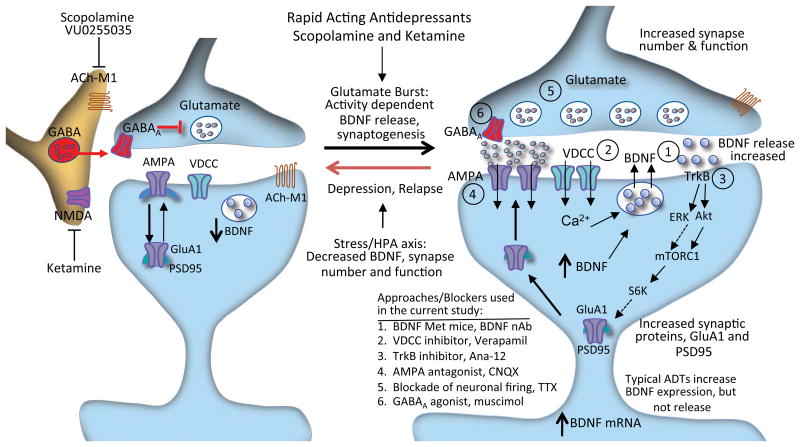
Figure 6. Mechanisms underlying the rapid antidepressant actions of scopolamine: ACh-M1 receptor regulation of BDNF release and TrkB signaling.
Scopolamine or VU0255035 blockade of ACh-M1 receptors located on GABAergic interneurons results in disinhibition and a burst of glutamate that activates AMPA receptors. This leads to stimulation of voltage dependent calcium channels (VDCC) and influx of Ca2+ that activates BDNF release, which then stimulates TrkB signaling, including activation of the ERK1/2-mTORC1 pathway. This pathway controls the translation and synthesis of synaptic proteins (such as GluA1 and PSD95), which are required for the formation of new synapses, which is associated with the rapid antidepressant actions of scopolamine. Numbers indicate points in these pathways that were targeted by mutant and pharmacological approaches, including analysis of (1) BDNF Met knock in mice in which BDNF release is blocked or infusion of BDNF neutralizing antibody, (2) VDCC inhibitor, verapamil, (3) selective TrkB inhibitor, Ana-12, (4) AMPA receptor antagonist, CNQX, (5) blockade of neuronal firing, TTX, and (6) a neuronal silencing agent, the GABAA receptor agonist muscimol.
Scopolamine fails to induce antidepressant responses in mice with a knockin of the BDNF Met allele, which blocks the processing and activity dependent release of mature BDNF. Moreover, neutralizing BDNF within the mPFC is sufficient to prevent the antidepressant behavioral actions of scopolamine, indicating that the release of BDNF in the mPFC is essential for the antidepressants actions of scopolamine. Previously, we showed that the rapid and long lasting synaptic and behavioral effects of scopolamine require stimulation of glutamate AMPA receptor and mTORC1 signaling (6). Interestingly, in vitro studies show that stimulation of AMPA receptors causes activity dependent BDNF release, which in turn leads to activation of TrkB and increased mTORC1-signaling (24). These findings are consistent with the hypothesis that scopolamine leads to increased glutamate-AMPA activation, activity dependent BDNF release, and induction of TrkB-mTORC1 signaling (Figure 6).
The results also demonstrate that pretreatment with verapamil prevents scopolamine-induced behavioral actions and activation of mTORC1 signaling, indicating that stimulation of AMPA receptors and subsequent depolarization-induced activation of L-type calcium channels underlies scopolamine-induced BDNF release. These findings, combined with results demonstrating blockade of scopolamine responses in BDNF Met knockin mice indicate that processing and release of BDNF are required for the antidepressant actions of scopolamine. This possibility is further supported by studies demonstrating that scopolamine stimulates BDNF release in primary cultured neurons, and this effect is blocked by incubation with an AMPA receptor antagonist (CNQX), a GABAA receptor agonist (muscimol), a glutamate release inhibitor (TTX), or a VDCC blocker (verapamil). Consistent with our previous studies (16, 17), these blockers alone did not influence basal BDNF release, demonstrating that activation of glutamate AMPA receptors and L-type VDCCs represent integral, cellular determinants of scopolamine-stimulated BDNF release and antidepressant responses (Figure 6). These findings are consistent with studies of ketamine that also show a requirement for BDNF release and TrkB-mTORC1 signaling (13, 16) demonstrating convergence of these pathways for rapid acting antidepressants (Figure 6).
Scopolamine is a non-selective antagonist that acts on all five muscarinic receptor subtypes (30). We previously demonstrated that VU0255035, a muscarinic receptor antagonist with much higher selectivity for M1-AChR (∼70 fold) (26, 31), produces antidepressant behavioral responses in the FST and stimulates mTORC1 signaling similar to scopolamine (7). Here we demonstrate that VU0255035 rapidly stimulates BDNF release in cortical neurons. Collectively, these studies support the hypothesis that selective M1-AChR blockade produces effects similar to scopolamine and could be useful for the treatment of depression. However, BDNF loss of function studies are needed to further verify the necessity of BDNF release in mediating the antidepressant effects of M1 selective blockade. Although M1-AChR blockade could have side effects, notably memory impairment reported in rats (32), there is evidence that VU0255035 does not cause cognitive deficits (26). Moreover, only very low, intermittent dosing is required to produce long lasting synaptic and antidepressant behavioral responses. In any case, VU0255035 may useful pharmacological tool for further studies of the cellular basis of the antidepressant actions of muscarinic receptor blockade.
The requirement for AMPA receptor activity in scopolamine- and VU0255035-stimulation of BDNF release indicates that these agents increase glutamate release and neuronal depolarization. Increased release of glutamate could occur via blockade of tonic firing GABA neurons and the resulting disinhibition of glutamate transmission (9, 11). The presence of GAD67-immunolabeled GABA neurons in the primary cortical cultures is consistent with the possibility that disinhibition underlies scopolamine-stimulated BDNF release. In agreement with previous reports (33-35), our results show cortical neurons contains ChAT-positive cells, and that ChAT co-localizes with GABA interneurons (36). GABA interneuron subtypes in the mPFC show disparate M1-AChR expression and differential responses to cholinergic stimulation that are thought to be important determinants of cortical network activity (37, 38), could therefore influence the actions of scopolamine. This possibility is further supported by recent studies demonstrating that M1-AChRs on somatostatin (SST)-positive-GABAergic interneurons in the mPFC are required for the rapid antidepressant effects of scopolamine (8). Importantly, SST interneurons in the mPFC are reported to be vulnerable in conditions of decreased BDNF support (39), and it is possible that scopolamine-mediated BDNF release may provide neurotrophic support not only for pyramidal neuron, but also for SST interneurons to restore GABA synthesis and signaling. Together these findings raise the possibility that BDNF signaling on SST GABA interneurons also play an integral role in mediating the action of scopolamine.
Neuroimaging studies show that depressed patients have reduced gray matter volume and altered blood flow in PFC (40), and rodent chronic stress models consistently report reductions in dendrite complexity and spine synapse number and function in mPFC (9). Conversely, scopolamine and other rapid acting antidepressants (i.e., ketamine) increase spine synapse number and function in the mPFC (9). The ability of rapid-acting antidepressants to cause fast release of BDNF could contribute to increased synapse formation in vivo. Our studies in primary neuronal cultures are consistent with this hypothesis demonstrating that scopolamine increases dendrite complexity. In the mPFC, scopolamine- and ketamine-induction of spines is reported in glutamatergic pyramidal neurons that are identified by electrophysiological characteristics, and that show corresponding increases in synaptic function. We have reported that blockade of ketamine-induction of spine number and function also blocks antidepressant behavioral responses (13). Together these data indicate that increased spine number and function are associated with the rapid action of scopolamine, and is dependent on BDNF-TrkB signaling in primary neuronal cultures. However, similar studies showing that blockade of scopolamine-induction of synapse formation also block antidepressant behavioral responses are required to provide further evidence of this association for scopolamine.
Conclusions
Collectively, the data point to convergent cellular and molecular mechanisms that underlie the actions of scopolamine and VU0255035, as well as ketamine, demonstrating that BDNF release is required for the rapid antidepressant effects of these agents. These results also provide evidence that increased BDNF release differentiates the rapid actions of scopolamine and ketamine from conventional antidepressants (e.g., monoamine reuptake inhibitors), which increase the expression but not the release of BDNF (9, 11). Recent studies have demonstrated that activity-dependent, postsynaptic release of BDNF functions in an autocrine manner to enhance synapse formation (41). Moreover, BDNF could be released from presynaptic sites that originate from neurons in other regions, including subcortical sites. Additional studies will be needed to further elucidate the role of pre- vs. postsynaptic release of BDNF in the synaptic and antidepressant behavioral actions of scopolamine and other rapid-acting antidepressants.
Supplementary Material
Supplementary Figure S1. TrkB and ERK1/2 phosphorylation after scopolamine administration in BDNF mutant mice. BDNF WT and BDNF Met/Met mice (n=3-5/group) were administered scopolamine (25 μg/kg), and pTrkB and pERK 1/2 protein levels were assessed. BDNF Met/Met mice showed significantly less pTrkB and pERK 1/2 protein levels as compared to littermate WT mice. (WT vs. BDNF met/met p<0.05 for both pTrkB and pERK). Data are represented as mean ± SEM.
Supplementary Figure S2. BDNF protein levels within the prefrontal cortex 1 hour after scopolamine administration in wild type mice. Wild type (C57/BL6) mice (n=3-4/group) were administered scopolamine (25 μg/kg), and BDNF protein levels were detected by western blots. There was a main effect of drug (F(1,12)= 4.89, p=0.04). Multiple comparisons revealed that scopolamine treatment increased levels of the mature from of BDNF (14 KD band using rabbit anti BDNF antibody (N-20), Santacruz Biotechnology). Scopolamine did not change proBDNF (32 KD band, also detected by the N-20 antibody) (WT saline vs. WT scop p<0.01). Blots were reprobed for levels of β-actin and the results are represented as mean ± SEM., over β-actin.
Supplementary Figure S3. Phosphorylation of ERK 1 (44 KD) vs ERK 2 (42 KD) within the prefrontal cortex 1 hour after scopolamine administration. Phosphorylation of ERK 1 (44 KD) vs. ERK 2 (42 KD) was detected by western blots with the entire PFC 1 hour after scopolamine (25 μg/kg) administration. The phosphorylation sites that were tested for ERK1/2 were (T202/Y204) (Cell Signaling). Scopolamine administration produces a significant increase in levels of phospho-ERK 1 and ERK 2. Blots were reprobed for levels of total ERK (Cell Signaling) and the results are represented as mean ± SEM., over total ERK 1 or ERK 2 (n= 9-10 /group).
Acknowledgments
This research was supported by NIMH Grants MH045481 and MH093897 to R.S.D and the State of CT. We would like to thank Xiao-Yuan Li for excellent technical assistance.
Footnotes
Author Contributions: S.G., E.B., and R.S.D. designed research studies; S.G., E.B., W.Y., B.H., A.E.L., and M.J.G. conducted experiments and acquired data; R.S.D. provided resources and materials; S.G., E.B., A.E.L., and R.S.D. analyzed data and wrote the manuscript.
Conflict of Interest and Disclosures: R.S.D. has received consulting fees from Taisho, Johnson & Johnson, and Naurex, and grant support from Taisho, Johnson & Johnson, Naurex, Allergan, Navitor, Lundbeck, and Lilly. SG, EB, WY, BH, AEL, MJG, report no biomedical financial interests or potential conflicts of interest. None of the above-listed companies or funding agencies have had any influence on the content of this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 2.Fournier JC, DeRubeis RJ, Hollon SD, Dimidjian S, Amsterdam JD, Shelton RC, et al. Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA. 2010;303:47–53. doi: 10.1001/jama.2009.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papakostas GI. Limitations of contemporary antidepressants: tolerability. J Clin Psychiatry. 2007;68(Suppl 10):11–17. [PubMed] [Google Scholar]
- 4.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 5.Furey ML, Drevets WC. Antidepressant efficacy of the antimuscarinic drug scopolamine: a randomized, placebo-controlled clinical trial. Arch Gen Psychiatry. 2006;63:1121–1129. doi: 10.1001/archpsyc.63.10.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Voleti B, Navarria A, Liu RJ, Banasr M, Li N, Terwilliger R, et al. Scopolamine rapidly increases mammalian target of rapamycin complex 1 signaling, synaptogenesis, and antidepressant behavioral responses. Biol Psychiatry. 2013;74:742–749. doi: 10.1016/j.biopsych.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Navarria A, Wohleb ES, Voleti B, Ota KT, Dutheil S, Lepack AE, et al. Rapid antidepressant actions of scopolamine: Role of medial prefrontal cortex and M1-subtype muscarinic acetylcholine receptors. Neurobiol Dis. 2015;82:254–261. doi: 10.1016/j.nbd.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wohleb ES, Wu M, Gerhard DM, Taylor SR, Picciotto MR, Alreja M, et al. GABA interneurons mediate the rapid antidepressant-like effects of scopolamine. J Clin Invest. 2016;126:2482–2494. doi: 10.1172/JCI85033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duman RS, Aghajanian GK, Sanacora G, Krystal JH. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med. 2016;22:238–249. doi: 10.1038/nm.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duman RS, Aghajanian GK. Neurobiology of rapid acting antidepressants: role of BDNF and GSK-3beta. Neuropsychopharmacology. 2014;39:233. doi: 10.1038/npp.2013.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu RJ, Lee FS, Li XY, Bambico F, Duman RS, Aghajanian GK. Brain-derived neurotrophic factor Val66Met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biol Psychiatry. 2012;71:996–1005. doi: 10.1016/j.biopsych.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 16.Lepack AE, Fuchikami M, Dwyer JM, Banasr M, Duman RS. BDNF Release Is Required for the Behavioral Actions of Ketamine. Int J Neuropsychopharmacol. 2014;18(1):1–6. doi: 10.1093/ijnp/pyu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laje G, Lally N, Mathews D, Brutsche N, Chemerinski A, Akula N, et al. Brain-derived neurotrophic factor Val66Met polymorphism and antidepressant efficacy of ketamine in depressed patients. Biol Psychiatry. 2012;72:e27–28. doi: 10.1016/j.biopsych.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lepack AE, Bang E, Lee B, Dwyer JM, Duman RS. Fast-acting antidepressants rapidly stimulate ERK signaling and BDNF release in primary neuronal cultures. Neuropharmacology. 2016;111:242–252. doi: 10.1016/j.neuropharm.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cazorla M, Premont J, Mann A, Girard N, Kellendonk C, Rognan D. Identification of a low-molecular weight TrkB antagonist with anxiolytic and antidepressant activity in mice. J Clin Invest. 2011;121:1846–1857. doi: 10.1172/JCI43992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang C, Shirayama Y, Zhang Jc, Ren Q, Yao W, Ma M, et al. R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl Psychiatry. 2015;5 doi: 10.1038/tp.2015.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dwyer JM, Maldonado-Aviles JG, Lepack AE, DiLeone RJ, Duman RS. Ribosomal protein S6 kinase 1 signaling in prefrontal cortex controls depressive behavior. Proc Natl Acad Sci U S A. 2015;112:6188–6193. doi: 10.1073/pnas.1505289112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borroni AM, Fichtenholtz H, Woodside BL, Teyler TJ. Role of voltage-dependent calcium channel long-term potentiation (LTP) and NMDA LTP in spatial memory. J Neurosci. 2000;20:9272–9276. doi: 10.1523/JNEUROSCI.20-24-09272.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jourdi H, Hsu YT, Zhou M, Qin Q, Bi X, Baudry M. Positive AMPA receptor modulation rapidly stimulates BDNF release and increases dendritic mRNA translation. J Neurosci. 2009;29:8688–8697. doi: 10.1523/JNEUROSCI.6078-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Witkin JM, Overshiner C, Li X, Catlow JT, Wishart GN, Schober DA, et al. M1 and m2 muscarinic receptor subtypes regulate antidepressant-like effects of the rapidly acting antidepressant scopolamine. J Pharmacol Exp Ther. 2014;351:448–456. doi: 10.1124/jpet.114.216804. [DOI] [PubMed] [Google Scholar]
- 26.Sheffler DJ, Williams R, Bridges TM, Xiang Z, Kane AS, Byun NE, et al. A novel selective muscarinic acetylcholine receptor subtype 1 antagonist reduces seizures without impairing hippocampus-dependent learning. Molecular pharmacology. 2009;76:356–368. doi: 10.1124/mol.109.056531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kato-Negishi M, Muramoto K, Kawahara M, Kuroda Y, Ichikawa M. Developmental changes of GABAergic synapses formed between primarycultured cortical neurons. Brain Res Dev Brain Res. 2004;152:99–108. doi: 10.1016/j.devbrainres.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 28.Weir K, Blanquie O, Kilb W, Luhmann H, Sinning A. Comparison of spike parameters from optically identified GABAergic and glutamatergic neurons in sparse cortical cultures. Front Cell Neurosci. 2015;8:460. doi: 10.3389/fncel.2014.00460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Obrietan K, van den Pol A. GABAB receptor-mediated inhibition of GABAA receptor calcium elevations in developing hypothalamic neurons. J Neurophysiol. 1998;79:1360–1370. doi: 10.1152/jn.1998.79.3.1360. [DOI] [PubMed] [Google Scholar]
- 30.Golding JF, Stott JR. Comparison of the effects of a selective muscarinic receptor antagonist and hyoscine (scopolamine) on motion sickness, skin conductance and heart rate. Br J Clin Pharmacol. 1997;43:633–637. doi: 10.1046/j.1365-2125.1997.00606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiang Z, Thompson AD, Jones CK, Lindsley CW, Conn PJ. Roles of the M1 muscarinic acetylcholine receptor subtype in the regulation of basal ganglia function and implications for the treatment of Parkinson's disease. The Journal of pharmacology and experimental therapeutics. 2012;340:595–603. doi: 10.1124/jpet.111.187856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roldan G, Bolanos-Badillo E, Gonzalez-Sanchez H, Quirarte GL, Prado-Alcala RA. Selective M1 muscarinic receptor antagonists disrupt memory consolidation of inhibitory avoidance in rats. Neuroscience letters. 1997;230:93–96. doi: 10.1016/s0304-3940(97)00489-8. [DOI] [PubMed] [Google Scholar]
- 33.Kasashima S, Kawashima A, Muroishi Y, Futakuchi H, Nakanishi I, Oda Y. Neurons with choline acetyltransferase immunoreactivity and mRNA are present in the human cerebral cortex. Histochem Cell Biol. 1999;111:197–207. doi: 10.1007/s004180050349. [DOI] [PubMed] [Google Scholar]
- 34.Lewis DA. Distribution of choline acetyltransferase-immunoreactive axons in monkey frontal cortex. Neuroscience. 1991;40:363–374. doi: 10.1016/0306-4522(91)90126-9. [DOI] [PubMed] [Google Scholar]
- 35.Mechawar N, Descarries L. The cholinergic innervation develops early and rapidly in the rat cerebral cortex: a quantitative immunocytochemical study. Neuroscience. 2001;108:555–567. doi: 10.1016/s0306-4522(01)00389-x. [DOI] [PubMed] [Google Scholar]
- 36.Kosaka T, Tauchi M, Dahl J. Cholinergic neurons containing GABA-like and/or glutamic acid decarboxylase-like immunoreactivities in various brain regions of the rat. Exp Brain Res. 1988;70:605–617. doi: 10.1007/BF00247609. [DOI] [PubMed] [Google Scholar]
- 37.Kawaguchi Y. Selective cholinergic modulation of cortical GABAergic cell subtypes. J Neurophysiol. 1997;78:1743–1747. doi: 10.1152/jn.1997.78.3.1743. [DOI] [PubMed] [Google Scholar]
- 38.Neske GT, Patrick SL, Connors BW. Contributions of diverse excitatory and inhibitory neurons to recurrent network activity in cerebral cortex. J Neurosci. 2015;35:1089–1105. doi: 10.1523/JNEUROSCI.2279-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tripp A, Oh H, Guilloux JP, Martinowich K, Lewis DA, Sibille E. Brain-derived neurotrophic factor signaling and subgenual anterior cingulate cortex dysfunction in major depressive disorder. Am J Psychiatry. 2012;169:1194–1202. doi: 10.1176/appi.ajp.2012.12020248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harward SC, Hedrick NG, Hall CE, Parra-Bueno P, Milner TA, Pan EH, et al. Autocrine BDNF-TrkB signalling within a single dendritic spine. Nature. 2016;538:99. doi: 10.1038/nature19766. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1. TrkB and ERK1/2 phosphorylation after scopolamine administration in BDNF mutant mice. BDNF WT and BDNF Met/Met mice (n=3-5/group) were administered scopolamine (25 μg/kg), and pTrkB and pERK 1/2 protein levels were assessed. BDNF Met/Met mice showed significantly less pTrkB and pERK 1/2 protein levels as compared to littermate WT mice. (WT vs. BDNF met/met p<0.05 for both pTrkB and pERK). Data are represented as mean ± SEM.
Supplementary Figure S2. BDNF protein levels within the prefrontal cortex 1 hour after scopolamine administration in wild type mice. Wild type (C57/BL6) mice (n=3-4/group) were administered scopolamine (25 μg/kg), and BDNF protein levels were detected by western blots. There was a main effect of drug (F(1,12)= 4.89, p=0.04). Multiple comparisons revealed that scopolamine treatment increased levels of the mature from of BDNF (14 KD band using rabbit anti BDNF antibody (N-20), Santacruz Biotechnology). Scopolamine did not change proBDNF (32 KD band, also detected by the N-20 antibody) (WT saline vs. WT scop p<0.01). Blots were reprobed for levels of β-actin and the results are represented as mean ± SEM., over β-actin.
Supplementary Figure S3. Phosphorylation of ERK 1 (44 KD) vs ERK 2 (42 KD) within the prefrontal cortex 1 hour after scopolamine administration. Phosphorylation of ERK 1 (44 KD) vs. ERK 2 (42 KD) was detected by western blots with the entire PFC 1 hour after scopolamine (25 μg/kg) administration. The phosphorylation sites that were tested for ERK1/2 were (T202/Y204) (Cell Signaling). Scopolamine administration produces a significant increase in levels of phospho-ERK 1 and ERK 2. Blots were reprobed for levels of total ERK (Cell Signaling) and the results are represented as mean ± SEM., over total ERK 1 or ERK 2 (n= 9-10 /group).