Abstract
Objective
Children with overweight/obesity have elevated eating disorder (ED) pathology, which may increase their risk for clinical EDs. The current study identified patterns of ED pathology in children with overweight/obesity entering family-based behavioral weight loss treatment (FBT), and examined whether children with distinct patterns differed in their ED pathology and zBMI change across FBT.
Methods
Before participating in 16-session FBT, children (N=241) completed surveys/interviews assessing ED pathology [emotional eating, shape/weight/eating concerns, restraint, and loss of control (LOC)]. Shape/weight concerns and LOC were also assessed post-treatment. Child height/weight were measured at baseline and post-treatment. Latent class analysis identified patterns of ED pathology. Repeated-measures ANOVA examined changes in zBMI and ED pathology.
Results
Four patterns of ED pathology were identified: Low ED Pathology, Shape and Weight Concerns, Only Loss of Control, and High ED Pathology. Shape/weight concerns decreased across treatment, with highest decreases in patterns characterized by high shape and weight concerns. All groups experienced significant decreases in zBMI; however, children with the highest ED pathology did not achieve clinically significant weight loss.
Conclusions
ED pathology decreased after FBT, decreasing ED risk. While all children achieved zBMI reductions, further research is needed to enhance outcomes for children with high ED pathology.
Keywords: childhood obesity, family based interventions, disordered eating, eating disorders
Introduction
Children with overweight/obesity have higher rates of eating disorder (ED) pathology than their peers without overweight/obesity [1–4], and thus are at an increased risk for developing a clinical ED. ED pathology can include disordered attitudes/perceptions regarding shape, weight, and eating [5, 6]. ED pathology can also involve disordered eating behaviors such as extreme dietary restraint (i.e., attempting rigid/restrictive eating to control one’s weight), loss of control (LOC) eating (i.e., an inability to control what/how much one is eating), and emotional eating (i.e., desire to eat in response to emotions) [5–7]. Varying features of ED pathology may be observed across children in obesity treatment-seeking samples. For example, a high percentage show elevated shape and weight concerns [8], whereas only roughly 20–35% experience LOC eating (i.e., objective or subjective binge episodes) [9]. Notably, elevated shape and weight concerns are one of the most well-established risk factors for the development of EDs [10]; it is thus striking that such a high percentage of obesity treatment-seeking youth endorse these concerns. Given these considerations, it is critical to examine a wide variety of ED pathology in children with overweight/obesity.
Features of ED pathology among obesity treatment-seeking youth may impact weight-loss outcomes; however, the existing studies show mixed results when evaluating a range of ED symptoms/pathology. Some show no weight loss differences between adolescents with and without baseline ED symptoms [11, 12], whereas studies both in a combined school-aged and adolescent sample [13] and in a primarily school-aged sample [14] have shown that youth with baseline ED pathology lose less weight. Thus, it remains unclear the extent to which ED pathology impacts weight loss in the context of behavioral intervention.
Differences in individual symptom patterns of ED pathology may help explain these inconsistent findings. For example, some degree of flexible dietary restraint (i.e., moderate restraint that limits, but does not eliminate, the intake of particular foods) and some concern for weight and/or shape may be beneficial for weight loss [15], whereas symptoms that can cause overeating (e.g., LOC eating, emotional eating) may be detrimental. Previous research has identified patterns of disinhibited eating behaviors in a community sample [16] and overeating phenotypes in obesity treatment-seeking children [17]; however, these studies did not classify a broad range of ED pathology nor did they examine how distinct profiles of ED pathology differentially impact weight loss during behavioral treatment. As such, this study aims to (a) classify 7- to 11-year-old children with overweight/obesity entering family-based treatment (FBT) into groups with distinct patterns of ED pathology and then (b) examine ED pattern differences in: 1) baseline weight status and demographics; 2) treatment attendance/completion; and 3) treatment outcomes (i.e., change in weight and ED pathology). It was hypothesized that multiple patterns of ED pathology exist in the current sample, ranging from low to high levels of ED pathology, with these patterns impacting response to FBT.
Methods
Study design
Data for this study came from a larger, multi-site randomized controlled trial (RCT) in Saint Louis, MO and Seattle, WA which investigated the effectiveness of weight-loss maintenance programs after completion of FBT. The current analysis only includes data from the initial weight-loss phase in which all families received the same 16-session FBT. In brief, FBT promotes a reduced-energy-dense diet and an increase in consumption of nutritious foods, increased physical activity, and responsive parenting skills such as routines, limit-setting, and positive reinforcement (see [18] for a detailed description of the program). While not designed to treat ED pathology, FBT included content that targeted aspects of ED pathology (e.g., emotional eating). Child anthropometrics and ED pathology were assessed before and after FBT. Trained research assistants and interventionists administered the interview/questionnaires and delivered the FBT intervention, respectively.
Participants
Participants were 241 children, 7–11 years old, with overweight/obesity (body mass index [BMI] ≥ 85th percentile based on their sex and age). RCT inclusion criteria included at least one parent having a BMI ≥ 25 kg/m2, and at least one parent was required to participate. Parent-child dyads were recruited through weight-loss clinics, schools, media, and pediatrician referrals. Exclusion criteria included the child or participating parent having: alcohol or drug dependence; low English comprehension; a diagnosed ED, other mental illness, or a physical condition that interfered with engaging in physical activity or maintaining the program dietary goals; or taking medication that affected weight status. Children provided written assent, and parents provided written informed consent. The study was approved by each university’s Institutional Review Board. All 241 children who began FBT were included in the latent class analysis (LCA) and baseline analyses. Of these 241 children, 183 completed FBT, 182 had post-treatment weights, and 175 had all post-treatment measures.
Measures
Demographics
Parents completed demographic questionnaires measuring parent and child sex, age, race, and ethnicity. Annual household income was reported in categories ranging from 1 (under $9,999) to 11 (over $100,000).
Treatment attendance and completion
Treatment attendance was assessed as the number of sessions completed out of 16 possible sessions, including phone/make-up sessions. Treatment completion was defined as attending the 12th or later session.
Anthropometrics
Child weight was measured to the nearest 0.1 kg (without shoes, in light clothing) on a calibrated electronic scale. Height was measured to the nearest 0.1 cm on a stadiometer. Child BMI z-score (z-BMI; [19]) was calculated.
ED pathology variables
Emotional Eating Behaviors
The Emotional Eating Scale for Children and Adolescents (EES-C) [20] is a 25-item questionnaire modified from the adult Emotional Eating Scale [7]. Respondents rate their desire to eat in the face of each emotion (e.g., anger) on a scale of 0–4 (i.e., “no desire” to “overwhelming urge to eat”), and a total score is computed as an average across all items [21]. Cronbach’s alpha for EES-C in the current study was 0.95 for all children (0.94 for the 7 year-olds). Scores were dichotomized for use in LCA; children were coded a “no” if their total score was < 1 and a “yes” if their total score was ≥ 1 to indicate that they have at least a “small desire to eat” across emotions. These cut-offs were chosen because samples with clinically-relevant eating pathology have previously scored at or above 1 on this scale [20].
Loss of Control (LOC)
The ChEDE [5], which assesses ED features in children, is a semi-structured interview adapted from the adult Eating Disorder Examination [6] and was used to assess LOC. Children indicated if they had experienced LOC eating in the last three months. If yes, children indicated the number of LOC episodes they had experienced in the past one and three months. Findings from Kass et al. [22] indicate that children who report any LOC eating are distinct from children who do not report any LOC eating. Thus, to dichotomize LOC eating for use in LCA, children were coded a “yes” or “no,” respectively, if they did or did not report experiencing any LOC eating in the past three months.
Shape, Weight, and Eating Concern, and Dietary Restraint
The YEDE-Q [23] is a 39-item child version of the adult Eating Disorder Examination Questionnaire (EDE-Q; [24]) with child-friendly language and adapted instructions based on those developed by Goldfein and colleagues [25]. The YEDE-Q demonstrates strong reliability and validity [23]; it has four subscales: shape concern, weight concern, eating concern, and restraint. Cronbach’s alpha for shape concern, weight concern, eating concern, and restraint were 0.90, 0.78, 0.74, and 0.68, respectively, with similar internal consistencies among 7-year-olds. Prior work in adults has indicated 2.3 as a clinically meaningful cut-off on the EDE-Q [26]. Thus, to dichotomize the YEDE-Q subscales for use in LCA, due to the absence of an existing clinically-meaningful cut-off in youth, children were coded a “no” or “yes” if the subscale score was < 2.3 or ≥ 2.3, respectively. Of the four subscales, only shape concern and weight concern were assessed post-treatment.
Statistical Analyses
All data analyses were performed using SPSS software, version 22, unless otherwise indicated. Statistical significance was defined as p ≤ 0.05 and adjusted for multiple comparisons when appropriate. LCA was conducted using SAS version 9.4 and used to identify the patterns of ED pathology using emotional eating, shape concern, eating concern, weight concern, restraint, and LOC, all dichotomized as described above. A detailed description of this LCA procedure is described elsewhere [27]. Item-response probabilities are defined as the probability of endorsing each item given class membership and range from 0–1 [27]. The final model was identified by comparing latent class models with one to six latent classes to select the model with the optimal fit (e.g., Akaike’s Information Criterion) and best model interpretation using criteria specified by Lanza et al. [27]; the four-class model was identified as the best one. The average posterior probabilities for the four classes were: 0.97, 0.84, 0.97, and 0.98, indicating excellent model fit.
After the four-class model was identified, differences among the four ED pathology groups were examined in relation to baseline variables, treatment adherence, and treatment outcomes. First, each child was assigned to a group using the classify-analyze approach [28]. Next, a series of (a) ANOVAs with Tukey’s post-hoc tests (continuous variables) and (b) chi-square tests (categorical variables) were conducted to examine group differences on the baseline variables. Finally, changes in zBMI and ED pathology across FBT were examined via repeated-measures ANOVA, adjusting for child sex, age, race, ethnicity, and income; post-hoc tests examined differences between the groups, with LOW (the group with lowest ED pathology; see below) as the reference group.
Results
The percent of the total sample with each dichotomized ED pathology variable ranged from 16% to 56%. Roughly one-third or less of the sample reported eating concern, restraint, emotional eating, and/or LOC (16%, 26%, 33%, and 34%, respectively). Shape concern and weight concern were reported by 46% and 56% of the sample, respectively.
Identification of ED pathology patterns
Shown in Figure 1, the four derived patterns of ED pathology are: Low ED Pathology (LOW), Shape and Weight Concerns (SWC), Only Loss of Control (OLOC), and High ED Pathology (HIGH).
Figure 1.
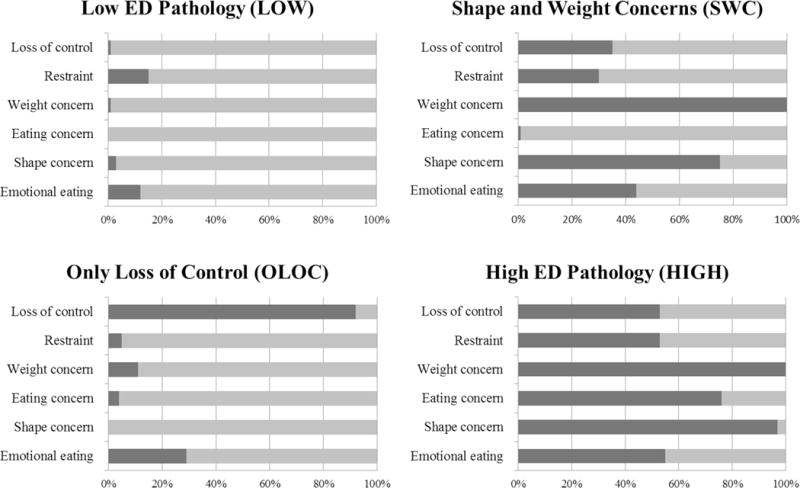
Four ED pathology patterns identified from Latent Class Analysis, and each group’s probability (dark gray = yes, light gray = no) of reporting each of the ED pathology variables
The LOW group was characterized by a low probability of endorsing any of the ED pathology variables. The SWC group was characterized by a high probability of reporting shape concerns and weight concerns, and a lower probability of endorsing other ED pathology. The OLOC group was characterized by a high probability of endorsing LOC eating and a lower probability of endorsing other ED pathology. The HIGH group was characterized by a high probability of endorsing all the ED pathology variables. Means for each ED pathology variable are provided in Table 1.
Table 1.
ED pathology variable levels at baseline by ED pathology group
| ED pathology group | |||||
|---|---|---|---|---|---|
| Total Sample (N = 241) |
Low ED Pathology (LOW; n=83) |
Shape and Weight Concerns (SWC; n=97) |
Only Loss of Control (OLOC; n=24) |
High ED Pathology (HIGH; n=37) |
|
| ED pathology | |||||
| Emotional eating | 0.78 (0.73) | 0.38 (0.44)a | 0.90 (0.71)b | 0.85 (0.74)b | 1.29 (0.87)c |
| Shape concern | 2.39 (1.55) | 1.07 (0.54)a | 2.99 (1.11)b | 1.25 (0.49)a | 4.51 (1.17)c |
| Eating concern | 1.30 (1.13) | 0.60 (0.47)a | 1.21 (0.55)b | 0.70 (0.59)a | 3.49 (0.84)c |
| Weight concern | 2.67 (1.41) | 1.34 (0.53)a | 3.40 (0.80)b | 1.51 (0.41)a | 3.42 (0.81)c |
| Restraint | 1.55 (1.15) | 1.16 (1.02)a | 1.74 (1.18)b | 0.88 (0.72)a | 2.38 (0.98)c |
| Any LOC – last 3 months | 82 (34%) | 0 (0%)a | 38 (39%)b | 24 (100%)c | 19 (51%)b |
| Number of LOC episodes – last month | 1.40 (3.82) | 0 (0)a | 1.54 (4.03)b | 2.54 (2.30)bc | 3.46 (6.36)c |
Values are mean (SD) or N (%).
ED pathology group means for a given variable sharing the same superscript are not significantly different from each other (p < 0.05).
Abbreviations: ED, Eating disorder; LOC, Loss of control over eating.
Demographics and baseline weight metrics
Demographics and baseline weight metrics are shown in Table 2. LOW had a significantly higher household income than HIGH, with no other group differences. Children in the OLOC group were younger than children in the other three groups. A greater proportion of children in the HIGH group were female compared to the LOW group, with no other group differences. The proportion of children self-identifying as white, black, or other differed between the HIGH and OLOC groups, with no other differences among groups. There were no group differences in ethnicity or baseline child zBMI.
Table 2.
Baseline demographics and zBMI by ED pathology group
| ED pathology group | |||||
|---|---|---|---|---|---|
| Total Sample (N = 241) |
Low ED Pathology (LOW; n=83) |
Shape and Weight Concerns (SWC; n=97) |
Only Loss of Control (OLOC; n=24) |
High ED Pathology (HIGH; n=37) |
|
| Demographics | |||||
| Annual household income1 | 7.65 (3.15) | 8.18 (2.97)a | 7.40 (3.22)ab | 8.32 (2.36)ab | 6.73 (3.59)b |
| Age (years) | 9.93 (1.32) | 9.96 (1.37)a | 10.03 (1.31)a | 9.18 (1.20)b | 10.07 (1.17)a |
| Child sex | |||||
| Female | 151 (63%) | 45 (54%)a | 61 (63%)ab | 16 (67%)ab | 29 (78%)b |
| Male | 90 (37%) | 38 (46%) | 36 (37%) | 8 (33%) | 8 (22%) |
| Race | |||||
| White | 172 (71%) | 57 (69%)ab | 71 (73%)ab | 19 (79%)a | 25 (68%)b |
| Black | 38 (16%) | 11 (13%) | 16 (17%) | 1 (4%) | 10 (27%) |
| Other | 31 (13%) | 15 (18%) | 10 (10%) | 4 (17%) | 2 (5%) |
| Ethnicity | |||||
| Non-Hispanic/Latino | 216 (90%) | 76 (92%)a | 88 (91%)a | 20 (83%)a | 32 (86%)a |
| Hispanic/Latino | 25 (10%) | 7 (8%) | 9 (9%) | 4 (17%) | 5 (14%) |
| Weight metric | |||||
| zBMI | 2.19 (0.38) | 2.15 (0.40)a | 2.22 (0.37)a | 2.08 (0.39)a | 2.26 (0.35)a |
Values are mean (SD) or N (%).
ED pathology group means for a given variable sharing the same superscript are not significantly different from each other (p < 0.05).
Annual household income is measured on a scale from 1 (under $9,999) to 11 (over $100,000).
Abbreviations: ED, Eating disorder; zBMI, Body mass index z-score.
Treatment attendance and completion
FBT treatment completion rate and session attendance are shown in Table 3. There were no differences among the ED pathology groups in attendance or FBT treatment completion.
Table 3.
Treatment attendance and completion by ED pathology group
| ED pathology group | |||||
|---|---|---|---|---|---|
| Total Sample (N = 241) |
Low ED Pathology (LOW; n=83) |
Shape and Weight Concerns (SWC; n=97) |
Only Loss of Control (OLOC; n=24) |
High ED Pathology (HIGH; n=37) |
|
| Treatment completion | |||||
| % FBT completers | 183 (75.9) | 66 (79.5)a | 70 (72.2)a | 19 (79.2)a | 28 (75.7)a |
| Attendance1 | |||||
| All participants | 11.8 (4.9) | 12.0 (4.8)a | 11.3 (5.1)a | 12.3 (5.2)a | 11.5 (4.5)a |
| Only those who completed FBT | 14.2 (1.9) | 14.1 (2.2)a | 14.1 (1.7)a | 14.8 (1.1)a | 13.8 (2.0)a |
Values are mean (SD) or N (%).
ED pathology group means for a given variable sharing the same superscript are not significantly different from each other (p < 0.05).
Out of a possible 16 sessions
Abbreviations: ED, Eating disorder; FBT, Family-based behavioral weight loss treatment.
Change in ED pathology across treatment
There was a significant reduction in weight concern from baseline to post-treatment for the entire sample (F(1, 167) = 6.80, p < 0.01), with a significant time by group interaction (F(1, 167) = 8.30, p < 0.001). Compared to children in LOW, children in HIGH (2.86, 95% CI: 2.54, 3.18, p < 0.001), and SWC (1.76, 95% CI: 1.54, 1.99, p < 0.001) saw greater reductions in weight concern, with no differences between children in LOW and OLOC. There was not a significant change in shape concern from baseline to post-treatment for the entire sample (F(1, 167) = 1.04, p > 0.10). However, there was a significant time by group interaction (F(1, 167) = 8.40, p < 0.001). Compared to children in LOW, those in HIGH (2.94, 95% CI: 2.54, 3.18, p < 0.001) and SWC (1.65, 95% CI: 1.54, 1.99, p < 0.001) saw greater reductions in shape concern, with no differences between children in LOW and OLOC. There was not a significant change in number of LOC eating episodes over the past month for the entire sample (F(1, 167) = 0.45, p > 0.10), nor was there a significant time by group interaction (F(1, 167) = 1.96, p > 0.10). Means for the ED pathology variables are listed in Table 4.
Table 4.
ED pathology variables assessed at both pre- and post-FBT for the total sample and by ED pathology group
| ED pathology group | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total sample (n=175) |
Low ED Pathology (LOW; n=64; 36%) |
Shape and Weight Concerns (SWC; n=68; 39%) |
Only Loss of Control (OLOC; n=19; 11%) |
High ED Pathology (HIGH; n=24; 14%) |
||||||
| Pre-FBT | Post-FBT | Pre-FBT | Post-FBT | Pre-FBT | Post-FBT | Pre-FBT | Post-FBT | Pre-FBT | Post-FBT | |
| Weight concern | 2.59 (1.45) |
2.17 (1.28) |
1.29 (0.54) |
1.30 (0.68) |
3.42 (0.83) |
2.65 (1.15) |
1.47 (0.44) |
1.51 (0.80) |
4.62 (1.02) |
3.64 (1.34) |
| Shape concern | 2.36 (1.58) |
1.91 (1.34) |
1.07 (0.55) |
1.10 (0.68) |
3.08 (1.15) |
2.38 (1.29) |
1.26 (0.48) |
1.05 (0.52) |
4.66 (1.15) |
3.44 (1.27) |
| # LOC episodes | 1.28 (3.68) |
0.57 (1.76) |
0.00 (0.00) |
0.18 (0.53) |
1.40 (3.89) |
0.67 (2.06) |
2.53 (2.12) |
0.79 (2.32) |
3.35 (6.50) |
1.12 (2.25) |
Values are mean (SD).
Number of LOC episodes is measured over the previous month.
Abbreviations: ED, Eating disorder; FBT, Family-based behavioral weight loss treatment; LOC, Loss of control over eating.
Change in zBMI across treatment
There was a significant reduction in zBMI from baseline to post-treatment for the entire sample (F(1, 172) = 20.42, p < 0.001), with the overall sample achieving a 0.28 unit reduction in zBMI. Additionally, there was a significant time by group interaction (F(1, 172) = 3.99, p < 0.01). Relative to the LOW group, both the SWC (0.20, 95% CI: 0.05, 0.35, p < 0.05) and HIGH (0.27, 95% CI: 0.06, 0.47, p < 0.05) groups experienced less zBMI change, with no differences between LOW and OLOC. Baseline and post-treatment zBMI across groups are shown in Table 5.
Table 5.
zBMI assessed at both baseline and post-treatment for the total sample and by ED pathology group
| ED pathology group | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total sample (n=182) |
Low ED Pathology (LOW; n=66, 34.4%) |
Shape and Weight Concerns (SWC; n=70, 40.2%) |
Only Loss of Control (OLOC; n=19, 10.0%) |
High ED Pathology (HIGH; n=27, 15.4%) |
||||||
| Pre-FBT | Post-FBT | Pre-FBT | Post-FBT | Pre-FBT | Post-FBT | Pre-FBT | Post-FBT | Pre-FBT | Post-FBT | |
| zBMI | 2.17 (0.39) |
1.89 (0.55) |
2.10 (0.39) |
1.75 (0.60) |
2.24 (0.36) |
1.98 (0.48) |
2.08 (1.74) |
1.74 (0.60) |
2.24 (0.38) |
2.08 (0.49) |
Values are mean (SD).
Abbreviations: ED, Eating disorder; FBT, Family-based behavioral weight loss treatment; zBMI, Body mass index z-score.
Relation between changes in zBMI and weight and shape concerns
For the whole sample, change in zBMI was weakly correlated with change in both weight concern (r = 0.17; p < 0.05) and shape concern (r = 0.20; p < .01). When explored by group using within-class correlations, the relation between changes in zBMI and weight and/or shape concerns was only significant for the SWC group (r = 0.26 and 0.25, respectively; ps < .05) and was not significant for the other groups.
Discussion
Four patterns of ED pathology were identified in a treatment-seeking sample of children with overweight/obesity. One group had a very low level of ED pathology (LOW), and three groups presented with unique patterns of ED pathology (SWC, OLOC, and HIGH). These groups are similar to previous findings, in which identified patterns of overeating and disinhibited eating varied from low to high levels [16, 17]. Shape and weight concerns decreased for the overall sample across FBT, with children in SWC and HIGH experiencing greater decreases than children in LOW. All groups experienced significant decreases in zBMI, with the sample overall achieving a clinically significant weight loss (i.e., zBMI changes ≥ 0.25 units; [29]). However, children in HIGH and SWC reduced their zBMI less than children in LOW.
Only one-third of the sample was classified as having low ED pathology. Other studies of treatment-seeking samples have found higher rates of children without ED pathology [11, 14, 30], but this may reflect less comprehensive measures (e.g., not assessing shape/weight concerns, LOC). The SWC group, the largest group at 40% of the sample, reported high levels of shape and weight concerns but no other ED pathology, which is not surprising given the positive relation between elevated weight status and shape/weight concerns [3]. The OLOC group, which was the smallest group at 10% of the sample, reported only LOC eating. Finally, the HIGH group was characterized by high levels of emotional eating, dietary restraint, shape, weight, and eating concerns, and LOC eating. Given their high and varied presentation of ED pathology, this group may be at the greatest risk of developing an ED.
There were some demographic differences across the groups. There was a greater proportion of females in the HIGH group compared to the LOW group. This is consistent with the extant literature in that ED pathology/EDs are more common among females than males, although this difference is much less marked in terms of binge eating in the context of obesity [31]. However, it is important to note that 8 of the 37 children (22%) in the HIGH group were male, in line with research suggesting that males, and particularly males with overweight/obesity and/or seeking weight loss treatment, also experience high levels of ED pathology [32]. Indeed, in an FBT-seeking sample, no differences were found between male and female children’s scores on the Kids’ Eating Disorders Survey [33]. Children in the OLOC group were younger than children in the other groups; their younger age may partially explain why they only experienced LOC eating and not other ED pathology. However, this contrasts with the finding that children as young as five experience shape and weight concerns [34]; as such, future work is needed to explore this finding. There were no differences among the groups in zBMI at baseline, indicating that while elevated weight is an established risk factor for the development of EDs [3], there are other risk factors that also contribute to the development of ED pathology among youth with obesity. Alternatively, it may be that once weight status is above a certain threshold, there is less of a relation between weight and ED pathology.
There were no differences among the groups in treatment completion or attendance. The extant literature is mixed regarding the relation between ED pathology and treatment completion, with some studies finding that children with ED pathology are more likely to drop out of obesity treatment [30] and others seeing no relation [35]. Findings from the current study indicate that children will participate in FBT, regardless of level of ED pathology.
Self-reported dieting has been shown to increase risk for weight gain [36], obesity, and EDs [37] in children and adolescents. As such, one frequent concern about obesity treatment is that it will cause disordered eating/clinical EDs. The current study confirms earlier work indicating that this is not the case [33]. In fact, levels of ED pathology decreased from baseline to post-treatment in the overall sample and specifically in the HIGH group. Notably, in the HIGH group, the changes in ED pathology were not significantly correlated with reductions in weight, indicating that for children with comorbid obesity and ED pathology, large reductions in weight status are not necessary to see changes in ED pathology. The discrepancy in findings between the dieting and obesity-treatment literatures is likely due to differences in energy-reduction method; while most self-initiated dieting is often accompanied by extreme restriction, particularly of specific foods and/or food groups [1], supervised obesity treatment focuses on encouraging more sustainable lifestyle modifications. In addition, FBT teaches parents to model healthy eating practices, use praise/positive reinforcement for healthy behaviors rather than focusing on weight or criticizing less healthy choices, and provide support (e.g., assisting with self-monitoring) for their child’s efforts. These parenting strategies may minimize children’s feelings of deprivation or guilt that might contribute to risk for EDs that are seen among self-initiated dieters [38].
All four ED pathology groups lost weight during FBT, with the overall sample losing a clinically significant amount of weight. While children in SWC lost less weight than children in LOW, they still achieved a clinically significant amount of weight loss. On the other hand, children in HIGH lost less weight than children in LOW, with their weight loss not meeting clinical significance. This suggests that it may be necessary to supplement treatment activities for children who present with obesity and high, varied ED pathology. Additional treatment content may include concepts from cognitive-behavioral therapy for body image and EDs [39] and/or interpersonal psychotherapy for EDs [40]. Given that the current trial only examined post-treatment weight change, future work is necessary to examine long-term outcomes.
Strengths of the current study include a large sample size, availability of data on varied aspects of ED pathology, and the ability to examine impact on changes in children’s weight status and ED pathology. Given that this is a FBT-seeking sample, children in the current study might have a different presentation and/or prevalence of ED pathology than non-treatment-seeking samples or those seeking other forms of behavioral weight loss treatment; thus, these findings might not generalize to other samples. Finally, for the LCA, the current study dichotomized continuous variables, which may limit statistical power. While clinically-relevant cut-offs were utilized, given the continuous nature of these variables, it is recommended that a latent profile analysis be used in future studies. Additionally, future work is needed to confirm the clinically-meaningful cut-off on the YEDE-Q (i.e., lower levels in children may indicate heightened risk).
Conclusion
In conclusion, findings from the current study advance the understanding of what ED pathology looks like in treatment-seeking children with overweight/obesity. ED pathology is prevalent among children with overweight/obesity, but has varying presentations. Four distinct patterns of ED pathology were identified, ranging from low ED pathology to high, varied ED pathology. Additionally, levels of ED pathology decreased across FBT among the full sample, including in the group with the highest ED pathology, indicating that obesity treatment does not increase risk for EDs and in fact likely lowers it. ED pathology pattern was related to treatment response, and children with the highest ED pathology lost less weight than children with low/no ED pathology. Future work is needed to identify how best to tailor FBT for children with concurrent obesity and high ED pathology to enhance weight loss success.
What is already known about this subject?
Children with overweight/obesity have higher levels of eating disorder (ED) pathology than their peers without obesity, which increases their ED risk
Previous findings have been mixed with respect to whether ED pathology impacts obesity treatment response
What does this study add?
Patterns of ED pathology varied in an obesity treatment-seeking sample, ranging from low ED pathology to high and varied ED pathology
Children experienced reductions in shape and weight concerns during obesity treatment
Obesity treatment was successful at inducing weight change in all children; however, children with high and varied ED pathology may have poorer treatment response
Acknowledgments
Funding: This work was supported by several National Institutes of Health (NIH) Grants: R01HD036904 (National Institute of Child Health and Human Development), UL1TR002345 (National Center for Advancing Translational Sciences), and K24MH070446 (National Institute of Mental Health). KN Balantekin was supported by T32HL130357 (National Heart, Lung, and Blood Institute), JF Hayes was supposed by NIH Grant T32HL007456 (National Heart, Lung, and Blood Institute), and RI Stein was supported by NIH Grant KL2RR024994 (National Center for Research Resources).
Footnotes
Disclosure: Dr. Epstein reports involvement with Kurbo/Datri Health outside the submitted work; Dr. Wilfley reports consulting for Shire Pharmaceuticals and Sunovian Pharmaceuticals outside the submitted work. No other authors report any disclosures or conflicts of interest.
References
- 1.Balantekin KN, Birch LL, Savage JS. Patterns of weight-control behavior among 15 year old girls. Int J Eat Disord. 2015;48(6):589–600. doi: 10.1002/eat.22426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burrows A, Cooper M. Possible risk factors in the development of eating disorders in overweight pre-adolescent girls. Int J Obes. 2002;26(9):1268–1273. doi: 10.1038/sj.ijo.0802033. [DOI] [PubMed] [Google Scholar]
- 3.Striegel-Moore RH, Silberstein LR, Rodin J. Toward an understanding of risk factors for bulimia. Am Psychol. 1986;41(3):246–263. doi: 10.1037//0003-066x.41.3.246. [DOI] [PubMed] [Google Scholar]
- 4.Vander Wal JS, Thelen MH. Eating and body image concerns among obese and average-weight children. Addict Behav. 2000;25(5):775–758. doi: 10.1016/s0306-4603(00)00061-7. [DOI] [PubMed] [Google Scholar]
- 5.Bryant-Waugh RJ, Cooper PJ, Taylor CL, Lask BD. The use of the eating disorder examination with children: A pilot study. Int J Eat Disord. 1996;19(4):391–397. doi: 10.1002/(SICI)1098-108X(199605)19:4<391::AID-EAT6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 6.Cooper Z, Fairburn C. The eating disorder examination: A semi-structured interview for the assessment of the specific psychopathology of eating disorders. Int J Eat Disord. 1987;6(1):1–8. [Google Scholar]
- 7.Arnow B, Kenardy J, Agras WS. The emotional eating scale: The development of a measure to assess coping with negative affect by eating. Int J Eat Disord. 1995;18(1):79–90. doi: 10.1002/1098-108x(199507)18:1<79::aid-eat2260180109>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 8.Doyle AC, le Grange D, Goldschmidt A, Wilfley DE. Psychosocial and physical impairment in overweight adolescents at high risk for eating disorders. Obesity. 2007;15(1):145–154. doi: 10.1038/oby.2007.515. [DOI] [PubMed] [Google Scholar]
- 9.Eddy KT, Tanofsky-Kraff M, Thompson-Brenner H, Herzog DB, Brown TA, Ludwig DS. Eating disorder pathology among overweight treatment-seeking youth: Clinical correlates and cross-sectional risk modeling. Behav Res Ther. 2007;45(10):2360–2371. doi: 10.1016/j.brat.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 10.Killen JD, Taylor CB, Hayward C, et al. Weight concerns influence the development of eating disorders: A 4-year prospective study. J Consult Clin Psychol. 1996;64(5):936–940. doi: 10.1037//0022-006x.64.5.936. [DOI] [PubMed] [Google Scholar]
- 11.Bishop-Gilyard CT, Berkowitz RI, Wadden TA, Gehrman CA, Cronquist JL, Moore RH. Weight reduction in obese adolescents with and without binge eating. Obesity. 2011;19(5):982–987. doi: 10.1038/oby.2010.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giel KE, Zipfel S, Schweizer R, et al. Eating disorder pathology in adolescents participating in a lifestyle intervention for obesity: Associations with weight change, general psychopathology and health-related quality of life. Obes Facts. 2013;6(4):307–316. doi: 10.1159/000354534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braet C. Patient characteristics as predictors of weight loss after an obesity treatment for children. Obesity. 2006;14(1):148–155. doi: 10.1038/oby.2006.18. [DOI] [PubMed] [Google Scholar]
- 14.Wildes JE, Marcus MD, Kalarchian MA, Levine MD, Houck PR, Cheng Y. Self-reported binge eating in severe pediatric obesity: Impact on weight change in a randomized controlled trial of family-based treatment. Int J Obes. 2010;34(7):1143–1148. doi: 10.1038/ijo.2010.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teixeira PJ, Silva MN, Coutinho SR, et al. Mediators of weight loss and weight loss maintenance in middle-aged women. Obesity. 2010;18(4):725–735. doi: 10.1038/oby.2009.281. [DOI] [PubMed] [Google Scholar]
- 16.Vannucci A, Tanofsky-Kraff M, Crosby RD, et al. Latent profile analysis to determine the typology of disinhibited eating behaviors in children and adolescents. J Consult Clin Psychol. 2013;81(3):494–507. doi: 10.1037/a0031209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boutelle KN, Peterson CB, Crosby RD, et al. Overeating phenotypes in overweight and obese children. Appetite. 2014;76:95–100. doi: 10.1016/j.appet.2014.01.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayes JF, Altman M, Kolko RP, et al. Decreasing food fussiness in children with obesity leads to greater weight loss in family-based treatment. Obesity. 2016;24(10):2158–2163. doi: 10.1002/oby.21622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000;(314):1–27. [PubMed] [Google Scholar]
- 20.Tanofsky-Kraff M, Theim KR, Yanovski SZ, et al. Validation of the emotional eating scale adapted for use in children and adolescents (EES-C) Int J Eat Disord. 2007;40(3):232–240. doi: 10.1002/eat.20362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vannucci A, Tanofsky-Kraff M, Shomaker LB, et al. Construct validity of the emotional eating scale adapted for children and adolescents. Int J Obes. 2012;36(7):938–943. doi: 10.1038/ijo.2011.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kass AE, Theim Hurst K, Kolko RP, et al. Psychometric evaluation of the youth eating disorder examination questionnaire in children with overweight or obesity. Int J Eat Disord. 2017;50(7):775–780. doi: 10.1002/eat.22693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldschmidt AB, Doyle AC, Wilfley DE. Assessment of binge eating in overweight youth using a questionnaire version of the child eating disorder examination with instructions. Int J Eat Disord. 2007;40(5):460–467. doi: 10.1002/eat.20387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fairburn CG, Beglin SJ. Assessment of eating disorders: Interview or self-report questionnaire? Int J Eat Disord. 1994;16(4):363–370. [PubMed] [Google Scholar]
- 25.Goldfein JA, Devlin MJ, Kamenetz C. Eating Disorder Examination-Questionnaire with and without instruction to assess binge eating in patients with binge eating disorder. Int J Eat Disord. 2005;37(2):107–111. doi: 10.1002/eat.20075. [DOI] [PubMed] [Google Scholar]
- 26.Mond JM, Hay PJ, Rodgers B, Owen C, Beumont PJ. Validity of the Eating Disorder Examination Questionnaire (EDE-Q) in screening for eating disorders in community samples. Behav Res Ther. 2004;42(5):551–567. doi: 10.1016/S0005-7967(03)00161-X. [DOI] [PubMed] [Google Scholar]
- 27.Lanza ST, Collins LM, Lemmon DR, Schafer JL. PROC LCA: A SAS procedure for latent class analysis. Struct Equ Modeling. 2007;14(4):671–694. doi: 10.1080/10705510701575602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bray BC, Lanza ST, Tan X. Eliminating bias in classify-analyze approaches for latent class analysis. Struct Equ Modeling. 2015;22(1):1–11. doi: 10.1080/10705511.2014.935265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ford AL, Hunt LP, Cooper A, Shield JP. What reduction in BMI SDS is required in obese adolescents to improve body composition and cardiometabolic health? Arch Dis Child. 2010;95(4):256–261. doi: 10.1136/adc.2009.165340. [DOI] [PubMed] [Google Scholar]
- 30.Levine MD, Ringham RM, Kalarchian MA, Wisniewski L, Marcus MD. Overeating among seriously overweight children seeking treatment: Results of the children’s eating disorder examination. Int J Eat Disord. 2006;39(2):135–140. doi: 10.1002/eat.20218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patton G, Selzer R, Coffey C, Carlin J, Wolfe R. Onset of adolescent eating disorders: Population based cohort study over 3 years. BMJ. 1999;318(7186):765–768. doi: 10.1136/bmj.318.7186.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glasofer DR, Tanofsky-Kraff M, Eddy KT, et al. Binge eating in overweight treatment-seeking adolescents. J Pediatr Psychol. 2007;32(1):95–105. doi: 10.1093/jpepsy/jsl012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Epstein LH, Paluch RA, Saelens BE, Ernst MM, Wilfley DE. Changes in eating disorder symptoms with pediatric obesity treatment. J Pediatr. 2001;139(1):58–65. doi: 10.1067/mpd.2001.115022. [DOI] [PubMed] [Google Scholar]
- 34.Davison KK, Markey CN, Birch LL. A longitudinal examination of patterns in girls’ weight concerns and body dissatisfaction from ages 5 to 9 years. Int J Eat Disord. 2003;33(3):320–332. doi: 10.1002/eat.10142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goossens L, Braet C, Decaluwé V. Loss of control over eating in obese youngsters. Behav Res Ther. 2007;45(1):1–9. doi: 10.1016/j.brat.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 36.Balantekin KN, Savage JS, Marini ME, Birch LL. Parental encouragement of dieting promotes daughters’ early dieting. Appetite. 2014;80:190–196. doi: 10.1016/j.appet.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haines J, Neumark-Sztainer D. Prevention of obesity and eating disorders: A consideration of shared risk factors. Health Edu Res. 2006;21(6):770–782. doi: 10.1093/her/cyl094. [DOI] [PubMed] [Google Scholar]
- 38.Neumark-Sztainer D, Wall M, Guo J, Story M, Haines J, Eisenberg M. Obesity, disordered eating, and eating disorders in a longitudinal study of adolescents: How do dieters fare 5 years later? J Am Diet Assoc. 2006;106(4):559–568. doi: 10.1016/j.jada.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 39.Jones M, Luce KH, Osborne MI, et al. Randomized, controlled trial of an internet-facilitated intervention for reducing binge eating and overweight in adolescents. Pediatrics. 2008;121(3):453–462. doi: 10.1542/peds.2007-1173. [DOI] [PubMed] [Google Scholar]
- 40.Tanofsky-Kraff M, Wilfley DE, Young JF, et al. Preventing excessive weight gain in adolescents: Interpersonal psychotherapy for binge eating. Obesity. 2007;15(6):1345–1355. doi: 10.1038/oby.2007.162. [DOI] [PMC free article] [PubMed] [Google Scholar]


