Abstract
The most important margin in determining the prognosis of rectal cancer is circumferential resection margin (CRM). But, the type of surgery is determined by distal rectal margin (DRM), whether sphincter saving procedure is possible or patient needs an abdominoperineal resection. There are no standardized uniform guidelines for measurement of DRM. The purpose of this study is to assess the distal microscopic spread beyond gross margin after neoadjuvant concurrent chemoradiation (CCRT) in rectal cancers, the factors influencing the distal microscopic spread, the shrinkage of the distal margin in pinned and unpinned fresh and fixed specimen, and to find out the best method of measurement of distal rectal margin. A prospective analytical study was conducted from May 2013 through February 2015 in 47 cases of carcinoma rectum (both AR and APR) who had received neoadjuvant CCRT. Fresh specimen was collected within 30 min of specimen retrieval and a longitudinal cut was made in the distal margin of all specimens. One side of the specimen was pinned onto a cork board and the other side was left unpinned. Measurements were made from the distal end of clinical gross tumor. DRM was determined in both pinned and unpinned sides in fresh and fixed specimen. Of the 47 patients, 2 patients (4.2%) had small focus of tumor beyond gross margins, 1 at 6 mm and another at 3.5 mm on the unpinned side. The average margin for fresh and fixed pinned specimens was 3.67 and 3.47 cm, respectively, with percentage shrinkage of 5.4% for the pinned specimens. The average margin for fresh and fixed unpinned specimens was 3.32 and 2.84 cm, respectively, with percentage shrinkage of 14.4% for the unpinned specimens. Six patients (12.7%) had complete pathological response. Correlation of distal margin was better in pinned specimen. A correction factor of 15% for shrinkage needs to be taken into account while assessing unpinned specimen. Only in 4.2% of patients, there was distal submucosal spread beyond gross margin. Long-term follow up is required for assessing adequacy of DRM post neoadjuvant CCRT.
Keywords: Distal rectal margin, Pinned and unpinned specimen, Formaline fixation
Introduction
The success of management in locally advanced rectal cancer depends upon neoadjuvant treatment followed by a good surgery and adjuvant treatment based on the pathological stage. In patients with rectal cancer, good surgery consists of adequacy of the distal rectal margin which is in turn dependent on the risk for intramural tumor spread, on the distal mesorectal lymphatic spread and good circumferential margin. Tumor cell deposits within mesorectal lymph nodes have been identified up to 5 cm distal to the inferior aspect of the tumor, emphasizing the need to adhere to the principles of total mesorectal excision and giving rise to the concept of tumor-specific mesorectal excision (mesorectal transection 5 cm distal to the inferior border of the tumor) for more proximal rectal cancers [1–3]. In such circumstances, ensuring an adequate distal margin does not jeopardize the potential for sphincter preservation. The quality of the surgical technique and the status of the circumferential radial margin (CRM) is one of most important predictive factors for both local and distant recurrence as well as survival [4–6]. For patients with low-lying tumors treated with total mesorectal excision, the primary concern in the absence of lateral or inguinal lymphatic metastases is distal intramural spread. Here, the clinical evidence is less clear regarding what constitutes an adequate distal margin, particularly in the setting of neoadjuvant chemoradiotherapy [7–9]. The method in which the distal rectal margin (DRM) has been measured, i.e., fresh specimen or a formalin-fixed specimen and pinned stretched or unpinned specimen, has not been standardized. The purpose of this study is to assess the distal microscopic intramural spread beyond gross margins after neoadjuvant chemoradiation, the factors influencing the distal microscopic spread, the distal margin in pinned and unpinned, fresh and fixed specimen, and to find out the best method of measurement.
Materials and Methods
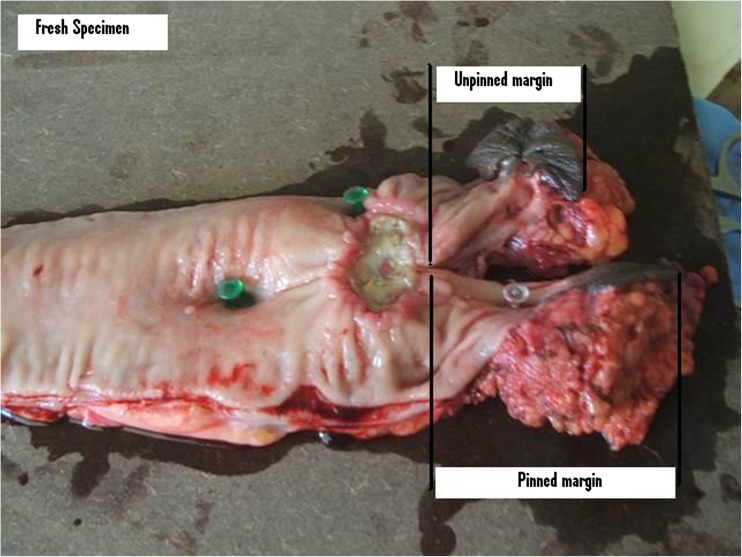
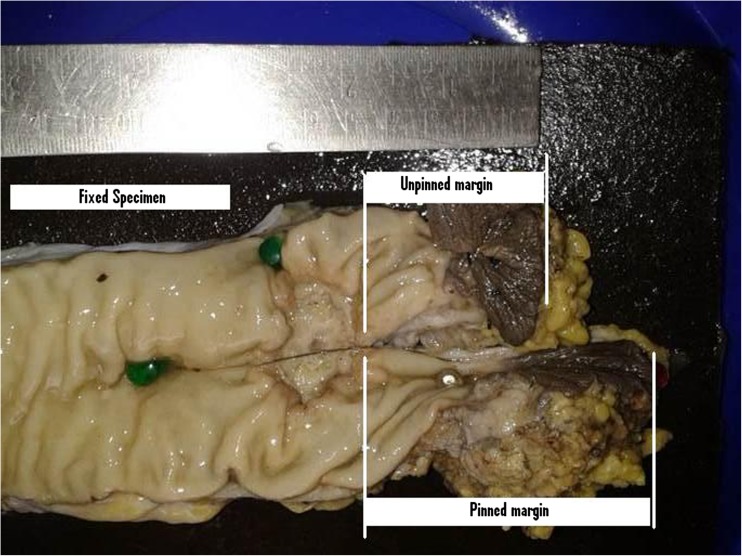
It was a prospective analytical study from May 2013 through Feb 2015 at Regional Cancer Centre Thiruvananthapuram Kerala India. Study population consisted of all cases of carcinoma rectum within 12 cm from anal verge, AJCC 7 stages II and III cancers operated in above period who had received neoadjuvant concurrent chemoradiation. A total of 47 patients underwent preoperative long-course radiotherapy (5040 cGy in 28 fractions) with concurrent fluorouracil (FU)-based chemotherapy for locally advanced (T3–4 and/or N+) rectal cancer. Chemotherapy was delivered according to one of the following regimens: (1) continuous infusion i.v. of 5-FU alone (225 mg/m2/day); (2) continuous infusion i.v. of 5-FU (225 mg/m2/day) plus a weekly bolus of oxaliplatin 60 mg/m2 for six times; and (3) oral capecitabine (1300 mg/m2/day). After 6 weeks post neoadjuvant concurrent chemoradiation, they underwent either anterior resection (AR) or abdominoperineal resection (APR). Complete clinical response post chemoradiation with no palpable tumor or residual scar were excluded from the study. Fresh specimen was collected within 30 min of specimen retrieval. A longitudinal cut was made in the distal margin of all specimens of AR and APR. One half side of the specimen was pinned onto a hard board and the other half side was left unpinned (Figs. 1 and 2). Measurements were made from the distal end of clinical gross tumor to the specimen edge. Distal rectal margin was recorded in both pinned and unpinned sides in both fresh and fixed specimen. Further distal mural spread was assessed with 2 mm cuts on either side for 1 cm.
Fig. 1.
Fresh specimen
Fig. 2.
Fixed specimen
Results
There were 25 (53.2%) males and 22 (46.8%) females with a total of 47 patients who underwent surgery for Ca rectum post CCRT of which 15 (31.9%), 9 (19.1%), and 22 (46.8%) of them underwent AR, LAR, and APR, respectively. There were 14 (29.8%) of them who were less than or equal to 50 years and 33 (70.2%) of them who were more than 50 years of age. Most common presenting symptom was bleeding per rectum in 25 (53.2%) patients and second most common presentation was altered bowel habits (40.4%). Twenty-five (53%) patients were having normal body mass index (18.5 to 24.9) followed by 11 (23.4%) patients who were overweight (25 to 29.9) and 4 (8.4%) patients who were obese (30 to 39.9). There were 7 (14.9) patients who were underweight (<18.5). The mean distance of the growth from the anal verge was 4.1 and 4.2 cm pre CCRT and post CCRT, respectively. Mean distance of the growth from the verge as measured by flexible endoscopy was 4.9 and 5.3 cm, respectively (Table 1).
Table 1.
Epidemiologic factors
| (%) | Total | |||
|---|---|---|---|---|
| Sex distribution | m 25(53.2) | f 22(46.8) | 47(100) | |
| Age | ≤50 years = 14 (29.8) | >50 years = 33 (70.2) | ||
| Type of surgery | AR = 15 (31.9) | LAR = 9 (19.1) | APR = 46.8 | |
| Presenting symptom | Bleeding PR = 25 (53.2) | Altered bowel habits = 19 (40.4) | ||
| BMI | <18.5 = 7 (14.9) | 18.5 to 24.9 = 25 (53) | 25 to 29.9 = 11 (23.4) | 30 to 39.9 = 4 (8.4) |
| Mean distance from anal verge on PR (cm) | Pre CCRT = 4.1 | Post CCRT = 4.2 | ||
| Flexible endoscopy mean distance from anal verge (cm) | Pre CCRT = 4.9 | Post CCRT = 5.3 | ||
When gross margins were measured from the cut margin of the specimen in the pinned side, the mean length was 3.67 cm in fresh and was 3.47 cm after 48 h of formalin fixation, respectively. The same margins when measured from cut margin of the specimen on unpinned side were 3.32 and 2.84 cm in length in fresh and fixed specimen, respectively. The percentage of shrinkage of the length of the margin was 5.4% on pinned side compared to 14.4% on the unpinned side due to formalin fixation. Two patients had small focus of tumor beyond gross margins, one at 6 mm and another at 3.5 mm on the unpinned side of the specimen accounting for 4.2% of distal mucosal spread. The CRM positivity was seen in 7 (15%) patients. There were 35 (74.5%) patients with moderately differentiated carcinoma and 6 (12.8%) patients with poorly differentiated carcinoma and complete pathological response each. Average number of lymph nodes harvested was 6 which ranged from 0 to 15 in number. There was complete pathological response in six (12.8%) patients. Tumor regression grade showed grade 0, 1, 2, and 3 in 6 (12.8%), 5 (10.6%), 15 (31.9%), and 21 (44.7%), respectively (Table 2).
Table 2.
Pathological factors
| Pinned margin (mean in cm) | Fresh = 3.67 | Fixed = 3.47 | ||
| Unpinned margin (mean in cm) | Fresh = 3.32 | Fixed = 2.84 | ||
| Percentage shrinkage | Fresh = 5.4 | Fixed = 14.4 | ||
| Positive CRM | 7 (15%) | |||
| Differentiation | MD = 35 (74.5) | PD = 6 (12.8) | pCR = 6 (12.8) | |
| Avg no of LNs | 6 | |||
| TRG | 0 = 6 (12.8) | 1 = 5 (10.6) | 2 = 15 (31.9) | 3 = 21 (44.7) |
Discussion
Traditionally, rectal cancer specimens were grossed with cut opening of the specimen longitude at uninvolved part of circumference or at 12 o’clock position when whole of circumference is involved. Quirke et al. [10] and Nagtegaal et al. [11] have developed an approach for the assessment and processing of the TME specimen which is most commonly followed now. In our institution, we still follow the traditional grossing method. Sondenna et al. [12, 13] compared DRM based on the different methods of measurement by pathologist. The margin was significantly less in unpinned specimen than the pinned specimen which shows the importance of processing the specimen for margin evaluation. The specimen was studied in non neoadjuvant treatment era. Here, we have tried to evaluate the DRM post CCRT and find the amount of shrinkage during fixation which may carry a major impact on DRM when the margins are close.
The amount of shrinkage of the colorectal specimen and its impact on DRM has been studied by Goldstein et al. [14]. After fixation, the segments of bowel shrank 57% of the in vivo length of which approximately 70% of the shrinkage occurred during the first 10 to 20 min after removal, and the remaining 30% occurred after fixation. They concluded that for optimal accuracy, margin distance must be obtained immediately after surgical removal. Once the specimen has been removed for several minutes, the difference between unfixed and fixed margin lengths is 30%. A correction factor of approximately 2× should be applied when interpreting the margin length. In our study, we have pinned the specimen on to the board within 30 min of specimen retrieval and have processed it and fixed to the board as done by Sondenna et al. [12] and measured the pinned and unpinned side, fresh, and fixed. The average shrinkage was 5% on the pinned side and 15% on the unpinned side after fixation. As Goldstein et al. [14] concluded, the fixation of fresh specimen within first 10 min would have increased discrepancy even more. But the amount of shrinkage was only 15% in our study compared to 30% in later half as shown by them.
There has been a lot of heterogeneity in recording of DRM in post CCRT ca rectum. As shown by systematic review by Bujko et al. [15], there have been four studies in which the method of measurement was pinned fixed, unpinned fixed, pathologist recorded, and not defined in each of them has been compared which makes it difficult to conclude especially for close margins <5 mm. In our observation, there is better correlation when the specimen is pinned and fixed for histopathology and if unpinned and fixed, a correction factor of 15% has to be taken into account for shrinkage. There has to be standard guidelines for proper fixation of the specimen and uniformity in reporting of the same.
Adequate DRM after CCRT is an ongoing debate. Bujko et al. [15] have published a systematic review consisting of 17 studies identified from search of PubMed database 1982 to 2011(TME era). The decisions of AR or APR had been based not only on the distance between tumor and anal sphincter but also on a variety of other clinical factors. There was inherent selection bias in favor of patients with short margins. Only one study was prospective, remaining were retrospective studies. Two studies gathered data from multicentre randomized studies. The rest of the studies presented single-center data. They concluded that findings support the practice of sphincter preservation in selected settings of close distal margins (<1 cm) after total mesorectal excision for distal rectal cancer. There was no statistically significant difference in either local control or survival with margins of <1 cm. Indeed, negative margin as close as ≤5 mm is acceptable. The precise rules for this selection had not been defined and therefore, they recommended further studies to identify the criteria for selecting patients to an approach of close distal margins for sphincter preservation.
Recently, the role of frozen section in evaluating the distal rectal margin has been studied by Gomes et al. [16] while performing sphincter-saving rectal surgery and to identify the subgroups that would benefit the most from intraoperative frozen section (IOFS) analysis. IOFS had a sensitivity of 85.17% with a specificity of 100% and a negative predictive value of 99.16%. Specimens with a positive IOFS were lower rectal (P < 0.05), poorly differentiated, and post CCRT locally advanced tumors. They concluded that IOFS to confirm negative DRM is recommended in lower rectal tumors. It can be considered for locally advanced post CCRT poorly differentiated mid-rectal tumors and avoided for upper rectal tumors.
Close margins as close as 2 mm pooled analysis from five studies have shown a local recurrence rate of 2.7% (95% CI 0 to 6.4) with no much change in long-term survival compared to larger margins [7, 8, 17–19]. Our study showed two (4.2%) patients with one at 6 mm and another at 3.5 mm; there was distal submucosal spread beyond gross margin which would be missed if 2 mm is taken as adequate margin. But long-term results are required of larger studies to know the outcome for <5 mm margins.
CRM involvement occurs at a rate ranging from 7.3 to 25% [20–22]. Many studies have demonstrated that CRM involvement is able to predict local recurrence and poor prognosis in patients with rectal cancer [10, 21–24]. In our study, CRM positivity was seen in seven (15%) patients.
Complete pathological response (CPR) of 15.6% was observed in systematic review conducted by Zorcolo et al. [25] in combined modality treatment after neoadjuvant CCRT which showed CPR for rectal cancer is associated with improved local and distal control as well as better OS and DFS, i.e., tumor regression grade (TRG) of 0 was seen in six (12.8%) patients in our study.
Conclusion
The measurement of DRM has to be standardized. Correlation of distal margin was better in pinned specimen. A correction factor of 15% for shrinkage needs to be taken into account while assessing unpinned specimen. Only in 4.2% of patients, there was distal submucosal spread beyond gross margin. Long-term follow up is required for assessing adequacy of DRM post neoadjuvant CCRT.
References
- 1.Scott N, Jackson P, Al-Jaberi T, Dixon MF, Quirke P, Finan PJ. Total mesorectal excision and local recurrence: a study of tumour spread in the mesorectum distal to rectal cancer. Br J Surg. 1995;82:1031–1033. doi: 10.1002/bjs.1800820808. [DOI] [PubMed] [Google Scholar]
- 2.Hida J, Yasutomi M, Maruyama T, Fujimoto K, Uchida T. Okuno lymph node metastases detected in the mesorectum distal to carcinoma of the rectum by the clearing method: justification of total mesorectal excision. J Am Coll Surg. 1997;184:584–588. [PubMed] [Google Scholar]
- 3.Reynolds JV, Joyce WP, Dolan J, Sheahan K, Hyland JM. Pathological evidence in support of total mesorectal excision in the management of rectal cancer. Br J Surg. 1996;83:1112–1115. doi: 10.1002/bjs.1800830826. [DOI] [PubMed] [Google Scholar]
- 4.Nagtegaal ID, Quirke P. What is the role for the circumferential margin in the modern treatment of rectal cancer? J Clin Oncol. 2008;26:303. doi: 10.1200/JCO.2007.12.7027. [DOI] [PubMed] [Google Scholar]
- 5.Quirke P, Durdey P, Dixon MF, Williams NS. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumour spread and surgical excision. Lancet. 1986;2:996. doi: 10.1016/S0140-6736(86)92612-7. [DOI] [PubMed] [Google Scholar]
- 6.Gosens MJ, Klaassen RA, Tan-Go I, et al. Circumferential margin involvement is the crucial prognostic factor after multimodality treatment in patients with locally advanced rectal carcinoma. Clin Cancer Res. 2007;13:6617. doi: 10.1158/1078-0432.CCR-07-1197. [DOI] [PubMed] [Google Scholar]
- 7.Nash GM, Weiss A, Dasgupta R, Gonen M, Guillem JG, Wong WD (2010) Close distal margin and rectal cancer recurrence after sphincter-preserving rectal resection. Dis Colon rectum 53:1365–1373 [DOI] [PubMed]
- 8.Kiran RP, Lian I, Lavery IC (2011) Does a subcentimeter distal resection margin adversely influence oncologic outcomes in patients with rectal cancer undergoing restorative proctectomy? Dis Colon rectum 54:157–163 [DOI] [PubMed]
- 9.Rutkowski A, Nowacki MP, Chwalinski M, et al. Acceptance of a 5 mm distal bowel resection margin for rectal cancer: is it safe? Color Dis. 2010 doi: 10.1111/j.1463-1318.2010.02542.x. [DOI] [PubMed] [Google Scholar]
- 10.Quirke P, Durdey P, Dixon MF, Williams NS. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumour spread and surgical excision. Lancet. 1986;2(8514):996–999. doi: 10.1016/S0140-6736(86)92612-7. [DOI] [PubMed] [Google Scholar]
- 11.Nagtegaal ID, van de Velde CJH, van der Worp E, Kapiteijn E, Quirke P, van Krieken JHJM. Macroscopic evaluation of rectal cancer resection specimen: clinical significance of the pathologist in quality control. J Clin Oncol. 2002;20(7):1729–1734. doi: 10.1200/JCO.2002.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Park IJ, Kim JC. Adequate length of the distal resection margin in rectal cancer: from the oncological point of view. J Gastrointest Surg. 2010;14(8):1331–1337. doi: 10.1007/s11605-010-1165-3. [DOI] [PubMed] [Google Scholar]
- 13.Deo SVS, Kapali AS, Gupta M, Shukla NK. A review of controversies in the management of colorectal cancers. The Indian Journal of Surgery. 2012;74(3):221–227. doi: 10.1007/s12262-012-0586-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldstein NS, Soman A, Sacksner J. Disparate surgical margin lengths of colorectal resection specimens between in vivo and in vitro measurements: the effects of surgical resection and formalin fixation on organ shrinkage. Am J Clin Pathol. 1999;111(3):349–351. doi: 10.1093/ajcp/111.3.349. [DOI] [PubMed] [Google Scholar]
- 15.Bujko K, Rutkowski A, Chang GJ, Michalski W, Chmielik E, Kusnierz J. Is the 1-cm rule of distal bowel resection margin in rectal cancer based on clinical evidence? A systematic review. Ann Surg Oncol. 2012;19(3):801–808. doi: 10.1245/s10434-011-2035-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gomes RM, Bhandare M, Desouza A, Bal M, Saklani AP. Role of intraoperative frozen section for assessing distal resection margin after anterior resection. Int J Color Dis. 2015;30(8):1081–1089. doi: 10.1007/s00384-015-2244-4. [DOI] [PubMed] [Google Scholar]
- 17.Rutkowski A, Bujko K, Nowacki MP, Chmielik E, Nasierowska-Guttmejer A, Wojnar A. Polish colorectal study group distal bowel surgical margin shorter than 1 cm after preoperative radiation for rectal cancer: is it safe? Ann Surg Oncol. 2008;15:3124–3131. doi: 10.1245/s10434-008-0125-6. [DOI] [PubMed] [Google Scholar]
- 18.Rutkowski A, Nowacki MP, Chwalinski M, et al. Acceptance of a 5 mm distal bowel resection margin for rectal cancer: is it safe? Color Dis. 2010 doi: 10.1111/j.1463-1318.2010.02542.x. [DOI] [PubMed] [Google Scholar]
- 19.Andreola S, Leo E, Belli F, et al. Adenocarcinoma of the lower third of the rectum surgically treated with a <10-mm distal clearance: preliminary results in 35 N0 patients. Ann Surg Oncol. 2001;8:611–615. doi: 10.1245/aso.2001.8.7.611. [DOI] [PubMed] [Google Scholar]
- 20.Hermanek P, Junginger T. The circumferential resection margin in rectal carcinoma surgery. Tech Coloproctol. 2005;9:193–199. doi: 10.1007/s10151-005-0226-1. [DOI] [PubMed] [Google Scholar]
- 21.Adam IJ, Mohamdee MO, Martin IG, Scott N, Finan PJ, Johnston D, et al. Role of circumferential margin involvement in the local recurrence of rectal cancer. Lancet. 1994;344:707–711. doi: 10.1016/S0140-6736(94)92206-3. [DOI] [PubMed] [Google Scholar]
- 22.Wibe A, Syse A, Andersen E, Tretli S, Myrvold HE, Søreide O (2004) Norwegian rectal cancer group. Oncological outcomes after total mesorectal excision for cure for cancer of the lower rectum: anterior vs. abdominoperineal resection. Dis Colon rectum 47:48–58 [DOI] [PubMed]
- 23.de Haas-Kock DF, Baeten CG, Jager JJ, Langendijk JA, Schouten LJ, Volovics A, et al. Prognostic significance of radial margins of clearance in rectal cancer. Br J Surg. 1996;83:781–785. doi: 10.1002/bjs.1800830617. [DOI] [PubMed] [Google Scholar]
- 24.Baik SH, Kim NK, Lee YC, Kim H, Lee KY, Sohn SK, et al. Prognostic significance of circumferential resection margin following total mesorectal excision and adjuvant chemoradiotherapy in patients with rectal cancer. Ann Surg Oncol. 2007;14:462–469. doi: 10.1245/s10434-006-9171-0. [DOI] [PubMed] [Google Scholar]
- 25.Zorcolo L, Rosman AS, Restivo A, Pisano M, Nigri GR, Fancellu A, Melis M. Complete pathologic response after combined modality treatment for rectal cancer and long-term survival: a meta-analysis. Ann Surg Oncol. 2012;19(9):2822–2832. doi: 10.1245/s10434-011-2209-y. [DOI] [PubMed] [Google Scholar]