Abstract
Malignant gastrointestinal neuroectodermal tumor (GNET) is a rare malignant tumor. It is also referred to as clear cell sarcoma-like gastrointestinal tumor (CCSLGT). It is an aggressive tumor with a high rate of local recurrence, metastases, and early death from disease. Its pathogenesis is not known. It shows evidence of neural differentiation and lacks immunohistochemical and ultrastructural evidence of melanocytic differentiation. It needs to be distinguished from various mimickers owing to its aggressive course. Herein, we report a case of GNET in a 55-year-old female patient.
Keywords: Clear cell sarcoma-like gastrointestinal tumor, CCSLGT, Gastrointestinal neuroectodermal tumor, GNET, Jejunum
Introduction
Malignant gastrointestinal neuroectodermal tumor (GNET) is an extremely rare tumor with only less than 40 reported cases. Previously GNET has been referred to as clear cell sarcoma-like gastrointestinal tumor (CCSLGT) [1, 2] or clear cell sarcoma-like tumor with osteoclast-like giant cells of the gastrointestinal tract [3]. Stockman et al. [1] reviewed largest series of these tumors in 2012 and suggested the term GNET, which is now a well-accepted terminology for this tumor.
It is an aggressive form of neuroectodermal tumor that should be differentiated from other primitive epithelioid and spindle cell tumors of gastrointestinal tract (GIT) [1]. The most important differential of GNET is the clear cell sarcoma of tendons and aponeuroses involving the GIT also known as clear cell sarcoma-gastrointestinal (CCS-GI). The two share similar morphological as well as molecular features and show S100 positivity. However, the distinctive ultrastructural characteristics and lack of melanocytic differentiation in GNET distinguish it from CCS-GI [2].
Because of the similarities between the two entities, it has been debated as to whether they might represent variants of the same entity [4]. However, more recent evidence points to GNET and CCS-GI representing two distinct tumor types [1].
Case Report
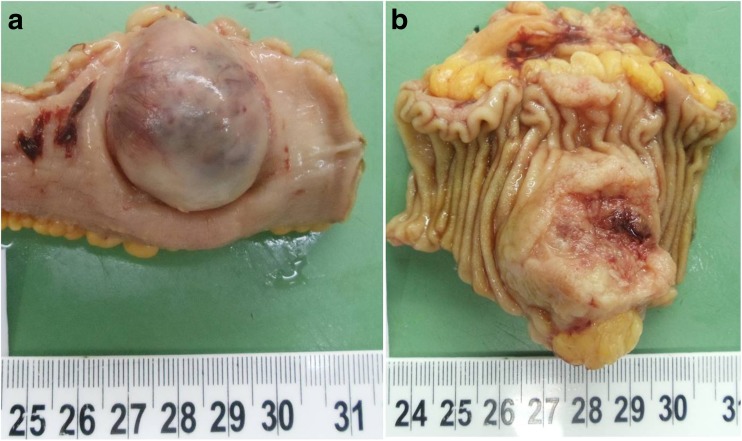
A 55-year-old lady presented to the GI surgery unit with abdominal distension and melena. On evaluation, she was found to have a jejunal mass on CECT. The tumor was resected and was sent to the histopathology department with a clinical diagnosis of gastrointestinal stromal tumor (GIST). We received an intestinal segment measuring 7 cm in length. Outer surface showed a nodular bulge with stretched out and congested serosa over it. No breach was present. Cut surface showed a nodular growth measuring 4.5 × 2.5 × 2 cm corresponding to the outer bosselated area [Fig. 1]. Tumor was present in the gut wall; overlying mucosa was stretched out and showed large areas of ulceration, hemorrhage, and necrosis. The resected ends were free.
Fig. 1.
a, b Nodular bosselation by the tumor over the serosal aspect (a). Mucosal aspect of the specimen showing the tumor growth (b)
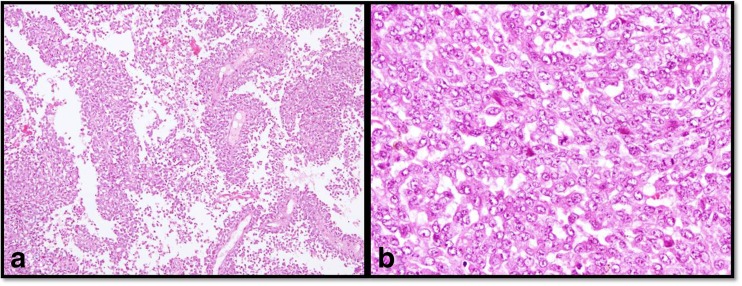
Microscopic examination revealed a poorly differentiated malignant tumor showing prominent pseudopapillary arrangement along with solid and nested pattern [Fig. 2a]. Tumor cells were large polygonal having eosinophilic to clear cytoplasm, ovoid nuclei with dispersed chromatin and conspicuous 1–2 nucleoli [Fig. 2b]. Focal spindle cell component with fascicular arrangement was also seen. Tumor was mitotically active (6–7/10hpf) and showed focal areas of necrosis. Overlying mucosa was largely ulcerated and tumor was present within the submucosa and muscularis propria of the gut.
Fig. 2.
a, b Tumor showing prominent pseudopapillary arrangement (a; H&E, ×10). Large polygonal cells arranged in solid pattern with ovoid nuclei, dispersed chromatin and conspicuous 1–2 nucleoli
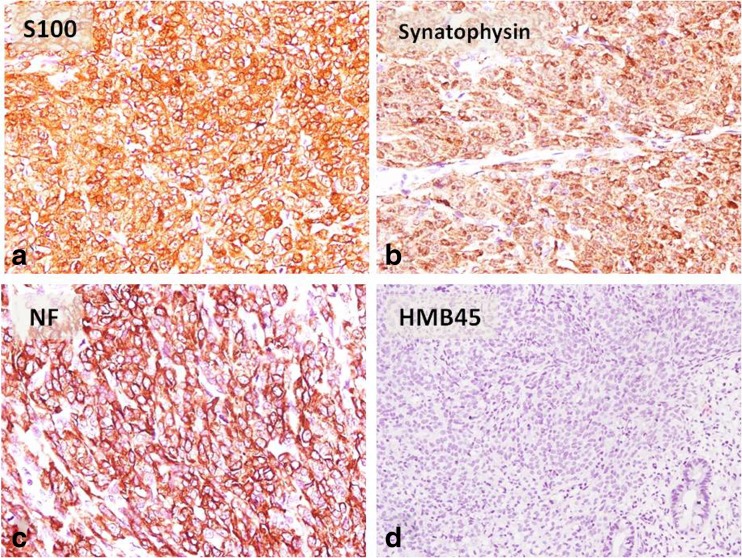
On immunohistochemistry (IHC), tumor cells were positive for vimentin, S100, synaptophysin, and neurofilament and were negative for CK, CD117, DOG-1, HMB 45, CD 34, SMA, and chromogranin [Fig. 3]. Ki67 proliferation index was 8–9%.
Fig. 3.
a–d On IHC, tumor cells were diffusely positive for S100 (a), synaptophysin (b), neurofilament (c), and negative for HMB45 (d)
Based on the microscopic and IHC findings, diagnosis of malignant GNET was made.
Discussion
GNET is a rare tumor and typically arises in young to middle-aged adults and most often involves small intestine, although any part of gastrointestinal tract may be affected [1]. Review of literature revealed reports of cases involving the stomach, ileum, jejunum, and colon [1, 3, 5–12].
Histologically, GNET is characterized by sheets or nests of primitive epithelioid-to-oval or occasionally spindle-shaped tumor cells. Pseudopapillary and alveolar formation is also seen. The tumor cells have moderate amount of clear to lightly eosinophilic cytoplasm. The nuclei are mostly centrally located and round with irregularly dispersed chromatin and either inapparent or small nucleoli, in most cases. Osteoclast type giant cells are a common feature and have been reported in 50% cases [1]. In our case, similar morphology was seen; however, no giant cells were present.
IHC profile is indicative of neural differentiation as evident by positive staining with S100 (100%), SOX 10 (100%), CD56 (70%), synaptophysin (56%), NB84 (50%), NSE (45%), and neurofilament (14%) [1]. They stain negative for melanocytic markers like HMB45, melan A, and tyrosinase. Ultrastructurally also they lack melanocytic differentiation and show features of neural differentiation, including multiple interdigitating cell processes containing dense core granules and clear vesicles resembling synaptic bulbs [1–3].
Antonescu et al. (2006) initially suggested a gastrointestinal neuroectodermal precursor cell as the cell of origin of GNET that has lost the potential to differentiate along the melanocytic lineage [5]. However, recently Stockman et al. (2012) suggested origin of these tumors from an autonomic nervous system-related primitive cell of neural crest derivation that shows a neural line of differentiation with complete absence of melanocytic features. [1].
At the molecular genetic level, GNET is associated with EWSR1 gene rearrangements, which results in the fusion of EWSR1 and ATF1, or EWSR1 and CREB1 [1, 5]. However, molecular genetic rearrangements are not regarded as diagnostic or pathognomic of these tumors as are known to be present in several other tumors including CCS-GI [1].
GNET should be suspected in any tumor arising in the wall of the gastrointestinal tract (GIT) displaying an epithelioid or spindle cell population. Morphological differentials include CCS-GI, metastatic melanoma, gastrointestinal stromal tumor (GIST) and synovial sarcoma and metastatic clear cell carcinoma.
GNET is often difficult to distinguish from CCS-GI because they share some morphological features, such as clear to lightly eosinophilic cytoplasm and a tendency toward nested growth. Presence of features like sheet like, pseudoalveolar or pseudopapillary growth pattern, inconspicuous or small nucleoli and osteoclast like giant cells are more indicative of GNET whereas more uniform appearance, with cells arranged in defined nests separated by thin fibrous septa, that are relatively monotonous, and contain macronucleoli point toward CCS-GI. Immunohistochemically, both express S100, however differ in expression of melanocytic markers which are positive in CCS-GI and characteristically negative in GNET. Ultrastructurally, also CCS-GI shows melanocytic differentiation (melanosomes). Melanocytic differentiation has never been reported in GNETs. At molecular level, both harbor EWSR1 gene rearrangements and cannot be distinguished on this basis. Prognostically, GNET is a more aggressive tumor with poor outcome in comparison to CCS-GI [1, 5, 13].
Malignant melanoma is differentiated on the basis of immunohistochemical markers. These are positive for HMB45 or Melan-A and exhibit no EWSR1 mutations. Instead, they show BRAF mutations [1, 14].
GIST enters the differential diagnoses because of its intramural location and similar histological features. Positive immunostaining with CD117, DOG1, and/or CD34 is reliable to diagnose GIST. These markers are universally negative in GNET. It is important to differentiate above two because effective target therapy for GIST is available. Also GNET is associated with a poorer prognosis and aggressive course [15].
Monophasic synovial sarcoma, though rare in the gastrointestinal wall, histologically resemble GNET. Since S100 has been reported in 30% of synovial sarcoma of soft tissue, epithelial markers should be done to rule it out. GNET stain negative for epithelial markers like CK and EMA. Also synovial sarcoma shows characteristic SYT rearrangement in t(X;18), unlike GNET which demonstrates rearrangements of EWSR1 gene [16].
Metastatic clear cell carcinoma, generally those of the kidney or ovary, resemble GNET morphologically; however, they stain for epithelial markers like cytokeratin and EMA [13].
There is no consensus regarding the mode of treatment. However, the most common treatment for patients with GNET is excision of the tumor. Metastases and recurrence have been reported after surgical excision of the tumor. One of the case reports describes the use of chemotherapy post surgical resection [5]. GNET is a rare and recently characterized neoplasm, which typically behaves in an aggressive manner with a high rate of local recurrence, metastases (often at presentation), and early death from disease [2, 3, 5].
EWSR1-CREB1 activates the melanocyte transcription factor MITF, which in turn activates transcription of c-MET, an oncogenic receptor tyrosine kinase recently shown to be activated in clear cell sarcoma and GNET [17]. Inhibitors of MET are currently being studied in early clinical trials [18]. As yet there is no consensus on the use or benefit of adjuvant or targeted therapies.
Considering the limited literature available on GNET and rarity of this neoplasm documentation of any such case is important to increase the data base. It also seems likely that the true incidence of GNET is underrepresented because it has been misdiagnosed due to unfamiliarity of surgical pathologists with the entity and morphological overlap with other tumors in intra-abdominal sites.
A combined approach utilizing a comprehensive panel of immunohistochemical markers along with molecular and ultrastructural analyses is suggested for identification of this rare aggressive tumor and distinguishing it from its mimickers.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Stockman DL, Miettinen M, Suster S, Spagnolo D, Malagon HD, Hornick JL, et al. Malignant gastrointestinal neuroectodermal tumor: clinicopathologic, immunohistochemical, ultrastructural, and molecular analysis of 16 cases with a reappraisal of clear cell sarcoma-like tumors of the gastrointestinal tract. Am J Surg Pathol. 2012;36:857–868. doi: 10.1097/PAS.0b013e31824644ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kosemehmetoglu K, Folpe AL. Clear cell sarcoma of tendons and aponeuroses, and osteoclast-rich tumour of the gastrointestinal tract with features resembling clear cell sarcoma of soft parts: a review and update. J Clin Pathol. 2010;63:416–423. doi: 10.1136/jcp.2008.057471. [DOI] [PubMed] [Google Scholar]
- 3.Huang W, Zhang X, Li D, Chen J, Meng K, Wang Y, et al. Osteoclast-rich tumor of the gastrointestinal tract with features resembling those of clear cell sarcoma of soft parts. Virchows Arch. 2006;448:200–203. doi: 10.1007/s00428-005-0051-y. [DOI] [PubMed] [Google Scholar]
- 4.Rosai J. Editorial: clear cell sarcoma and osteoclast-rich clear cell sarcoma-like tumor of the gastrointestinal tract: one tumor type or two?: melanoma or sarcoma? Int J Surg Pathol. 2005;13:309–311. doi: 10.1177/106689690501300401. [DOI] [PubMed] [Google Scholar]
- 5.Antonescu CR, Nafa K, Segal NH, Dal Cin P, Ladanyi M. EWS-CREB1: a recurrent variant fusion in clear cell sarcoma—association with gastrointestinal location and absence of melanocytic differentiation. Clin Cancer Res. 2006;12:5356–5362. doi: 10.1158/1078-0432.CCR-05-2811. [DOI] [PubMed] [Google Scholar]
- 6.Balkaransingh P, Saad SA, Govil SC, Thind PK, Ballance CM, Weiss AR. Clear cell sarcoma of the gastrointestinal tract presenting as a second malignant neoplasm following neuroblastoma in infancy. Pediatr Blood Cancser. 2012;58:481–482. doi: 10.1002/pbc.23330. [DOI] [PubMed] [Google Scholar]
- 7.Comin CE, Novelli L, Tornaboni D, Messerini L. Clear cell sarcoma of the ileum: report of a case and review of literature. Virchows Arch. 2007;451:839–845. doi: 10.1007/s00428-007-0454-z. [DOI] [PubMed] [Google Scholar]
- 8.Donner LR, Trompler RA, Dobin S. Clear cell sarcoma of the ileum: the crucial role of cytogenetics for the diagnosis. Am J Surg Pathol. 1998;22:121–124. doi: 10.1097/00000478-199801000-00016. [DOI] [PubMed] [Google Scholar]
- 9.Friedrichs N, Testi MA, Moiraghi L, Modena P, Paggen E, Plötner A, et al. Clear cell sarcoma-like tumor with osteoclast-like giant cells in the small bowel: further evidence for a new tumor entity. Int J Surg Pathol. 2005;13:313–318. doi: 10.1177/106689690501300402. [DOI] [PubMed] [Google Scholar]
- 10.Lagmay JP, Ranalli M, Arcila M, Baker P. Clear cell sarcoma of the stomach. Pediatr Blood Cancer. 2009;53:214–216. doi: 10.1002/pbc.22014. [DOI] [PubMed] [Google Scholar]
- 11.Pauwels P, Debiec-Rychter M, Sciot R, Vlasveld T, den Butter B, Hagemeijer A, et al. Clear cell sarcoma of the stomach. Histopathology. 2002;41:526–530. doi: 10.1046/j.1365-2559.2002.01509.x. [DOI] [PubMed] [Google Scholar]
- 12.Shenjere P, Salman WD, Singh M, Mangham DC, Williams A, Eyden BP, et al. Intra-abdominal clear-cell sarcoma: a report of 3 cases, including 1 case with unusual morphological features, and review of the literature. Int J Surg Pathol. 2012;20:378–385. doi: 10.1177/1066896911425485. [DOI] [PubMed] [Google Scholar]
- 13.Wang J, Thway K. Clear cell sarcoma-like tumor of the gastrointestinal tract: an evolving entity. Arch Pathol Lab Med. 2015;139:407–412. doi: 10.5858/arpa.2013-0547-RS. [DOI] [PubMed] [Google Scholar]
- 14.Panagopoulos I, Mertens F, Isaksson M, Mandahl N. Absence of mutations of the BRAF gene in malignant melanoma of soft parts (clear cell sarcoma of tendons and aponeuroses) Cancer Genet Cytogenet. 2005;156:74–76. doi: 10.1016/j.cancergencyto.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Miettinen M, Wang ZF, Lasota J. DOG1 antibody in the differential diagnosis of gastrointestinal stromal tumors: a study of 1840 cases. Am J Surg Pathol. 2009;33:1401–1408. doi: 10.1097/PAS.0b013e3181a90e1a. [DOI] [PubMed] [Google Scholar]
- 16.Makhlouf HR, Ahrens W, Agarwal B, Dow N, Marshalleck JJ, Lee EL, et al. Synovial sarcoma of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 10 cases. Am J Surg Pathol. 2008;32:275–278. doi: 10.1097/PAS.0b013e31812e6a58. [DOI] [PubMed] [Google Scholar]
- 17.Davis IJ, McFadden AW, Zhang Y, Coxon A, Burgess TL, Wagner AJ, et al. Identification of the receptor tyrosine kinase c-Met and its ligand, hepatocyte growth factor, as therapeutic targets in clear cell sarcoma. Cancer Res. 2010;70:639–645. doi: 10.1158/0008-5472.CAN-09-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wagner AJ, Goldberg JM, Dubois SG, Choy E, Rosen L, Pappo A, et al. Tivantinib (ARQ 197), a selective inhibitor of MET, in patients with microphthalmia transcription factor-associated tumors: results of a multicenter phase 2 trial. Cancer. 2012;118:5894–5902. doi: 10.1002/cncr.27582. [DOI] [PubMed] [Google Scholar]