Abstract
Borderline ovarian tumors (BOTs) are a heterogeneous group of non-invasive epithelial ovarian tumors that occur at a younger age, present in early stage, frequently associated with infertility but are easily curable. Although they may have symptomatic long-term recurrences, they have an excellent prognosis in spite of peritoneal spread. Among the epithelial tumors of the ovary, BOTs fall in the spectrum lying between cystadenomas (benign) and cystadenocarcinomas (malignant). Their oncological behavior is more aggressive than benign ovarian tumors but relatively less than that of malignant ovarian tumors. Since the age group affected is usually young females, preservation of fertility is an important aspect of treatment protocol. Although the management of these tumors has been extensively discussed, it still remains a controversial gray zone. In this review, epidemiology, pathogenesis, histologic subtypes, various surgical approaches, follow-up, and management of recurrence have been discussed. Choosing the best treatment still poses a challenge for the treating oncosurgeon.
Keywords: Borderline ovarian tumors, Fertility-sparing surgery, Restaging surgery
Introduction
Borderline ovarian tumors (BOTs) are defined histologically by atypical epithelial proliferation without destructive stromal invasion [1]. BOTs were first described as a separate group in 1929 by Taylor as “semi-malignant disease” [2]. BOTs have histopathologic features and biologic behavior intermediate between clearly benign and frankly malignant ovarian tumors. The outcome of patients with BOT is relatively favorable even in the presence of peritoneal disease. A wide variety of terms and classifications have been used for these lesions. BOT was recognized as a separate entity by FIGO (The International Federation of Gynecology and Obstetrics) in 1961 as tumors of low malignant potential. The World Health Organization (WHO) coined the term “borderline” in 1973. Presently, three terms are used to refer to these tumors: borderline tumor, tumor of low malignant potential, and atypical proliferative tumor.
Incidence
Borderline ovarian tumors account for 10–20% of all epithelial ovarian tumors with an incidence of 1.8–5.5 per 100,000 women per year [1]. In authors’ experience, the incidence of BOT was only 9.3% (8 out of 86) among patients treated over last 21 months.
Epidemiology
Apart from age, women with BOT usually do not differ from women with ovarian carcinoma in epidemiologic characteristics. Average age of patients with borderline tumors is approximately 46 years [3] (10–20 years younger than that of invasive ovarian cancer). One third of patients with BOT are younger than 40 years at presentation [4, 5].
Primary infertility and nulliparity increase the risk of BOT, whereas oral contraceptives, multiple pregnancies, and breastfeeding are protective. A case control study by Riman et al. in Sweden on 3899 patients showed that women who had given birth more than once had a lower risk of developing borderline ovarian tumors compared with those women who had not given birth at all (odds ratio (OR): 0.44, confidence interval (CI): 0.26–0.75 for serous tumors and OR: 0.63, CI: 0.34–1.19 for mucinous tumors) [6].
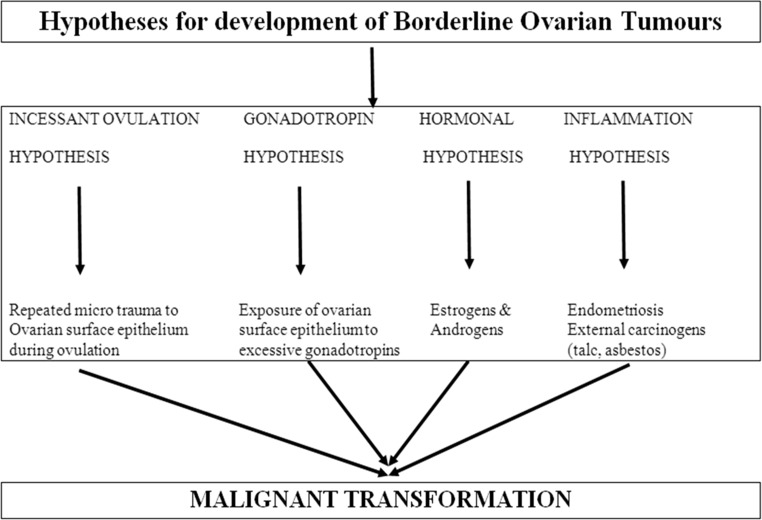
Different hypotheses have been described to corroborate the reproductive risk factors. According to the incessant ovulation hypothesis, the development of ovarian malignancy is a consequence of repeated microtrauma to the ovarian surface epithelium during ovulation [7]. However, the gonadotropin hypothesis states that malignant transformation can be caused by the exposure of ovarian surface epithelium to excessive gonadotropin levels. Some case control studies noted a two- to fourfold increased risk of BOT after the use of fertility drugs followed by ovarian stimulation and multiple ovarian punctures [8]. The hormonal hypothesis explains a protective role of progesterone while implicating estrogens and androgens in promoting tumor cell growth [6]. A fourth hypothesis put forward is the inflammation hypothesis implicating the association of endometriosis and external carcinogens (e.g., talc, asbestos) in carcinogenesis particularly endometrioid and clear cell type of BOTs [9] (Fig. 1).
Fig. 1.
Hypotheses for development of borderline ovarian tumors
Infertility is reported in approximately 10–35% of patients with BOT [10]. Women undergoing IVF treatment are at increased risk of being diagnosed with borderline ovarian tumors [11]. Borderline tumors are rarely seen in women with BRCA mutations [12].
Serum tumor markers do not usually help in the diagnosis of borderline ovarian tumors. Van Calster and colleagues showed the amounts of CA 125 in serum overlapped between patients with borderline ovarian tumors and early-stage ovarian carcinoma [13]. Abnormal concentrations of CA 125 were noted in about 40% of patients with stage I borderline ovarian tumors and reached 83% in women with advanced-stage disease [14]. No data support the relevance of serum tumor markers, exception being advanced-stage BOT.
Pathogenesis
Two pathways have been proposed in the pathogenesis of serous borderline ovarian tumors. First is the “low-grade” pathway that involves BRAF and KRAS mutations. According to this pathway, serous ovarian cystadenomas progress to serous BOTs which eventually lead to low-grade serous epithelial ovarian carcinoma through a continuum of histological precursor lesions [15]. Only 2% of all serous BOTs progress to carcinoma via this “low-grade” pathway. Second is the “high-grade” pathway that involves mutations in the p53 gene. Most serous ovarian carcinomas belong to this high-grade pathway, with no known precursor. Serous BOTs are characterized by activation of specific tumor suppressor genes (SERPINA 5 and dual specificity phosphatase 4 [DUSP4]) that inhibit degradation of the extracellular matrix, a key event in the pathogenesis of invasive growth [16].
Mucinous carcinogenesis encompasses a sequence of malignant transformations from benign mucinous tumors to carcinomas. There are three types of ras oncogenes namely K, N, and H. It has been observed that mucinous BOTs have a higher frequency of K-ras mutations than that of mucinous cystadenoma, but a lower rate than that of mucinous carcinoma. It is still not known whether BRCA1 and BRCA2 mutations increase the risk of BOT [5]. In contrast to serous and mucinous borderline ovarian tumors, endometrioid borderline ovarian tumors are characterized by mutations involving the beta catenin gene (50%), PTEN gene (20%), and microsatellite instability gene (up to 50%) [17].
Presenting Complaints
Most women with BOT are asymptomatic at presentation. Pelvic mass may be an incidental finding on routine pelvic examination. Around 50–60% of patients present with non-specific symptoms such as abdominal pain or discomfort, abdominal distension, bowel irregularity, persistent fatigue, or weight loss. Ten percent of patients present with abnormal uterine bleeding [18]. Sometimes, ovarian mass may be detected on a screening abdominal ultrasound.
Diagnosis
Borderline tumors are difficult to detect clinically until they are huge in size or advanced in stage. Pelvic ultrasound helps in identifying the ovarian mass but it is neither sensitive nor specific enough to be used as a screening tool in normal population. As opposed to ovarian carcinoma, BOTs are characterized by the absence of ascites. Serum CA 125 levels neither aid in the diagnosis nor follow up care of patients with borderline tumors. In the systematic review by du Bois et al., CA 125 levels were negative (CA 125 </= 35 U/ml) in 53.8% of patients with borderline tumors [18]. However, it may give a rough idea of benign vs. malignant nature of the tumor and can be taken into account during pre-operative counseling of the patient as to what to expect in the operating room. On imaging, borderline ovarian tumors can be seen as complex cystic masses with mural nodules and septations. CT does not have any key distinguishing features that would enable differentiating borderline from malignant ovarian tumors; however, it should always be done pre-operatively as a part of workup protocol of all ovarian masses to identify the possible foci of metastasis. The findings of MRI also cannot predict whether an ovarian mass is of borderline or of malignant nature. Borderline ovarian tumors are not PET-avid and hence are interpreted as “benign” tumors on PET [19]. Ovarian masses that show complex features on MRI that are concerning for malignancy but appear as “benign” on PET are said to be characteristic of borderline ovarian tumors [19]. However, the diagnosis of borderline ovarian tumor is established intra-operatively by frozen section analysis of the ovarian mass or postoperatively. Pathologic criteria for diagnosis include the absence of stromal invasion in the ovary and at least two of the following characteristics: epithelial tufting, multilayering of the epithelium, mitotic activity, and nuclear atypia.
Histopathology
The two major histologic subtypes of BOTs are serous and mucinous, serous type being more common. Serous and mucinous BOTs constitute 43–53% and 42.5–52% of all BOTs, respectively [18, 20]. The ratio in Asia is different, with an equivalent or higher rate of mucinous borderline ovarian tumors [21]. Rare entities such as endometrioid, clear cell, mixed, transitional cell, or Brenner type account for less than 5% of borderline ovarian tumors.
Histologically, serous BOTs are divided into typical subtype (90%) and micropapillary subtype (10%) [22]. Serous BOTs are bilateral in 15–40% of cases and 15–40% of serous BOTs are associated with extraovarian disease (peritoneal implants or nodal disease) [23]. The extraovarian peritoneal disease in pts. with BOT was defined as “implants” (and not metastases) by WHO because of their indolent nature. The peritoneal implants of BOT were classified as invasive vs. non-invasive in 1988 by Bell and colleagues [24]. If the implants have merely “stuck on” the peritoneal surfaces, they are referred to as non-invasive, but if they have invaded the underlying tissues such as omentum or bowel wall, they are referred to as invasive.
These implants are non-invasive in 85% of cases and invasive in only 15%. BOTs with invasive implants have a poorer prognosis. The concept of serous BOTs displaying micropapillary patterns was introduced in 1996. These lesions were characterized by a greater frequency of bilateral tumors, surface involvement and a higher rate of invasive peritoneal implants compared with serous BOTs without micropapillary patterns [25]. A small subset of implants also may originate de novo from nodal endosalpingiosis (spectrum of secondary mullerian system involvement in the pelvis) [26].
Mucinous BOTs are divided into two subtypes, intestinal (or gastrointestinal) (85–90%) and mullerian (or endocervical/seromucinous) lesions. The intestinal type is usually unilateral while endocervical type is bilateral in as many as 40% cases. For many years, pseudomyxoma peritonei (i.e., the presence of mucinous ascites or mucoid nodules adherent to peritoneal surfaces) was thought to result from ovarian borderline tumors, but it has recently been revealed that virtually, all ovarian tumors associated with pseudomyxoma peritonei represent metastases from ruptured primary low-grade mucinous tumors of the appendix [27]. All patients with bilateral ovarian masses should be evaluated for a primary intestinal tumor.
Treatment
The principal treatment of borderline ovarian tumors is surgical resection of the primary tumor. There has been a paradigm shift in the treatment of BOTs with a radical surgery about a decade ago to a more conservative treatment since BOT has a predilection to affect younger women.
Frozen Section Analysis
The diagnosis of BOT cannot be determined before surgery. Intra-operative frozen section analysis is therefore essential in the management of suspicious ovarian masses to tailor the extent of the surgery. However, frozen section has limitations in diagnostic accuracy, and at times, frozen section findings may not corroborate with the final histopathological report. Borderline tumors are correctly diagnosed 58–86% of the time by frozen section depending on the experience of the histopathologist. The tumor is underdiagnosed as a benign one in 31% of patients on intra-operative frozen section. A sub-diagnosis of 25–30% has been shown in differentiating a BOT from a malignant tumor [28] which means that the patient is unfortunately subjected to a second surgery to stage the disease if frozen was reported as borderline and the final histopathological diagnosis comes as a malignant ovarian tumor.
Fertility-Sparing Surgery
BOTs are usually found in younger population as compared to invasive ovarian cancer, so the preservation of fertility is an important issue in deciding the management of BOTs. Young women who desire to preserve fertility may be candidates for fertility-sparing approach, which is defined as preservation of the uterus and at least part of one ovary. Thus, in patients with tumor confined to one ovary, it includes unilateral salpingo-oophorectomy or ovarian cystectomy with complete surgical staging. Biopsy from the normal-looking contralateral ovary is not required as it may cause unnecessary damage to the ovarian reserve and/or peritoneal adhesions [29]. In case of bilateral ovarian involvement, unilateral or bilateral ovarian cystectomy or a unilateral salpingo-oophorectomy with contralateral cystectomy may be considered. Recent studies have indicated that the fertility-sparing treatment for BOTs is well tolerated and able to permit future pregnancy [30]. Fertility-sparing treatment may however increase the risk of relapse [31]. Studies from the Norwegian Radium Hospital [20] and Gynecologic Oncology Group [32] have shown that relapse rates after bilateral salpingo-oophorectomy range between 0 and 20%. This rate varies between 12 and 58% for cystectomy and between 2.5 and 5.7% for radical surgery. Thus, the gynecologic oncologist has to adequately weigh the pros and cons of a fertility-preserving approach in women with borderline ovarian tumors and counsel the patient regarding the advantages and disadvantages of same with the advice of a regular and long-term follow-up.
In postmenopausal women or in women who do not wish to preserve fertility, the complete staging procedure includes peritoneal washing, type I hysterectomy with bilateral salpingo-oophorectomy, multiple peritoneal biopsies, total omentectomy, resection of grossly visible metastases, and inspection and palpation of the entire abdominal cavity. Appendectomy for mucinous tumors is strongly recommended to exclude synchronous or primitive appendiceal tumor. Lymph node involvement has been reported in about 25% patients with advanced-stage BOT (FIGO stage III or IV) [33]. In spite of this, lymphadenectomy can be omitted even for stage II and III disease, as there is no difference in the recurrence or survival rate [34]. Moreover, pelvic and para-aortic lymphadenectomy do not have a prognostic value in BOTs indicating that involvement of the lymph nodes does not decrease survival; neither does lymphadenectomy increase it.
Role of Laparoscopy
In the present era, benign-looking ovarian masses are increasingly dealt via laparoscopic route. The use of endo bags decreases the risk of spillage during surgery and also reduces the likelihood of port site metastases. Young women who wish to preserve fertility can be given the option of laparoscopic approach since it has the advantage of shorter hospitalization, shorter postoperative recovery period, less adhesion formation, and improved cosmesis [35]. Cyst rupture and incomplete staging occur significantly more frequently by laparoscopy compared with laparotomy (33.9 vs. 12.4%) [14, 36]. Hence, laparoscopic surgery for BOTs should be reserved for experienced centers to reduce the risk of intra-abdominal tumor rupture and incomplete staging, thus reducing the rate of recurrence.
Restaging Surgery
Surgical treatment is a crucial component of BOT treatment. In patients in whom an oophorectomy or cystectomy has been performed and a borderline tumor is later documented in the permanent pathology, no additional staging surgery is necessary, but the patient should be monitored with transvaginal ultrasonography. Shim et al. [37] systematically reviewed published studies comparing complete surgical staging (CSS) with incomplete surgical staging (ISS) in BOT patients through April 2015 and concluded that restaging should be individualized and be based on clinician’s advice and patient’s wishes. They concluded that restaging surgery is recommended if (1) there are histologic features suggestive of invasive recurrence (an invasive peritoneal implant or micropapillary pattern) [38, 39], (2) the peritoneum is not clearly reported as “normal” or if there was no systematic exploration during initial surgery, (3) if macroscopic peritoneal implants are found in the initial surgery, (4) if gross lesions remain after initial surgery, and (5) if the patients are less likely to come for regular follow-up. CSS was associated with a reduced recurrence risk, although no significant association with survival was observed.
Overall, patients with BOT should be thoroughly evaluated by experienced gynecological oncologist, and the pros and cons of a fertility-sparing surgery should be explained in detail. These patients require a close and a long-term follow-up after conservative surgery. Balancing radicality between oncologic safety and treatment burden has already led to remarkable changes in the management pattern over the last decades and is still a challenging task.
FIGO Stage
Staging for BOT is similar to the FIGO staging system used for epithelial ovarian cancers. BOTs are diagnosed at stage I in 82% of cases [40]. Patients have 60% chance of having stage I disease when diagnosed. Diagnosis of BOT in stage II and III is rare with stage IV being an exception. In a systematic review of 6362 patients by du Bois et al., 78.9% patients with BOT were diagnosed in FIGO stage I and 21.1% with FIGO stages II–IV, although FIGO stage IV is very rare [18].
Adjuvant Treatment
There is no data to suggest any role of adjuvant chemotherapy or radiation therapy in order to improve survival [41–43]. Six randomized controlled trials [44–46] have compared different forms of adjuvant therapy in women who had undergone radical surgery for borderline ovarian tumors. All these studies did not show any definitive advantage of adjuvant therapy given postoperatively. Platinum-based adjuvant chemotherapy can be considered in patients with invasive epithelial implants due to the known poor prognosis of this pathological entity; however, there is no level I evidence to suggest overall survival advantage. A retrospective study from the Gynecologic Oncologic Group (GOG) analyzed 988 adequately staged patients with stage I BOT who did not receive adjuvant treatment and observed a 5-year mortality rate of only 0.7% [47].
Fertility After Conservative Treatment of BOT
Following fertility preserving surgery for borderline ovarian tumors, the pregnancy rate is nearly 50%, and most are achieved spontaneously [14, 29]. Ovulation induction is often required in the remaining in order to conceive with the general recommendation to use the minimum number of stimulation cycles. Egg retrieval and egg freezing are alternative options for women with reduced fertility after conservative surgery. This requires a close collaboration of gynecologic oncologists and reproductive endocrinologists. The presence of postsurgical adnexal adhesions is associated with a 20–30% reduction of pregnancy rate [48]. Fasouliotis et al. found no perceptible negative effect of previous BOT on pregnancy rates after IVF [49]. Women who have completed child bearing need not get their preserved ovary removed provided they agree for a close and regular follow-up. However, the decision of removal of the remaining ovary after family is complete depends on the histologic subtype, FIGO stage of the disease, type of conservative surgery, and patient’s own wishes. Some women refuse to undergo the psychological stress of waiting for relapse since there exists a risk of recurrence with invasive ovarian tumor. Therefore, some authors recommend definitive surgery after child bearing is complete [50]. To achieve low morbidity related just to the completion of salpingo-oophorectomy, a concurrent hysterectomy can be avoided because no solitary recurrences in the uterus have been observed [51].
Follow-Up
Follow-up should be long-term and lifelong as recurrences have been reported as late as 39 years after initial treatment [52]. The general recommendation is follow-up every 3 months during first 2 years, every 6 months between 2 and 5 years and yearly thereafter. Clinical examination should be supplemented with TVS and CA 125 levels. Patients who have been treated conservatively should have a close follow-up with special attention to the remaining ovary. MRI should be done if there is suspicion of local recurrence while a contrast enhanced CT is helpful if there is suspicion of extrapelvic recurrent disease.
Prognosis
Patients with BOT have an excellent overall prognosis. Features that carry a poor prognosis include advanced stage, invasive peritoneal implants, macroscopic residual disease, and micropapillary architecture for serous BOT. The tumor tends to behave more aggressively if the micropapillary architecture is associated with invasive implants. Ninety five percent borderline tumors have diploid DNA, a finding almost always associated with a good prognosis. Aneuploid tumors have a worse prognosis and are associated with a high recurrence risk. In large flow cytometric analysis on 370 pts. of BOT reported by Kaern et al., aneuploidy in BOT was associated with a 15-year survival rate of only 15%, despite the 85% survival rate in the patients with diploid tumors [53].
Recurrence
The FIGO stage at presentation, presence, or absence of invasive implants and macroscopic residual disease after surgery are the most important predictors of recurrence. Most recurrences are of borderline nature and can be properly salvaged with surgical treatment alone. Conservative treatment can again be considered in the setting of recurrent disease, if the patient is desirous of fertility preservation. The patient should be properly counseled about the risk of future recurrence. However, conservative treatment should not be recommended if invasive implants have been found and complete surgical debulking should be done without sparing fertility. Patients with stage I disease have a recurrence rate of approximately 15%. FIGO stage (II–IV), younger age, bilateral tumors, serous tumors, presence of peritoneal implants especially invasive implants, and type of surgery (laparoscopic approach, fertility sparing) are the most significant predictive factors of relapse [54, 55]. About 70% of recurrent lesions show borderline histology and can be effectively cured by surgery without any impact on the long-term survival [40, 56, 57]. BOT may relapse as malignant disease although very rarely. Patients who recur with invasive ovarian cancer often carry a grave prognosis. Therefore, avoiding recurrent disease should be an important goal while planning overall surgical treatment of all patients with borderline ovarian tumors.
Survival
Survival of patients with borderline tumors is excellent. Overall 5-year and 10-year survival rates for stage I, II, and III disease are 99 and 97, 98 and 90, and 96 and 88%, respectively [58].
BOTs have a 5-year survival rate of more than 90% across all tumor stages, with a considerable number of patients cured [40]. If all stages of borderline tumors are included, the 5-year survival rate is 87% [59]. Seidman and Kurman summarized 97 reports, including a total of 4129 women with serous BOT and found a 5-year survival rate of 98% for women with non-invasive implants and 33% for those with invasive implants [60].
Future Research
Pharmacologic inhibitors of the BRAF–KRAS pathway are being considered to treat patients with advanced serous borderline ovarian tumors to improve patient survival.
Conclusion
Borderline ovarian tumors are usually diagnosed at an early stage and have more indolent behavior, excellent prognosis, longer survival, and later recurrence compared with invasive ovarian cancer. Fertility-sparing surgery is the treatment of choice in young females who desire motherhood with adequate counseling for close and long-term follow-up. Surgery with no macroscopic residual remains the mainstay of treatment. There is no benefit of adjuvant chemotherapy, radiation therapy, hormonal, or targeted therapy in borderline ovarian tumors. Removal of the preserved ovary, though not mandatory, should be done after completion of child bearing in order to save the patient from the psychological stress of waiting for relapse since there is always a risk of development of invasive ovarian tumor.
Compliance with Ethical Standards
Conflicts of Interest
The authors declare that they have no conflict of interest.
Contributor Information
Nidhi Nayyar, Phone: +91- 8802600875, Email: drnidhi_nayyar@rediffmail.com.
Prerna Lakhwani, Phone: +91-9910550179, Email: prernalakhwani@gmail.com.
Ashish Goel, Phone: +91-9818714549, Email: dr_ashishgoel@yahoo.com.
Pankaj Kr. Pande, Phone: +91-9810441065, Email: pankaj.pande@hotmail.com
Kapil Kumar, Phone: +91-9810065202, Email: kdrkapil@yahoo.in.
References
- 1.Skirnisdottir I, Garmo H, Wilander E, Holmberg L. Borderline ovarian tumours in Sweden 1960-2005: trends in incidence and age at diagnosis compared to ovarian cancer. Int J Cancer. 2008;123:1897–1901. doi: 10.1002/ijc.23724. [DOI] [PubMed] [Google Scholar]
- 2.Taylor HC. Malignant and semi-malignant tumours of the ovary. Surg Gynecol Obstet. 1929;48:204–230. [Google Scholar]
- 3.Scully RE, Young RH, Clement PB (1998) Tumors of the ovary, maldeveloped gonads, fallopian tube, and broad ligament. In: Atlas of Tumor Pathology. 3rd Series, Fascicle 23. Washington, DC: Armed Forces Institute of Pathology 1–168
- 4.Sherman ME, Berman J, Birrer MJ, et al. Current challenges and opportunities for research on borderline ovarian tumors. Hum Pathol. 2004;35:961–970. doi: 10.1016/j.humpath.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Sherman ME, Mink PJ, Curtis R, et al. Survival among women with borderline ovarian tumors and ovarian carcinoma: a population-based analysis. Cancer. 2004;100:1045–1052. doi: 10.1002/cncr.20080. [DOI] [PubMed] [Google Scholar]
- 6.Riman T, Dickman PW, Nilsson S. Risk factors for epithelial borderline ovarian tumours : results of a Swedish case control study. Gynecol Oncol. 2001;83:575–585. doi: 10.1006/gyno.2001.6451. [DOI] [PubMed] [Google Scholar]
- 7.Fathalla MF. Incessant ovulation : a factor in ovarian neoplasia? Lancet. 1971;2:163. doi: 10.1016/S0140-6736(71)92335-X. [DOI] [PubMed] [Google Scholar]
- 8.Van Leeuwen FE, Klip H, Mooji TM, et al. Risk of borderline and invasive ovarian tumours after ovarian stimulation for in vitro fertilization in a large Dutch cohort. Hum Reprod. 2011;26:3456–3465. doi: 10.1093/humrep/der322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seidman JD, Cho KR, Ronett BM et al (2011) Surface epithelial tumours of the ovary. In : Kurman RJ, Ellenson LH, Ronett BM, editors. Blaustein’s Pathology of the Female Genital Tract. 6th ed. New York : Springer Science + Bussiness Medica Pp. 680–772
- 10.Fauvet R, Poncelet C, Booccara J, Descamps P, Fondrinier E, Darai E. Fertility after conservative treatment for borderline ovarian tumours: a French multicenter study. Fertil Steril. 2005;83:284–290. doi: 10.1016/j.fertnstert.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Stewart LM, Holman CDJ, Finn JC, Preen DB, Hart R. In vitro fertilization is associated with increased risk of borderline ovarian tumours. Gynecol Oncol. 2013;129:372–376. doi: 10.1016/j.ygyno.2013.01.027. [DOI] [PubMed] [Google Scholar]
- 12.Bjorge T, Lie AK, Hovig E, et al. BRCA1 mutations in ovarian cancer and borderline tumours in Norway : a nested case-control study. Br J Cancer. 2004;91:1829–1834. doi: 10.1038/sj.bjc.6602199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Calster B, Timmerman D, Bourne T, et al. Discrimination between benign and malignant adnexal masses by specialist ultrasound examination versus serum Ca 125. J Natl Cancer Inst. 2007;99:1706–1714. doi: 10.1093/jnci/djm199. [DOI] [PubMed] [Google Scholar]
- 14.Fauvet R, Boccara J, Dufournet C, Poncelet C, Darai E. Laparoscopic management of borderline ovarian tumours : results of a French multicenter study. Ann Oncol. 2005;16:403–410. doi: 10.1093/annonc/mdi083. [DOI] [PubMed] [Google Scholar]
- 15.Kurman RJ, Visvanathan K, Roden R, et al. Early detection and treatment of ovarian cancer: shifting from early stage to minimal volume of disease based on a new model of carcinogenesis. Am J Obstet Gynecol. 2008;198:351–356. doi: 10.1016/j.ajog.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sieben NL, Oosting J, Flanagan AM, et al. Differential gene expression in ovarian tumours reveals Dusp 4 and Serpina 5 as key regulators for benign behavior of serous borderline ovarian tumours. J Clin Oncol. 2005;23:7257–7264. doi: 10.1200/JCO.2005.02.2541. [DOI] [PubMed] [Google Scholar]
- 17.Seong SJ, Kim DH, Kim MK, Song T. Controversies in borderline ovarian tumours. J Gynaecol Oncol. 2015;26(4):343. doi: 10.3802/jgo.2015.26.4.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.du Bois A, Ewald-Riegler N, du Bois O, et al. Borderline tumours of the ovary: a systematic review. Geburtshilfe Frauenheilkd. 2009;69:807–833. doi: 10.1055/s-0029-1186007. [DOI] [Google Scholar]
- 19.Risum S, Hogdall C, Loft A, et al. The diagnostic value of PET/CT for primary ovarian cancer : a prospective study. Gynecol Oncol. 2007;105:145–149. doi: 10.1016/j.ygyno.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 20.Kaern J, Trope CG, Abeler VM. A retrospective study of 370 borderline tumours of the ovary treated at Norwegian Radium Hospital from 1970 to 1982. A review of clinicopathologic features and treatment modalities. Cancer. 1993;71:1810–1820. doi: 10.1002/1097-0142(19930301)71:5<1810::AID-CNCR2820710516>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 21.Yokoyama Y, Moriya T, Takano T, et al. Clinical outcomes and risk factors for recurrence in borderline ovarian tumours. Br J Cancer. 2006;94:1586–1591. doi: 10.1038/sj.bjc.6603139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tavassoli FA, Devilee P. World Health Organization classification of tumours: pathology and genetics of tumours of the breast and female genital organs. Lyon: IARC Press; 2003. [Google Scholar]
- 23.Kane A, Uzan C, Rey A, et al. Prognostic factors in patients with ovarian serous low malignant potential (borderline) tumours with peritoneal implants. Oncologist. 2009;14:591–600. doi: 10.1634/theoncologist.2008-0263. [DOI] [PubMed] [Google Scholar]
- 24.Bell DA, Weinstock MA, Sculy RE. Peritoneal implants of ovarian serous borderline tumours: histologic features and prognosis. Cancer. 1988;62:2212–2222. doi: 10.1002/1097-0142(19881115)62:10<2212::AID-CNCR2820621024>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 25.Burks R, Sherman M, Kurman R. Micropapillary serous carcinoma of the ovary: a distinctive low-grade carcinoma related to serous borderline tumours. Am J Surg Pathol. 1996;20:1319–1330. doi: 10.1097/00000478-199611000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Silverberg S. Borderline ovarian tumours: concensus, controversy, and continuing challenges. Pathol Case Rev. 2006;11:9–17. doi: 10.1097/01.pcr.0000196556.41743.cd. [DOI] [Google Scholar]
- 27.Ronett BM, Kajdacsy-Balla A, Gilks CB, et al. Mucinous borderline ovarian tumours: points of general agreement and persistent controversies regarding nomenclature, diagnostic criteria, and behavior. Hum Pathol. 2004;35:949–960. doi: 10.1016/j.humpath.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 28.Tempfer CB, Polterauer S, Bentz EK. Accuracy of intra-operative frozen section analysis in borderline tumours of the ovary: a retrospective analysis of 96 cases and review of the literature. Gynecol Oncol. 2007;107:248–252. doi: 10.1016/j.ygyno.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 29.Morice P, Camatte S, El Hassan J, Pautier P, Duvillard P, Castaigne D. Clinical outcomes and fertility after conservative treatment of ovarian borderline tumours. Fertil Steril. 2001;75:92–96. doi: 10.1016/S0015-0282(00)01633-2. [DOI] [PubMed] [Google Scholar]
- 30.Frega A, Coluccia AC, Di Martino G, Catalano A, Milazzo GN, Assorgi C, Manzara F, Romeo GD, Gentile M, Marziani R, Moscarini M. Borderline ovarian tumors, fertility-sparing surgery and pregnancy outcome. Eur Rev Med Pharmacol Sci. 2014;18:281–284. [PubMed] [Google Scholar]
- 31.Uzan C, Muller E, Kane A, Rey A, Gouy S, Bendiffallah S, Duvillard P, Fauvet R, Darai E, Morice P. Prognostic factors for recurrence after conservative treatment in a series of 119 patients with stage I serous borderline tumors of the ovary. Ann Oncol. 2014;25:166–171. doi: 10.1093/annonc/mdt430. [DOI] [PubMed] [Google Scholar]
- 32.Gershenson DM. Clinical management potential tumours of low malignancy. Best Pract Res Clin Obstet Gynecol. 2002;16:513–527. doi: 10.1053/beog.2002.0308. [DOI] [PubMed] [Google Scholar]
- 33.Lesieur B, Kane A, Duvillard P, et al. Prognostic value of lymph node involvement in ovarian serous borderline tumours. Am J Obstet Gynecol. 2011;204:438.e1–438.e7. doi: 10.1016/j.ajog.2010.12.055. [DOI] [PubMed] [Google Scholar]
- 34.Carnatte S, Morice P, Thoury A, et al. Impact of surgical staging in patients with macroscopic ‘stage I’ ovarian borderline tumours: analysis of a continuous series of 101 cases. Eur J Cancer. 2004;40:1842–1849. doi: 10.1016/j.ejca.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 35.Deffieux X, Morice P, Camette S, et al. Results after laparoscopic management of serous borderline tumour of the ovary with peritoneal implants. Gynecol Oncol. 2005;97:84–89. doi: 10.1016/j.ygyno.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 36.Vandenput I, Amant F, Vergote I. Peritoneal recurrences might be less common in advanced stage serous borderline ovarian tumours that were treated by laparotomy. Gynecol Oncol. 2005;98:523. doi: 10.1016/j.ygyno.2005.05.037. [DOI] [PubMed] [Google Scholar]
- 37.Shim S-H, Kim S-N, Jung P-S, Dong M, Kim JE, Lee SJ. Impact of surgical staging on prognosis in patients with borderline ovarian tumours: a meta-analysis. Eur J Cancer. 2016;54:84–95. doi: 10.1016/j.ejca.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 38.Fauvet R, Boccara J, Dufournet C, David-Montefiore E, Poncelet C, Darai E. Restaging surgery for women with borderline ovarian tumours: results of a French multicenter study. Cancer. 2004;100:1145–1151. doi: 10.1002/cncr.20098. [DOI] [PubMed] [Google Scholar]
- 39.Morgan RJ Jr, Alvarez RD, Armstrong DK, Burger RA, Chen LM, Copeland L et al (2013) Ovarian cancer, version 2.2013. J Natl Copmr Canc Netw 11:1199–1209 [DOI] [PubMed]
- 40.du Bois A, Ewald-Riegler N, de Gregorio N, Reuss A, Mahner S, Fotopapoulou C, et al. Borderline tumours of the ovary: a cohort study of the Arbeitsgmeinschaft Gynaekologische Onkologie (AGO) Study Group. Eur J Cancer. 2013;49:1905–1914. doi: 10.1016/j.ejca.2013.01.035. [DOI] [PubMed] [Google Scholar]
- 41.Sutton GP, Bundy GN, Omura GA, et al. Stage III ovarian tumors of low malignant potential treated with cisplatin combination therapy: a gynecologic oncology group study. Gynecol Oncol. 1991;41:230–233. doi: 10.1016/0090-8258(91)90314-U. [DOI] [PubMed] [Google Scholar]
- 42.Barakat RR, Benjamin IB, Lewis JL, Jr, et al. Platinum based chemotherapy for advanced stage serous ovarian tumors of low malignant potential. Gynecol Oncol. 1995;59:390–393. doi: 10.1006/gyno.1995.9956. [DOI] [PubMed] [Google Scholar]
- 43.Ronnett BM, Kurman RJ, Shmookler BM, et al. Pseudomyxoma peritonei in women: a clinicopathologic analysis of 30 cases with emphasis on site of origin, prognosis, and relationship to ovarian mucinous tumors of low malignant potential. Hum Pathol. 1995;26:509–524. doi: 10.1016/0046-8177(95)90247-3. [DOI] [PubMed] [Google Scholar]
- 44.Creasman WT, Park R, Norris H, Disaia PJ, Morrow CP, Hreshchyshyn MM. Stage I borderline ovarian tumors. Obstet Gynecol. 1982;59(1):93–96. [PubMed] [Google Scholar]
- 45.Sutton GP, Bundy BN, Omura GA, Yordan EL, Beecham JB, Bonfiglio T. Stage III ovarian tumors of low malignant potential treated with cisplatin combination therapy (a Gynecologic Oncology Group study) Gynecol Oncol. 1991;41(3):230–233. doi: 10.1016/0090-8258(91)90314-U. [DOI] [PubMed] [Google Scholar]
- 46.Trope C, Kaern J, Vergote IB, Kristensen G, Abeler V. Are borderline tumors of the ovary overtreated both surgically and systemically? A review of four prospective randomized trials including 253 patients with borderline tumors. [review] [27 refs] Gynecol Oncol. 1993;51(2):236–243. doi: 10.1006/gyno.1993.1279. [DOI] [PubMed] [Google Scholar]
- 47.Barnhill DR, Kurman RJ, Brady MF, et al. Preliminary analysis of the behavior of stage I ovarian serous tumors of low malignant potential: a Gynecologic Oncology Group Study. J Clin Oncol. 1995;13:2752–2756. doi: 10.1200/JCO.1995.13.11.2752. [DOI] [PubMed] [Google Scholar]
- 48.Practice Committee of the American Society for Reproductive Medicine: Society of Reproductive Surgeons Pathogenesis, consequences and control of peritoneal adhesions in gynecologic surgery. Fertil Steril. 2007;88:21–26. doi: 10.1016/j.fertnstert.2007.04.066. [DOI] [PubMed] [Google Scholar]
- 49.Fasouliotis SJ, Davis O, Schattman G, et al. Safety and efficacy of infertility treatment after conservative management of borderline ovarian tumours: a preliminary report. Fertil Steril. 2004;82:568–572. doi: 10.1016/j.fertnstert.2004.02.114. [DOI] [PubMed] [Google Scholar]
- 50.Tinelli R, Tinelli A, Tinelli FG, Cicinelli E, Malvasi A. Conservative surgery for borderline ovarian tumors: a review. Gynecol Oncol. 2006;100:185–191. doi: 10.1016/j.ygyno.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 51.Kurman RJ, Trimble CL. The behavior of serous tumours of low malignant potential : are they ever malignant? Int J Gynecol Pathol. 1993;12:120–127. doi: 10.1097/00004347-199304000-00006. [DOI] [PubMed] [Google Scholar]
- 52.Silva EG, Gershenson DM, Malpica A, et al. The recurrence and the overall survival rates of ovarian serous borderline neoplasms with non-invasive implants is time dependent. Am J Surg Pathol. 2006;30:1367–1371. doi: 10.1097/01.pas.0000213294.81154.95. [DOI] [PubMed] [Google Scholar]
- 53.Kaern J, Trope C, Kjorstad KE, et al. Cellular DNA content as a new prognostic tool in patients with borderline tumours of the ovary. Gynecol Oncol. 1990;38:452–457. doi: 10.1016/0090-8258(90)90090-8. [DOI] [PubMed] [Google Scholar]
- 54.Harter P, Gershenson D, Lhomme C, Lecuru F, Ledermann J, Provencher DM, et al. Gynecologic Cancer Intergroup (GCIG) consensus review for ovarian tumours of low malignant potential (borderline ovarian tumours) Int J Gynecol Cancer. 2014;24:S5–S8. doi: 10.1097/IGC.0000000000000282. [DOI] [PubMed] [Google Scholar]
- 55.Trillsch F, Mahner S, Woelber L, Vettorazzi E, Reuss A, Ewald-Riegler N, et al. Age-dependent differences in borderline ovarian tumours (BOT) regarding clinical characteristics and outcome: results from a sub-analysis of the Arbeitsgmeinschaft Gynaekologische Onkologie (AGO) ROBOT study. Ann Oncol. 2014;25:1320–1327. doi: 10.1093/annonc/mdu119. [DOI] [PubMed] [Google Scholar]
- 56.Zanetta G, Rota S, Chiari S, Bonazzi C, Bratina G, Mangioni C. Behaviour of borderline tumours with particular interest to persistence, recurrence and progression to invasive carcinoma: a prospective study. J Clin Oncol. 2001;19:2658–2664. doi: 10.1200/JCO.2001.19.10.2658. [DOI] [PubMed] [Google Scholar]
- 57.Park JY, Kim DY, Kim JH, Kim YM, Kim YT, Nam JH. Surgical management of borderline ovarian tumours: the role of fertility-sparing surgery. Gynecol Oncol. 2009;113:75–82. doi: 10.1016/j.ygyno.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 58.Trimble CL, Kosary C, Trimble EL. Long term survival and patterns of care in women with ovarian carcinoma: a population based analysis. Gynecol Oncol. 2002;86:34–37. doi: 10.1006/gyno.2002.6711. [DOI] [PubMed] [Google Scholar]
- 59.APM H, Odicino F, Maisonneuve P, et al. Carcinoma of the ovary. 26th Annual Report on the Results of Treatment in Gynaecological Cancer. Int J Gynaecol Obstet. 2006;95(suppl 1):S161–S192. doi: 10.1016/S0020-7292(06)60033-7. [DOI] [PubMed] [Google Scholar]
- 60.Seidman JD, Kurman RJ. Subclassification of serous borderline tumors of the ovary into benign and malignant types: a clinicopathologic study of 65 advanced stage cases. Am J Surg Pathol. 1996;20:1331–1345. doi: 10.1097/00000478-199611000-00004. [DOI] [PubMed] [Google Scholar]