1. Introduction
Cancer immunotherapy has generated a paradigm shift in the way that cancer is treated. However, not only have high response rates to immunotherapy been observed only in certain cancer types, but many patients fail to mount effective antitumor immune responses.[1] Multiple lines of evidence indicate that the presence of tumor-infiltrating lymphocytes (TILs) serves as a prognostic marker and predicts antitumor immune responses to different therapies, including immunotherapy and chemotherapy.[2] Tumors lacking TILs have been characterized as “non-inflamed”, and generally correlate with treatment failure and poor prognosis.[3] For example, the efficacy of one type of cancer immunotherapy, immune checkpoint blockade antibodies, in patients with breast cancer, which has relatively less TILs (mean percentage of 10%), [4] is far less effective compared to that in patients with melanoma or non-small cell lung carcinoma, characterized as “inflamed” tumor types, which are abundant with TILs.[5] Thus, how to promote the transport, activity, and persistence of TILs in the tumor microenvironment is crucial for developing effective immunotherapies, especially for the “non-inflamed” tumor types.
Intratumoral accumulation of cytotoxic immune cells (e.g. TILs) and cancer therapies are crucial for enhanced anti-tumor responses. Yet, successful transport of cancer therapies depends on their sequential negotiation of biological barriers, [6, 7] including non-specific distribution into non-lymphatic or non-tumor tissue compartments, limitations in hemorheological/blood vessel flow and pressure gradients within tumors, the density and composition of the tumor stroma, [8] and the dynamics in intratumoral cell-cell and cell-matrix interfaces affecting tensile forces.[6, 9] Although these physical spatio-temporal peculiarities and aberrations of tumors have been less studied, it is becoming clear that intratumoral processes may be more indicative of therapeutic efficacy.[10–13]. Furthermore, it is becoming clear that as the tumor progresses, intratumoral transport properties change.[14] These intratumoral transport property changes may also be heterogeneous within the tumor as well as between patients, and a greater understanding of how these changes influence therapeutic efficacy will ultimately lead to fine-tuning of the tumor microenvironment. This fine-tuning would then tip the balance towards a phenotype that is amenable to immune cells and immunotherapy transport. Thus, the impact of transport phenomena on immunotherapeutic efficacy (and therapeutic resistance) should be considered when developing strategies for new immunotherapies.
Application of nanotechnologies can facilitate the transport of therapeutics into tumors. For the purposes of this review, the “operational definition for nanotechnology involves three ingredients: 1) nanoscale sizes in the device or its crucial components; 2) the man-made nature; and 3) having properties that only arise because of the nanoscopic dimensions”.[15] However, we recognize that there are other acceptable definitions in the scientific literature. Applying nanotechnology to package drugs, small molecules, oligonucleotides, immunomodulatory compounds, etc. into nanometer- or micrometer-size particles allows these therapeutics to pass sequential physical and biological barriers and enrich in tumor tissues.[16–21] The released therapeutics can affect not only cancer cells but also immune cells, consequently modifying the tumor microenvironment.[22] Nanotechnology-based cancer vaccines promote rapid expansion of tumor-specific T cells, and various forms of nanoparticles (NPs) have been utilized in the generation of therapeutic T cells for adoptive T cell therapies. Furthermore, multiple laboratories have applied nanotechnology-based approaches to unleash the activities of TILs by suppressing the activities of immune checkpoint inhibitor proteins, regulatory T (Treg) cells, and immunosuppressive myeloid cells (IMCs), by mimicking tumor-associated leukocytes, and by altering the tumor extracellular matrix (ECM). However, the development of new nanotechnologies for cancer treatment will ultimately depend on overcoming biological transport barriers to enhance cancer immunotherapy.[7] This review summarizes advances in two areas of nanotechnology-based cancer immunotherapy: 1) generation of tumor antigen-specific T cells, and 2) bypassing the transport barriers in facilitating antitumor immunity.
2. Promoting generation and tissue infiltration of T lymphocytes using NP-based immunotherapies
2.1. Nanotherapeutic cancer vaccines
Immunotherapy with cancer vaccines offers the potential for highly specific cancer cell cytotoxicity, superlative T cell memory response, and minimal systemic toxicity. Therefore, it is a very attractive approach for cancer treatment. Cancer vaccines typically include a tumor antigen and an adjuvant to enhance immune responses. Since dendritic cells (DCs) are the major antigen-presenting cells (APCs), DC vaccines have also been developed, through the use of both circulating and bone marrow-derived DCs, in order to maximize antitumor immunity. The first therapeutic DC vaccine, Sipuleucel-T (Provenge®), generated from autologous peripheral blood mononuclear cells pulsed with a prostatic acid phosphatase–GM-CSF recombinant fusion protein, [23] was approved for treatment of metastatic castration-resistant prostate cancer by the U.S. Food and Drug Administration (FDA) in 2010. A once promising non-DC vaccine, nelipepimut-S (E75) vaccine (NeuVax™), for human epidermal growth factor receptor 2 (HER2)+ breast cancer that contains the E75 antigen peptide mixed with the adjuvant, granulocyte-macrophage colony-stimulating factor (GM-CSF), [24, 25] was recently tested in a Phase III clinical trial, sponsored by Galena Biopharma, Inc.. However, the clinical trial was discontinued based on negative data from a planned safety and futility interim analysis. With the recent advancements in next-generation sequencing, therapeutic vaccines can now be tailored to target a group of patient-specific mutant neoantigen epitopes, as evidenced by the success in treatment of melanoma patients with therapeutic cancer vaccines.[26–28] More vaccines are expected to reach the clinic in the coming years.
Despite recent successes, cancer vaccine development still faces a number of challenges. One key factor in determining DC vaccine or non-DC vaccine efficacy is transport of the vaccine-internalized DCs to lymphatic tissues; more specifically, transport to the T cell-rich paracortex of the lymph nodes, where stimulation of antigen-specific T cells occurs. Animal studies have shown that the route of administration determines biodistribution and, consequently, vaccine efficacy. For example, intravenously injected DC vaccines mainly accumulate in the spleen, whereas subcutaneously injected DCs preferentially home to the T cell areas of the draining lymph nodes.[29] Clinical studies have revealed that regardless of vaccine injection site, less than 5% of the DCs can reach the lymph nodes.[30] In addition, the stimulatory signals of ex vivo matured DCs, used for DC vaccine generation, cannot be maintained in vivo. Therefore, designing strategies to transport response-eliciting DC vaccine or non-DC vaccines and overcoming the sequential physical and biological barriers for this transport are critical for the success of cancer vaccines.
NPs and microparticles have been incorporated into cancer vaccines to deliver tumor antigens. NPs can be loaded with more than a single antigen epitope, can improve antigen stability, can slow the release of antigens for sustained T cell responses, and can be targeted to specific sites. Injecting NPs that contain antigens and immunomodulatory compounds leads to the accumulation of APCs, such as DCs, at the injection site, followed by APC transport into lymph nodes for antigen presentation to T cells.[31–33] However, due to the lack of optimization for direct transport through lymphatic vessels, NP-based vaccines have fallen short.[34, 35] Thus, a recent strategy to develop lymph-node targeted NP-based vaccines, included not only size-tuning and covalent and non-covalent attachment of polyethylene glycol (i.e., PEGylation) to reduce NP-mediated immunogenicity, but also the hitch-hiking of NPs, specifically liposomes, onto albumin proteins, which migrate to lymph nodes.[36, 37] Therefore, the ability to directly transport NPs and microparticles to the tumor will ultimately produce a more potent vaccine.
NPs and microparticles can also serve as adjuvants in order to boost antitumor immunity. Although various forms of aluminum salt precipitates (alum, 1–50 μm) have been widely used as adjuvants in prophylactic vaccines for infectious diseases, these T helper 2 (Th2) cell-biased adjuvants are not effective in activating TILs, specifically, CD8+ cytotoxic T cells.[38, 39] Interestingly, porous silicon microparticles (PSMs), which not only serve as adjuvants but also aide in adjuvant and tumor antigen delivery, are effective in triggering DC production of type I interferon (IFN-I; including IFN-α and β), which is essential for the cytotoxic activity of CD8+ cytotoxic T cells.[40] It has been well documented that IFN-I production by host APCs serves as the bridge to connect innate and adaptive immune responses.[41] In addition, the micrometer-size particles can also serve as a reservoir for sustained release of tumor antigen peptides and facilitate antigen processing inside the DCs. Treatment with a DC vaccine carrying PSMs, serving as an adjuvant and loaded with HER2-specific peptides (Nano-DC vaccine), modulated the tumor immune microenvironment, as indicated by elevated levels of intratumoral inflammatory cytokines and tumor-infiltrating, antigen-presenting CD11c+ DCs in a murine model of HER2+ breast cancer. Nano-DC vaccine treatment completely inhibited tumor growth. Importantly, antitumor immunity was CD8+ cytotoxic T cell-dependent, as depletion of this subtype of T lymphocytes completely abolished inhibition of tumor growth.[40] Polymer-based nanovaccines have also been developed for cancer treatment. Gao and colleagues recently reported a STING-activating nanovaccine, consisting of a synthetic polymeric NP (PC7A NP) with an antigen. This vaccine generated a strong cytotoxic T cell response.[42] The enhancement of the transport of cancer vaccines and DCs by nanotechnologies will undoubtedly lead to improved effectiveness, but the therapeutic components of cancer vaccines are also key for this efficacy.
Nanotechnology has played a very significant role in the development of next-generation messenger (m)RNA-based therapeutic cancer vaccines. In contrast to the peptide vaccines, mRNA vaccines have the advantage of incorporating multiple antigen epitopes in one minigene construct, and thus, can be customized to fit the needs of individual patients, based on the unique mutation spectrum in their cancer genome. In addition, the mRNA molecules can serve as self-adjuvants, once in complex with selected proteins on polymers, by stimulating innate immune Toll-like receptor (TLR) 7 and 8 signaling.[43–45] The mRNA vaccines also differ from the traditional DNA plasmid vaccines in that, among other advantages, they function in both dividing and non-dividing cells, and there is no risk for genomic integration.[46, 47] Still, mRNA molecules are vulnerable to degradation by plasma and tissue enzymes. In addition, they cannot enter APCs by default and need to be transfected into these cells ex vivo (DC vaccine) or delivered by NPs in vivo (non-DC vaccine). Various forms of NPs have been generated by both academic laboratories and biopharmaceutical companies to achieve maximum efficiency for mRNA vaccines. CureVac developed a two-component mRNA vaccine: a free mRNA encoding the tumor antigen mixed with a 250–350 nm protamine/mRNA complex for additional immune stimulation.[43] The ratio of these free and complexed mRNA molecules can be optimized to ensure both effective antigen expression and potent immunostimulation. Su and colleagues applied biodegradable NPs to deliver mRNA.[44] In this construct, a pH-responsive poly-(β-amino ester) core is enveloped by a phospholipid bilayer to minimize potential toxicity from the polymer, and mRNA molecules are adsorbed onto the surface of the phospholipids through electrostatic interactions. The authors found that intranasal administration of the mRNA NPs could trigger mRNA expression in mice as soon as six hours after treatment. We have taken a different approach to develop therapeutic NP- and mRNA-based vaccines. Instead of exposing the antigen-encoding mRNA molecules to the harsh physiological environment, we packaged mRNA into a core structure and wrapped it with a lipid shell to generate lipopolyplex mRNA vaccines.[45] Inside the lipopolyplex, mRNA molecules are shielded from cellular RNases. Once the intradermally administered mRNA vaccine NPs are taken up by the APCs, tumor antigens are effectively expressed, and the APCs are potently stimulated. We have demonstrated excellent therapeutic efficacy of this mRNA vaccine in murine tumor models. Further, a recent study has demonstrated that the formulation of mRNA vaccines can be tailored to target the lymphatic system by simply adjusting the net charge of the NPs constituted with mRNA and cationic liposomes (i.e., DOTMA/DOPE).[48] The intravenously injected RNA-lipopolyplexes were captured by DCs, and they stimulated IFN-α expression. Therapeutic efficacy was demonstrated both in murine tumor models and in a Phase I dose-escalation clinical trial. It is important to point out that the application of NP-based mRNA vaccines is not limited to cancer treatment. A recent study showed successful application of this lipid NP-encapsulated modified mRNA vaccine in the treatment of Zika virus infection.[49] Thus, it is clear that NPs can provide a significant advantage in bridging innate immune responses with adaptive immune responses for the development of anti-infectious agents as well as cancer immunotherapies.
2.2. Nanotherapeutic adoptive T cell therapy
Nanotechnology has been incorporated in the design of several cancer therapies to enhance their physical, chemical, and/or biological properties, and recently, nanotechnology is being tested in the design, generation, and use in adoptive T cell therapy.[50] In adoptive T cell therapy, tumor-specific cytotoxic T cells, cultured from patient-harvested T cells, are infused back into the patient, with the intent to recognize, target, and destroy tumor cells.[51] Adoptive T cell therapy, using engineered Chimeric Antigen Receptors (CAR) and T Cell Receptors (TCR), is promising for treating a variety of cancers.[52–59] Recent clinical trials using T cells expressing CARs have shown unprecedented success in treating multiple myeloma, [60] leukemia, [61–63] sarcoma, [64] and neuroblastoma, [65–67] and there are currently over 300 CAR-T cell clinical trials being conducted. Recent clinical trials of adoptive T cell therapy using TCR-engineered T cells have also proven successful for the treatment of patients with synovial sarcoma[68] and metastatic melanoma.[68, 69] Adoptive TCR-engineered T cell therapy is currently being tested in patients with bladder carcinoma, breast cancer, esophagus carcinoma, lung cancer, multiple myeloma, neuroblastoma, and ovarian cancer. However, some common limitations with adoptive T cell therapy include not only the time restraints and costs of T cell generation but also the subsequent rapid decline in viability and function of the transplanted T cells.
Recent advancements have addressed these limitations by incorporating nanotechnology with adoptive T cell therapy. For example, through the use of paramagnetic, nanoscale artificial APCs (nano-aAPC), tumor-specific T cells can be efficiently enriched and expanded in vitro, conferring a proliferation advantage after adoptive T cell transfer in vivo.[70] Furthermore, NPs have been used to enhance the functional activity of T cells. The transfer of autologous T cells carrying NPs loaded with NSC-878777, a dual inhibitor of two key phosphatases (Shp1 and Shp2), which normally downregulate TCR activation in the immunological synapse between APCs and T cells, enhanced survival in mice with advanced prostate cancer.[71] Furthermore, nanotechnology has also been incorporated into adoptive T cell transfer to deliver potent drugs to tumor sites. Autologous nanocapsule-functionalized T cells that carried Sn-38-loaded nanocapsules on their surfaces, basically serving as living chaperones, successfully delivered chemotherapeutics directly to tumor sites. This live T cell delivery approach effectively reduced tumor burden after two weeks of treatment and enhanced survival under conditions where free SN-38– and SN-38–loaded nanocapsules alone were ineffective.[72] Thus, by incorporating nanotechnology tools, the process of T cell generation for adoptive transfer can become more streamlined, T cell functional activity can be more long-lasting in vivo after adoptive transfer, and adoptively transferred T cells can be used to deliver other therapeutics.
The potent clinical responses of adoptive T cell therapy suggest that at least a portion of engineered T cells can be transported to the tumor site. However, once CAR-engineered T cells or TCR-engineered T cells reach tumor sites, the question is whether these cancer antigen-specific T cells can more efficiently and effectively perform their designed function to eliminate cancer cells. Recent evidence suggests that when combined with nanotechnology, this may be feasible. For example, in a melanoma adoptive immunotherapy model, T cells activated by nano-aAPC induced greater activation of previously activated T cells compared to naïve T cells, resulting in a lower threshold for activation. Further, application of an external magnetic field induced nano-aAPC aggregation; thereby, enhancing T cell proliferation in vitro. The in vivo adoptive transfer of nano-aAPC aggregated T cells inhibited B16 melanoma growth.[73] Overall, these promising results indicate that the inclusion of nanotechnology in adoptive T cell therapy has many beneficial clinical applications. Through the combination of nanotechnology and adoptive T cell therapy, many promising advancements have been made with this type of cancer immunotherapy. The ability to use autologous cancer antigen-specific T cells to directly transport inhibitors via NPs into the tumor site appears to be effective in the few reported cases. However, it is possible that loading the nanocapsule or NP “backpacks” on T cells with additional cancer treatments, such as mRNA vaccines or checkpoint inhibitors, could be the next stage in maximizing therapeutic efficacy.
2.3. Nanotherapeutic agents at the interface between innate and adaptive immune responses
Whereas cancer vaccines and engineered T cells target specific cell types in the innate and adaptive immune systems, there are other immunotherapeutic agents that connect innate and adaptive immune responses. Bispecific T cell engager (BiTE) antibodies and NPs are just some examples in this group. The BiTE antibodies are heterodimers of IgG single chain variable fragments (scFv) that have dual specificities for a tumor-associated antigen presented by innate immune APCs and for adaptive immune T cells, and can recruit cytotoxic T cells to tumor cells.[74, 75] This group of therapeutic agents has been successfully used as a cancer immunotherapy in animal studies and clinical applications.[76–78] In fact, Blinatumomab, a BiTE antibody, has been approved by the U.S. FDA as the first drug in this class to treat Philadelphia chromosome-negative relapse or refractory acute lymphoblastic leukemia. Furthermore, in a recent study, Yuan and colleagues applied colloidal NPs to develop a multivalent bispecific nanobioconjugate engager (mBiNE) composed of colloidal NPs conjugated with a HER2-specific antibody and a calreticulin-binding low-density lipoprotein receptor-related protein 1 (LRP1) that specifically binds the cell surface HER2 protein and pro-phagocytosis-mediating calreticulin.[79] Treatment of tumor-bearing mice with mBiNE promoted receptor-targeted phagocytosis of cancer cells by macrophages and, consequently, enhanced antigen processing and presentation by APCs. Interestingly, the initial response to mBiNE treatment was dependent on HER2 expression, but the subsequent antitumor immunity was also effective on HER2− cells, indicating an antigen-spreading effect.
Antigen-capturing NPs (AC-NPs), which are composed of chemically modified poly(lactic-co-glycolic acid) (PLGA) that binds to tumor-derived protein antigens with specificity, represent another group of immunotherapeutic agents in this class. Min and colleagues recently demonstrated that not only can NPs capture tumor antigens shed from the tumor cell debris in mice after radiation therapy, but they can also deliver these tumor-specific proteins to innate immune APCs. Further, their results indicated that surface properties of the NPs determine the types of protein antigen that can be captured.[80] The antigen-captured AC-NPs induced expansion of adaptive immune cytotoxic T cells and provided synergistic inhibition of tumor growth when they were applied in combination with anti-programmed cell death 1 (anti-PD-1) antibody in irradiated, B16F10 melanoma-bearing mice.
Nanotechnology has also been applied to overcome challenges in production, processing, and storage of the traditional therapeutic antibodies. Applying the same design strategy of mRNA vaccines, Stadler and colleagues have recently shown that a polymer/lipid-based formulation of mRNA can be used to effectively produce a bispecific antibody, targeting the adaptive immune TCR-associated molecule CD3 and the innate immune APC-presented tumor-associated antigen claudin 6 (CLDN6) in vivo.[81] This therapeutic mRNA was effective at eliminating large tumors in murine tumor models. In summary, it is clear that nanotechnology can be used to bridge the innate and adaptive immune responses; thus, promoting homing of TILs to tumors. However, for maximal antitumor immunity, these TILs must overcome additional physical and biological transport barriers to fully function inside tumors.
3. Breaking transport barriers to enhance cancer immunotherapy using NP-based immunotherapies
3.1. Nanotherapeutic immune checkpoint blockade
A major barrier of cancer immunotherapy is negative regulators (or checkpoints) in the tumor microenvironment. Although negative immune regulation is fundamentally important for maintaining a homeostatic balance between host immunity and tolerance, T-cell co-inhibitory molecules, cytotoxic lymphocyte-associated molecule-4 (CTLA-4) and PD-1, inhibit T cell activation and proliferation, which normally augment the antitumor immune activity of T cells. PD-1, expressed on T cells, interacts with its ligands, PD-L1 (B7-h1) and PD-L2, which are expressed on tumor cells and stromal cells.[82, 83] CTLA-4 inhibits T cell activity by competing with the co-stimulatory molecule CD28 for binding to the shared ligand CD80.[84] Several therapeutics have been designed to inhibit these immune checkpoint molecules in order to allow a patient’s own immune cells to kill tumors.
Recent clinical trials using the anti-CTLA-4 monoclonal antibody ipilimumab, [85] the anti-PD-1 antibody pembrolizumab, [86] and most recently, the anti-programmed death ligand 1 (PD-L1) antibody MPDL3280A[4, 87] have led to long-term patient survival and sometimes a cure in patients with “inflamed” solid tumor types (e.g., melanoma, non-small cell lung cancer, renal cell cancer carcinoma, urothelial carcinoma, and head-and-neck squamous cell carcinoma). Still, immune checkpoint blockade-based immunotherapies rely on the high expression of PD-L1 on tumors and/or pre-existing tumor-infiltrating CD8+ T cells expressing PD-1, [88] which are evident in “inflamed”, but not “non-inflamed” cancer types. In fact, pancreatic cancer and some types of breast cancer may have lower levels of immune checkpoint proteins and ligands expressed on tumors and intratumoral immune cells, respectively, [4] and therefore, immune checkpoint inhibitors may have a low success rate in these cancer types.
Thus, recent strategies have attempted to further enhance the effectiveness of immune checkpoint blockade-based immunotherapies, including the combination of immunotherapies with NPs. This combination would allow for a targeted, sustained release of immune checkpoint antibodies in a controllable manner, possibly enhancing the transport of cytotoxic, effector T cells to tumors.[89] Wang, C. et al. used a microneedle patch to deliver anti-PD-1 via self-dissociating NPs, which released anti-PD-1 in a sustained manner in a B16F10 mouse melanoma model.[90] In another recent study, cationic lipid-assisted polyethylene glycol–polylactic acid (PEG–PLA)-based NPs were used to deliver small interfering RNA (siRNA) specific to CTLA-4 into T cells. This system allowed for the transport of CTLA-4-siRNA-possessing cytotoxic, effector T cells into tumor sites in tumor-bearing mice.[91] The combination of NPs and immunotherapy allows for delivery of checkpoint-based immunotherapies into T cells, thereby manipulating the immunosuppressive tumor microenvironment from the inside out at the same time as enhancing T cell-mediated antitumor immunity.
Strategies combining NPs, immune checkpoint blockade, and other immunomodulatory compounds with photodynamic therapy (PDT) have shown significant immunological antitumor responses, including the development of immunological memory.[92, 93] In PDT treatment, photosensitizing agents are exposed to a particular wavelength of light, generating reactive oxygen species (ROS) that kill nearby cells.[94] Through the use of nanosized carriers, photosensitizing agents can be transported to the tumor site, and subsequently activated. Indeed, administration of zinc pyrophosphate (ZnP) NPs loaded with the photosensitizer pyrolipid (ZnP@pyro) produced an immunogenic environment in tumors. The result was the sensitization of tumors to PD-L1 checkpoint blockade therapy. Thus, the combination of ZnP@pyro, PDT, and anti-PD-L1 was successful at not only eradicating primary 4T1 breast tumors but also significantly preventing metastasis to the lung.[88] Additionally, core-shell NPs, carrying oxaliplatin in the core and PDT-activatable pyrolipid in the shell (NCP@pyrolipid) have proven, when combined with anti-PD-L1 therapy, to be effective at inducing potent tumor-specific immune responses.[93] PDT combined with anti-PD-L1 therapy appears to have a synergistic effect. Furthermore, PDT with NPs and CTLA-4 blockade eliminated tumors upon exposure to near-infrared irradiation.[95]
The combination of checkpoint blockade-based immunotherapies with nanotechnology provides an effective approach for cancer treatment. Still, it is likely that NP-enhanced immune checkpoint blockade is not sufficient to treat many cancer types. Therefore, additional strategies will be required to transport these drugs directly to the tumor site for maximal efficacy. Given that for many patients, checkpoint-based immunotherapies alone are not successful in combatting cancer, it is important to recognize that other treatments such as Nano-DC vaccines and adoptive T cell therapy could be incorporated alongside checkpoint-based immunotherapies to develop optimized cancer treatment regimens, and perhaps be more universally effective in treating all types of cancer.
3.2. Targeting the tumor microenvironment with nanoparticles
The tumor microenvironment contains numerous cells of the innate and adaptive immune system as well as matricellular proteins that modulate the intratumoral transport of TILs. Fine-tuning the tumor microenvironment using NPs may tip the balance towards a phenotype that is amenable to immune cell and immunotherapy transport. Specific modulation of Treg cells, IMCs, or matricellular proteins by NPs offers a promising approach to convert “non-inflamed” tumors to “inflamed” tumors.
Treg cells mediate immunosuppression and pose a major obstacle for effective cancer immunotherapy.[96–99] In fact, cancers commonly associated with low numbers of specific TILs, such as pancreatic cancer and breast cancer, have an increased prevalence of peripheral blood and intratumoral Treg cells.[100] High levels of Treg cells correlate with cancer progression and poor patient prognosis.[101, 102] Most studies using NPs loaded with drugs, small molecules, oligonucleotides, immunomodulatory compounds, etc. have not directly targeted Treg cells but have indirectly decreased or increased Treg cell levels. A doxorubicin-loaded NP-based zoledronic acid-containing formulation decreased the number of immunosuppressive Treg cells infiltrating into breast tumors.[103] Immunomodulatory molecules conjugated to the surfaces of PEGylated liposomes also indirectly resulted in reduced Treg cell numbers in an in vivo melanoma model.[104] Indirect targeting of Treg cells with siRNA-loaded chitosan NPs via inhibition of the matricellular protein, Galactin-1, a protein that modulates cell-cell and cell-matrix interactions, also reduced Treg cell numbers.[105] Furthermore, targeting NPs, loaded with CTLA-4-siRNA, to both cytotoxic CD4+ and CD8+ T cells significantly increased the percentage of antitumor CD8+ T cells, while it decreased the percentage of Treg cells.[91]
In addition to Treg cells, tumor-associated leukocytes, specifically IMCs, also mediate immune suppression, thus, limiting the effectiveness of cancer immunotherapies. Tumor-associated myeloid cells including myeloid-derived suppressor cells (MDSCs), tumor-associated neutrophils, regulatory tumor-associated dendritic cells, and tumor-associated macrophages constitute a significant part of the immunosuppressive tumor microenvironment. In fact, different types of cancer therapies (e.g., sunitinib [a receptor tyrosine kinase inhibitor] and radiation therapy) can reduce accumulation and immunosuppressive activity of MDSCs.[106] Although it has been proposed that, due to their high tropism to tumors, these cells could be used as Trojan horses for the tumor-targeted delivery of anticancer therapeutics, [107–111] NP targeting of these cells poses significant challenges due to their phenotypic heterogeneity and their ability to transdifferentiate into other cell types. Alternatively, NP-based biomimetics of IMCs may overcome these challenges. Specifically, transfer of leukocyte membranes, containing more than 150 leukocyte membrane-associated proteins, onto nanoporous silicon particles can mimic the function of tumor-associated leukocytes.[112–115] These Leukolike Vectors (LLVs) contain multiple receptors for mediating adherence to the tumor vasculature, [114] and LLVs could be used to deliver drugs, small molecules, oligonucleotides, immunomodulatory compounds, etc. to the tumor site.
As part of the tumor microenvironment, the ECM, which is composed of matricellular proteins, not only plays a key role in tumor development and progression, but also is a source of resistance to cancer drug delivery.[116] Therefore, identifying and NP-targeting matricellular proteins that are critical for the control of ECM signaling in tumors will be essential in the development of more efficacious immunotherapies. A recent study strategically used a liposome-protamine-hyaluronic acid (LPH) NP to deliver TGFβ siRNA in advanced melanoma tumors, in which a cancer vaccine, composed of a lipid-calcium-phosphate (LCP) NP, a tumor antigen, and an adjuvant, had become less effective. The manipulation of the tumor ECM in the late stage of tumor progression boosted the response to the NP-based cancer vaccine by increasing levels of tumor infiltrating CD8+ cytotoxic T cells and decreasing levels of Treg cells.[117] Another potential target of NP-based therapeutics is fibronectin signaling. FBln5, through its competition with fibronectin, reduces fibronectin signaling. Fbln5−/− mice, when compared to their WT littermates, demonstrated suppressed tumor growth and angiogenesis.[118] Hence, development of an NP-based Fbln5-specific cancer vaccine or an NP-containing Fbln5 siRNA may prove highly therapeutic. Indeed, using the fibronectin-targeting moiety CLT1 peptide, conjugated to PEG-PLA NPs, Zhang and colleagues showed enhanced therapeutic penetration and retention in glioma-bearing mice.[119] Other strategies, such as targeting albumin NPs (e.g., Abraxane) to matricellular proteins, have also been used to enhance the efficacy of cancer treatment.[120] NPs can also indirectly remodel the ECM via tumor-endothelial cell siRNA encapsulated lipid NPs.[121] In addition, NPs can be loaded with enzymes to degrade the ECM of tumors. Mesoporous silica nanoparticles conjugated to Bromelain, a crude enzymatic complex, were shown to digest tumor ECM.[122] Alternatively, NPs can be conjugated with antibodies that target ECM-modifying enzymes. For example, poly(d, l-lactide-co-glycolide) NPs were coated with an antibody to the ECM-modifying enzyme lysyl oxidase to alter the tumor microenvironment.[123] Thus, modulation of the tumor ECM could structurally remodel tumors for enhanced transport of TILs; subsequently, changing “non-inflamed” tumor types into “inflamed” types.
4. Conclusions and future perspectives
Nanotechnology is a promising approach to facilitate the transport of immune cells and cancer immunotherapies to tumor sites via the delivery of drugs, small molecules, oligonucleotides, immunomodulatory compounds, etc. or through remodeling of the tumor microenvironment. A minimum threshold of antigen-specific TILs that are not blocked by the tumor microenvironment must be achieved to induce clinical responsiveness to cancer immunotherapy. Clinical responses of cancer patients to cancer vaccines, immune checkpoint blockade, and adoptive T cell therapy rely on the presence of TILs. Appropriate TIL transport to tumors is dependent on communication between innate (e.g., APCs, IMCs) and adaptive (e.g., effector T cells, Treg cells) immune cells, in which both can be modulated by NPs.
Not only can surface modifications of NPs facilitate APC (i.e., DC) transport through the lymphatic system but also loading of NPs with multiple antigens for DC presentation to T cells can potentiate the effectiveness of cancer immunotherapies (Fig. 1). These NP-based cancer vaccines can be tailored to the unique mutations of the individual patient, thus, offering a personalized immunotherapeutic approach. However, to maintain an effective antitumor response throughout the patient’s lifetime, NP-based cancer vaccines must be both flexible and adaptable in order to rapidly incorporate any newly discovered patient-specific tumor antigen mutations. Ideally these vaccines would establish T cell memory, inhibiting metastasis and preventing relapse. In addition, NPs can be applied to intracellularly inhibit immune checkpoint protein expression in endogenous as well as adoptively transferred T cells. This would limit the non-specificity of systemic immune checkpoint antibody-mediated inhibition and limit possible adverse side effects (e.g., autoimmune reactions). Furthermore, NPs can expand adoptively transferred TCR-engineered or CAR-engineered T cells in vivo. These NPs could be sequentially or periodically dosed in a patient to ensure sufficient numbers of transferred T cells for lifelong therapy. However, a more curative approach may encompass a combination of therapies, wherein NP-based cancer vaccines are combined along with chemotherapy, radiation therapy, targeted therapies, or other cancer immunotherapies, including NP-based cancer immunotherapies. The timing of administration, the dosing schedule, and the sequence of these therapies could be optimized for each individual patient, leading to a cure for some.
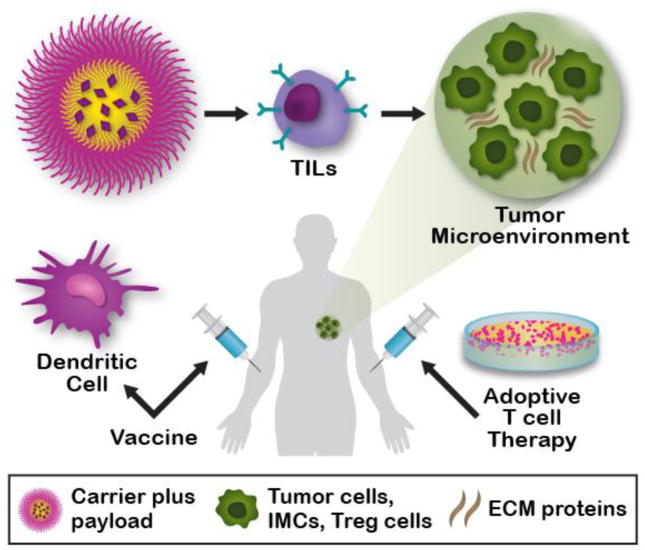
Fig. 1.
Nanotechnology-based immunotherapies overcome transport barriers to promote tissue infiltration of immune cells. Nanoparticles and microparticles can be loaded with multiple payloads, such as drugs, small molecules, oligonucleotides, immunomodulatory compounds, etc. These nanotechnologies can be directly injected into cancer patients as non-dendritic cell (DC) cancer vaccines, subsequently stimulating DCs in vivo and leading to the physical transport of DCs into lymph nodes for T-cell stimulation. Alternatively, these particles can be used to generate DC vaccines ex vivo for subsequent injection into cancer patients for T-cell stimulation. Nanotechnologies can also be applied to enhance T-cell expansion in vitro for subsequent adoptive T cell therapy, incorporated into adoptive T cell therapies to deliver potent anticancer therapeutics directly to tumor sites by tumor infiltration of T lymphocytes, indirectly remodel the tumor extracellular matrix (ECM), or directly target matricellular proteins.
Alternative to actively targeting immune cells, NPs can be applied to target the tumor microenvironment, thereby changing “non-inflamed” tumors to become more conducive to T cell intratumoral transport (Fig. 1). Current NP-based therapies indirectly modulate Treg cell number and function and could be used to prevent T cell differentiation into Treg cells. Furthermore, NP-based biomimetics of IMCs as well as direct NP-based targeting of matricellular proteins are other possible approaches to change the tumor microenvironment structure and composition, thus, facilitating the transport of TILs. Overcoming physical and biological barriers (at the systemic and intratumoral levels) of NP and cancer immunotherapy transport and testing the targetability of cells of the innate and adaptive immune system may be the future focus for NP-based cancer immunotherapeutics, which could be used as strategies for controlling tumor progression, promoting immune surveillance, and blocking metastasis.
Acknowledgments
The authors acknowledge funding support from the National Institutes of Health (U54CA210181 and R01CA193880-01) and US Department of Defense (W81XWH-12-1-0414). MF is the Ernest Cockrell Jr. Presidential Distinguished Chair at Houston Methodist Research Institute. EAM is a R. Lee Clark Fellow at the University of Texas MD Anderson Cancer Center, supported by the Jeanne F. Shelby Scholarship Fund. Matthew Landry (Houston Methodist Research Institute, Office of Strategic Research Initiatives) contributed to the figure schematic.
Abbreviations
- TILs
tumor-infiltrating lymphocytes
- carrier
nanotechnologies
- payload
drugs, small molecules, oligonucleotides, immunomodulatory compounds, etc
- IMCs
immunosuppressive myeloid cells
- Treg cells
regulatory T cells
- ECM
extracellular matrix
Footnotes
Competing interests: None to declare
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell. 2017;168(4):707–723. doi: 10.1016/j.cell.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 3.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14(10):1014–22. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HE, Horn L, Lawrence DP, Rost S, Leabman M, Xiao Y, Mokatrin A, Koeppen H, Hegde PS, Mellman I, Chen DS, Hodi FS. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–7. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smyth MJ, Ngiow SF, Ribas A, Teng MW. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat Rev Clin Oncol. 2016;13(3):143–58. doi: 10.1038/nrclinonc.2015.209. [DOI] [PubMed] [Google Scholar]
- 6.Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33(9):941–51. doi: 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrari M. Frontiers in cancer nanomedicine: directing mass transport through biological barriers. Trends in biotechnology. 2010;28(4):181–8. doi: 10.1016/j.tibtech.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yokoi K, Kojic M, Milosevic M, Tanei T, Ferrari M, Ziemys A. Capillary-wall collagen as a biophysical marker of nanotherapeutic permeability into the tumor microenvironment. Cancer Res. 2014;74(16):4239–46. doi: 10.1158/0008-5472.CAN-13-3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Michor F, Liphardt J, Ferrari M, Widom J. What does physics have to do with cancer? Nat Rev Cancer. 2011;11(9):657–70. doi: 10.1038/nrc3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muller M, dela Pena A, Derendorf H. Issues in pharmacokinetics and pharmacodynamics of anti-infective agents: distribution in tissue. Antimicrobial agents and chemotherapy. 2004;48(5):1441–53. doi: 10.1128/AAC.48.5.1441-1453.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolf W, Presant CA. Tumor-based pharmacokinetics has greater significance for anticancer drugs than does blood-based pharmacokinetics. Clinical pharmacology and therapeutics. 2004;76(5):508. doi: 10.1016/j.clpt.2004.08.012. author reply 508–9. [DOI] [PubMed] [Google Scholar]
- 12.Ziemys A, Klemm S, Milosevic M, Yokoi K, Ferrari M, Kojic M. Computational analysis of drug transport in tumor microenvironment as a critical compartment for nanotherapeutic pharmacokinetics. Drug delivery. 2016;23(7):2524–2531. doi: 10.3109/10717544.2015.1022837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yokoi K, Chan D, Kojic M, Milosevic M, Engler D, Matsunami R, Tanei T, Saito Y, Ferrari M, Ziemys A. Liposomal doxorubicin extravasation controlled by phenotype-specific transport properties of tumor microenvironment and vascular barrier. J Control Release. 2015;217:293–9. doi: 10.1016/j.jconrel.2015.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiseliovas V, Milosevic M, Kojic M, Mazutis L, Kai M, Liu YT, Yokoi K, Ferrari M, Ziemys A. Tumor progression effects on drug vector access to tumor-associated capillary bed. J Control Release. 2017;261:216–222. doi: 10.1016/j.jconrel.2017.05.031. [DOI] [PubMed] [Google Scholar]
- 15.Theis T, Parr D, Binks P, Ying J, Drexler KE, Schepers E, Mullis K, Bai C, Boland JJ, Langer R, Dobson P, Rao CN, Ferrari M. nan’o.tech.nol’o.gy n. Nat Nanotechnol. 2006;1(1):8–10. doi: 10.1038/nnano.2006.77. [DOI] [PubMed] [Google Scholar]
- 16.Xu R, Zhang G, Mai J, Deng X, Segura-Ibarra V, Wu S, Shen J, Liu H, Hu Z, Chen L, Huang Y, Koay E, Huang Y, Liu J, Ensor JE, Blanco E, Liu X, Ferrari M, Shen H. An injectable nanoparticle generator enhances delivery of cancer therapeutics. Nat Biotechnol. 2016;34(4):414–8. doi: 10.1038/nbt.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen H, Rodriguez-Aguayo C, Xu R, Gonzalez-Villasana V, Mai J, Huang Y, Zhang G, Guo X, Bai L, Qin G, Deng X, Li Q, Erm DR, Aslan B, Liu X, Sakamoto J, Chavez-Reyes A, Han HD, Sood AK, Ferrari M, Lopez-Berestein G. Enhancing chemotherapy response with sustained EphA2 silencing using multistage vector delivery. Clin Cancer Res. 2013;19(7):1806–15. doi: 10.1158/1078-0432.CCR-12-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mu C, Wu X, Ma H, Tao W, Zhang G, Xia X, Shen J, Mai J, Sun T, Sun X, Arlinghaus RB, Shen H. Effective Concentration of a Multikinase Inhibitor within Bone Marrow Correlates with In Vitro Cell Killing in Therapy-Resistant Chronic Myeloid Leukemia. Molecular cancer therapeutics. 2016;15(5):899–910. doi: 10.1158/1535-7163.MCT-15-0577-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mai J, Huang Y, Mu C, Zhang G, Xu R, Guo X, Xia X, Volk DE, Lokesh GL, Thiviyanathan V, Gorenstein DG, Liu X, Ferrari M, Shen H. Bone marrow endothelium-targeted therapeutics for metastatic breast cancer. J Control Release. 2014;187:22–9. doi: 10.1016/j.jconrel.2014.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dave B, Granados-Principal S, Zhu R, Benz S, Rabizadeh S, Soon-Shiong P, Yu KD, Shao Z, Li X, Gilcrease M, Lai Z, Chen Y, Huang TH, Shen H, Liu X, Ferrari M, Zhan M, Wong ST, Kumaraswami M, Mittal V, Chen X, Gross SS, Chang JC. Targeting RPL39 and MLF2 reduces tumor initiation and metastasis in breast cancer by inhibiting nitric oxide synthase signaling. Proc Natl Acad Sci U S A. 2014;111(24):8838–43. doi: 10.1073/pnas.1320769111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zong H, Sen S, Zhang G, Mu C, Albayati ZF, Gorenstein DG, Liu X, Ferrari M, Crooks PA, Roboz GJ, Shen H, Guzman ML. In vivo targeting of leukemia stem cells by directing parthenolide-loaded nanoparticles to the bone marrow niche. Leukemia. 2016;30(7):1582–6. doi: 10.1038/leu.2015.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ban Y, Mai J, Li X, Mitchell-Flack M, Zhang T, Zhang L, Chouchane L, Ferrari M, Shen H, Ma X. Targeting Autocrine CCL5-CCR5 Axis Reprograms Immunosuppressive Myeloid Cells and Reinvigorates Antitumor Immunity. Cancer Res. 2017;77(11):2857–2868. doi: 10.1158/0008-5472.CAN-16-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF Investigators IS. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. The New England journal of medicine. 2010;363(5):411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 24.Mittendorf EA, Clifton GT, Holmes JP, Clive KS, Patil R, Benavides LC, Gates JD, Sears AK, Stojadinovic A, Ponniah S, Peoples GE. Clinical trial results of the HER-2/neu (E75) vaccine to prevent breast cancer recurrence in high-risk patients: from US Military Cancer Institute Clinical Trials Group Study I-01 and I-02. Cancer. 2012;118(10):2594–602. doi: 10.1002/cncr.26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clifton GT, Gall V, Peoples GE, Mittendorf EA. Clinical Development of the E75 Vaccine in Breast Cancer. Breast Care (Basel) 2016;11(2):116–21. doi: 10.1159/000446097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J, Bozym DJ, Zhang W, Luoma A, Giobbie-Hurder A, Peter L, Chen C, Olive O, Carter TA, Li S, Lieb DJ, Eisenhaure T, Gjini E, Stevens J, Lane WJ, Javeri I, Nellaiappan K, Salazar AM, Daley H, Seaman M, Buchbinder EI, Yoon CH, Harden M, Lennon N, Gabriel S, Rodig SJ, Barouch DH, Aster JC, Getz G, Wucherpfennig K, Neuberg D, Ritz J, Lander ES, Fritsch EF, Hacohen N, Wu CJ. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017 doi: 10.1038/nature22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sahin U, Derhovanessian E, Miller M, Kloke BP, Simon P, Lower M, Bukur V, Tadmor AD, Luxemburger U, Schrors B, Omokoko T, Vormehr M, Albrecht C, Paruzynski A, Kuhn AN, Buck J, Heesch S, Schreeb KH, Muller F, Ortseifer I, Vogler I, Godehardt E, Attig S, Rae R, Breitkreuz A, Tolliver C, Suchan M, Martic G, Hohberger A, Sorn P, Diekmann J, Ciesla J, Waksmann O, Bruck AK, Witt M, Zillgen M, Rothermel A, Kasemann B, Langer D, Bolte S, Diken M, Kreiter S, Nemecek R, Gebhardt C, Grabbe S, Holler C, Utikal J, Huber C, Loquai C, Tureci O. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. 2017 doi: 10.1038/nature23003. [DOI] [PubMed] [Google Scholar]
- 28.Carreno BM, Magrini V, Becker-Hapak M, Kaabinejadian S, Hundal J, Petti AA, Ly A, Lie WR, Hildebrand WH, Mardis ER, Linette GP. Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science. 2015;348(6236):803–8. doi: 10.1126/science.aaa3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eggert AA, Schreurs MW, Boerman OC, Oyen WJ, de Boer AJ, Punt CJ, Figdor CG, Adema GJ. Biodistribution and vaccine efficiency of murine dendritic cells are dependent on the route of administration. Cancer Res. 1999;59(14):3340–5. [PubMed] [Google Scholar]
- 30.Verdijk P, Aarntzen EH, Lesterhuis WJ, Boullart AC, Kok E, van Rossum MM, Strijk S, Eijckeler F, Bonenkamp JJ, Jacobs JF, Blokx W, Vankrieken JH, Joosten I, Boerman OC, Oyen WJ, Adema G, Punt CJ, Figdor CG, de Vries IJ. Limited amounts of dendritic cells migrate into the T-cell area of lymph nodes but have high immune activating potential in melanoma patients. Clin Cancer Res. 2009;15(7):2531–40. doi: 10.1158/1078-0432.CCR-08-2729. [DOI] [PubMed] [Google Scholar]
- 31.Smith DM, Simon JK, Baker JR., Jr Applications of nanotechnology for immunology. Nat Rev Immunol. 2013;13(8):592–605. doi: 10.1038/nri3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol. 2005;5(8):617–28. doi: 10.1038/nri1670. [DOI] [PubMed] [Google Scholar]
- 33.Bousso P. T-cell activation by dendritic cells in the lymph node: lessons from the movies. Nat Rev Immunol. 2008;8(9):675–84. doi: 10.1038/nri2379. [DOI] [PubMed] [Google Scholar]
- 34.Reddy ST, van der Vlies AJ, Simeoni E, Angeli V, Randolph GJ, O’Neil CP, Lee LK, Swartz MA, Hubbell JA. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat Biotechnol. 2007;25(10):1159–64. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- 35.Reddy ST, Rehor A, Schmoekel HG, Hubbell JA, Swartz MA. In vivo targeting of dendritic cells in lymph nodes with poly(propylene sulfide) nanoparticles. J Control Release. 2006;112(1):26–34. doi: 10.1016/j.jconrel.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 36.Liu H, Moynihan KD, Zheng Y, Szeto GL, Li AV, Huang B, Van Egeren DS, Park C, Irvine DJ. Structure-based programming of lymph-node targeting in molecular vaccines. Nature. 2014;507(7493):519–22. doi: 10.1038/nature12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim SY, Noh YW, Kang TH, Kim JE, Kim S, Um SH, Oh DB, Park YM, Lim YT. Synthetic vaccine nanoparticles target to lymph node triggering enhanced innate and adaptive antitumor immunity. Biomaterials. 2017;130:56–66. doi: 10.1016/j.biomaterials.2017.03.034. [DOI] [PubMed] [Google Scholar]
- 38.Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33(4):492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lindblad EB. Aluminium compounds for use in vaccines. Immunol Cell Biol. 2004;82(5):497–505. doi: 10.1111/j.0818-9641.2004.01286.x. [DOI] [PubMed] [Google Scholar]
- 40.Xia X, Mai J, Xu R, Perez JE, Guevara ML, Shen Q, Mu C, Tung HY, Corry DB, Evans SE, Liu X, Ferrari M, Zhang Z, Li XC, Wang RF, Shen H. Porous silicon microparticle potentiates anti-tumor immunity by enhancing cross-presentation and inducing type I interferon response. Cell Rep. 2015;11(6):957–66. doi: 10.1016/j.celrep.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fuertes MB, Woo SR, Burnett B, Fu YX, Gajewski TF. Type I interferon response and innate immune sensing of cancer. Trends Immunol. 2013;34(2):67–73. doi: 10.1016/j.it.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luo M, Wang H, Wang Z, Cai H, Lu Z, Li Y, Du M, Huang G, Wang C, Chen X, Porembka MR, Lea J, Frankel AE, Fu YX, Chen ZJ, Gao J. A STING-activating nanovaccine for cancer immunotherapy. Nat Nanotechnol. 2017;12(7):648–654. doi: 10.1038/nnano.2017.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fotin-Mleczek M, Duchardt KM, Lorenz C, Pfeiffer R, Ojkic-Zrna S, Probst J, Kallen KJ. Messenger RNA-based vaccines with dual activity induce balanced TLR-7 dependent adaptive immune responses and provide antitumor activity. Journal of immunotherapy (Hagerstown, Md: 1997) 2011;34(1):1–15. doi: 10.1097/CJI.0b013e3181f7dbe8. [DOI] [PubMed] [Google Scholar]
- 44.Su X, Fricke J, Kavanagh DG, Irvine DJ. In vitro and in vivo mRNA delivery using lipid-enveloped pH-responsive polymer nanoparticles. Mol Pharm. 2011;8(3):774–87. doi: 10.1021/mp100390w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Persano S, Guevara ML, Li Z, Ferrari M, Pompa PP, Shen H. Lipopolyplex potentiates anti-tumor immunity of mRNA-based vaccination. Biomaterials (accepted) 2017 doi: 10.1016/j.biomaterials.2017.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sahin U, Kariko K, Tureci O. mRNA-based therapeutics--developing a new class of drugs. Nat Rev Drug Discov. 2014;13(10):759–80. doi: 10.1038/nrd4278. [DOI] [PubMed] [Google Scholar]
- 47.McNamara M, Nair S, Holl E. RNA-based vaccines in cancer immunotherapy. J Immunol Res. 2015;2015:794528. doi: 10.1155/2015/794528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kranz LM, Diken M, Haas H, Kreiter S, Loquai C, Reuter KC, Meng M, Fritz D, Vascotto F, Hefesha H, Grunwitz C, Vormehr M, Husemann Y, Selmi A, Kuhn AN, Buck J, Derhovanessian E, Rae R, Attig S, Diekmann J, Jabulowsky RA, Heesch S, Hassel J, Langguth P, Grabbe S, Huber C, Tureci O, Sahin U. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature. 2016 doi: 10.1038/nature18300. [DOI] [PubMed] [Google Scholar]
- 49.Richner JM, Himansu S, Dowd KA, Butler SL, Salazar V, Fox JM, Julander JG, Tang WW, Shresta S, Pierson TC, Ciaramella G, Diamond MS. Modified mRNA Vaccines Protect against Zika Virus Infection. Cell. 2017;169(1):176. doi: 10.1016/j.cell.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 50.Goldberg MS. Immunoengineering: how nanotechnology can enhance cancer immunotherapy. Cell. 2015;161(2):201–4. doi: 10.1016/j.cell.2015.03.037. [DOI] [PubMed] [Google Scholar]
- 51.Perica K, Varela JC, Oelke M, Schneck J. Adoptive T cell immunotherapy for cancer. Rambam Maimonides Med J. 2015;6(1):e0004. doi: 10.5041/RMMJ.10179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Turtle CJ. Chimeric antigen receptor modified T cell therapy for B cell malignancies. International journal of hematology. 2014;99(2):132–40. doi: 10.1007/s12185-013-1490-x. [DOI] [PubMed] [Google Scholar]
- 53.Jensen MC, Riddell SR. Design and implementation of adoptive therapy with chimeric antigen receptor-modified T cells. Immunol Rev. 2014;257(1):127–44. doi: 10.1111/imr.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dotti G, Gottschalk S, Savoldo B, Brenner MK. Design and development of therapies using chimeric antigen receptor-expressing T cells. Immunol Rev. 2014;257(1):107–26. doi: 10.1111/imr.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chmielewski M, Hombach AA, Abken H. Of CARs and TRUCKs: chimeric antigen receptor (CAR) T cells engineered with an inducible cytokine to modulate the tumor stroma. Immunol Rev. 2014;257(1):83–90. doi: 10.1111/imr.12125. [DOI] [PubMed] [Google Scholar]
- 56.Barrett DM, Singh N, Porter DL, Grupp SA, June CH. Chimeric antigen receptor therapy for cancer. Annual review of medicine. 2014;65:333–47. doi: 10.1146/annurev-med-060512-150254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Riches JC, Gribben JG. Advances in chimeric antigen receptor immunotherapy for chronic lymphocytic leukemia. Discovery medicine. 2013;16(90):295–302. [PubMed] [Google Scholar]
- 58.Heczey A, Louis CU. Advances in chimeric antigen receptor immunotherapy for neuroblastoma. Discovery medicine. 2013;16(90):287–94. [PMC free article] [PubMed] [Google Scholar]
- 59.Chmielewski M, Hombach AA, Abken H. Antigen-Specific T-Cell Activation Independently of the MHC: Chimeric Antigen Receptor-Redirected T Cells. Frontiers in immunology. 2013;4:371. doi: 10.3389/fimmu.2013.00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garfall AL, Maus MV, Hwang WT, Lacey SF, Mahnke YD, Melenhorst JJ, Zheng Z, Vogl DT, Cohen AD, Weiss BM, Dengel K, Kerr ND, Bagg A, Levine BL, June CH, Stadtmauer EA. Chimeric Antigen Receptor T Cells against CD19 for Multiple Myeloma. The New England journal of medicine. 2015;373(11):1040–7. doi: 10.1056/NEJMoa1504542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, Mahnke YD, Melenhorst JJ, Rheingold SR, Shen A, Teachey DT, Levine BL, June CH, Porter DL, Grupp SA. Chimeric antigen receptor T cells for sustained remissions in leukemia. The New England journal of medicine. 2014;371(16):1507–17. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, Fry TJ, Orentas R, Sabatino M, Shah NN, Steinberg SM, Stroncek D, Tschernia N, Yuan C, Zhang H, Zhang L, Rosenberg SA, Wayne AS, Mackall CL. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet (London, England) 2015;385(9967):517–28. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. The New England journal of medicine. 2011;365(8):725–33. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ahmed N, Brawley VS, Hegde M, Robertson C, Ghazi A, Gerken C, Liu E, Dakhova O, Ashoori A, Corder A, Gray T, Wu MF, Liu H, Hicks J, Rainusso N, Dotti G, Mei Z, Grilley B, Gee A, Rooney CM, Brenner MK, Heslop HE, Wels WS, Wang LL, Anderson P, Gottschalk S. Human Epidermal Growth Factor Receptor 2 (HER2) -Specific Chimeric Antigen Receptor-Modified T Cells for the Immunotherapy of HER2-Positive Sarcoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2015;33(15):1688–96. doi: 10.1200/JCO.2014.58.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, Dotti G, Huls MH, Liu E, Gee AP, Mei Z, Yvon E, Weiss HL, Liu H, Rooney CM, Heslop HE, Brenner MK. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nature medicine. 2008;14(11):1264–70. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, Chung SS, Stefanski J, Borquez-Ojeda O, Olszewska M, Qu J, Wasielewska T, He Q, Fink M, Shinglot H, Youssif M, Satter M, Wang Y, Hosey J, Quintanilla H, Halton E, Bernal Y, Bouhassira DC, Arcila ME, Gonen M, Roboz GJ, Maslak P, Douer D, Frattini MG, Giralt S, Sadelain M, Brentjens R. Efficacy and toxicity management of 19–28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Science translational medicine. 2014;6(224):224ra25. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Louis CU, Savoldo B, Dotti G, Pule M, Yvon E, Myers GD, Rossig C, Russell HV, Diouf O, Liu E, Liu H, Wu MF, Gee AP, Mei Z, Rooney CM, Heslop HE, Brenner MK. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;118(23):6050–6. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Robbins PF, Morgan RA, Feldman SA, Yang JC, Sherry RM, Dudley ME, Wunderlich JR, Nahvi AV, Helman LJ, Mackall CL, Kammula US, Hughes MS, Restifo NP, Raffeld M, Lee CC, Levy CL, Li YF, El-Gamil M, Schwarz SL, Laurencot C, Rosenberg SA. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2011;29(7):917–24. doi: 10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chodon T, Comin-Anduix B, Chmielowski B, Koya RC, Wu Z, Auerbach M, Ng C, Avramis E, Seja E, Villanueva A, McCannel TA, Ishiyama A, Czernin J, Radu CG, Wang X, Gjertson DW, Cochran AJ, Cornetta K, Wong DJ, Kaplan-Lefko P, Hamid O, Samlowski W, Cohen PA, Daniels GA, Mukherji B, Yang L, Zack JA, Kohn DB, Heath JR, Glaspy JA, Witte ON, Baltimore D, Economou JS, Ribas A. Adoptive transfer of MART-1 T-cell receptor transgenic lymphocytes and dendritic cell vaccination in patients with metastatic melanoma. Clin Cancer Res. 2014;20(9):2457–65. doi: 10.1158/1078-0432.CCR-13-3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Perica K, Bieler JG, Schutz C, Varela JC, Douglass J, Skora A, Chiu YL, Oelke M, Kinzler K, Zhou S, Vogelstein B, Schneck JP. Enrichment and Expansion with Nanoscale Artificial Antigen Presenting Cells for Adoptive Immunotherapy. ACS Nano. 2015;9(7):6861–71. doi: 10.1021/acsnano.5b02829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stephan MT, Stephan SB, Bak P, Chen J, Irvine DJ. Synapse-directed delivery of immunomodulators using T-cell-conjugated nanoparticles. Biomaterials. 2012;33(23):5776–87. doi: 10.1016/j.biomaterials.2012.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang B, Abraham WD, Zheng Y, Bustamante López SC, Luo SS, Irvine DJ. Active targeting of chemotherapy to disseminated tumors using nanoparticle-carrying T cells. Science translational medicine. 2015;7(291):291ra94–291ra94. doi: 10.1126/scitranslmed.aaa5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Perica K, Tu A, Richter A, Bieler JG, Edidin M, Schneck JP. Magnetic field-induced T cell receptor clustering by nanoparticles enhances T cell activation and stimulates antitumor activity. ACS Nano. 2014;8(3):2252–60. doi: 10.1021/nn405520d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brischwein K, Parr L, Pflanz S, Volkland J, Lumsden J, Klinger M, Locher M, Hammond SA, Kiener P, Kufer P, Schlereth B, Baeuerle PA. Strictly target cell-dependent activation of T cells by bispecific single-chain antibody constructs of the BiTE class. J Immunother. 2007;30(8):798–807. doi: 10.1097/CJI.0b013e318156750c. [DOI] [PubMed] [Google Scholar]
- 75.Frankel SR, Baeuerle PA. Targeting T cells to tumor cells using bispecific antibodies. Curr Opin Chem Biol. 2013;17(3):385–92. doi: 10.1016/j.cbpa.2013.03.029. [DOI] [PubMed] [Google Scholar]
- 76.Dao T, Pankov D, Scott A, Korontsvit T, Zakhaleva V, Xu Y, Xiang J, Yan S, de Morais Guerreiro MD, Veomett N, Dubrovsky L, Curcio M, Doubrovina E, Ponomarev V, Liu C, O’Reilly RJ, Scheinberg DA. Therapeutic bispecific T-cell engager antibody targeting the intracellular oncoprotein WT1. Nat Biotechnol. 2015;33(10):1079–86. doi: 10.1038/nbt.3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kantarjian H, Jabbour E, Topp MS. Blinatumomab for Acute Lymphoblastic Leukemia. The New England journal of medicine. 2017;376(23):e49. doi: 10.1056/NEJMc1704012. [DOI] [PubMed] [Google Scholar]
- 78.Martinelli G, Boissel N, Chevallier P, Ottmann O, Gokbuget N, Topp MS, Fielding AK, Rambaldi A, Ritchie EK, Papayannidis C, Sterling LR, Benjamin J, Stein A. Complete Hematologic and Molecular Response in Adult Patients With Relapsed/Refractory Philadelphia Chromosome-Positive B-Precursor Acute Lymphoblastic Leukemia Following Treatment With Blinatumomab: Results From a Phase II, Single-Arm, Multicenter Study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2017;35(16):1795–1802. doi: 10.1200/JCO.2016.69.3531. [DOI] [PubMed] [Google Scholar]
- 79.Yuan H, Jiang W, von Roemeling CA, Qie Y, Liu X, Chen Y, Wang Y, Wharen RE, Yun K, Bu G, Knutson KL, Kim BYS. Multivalent bi-specific nanobioconjugate engager for targeted cancer immunotherapy. Nat Nanotechnol. 2017 doi: 10.1038/nnano.2017.69. [DOI] [PubMed] [Google Scholar]
- 80.Min Y, Roche KC, Tian S, Eblan MJ, McKinnon KP, Caster JM, Chai S, Herring LE, Zhang L, Zhang T, DeSimone JM, Tepper JE, Vincent BG, Serody JS, Wang AZ. Antigen-capturing nanoparticles improve the abscopal effect and cancer immunotherapy. Nat Nanotechnol. 2017 doi: 10.1038/nnano.2017.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stadler CR, Bahr-Mahmud H, Celik L, Hebich B, Roth AS, Roth RP, Kariko K, Tureci O, Sahin U. Elimination of large tumors in mice by mRNA-encoded bispecific antibodies. Nature medicine. 2017 doi: 10.1038/nm.4356. [DOI] [PubMed] [Google Scholar]
- 82.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nature medicine. 2002;8(8):793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 83.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nature medicine. 1999;5(12):1365–9. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 84.Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541(7637):321–330. doi: 10.1038/nature21349. [DOI] [PubMed] [Google Scholar]
- 85.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. The New England journal of medicine. 2010;363(8):711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. The New England journal of medicine. 2012;366(26):2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng SL, Shen X, Boyd Z, Hegde PS, Chen DS, Vogelzang NJ. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515(7528):558–62. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 88.Duan X, Chan C, Guo N, Han W, Weichselbaum RR, Lin W. Photodynamic Therapy Mediated by Nontoxic Core-Shell Nanoparticles Synergizes with Immune Checkpoint Blockade To Elicit Antitumor Immunity and Antimetastatic Effect on Breast Cancer. J Am Chem Soc. 2016;138(51):16686–16695. doi: 10.1021/jacs.6b09538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Peng W, Liu C, Xu C, Lou Y, Chen J, Yang Y, Yagita H, Overwijk WW, Lizée G, Radvanyi L, Hwu P. PD-1 BLOCKADE ENHANCES T CELL MIGRATION TO TUMORS BY ELEVATING IFN-γ INDUCIBLE CHEMOKINES. Cancer research. 2012;72(20):5209–5218. doi: 10.1158/0008-5472.CAN-12-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang C, Ye Y, Hochu GM, Sadeghifar H, Gu Z. Enhanced Cancer Immunotherapy by Microneedle Patch-Assisted Delivery of Anti-PD1 Antibody. Nano letters. 2016;16(4):2334–2340. doi: 10.1021/acs.nanolett.5b05030. [DOI] [PubMed] [Google Scholar]
- 91.Li SY, Liu Y, Xu CF, Shen S, Sun R, Du XJ, Xia JX, Zhu YH, Wang J. Restoring anti-tumor functions of T cells via nanoparticle-mediated immune checkpoint modulation. J Control Release. 2016;231:17–28. doi: 10.1016/j.jconrel.2016.01.044. [DOI] [PubMed] [Google Scholar]
- 92.Chen Q, Xu L, Liang C, Wang C, Peng R, Liu Z. Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nature communications. 2016;7:13193. doi: 10.1038/ncomms13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.He C, Duan X, Guo N, Chan C, Poon C, Weichselbaum RR, Lin W. Core-shell nanoscale coordination polymers combine chemotherapy and photodynamic therapy to potentiate checkpoint blockade cancer immunotherapy. 2016;7:12499. doi: 10.1038/ncomms12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dolmans DEJGJ, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003;3(5):380–387. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- 95.Xu J, Xu L, Wang C, Yang R, Zhuang Q, Han X, Dong Z, Zhu W, Peng R, Liu Z. Near-Infrared-Triggered Photodynamic Therapy with Multitasking Upconversion Nanoparticles in Combination with Checkpoint Blockade for Immunotherapy of Colorectal Cancer. ACS Nano. 2017;11(5):4463–4474. doi: 10.1021/acsnano.7b00715. [DOI] [PubMed] [Google Scholar]
- 96.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nature medicine. 2004;10(9):942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 97.Wang HY, Lee DA, Peng G, Guo Z, Li Y, Kiniwa Y, Shevach EM, Wang RF. Tumor-specific human CD4+ regulatory T cells and their ligands: implication for immunotherapy. Immunity. 2004;20:107–118. doi: 10.1016/s1074-7613(03)00359-5. [DOI] [PubMed] [Google Scholar]
- 98.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6(4):295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 99.Wang HY, Wang RF. Regulatory T cells and cancer. Current opinion in immunology. 2007;19(2):217–23. doi: 10.1016/j.coi.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 100.Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, Drebin JA, Strasberg SM, Eberlein TJ, Goedegebuure PS, Linehan DC. Prevalence of Regulatory T Cells Is Increased in Peripheral Blood and Tumor Microenvironment of Patients with Pancreas or Breast Adenocarcinoma. The Journal of Immunology. 2002;169(5):2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 101.Tang Y, Xu X, Guo S, Zhang C, Tang Y, Tian Y, Ni B, Lu B, Wang H. An Increased Abundance of Tumor-Infiltrating Regulatory T Cells Is Correlated with the Progression and Prognosis of Pancreatic Ductal Adenocarcinoma. PLOS ONE. 2014;9(3):e91551. doi: 10.1371/journal.pone.0091551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li Z, Dong P, Ren M, Song Y, Qian X, Yang Y, Li S, Zhang X, Liu F. PD-L1 Expression Is Associated with Tumor FOXP3(+) Regulatory T-Cell Infiltration of Breast Cancer and Poor Prognosis of Patient. J Cancer. 2016;7(7):784–93. doi: 10.7150/jca.14549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kopecka J, Porto S, Lusa S, Gazzano E, Salzano G, Pinzon-Daza ML, Giordano A, Desiderio V, Ghigo D, De Rosa G, Caraglia M, Riganti C. Zoledronic acid-encapsulating self-assembling nanoparticles and doxorubicin: a combinatorial approach to overcome simultaneously chemoresistance and immunoresistance in breast tumors. Oncotarget. 2016;7(15):20753–72. doi: 10.18632/oncotarget.8012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kwong B, Gai SA, Elkhader J, Wittrup KD, Irvine DJ. Localized immunotherapy via liposome-anchored Anti-CD137 + IL-2 prevents lethal toxicity and elicits local and systemic antitumor immunity. Cancer Res. 2013;73(5):1547–58. doi: 10.1158/0008-5472.CAN-12-3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Van Woensel M, Mathivet T, Wauthoz N, Rosiere R, Garg AD, Agostinis P, Mathieu V, Kiss R, Lefranc F, Boon L, Belmans J, Van Gool SW, Gerhardt H, Amighi K, De Vleeschouwer S. Sensitization of glioblastoma tumor micro-environment to chemo- and immunotherapy by Galectin-1 intranasal knock-down strategy. Sci Rep. 2017;7(1):1217. doi: 10.1038/s41598-017-01279-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen HM, Ma G, Gildener-Leapman N, Eisenstein S, Coakley BA, Ozao J, Mandeli J, Divino C, Schwartz M, Sung M, Ferris R, Kao J, Wang LH, Pan PY, Ko EC, Chen SH. Myeloid-Derived Suppressor Cells as an Immune Parameter in Patients with Concurrent Sunitinib and Stereotactic Body Radiotherapy. Clin Cancer Res. 2015;21(18):4073–85. doi: 10.1158/1078-0432.CCR-14-2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pan PY, Chen HM, Chen SH. Myeloid-derived suppressor cells as a Trojan horse: A cellular vehicle for the delivery of oncolytic viruses. Oncoimmunology. 2013;2(8):e25083. doi: 10.4161/onci.25083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu L, Yi H, Wang C, He H, Li P, Pan H, Sheng N, Ji M, Cai L, Ma Y. Integrated Nanovaccine with MicroRNA-148a Inhibition Reprograms Tumor-Associated Dendritic Cells by Modulating miR-148a/DNMT1/SOCS1 Axis. Journal of immunology (Baltimore, Md: 1950) 2016;197(4):1231–41. doi: 10.4049/jimmunol.1600182. [DOI] [PubMed] [Google Scholar]
- 109.Chu D, Gao J, Wang Z. Neutrophil-Mediated Delivery of Therapeutic Nanoparticles across Blood Vessel Barrier for Treatment of Inflammation and Infection. ACS Nano. 2015;9(12):11800–11. doi: 10.1021/acsnano.5b05583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Anselmo AC, Mitragotri S. Cell-mediated delivery of nanoparticles: taking advantage of circulatory cells to target nanoparticles. J Control Release. 2014;190:531–41. doi: 10.1016/j.jconrel.2014.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dong X, Chu D, Wang Z. Leukocyte-mediated Delivery of Nanotherapeutics in Inflammatory and Tumor Sites. Theranostics. 2017;7(3):751–763. doi: 10.7150/thno.18069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Parodi A, Quattrocchi N, van de Ven AL, Chiappini C, Evangelopoulos M, Martinez JO, Brown BS, Khaled SZ, Yazdi IK, Enzo MV, Isenhart L, Ferrari M, Tasciotti E. Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions. Nat Nanotechnol. 2013;8(1):61–8. doi: 10.1038/nnano.2012.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Corbo C, Parodi A, Evangelopoulos M, Engler DA, Matsunami RK, Engler AC, Molinaro R, Scaria S, Salvatore F, Tasciotti E. Proteomic Profiling of a Biomimetic Drug Delivery Platform. Current drug targets. 2015;16(13):1540–7. doi: 10.2174/1389450115666141109211413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Palomba R, Parodi A, Evangelopoulos M, Acciardo S, Corbo C, de Rosa E, Yazdi IK, Scaria S, Molinaro R, Furman NE, You J, Ferrari M, Salvatore F, Tasciotti E. Biomimetic carriers mimicking leukocyte plasma membrane to increase tumor vasculature permeability. Sci Rep. 2016;6:34422. doi: 10.1038/srep34422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Molinaro R, Corbo C, Martinez JO, Taraballi F, Evangelopoulos M, Minardi S, Yazdi IK, Zhao P, De Rosa E, Sherman MB, De Vita A, Toledano Furman NE, Wang X, Parodi A, Tasciotti E. Biomimetic proteolipid vesicles for targeting inflamed tissues. Nature materials. 2016;15(9):1037–46. doi: 10.1038/nmat4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Netti PA, Berk DA, Swartz MA, Grodzinsky AJ, Jain RK. Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer Res. 2000;60(9):2497–503. [PubMed] [Google Scholar]
- 117.Xu Z, Wang Y, Zhang L, Huang L. Nanoparticle-delivered transforming growth factor-beta siRNA enhances vaccination against advanced melanoma by modifying tumor microenvironment. ACS Nano. 2014;8(4):3636–45. doi: 10.1021/nn500216y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schluterman MK, Chapman SL, Korpanty G, Ozumi K, Fukai T, Yanagisawa H, Brekken RA. Loss of fibulin-5 binding to beta1 integrins inhibits tumor growth by increasing the level of ROS. Dis Model Mech. 2010;3(5–6):333–42. doi: 10.1242/dmm.003707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang B, Shen S, Liao Z, Shi W, Wang Y, Zhao J, Hu Y, Yang J, Chen J, Mei H, Hu Y, Pang Z, Jiang X. Targeting fibronectins of glioma extracellular matrix by CLT1 peptide-conjugated nanoparticles. Biomaterials. 2014;35(13):4088–98. doi: 10.1016/j.biomaterials.2014.01.046. [DOI] [PubMed] [Google Scholar]
- 120.Kouchakzadeh H, Safavi MS, Shojaosadati SA. Efficient delivery of therapeutic agents by using targeted albumin nanoparticles. Advances in protein chemistry and structural biology. 2015;98:121–43. doi: 10.1016/bs.apcsb.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 121.Sakurai Y, Hada T, Yamamoto S, Kato A, Mizumura W, Harashima H. Remodeling of the Extracellular Matrix by Endothelial Cell-Targeting siRNA Improves the EPR-Based Delivery of 100 nm Particles. Molecular therapy: the journal of the American Society of Gene Therapy. 2016;24(12):2090–2099. doi: 10.1038/mt.2016.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Parodi A, Haddix SG, Taghipour N, Scaria S, Taraballi F, Cevenini A, Yazdi IK, Corbo C, Palomba R, Khaled SZ, Martinez JO, Brown BS, Isenhart L, Tasciotti E. Bromelain surface modification increases the diffusion of silica nanoparticles in the tumor extracellular matrix. ACS Nano. 2014;8(10):9874–83. doi: 10.1021/nn502807n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kanapathipillai M, Mammoto A, Mammoto T, Kang JH, Jiang E, Ghosh K, Korin N, Gibbs A, Mannix R, Ingber DE. Inhibition of mammary tumor growth using lysyl oxidase-targeting nanoparticles to modify extracellular matrix. Nano letters. 2012;12(6):3213–7. doi: 10.1021/nl301206p. [DOI] [PubMed] [Google Scholar]