Abstract
Cytokines are small, secreted proteins associated with the maintenance of immune homeostasis but also implicated with the pathogenesis of several autoimmune and inflammatory diseases. Biologic agents blocking cytokines or their receptors have revolutionized the treatment of such pathologies. Nonetheless, some patients fail to respond to these drugs or do not achieve complete remission. The signal transduction originating from membrane-bound cytokine receptors is an intricate network of events that lead to gene expression and ultimately regulate cellular functionality. Our understanding of the intracellular actions that molecules such as interleukins, inteferons (IFNs) and tumor necrosis factor (TNF) set into motion has greatly increased in the past few years, making it possible to interfere with cytokines’ signaling cascades. The Janus kinase (JAK)/signal transducer and activator of transcription (STAT), the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB), the mitogen activated protein kinase (MAPK) and the Phosphatidylinositol-3’-kinases (PI3K) pathways have all been intensively studied and key steps as well as molecules have been identified. These research efforts have led to the development of a new generation of small molecule inhibitors. Drugs capable of blocking JAK enzymatic activity or interfering with the proteasome-mediated degradation of intermediates in the NF-kB pathway have already entered the clinical arena confirming the validity of this approach. In this review, we have recapitulated the biochemical events downstream of cytokine receptors and discussed some of the drugs which have already been successfully utilized in the clinic. Moreover, we have highlighted some of the new molecules that are currently being developed for the treatment of immune-mediated pathologies and malignancies.
Keywords: Cytokines, Signal Transduction, Drug development, Inflammation, Autoimmunity, Therapy
1. Introduction
The term cytokine defines an extensive range of soluble factors that serve as intercellular communication tools of the immune system. These molecules are critical for developmental and homeostatic immune processes including host defense, inflammation, trauma and cancer. When their expression or their intracellular pathways are dysregulated, immune homeostasis is altered leading to the development of pathologies such as chronic inflammation, autoimmune syndromes as well as malignancies [1].
Produced by a plethora of cell types and having diverse functions which are not limited to just the immune response, cytokines are better classified based on the type of receptors they bind. This classification also allows us to highlight similarities in their signal transduction cascades. There are five unique classes of receptors: the so-called type I and type II cytokine receptors, the tumor necrosis factor (TNF) receptor family, the interleukin (IL)-1 receptor and the IL-17 family of receptors. The final subgroup of cytokines is the transforming growth factor receptor superfamily which will not be covered by this review.
Type I cytokines include molecules such as IL-2 through 7, colony stimulating factors (CSFs), hematopoietic factors and many others. Notably, they have critical functions in lymphocyte development and can act as positive as well as negative regulators of the immune responses.
Interferons (IFNs) and cytokines of the IL-10 family are all members of the group designated as type II cytokines. There are over 20 IFNs subdivided into three major groups, and their primary activity is to inhibit viral infections and stimulate the immune system to fight pathogens. The IL-10 family includes IL-10, IL-19 through 24, and IL-26. IL-10 is the most extensively studied and plays an essential role in both anti-inflammatory and immunosuppressive functions.
The TNF family contains a large number of molecules which are also potent modulators of either immune cell development or pro-inflammatory responses. Overall, these molecules comprise a structurally related group of ligands, receptors, and inhibitory decoy receptors, with tissue-specific expression, ligand-receptor binding and biological functions [2, 3].
Similarly to TNF, the IL-1 family also includes inhibitory decoy receptors and ligands of this IL-1 family have been shown to have pro- as well as anti-inflammatory effects. Indeed, IL-1, IL-18, and IL-36 are potent inducers of other pro-inflammatory cytokines whereas IL-37 and IL-38 act as negative regulators.
The IL-17 family includes IL-17A through IL-17E, which is also known as IL-25. Members of this family induce the production of other cytokines including TNF and IL-1, chemokines (e.g IL-8), prostaglandins and antimicrobial peptides. Moreover, they are critical for host defense against gram-negative extracellular bacteria like Klebsiella pneumoniae and fungi like Candida albicans [4].
Several studies have shown that these molecules or their receptors can be targeted therapeutically to treat chronic inflammatory conditions and immune related disorders. Therefore, it is not surprising that blocking cytokines and their receptors with biologic disease modifying antirheumatic drugs (DMARDs) has revolutionized the treatment of the above-mentioned pathologies. Nonetheless, blocking the action of a single cytokine is sometimes not sufficient. Moreover, parenteral or endovenous administration are often required; thus, the development of novel therapeutic strategies is needed. In the past few years, our understanding of the cytokine signaling cascades has greatly expanded and inhibition of the enzymatic activity of intracellular molecules, such as receptor-associated kinases and of transcription factors (TFs) is not only very attractive but more importantly, feasible. The aim of this review is to briefly describe the signaling cascades downstream of cytokine receptors and present the molecules which have been pharmacologically targeted or being considered as possible targets for the development of novel ad hoc therapies for inflammatory, immune-related disorders as well as malignancies.
2. Type I and type II cytokines and the JAK/STAT pathway
Type I and type II cytokine receptors do not possess intrinsic enzymatic activities but instead rely on specific cytosolic kinases, known as the JAKs, to transmit the signal inside the cell. The family is constituted of four members: JAK1, JAK2, JAK3, and TYK2. They were all discovered in the early 1990’s [5] and are named after the Roman god Janus Bifrons. Similar to the two-faced god, JAK C-terminus architecture is constituted by a kinase domain preceded by a pseudokinase domain that are structurally very similar to each other. Although initially thought to be deprived of a clear enzymatic activity, the pseudokinase domain is instead catalytically active and can phosphorylate and activate the kinase domain therefore serving an important regulatory role. Moreover, Janus was the god of beginnings and entryways; likewise, JAKs oversee the start of the signaling cascade which originates outside the cell and continues in the cytoplasm.
The binding of a cytokine to its cognate receptor results in alteration of the conformational structure of the receptor chains bringing the associated JAKs in close proximity to each other and ultimately resulting in activation of their phosphotransferase activity. Thus, JAKs, which work in pairs, phosphorylate themselves and, in turn, the intracellular portion of the receptors. The phosphorylated receptors become substrate for the binding of several intracellular molecules including the SH2 domain-containing latent cytoplasmic transcription factors known as STATs. This family of DNA-binding proteins is composed of seven family members, namely, STAT1, STAT2, STAT3, STAT4, STAT5A, STAT5B, and STAT6. Binding to the receptors results in their phosphorylation by the JAKs upon which they detach from the receptor chains, dimerize and translocate to the nucleus to regulate transcription of specific target genes [6]. Different cytokine receptors associate with different JAK pairs and preferentially recruit specific STATs, achieving a large number of molecular combinations that ultimately lead to cytokine-specific intracellular molecular responses. JAK1, JAK2 and TYK2 are expressed in various cell types whereas JAK3 is selectively expressed in hematopoietic cells [7]. JAK1 and JAK2 are the critical JAKs for the signaling of cytokines which utilize the receptor subunit gp130, like IL-6, and IL-11, but also for IL-3 and IL-5 which share the common β chain subunit in their receptor complexes. Cytokines such as CSFs, erythropoietin and growth hormone solely rely on JAK2. Notably, JAK3 is exclusively utilized by cytokines that share the receptor subunit known as the common γ chain (γc) which include: IL-2, IL-4, IL-7, IL-9, IL-15 and IL-21. Finally, TYK2 is mainly activated by type I IFNs and by interleukins such as IL-12 and IL-23.
3. JAKs-associated diseases
The biological importance of the JAKs has also been shown with the generation of animals lacking their expression. Both JAK1 and JAK2 nullizygous mice are embryonically lethal due to major developmental defects including compromised lymphopoiesis and erythropoiesis. JAK1 mutations in humans cause B, T and myeloid cell leukemias and have been sporadically observed in solid cancers [1], whereas JAK2 protein alterations have been associated with several human diseases. It was originally found that JAK2 could fuse to a part of the transcription factor TEL to generate a fusion protein responsible for Sézary syndrome, a type of cutaneous T cell lymphoma [8]. Other fusion proteins of JAK2 with PCM1 and SEC1A have been reported as causes of leukemias and lymphomas [9, 10]. Moreover, JAK2 mutations were found to be the cause underlying over 50% of myeloproliferative diseases such as polycythemia vera (PV), primary myelofibrosis and essential thrombocytopenia [11, 12]. The most common mutation associated with these diseases is the V617F mutation in the pseudokinase domain of JAK2, which results in a constitutively active molecule, leading to an uncontrolled, cytokine-independent erythrocyte proliferation. Mutations in the common γc result in impaired signaling for cytokines critical for hematopoietic cell development leading to a condition known as X-linked severe combined immunodeficiency (X-SCID), in which affected patients do not have T or NK cells and have B cells with severely impaired functionality. Similarly, autosomal recessive mutations in JAK3 cause SCID [13]. Interestingly, some JAK3-deficient patients have been described as having few, poorly functioning, T cells and exhibit autoimmune features [14, 15].
Intriguingly, TYK2 deficiency in mice only appears to affect Type I and Type II IFNs’ activity as TYK2 deficient mice showed increased susceptibility to viral and intracellular infections. TYK2 deficiency has also been reported in humans. Autosomal recessive TYK2 deficiency causes a disease with characteristic features, at least in some patients, of both hyper-IgE syndrome (HIES) and susceptibility to mycobacterial disease (following BCG vaccination). Other patients instead developed neurobrucellosis and viral (herpes zoster) infections but did not show any signs of atopy, asthma, or fungal skin infections [16–18].
4. JAK inhibitors
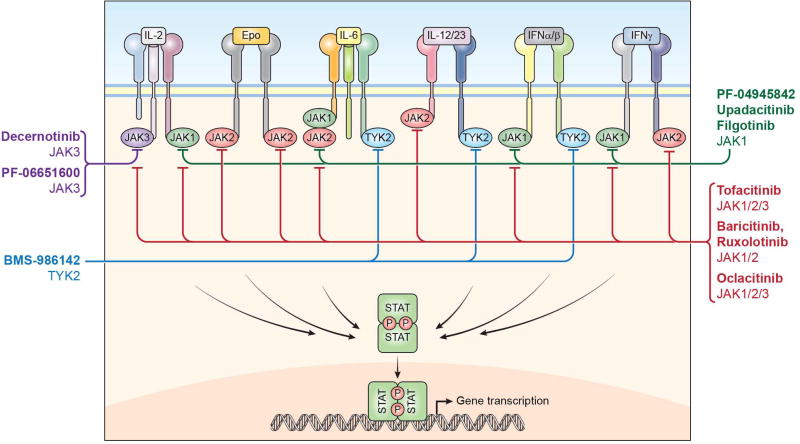
Phosphotransferases, like the JAKs, are enzymatic switches which have been considered as ideal therapeutic targets. Almost all current kinase inhibitors are ATP competitors. They block the ATP binding site preventing the enzyme’s activity, ultimately halting the downstream signaling cascade and the subsequent cellular responses. Indeed, targeting of the JAKs has been quite successful, and JAK inhibitors are now approved to be used in humans and dogs with several new molecules currently being developed (Fig. 1). Because of its immune-specific expression, JAK3 was initially considered to be the best target for the development of specific inhibitors [19, 20].
Figure 1.
Type 1 and type 2 cytokines signaling cascade is dependent on the enzymatic activity of members of the Janus kinase family: JAK1, JAK2, JAK3 and TYK2. These enzymes phosphorylate STATs. First-generation JAK inhibitors (red line) block more than one JAK and consequently, inhibit the effect of several cytokines. Selective JAK inhibitors (green line: JAK1; purple line: JAK3, blue line: TYK2), instead have the potential to limit the activity of only selected cytokines.
Nonetheless, ruxolitinib (trade name, Jakafi®) which exhibits selectivity towards JAK1 and JAK2, was the first JAK inhibitor to be approved by the regulatory agencies. With the recognition of JAK2 mutations being responsible for hematologic disorders such as PV and primary myelofibrosis, for which very few therapeutic approaches were available, ruxolitinib was quickly approved. In these patients, treatment with the JAK inhibitor resulted in reduced spleen size and amelioration of the associated symptoms. Given its JAK1-JAK2 specificity, ruxolitinib inhibits intracellular signaling of multiple pro-inflammatory cytokines including IL-6, IL-12, IL-23 and IFN-γ but also erythropoietin, growth hormone and CSFs. Hence, besides its use in myeloproliferative neoplasms, ruxolitinib has also been tested in several other pathologies. In a phase II clinical trial performed in patients with rheumatoid arthritis (RA), ruxolitinib improved the disease symptoms (ClinicalTrials.gov NCT00550043). Individual cases of successful administration of ruxolitinib have been reported for Chilblain lupus erythematosus as well as dermatomyositis in a patient that also developed JAK2 V617F–positive myelofibrosis [21, 22]. In addition, ruxolitinib has also demonstrated efficacy in the treatment of plaque psoriasis and other dermatologic disorders such as alopecia areata (AA) and alopecia universalis (AU). For these two pathologies, efficacy has been shown following oral administration as well as in the topical formulation [23, 24]. Notably, the appearance of new mutations in the JAK2 protein, resulting in resistance to ruxolitinib and other JAK2 inhibitors, has been documented [25]. An alternative mechanism leading to resistance has been proposed to involve heterodimerization between JAK2 and JAK1 or TYK2 [26].
Tofacitinib (trade name, Xeljianz®) was the second JAK inhibitor approved for clinical use. It was originally developed as a JAK3 inhibitor but, like all the first-generation JAK inhibitors, it also interferes with the activity of JAK1 and to a lesser extent, JAK2. Because of its capability to inhibit three out of four JAKs, tofacitinib blocks signaling originating from a wide variety of cytokine receptors including the γc dependent cytokines (IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21) but also cytokines sharing the gp130 subunit such as IL-6, IL-11 and IL-27, oncostatin M, Leukemia Inhibitory Factor as well as type I and type II IFNs and many others. Furthermore, it can indirectly inhibit the production of cytokines which do not signal via the JAK/STAT pathway, such as TNF and IL-1. Although it was initially considered for the prevention of transplant rejection its clinical applicability in transplantation was abandoned because of safety considerations due to the required high doses, eventually leading to BK viremia, nephropathy, and post-transplant lymphoproliferative diseases [27, 28]. Despite this broad spectrum of activity, safety and efficacy of tofacitinib was demonstrated in several phase III clinical trials and long term extension studies in which it was tested as a monotherapy as well as in combination with other DMARDs and proven to be superior to methotrexate (MTX) and non-inferior to an anti-TNF biologic. Moreover, it has been shown to be efficacious in patients who did not respond to biologics [29–34]. So far, approval has been granted for the treatment of RA patients intolerant or unresponsive to methotrexate. Besides RA, the FDA is currently considering approval for its use in the treatment of psoriasis and psoriatic arthritis (PsA). Advanced clinical trials are also ongoing for the treatment of inflammatory bowel disease (IBD), PsA, juvenile idiopathic arthritis, alopecia (both AA and AU) and atopic dermatitis (AD) (NCT01877668; NCT01500551; NCT02299297; NCT02001181). Moreover, tofacitinib has been sporadically utilized in the treatment of vasculitis [35] and we have shown it to be efficacious in preclinical models of graft versus host disease (GVHD) and systemic lupus erythematosus (SLE) [36, 37].
The third JAK inhibitor to be approved was oclatinib (trade name, Apoquel®), another pan-JAK inhibitor, for the veterinary treatment of canine atopic dermatitis and eczema [38, 39]. It is therefore not surprising that, upon the approval of oclacitinib, the possibility to inhibit JAKs is being considered for the treatment of allergic inflammation [40].
The EMA has recently approved another JAK1-JAK2 inhibitor, baricitinib (trade name, Olumiant®) for patients with moderate to severe RA which have not responded or are unable to tolerate treatment with DMARDs or biologics. Structurally, baricitinib is very similar to ruxolitinib but unlike ruxolitinib and tofacitinib, which are metabolized via the cytochrome P450 system, it is cleared by the kidney. Its activity overlaps with ruxolitinib and, besides RA, where it has been shown to be superior to MTX and the TNF inhibitor adalimumab, baricitinib showed efficacy in patients with psoriasis and is currently being assessed in AD and giant cell arteritis (NCT02576938; NCT03026504). Importantly, baricitinib has been used, albeit at doses higher than what was approved for RA, in the treatment of mendelian autoinflammatory disorders characterized by an activation of interferon-dependent genes such as Chronic Atypical Neutrophilic Dermatosis with Lipodystrophy and Elevated temperature (CANDLE) and Stimulator of Interferon Genes (STING)-Associated Vasculopathy with Onset in Infancy (SAVI) (NCT01724580). A clinical trial is also ongoing in which baricitinib is assessed for GVHD (NCT02759731).
Other pan-JAK inhibitors such as peficitinib, itacitinib, momelotinib and VR588 are being investigated for several inflammatory pathologies such as RA, psoriasis and asthma but also for malignancies like B-cell lymphoma, Hodgkin lymphoma as well as solid tumors.
The next-generation of JAK inhibitors include not only molecules that compete with ATP, but also compounds which inhibit via an allosteric mechanism. These drugs are being developed to selectively block only one specific JAK which should result in less side effects while maintaining efficacy in selected pathologies.
JAK1-selective inhibitors like filgotinib (which maintains minimal activity on JAK2), Upadacitinib and PF-04965842 are all in advanced phases of development. Filgotinib has been tested in RA and has shown promising results in the treatment of Crohn’s disease, ulcerative colitis (UC), ankylosing spondylitis (AS), RA and PsA (NCT02914600; NCT02914535; NCT03117270; NCT02873936; NCT03101670). Upadacitinib binds JAK1 outside the ATP-binding pocket on a region structurally not shared by JAK2 and it is, therefore, the first JAK inhibitor that utilizes an allosteric control to regulate JAK activity. Currently, upadacitinib is being investigated for the treatment of RA and PsA as well as UC and AD (NCT03086343; 0310440; NCT03006068; NCT02925117). PF-04965842 is also being investigated in AD (NCT02780167).
Decernotinib and PF-06651600 are JAK3-selective drugs and therefore, given the aforementioned JAK3 pattern of expression, designed to act solely on immune cells. Both are in late phase of development for the treatment of RA and, in the case of PF-06651600, alopecia, and UC (NCT02974868; NCT02969044; NCT02958865).
Given the well described IFN-signature associated with SLE, TYK2 inhibitors are being developed for that clinical application. Moreover, since this kinase is utilized by IL-12 and IL-23, there is also the possibility to use selective TYK2 inhibitors for the treatment of psoriasis. Indeed, a phase II clinical trial in psoriasis is currently underway with BMS986165 (NCT02931838) for which preclinical studies are also ongoing in SLE and IBD.
Inhibition of cytokines that are critical for biological functions comes with a price. Side effects are dependent on which specific JAK (or JAKs) are blocked at specific concentrations. Although the drugs which have so far been approved display an acceptable safety profile, suppression of the immune response, along with effects on hematopoiesis, resulting in anemia and leukopenia, have been observed. Alteration of lipid metabolism and few gastrointestinal perforations have also been reported.
Despite the fact that red blood cell reduction is a desired effect for ruxolitinib in the context of myeloproliferative diseases, JAK2 inhibition results in mild anemia, thrombocytopenia and neutropenia. The side effects of tofacitinib are similar to what have been observed in patients treated with just adalimumab or other anti-TNF biologics. The most common adverse effect is the reduction of neutrophil numbers possibly resulting in upper respiratory infections, nasopharyngeal inflammation, and anemia. Opportunistic infections such as herpes zoster, esophageal candidiasis, pneumocystis, cytomegalovirus infections and tuberculosis have also been reported. Notably, reactivation of varicella zoster virus is substantially higher in tofacitinib-treated patients [41]. Moreover, tofacitinib inhibition of the JAK3-dependent cytokine IL-15, which is critical for NK cell development, could be responsible for the increase in viral infections. Although NK cells are also important for anti-tumor responses, interestingly, the risk of developing malignancies in tofacitinib-treated patients does not seem to be increased [42]. Anti-IL-6 antibody used in the treatment of rheumatic diseases results in increased cholesterol levels and this side effect has been observed for tofacitinib which also reduces cholesterol ester catabolism, increases serum LDL and HDL concentrations and normalizes the low levels of circulating cholesterol typical of RA patients [43]. In addition, recent preclinical data indicate that tofacitinib improves vascular dysfunction in a mouse model of SLE [36]. The safety profile of baricitinib is similar to what has already been mentioned for tofacitnib and ruxolitinib with mild anemia and neutropenia being observed. Elevations in hepatic transaminases and serum creatinine are also common in patients treated with JAK inhibitors. Interestingly, despite having been developed to achieve a better safety profile, JAK-selective inhibitors currently investigated display side effects somewhat overlapping with first-generation compounds. Filgotinib-treated patients develop neutropenia, show increased hemoglobin levels as well as serum lipoproteins. Upadacitnib administration results in an increase in serum lipids as well as hepatic transaminases [44] and RA patients treated with decernotinib unexpectedly presented reduced neutrophil counts suggesting an effect beyond JAK3 inhibition [45].
Notably, type I and type II cytokines are also capable of activating other signaling pathways including the mitogen activated protein kinase (MAPK) and the Phosphatidylinositol-3’-kinase (PI3K) pathways, which will be discuss in greater details below.
5. TNF and its family members
The TNF, IL-1 and IL-17 receptor families are very distinct transmembrane receptors responsible, upon ligand binding, for a wide array of cellular functions. Nonetheless, their signaling cascades partially overlap and utilize some of the same mediators leading to the activation of some common TFs. Although these receptor families share signaling pathways, regulatory mechanisms allow each ligand to perform distinct and specialized functions. For example, expression of both ligands and receptors is tightly regulated in cells of the immune systems as well as other cell types.
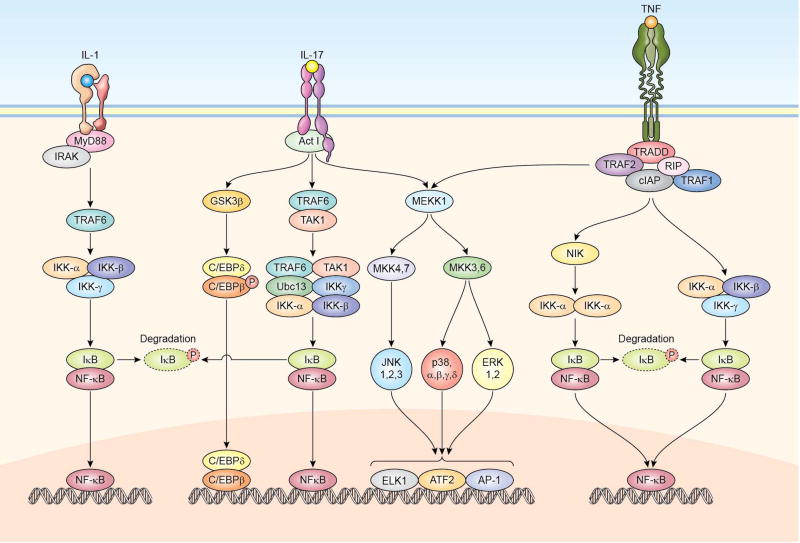
TNF receptors (TNFRs) are type-I transmembrane glycoproteins which, although share an overall low sequence similarity, once engaged by their ligands, can drive common signaling pathways [46, 47] (Fig. 2). Lacking an intrinsic kinase activity, the majority of TNFRs signal through the recruitment, on their cytoplasmic tails, of multifunctional adapter proteins: TNFR-associated factors 1 to 6 (TRAF1 to 6), TNFR-associated death domain protein (TRADD) and Fas-associated death domain protein (FADD) [48–50] (Fig. 2). In general, TNFRs that signal through TRAFs can be regarded as pro-inflammatory but, according to the responding cell type, they can also induce proliferation, cell survival (anti-apoptotic stimuli), differentiation as well as synthesis and release of inflammatory cytokines and chemokines. TRAF proteins can activate one or both the NF-kB and the MAPKs cascades. Engagement of these signaling pathways by TNF family members leads to the activation of specific genes, ultimately resulting in activities which range from amplification of innate inflammatory responses and septic shock to regulation of lymphocyte costimulation as well as control of lymphoid structure development. Accordingly, alterations in the receptor or the signaling pathways lead to aberrant immune responses. Mutations affecting the TNFR1 protein are associated with periodic fever syndromes [51]. Heterozygous dominant negative mutations in the FAS gene result in defective apoptosis and development of the autoimmune lymphoproliferative syndrome [52]. Moreover, mutations in the gene encoding for CD40 ligand result in X-linked hyper-IgM (X-HIM) syndrome, characterized by frequent opportunistic infections, usually bacterial, and increased susceptibility to cancer [53].
Figure 2.
Schematic representation of signaling pathways that are activated by IL-1, IL-17, and TNF. The major pathways activated by these cytokines are the NF-kB, MAPK and the GSK3β–C/EBPδ/β pathways, ultimately leading to the regulation of gene expression.
6. The IL-1 family
The IL-1 receptor (IL-1R) family is composed of 11 members which includes the IL-1RI and II, the IL-1R–accessory protein (IL-1RAcP), which pairs with the IL-1R, IL-18 and IL-33 receptors, IL-33R (T1/ST2) and a few others. The majority of the IL-1 family ligands, also constituted of 11 members, have prodomains which are proteolytically removed in order for the cytokine to be biologically active. The cleavage occurs within a multi-protein complex known as the inflammasome and is followed by secretion of the active cytokine. The cytoplasmic domains contain a conserved fold known as the Toll, IL-1R, plant Resistance genes (TIR) domain, which is also present in Toll-like receptors (TLR) [54, 55]. In the case of IL-1, ligand binding results in a signaling cascade initiated by the recruitment of the adaptor protein MyD88 to the IL-1R–IL-1RAcP complex via TIR-TIR domain interactions. MyD88, in turn, recruits the kinase IRAK-4, which leads to the formation of protein complexes that include molecules such as IRAK-1 or IRAK-2, TRAF6, TAK1, TAB1 and TAB2. This results in activation of the NF-kB, MAPK and PI3K pathways (Fig. 2). Members of the IL-1 family have diverse functions within and outside the context of the immune system. These functions include the induction of acute-phase protein synthesis, cachexia and fever which are tempered by the actions of the natural cytokine antagonist, IL-1 receptor antagonist IL-1Ra, encoded by the IL1RN gene. Mutations in IL1RN cause a systemic autionflammatory disease denoted Deficiency of IL-1Ra or DIRA [56]. Moreover, mutations in the inflammasome key component NLR Family Pyrin Domain Containing 3 (NLRP3), also called cryopyrin, result in hereditary periodic fever syndromes, such as familial cold autoinflammatory syndrome, Muckle-Wells syndrome, and neonatal onset multi-organ inflammatory disease [57]. Other NLR proteins, including NLRP1, NLR family CARD domain-containing protein 4 (NLRC4), ICE-Protease Activating Factor (IPAF), NLR Family Apoptosis Inhibitory Protein (NAIP), and absent in melanoma 2 (AIM2), are involved in the formation of other types of inflammasomes. Mutations in NLRP1 are associated with vitiligo [58] and several SNPs in this gene have been associated with the development of SLE. Mutations in NLRC4 instead, result in excessive IL-18 production and macrophage activation syndrome [59]. In addition, several SNPs in the IL-38 locus (another family member), have been associated with RA and spondyloarthropaties. Furthermore, mutations in the key adaptor MyD88 which connects the IL-1R, IL-18R and IL-33R to the IRAK complex result in defective NF-kB activation and decreased production of pro-inflammatory cytokines. These patients are susceptible to recurrent invasive pyogenic bacterial infections and often develop meningitis and sepsis [60]. A similar, but not completely overlapping, clinical disorder was observed in patients carrying hypomorphic mutations in the X-linked IKBKG gene (which codifies for the IKKγ protein) and hypermorphic mutations in the IKBA gene (which codifies for the IkB kinase) cause X-linked recessive and autosomal dominant anhidrotic ectodermal dysplasia with immunodeficiency (EDA-ID) syndromes. These patients have impaired antibody responses and are susceptible not only to invasive pyogenic bacteria but also mycobacteria, viruses, fungi, and parasites [61].
7. The IL-17 family
The cytoplasmic tails of both IL-17R subunits bind the adaptor and E3 ubiquitin ligase molecule Act-1 which mediates the K63-linked ubiquitylation of TRAF6, resulting in downstream activation of TAK1, formation of the IKK complex and activation of the canonical NF-kB pathway. IL-17 also activates the MAPK pathway and, through GSK3β, leads to activation of the TFs complex constituted by C/EBPβ and C/EBPδ dimers (Fig. 2). IL-17A has been shown to mediate inflammatory responses both in animal models and in humans. Deficiency in the IL-17 pathway or high titers of neutralizing autoantibodies against IL-17A and IL-17F results in chronic mucocutaneous candidiasis [62]. Moreover, IL-17A and IL-17F are important regulators of stress granulopoiesis and recruitment of myeloid cells to sites of inflammation and therefore promote and maintain inflammation by inducing the production of chemokines, G-CSF and GM-CSF. IL-17E (IL-25) is essential for elimination of helminthic parasites and is considered a Th2 cytokine. It regulates the inflammatory response characterized by overproduction of other Th2 cytokines such as IL-4 and IL-5, mucus production, and eosinophilia [63].
8. Targeting the NF-kB pathway
NF-kB represents a family of five ubiquitously expressed and closely related TFs comprising NF-kB1 (p50/p105), NF-κB2 (p52/p100), RelA (p65), c-Rel and RelB that share a Rel homology domain in their N-terminus with broad roles in gene induction and regulation of crucial biological functions such as survival, proliferation, differentiation and inflammation. Besides cytokines, NF-κB factors are activated by growth factors and receptor tyrosine kinases and therefore, are considered one of the major regulators of the immune response. In resting cells, NF-kB is inactive and retained in the cytoplasm due to its interaction with the inhibitors of NF-kB, IkB (which is comprised of three proteins, IkBα, IkBβ and IkBε). Cytokine receptor engagement leads to activation and recruitment of TRAF1 and 2, cellular inhibitor of apoptosis (cIAP) and activation of the NF-kB inducing kinase (NIK) and, subsequently, of the serine/threonine kinase IKKα (inhibitor of nuclear factor kappa-B kinase) (Fig. 2). Through phosphorylation, IKKα either inhibits IkB leading to its dissociation from NF-kB (canonical pathway) or directly activates NIK (non canonical pathway). In both cases, the result is the downstream activation of NF-kB, its nuclear translocation, and consequent binding as homo- or heterodimers to specific DNA sequences.
Notably, a significant number of genetic variants within the NF-kB signaling cascade genes have been identified in patients with autoimmune diseases in genome-wide association studies (GWAS) [64]. For example, variants have been demonstrated to be strongly associated with high risk of multiple sclerosis (MS) through an aberrant enhancement of NF-kB activation and consequently, increased responsiveness to inflammatory stimuli [65]. NF-kB-mediated dysregulation has been shown to be crucial for the development of numerous pathological conditions beyond inflammation and/or autoimmunity: from AIDS and atherosclerosis to cancer and muscular dystrophy. Therefore, the signaling cascade involving NF-kB has been considered a primary target for pharmacological intervention [66–68] and a wide range of drugs targeting crucial steps of this pathway, such as kinases activation, phosphatase enzymatic activity, ubiquitination, nuclear translocation, DNA binding and post translational modifications, have been tested [67, 69].
Considering that IKK activation and consequent IκB phosphorylation and proteasome-mediated degradation represent the first common step of many NF-kB-activating pathways, the most prominent strategy, which has already been shown to be efficacious in a variety of cancer treatments, is to block proteasome degradation [70, 71]. Bortezomib (trade name Velcade®) has been the first proteasome inhibitor approved for the clinical treatment of multiple myeloma [67, 69]. Moreover, preclinical studies in murine models of lupus nephritis, colitis, MS and myasthenia gravis have shown bortezomib efficacy [72–76]. It also reduces the inflammation severity in a rat model of RA [77]. Notably, a clinical trial is currently recruiting SLE patients (NCT02102594). Similarly, ixazomib (trade name Ninlaro®), which selectively and reversibly inhibits the proteasome subunit beta type-5 and it is used to treat multiple myeloma, is currently in phase I for the treatment of lupus nephritis (NCT02176486) [78].
In addition to these strategies, the usage of selective inhibitors of IkB nuclear export (SINE) like KPT-350, has been showing promising results in preclinical models of SLE and RA as well as amyotrophic lateral sclerosis (ALS), traumatic brain injury, MS and Duchenne muscular dystrophy [79]. Alternative approaches using ATP analog competitors and compounds that activate specific phosphatases or drugs with allosteric effects on IKK structure or inhibitors have also been tested in preclinical models [80]. Unfortunately, these studies have shown considerable adverse “off-target” effects.
An interesting approach, although still distant from being utilized in the clinic, is to selectively block the NF-kB binding to DNA either using decoy oligonucleotides or molecules that create a steric impediment for the DNA binding [81, 82]. Indeed, the DNA binding inhibitor Dehydroxymethylepoxyquinomicin (DHMEQ) has been tested with some success in models of multiple autoimmune diseases, including RA, SLE and colitis [83–85]. Direct inhibition of NF-kB-DNA binding has also been explored with the usage of synthetic double-stranded DNA oligodeoxynucleotides, “decoy” molecules that covalently interact with NF-kB dimers [86, 87]. It has to be noted that the short half-life of these molecules, which require frequent administration, may diminish their therapeutic potential. Finally, NF-kB subunits undergo extensive posttranslational modifications, including phosphorylation, acetylation, nitrosylation, glycosylation, ubiquitination, and sumoylation, that modulate both the strength and duration of the NF-kB activity [88] and, therefore, some attempts to modulate these posttranslational modifications have been made [89].
9. Targeting the MAPK pathway
MAPKs are a group of highly conserved cytoplasmic serine/threonine enzymes involved in the control of a wide range of fundamental biological processes and represent a converging point for many upstream signaling pathways initiated, not only by cytokines, but also by receptor tyrosine kinases, immunoreceptors as well as G protein-coupled receptors [90–92]. Inappropriate activation of MAPKs can be linked to the development of several pathologies including SLE, RA, and IBD as well as cancer [93].
Three distinct MAPK families have been described, each with a specific role: the c-Jun N-terminal kinases 1–3 (JNK1–3), the extracellular signal-regulated kinases 1 and 2 (ERK1 and ERK2), and p38 MAPK isoforms (p38α, β, γ, and δ) (Fig. 2). Lesser-studied atypical MAPKs include ERK3/ERK4, ERK7 and Nemo-like kinase [94]. ERK1 and ERK2 control cell proliferation and differentiation [95] whereas JNK1, JNK2 and JNK3 also mediate apoptosis, cytokine production and the production of metalloproteinases involved in the regulation of the extracellular matrix [96]. Drugs like cobimetnib and trametinib inhibit MEK1 and MEK2, upstream of ERKs and have already reached the market for the treatment of melanoma while several others inhibitors, including molecules acting via an allosteric mechanism, are in late stage of development. Brimaptide (also known as XG-102) is a non-competitive JNK inhibitor for which positive phase II results have been observed in inflammatory eye disease (NCT02508337). Furthermore, a phase II is about to be started in patients with idiopathic pulmonary fibrosis with CC-90001, a JNK1-selective inhibitor (NCT02110420). Crucial in the immune response, the p38 family (α, γ and δ) are instead potent regulators of pro-inflammatory cytokines expression including of TNF-α, IL-1, and IL-6 [95]. In the late 1990’s considerable efforts were made to develop p38 inhibitors. Some have progressed to late stage clinical development, mostly for the treatment of RA, but despite their therapeutic appeal and promising preclinical early results [97–99], all clinical trials generally failed due to either poor efficacy or major side effects such as hepatotoxicity and other liver function abnormalities [95, 100–102]. It also appears that the inhibition of MAPKs triggers compensatory mechanisms, involving other MAPK members leading to alternative activation of inflammatory pathways [103]. However, development of better p38 inhibitors has continued and one of the newest molecules, acumapimod, is currently in phase II for the treatment of chronic obstructive pulmonary disease (NCT02700919) [104].
10. Targeting the PI3K pathway
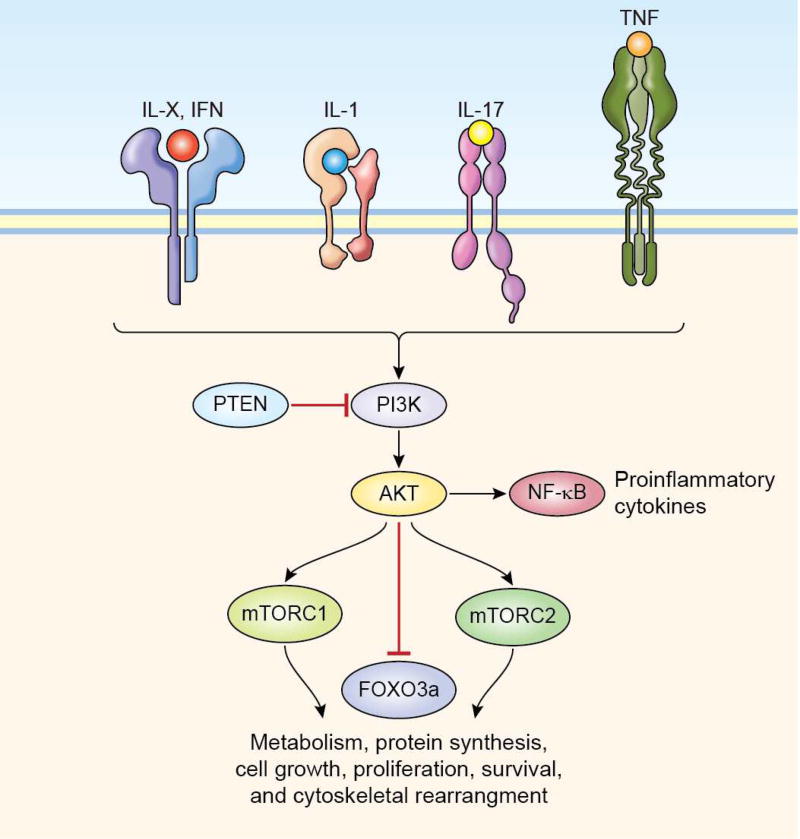
PI3K are lipid kinases divided into four different classes, grouped based on structure and substrate similarities. Inactive PI3Ks are rapidly activated in the presence of extracellular stimuli such as cytokines, growth factors, insulin, and G-protein coupled receptors. Class I is further subdivided in IA and IB. Class IA PI3K is composed of a heterodimer between a p110 catalytic subunit and a p85 regulatory subunit. There are three types of p110 catalytic subunit: p110α , p110β and p110δ and five different regulatory subunits, namely p85α, p55α and p50α which are splice variants of the same gene and p85β and p55γ which are encoded by different genes. Whereas most of these subunits are broadly expressed, the catalytic p110δ and the class IB p110γ are preferentially expressed in the immune system. Class I PI3Ks phosphorylate phosphatidylinositol-(4,5)-phosphate (PIP2) to produce the second lipid messenger phosphatidylinositol-(3,4,5)-phosphate (PIP3). Rising PIP3 levels result in recruitment to the plasma member of Protein kinase B (also known as AKT) a serine/threonine phosphotransferase. This results in activation of AKT, which triggers most of the downstream biological activities (Fig. 3). Moreover, the PI3K–AKT cascade orchestrates both pro-inflammatory (promoting cytokine production through AKT-mediated IkB phosphorylation and consequent NF-kB activation), and anti-inflammatory signaling to maintain effective immunity while protecting host tissues [105]. Given that these enzymes are critical for cell differentiation, growth, proliferation and survival, their activity or expression is frequently altered in human cancers [106, 107]. Indeed, mutations in tyrosine kinases receptors upstream of PI3K, the PI3Ks, AKT and the negative regulator tumor suppressor phosphatase and tensin homolog (PTEN) are associated with tumor development and may be responsible for drug sensitivity or resistance to specific therapeutic agents [108–110]. For this reason, crucial players in this pathway have been studied as potential targets. PI3K inhibitors include isoform-specific and pan-class I PI3K inhibitors or drug acting on downstream molecules such as AKT as well as antagonist of PI3K/AKT signaling like PTEN.
Figure 3.
PI3K is rapidly activated upon the engagement of different cytokine receptors. AKT is a PI3K substrate which, in turn, activates the mTOR complexes thereby regulating numerous cellular functions such as metabolism, protein synthesis, cell growth, proliferation, and survival as well as cytoskeletal rearrangement.
To date, there are about 30 or more different PI3K inhibitors currently investigated in more than 300 clinical trials around the world, mostly focused on the treatment of malignancies. The most advanced are copanlisib, currently in pre-registration stage for the treatment of lymphomas, buparlisib and taselisib, two compounds with potential antineoplastic activity in phase II and III clinical trials for various malignancies [106, 111]. Pan-PI3K inhibitors display serious adverse effects whereas; isoform-specific PI3K inhibitors seem to have a more favorable safety profile [112]. One example is represented by the PI3Kα-selective inhibitor alpelisib currently in clinical development (phase II) for breast cancer (NCT03056755).
Considering that PI3K-δ and PI3K-γ are the isoforms preferentially expressed in immune cells, inhibitors interfering with their enzymatic activity are being considered, not only for malignancies but also for inflammatory and autoimmune diseases. Duvelisib is a PI3K-δ and PI3K-γ inhibitor currently in phase III for B-cell lymphoma and chronic lymphocytic leukemia (NCT02576275) and for which a phase IIb trial for asthma was recently completed (NCT01653756). This compound has also been shown to be efficacious in murine models of arthritis, SLE and asthma [113]. It ameliorates glomerulonephritis, proteinuria, and reduces anti-dsDNA serum titers in the NZBWF1/J SLE mouse model [113]. Its clinical development for these pathologies, though, appears to have been stopped. Notably, the PI3K-δ-specific compounds CDZ-173 and UCB5857 are currently in phase II for Sjögren’s syndrome, which is considered an “unmet need’ disease by the FDA (NCT02775916; NCT02610543).
AKT inhibition has also been considered for solid cancers. Ipataserib is an ATP competitor, which is currently being investigated for prostate (phase Ib; NCT01485861), gastrointestinal and oesophageal (phase II; NCT01896531), and breast cancers (phase II; NCT02301988). Afuresertib and AZD-5363 are other ATP competitors currently in phase I and II stage for ovarian and breast cancer (NCT02208375) as well as myeloma (NCT02177682). Interestingly, the allosteric inhibitor MK-2206 has already reached phase II of clinical development for solid cancers (NCT01283035). A novel approach to inhibit AKT activity has been exploited with RX-0201, which is an anti-sense oligonucleotide that binds to AKT mRNA inhibiting its translation. Formulated for a nano-liposomal delivery, this drug is in phase II of clinical development for pancreatic and renal cancer (NCT01028495; NCT02089334).
Downstream of PI3K and AKT, the mammalian target of rapamycin (mTOR) is a serine/threonine kinase member of the phosphatidylinositol-3 kinase (PI3K)-related kinases (PIKKs) family. mTOR is the catalytic subunit shared by the two complexes mTORC1 and mTORC2 which regulate metabolism, protein synthesis, survival, as well as the cytoskeletal rearrangements needed for antigen presentation [114] (Fig. 3). Since they are directly involved in metabolism and cell growth control, the mTOR complexes have been associated with nearly all human cancers as well as autoimmune disorders such as SLE [114, 115]. Rapamycin was, of course, the first drug discovered to block mTOR activity and is currently approved for the treatment of breast, renal, lung and several neoplastic diseases. It is also clinically utilized for restenosis and specific formulations of the drug are in advanced stages of clinical development for uveitis [116]. Newer inhibitors, such as sapanisertib and voxtalisib, which inhibit mTOR but also the p110γ isoform of PI3K and the Mitogen-activated protein kinase kinase MEK, are in phase II for the treatment of solid tumors (NCT01390818). The only mTOR inhibitors currently under development, albeit still at a preclinical level, for autoimmune diseases, are TAM-01 and CZ-415, which are under consideration for SLE and RA, respectively.
As mentioned above, IRAKs are crucial mediators of IL-1 but also for IL-18R and TLRs signaling. Given the role that all these molecules have in the immune and inflammatory response they are attractive targets for the development of immunomodulatory drugs [117]. The IRAK family comprises four members, IRAK-1, IRAK-2, IRAK-3 (also known as IRAK-M) and IRAK-4, and has a role in both positive and negative regulation of signal transduction. Recently, it has been shown that IRAK-1 may act as an oncogene in the development of hepatocellular carcinoma [118]. IRAK-4 appears to be indispensable for IL-1R (and TLR) signaling. Indeed, similar to what has been described for MYD88, autosomal recessive mutations in the IRAK4 gene lead to susceptibility to pyogenic bacterial infections often caused by Streptococcus pneumoniae and Pseudomonas aeruginosa [119, 120]. Moreover, these patients often present with arthritis and osteomyelitis. The IRAK-4 selective inhibitor PF-06650833 is currently being assessed in a phase II clinical trial in RA patients (NCT02996500) as well as in preclinical models of SLE and IBD. Preclinical studies are also ongoing with BMS-986126, ND-346 and CA-4948 in models of SLE, lymphomas and other malignancies [121, 122].
11. Targeting transcription factors: The new frontier?
Blocking the activity of TFs downstream of cytokine receptors could be a very effective mechanism to interfere with dysregulated cytokine signaling. On the other hand, TFs do not have an enzymatic activity like kinases and exert their functions within the nucleus with the result of being a less amenable substrate. Nonetheless, given the importance of some of these molecules in the regulation of inflammatory pathologies, they are still attractive targets.
Downstream of JAKs for example, the block of STAT activation could be achieved in several manners. Since STATs directly bind to the intracellular portion of cytokine receptors, interfering with the STAT-receptor interaction could potentially be exploited. Similarly, the action of these TFs could be hindered by preventing STAT dimerization or STAT-DNA interaction. These strategies have been considered, especially in cancer, in which constitutive activation of STAT3 and STAT5 have frequently been observed. Interestingly, three well-known drugs, the antipsychotic pimozide, the lipoprotein-lowering drug pravastatin and the immunomodulatory leflunomide have been shown to inhibit the phosphorylation of STAT5, STAT1 and STAT6 respectively [123–125]. The exact mechanism by which a dopamine D2 receptor, a hydroxymethylglutaryl-CoA reductase and a dihydroorotate dehydrogenase inhibitor block STAT phosphorylation, however, is still unclear. Cytokines activating STAT3, such as IL-6, have also been shown to regulate the development of Th cells, which secrete IL-17, known as Th17, which have been associated with chronic inflammation. On the other hand, STAT3 and STAT1 are very homologous molecules and, given that STAT1 is utilized by cytokines with critical roles against pathogens and tumor growth, the blocking of STAT activity presents several challenges. Nonetheless, a few small molecules targeting STAT3 have been tested in a clinical setting. The development of napabucasin has reached phase III for oseophageal and gastrointestinal cancers (NCT02178956) and phase II for several other solid tumors (NCT02467361). STA-21 is a natural antibiotic that inhibits STAT3 dimerization and has been tested in phase I and II clinical trials for the treatment of psoriasis (NCT01047943) [126]. STAT3 decoy oligonucleotides bind their DNA-binding domain with a higher degree of selectivity, but they are quickly degraded in vivo. AZD9150 is a next-generation antisense oligonucleotide inhibitor of STAT3 that has demonstrated clinical efficacy (currently in phase I and II) in solid tumors, such as liver cancer, as well as B cell lymphoma (NCT02499328; NCT01839604; NCT02549651). GLG-801 is a re-purposed drug which inhibits DNA binding of phosphorylated STAT3 and has reached phase II as well. Several other compounds targeting STAT3 are still at the preclinical stage. Th2 cytokines like IL-4 and IL-13, with a critical role in allergy and atopy, signal via STAT6 the blocking of which is therefore an attractive goal for asthma and other allergic diseases. To this end, phosphopeptides that can block the STAT6 SH2 domain-cytokine receptor interaction have been developed [127].
Because of its key role in the differentiation and development of Th17 as well as γδTcells, the retinoic-acid-orphan-receptor (ROR) C (also known as RORγ) is being actively investigated. ROR is a nuclear hormone receptor of which two isoforms, RORγ1 and RORγ2 (also known as RORγt), exist. Current low-molecular-weight compunds, which target the identical ligand binding domain shared by both RORC isoforms, have been reported to block Th17 differentiation in vitro as well as in vivo in various animal models of autoimmune diseases [128–130]. Following these promising preclinical results, several clinical trials with oral and topical RORγ inhibitors such as: VTP-43742, GSK2981278, ABBV-553, ARN-6039 and AZD-0284 have been started in psoriasis, MS, RA and uveitis patients (NCT02555709; NCT03004846; NCT03145948; NCT02976831). While several other inhibitors are still at the preclinical stage, an antagonist that inhibits RORγt with an allosteric mechanism, which could lead to increased selectivity, has also been reported to have promising in vitro activity [131].
As a note of caution, mice in which Rorc deficiency was induced when the animals had already reached the adult stage, developed thymic lymphoblastic lymphoma similarly to what had been observed for constitutive Rorc−/− mice [132]. Moreover, patients with bi-allelic RORC mutations presented immunological alterations similar to the nullizygous mice, including loss of axillary and cervical lymph nodes, lack of iNKT and LTi cells, reduced thymus size and absence of IL-17A/F-producing T cells, ultimately resulting in impaired immune response to Mycobacterium and Candida [133]. To date, it is not yet known if patients enrolled in the clinical trials mentioned above will develop any thymic aberration but it is possible that long-term therapy with these drugs may have unwanted consequences.
12. Conclusions
In this review article, we have summarized the current knowledge of the events that occur following the binding of four, very broad, groups of cytokines to their specific receptors. Furthermore we have highlighted the recent advances in the development of molecules which interfere with the signaling cascades set into motion by these cytokines. The targeting of cytokines, with the drugs commonly referred as biologics, led to tremendous benefits for patients suffering from various inflammatory and rheumatologic diseases. With a better understanding of the intracellular signals induced by these soluble factors and their alteration in diseases, we can now exploit a larger number of targets and, at the same time, achieve better specificity while often reducing undesired effects.
Our review pays tribute to a “Maestro” of inflammation research, Professor Alberto Mantovani. Alberto’s breakthroughs range from the discovery of a chemokine such as CCL2, to the recognition that some cytokine receptors possess decoy functions and help in “fine tuning” cytokine activity. We could write several more pages listing all the groundbreaking contributions that Alberto has made to our understanding of the immune response, inflammation and tumor biology. He also has contributed tremendously to the dissemination of science to the general public.
I (Massimo Gadina) had the privilege, as a young pre-doctoral student, to spend one year in the Laboratory of Immunology at the Istituto di Ricerche Farmacologiche "Mario Negri" in Milan under the leadership of Dr. Mantovani. Having previously worked on monoclonal antibodies as tools for the treatment of cancer, it was in Alberto’s group that I discovered the world of cytokines which has then kept me busy for the last 28 years. It is, therefore, a great honor for my collaborators and I, to contribute to this special issue of the Journal of Autoimmunity that recognizes Professor Manovani’s career, his work and his role in nurturing and mentoring a large number of successful scientists.
Highlights.
Cytokines regulates the immune response but are also implicated in the development of several immune mediated diseases.
Stopping cytokines action by blocking cytokines themselves or their receptors has been successfully employed to treat patients.
Targeting the signal transduction cascades downstream of cytokine receptors has now been employed to generate new and effective drugs.
Acknowledgments
We would like to thank Dr. Giuseppe Sciumè, Dr. Roberta Visconti, Ms. Mimi Le and Miss Anna Trier for the critical revision of the manuscript
Abbreviations
- JAK
Janus Kinase
- STAT
Signal transducer and activator of transcription
- NF-kB
nuclear factor kappa-light-chain-enhancer of activated B cells
- MAPK
Mitogen activated protein kinase
- PI3K
Phosphatidylinositol-3’-kinases
- TNF
tumor necrosis factor
- IL
Interleukin
- CSFs
Colony stimulating factors
- IFNs
Interferons
- DMARDs
disease modifying anti rheumatic drugs
- TFs
Transcription factors
- X-SCID
X-linked severe combined immunodeficiency
- PV
polycythemia vera
- HIES
hyper-IgE syndrome
- RA
rheumatoid arthritis
- AA
alopecia areata
- AU
alopecia universalis
- MTX
methotrexate
- IBD
inflammatory bowel disease
- PsA
psoriatic arthritis
- AD
atopic dermatitis
- GVHD
graft versus host disease
- SLE
systemic lupus erythematosus
- CANDLE
Chronic Atypical Neutrophilic Dermatosis with Lipodystrophy and Elevated temperature
- STING
Stimulator of Interferon Genes
- SAVI
STING-associated Vasculopathy with Onset in Infancy
- UC
ulcerative colitis
- AS
ankylosing spondylitis
- TNFRs
TNF receptors
- TRADD
TNFR-associated death domain protein
- FADD
Fas-associated death domain protein
- X-HIM
X-Linked hyper-IgM
- EDA-ID
ectodermal dysplasia with immunodeficiency
- TLR
Toll-like receptors
- NLRP3
NLR family pyrin domain containing 3
- NLRC4
NLR family CARD domain-containing protein
- IPAF
ICE-protease activating factor
- NAIP
NLR family apoptosis inhibitory protein
- AIM2
melanoma 2
- cIAP
cellular inhibitor of apoptosis
- NIK
NF-kB-inducing kinase
- IKKγ
inhibitor of nuclear factor kappa-B kinase
- GWAS
genome-wide association studies
- MS
Multiple Sclerosis
- SINE
selective inhibitors of nuclear export
- PIP2
phosphatidylinositol-(4,5)-phosphate
- PIP3
phosphatidylinositol-(3,4,5)-phosphate
- PTEN
phosphatase and tensin homolog
- mTOR
mammalian target of rapamycin
- ROR
retinoic-acid-orphan-receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.O’Shea JJ, Holland SM, Staudt LM. JAKs and STATs in immunity, immunodeficiency, and cancer. N Engl J Med. 2013;368:161–70. doi: 10.1056/NEJMra1202117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalliolias GD, Ivashkiv LB. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat Rev Rheumatol. 2016;12:49–62. doi: 10.1038/nrrheum.2015.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Croft M, Siegel RM. Beyond TNF: TNF superfamily cytokines as targets for the treatment of rheumatic diseases. Nat Rev Rheumatol. 2017;13:217–33. doi: 10.1038/nrrheum.2017.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curtis MM, Way SS. Interleukin-17 in host defence against bacterial, mycobacterial and fungal pathogens. Immunology. 2009;126:177–85. doi: 10.1111/j.1365-2567.2008.03017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Shea JJ, Murray PJ. Cytokine signaling modules in inflammatory responses. Immunity. 2008;28:477–87. doi: 10.1016/j.immuni.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamaoka K, Saharinen P, Pesu M, Holt VE, 3rd, Silvennoinen O, O’Shea JJ. The Janus kinases (Jaks) Genome Biol. 2004;5:253. doi: 10.1186/gb-2004-5-12-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leonard WJ, O’Shea JJ. Jaks and STATs: biological implications. Annu Rev Immunol. 1998;16:293–322. doi: 10.1146/annurev.immunol.16.1.293. [DOI] [PubMed] [Google Scholar]
- 8.Lacronique V, Boureux A, Valle VD, Poirel H, Quang CT, Mauchauffe M, et al. A TEL-JAK2 fusion protein with constitutive kinase activity in human leukemia. Science. 1997;278:1309–12. doi: 10.1126/science.278.5341.1309. [DOI] [PubMed] [Google Scholar]
- 9.Rodig SJ, Meraz MA, White JM, Lampe PA, Riley JK, Arthur CD, et al. Disruption of the Jak1 gene demonstrates obligatory and nonredundant roles of the Jaks in cytokine-induced biologic responses. Cell. 1998;93:373–83. doi: 10.1016/s0092-8674(00)81166-6. [DOI] [PubMed] [Google Scholar]
- 10.Van Roosbroeck K, Cox L, Tousseyn T, Lahortiga I, Gielen O, Cauwelier B, et al. JAK2 rearrangements, including the novel SEC31A–JAK2 fusion, are recurrent in classical Hodgkin lymphoma. Blood. 2011;117:4056–64. doi: 10.1182/blood-2010-06-291310. [DOI] [PubMed] [Google Scholar]
- 11.Kiladjian JJ. The spectrum of JAK2-positive myeloproliferative neoplasms. Hematology Am Soc Hematol Educ Program. 2012;2012:561–6. doi: 10.1182/asheducation-2012.1.561. [DOI] [PubMed] [Google Scholar]
- 12.Pinilla-Ibarz J, Sweet KL, Corrales-Yepez GM, Komrokji RS. Role of tyrosine-kinase inhibitors in myeloproliferative neoplasms: comparative lessons learned. Onco Targets Ther. 2016;9:4937–57. doi: 10.2147/OTT.S102504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kovanen PE, Leonard WJ. Cytokines and immunodeficiency diseases: critical roles of the gamma(c)-dependent cytokines interleukins 2, 4, 7, 9, 15, and 21, and their signaling pathways. Immunol Rev. 2004;202:67–83. doi: 10.1111/j.0105-2896.2004.00203.x. [DOI] [PubMed] [Google Scholar]
- 14.Frucht DM, Gadina M, Jagadeesh GJ, Aksentijevich I, Takada K, Bleesing JJ, et al. Unexpected and variable phenotypes in a family with JAK3 deficiency. Genes Immun. 2001;2:422–32. doi: 10.1038/sj.gene.6363802. [DOI] [PubMed] [Google Scholar]
- 15.Brugnoni D, Notarangelo LD, Sottini A, Airo P, Pennacchio M, Mazzolari E, et al. Development of autologous, oligoclonal, poorly functioning T lymphocytes in a patient with autosomal recessive severe combined immunodeficiency caused by defects of the Jak3 tyrosine kinase. Blood. 1998;91:949–55. [PubMed] [Google Scholar]
- 16.Minegishi Y, Saito M, Morio T, Watanabe K, Agematsu K, Tsuchiya S, et al. Human tyrosine kinase 2 deficiency reveals its requisite roles in multiple cytokine signals involved in innate and acquired immunity. Immunity. 2006;25:745–55. doi: 10.1016/j.immuni.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Kilic SS, Hacimustafaoglu M, Boisson-Dupuis S, Kreins AY, Grant AV, Abel L, et al. A patient with tyrosine kinase 2 deficiency without hyper-IgE syndrome. J Pediatr. 2012;160:1055–7. doi: 10.1016/j.jpeds.2012.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kreins AY, Ciancanelli MJ, Okada S, Kong XF, Ramirez-Alejo N, Kilic SS, et al. Human TYK2 deficiency: Mycobacterial and viral infections without hyper-IgE syndrome. J Exp Med. 2015;212:1641–62. doi: 10.1084/jem.20140280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Russell SM, Tayebi N, Nakajima H, Riedy MC, Roberts JL, Aman MJ, et al. Mutation of Jak3 in a patient with SCID: essential role of Jak3 in lymphoid development. Science. 1995;270:797–800. doi: 10.1126/science.270.5237.797. [DOI] [PubMed] [Google Scholar]
- 20.Macchi P, Villa A, Giliani S, Sacco MG, Frattini A, Porta F, et al. Mutations of Jak-3 gene in patients with autosomal severe combined immune deficiency (SCID) Nature. 1995;377:65–8. doi: 10.1038/377065a0. [DOI] [PubMed] [Google Scholar]
- 21.Wenzel J, van Holt N, Maier J, Vonnahme M, Bieber T, Wolf D. JAK1/2 Inhibitor Ruxolitinib Controls a Case of Chilblain Lupus Erythematosus. J Invest Dermatol. 2016;136:1281–3. doi: 10.1016/j.jid.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 22.Hornung T, Janzen V, Heidgen FJ, Wolf D, Bieber T, Wenzel J. Remission of recalcitrant dermatomyositis treated with ruxolitinib. N Engl J Med. 2014;371:2537–8. doi: 10.1056/NEJMc1412997. [DOI] [PubMed] [Google Scholar]
- 23.Craiglow BG, Tavares D, King BA. Topical Ruxolitinib for the Treatment of Alopecia Universalis. JAMA Dermatol. 2016;152:490–1. doi: 10.1001/jamadermatol.2015.4445. [DOI] [PubMed] [Google Scholar]
- 24.Xing L, Dai Z, Jabbari A, Cerise JE, Higgins CA, Gong W, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med. 2014;20:1043–9. doi: 10.1038/nm.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weigert O, Lane AA, Bird L, Kopp N, Chapuy B, van Bodegom D, et al. Genetic resistance to JAK2 enzymatic inhibitors is overcome by HSP90 inhibition. J Exp Med. 2012;209:259–73. doi: 10.1084/jem.20111694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koppikar P, Bhagwat N, Kilpivaara O, Manshouri T, Adli M, Hricik T, et al. Heterodimeric JAK-STAT activation as a mechanism of persistence to JAK2 inhibitor therapy. Nature. 2012;489:155–9. doi: 10.1038/nature11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vincenti F, Tedesco Silva H, Busque S, O’Connell P, Friedewald J, Cibrik D, et al. Randomized phase 2b trial of tofacitinib (CP-690,550) in de novo kidney transplant patients: efficacy, renal function and safety at 1 year. Am J Transplant. 2012;12:2446–56. doi: 10.1111/j.1600-6143.2012.04127.x. [DOI] [PubMed] [Google Scholar]
- 28.Vincenti F, Silva HT, Busque S, O’Connell PJ, Russ G, Budde K, et al. Evaluation of the effect of tofacitinib exposure on outcomes in kidney transplant patients. Am J Transplant. 2015;15:1644–53. doi: 10.1111/ajt.13181. [DOI] [PubMed] [Google Scholar]
- 29.Lee EB, Fleischmann R, Hall S, Wilkinson B, Bradley JD, Gruben D, et al. Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med. 2014;370:2377–86. doi: 10.1056/NEJMoa1310476. [DOI] [PubMed] [Google Scholar]
- 30.Singh JA, Hossain A, Tanjong Ghogomu E, Mudano AS, Maxwell LJ, Buchbinder R, et al. Biologics or tofacitinib for people with rheumatoid arthritis unsuccessfully treated with biologics: a systematic review and network meta-analysis. Cochrane Database Syst Rev. 2017;3:CD012591. doi: 10.1002/14651858.CD012591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Heijde D, Tanaka Y, Fleischmann R, Keystone E, Kremer J, Zerbini C, et al. Tofacitinib (CP-690,550) in patients with rheumatoid arthritis receiving methotrexate: twelve-month data from a twenty-four-month phase III randomized radiographic study. Arthritis Rheum. 2013;65:559–70. doi: 10.1002/art.37816. [DOI] [PubMed] [Google Scholar]
- 32.Yuan K, Chen J, Xu A. Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med. 2014;371:1163–4. doi: 10.1056/NEJMc1408607. [DOI] [PubMed] [Google Scholar]
- 33.Burmester GR, Blanco R, Charles-Schoeman C, Wollenhaupt J, Zerbini C, Benda B, et al. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet. 2013;381:451–60. doi: 10.1016/S0140-6736(12)61424-X. [DOI] [PubMed] [Google Scholar]
- 34.van Vollenhoven RF, Fleischmann R, Cohen S, Lee EB, Garcia Meijide JA, Wagner S, et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med. 2012;367:508–19. doi: 10.1056/NEJMoa1112072. [DOI] [PubMed] [Google Scholar]
- 35.Salvarani C, Magnani L, Catanoso M, Pipitone N, Versari A, Dardani L, et al. Tocilizumab: a novel therapy for patients with large-vessel vasculitis. Rheumatology (Oxford) 2012;51:151–6. doi: 10.1093/rheumatology/ker296. [DOI] [PubMed] [Google Scholar]
- 36.Furumoto Y, Smith CK, Blanco L, Zhao W, Brooks SR, Thacker SG, et al. Tofacitinib Ameliorates Murine Lupus and Its Associated Vascular Dysfunction. Arthritis Rheumatol. 2017;69:148–60. doi: 10.1002/art.39818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okiyama N, Furumoto Y, Villarroel VA, Linton JT, Tsai WL, Gutermuth J, et al. Reversal of CD8 T-cell-mediated mucocutaneous graft-versus-host-like disease by the JAK inhibitor tofacitinib. J Invest Dermatol. 2014;134:992–1000. doi: 10.1038/jid.2013.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saridomichelakis MN, Olivry T. An update on the treatment of canine atopic dermatitis. Vet J. 2016;207:29–37. doi: 10.1016/j.tvjl.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 39.Cosgrove SB, Wren JA, Cleaver DM, Walsh KF, Follis SI, King VI, et al. A blinded, randomized, placebo-controlled trial of the efficacy and safety of the Janus kinase inhibitor oclacitinib (Apoquel(R)) in client-owned dogs with atopic dermatitis. Vet Dermatol. 2013;24:587–97. e141–2. doi: 10.1111/vde.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gonzales AJ, Bowman JW, Fici GJ, Zhang M, Mann DW, Mitton-Fry M. Oclacitinib (APOQUEL((R))) is a novel Janus kinase inhibitor with activity against cytokines involved in allergy. J Vet Pharmacol Ther. 2014;37:317–24. doi: 10.1111/jvp.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Winthrop KL, Yamanaka H, Valdez H, Mortensen E, Chew R, Krishnaswami S, et al. Herpes zoster and tofacitinib therapy in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66:2675–84. doi: 10.1002/art.38745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strand V, van Vollenhoven RF, Lee EB, Fleischmann R, Zwillich SH, Gruben D, et al. Tofacitinib or adalimumab versus placebo: patient-reported outcomes from a phase 3 study of active rheumatoid arthritis. Rheumatology (Oxford) 2016;55:1031–41. doi: 10.1093/rheumatology/kev442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Charles-Schoeman C, Fleischmann R, Davignon J, Schwartz H, Turner SM, Beysen C, et al. Potential mechanisms leading to the abnormal lipid profile in patients with rheumatoid arthritis versus healthy volunteers and reversal by tofacitinib. Arthritis Rheumatol. 2015;67:616–25. doi: 10.1002/art.38974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kremer JM, Emery P, Camp HS, Friedman A, Wang L, Othman AA, et al. A Phase IIb Study of ABT-494, a Selective JAK-1 Inhibitor, in Patients With Rheumatoid Arthritis and an Inadequate Response to Anti-Tumor Necrosis Factor Therapy. Arthritis Rheumatol. 2016;68:2867–77. doi: 10.1002/art.39801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gadina M, Schwartz DM, O’Shea JJ. Decernotinib: A Next-Generation Jakinib. Arthritis Rheumatol. 2016;68:31–4. doi: 10.1002/art.39463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 47.Sedger LM, McDermott MF. TNF and TNF-receptors: From mediators of cell death and inflammation to therapeutic giants - past, present and future. Cytokine Growth Factor Rev. 2014;25:453–72. doi: 10.1016/j.cytogfr.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 48.Silke J, Brink R. Regulation of TNFRSF and innate immune signalling complexes by TRAFs and cIAPs. Cell Death Differ. 2010;17:35–45. doi: 10.1038/cdd.2009.114. [DOI] [PubMed] [Google Scholar]
- 49.Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3:745–56. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 50.Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–90. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- 51.Aksentijevich I, Kastner DL. Genetics of monogenic autoinflammatory diseases: past successes, future challenges. Nat Rev Rheumatol. 2011;7:469–78. doi: 10.1038/nrrheum.2011.94. [DOI] [PubMed] [Google Scholar]
- 52.Li P, Huang P, Yang Y, Hao M, Peng H, Li F. Updated Understanding of Autoimmune Lymphoproliferative Syndrome (ALPS) Clin Rev Allergy Immunol. 2016;50:55–63. doi: 10.1007/s12016-015-8466-y. [DOI] [PubMed] [Google Scholar]
- 53.Fuleihan RL. The X-linked hyperimmunoglobulin M syndrome. Semin Hematol. 1998;35:321–31. [PubMed] [Google Scholar]
- 54.Palomo J, Dietrich D, Martin P, Palmer G, Gabay C. The interleukin (IL)-1 cytokine family--Balance between agonists and antagonists in inflammatory diseases. Cytokine. 2015;76:25–37. doi: 10.1016/j.cyto.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 55.Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity. 2013;39:1003–18. doi: 10.1016/j.immuni.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aksentijevich I, Masters SL, Ferguson PJ, Dancey P, Frenkel J, van Royen-Kerkhoff A, et al. An autoinflammatory disease with deficiency of the interleukin-1-receptor antagonist. N Engl J Med. 2009;360:2426–37. doi: 10.1056/NEJMoa0807865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Jesus AA, Canna SW, Liu Y, Goldbach-Mansky R. Molecular mechanisms in genetically defined autoinflammatory diseases: disorders of amplified danger signaling. Annu Rev Immunol. 2015;33:823–74. doi: 10.1146/annurev-immunol-032414-112227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Levandowski CB, Mailloux CM, Ferrara TM, Gowan K, Ben S, Jin Y, et al. NLRP1 haplotypes associated with vitiligo and autoimmunity increase interleukin-1beta processing via the NLRP1 inflammasome. Proc Natl Acad Sci U S A. 2013;110:2952–6. doi: 10.1073/pnas.1222808110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Canna SW, de Jesus AA, Gouni S, Brooks SR, Marrero B, Liu Y, et al. An activating NLRC4 inflammasome mutation causes autoinflammation with recurrent macrophage activation syndrome. Nat Genet. 2014;46:1140–6. doi: 10.1038/ng.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maglione PJ, Simchoni N, Black S, Radigan L, Overbey JR, Bagiella E, et al. IRAK-4 and MyD88 deficiencies impair IgM responses against T-independent bacterial antigens. Blood. 2014;124:3561–71. doi: 10.1182/blood-2014-07-587824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bustamante J, Picard C, Boisson-Dupuis S, Abel L, Casanova JL. Genetic lessons learned from X-linked Mendelian susceptibility to mycobacterial diseases. Ann N Y Acad Sci. 2011;1246:92–101. doi: 10.1111/j.1749-6632.2011.06273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Puel A, Picard C, Cypowyj S, Lilic D, Abel L, Casanova JL. Inborn errors of mucocutaneous immunity to Candida albicans in humans: a role for IL-17 cytokines? Curr Opin Immunol. 2010;22:467–74. doi: 10.1016/j.coi.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–95. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 64.Hussman JP, Beecham AH, Schmidt M, Martin ER, McCauley JL, Vance JM, et al. GWAS analysis implicates NF-kappaB-mediated induction of inflammatory T cells in multiple sclerosis. Genes Immun. 2016;17:305–12. doi: 10.1038/gene.2016.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Housley WJ, Fernandez SD, Vera K, Murikinati SR, Grutzendler J, Cuerdon N, et al. Genetic variants associated with autoimmunity drive NFkappaB signaling and responses to inflammatory stimuli. Sci Transl Med. 2015;7:291ra93. doi: 10.1126/scitranslmed.aaa9223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bacher S, Schmitz ML. The NF-kappaB pathway as a potential target for autoimmune disease therapy. Curr Pharm Des. 2004;10:2827–37. doi: 10.2174/1381612043383584. [DOI] [PubMed] [Google Scholar]
- 67.Herrington FD, Carmody RJ, Goodyear CS. Modulation of NF-kappaB Signaling as a Therapeutic Target in Autoimmunity. J Biomol Screen. 2016;21:223–42. doi: 10.1177/1087057115617456. [DOI] [PubMed] [Google Scholar]
- 68.Zhang Q, Lenardo MJ, Baltimore D. 30 Years of NF-kappaB: A Blossoming of Relevance to Human Pathobiology. Cell. 2017;168:37–57. doi: 10.1016/j.cell.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nakanishi C, Toi M. Nuclear factor-kappaB inhibitors as sensitizers to anticancer drugs. Nat Rev Cancer. 2005;5:297–309. doi: 10.1038/nrc1588. [DOI] [PubMed] [Google Scholar]
- 70.Arepalli SK, Choi M, Jung JK, Lee H. Novel NF-kappaB inhibitors: a patent review (2011 – 2014) Expert Opin Ther Pat. 2015;25:319–34. doi: 10.1517/13543776.2014.998199. [DOI] [PubMed] [Google Scholar]
- 71.Gupta SC, Sundaram C, Reuter S, Aggarwal BB. Inhibiting NF-kappaB activation by small molecules as a therapeutic strategy. Biochim Biophys Acta. 2010;1799:775–87. doi: 10.1016/j.bbagrm.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Neubert K, Meister S, Moser K, Weisel F, Maseda D, Amann K, et al. The proteasome inhibitor bortezomib depletes plasma cells and protects mice with lupus-like disease from nephritis. Nat Med. 2008;14:748–55. doi: 10.1038/nm1763. [DOI] [PubMed] [Google Scholar]
- 73.Gomez AM, Vrolix K, Martinez-Martinez P, Molenaar PC, Phernambucq M, van der Esch E, et al. Proteasome inhibition with bortezomib depletes plasma cells and autoantibodies in experimental autoimmune myasthenia gravis. J Immunol. 2011;186:2503–13. doi: 10.4049/jimmunol.1002539. [DOI] [PubMed] [Google Scholar]
- 74.Fissolo N, Kraus M, Reich M, Ayturan M, Overkleeft H, Driessen C, et al. Dual inhibition of proteasomal and lysosomal proteolysis ameliorates autoimmune central nervous system inflammation. Eur J Immunol. 2008;38:2401–11. doi: 10.1002/eji.200838413. [DOI] [PubMed] [Google Scholar]
- 75.Schmidt N, Gonzalez E, Visekruna A, Kuhl AA, Loddenkemper C, Mollenkopf H, et al. Targeting the proteasome: partial inhibition of the proteasome by bortezomib or deletion of the immunosubunit LMP7 attenuates experimental colitis. Gut. 2010;59:896–906. doi: 10.1136/gut.2009.203554. [DOI] [PubMed] [Google Scholar]
- 76.Basler M, Dajee M, Moll C, Groettrup M, Kirk CJ. Prevention of experimental colitis by a selective inhibitor of the immunoproteasome. J Immunol. 2010;185:634–41. doi: 10.4049/jimmunol.0903182. [DOI] [PubMed] [Google Scholar]
- 77.Palombella VJ, Conner EM, Fuseler JW, Destree A, Davis JM, Laroux FS, et al. Role of the proteasome and NF-kappaB in streptococcal cell wall-induced polyarthritis. Proc Natl Acad Sci U S A. 1998;95:15671–6. doi: 10.1073/pnas.95.26.15671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Furie R, Toder K, Zapantis E. Lessons Learned From the Clinical Trials of Novel Biologics and Small Molecules in Lupus Nephritis. Semin Nephrol. 2015;35:509–20. doi: 10.1016/j.semnephrol.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 79.Haines JD, Herbin O, de la Hera B, Vidaurre OG, Moy GA, Sun Q, et al. Nuclear export inhibitors avert progression in preclinical models of inflammatory demyelination. Nat Neurosci. 2015;18:511–20. doi: 10.1038/nn.3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Witt J, Barisic S, Schumann E, Allgower F, Sawodny O, Sauter T, et al. Mechanism of PP2A–mediated IKK beta dephosphorylation: a systems biological approach. BMC Syst Biol. 2009;3:71. doi: 10.1186/1752-0509-3-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Morishita R, Sugimoto T, Aoki M, Kida I, Tomita N, Moriguchi A, et al. In vivo transfection of cis element “decoy” against nuclear factor-kappaB binding site prevents myocardial infarction. Nat Med. 1997;3:894–9. doi: 10.1038/nm0897-894. [DOI] [PubMed] [Google Scholar]
- 82.Logeat F, Israel N, Ten R, Blank V, Le Bail O, Kourilsky P, et al. Inhibition of transcription factors belonging to the rel/NF-kappa B family by a transdominant negative mutant. EMBO J. 1991;10:1827–32. doi: 10.1002/j.1460-2075.1991.tb07708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wakamatsu K, Nanki T, Miyasaka N, Umezawa K, Kubota T. Effect of a small molecule inhibitor of nuclear factor-kappaB nuclear translocation in a murine model of arthritis and cultured human synovial cells. Arthritis Res Ther. 2005;7:R1348–59. doi: 10.1186/ar1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Qu H, Bian W, Xu Y. A novel NF-kappaB inhibitor, DHMEQ, ameliorates pristane-induced lupus in mice. Exp Ther Med. 2014;8:100–4. doi: 10.3892/etm.2014.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Funakoshi T, Yamashita K, Ichikawa N, Fukai M, Suzuki T, Goto R, et al. A novel NF-kappaB inhibitor, dehydroxymethylepoxyquinomicin, ameliorates inflammatory colonic injury in mice. J Crohns Colitis. 2012;6:215–25. doi: 10.1016/j.crohns.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 86.Ozaki K, Makino H, Aoki M, Miyake T, Yasumasa N, Osako MK, et al. Therapeutic effect of ribbon-type nuclear factor-kappaB decoy oligonucleotides in a rat model of inflammatory bowel disease. Curr Gene Ther. 2012;12:484–92. doi: 10.2174/156652312803519814. [DOI] [PubMed] [Google Scholar]
- 87.De Stefano D. Oligonucleotides decoy to NF-kappaB: becoming a reality? Discov Med. 2011;12:97–105. [PubMed] [Google Scholar]
- 88.Huang B, Yang XD, Lamb A, Chen LF. Posttranslational modifications of NF-kappaB: another layer of regulation for NF-kappaB signaling pathway. Cell Signal. 2010;22:1282–90. doi: 10.1016/j.cellsig.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Won M, Byun HS, Park KA, Hur GM. Post-translational control of NF-kappaB signaling by ubiquitination. Arch Pharm Res. 2016;39:1075–84. doi: 10.1007/s12272-016-0772-2. [DOI] [PubMed] [Google Scholar]
- 90.Cargnello M, Roux PP. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev. 2011;75:50–83. doi: 10.1128/MMBR.00031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Morrison DK. MAP kinase pathways. Cold Spring Harb Perspect Biol. 2012:4. doi: 10.1101/cshperspect.a011254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sabio G, Davis RJ. TNF and MAP kinase signalling pathways. Semin Immunol. 2014;26:237–45. doi: 10.1016/j.smim.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chuang HC, Wang X, Tan TH. MAP4K Family Kinases in Immunity and Inflammation. Adv Immunol. 2016;129:277–314. doi: 10.1016/bs.ai.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 94.Coulombe P, Meloche S. Atypical mitogen-activated protein kinases: structure, regulation and functions. Biochim Biophys Acta. 2007;1773:1376–87. doi: 10.1016/j.bbamcr.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 95.Sweeney SE, Firestein GS. Mitogen activated protein kinase inhibitors: where are we now and where are we going? Ann Rheum Dis. 2006;65(Suppl 3):iii83–8. doi: 10.1136/ard.2006.058388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Davies C, Tournier C. Exploring the function of the JNK (c-Jun N-terminal kinase) signalling pathway in physiological and pathological processes to design novel therapeutic strategies. Biochem Soc Trans. 2012;40:85–9. doi: 10.1042/BST20110641. [DOI] [PubMed] [Google Scholar]
- 97.Badger AM, Bradbeer JN, Votta B, Lee JC, Adams JL, Griswold DE. Pharmacological profile of SB 203580, a selective inhibitor of cytokine suppressive binding protein/p38 kinase, in animal models of arthritis, bone resorption, endotoxin shock and immune function. J Pharmacol Exp Ther. 1996;279:1453–61. [PubMed] [Google Scholar]
- 98.Fijen JW, Zijlstra JG, De Boer P, Spanjersberg R, Tervaert JW, Van Der Werf TS, et al. Suppression of the clinical and cytokine response to endotoxin by RWJ-67657, a p38 mitogen-activated protein-kinase inhibitor, in healthy human volunteers. Clin Exp Immunol. 2001;124:16–20. doi: 10.1046/j.1365-2249.2001.01485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jackson JR, Bolognese B, Hillegass L, Kassis S, Adams J, Griswold DE, et al. Pharmacological effects of SB 220025, a selective inhibitor of P38 mitogen-activated protein kinase, in angiogenesis and chronic inflammatory disease models. J Pharmacol Exp Ther. 1998;284:687–92. [PubMed] [Google Scholar]
- 100.Kontzias A, Laurence A, Gadina M, O’Shea JJ. Kinase inhibitors in the treatment of immune-mediated disease. F1000 Med Rep. 2012;4:5. doi: 10.3410/M4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee MR, Dominguez C. MAP kinase p38 inhibitors: clinical results and an intimate look at their interactions with p38alpha protein. Curr Med Chem. 2005;12:2979–94. doi: 10.2174/092986705774462914. [DOI] [PubMed] [Google Scholar]
- 102.Hammaker D, Firestein GS. “Go upstream, young man”: lessons learned from the p38 saga. Ann Rheum Dis. 2010;69(Suppl 1):77–82. doi: 10.1136/ard.2009.119479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Patterson H, Nibbs R, McInnes I, Siebert S. Protein kinase inhibitors in the treatment of inflammatory and autoimmune diseases. Clin Exp Immunol. 2014;176:1–10. doi: 10.1111/cei.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Norman P. Investigational p38 inhibitors for the treatment of chronic obstructive pulmonary disease. Expert Opin Investig Drugs. 2015;24:383–92. doi: 10.1517/13543784.2015.1006358. [DOI] [PubMed] [Google Scholar]
- 105.Stark AK, Sriskantharajah S, Hessel EM, Okkenhaug K. PI3K inhibitors in inflammation, autoimmunity and cancer. Curr Opin Pharmacol. 2015;23:82–91. doi: 10.1016/j.coph.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Massacesi C, Di Tomaso E, Urban P, Germa C, Quadt C, Trandafir L, et al. PI3K inhibitors as new cancer therapeutics: implications for clinical trial design. Onco Targets Ther. 2016;9:203–10. doi: 10.2147/OTT.S89967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Koren S, Bentires-Alj M. Tackling Resistance to PI3K Inhibition by Targeting the Epigenome. Cancer Cell. 2017;31:616–8. doi: 10.1016/j.ccell.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 108.Mayer IA. Clinical Implications of Mutations in the PI3K Pathway in HER2+ Breast Cancer: Prognostic or Predictive? Curr Breast Cancer Rep. 2015;7:210–4. doi: 10.1007/s12609-015-0197-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Miller TW, Rexer BN, Garrett JT, Arteaga CL. Mutations in the phosphatidylinositol 3-kinase pathway: role in tumor progression and therapeutic implications in breast cancer. Breast Cancer Res. 2011;13:224. doi: 10.1186/bcr3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ali IU, Schriml LM, Dean M. Mutational spectra of PTEN/MMAC1 gene: a tumor suppressor with lipid phosphatase activity. J Natl Cancer Inst. 1999;91:1922–32. doi: 10.1093/jnci/91.22.1922. [DOI] [PubMed] [Google Scholar]
- 111.LoRusso PM. Inhibition of the PI3K/AKT/mTOR Pathway in Solid Tumors. J Clin Oncol. 2016 doi: 10.1200/JCO.2014.59.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mayer IA, Arteaga CL. The PI3K/AKT Pathway as a Target for Cancer Treatment. Annu Rev Med. 2016;67:11–28. doi: 10.1146/annurev-med-062913-051343. [DOI] [PubMed] [Google Scholar]
- 113.Winkler DG, Faia KL, DiNitto JP, Ali JA, White KF, Brophy EE, et al. PI3K–delta and PI3K–gamma inhibition by IPI-145 abrogates immune responses and suppresses activity in autoimmune and inflammatory disease models. Chem Biol. 2013;20:1364–74. doi: 10.1016/j.chembiol.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 114.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–93. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kim LC, Cook RS, Chen J. mTORC1 and mTORC2 in cancer and the tumor microenvironment. Oncogene. 2017;36:2191–201. doi: 10.1038/onc.2016.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nguyen QD, Merrill PT, Clark WL, Banker AS, Fardeau C, Franco P, et al. Intravitreal Sirolimus for Noninfectious Uveitis: A Phase III Sirolimus Study Assessing Double-masKed Uveitis TReAtment (SAKURA) Ophthalmology. 2016;123:2413–23. doi: 10.1016/j.ophtha.2016.07.029. [DOI] [PubMed] [Google Scholar]
- 117.Wang Z, Wesche H, Stevens T, Walker N, Yeh WC. IRAK-4 inhibitors for inflammation. Curr Top Med Chem. 2009;9:724–37. doi: 10.2174/156802609789044407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ye ZH, Gao L, Wen DY, He Y, Pang YY, Chen G. Diagnostic and prognostic roles of IRAK1 in hepatocellular carcinoma tissues: an analysis of immunohistochemistry and RNA-sequencing data from the cancer genome atlas. Onco Targets Ther. 2017;10:1711–23. doi: 10.2147/OTT.S132120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Picard C, von Bernuth H, Ku CL, Yang K, Puel A, Casanova JL. Inherited human IRAK-4 deficiency: an update. Immunol Res. 2007;38:347–52. doi: 10.1007/s12026-007-0006-2. [DOI] [PubMed] [Google Scholar]
- 120.Grazioli S, Hamilton SJ, McKinnon ML, Del Bel KL, Hoang L, Cook VE, et al. IRAK-4 deficiency as a cause for familial fatal invasive infection by Streptococcus pneumoniae. Clin Immunol. 2016;163:14–6. doi: 10.1016/j.clim.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 121.Dudhgaonkar S, Ranade S, Nagar J, Subramani S, Prasad DS, Karunanithi P, et al. Selective IRAK4 Inhibition Attenuates Disease in Murine Lupus Models and Demonstrates Steroid Sparing Activity. J Immunol. 2017;198:1308–19. doi: 10.4049/jimmunol.1600583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kelly PN, Romero DL, Yang Y, Shaffer AL, 3rd, Chaudhary D, Robinson S, et al. Selective interleukin-1 receptor-associated kinase 4 inhibitors for the treatment of autoimmune disorders and lymphoid malignancy. J Exp Med. 2015;212:2189–201. doi: 10.1084/jem.20151074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nelson EA, Walker SR, Weisberg E, Bar-Natan M, Barrett R, Gashin LB, et al. The STAT5 inhibitor pimozide decreases survival of chronic myelogenous leukemia cells resistant to kinase inhibitors. Blood. 2011;117:3421–9. doi: 10.1182/blood-2009-11-255232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhou XX, Gao PJ, Sun BG. Pravastatin attenuates interferon-gamma action via modulation of STAT1 to prevent aortic atherosclerosis in apolipoprotein E-knockout mice. Clin Exp Pharmacol Physiol. 2009;36:373–9. doi: 10.1111/j.1440-1681.2008.05067.x. [DOI] [PubMed] [Google Scholar]
- 125.Siemasko K, Chong AS, Jack HM, Gong H, Williams JW, Finnegan A. Inhibition of JAK3 and STAT6 tyrosine phosphorylation by the immunosuppressive drug leflunomide leads to a block in IgG1 production. J Immunol. 1998;160:1581–8. [PubMed] [Google Scholar]
- 126.Cafferkey C, Chau I. Novel STAT 3 inhibitors for treating gastric cancer. Expert Opin Investig Drugs. 2016;25:1023–31. doi: 10.1080/13543784.2016.1195807. [DOI] [PubMed] [Google Scholar]
- 127.Mandal PK, Morlacchi P, Knight JM, Link TM, Lee GRt, Nurieva R, et al. Targeting the Src Homology 2 (SH2) Domain of Signal Transducer and Activator of Transcription 6 (STAT6) with Cell-Permeable, Phosphatase-Stable Phosphopeptide Mimics Potently Inhibits Tyr641 Phosphorylation and Transcriptional Activity. J Med Chem. 2015;58:8970–84. doi: 10.1021/acs.jmedchem.5b01321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Huh JR, Leung MW, Huang P, Ryan DA, Krout MR, Malapaka RR, et al. Digoxin and its derivatives suppress TH17 cell differentiation by antagonizing RORgammat activity. Nature. 2011;472:486–90. doi: 10.1038/nature09978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kojetin DJ, Burris TP. REV-ERB and ROR nuclear receptors as drug targets. Nat Rev Drug Discov. 2014;13:197–216. doi: 10.1038/nrd4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chang MR, Lyda B, Kamenecka TM, Griffin PR. Pharmacologic repression of retinoic acid receptor-related orphan nuclear receptor gamma is therapeutic in the collagen-induced arthritis experimental model. Arthritis Rheumatol. 2014;66:579–88. doi: 10.1002/art.38272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Scheepstra M, Leysen S, van Almen GC, Miller JR, Piesvaux J, Kutilek V, et al. Identification of an allosteric binding site for RORgammat inhibition. Nat Commun. 2015;6:8833. doi: 10.1038/ncomms9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liljevald M, Rehnberg M, Soderberg M, Ramnegard M, Borjesson J, Luciani D, et al. Retinoid-related orphan receptor gamma (RORgamma) adult induced knockout mice develop lymphoblastic lymphoma. Autoimmun Rev. 2016;15:1062–70. doi: 10.1016/j.autrev.2016.07.036. [DOI] [PubMed] [Google Scholar]
- 133.Okada S, Markle JG, Deenick EK, Mele F, Averbuch D, Lagos M, et al. IMMUNODEFICIENCIES. Impairment of immunity to Candida and Mycobacterium in humans with bi-allelic RORC mutations. Science. 2015;349:606–13. doi: 10.1126/science.aaa4282. [DOI] [PMC free article] [PubMed] [Google Scholar]