Abstract
This study aims to compare patient, tumor, treatment-related factors and survival between young (<45 years) and old (>45 years) Indian colorectal cancer (CRC) patients. Total 778 patients of CRC were registered at tertiary cancer center in India between 1 August 2013 and 31 July 2014. Patients were followed up for median period of 27.73 months. Data regarding patient, tumor, treatment and survival-related factors were collected. Patients were divided in young (≤45 years) and old (>45 years) age groups. Statistical analysis was done with SPSS software version 23. Young age group patients presented more commonly with poor histology, node-positive disease, and rectal site. Younger age group patients received multiple lines of neoadjuvant treatment. There was no significant overall survival difference in both groups of patients. On stratified stage-wise analysis, no significant overall survival (OS) difference was found between two groups (young vs old—1- and 3-year OS: 85.2 and 61.5% vs 81.5 and 64.5%, respectively; P = 0.881). On univariate analysis, gender, performance status, site, stage, differentiation, TRG, CRM status, signet ring type, and CEA level were significant prognostic factors. In disease-free survival (DFS) analysis, it is found that there is statistically significant difference in DFS (young vs old: 1 and 3 years; 77.6 and 62.8% vs 85.8 and 74.1%, respectively; P value, 0.02), but when OS was analyzed for same group of patient, there was no statistical difference (P = 0.302). This study confirms the high incidence rates of CRC in young Indian patients. There is no OS difference between two age groups. In operated group of patients, there is higher DFS in older patients but no OS advantage at 3 years follow-up. Further long-term follow-up is required to see any OS difference.
Keywords: Colorectal cancer, Disease-free survival, Overall survival, Young CRC
Introduction
Prevalence of colorectal cancer (CRC) in young patients is on the rise according to recent reports [1, 2]. Different reasons for this phenomenon have been proposed, but many questions remain unanswered. It is uncertain whether CRC in young patients is a disease with a different biology or there is any difference in clinical presentation and response to treatment, and if so, then whether it affects overall survival (OS) and disease-free survival (DFS). Many studies have tried to provide answers but with conflicting results [3–7]. Our study aimed to find the answers to these questions in 778 consecutive patients of CRC seen over a period of 1 year at a largest tertiary care cancer hospital in India.
Method
A total of 778 newly diagnosed patients with CRC were registered and seen in multidisciplinary team meeting at tertiary cancer care center in India, between 1 August 2013 and 31 July 2014 (12 months). All patients with diagnosis of CRC were evaluated, and demographic and disease-related data were entered in a prospective database regardless of intent of treatment and stage of the disease at the time of presentation. Tumor site was broadly divided into colon and rectum, with the rectosigmoid site included in the rectum group. The classification appearing in the seventh edition of AJCC was used for staging of the disease. Patients received treatment as per the prevailing standard guidelines after being evaluated by a multidisciplinary team. Patients were followed up for a median period of 27.73 months (reverse Kaplan–Meier method). Cutoff date for follow-up was 28 June 2016. Telephone calls and electronic medical records were used for follow-up and collection of data. Data regarding patient-related factors (e.g., age, gender, and performance status), tumor-related factors (e.g., subsite, histology, and stage), and treatment-related factors (e.g., intention, type of treatment, and response to treatment) were collected. All data were analyzed using descriptive statistics. Patients were divided into two groups comprising of young (<45 years) and old (>45 years) patients. Both these groups were compared with respect to earlier-mentioned factors using χ 2 test.
OS was calculated from the date of registration to the date of last follow-up. Of 778 patients, 186 (24%) were lost to follow-up, thus data of 559 patients were considered for OS analysis. DFS was calculated from the date of surgery to the date of recurrence in patients operated with curative intent. OS and DFS were assessed using the Kaplan–Meier method and were compared using the log rank test. Univariate and multivariate analyses were performed using the Cox proportional hazard model. Statistical analysis was done using SPSS Software, version 23 (SPSS Inc., Chicago, IL, USA).
Results
For analysis, 778 consecutive patients of CRC registered in 1-year period were selected. They were divided into two groups: ≤45 years (young) and >45 years (old). Of 778 patients, 351 (45.1%) belonged to the young age group and 427 (54.7%) to the old age group. Median age was 47 years with range of 11–85 years. Patient- and disease-related characteristics have been listed in Table 1. In both groups, no significant difference was observed between male and female population (young male vs old male: 63.81 vs 65.57%; young female vs old female: 36.18 vs 34.42%; P > 0.05).
Table 1.
Patient- and disease-related characteristics
| <45 years, N = 351 | >45 years, N = 427 | Total = 778 | |
|---|---|---|---|
| Age (%) | 351 (45.1%) | 427 (54.7%) | |
| Gender | P = 0.651 | ||
| Male | 224 (63.81%) | 280 (65.57%) | Total = 504 (64.8%) |
| Female | 127 (36.18%) | 147 (34.42%) | Total = 274 (35.2%) |
| Performance status | P = 0.647 | ||
| 1 | 283 (80.62%) | 334 (78.22%) | Total = 617 (79.30%) |
| 2 | 56 (15.95%) | 79 (18.50%) | Total = 135(17.35%) |
| 3 | 12 (3.41%) | 14 (3.27%) | Total = 26 (3.34%) |
| Subsite | P = 0.005 | ||
| Colon | 132 (37.60%) | 203 (47.54%) | Total = 335 (43.05%) |
| Rectum | 219 (62.39%) | 224 (52.45%) | Total = 443 (56.94%) |
| Histology | |||
| Signet ring | 72 (20.51%) | 33 (7.72%) | Total = 105; P = 0.000 |
| Mucinous | 72 (20.51%) | 59 (13.81%) | Total = 131; P = 0.013 |
| Histological differentiation | P = 0.000 | ||
| Moderate | 151 (43.01%) | 223 (52.22%) | Total = 374 |
| Poor | 97 (27.63%) | 63 (14.75%) | Total = 160 |
| Well | 3 (0.85%) | 17 (3.98%) | Total = 20 |
| CEA group | P = 0.395 | ||
| ≤5 | 165 (47%) | 187 (43.79%) | Total = 352 |
| >5 | 168 (47.86%) | 216 (50.58%) | Total = 384 |
| Stage | P = 0.036 | ||
| I | 12 (3.41%) | 26 (6.32%) | Total = 38 |
| II | 46 (13.10%) | 71 (16.86%) | Total = 117 |
| III | 189 (54.13%) | 191 (44.73%) | Total = 380 |
| IV | 104 (29.34%) | 139 (32.55%) | Total = 243 |
| Stage T | P = 0.71 | ||
| T1 | 4 (1.13%) | 9 (2.10%) | Total = 13 |
| T2 | 15 (4.27%) | 36 (8.43%) | Total = 51 |
| T3 | 244 (69.51%) | 283 (66.27%) | Total = 527 |
| T4 | 88 (25.07%) | 96 (22.48%) | Total = 184 |
| Stage N | P = 0.000 | ||
| N0 | 66 (18.80%) | 144 (33.72%) | Total = 210 |
| N+ | 285 (81.19%) | 280 (65.57%) | Total = 567 |
| Stage M | P = 0.381 | ||
| M0 | 247 (70.37%) | 288 (33.72%) | Total = 535 |
| M+ | 104 (29.62%) | 139 (32.78%) | Total = 243 |
| Metastasis | P = 0.792 | ||
| Peritoneum only | 18 (5.12%) | 25 (5.85%) | Total = 43 |
| Mixed peritoneal | 17 (4.84%) | 16 (3.74%) | Total = 33 |
| Nonperitoneal | 61 (17.37%) | 81 (18.96%) | Total = 142 |
| Metastases | P = 0.141 | ||
| Liver only | 15 (4.27%) | 21 (4.91%) | Total = 36 |
| Mixed hepatic | 22 (6.26%) | 44 (10.30%) | Total = 66 |
| Familial | P = 0.681 | ||
| Yes | 6 (1.7%) | 9 (2.10%) | Total = 15 |
| No | 345 (98.29%) | 416 (97.42%) | Total = 761 |
CEA carcinoembryonic antigen
Patients in the young age group presented more commonly with poor histology (mucinous type 20.51 vs 7.72%; signet ring type 20.51 vs 13.81%; P < 0.05), poor histological differentiation (27.63 vs 14.75%; P < 0.05), stage III (54.13 vs 44.73%, P < 0.05), and rectal site (62.39 vs 52.45%; P < 0.05).
Treatment-related characteristics have been listed in Table 2. In this study, no significant difference was observed between the two groups about intent of treatment. There was equal distribution of patients treated with curative and palliative intent. Patients in young age group received multiple lines of neoadjuvant treatment than those in the old age group (neoadjuvant chemoradiotherapy (NACTRT) f/b neoadjuvant chemotherapy: 13.10 vs 3.27%; P < 0.05) possibly due to progression of disease. Of the patients who underwent surgery, R0/R1 resection was found to achieve more commonly in patients of the old age group than their counterparts (53.56 vs 57.61%; P < 0.05).
Table 2.
Treatment-related characteristics
| <45 years N = 351 |
>45 years N = 427 |
||
|---|---|---|---|
| Intention of treatment | P = 0.666 | ||
| Curative | 254 (72.36%) | 303 (70.96%) | Total = 557 |
| Palliative | 97 (27.63%) | 124 (29.03%) | Total = 221 |
| NACTRT f/b NACT (rectum only) | P = 0.000 | ||
| Yes | 46 (13.10%) | 14 (3.27%) | Total = 60 |
| No | 304 (86.60%) | 413 (96.72%) | Total = 717 |
| Surgery | P = 0.024 | ||
| R0/R1 | 189 (53.84%) | 246 (57.61%) | Total = 435 |
| R2 | 1 (0.2%) | 3 (0.7%) | Total = 4 |
| Progression on neoadjuvant treatment | 40 (11.39) | 69 (16.15%) | Total = 109 |
| TRG | P = 0.02 | ||
| ≤2 | 52 (14.81%) | 38 (8.89%) | Total = 90 |
| >2 | 50 (14.24%) | 53 (12.41%) | Total = 103 |
| Circumferential resection margin (rectum only) | P = 0.235 | ||
| Positive | 3 (2.52%) | 5 (3.93%) | Total = 8 |
| Negative | 116 (97.47%) | 122 (96.06%) | Total = 238 |
| Distal resection margin | P = 0.738 | ||
| Positive | 2 (0.5%) | 1 (0.2%) | Total = 3 |
| Negative | 185 (52.70%) | 230 (53.86%) | Total = 415 |
NACTRT neoadjuvant chemoradiotherapy, NACT neoadjuvant chemotherapy
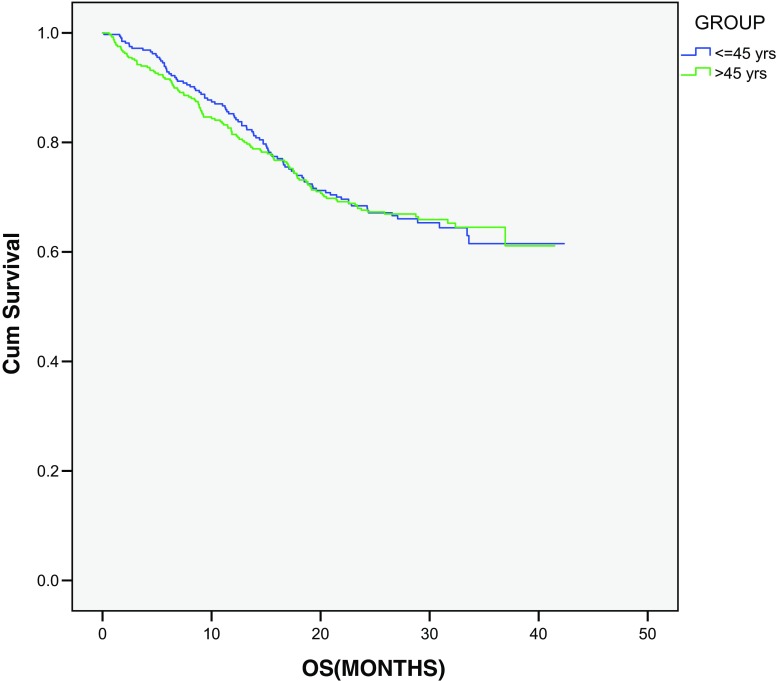
Figure 1 shows OS curves in all treated patients. In younger patients, 1- and 3-year OS is 85.2 and 61.5%, respectively. No significant OS difference was observed in both the groups (young vs old—1- and 3-year OS: 85.2 and 61.5% vs 81.5 and 64.5%, respectively; P = 0.881). On stratified stage-wise analysis, no significant OS difference was found between the two groups (P ≥ 0.05). On univariate analysis, gender, performance status, site, stage, differentiation, tumor regression grade (TRG), circumferential resection margin (CRM) status, signet ring type, and carcinoembryonic antigen level were found to be significant prognostic factors (Table 3).
Fig. 1.
Overall survival according to age groups
Table 3.
Univariate analysis of covariates affecting survival
| Hazard ratio | 95% CI | P value | |
|---|---|---|---|
| Age (years) | 0.929 | ||
| ≤45 | 0.988 | 0.75–1.29 | |
| >45 | 1 | Ref | |
| Gender | 0.051 | ||
| Male | 1.35 | 0.99–1.78 | |
| Female | 1 | Ref | |
| Performance status | |||
| 1 | 1 | Ref | |
| 2 | 2.5 | 1.83–3.43 | 0.000 |
| 3 | 10.9 | 6.39–18.73 | 0.000 |
| Site | 0.001 | ||
| Colon | 1.54 | 1.18–2.01 | |
| Rectum | 1 | Ref | |
| Stage | |||
| I | 0.00 | ||
| II | 1 | Ref | |
| III | 1.36 | 0.842–2.22 | 0.206 |
| IV | 5.917 | 3.66–9.55 | <0.05 |
| Grade | |||
| Well | 1 | Ref | |
| Moderate | 01.35 | 0.428–4.289 | 0.60 |
| Poor | 3.11 | 0.978–9.94 | 0.05 |
| Not available | 2.94 | 0.929–9.337 | 0.06 |
| Surgery | 0.000 | ||
| Yes | 1 | Ref | |
| No | 7.51 | 5.6–10.03 | |
| Histological type | |||
| Signet ring | 1.79 | 1.27–2.53 | 0.001 |
| Mucinous | 1.05 | 0.746–1.05 | 0.751 |
| TRG (rectum only) | 0.08 | ||
| ≤2 | 1 | Ref | |
| >2 | 2.28 | 0.88–5.88 | |
| CRM (rectum only) | |||
| Negative | 1 | Ref | |
| Positive | 3.34 | 1.04–10.71 | 0.04 |
| CEA | 0.00 | ||
| ≤5 | 1 | Ref | |
| >5 | 2.93 | 2.18–3.93 |
TRG tumor regression grade, CRM circumferential resection margin, CEA carcinoembryonic antigen
Results from the multivariate Cox proportional analysis showed that only male gender, rectum site, surgery, positive CRM status, stage IV, and poor performance status independently affected OS (Table 4).
Table 4.
Multivariate analysis of covariates affecting survival
| Hazard ratio | 95% CI | P value | |
|---|---|---|---|
| Gender | 0.002 | ||
| Male | 1 | Ref | |
| Female | 0.609 | 0.44–0.83 | |
| Subsite | 0.001 | ||
| Colon | 0.58 | 0.423–0.807 | |
| Rectum | 1 | Ref | |
| Surgery | 0.000 | ||
| Yes | 1 | Ref | |
| No | 3.41 | 2.13–5.41 | |
| CRM | 0.02 | ||
| Negative | 1 | Ref | |
| Positive | 4.05 | 1.17–13.98 | |
| Stage | 0.001 | ||
| Stage I | 1 | Ref | |
| Stage IV | 2.61 | 1.45–4.67 | |
| Performance status | |||
| 1 | 1 | Ref | |
| 2 | 1.55 | 1.10–2.18 | 0.011 |
| 3 | 2.54 | 1.29–5.02 | 0.007 |
CRM circumferential resection margin
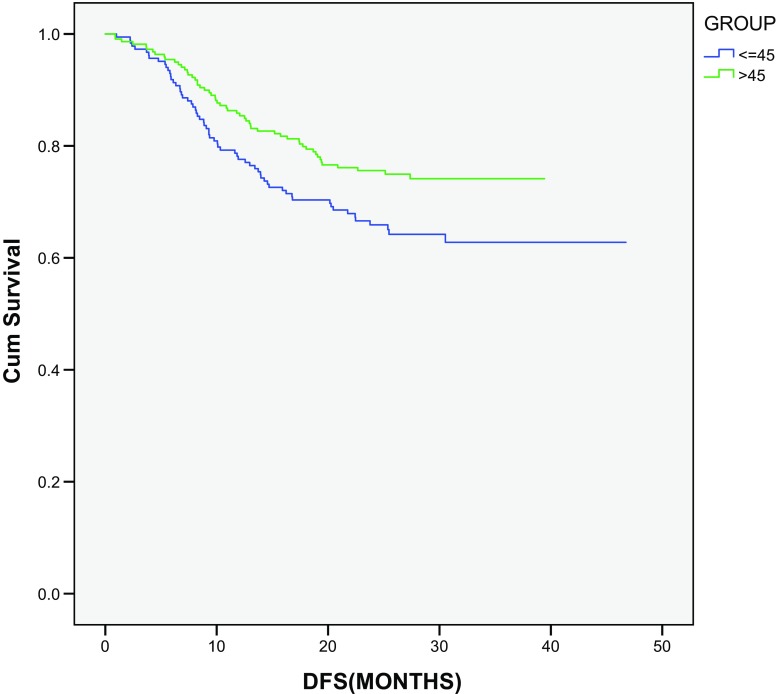
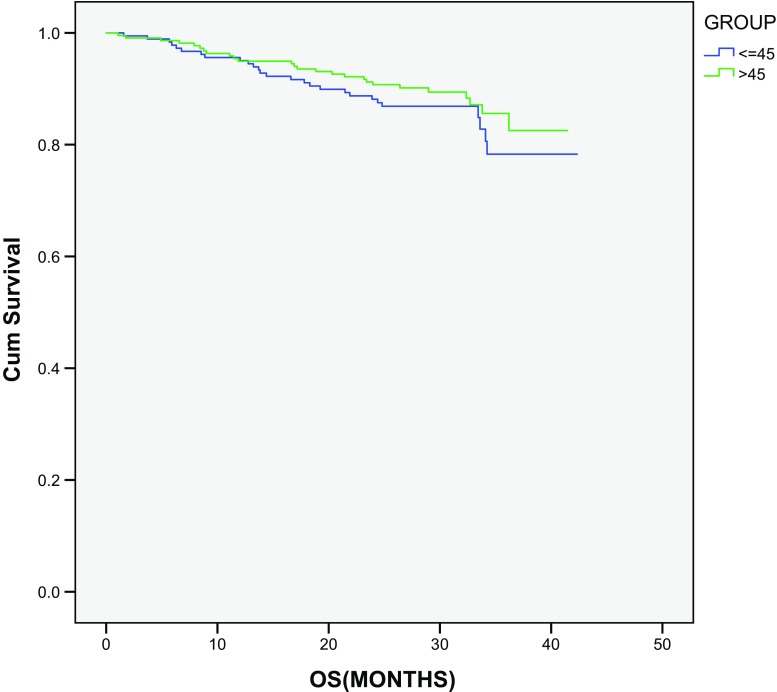
In patients operated with curative intent (total 403), the Kaplan–Meier method was used for determining DFS. In this study, statistically significant difference was observed in DFS for both groups (young vs old: 1 and 3 years; 77.6 and 62.8% vs 85.8 and 74.1%, respectively; P value, 0.02) (Fig. 2), whereas no statistical difference was observed in OS for the same groups (P = 0.302) (Fig. 3).
Fig. 2.
Disease-free survival analysis in operated group of patients
Fig. 3.
Overall survival analysis in operated group of patients
Discussion
Generally, CRC is a disease of older age, but recent studies have shown its increasing incidence in younger population [8, 9]. A reason for this phenomenon is not clear, but it has been suggested that increased awareness and better screening practices have resulted in higher detection rates in younger patients. Also, change in the dietary habits and increase in obesity in younger population may have some correlation with this trend. Recent analysis by Bailey et al. [9] of data from the Surveillance, Epidemiology, and End Results (SEER) CRC registry (1975–2010; n = 393,241) has shown increased incidence rates of CRC for patients in the age group of 20–49 years. For patients in the age groups of 20–34 and 35–49 years, incidence rates were found to increase by 1.99 and 0.41%, respectively. Previous retrospective studies have shown incidence of CRC among 4–10% of young patients, [8, 10] but our study has shown it to be in very high proportion of young patients (45.1%). The reason behind these unusually high rates could be institutional or referral bias. But previous studies in Indian patients have shown similar trends of higher incidence [11, 12].
Increased awareness or screening is unlikely to be the cause for increased number of young Indian patients with CRC due to the absence of a national screening program and also the fact that the cancers diagnosed in this subgroup are more advanced than those diagnosed in the elderly. More details about dietary factors and body mass index will help us clarify the association of westernization of lifestyle and eating habits with increasing incidence of CRC in the younger Indian population. In addition, increased number of young patients with CRC could be a reflection of high proportion of young population in India.
In this study, we have used 45 years as cutoff for the definition of young age. There is no consensus regarding the same worldwide. Some authors have used 50 years as a cutoff whereas others have used 40 years as a young-age criterion [5, 10, 13].
In our study, young patients have presented more commonly with node-positive disease than older patients. Also, less favorable histology types such as mucinous and signet ring have been found significantly more common in young age group. This way of presentation is consistent with other previous studies [10, 14, 15]. It is not clear why young patients generally present with more advanced disease and poor histology type and hence tend to have worse DFS. One reason for this advanced presentation could be failure to reach the diagnosis by physician due to less suspicion in view of young age or late consultation with doctor from the patient’s side. Few molecular studies have suggested that colorectal carcinomas are biologically different in young age group compared with older age group [16]. This may explain this different way of presentation. Whatever may be the reason but most important question lies ahead: does it affect survival? Many retrospective studies have been conducted to find the answer but with conflicting results. Our aim of this analysis was to answer this question in a cohort of Indian patients with CRC.
Along with demographic factors, we have analyzed the effect of treatment and related factors. It shows that in patients with rectal cancer more number of young patients has received additional chemotherapy regimen after NACTRT in view of poor response. The difference between two groups could be due to poor response to neoadjuvant treatment, which has been seen in our study where on postoperative histopathology more number of younger patients had higher TRG suggestive of poor response to neoadjuvant treatment. Also, one would assume that due to better performance status, young patients will receive and tolerate multiple lines of treatment. However, the performance status was similar in the two groups of our study.
In this study, no statistically significant difference was found in OS of both groups. Both groups have shown comparable 3-year OS (young vs old: 61.5 vs 64.5%, P = 0.881). Even with advanced presentation and poor response to neoadjuvant therapies, final OS was found to be comparable. This could be due to more aggressive therapy in younger patients. As younger patients have good performance status with less comorbidities, they generally tolerate chemotherapy better. How this factor influenced OS in our study is difficult to know as we have not included details of chemotherapy regimen and number of lines of chemotherapy received, which might have made a difference. In this study, no difference between performance statuses of both groups was observed.
We also conducted subgroup analysis on all operated patients who were treated with curative intent. Older patients were found to have significantly higher DFS than younger patients (3-year DFS young vs old: 62.8 vs 74.1%; P = 0.02), but on OS analysis, no statistical difference was observed in OS between the two groups (3-year OS, young vs old: 78.3 vs 85.6%; P = 0.302). Failure to see OS difference may be due to short follow-up period as 3-year DFS has been shown to correlate with 5-year OS in patients with stage III CRC [17]. We need to follow operated patients for longer duration to observe any OS difference. Younger patients may have a better performance status and less comorbidities, and thus may receive more lines of treatment with more chemotherapeutic drugs, which may explain the failure to observe OS difference.
Conclusion
This study showed that a significant proportion of patients with CRC are less than 45 years old. These patients were found to have disease with poor prognostic factors and a more advanced stage at presentation with a worse DFS after surgery, although no difference in 3-year OS was observed. Longer follow-up is needed to give us better information regarding long-term outcomes in these patients.
Contributor Information
Ashish B. Pokharkar, Email: ashish.pokharkar@gmail.com
Manish Bhandare, Email: manishbhandare@gmail.com.
Prachi Patil, Email: prachipatil@gmail.com.
Shaesta Mehta, Email: shaestamehta@yahoo.com.
Reena Engineer, Email: reena.engineer@gmail.com.
Avanish P. Saklani, Email: asaklani@hotmail.com
References
- 1.Fairley TL, Cardinez CJ, Martin J, Alley L, Friedman C, Edwards B, et al. Colorectal cancer in U.S. adults younger than 50 years of age, 1998–2001. Cancer. 2006;107(5 Suppl):1153–1161. doi: 10.1002/cncr.22012. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Jemal A, Ward EM. Increase in incidence of colorectal cancer among young men and women in the United States. Cancer Epidemiol Biomark Prev. 2009;18:1695–1698. doi: 10.1158/1055-9965.EPI-09-0186. [DOI] [PubMed] [Google Scholar]
- 3.Taylor MC, Pounder D, Ali-Ridha NH, Bodurtha A, MacMullin EC. Prognostic factors in colorectal carcinoma of young adults. Can J Surg. 1988;31:150–153. [PubMed] [Google Scholar]
- 4.Cusack JC, Giacco GG, Cleary K, Davidson BS, Izzo F, Skibber J, et al. Survival factors in 186 patients younger than 40 years old with colorectal adenocarcinoma. J Am Coll Surg. 1996;183:105–112. [PubMed] [Google Scholar]
- 5.Fu J, Yang J, Tan Y, Jiang M, Wen F, Huang Y, et al. Young patients (≤35 years old) with colorectal cancer have worse outcomes due to more advanced disease: a 30-year retrospective review. Medicine. 2014;93(23):e135. doi: 10.1097/MD.0000000000000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heys SD, Sherif A, Bagley JS, Brittenden J, Smart C, Eremin O. Prognostic factors and survival of patients aged less than 45 years with colorectal cancer. Br J Surg. 1994;81:685–688. doi: 10.1002/bjs.1800810519. [DOI] [PubMed] [Google Scholar]
- 7.Lee PY, Fletcher WS, Sullivan ES, Vetto JT. Colorectal cancer in young patients: characteristics and outcome. Am Surg. 1994;60:607–612. [PubMed] [Google Scholar]
- 8.Oconell JB, Maggard MA, Liu JH, Etzioni DA, Livingston EH, Ko CY. Rates of colon and rectal cancers are increasing in young adults. Am Surg. 2003;69:866–872. [PubMed] [Google Scholar]
- 9.Bailey CE, Hu C-Y, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975–2010. JAMA Surg. 2015;150:17–22. doi: 10.1001/jamasurg.2014.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Connell JB, Maggard MA, Livingston EH, Yo CK. Colorectal cancer in the young. Am J Surg. 2004;187:343–348. doi: 10.1016/j.amjsurg.2003.12.020. [DOI] [PubMed] [Google Scholar]
- 11.Laskar RS, Talukdar FR, Mondal R, Kannan R, Ghosh SK. High frequency of young age rectal cancer in a tertiary care centre of southern Assam, North East India. Indian J Med Res. 2014;139(2):314–318. [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta S, Bhattacharya D, Acharya AN, Majumdar S, Ranjan P, Das S. Colorectal carcinoma in young adults: a retrospective study on Indian patients: 2000-2008. Color Dis. 2010;12:e182–e189. doi: 10.1111/j.1463-1318.2010.02223.x. [DOI] [PubMed] [Google Scholar]
- 13.Schellerer VS, Merkel S, Schumann SC, Schlabrakowski A, Förtsch T, Schildberg C, et al. Despite aggressive histopathology survival is not impaired in young patients with colorectal cancer: CRC in patients under 50 years of age. Int J Colon Dis. 2012;27:71–79. doi: 10.1007/s00384-011-1291-8. [DOI] [PubMed] [Google Scholar]
- 14.Derwinger K, Kodeda K, Gerjy R. Age aspects of demography, pathology and survival assessment in colorectal cancer. Anticancer Res. 2010;30:5227–5231. [PubMed] [Google Scholar]
- 15.Ganapathi S, Kumar D, Katsoulas N, Melville D, Hodgson S, Finlayson C, et al. Colorectal cancer in the young: trends, characteristics and outcome. Int J Color Dis. 2011;26:927–934. doi: 10.1007/s00384-011-1174-z. [DOI] [PubMed] [Google Scholar]
- 16.Ballester V, Rashtak S, Boardman L. Clinical and molecular features of young onset colorectal cancer. World J Gastroenterol. 2016;22(5):1736–1744. doi: 10.3748/wjg.v22.i5.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sargent DJ, Wieand HS, Haller DG, Gray R, Benedetti JK, Buyse M, et al. Disease-free survival versus overall survival as a primary end point for adjuvant colon cancer studies: individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2005;23(34):8664–8670. doi: 10.1200/JCO.2005.01.6071. [DOI] [PubMed] [Google Scholar]