Abstract
Nanoparticles represent a new generation of drug delivery systems that can be engineered to harness optimal target selectivity for specific cells and tissues and high drug loading capacity, allowing for improved pharmacokinetics and enhanced bioavailability of therapeutics. The spontaneous propensity of both organic and colloidal nanoparticles to be captured by the cells of the reticuloendothelial system encouraged their utilization as passive targeting systems that can be preferentially directed to innate immune cells, such as macrophages, dendritic cells and neutrophils. The natural affinity for phagocytic cells suggests the possible implementation of nanoparticles as an immunotherapeutic platform for inflammatory diseases and autoimmune disorders. Here we discuss the recent advances in the application of nanotechnology to induce antigen-specific tolerance in autoimmunity and the use of nanoparticles for anti-inflammatory therapies, including treatment of inflammatory bowel diseases, psoriasis and rheumatoid arthritis.
Introduction
Nanomaterials within 1–100 nm have attracted much interest for biomedical applications thanks to a fortunate combination of their chemical and physical size-dependent properties and favorable interaction with the building blocks of life at the nanoscale. Nanoparticles (NPs) were eminently proposed as carriers endowed with inherent targeting properties to improve the available means for the cure of cancer (Ferrari, 2005). However, the impressive impact of nanotechnology in biomedicine has elicited the diffusion of NPs for the treatment of several diseases well beyond cancer research, in the attempt to find new solutions for currently unsolved problems. In particular, the design of high quality organic and/or inorganic nanocarriers represents a promising new road to the development of a novel generation of nanotools that match specific requirements for the management of different autoimmune and/or inflammatory disease conditions (Clemente-Casares & Santamaria, 2014). Indeed, NPs with carefully controlled chemistry, size, surface charge and tailorable functionalization with targeting ligands can convey drugs to previously considered inaccessible sites and give them new functions. Hence, nanoengineered drug carriers can target selectively cells and tissues, or preserve the drugs from the aggression of host defenses before they reach the desired destination (Xia, 2014).
The ability to fabricate NPs that fulfill exact requirements, and to adjust their size and morphology at the nanoscale with great precision, allows researchers to control their function and fate in a biological system. A number of nanodrugs have been announced in the marketplace in the past few years and many more are currently under clinical trials (Eifler & Thaxton, 2011). NP-based therapeutics, including nanoconjugates, nanoassemblies and nanosized formulations of approved drugs, can significantly improve the treatments of diseases, promising to reshape a versatile platform for pharmaceutical industries (Davis, Chen et al., 2008, Sun, Zhang et al., 2014).
In this review, we focus on nanomedicine-based treatments of autoimmune disorders and inflammatory diseases with emphasis on Inflammatory Bowel Diseases (IBDs), psoriasis and rheumatoid arthritis (RA).
Nanotechnology in inflammatory and autoimmune diseases
Compared to traditional drugs, “nano-drugs” present several advantages, including: 1) improving the delivery of insoluble drugs, maximizing the bioavailability and the treatment efficacy and reducing the side effects; 2) increasing the plasma half-life of peptide drugs, protecting them from degradation caused by the environment and by the high levels of proteases or other enzymes in the bloodstream; 3) co-delivering drugs and targeting agents for the efficient drug delivery and treatment of specific cells; 4) combining diagnostic tools with therapeutic mediators overcoming multidrug resistance mechanisms and resulting in “theranostic” agents; 5) controlling the release of drugs over a manageable period of time at precise dosages; 6) facilitating the drug transport across the biological barriers.
Although the number of different types of NPs intended for biomedical application is growing rapidly, most of them can be classified into two major classes: NPs that contain organic molecules and/or polymeric scaffolds as a main building material and those that use inorganic elements, usually colloidal metals, as a core (Fig. 1).
Figure 1.
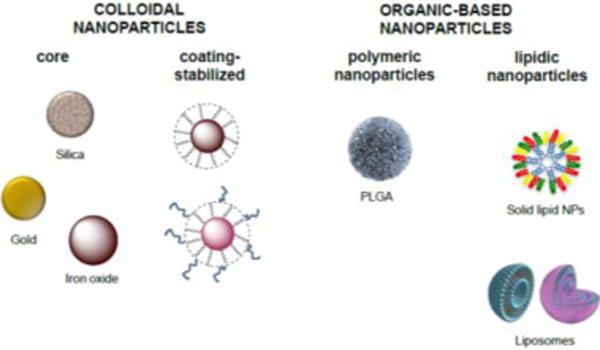
Examples of nanovectors used for the treatment of autoimmune diseases. Colloidal nanoparticles consist of a metal core (e.g., silica, gold, iron oxide) normally stabilized by an organic/polymeric coating. Organic nanoparticles can be either polymeric (e.g., PLGA) or lipid-based (e.g., SLNs or liposomes).
A key example of the first class is the biodegradable and biocompatible polymer poly(DL-lactide-co-glycolide acid) (PLGA) and its derivatives, which are approved by the Food and Drug Administration (FDA) and are generally considered as election products for the delivery of genic material, peptides and molecules in macrophages in vitro as well as in vivo (Brunner, Cohen et al., 2010, Mundargi, Babu et al., 2008). Another important family of drug nanocarriers are the lipid-based NPs, consisting of self-assembled nanoarchitectures primarily based on lipids as their building blocks (Khoury, Escriou et al., 2008, Moon, Huang et al., 2012). These include, although are not limited to, liposomes, solid lipid NPs and nanoemulsions, and are currently considered among the least toxic nanomaterials for in vivo applications. Extensive research has been conducted using lipid-based nanocarriers especially leading to progress in DNA/RNA and drug delivery (Puri, Loomis et al., 2009). On the other hand, most inorganic-based NPs share the same basic structure: a central core that defines the physical properties of the NP (including fluorescence, optical, magnetic, and electronic properties), with a protective (bio)organic coating on the surface, usually responsible for the biological recognition, for the improvement of the NP solubility, and for evading the clearance of the host immune system when it is undesired. This outer layer protects the core from degradation in a physiologically aggressive environment and can form electrostatic or covalent bonds, or both, with molecules that possess basic functional groups (Yeste, Takenaka et al., 2016).
Biodistribution and clearance of NPs in mammalians are governed by common processes mediated by the interaction with cells belonging to the macrophage family of the mononuclear phagocyte system (MPS), also referred to as reticuloendothelial system (RES), depending on their size and surface functionalization. For this reason, NPs exhibit a spontaneous propensity to be sequestered by innate immune cells. This feature makes drug nanocarriers particularly advantageous for targeted treatments directed toward immune diseases involving the contribution of circulating, as well as localized, macrophages, dendritic cells (DCs) and neutrophils.
The use of NPs to induce antigen-specific tolerance in autoimmune diseases
The possibility to control undesired immune responses has an enormous medical impact. Autoimmune diseases alone affect almost one every 20 individuals (Hayter & Cook, 2012) and, thus, represent a huge cohort of patients that might greatly benefit from NP-mediated approaches aimed at the induction of tolerance against specific antigens. Immune tolerance induction and maintenance is a very difficult task to achieve. This is partly due to the complexity of the immune system and partly to our poor understanding of its principles and mechanisms of functioning. Nevertheless, in the last decades, several approaches have been taken to induce antigen tolerance in the absence or in the presence of an active immune response. Autoreactivity and/or autoimmunity are often accompanied by the existence of an inflammatory environment and are characterized by the induction of autoreactive T cell and B cell memory formation. Inflammation allows the activation of the antigen presenting cells (APCs), for instance DCs, that preset the autoantigen(s) and lead to adaptive immune cell activation. The use of nanomaterials to target either the APCs or the autoreactive lymphocytes has had a tremendous impact on our capacity to control the development of autoimmunity, as modeled in various mouse systems. Two major approaches based on the use of NPs have been described so far: i) induction of tolerance by targeting the APCs ii) induction of tolerance by directly targeting the autoreactive lymphocytes (Fig. 2). These approaches have been used to block an autoreactive process via the activation of several possible mechanisms that, ultimately, affect adaptive lymphocyte functionality, and that lead to deletion, exhaustion, regulatory T cell (Treg) and B cell (Breg) induction, or a combination of these.
Figure 2.
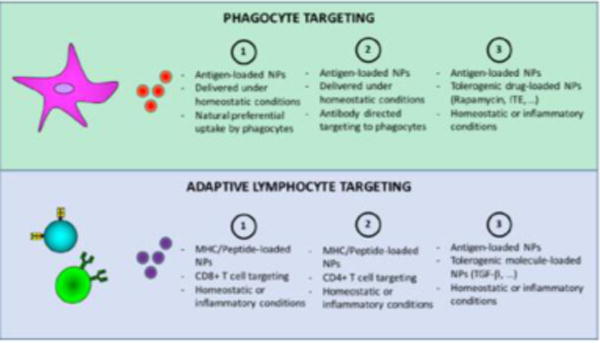
Alternative methods to induce antigen tolerance targeting phagocytes (upper panel) or T and B lymphocytes (lower panel).
Induction of tolerance by targeting the APCs under homeostatic conditions
Although enormous progresses have been made in the last decades, our understanding of the functioning of the immune system is still limited. The fundamental principles that drive inflammation, and consequently immune cell activation, and that lead to (adaptive) immune memory formation are still intensely debated. In 1989, Charles A. Janeway Jr. proposed the “Infectious non-self, non-infectious self-theory”(Janeway, 1989). In his theory Janeway proposed that the activation of the inflammatory process, which ultimately leads to the activation of antigen specific adaptive immune cells, is driven by the recognition of molecular patterns that are exclusively associated with exogenous, infectious non-self microorganisms (the so called PAMPs, pathogen associated molecular patterns). This idea has been extensively validated through the study of various families of pattern recognition receptors (PRRs), such as the Toll-like Receptors (TLRs), RIG-I like Receptors (RLRs), NOD-like Receptors (NLR) and C-type Lectin Receptors (CLRs) (Brubaker, Bonham et al., 2015). Starting from this evidence, it has been proposed that antigen presentation in the absence of inflammation (i.e.: in the absence of PRR ligands) leads to immune tolerance. Based on this assumption, it is possible to design tolerogenic approaches that target, via an ad hoc designed NP, the APCs responsible for adaptive immune cell activation. Among the possible APCs to be targeted, DCs represent an ideal candidate. DCs are at the crossroads of innate and adaptive immune responses. DCs are efficient in capturing NPs, presenting the antigen and deciding the fate of an immune response (Metcalfe & Fahmy, 2012). The capacity of nanoconjugates to specifically target DCs (via the use of anti-CD11c or other integrins) and/or specific DC-subpopulation (i.e.: DEC-205+ DCs) can be achieved using specific antibodies, as recently demonstrated (Lewis, Zaveri et al., 2012). This approach allows efficient uptake of the cargo both in vitro and in vivo and, consequently, increased antigen presentation under tolerogenic (i.e.: in the absence of PRR ligands) conditions. Besides a direct targeting of DCs, the administration of NPs able to deliver the antigen under non-inflammatory conditions has been shown to efficiently induce tolerance in the context of experimental autoimmune encephalomyelitis (EAE). The use of NPs coupled with the immunodominant myelin proteolipid protein has been shown to protect against EAE (Getts, Martin et al., 2012). Interestingly, tolerance was achieved via multiple mechanisms, and particularly via Treg activation, abortive T-cell activation, and T-cell anergy induction.
Induction of tolerance by targeting the APCs with a tolerogenic drug
Although presentation of the antigen in the absence of inflammation has been used, by itself, as an approach to induce antigen-specific tolerance, several reports demonstrated that the co-administration of an antigen with a tolerogenic drug might be a more efficient approach to control auotreactivity. The most common commercially available drugs used to control undesired immune responses, such as Cyclosporin A or Rapamycin (Cardenas, Zhu et al., 1995, Ho, Clipstone et al., 1996), usually require chronic administration. This often leads to a general immunosuppression that favors the development of infections or tumors. For these reasons, the above mentioned immunesuppressive drugs are only used when no other alternatives are possible (i.e.: during transplantation or autoimmune disorders not responsive to biologics or other drugs). The possibility to specifically target subset of immune cells with immune-dominant antigens in the presence of a tolerogenic drug is, therefore, a very intriguing alternative approach. NPs are extremely versatile materials able to incorporate both the antigen and the selected drug. Also, the natural ability of DCs to uptake NPs makes them an ideal tool to induce antigen specific tolerance (Bachmann & Jennings, 2010). Here, we report two strategies that have been used to induce tolerance by targeting DCs with an antigen and a tolerogenic drug. Maldonado et al., demonstrated that NPs that carry a peptide (or an entire protein) and rapamycin are able to induce a potent and durable tolerogenic state (Maldonado, LaMothe et al., 2015). This tolerance is restricted to the T and B cells that recognize the specific antigen and is resistant to subsequent multiple immunogenic challenges. Surprisingly the tolerogenic state upon exposure to these NPs can be achieved also in the presence of potent TLR agonists. This approach has been used to induce tolerance during allergic responses, EAE and to block the responses of antibody directed against factor VIII in hemophilia A mice. A second example is represented by the use of NPs that transport a peptide derived from the myelin oligodendrocyte glycoprotein (MOG) and co-deliver 2-(1′H-indole-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester (ITE) (Yeste, Nadeau et al., 2012). ITE is a ligand for the transcription factor aryl hydrocarbon receptor (AhR). AhR is one of the transcription factors that regulate Treg cell differentiation (Quintana, Basso et al., 2008). The use of this nanocomplex increased the capacity of DCs to induce Treg cell differentiation in vitro and reduced EAE development in vivo. The protective effect in vivo was due to an increased number of FoxP3+ Treg cells. Similarly to what was found for NP-mediated co-deliver of ITE and MOG, the co-deliver of the β cell antigen proinsulin and ITE also proved to efficiently induce the differentiation of Treg cells in vivo and protect against diabetes development in non-obese diabetic mice (Yeste et al., 2016).
Induction of tolerance by directly targeting the autoreactive lymphocytes
The NP-based approaches described so far efficiently induce antigen-specific lymphocyte tolerance via indirectly targeting APCs. Nevertheless, several approaches have also been undertaken to directly target adaptive lymphocytes to induce/expand regulatory populations. The use of NPs that transport disease-relevant peptide-major histocompatibility complexes (pMHC-NP) has proved to be particularly efficient to induce tolerance in several mouse models of autoimmune disorders. NPs bearing diabetes relevant peptide-MHC class I complexes have been shown to expand a population of autoregulatory low-avidity CD8+ T cells that protects against diabetes (Tsai, Shameli et al., 2010). This T cell population in turn suppresses antigen presentation by DCs in an interferon(IFN)-γ-, indolamine 2,3-dioxygenase-dependent manner. The activity of the expanded autoregulatory CD8+ T cells is particularly interesting because –although elicited against a known relevant antigen-the immunosuppressive effect is due to inhibition of multiple (unknown) autoantigen-loaded DCs. Ultimately, this approach has the potential to suppress multiple autoreactive lymphocyte clones. Similar to pMHC class I loaded NPs, also NPs conjugated with autoimmune relevant peptide-MHC class II complexes have been generated and successfully used (Clemente-Casares, Blanco et al., 2016). When MHC class II is used to present the antigen, NP administration allows the expansion of type 1 regulatory T cells (Tr1 cells), that on the one hand via TGF-β and IL-10, suppress DCs activation. On the other hand, these Tr1 cells also expand (via IL-21 production) a population of IL-10 producing Breg cells that further blocks autoimmune development. This approach was shown to potently dampen autoimmunity in mouse models of diabetes, arthritis as well as encephalomyelitis. Importantly, the administration of pMHC-NPs was effective also after disease induction, and not only when animals were pretreated with the nano-conjugate. Also, pMHC-NPs proved to be effective on both murine and human T cells, did not show off-target toxicity and appeared to be very well tolerated upon in vivo administration (Singha, Shao et al., 2017). All these characteristics make pMHC-NPs very valuable tools to develop future therapies and appear to be very important from a translational point of view.
Finally, it was also reported that the efficacy of Treg induction can be further ameliorated by administering NPs that transport biologically active moieties, such as TGF-β and IL-2 (McHugh, Park et al., 2015). Overall, these examples clearly demonstrate the translational potential of the use of NPs (differentially loaded, conjugated or directed) to induce antigen-specific tolerance and ameliorate the development of an autoimmune disorder.
The use of NPs for anti-inflammatory therapies
Not only autoimmune, but also chronic inflammatory diseases are generally treated with systemic administration of immunosuppressive or anti-inflammatory drugs. As also discussed above, the prolonged use of these drugs has severe toxic effects and limited therapeutic benefits with large numbers of patients that respond poorly or do not respond at all to therapies. For instance, therapies based on monoclonal antibodies (such as monoclonal antibodies to tumor necrosis factor (TNF)α or other inflammatory interleukins) have several disadvantages: i) antibody immunogenicity rendering subsequent therapies ineffective; ii) reactivation of tuberculosis, (anti-TNFα), liver and marrow toxicity (anti-IL-6R) and susceptibility to infectious diseases (all) and iii) heterogeneity in the therapeutic efficacy. Therapies based on the use of corticosteroids for long periods can cause impaired glucose metabolism, generalized immunosuppression, cardiovascular diseases, adrenal insufficiency, obesity, problems in wound repair and hypertension. Finally, therapies based on the use of immunosuppressors, like Cyclosporin A and Tacrolimus, have severe side effects which comprehend generalized immunosuppression, hypertension, paraesthesiae or tremor and headache, hypomagnesaemia, renal impairment, and gastrointestinal upset.
For the treatment of chronic inflammatory diseases, the development of efficient and selective ways of targeting specific cell and tissues and relevant pathways to minimize side effects represents an urgent need.
NPs for drug delivery in Inflammatory Bowel Disease
The use of NPs in anti-inflammatory therapies has been deeply investigated particularly for Inflammatory Bowel Diseases (IBDs).
IBDs are a group of inflammatory conditions of unknown etiology involving the colon and the small bowel. The major types of IBDs are Crohn’s disease (CD) and Ulcerative Colitis (UC) that affect approximately 1.4 million persons in the United States and 2.2 million persons in Europe (Baumgart & Carding, 2007). These two pathological entities, even if classified in the same family, differ in terms of location and nature. CD can affect almost any intestinal segment, from the mouth to the anus (the terminal ileum is the most affected segment), while UC is limited to the large intestine, starting always from the rectum with the potential of extending throughout the colon. Microscopically, CD has a typical transmural spread in the bowel wall, leading to stenosis, abscesses, and fistulas. UC is restricted to the mucosal layer and the main complication of the disease is colonic bleeding (Baumgart & Sandborn, 2007).
IBDs potently damage the bowel: almost all the patients with CD and half the patients with UC need at least one surgical procedure during their clinical history. Crohn’s patients are exposed to the risk of repeated surgery and thus to malabsorption, malnutrition, and finally to short bowel syndrome. UC patients have a definite risk of permanent stoma. Moreover, both CD and UC patients share the 20–30 times risk of developing colorectal cancer after 10–20 years of disease history. Finally, IBDs present with extraintestinal manifestations (more than one third of the patients) that are highly disabling and that affect the central and peripheral nervous system, the liver, the joint and bones, the eyes and the skin.
Available pharmacological treatments of IBDs are focused on the control of chronic inflammation through systemic immunosuppression, using thiopurines, cyclosporine or therapies with monoclonal antibodies. All these therapies have problems of safety and long-term efficacy, exposing patients to risk of opportunistic infections, the potential for cancer development, with a short-term and long-term efficacy not exceeding the 80% and 60%, respectively.
The ideal therapy in IBDs should be directed only against the diseased tissue, leaving unaltered the healthy intestine to avoid as much as possible the undesirable side effects. Moreover, oral administration has been identified as the most appropriate approach for colitis treatment (Isaacs, Lewis et al., 2005). Taking all this into account, the use of nanotherapeutics represents a promising new strategy for the treatment of IBD.
There are different obstacles that oral therapies must overcome to be effective: the acidic PH and the digestive enzymes in the stomach; the mucus in the colon and the barrier of epithelial cells. Ways for NPs to resist to acidic PH and to penetrate the mucus have been already set up (Date, Hanes et al., 2016) and new strategies will be probably developed in the next few years. Moreover, the strong phagocyte infiltration at the inflammatory sites, where the integrity of the epithelial barrier is compromised (Coskun, 2014), favors NP uptake by inflammatory cells specifically in the inflamed areas, thus restraining drug delivery at the diseased tissue. Nano-formulations of currently used drugs and potential new drugs, like small interfering RNAs (siRNAs) against inflammatory cytokines, have been shown to be suitable for oral administration. Various experimental animal models also demonstrated this treatment has a higher efficacy in reducing colitis symptoms compared to oral or systemic administration of free drugs.
PLGA have been used in rat models of acetic acid induced colitis to deliver mesalazine (5-ASA) to the colon via oral administration after colitis induction. PLGA favored the delivery of the drug at the inflammatory sites and minimized systemic effects (Mahajan, Sakarkar et al., 2011). The same preferential accumulation of NP at the inflamed colon was observed when 5-ASA-loaded silica NPs (SiNP) were used for the treatment of mice with TNBS-induced colitis (Moulari, Pertuit et al., 2008, Pertuit, Moulari et al., 2007).
Strategies to deliver siRNAs to inhibit TNFα production by phagocytes at the inflamed intestine have also been tested. A major issue in siRNA-based therapies is siRNA crossing of cell membrane. Numerous NP-based strategies have been investigated, ranging from the use of thioketal NPs that are specifically degraded by ROS (Wilson, Dalmasso et al., 2010), present in high concentrations at the inflammatory sites, to the use of PLA, efficiently taken up by inflammatory phagocytes present in the diseased tissue (Laroui, Theiss et al., 2011). To avoid possible NP and siRNA degradation in the gastrointestinal tract a strategy called NP-in-microparticle oral drug delivery system (NiMOS) (Bhavsar, Tiwari et al., 2006, Kriegel, Attarwala et al., 2013) has also been successfully tested in DSS-induced colitis mouse models. Gelatin NPs are encapsulated in poli-ε-caprolactone (PCL) microparticles (MP). MP protects NP form degradation until arrival in the colon. Here lipases digest the PLC and NPs are released and taken up by phagocytes (Bhavsar & Amiji, 2007, Bhavsar & Amiji, 2008). NiMOS carring anti-TNFα siRNAs proved to be very efficient in neutralize TNFα production and alleviate DSS induced colitis symptoms (Kriegel & Amiji, 2011).
Good results in the treatment of rat colitis models have also been obtained by oral administration of PLGA nanoformulations incorporating the immunosuppressant Tacrolimus. Efficient NP accumulation at the inflammatory site and effective therapeutic results have been described (Lamprecht, Yamamoto et al., 2005a, Lamprecht, Yamamoto et al., 2005b).
Distribution of non-functionalized PLGA NPs has also been analyzed in humans. Interestingly, a preferential accumulation of NPs at the sites of lesions has been found in a way dependent on the severity of the lesion, the higher the severity the higher the accumulation. Diversely, no binding of NP has been observed at the mucosa of healthy volunteers (Schmidt, Lautenschlaeger et al., 2013). Moreover, the capacity of NP to accumulate at the inflamed mucosa was further improved by conjugation with PEG (Lautenschlager, Schmidt et al., 2013).
In these studies, NP-based drug delivery has proven to be a promising approach given the capacity of NP to discriminate between diseased and non-diseased tissues.
NPs for drug delivery in psoriasis
Psoriasis is a chronic inflammatory disease of the skin that affects around 2% of the population worldwide (Boehncke & Schon, 2015). Psoriasis has different manifestations, but plaque psoriasis represents the commonest form of the disease affecting almost 90% of psoriatic patients (Diani, Altomare et al., 2015). Notably, psoriasis is considered a common risk factor for several diseases, including cardiovascular diseases and diabetes. Moreover, about 30% of psoriatic patients also develop arthritis (Chung, Eder et al., 2015, Golden, McCormick et al., 2013, Husted, Thavaneswaran et al., 2011). From an immunological point of view, Th17, Tc17 and γδ T cells have a major role in maintaining inflammation in the diseased skin (Benham, Norris et al., 2013, Laggner, Di Meglio et al., 2011a, Laggner, Di Meglio et al., 2011b). Topical treatments, systemic administration of anti-inflammatory medicaments, and phototherapies are the main approaches used to control the disease. Nevertheless, topical treatments are not particularly effective since they do not efficiently reach effector cells and systemic treatments are used only in severe forms of psoriasis given their important side effects.
Nanoformulations of corticosteroids for topic applications in plaque type psoriasis to inhibit the biosynthesis of eicosanoids have proved to be effective in animal models (Baboota, Alam et al., 2011). Incorporation of corticosteroids in SLNs, PLGA, microemulsions and liposomes has been demonstrated to be more efficient than the commercial corticosteroid formulations in skin penetration and reduction of inflammation (Badilli, Sen et al., 2011, Cevc & Blume, 2004, Korting, Zienicke et al., 1990, Zhang & Smith, 2011). Nanoformulations integrating compounds that reduce inflammatory cytokine production have been also tested. Nanostructured lipid carriers (NLPs) loaded with an analogue of vitamin D3, the calcipotriol, and methotrexate have shown good skin infiltration and no significant skin irritation (Lin, Huang et al., 2010).
Efforts have been made to generate NPs encapsulating Cyclosporin A or Tacrolimus for topic administration to improve the penetration of the skin, reduce the dosage and the systemic effects. NLPs incorporating Cyclosporin A have shown a more efficient anti-psoriatic effect in mouse models compared to the market formulations (Arora, Katiyar et al., 2017). Analogously, Tacrolimus incorporated into NPs self-assembled by the conjugations of hyaluronic acid with cholesterol (HA– Chol NPs) combined with nicotinamide (NIC), has proven to be more effective than the commercial Tacrolimus formulations in reducing the psoriatic lesions induced by imiquimod (Wan, Pan et al., 2017).
Metotrexate (MTX) is an acid folic inhibitor used for severe psoriasis. It prevents DNA synthesis in psoriatic lesions thus reducing keratinocytes proliferation. Moreover, it acts as an immunesuppressor by inducing T cell death. Numerous MTX incorporating nanoformulations have been produced and tested in human cadavers, animal models and in clinical trials. Generally, increased efficiency of drug absorption through the skin, reduction of diseased areas and decrease of the disease severity index have been observed (Ali, Salah et al., 2008, Lakshmi, Devi et al., 2007, Trotta, Peira et al., 2004, Trotta, Peira et al., 2002).
NPs for drug delivery in Rheumatoid Arthritis
Rheumatoid Arthritis (RA) is a complex, debilitating, chronic, systemic autoimmune disease. RA is characterized by an immunological, inflammatory and mesenchymal tissue reaction in the synovium accompanied by polyarticular synovitis, that ultimately leads to the progressive destruction of articular and periarticular structures (Harris, 1990). RA is a very diffuse autoimmune disease, affecting up to 0.1 to 3% of the adult population in Western countries and causing significant morbidity and disabilities. There are no curative therapies for RA, and the diverse physical and pharmacological strategies in use have the goal to relief pain and retard the progress of the irreversible articular damage. The current therapeutic strategy is aimed at reducing the inflammation in the synovia either with steroids or with immunesupressors, including MTX and biologic response modifiers (used in the most aggressive cases) such as monoclonal antibodies to TNFα, CD20 (B cell) and IL-6R.
As for the other chronic diseases, the severe side effects of current therapies impose the identification of new less toxic treatments.
Macrophages are passive targets of NPs given their phagocytic activity. Moreover, NPs administered systemically tend to accumulate at the inflamed joint due to the increased permeability of capillaries at sites of inflammation (Maeda, 2001). Since macrophages play a major role in RA, the use of NP as drug carriers to target macrophages at the diseased joints is a very promising approach for reduction of systemic side effects.
Multiple NP-based approaches have been tested to treat RA in animal models.
Liposomes encapsulated non-steroidal anti-inflammatory drugs (NSAIDs) administered intra venous have shown very potent anti-inflammatory effects, as proved by the reduction of serum levels of inflammatory cytokines. In some models, complete recovery from RA has also been observed especially if NPs were covered with PEG to increase their half-life (Bernardi, Zilberstein et al., 2009, Metselaar, Wauben et al., 2003). Clear reduction of RA clinical scores has been also obtained using NP to deliver siRNAs for down-modulating the expression of inflammatory cytokines such as TNFα or the Notch1 pathway, in animal models. Effective encapsulation of siRNAs has been obtained using thiolate glycol chitosan (tGC) NPs in aqueous solution. NP-delivered anti-Notch1 or anti-TNFα siRNAs showed a very potent inhibition of joint inflammation in the mouse model of collagen-induced arthritis (CIA) (Kim, Park et al., 2015, Lee, Lee et al., 2014). Superparamagnetic iron oxide NPs (SPION) have been used to deliver siRNAs to the inflamed joints via systemic administration. In this case siRNAs that downregulate the expression of the IL-2 receptor β chain have been used with good results in terms of accumulation at the in inflamed joints, and reduction of rat experimental arthritis symptoms (Duan, Dong et al., 2014). MTX efficiently delays joint destruction. Different nano-formulations with encapsulated MTX have been tested in RA models. Arginin-glycineaspartic acid (RGD)-attached gold (Au) half-shell NPs containing MTX injected iv have shown accumulation at the diseased joint in CIA mice. Moreover, by near-infrared (NIR) irradiation and local heat generation due to Au half-shells, enhanced local drug release is induced with a significant improved efficacy in terms of dosage and recovery form disease compared to the free drug administration (Lee, Kim et al., 2013).
Another interesting approach made use of folate NPs to target activated macrophages. Only activated macrophages express folate receptor β (FR β), therefore, only macrophages actively involved in the inflammatory process are targeted. Administration of folic acid and MTX-conjugated poly(amidoamine) dendrimer (generation 5 [G5]) NP covalently conjugated to polyvalent folic acid (FA) (G5-FA-MTX) into CIA rats could selectively target and kill inflammatory macrophages with an important reduction of inflammation and arthritis clinical score (Nogueira, Gomes et al., 2016, Thomas, Goonewardena et al., 2011). MTX was also used in combination with human serum albumin NPs showing a significant delay in cartilage disruption in a humanized mouse model of RA (Fiehn, Neumann et al., 2004).
Finally, Tacrolimus incorporating albumin nanoformulations have proven to be much more efficient in reducing arthritis clinical scores than other Tacrolimus formulations for iv or oral administration (Thao, Byeon et al., 2016).
Despite the availability of numerous preclinical studies demonstrating the efficacy of therapeutic approaches based on the use of nanocarriers, clinical confirmation is mostly lacking. Very few clinical trials using nanoformulations for the treatment of these inflammatory diseases have been conducted or are ongoing (Table 1). Clinical research to ascertain the fate and the efficacy of nanodrugs is now necessary to establish nanomedicine as a real perspective for patients.
Table 1.
Clinical trials to test the efficacy of nanomedicine for the treatment of inflammatory diseases
| Disease | Drug | Nanoparticle | Phase | Ref. |
|---|---|---|---|---|
| Type 1 Diabetes | Proinsulin derived peptide | gold nanoparticles | Phase I | https://clinicaltrials.gov/ |
| Plaque Psoriasis | Paclitaxel | uncoted nanoparticle ointment, SOR007 | Phase I | https://clinicaltrials.gov/ |
| Plaque Psoriasis | Methotrexate | Nisomes | PhaseI/II | Lakshmi, Devi et al., 2007 |
| RA | Prednisolone | Liposomes | Phase II | https://clinicaltrials.gov/ |
| RA | TNFα inhibitor | PEGylated nanomolecules | Phase III | https://clinicaltrials.gov/ |
Conclusions
The use of NPs for the treatment of autoimmune and inflammatory diseases will offer innovative solutions to improve the efficacy of current immunosuppressive therapies and will help to overcome side effects associated with such therapies. Several experimental examples support the increased efficacy of NP-based drug delivery compared to current therapies in the treatment of autoimmune and inflammatory diseases. Not only nanomedicines consent the reduction of the dose and the frequency of drug administration to reach the desired improvement of the clinical scores, but also allow the preferential targeting of diseased-, compared to healthy, tissues, with a sensitive reduction of side effects. A better understanding of the anatomical barriers and of the innate immune mechanisms underlying autoimmune and inflammatory diseases is necessary for the construction of increasingly efficient NP-based therapies. If confirmed by clinical trial, nanomedicine promises will represent a significant advance to current therapies and a real perspective for patients of a long-lasting disease control.
Acknowledgments
IZ is supported by NIH grant 1R01AI121066-01A1, 1R01DK115217, HDDC P30 DK034854 grant, Harvard Medical School Milton Found, CCFA Senior Research Awards, the Eleanor and Miles Shore 50th Anniversary Fellowship Program, and the Cariplo Foundation. FG is supported by Associazione Italiana per la Ricerca sul Cancro (IG 2016Id.18842), Cariplo Foundation (Grant 2014-0655), and Fondazione Regionale per la Ricerca Biomedica (FRRB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ali MFM, Salah M, Rafea M, Saleh N. Liposomal Methotrexate hydrogel for treatment of localized psoriasis: Preparation, characterization and laser targeting. Med Sci Monitor. 2008;14:Pi66–Pi74. [PubMed] [Google Scholar]
- Arora R, Katiyar SS, Kushwah V, Jain S. Solid lipid NPs and nanostructured lipid carrier-based nanotherapeutics in treatment of psoriasis: a comparative study. Expert opinion on drug delivery. 2017;14:165–177. doi: 10.1080/17425247.2017.1264386. [DOI] [PubMed] [Google Scholar]
- Baboota S, Alam MS, Sharma S, Sahni JK, Kumar A, Ali J. Nanocarrier-based hydrogel of betamethasone dipropionate and salicylic acid for treatment of psoriasis. International journal of pharmaceutical investigation. 2011;1:139–47. doi: 10.4103/2230-973X.85963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann MF, Jennings GT. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol. 2010;10:787–96. doi: 10.1038/nri2868. [DOI] [PubMed] [Google Scholar]
- Badilli U, Sen T, Tarimci N. Microparticulate Based Topical Delivery System of Clobetasol Propionate. Aaps Pharmscitech. 2011;12:949–957. doi: 10.1208/s12249-011-9661-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369:1627–40. doi: 10.1016/S0140-6736(07)60750-8. [DOI] [PubMed] [Google Scholar]
- Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007;369:1641–57. doi: 10.1016/S0140-6736(07)60751-X. [DOI] [PubMed] [Google Scholar]
- Benham H, Norris P, Goodall J, Wechalekar MD, FitzGerald O, Szentpetery A, Smith M, Thomas R, Gaston H. Th17 and Th22 cells in psoriatic arthritis and psoriasis. Arthritis research & therapy. 2013;15 doi: 10.1186/ar4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi A, Zilberstein ACCV, Jager E, Campos MM, Morrone FB, Calixto JB, Pohlmann AR, Guterres SS, Battastini AMO. Effects of indomethacin-loaded nanocapsules in experimental models of inflammation in rats. Brit J Pharmacol. 2009;158:1104–1111. doi: 10.1111/j.1476-5381.2009.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhavsar MD, Amiji MM. Gastrointestinal distribution and in vivo gene transfection studies with NPs-in-microsphere oral system (NiMOS) Journal of Controlled Release. 2007;119:339–348. doi: 10.1016/j.jconrel.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Bhavsar MD, Amiji MM. Development of novel biodegradable polymeric NPs-in-microsphere formulation for local plasmid DNA delivery in the gastrointestinal tract. Aaps Pharmscitech. 2008;9:288–294. doi: 10.1208/s12249-007-9021-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhavsar MD, Tiwari SB, Amiji MM. Formulation optimization for the NPs-in-microsphere hybrid oral delivery system using factorial design. Journal of Controlled Release. 2006;110:422–430. doi: 10.1016/j.jconrel.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Boehncke WH, Schon MP. Psoriasis. Lancet. 2015;386:983–994. doi: 10.1016/S0140-6736(14)61909-7. [DOI] [PubMed] [Google Scholar]
- Brubaker SW, Bonham KS, Zanoni I, Kagan JC. Innate Immune Pattern Recognition: A Cell Biological Perspective. Annu Rev Immunol. 2015 doi: 10.1146/annurev-immunol-032414-112240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner T, Cohen S, Monsonego A. Silencing of proinflammatory genes targeted to peritoneal-residing macrophages using siRNA encapsulated in biodegradable microspheres. Biomaterials. 2010;31:2627–36. doi: 10.1016/j.biomaterials.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Cardenas ME, Zhu D, Heitman J. Molecular mechanisms of immunosuppression by cyclosporine, FK506, and rapamycin. Curr Opin Nephrol Hypertens. 1995;4:472–7. doi: 10.1097/00041552-199511000-00002. [DOI] [PubMed] [Google Scholar]
- Cevc G, Blume G. Hydrocortisone and dexamethasone in very deformable drug carriers have increased biological potency, prolonged effect, and reduced therapeutic dosage. Bba-Biomembranes. 2004;1663:61–73. doi: 10.1016/j.bbamem.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Chung Y, Eder L, Chandran V, Gladman D. Cardiovascular Morbidity and Mortality of Cutaneous Psoriasis (PsC) and Psoriatic Arthritis (PsA) are well Documented, with Increased Risks of Myocardial Infarction, Ischemic Heart Disease, and the Metabolic Syndrome in these Patients. Journal of Rheumatology. 2015;42:1344–1345. [Google Scholar]
- Clemente-Casares X, Blanco J, Ambalavanan P, Yamanouchi J, Singha S, Fandos C, Tsai S, Wang J, Garabatos N, Izquierdo C, Agrawal S, Keough MB, Yong VW, James E, Moore A, Yang Y, Stratmann T, Serra P, Santamaria P. Expanding antigen-specific regulatory networks to treat autoimmunity. Nature. 2016;530:434–40. doi: 10.1038/nature16962. [DOI] [PubMed] [Google Scholar]
- Clemente-Casares X, Santamaria P. Nanomedicine in autoimmunity. Immunol Lett. 2014;158:167–74. doi: 10.1016/j.imlet.2013.12.018. [DOI] [PubMed] [Google Scholar]
- Coskun M. Intestinal epithelium in inflammatory bowel disease. Frontiers in medicine. 2014;1:24. doi: 10.3389/fmed.2014.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Date AA, Hanes J, Ensign LM. NPs for oral delivery: Design, evaluation and state-of-the-art. Journal of Controlled Release. 2016;240:504–526. doi: 10.1016/j.jconrel.2016.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7:771–82. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- Diani M, Altomare G, Reali E. T cell responses in psoriasis and psoriatic arthritis. Autoimmun Rev. 2015;14:286–292. doi: 10.1016/j.autrev.2014.11.012. [DOI] [PubMed] [Google Scholar]
- Duan JL, Dong JL, Zhang TT, Su ZY, Ding J, Zhang Y, Mao XH. Polyethyleneimine-functionalized iron oxide NPs for systemic siRNA delivery in experimental arthritis. Nanomedicine: nanotechnology, biology, and medicine. 2014;9:789–801. doi: 10.2217/nnm.13.217. [DOI] [PubMed] [Google Scholar]
- Eifler AC, Thaxton CS. Nanoparticle therapeutics: FDA approval, clinical trials, regulatory pathways, and case study. Methods Mol Biol. 2011;726:325–38. doi: 10.1007/978-1-61779-052-2_21. [DOI] [PubMed] [Google Scholar]
- Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer. 2005;5:161–71. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]
- Fiehn C, Neumann E, Wunder A, Krienke S, Gay S, Muller-Ladner U. Methotrexate (MTX) and albumin coupled with MTX (MTX-HSA) suppress synovial fibroblast invasion and cartilage degradation in vivo. Annals of the rheumatic diseases. 2004;63:884–886. doi: 10.1136/ard.2003.013748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getts DR, Martin AJ, McCarthy DP, Terry RL, Hunter ZN, Yap WT, Getts MT, Pleiss M, Luo X, King NJ, Shea LD, Miller SD. Microparticles bearing encephalitogenic peptides induce T-cell tolerance and ameliorate experimental autoimmune encephalomyelitis. Nat Biotechnol. 2012;30:1217–24. doi: 10.1038/nbt.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden JB, McCormick TS, Ward NL. IL-17 in psoriasis: Implications for therapy and cardiovascular co-morbidities. Cytokine. 2013;62:195–201. doi: 10.1016/j.cyto.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris ED., Jr Rheumatoid arthritis. Pathophysiology and implications for therapy. N Engl J Med. 1990;322:1277–89. doi: 10.1056/NEJM199005033221805. [DOI] [PubMed] [Google Scholar]
- Hayter SM, Cook MC. Updated assessment of the prevalence, spectrum and case definition of autoimmune disease. Autoimmun Rev. 2012;11:754–65. doi: 10.1016/j.autrev.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Ho S, Clipstone N, Timmermann L, Northrop J, Graef I, Fiorentino D, Nourse J, Crabtree GR. The mechanism of action of cyclosporin A and FK506. Clin Immunol Immunopathol. 1996;80:S40–5. doi: 10.1006/clin.1996.0140. [DOI] [PubMed] [Google Scholar]
- Husted JA, Thavaneswaran A, Chandran V, Eder L, Rosen CF, Cook RJ, Gladman DD. Cardiovascular and other comorbidities in patients with psoriatic arthritis: A comparison with patients with psoriasis. Arthrit Care Res. 2011;63:1729–1735. doi: 10.1002/acr.20627. [DOI] [PubMed] [Google Scholar]
- Isaacs KL, Lewis JD, Sandborn WJ, Sands BE, Targan SR. State of the art: IBD therapy and clinical trials in IBD. Inflammatory bowel diseases. 2005;11:S3–S12. doi: 10.1097/01.mib.0000184852.84558.b2. [DOI] [PubMed] [Google Scholar]
- Janeway CA., Jr Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- Khoury M, Escriou V, Courties G, Galy A, Yao R, Largeau C, Scherman D, Jorgensen C, Apparailly F. Efficient suppression of murine arthritis by combined anticytokine small interfering RNA lipoplexes. Arthritis Rheum. 2008;58:2356–67. doi: 10.1002/art.23660. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Park JS, Lee SJ, Jang J, Park JS, Back SH, Bahn G, Park JH, Kang YM, Kim SH, Kwon IC, Jo DG, Kim K. Notch1 targeting siRNA delivery NPs for rheumatoid arthritis therapy. Journal of Controlled Release. 2015;216:140–148. doi: 10.1016/j.jconrel.2015.08.025. [DOI] [PubMed] [Google Scholar]
- Korting HC, Zienicke H, Schaferkorting M, Braunfalco O. Liposome Encapsulation Improves Efficacy of Betamethasone Dipropionate in Atopic Eczema but Not in Psoriasis-Vulgaris. Eur J Clin Pharmacol. 1990;39:349–351. doi: 10.1007/BF00315408. [DOI] [PubMed] [Google Scholar]
- Kriegel C, Amiji M. Oral TNF-alpha gene silencing using a polymeric microsphere-based delivery system for the treatment of inflammatory bowel disease. Journal of Controlled Release. 2011;150:77–86. doi: 10.1016/j.jconrel.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegel C, Attarwala H, Amiji M. Multi-compartmental oral delivery systems for nucleic acid therapy in the gastrointestinal tract. Adv Drug Deliver Rev. 2013;65:891–901. doi: 10.1016/j.addr.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Laggner U, Di Meglio P, Perera GK, Hundhausen C, Lacy KE, Ali N, Smith CH, Hayday AC, Nickoloff BJ, Nestle FO. Clinical relevance of pro-inflammatory skin homing CLA+ V gamma 9V delta 2 T cells in psoriasis. Brit J Dermatol. 2011a;164:907–907. [Google Scholar]
- Laggner U, Di Meglio P, Perera GK, Hundhausen C, Lacy KE, Ali N, Smith CH, Hayday AC, Nickoloff BJ, Nestle FO. Identification of a Novel Proinflammatory Human Skin-Homing V gamma 9V delta 2 T Cell Subset with a Potential Role in Psoriasis. Journal of Immunology. 2011b;187:2783–2793. doi: 10.4049/jimmunol.1100804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshmi PK, Devi GS, Bhaskaran S, Sacchidanand S. Niosomal methotrexate gel in the treatment of localized psoriasis: Phase I and phase II studies. Indian J Dermatol Ve. 2007;73:157–161. doi: 10.4103/0378-6323.32709. [DOI] [PubMed] [Google Scholar]
- Lamprecht A, Yamamoto H, Takeuchi H, Kawashima Y. NPs enhance therapeutic efficiency by selectively increased local drug dose in experimental colitis in rats. J Pharmacol Exp Ther. 2005a;315:196–202. doi: 10.1124/jpet.105.088146. [DOI] [PubMed] [Google Scholar]
- Lamprecht A, Yamamoto H, Takeuchi H, Kawashima Y. A pH-sensitive microsphere system for the colon delivery of tacrolimus containing NPs. Journal of Controlled Release. 2005b;104:337–346. doi: 10.1016/j.jconrel.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Laroui H, Theiss AL, Yan Y, Dalmasso G, Nguyen HT, Sitaraman SV, Merlin D. Functional TNFalpha gene silencing mediated by polyethyleneimine/TNFalpha siRNA nanocomplexes in inflamed colon. Biomaterials. 2011;32:1218–28. doi: 10.1016/j.biomaterials.2010.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautenschlager C, Schmidt C, Lehr CM, Fischer D, Stallmach A. PEG-functionalized microparticles selectively target inflamed mucosa in inflammatory bowel disease. Eur J Pharm Biopharm. 2013;85:578–586. doi: 10.1016/j.ejpb.2013.09.016. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Lee A, Hwang SR, Park JS, Jang J, Huh MS, Jo DG, Yoon SY, Byun Y, Kim SH, Kwon IC, Youn I, Kim K. TNF-alpha Gene Silencing Using Polymerized siRNA/Thiolated Glycol Chitosan NPs for Rheumatoid Arthritis. Mol Ther. 2014;22:397–408. doi: 10.1038/mt.2013.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SM, Kim HJ, Ha YJ, Park YN, Lee SK, Park YB, Yoo KH. Targeted Chemo-Photothermal Treatments of Rheumatoid Arthritis Using Gold Half-Shell Multifunctional NPs. ACS nano. 2013;7:50–57. doi: 10.1021/nn301215q. [DOI] [PubMed] [Google Scholar]
- Lewis JS, Zaveri TD, Crooks CP, 2nd, Keselowsky BG. Microparticle surface modifications targeting dendritic cells for non-activating applications. Biomaterials. 2012;33:7221–32. doi: 10.1016/j.biomaterials.2012.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YK, Huang ZR, Zhuo RZ, Fang JY. Combination of calcipotriol and methotrexate in nanostructured lipid carriers for topical delivery. International journal of nanomedicine. 2010;5:117–28. doi: 10.2147/ijn.s9155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: The key role of tumor-selective macromolecular drug targeting. Adv Enzyme Regul. 2001;41:189–207. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- Mahajan N, Sakarkar D, Manmode A, Pathak V, Ingole R, Dewade D. Biodegradable NPs for Targeted Delivery in Treatment of Ulcerative Colitis. Adv Sci Lett. 2011;4:349–356. [Google Scholar]
- Maldonado RA, LaMothe RA, Ferrari JD, Zhang AH, Rossi RJ, Kolte PN, Griset AP, O’Neil C, Altreuter DH, Browning E, Johnston L, Farokhzad OC, Langer R, Scott DW, von Andrian UH, Kishimoto TK. Polymeric synthetic NPs for the induction of antigen-specific immunological tolerance. Proc Natl Acad Sci U S A. 2015;112:E156–65. doi: 10.1073/pnas.1408686111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh MD, Park J, Uhrich R, Gao W, Horwitz DA, Fahmy TM. Paracrine co-delivery of TGF-beta and IL-2 using CD4-targeted NPs for induction and maintenance of regulatory T cells. Biomaterials. 2015;59:172–81. doi: 10.1016/j.biomaterials.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe SM, Fahmy TM. Targeted nanotherapy for induction of therapeutic immune responses. Trends in molecular medicine. 2012;18:72–80. doi: 10.1016/j.molmed.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Metselaar JM, Wauben MHM, Wagenaar-Hilbers JPA, Boerman OC, Storm G. Complete remission of experimental arthritis by joint targeting of glucocorticoids with long-circulating liposomes. Arthritis and rheumatism. 2003;48:2059–2066. doi: 10.1002/art.11140. [DOI] [PubMed] [Google Scholar]
- Moon JJ, Huang B, Irvine DJ. Engineering nano- and microparticles to tune immunity. Adv Mater. 2012;24:3724–46. doi: 10.1002/adma.201200446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulari B, Pertuit D, Pellequer Y, Lamprecht A. The targeting of surface modified silica NPs to inflamed tissue in experimental colitis. Biomaterials. 2008;29:4554–60. doi: 10.1016/j.biomaterials.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Mundargi RC, Babu VR, Rangaswamy V, Patel P, Aminabhavi TM. Nano/micro technologies for delivering macromolecular therapeutics using poly(D,L-lactide-co-glycolide) and its derivatives. J Control Release. 2008;125:193–209. doi: 10.1016/j.jconrel.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Nogueira E, Gomes AC, Preto A, Cavaco-Paulo A. Folate-targeted NPs for rheumatoid arthritis therapy. Nanomed-Nanotechnol. 2016;12:1113–1126. doi: 10.1016/j.nano.2015.12.365. [DOI] [PubMed] [Google Scholar]
- Pertuit D, Moulari B, Betz T, Nadaradjane A, Neumann D, Ismaili L, Refouvelet B, Pellequer Y, Lamprecht A. 5-amino salicylic acid bound NPs for the therapy of inflammatory bowel disease. Journal of controlled release: official journal of the Controlled Release Society. 2007;123:211–8. doi: 10.1016/j.jconrel.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Puri A, Loomis K, Smith B, Lee JH, Yavlovich A, Heldman E, Blumenthal R. Lipid-based NPs as pharmaceutical drug carriers: from concepts to clinic. Crit Rev Ther Drug Carrier Syst. 2009;26:523–80. doi: 10.1615/critrevtherdrugcarriersyst.v26.i6.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Lautenschlaeger C, Collnot EM, Schumann M, Bojarski C, Schulzke JD, Lehr CM, Stallmach A. Nano- and microscaled particles for drug targeting to inflamed intestinal mucosa-A first in vivo study in human patients. Journal of Controlled Release. 2013;165:139–145. doi: 10.1016/j.jconrel.2012.10.019. [DOI] [PubMed] [Google Scholar]
- Singha S, Shao K, Yang Y, Clemente-Casares X, Sole P, Clemente A, Blanco J, Dai Q, Song F, Liu SW, Yamanouchi J, Umeshappa CS, Nanjundappa RH, Detampel P, Amrein M, Fandos C, Tanguay R, Newbigging S, Serra P, Khadra A, et al. Peptide-MHC-based nanomedicines for autoimmunity function as T-cell receptor microclustering devices. Nat Nanotechnol. 2017 doi: 10.1038/nnano.2017.56. [DOI] [PubMed] [Google Scholar]
- Sun T, Zhang YS, Pang B, Hyun DC, Yang M, Xia Y. Engineered NPs for drug delivery in cancer therapy. Angew Chem Int Ed Engl. 2014;53:12320–64. doi: 10.1002/anie.201403036. [DOI] [PubMed] [Google Scholar]
- Thao LQ, Byeon HJ, Lee C, Lee S, Lee ES, Choi HG, Park ES, Youn YS. Pharmaceutical potential of tacrolimus-loaded albumin NPs having targetability to rheumatoid arthritis tissues. Int J Pharmaceut. 2016;497:268–276. doi: 10.1016/j.ijpharm.2015.12.004. [DOI] [PubMed] [Google Scholar]
- Thomas TP, Goonewardena SN, Majoros IJ, Kotlyar A, Cao ZY, Leroueil PR, Baker JR. Folate-Targeted NPs Show Efficacy in the Treatment of Inflammatory Arthritis. Arthritis and rheumatism. 2011;63:2671–2680. doi: 10.1002/art.30459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotta M, Peira E, Carlotti ME, Gallarate M. Deformable liposomes for dermal administration of methotrexate. Int J Pharm. 2004;270:119–25. doi: 10.1016/j.ijpharm.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Trotta M, Peira E, Debernardi F, Gallarate M. Elastic liposomes for skin delivery of dipotassium glycyrrhizinate. Int J Pharm. 2002;241:319–27. doi: 10.1016/s0378-5173(02)00266-1. [DOI] [PubMed] [Google Scholar]
- Tsai S, Shameli A, Yamanouchi J, Clemente-Casares X, Wang J, Serra P, Yang Y, Medarova Z, Moore A, Santamaria P. Reversal of autoimmunity by boosting memory-like autoregulatory T cells. Immunity. 2010;32:568–80. doi: 10.1016/j.immuni.2010.03.015. [DOI] [PubMed] [Google Scholar]
- Wan T, Pan W, Long Y, Yu K, Liu S, Ruan W, Pan J, Qin M, Wu C, Xu Y. Effects of NPs with hydrotropic nicotinamide on tacrolimus: permeability through psoriatic skin and antipsoriatic and antiproliferative activities. International journal of nanomedicine. 2017;12:1485–1497. doi: 10.2147/IJN.S126210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DS, Dalmasso G, Wang L, Sitaraman SV, Merlin D, Murthy N. Orally delivered thioketal NPs loaded with TNF-alpha-siRNA target inflammation and inhibit gene expression in the intestines. Nature materials. 2010;9:923–8. doi: 10.1038/nmat2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y. Editorial: are we entering the nano era? Angew Chem Int Ed Engl. 2014;53:12268–71. doi: 10.1002/anie.201406740. [DOI] [PubMed] [Google Scholar]
- Yeste A, Nadeau M, Burns EJ, Weiner HL, Quintana FJ. Nanoparticle-mediated codelivery of myelin antigen and a tolerogenic small molecule suppresses experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2012;109:11270–5. doi: 10.1073/pnas.1120611109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeste A, Takenaka MC, Mascanfroni ID, Nadeau M, Kenison JE, Patel B, Tukpah AM, Babon JA, DeNicola M, Kent SC, Pozo D, Quintana FJ. Tolerogenic NPs inhibit T cell-mediated autoimmunity through SOCS2. Sci Signal. 2016;9:ra61. doi: 10.1126/scisignal.aad0612. [DOI] [PubMed] [Google Scholar]
- Zhang J, Smith E. Percutaneous permeation of betamethasone 17-valerate incorporated in lipid NPs. Journal of pharmaceutical sciences. 2011;100:896–903. doi: 10.1002/jps.22329. [DOI] [PubMed] [Google Scholar]


