Abstract
Background
Out-of-hospital cardiac arrest (OHCA) commonly presents with non-shockable rhythms (asystole and pulseless electrical activity (PEA)). Whether antiarrhythmic drugs are safe and effective when these evolve to shockable rhythms (ventricular fibrillation/pulseless ventricular tachycardia (VF/VT)) during resuscitation is not known.
Methods
Adults with non-traumatic OHCA, vascular access and VF/VT anytime after ≥1 shock(s) were prospectively randomized, double-blind, to receive amiodarone, lidocaine or placebo by paramedics. Patients presenting with initial shock-refractory VF/VT were previously reported. The current study was a pre-specified analysis in a separate cohort who initially presented with non-shockable OHCA and were randomized upon subsequently developing shock-refractory VF/VT. The primary outcome was survival to hospital discharge; secondary outcomes included discharge functional status and adverse drug-related effects.
Results
Of 37,889 patients with OHCA, 3,026 with initial VF/VT and 1,063 with initial non-shockable-turned-shockable rhythms were treatment-eligible, randomized and received their assigned drug. Baseline characteristics among non-shockable-turned-shockable patients were balanced across treatment arms except that placebo recipients included fewer men and were less likely to receive bystander-CPR. Active-drug recipients in this cohort required fewer shocks, supplemental doses of their assigned drug and ancillary antiarrhythmic drugs than placebo-recipients (p<0.05). In all, 16 (4.1%) amiodarone, 11 (3.1%) lidocaine and 6 (1.9%) placebo-treated patients survived to hospital discharge (p=0.24). There was no significant interaction of treatment assignment and discharge survival with the initiating OHCA rhythm (asystole, PEA, or VF/VT); survival in each of these categories was consistently higher with active-drugs, though the trends were not statistically significant. Adjusted absolute differences (95% confidence interval) in survival from non-shockable-turned-shockable arrhythmias with amiodarone vs placebo were 2.3% (−0.3, 4.8), p=0.08 and for lidocaine vs placebo 1.2% (−1.1, 3.6), p=0.30. Over one-half of these survivors were functionally independent or required minimal assistance. Drug-related adverse effects were infrequent.
Conclusions
Outcome from non-shockable-turned-shockable OHCA is poor, but not invariably fatal. Though not statistically significant, point estimates for survival were greater after amiodarone or lidocaine than placebo, without increased risk of adverse effects or disability, and consistent with previously observed favorable trends from treatment of initial shock-refractory VF/VT with these drugs. Together the findings may signal a clinical benefit that invites further investigation.
Keywords: cardiac arrest, resuscitation, amiodarone, lidocaine, placebo
Introduction
Sudden out-of-hospital cardiac arrest (OHCA) claims the lives of 347,000 persons each year in the North America, and hundreds of thousands more worldwide.1, 2 The epidemiology of OHCA has changed over recent years such that non-shockable rhythms (bradyasystole and pulseless electrical activity (PEA)) now predominate, and are deemed to be largely non-survivable.3, 4, 5
Non-shockable rhythms may evolve to shockable ventricular fibrillation or pulseless ventricular tachycardia (VF/VT) during the course of resuscitation in up to a quarter of patients with OHCA, for whom antiarrhythmic drug use has both pragmatic and public health importance.6, 7 Though antiarrhythmic medications are commonly administered for VF/VT, their specific role in treating non-shockable-turned-shockable rhythms has not been rigorously evaluated. Even if effective, their delayed deployment could prove too late to alter the clinical outcome from VF/VT that arises from a protracted period of asystole or PEA. Furthermore, the effects of antiarrhythmic drugs on conduction and tissue refractoriness could prove counterproductive were this to promote the recrudescence of bradyarrhythmias or PEA that originally provoked or accompanied OHCA, or result in other harmful effects. Conversely, effective pharmacologic suppression of recalcitrant VF/VT could help restore and stabilize circulation and in turn improve outcome. In short, the optimal approach to such patients is unknown.
The Amiodarone, Lidocaine or Placebo Study (ALPS) was a prospective, randomized, double-blind, placebo-controlled multicenter trial evaluating the effectiveness of amiodarone and lidocaine for OHCA due to shock-resistant VF/VT.8 While the trial’s main focus was on patients whose initial presenting OHCA rhythm was VF/VT, in actuality those with shock-resistant VF/VT at any time during resuscitation were eligible for randomization.9 Thus by design the trial randomized two cohorts – those with initial VF/VT (previously reported) and the complementary group with initially non-shockable OHCA arrhythmias (asystole or PEA) that subsequently turned shockable during the course of resuscitation. Accordingly, we undertook a pre-specified investigation of the clinical effects of amiodarone or lidocaine compared to placebo in the randomized cohort with initial non-shockable-turned-shockable OHCA. The primary outcome was survival to hospital discharge; secondary outcomes included functional status at discharge, and adverse drug-related effects.
Methods
Patients
The background, methods and primary outcome of the ALPS trial were previously described.8,9 This trial was conducted in compliance with all applicable regulatory requirements for exception from informed consent in emergency research. It involved paramedics from 55 emergency medical services (EMS) agencies across 10 North American sites participating in the Resuscitation Outcomes Consortium.10 The trial enrolled patients 18 years of age or older with atraumatic out-of-hospital cardiac arrest, established intravenous or intraosseous vascular access, and persistent (nonterminating) or recurrent (restarting after successful termination) VF/VT after one or more shocks. Patients were randomized to licensed parenteral preparations of lidocaine, normal saline, or Captisol-based formulation of amiodarone (Nexterone, Baxter Healthcare). Protected populations, patients who had already received open-label intravenous amiodarone or lidocaine during resuscitation, had known hypersensitivity to these drugs, advanced directives (“do not resuscitate” orders), or in whom VF/VT terminated before study drug could be administered were excluded from the trial.
The trial protocol specified that only patients who met clinical eligibility criteria and received any dose of study drug for ongoing shock-refractory VF/VT at any time during the resuscitation would be included in the analysis. Outcomes in the primary analysis population (comprised of study-eligible drug recipients with confirmed VF/VT as the presenting cause of OHCA) and in all randomized patients by intention to treat (regardless of whether study-eligible or having received drug) were previously reported, along with a listing of all participating EMS agencies and personnel.8 The focus of the current study, the population of randomized study-eligible drug recipients whose initial OHCA was non-shockable, but subsequently developed shock-refractory VF/VT, has not been previously characterized.
Definitions
Rhythms were identified from defibrillator recordings using cutaneous defibrillator electrodes that approximated a lead II configuration and were later reviewed manually by trained study personnel. A shockable versus non-shockable initial rhythm was determined by a shock versus no-shock advisory from an automated external defibrillator (AED) and/or by manual review of the electronic recording. PEA was defined as any organized ventricular rhythm (exclusive of ventricular tachycardia) with an absent pulse; asystole by absence of any ventricular rhythm or at most a single ventricular complex over a 6 second interval. VF was defined as irregular, disorganized ventricular electrical activity of variable amplitude and ventricular tachycardia as an organized rhythm with a wide QRS interval (≥120 msec) without associated P waves at a rate of more than 150 beats/minute. The incident call was defined as the initial contact with the Public Safety Answering Point that served as the emergency call center in each locality, and represented the initial activation of EMS for the OHCA event. The incident call to EMS arrival was defined as the time interval from this call to the first arriving EMS vehicle at the street address of the OHCA event.
Design and intervention
Trial drugs were packaged in sealed kits, each holding 3 identically formulated syringes of study drug, and each syringe containing 150 mg of amiodarone, 60 mg of lidocaine, or normal saline. Amiodarone, lidocaine and normal saline (placebo) kits and respective syringes were indistinguishable except by a numerical code and were randomly distributed to EMS providers in equal ratios of 1:1:1. Randomization was stratified by participating ROC site and EMS agency in permuted blocks of concealed size.
Patients were treated in accordance with local EMS protocols and American Heart Association (AHA) Advanced Life Support Guidelines that were in place at the time of the trial’s conduct.11 The subsequent opening of a study kit by EMS personnel constituted a patient’s enrollment in the trial, whose masked contents (amiodarone, lidocaine or placebo) determined their randomized treatment assignment. Two syringes were initially administered as a rapid bolus (one syringe if the estimated body weight was < 100 lbs (45 kg)), followed by standard resuscitation measures and shock(s). If VF/VT persisted, a single supplemental syringe of the same assigned study drug was administered, followed by standard interventions according to local practice, exclusive of any open label amiodarone or lidocaine before hospitalization.
All trial interventions were completed before patients’ hospital arrival. Hospital care providers were informed about the trial, but not treatment assignment unless emergency unblinding was requested, in which case it was provided strictly to the treating physician. Hospital care was not standardized, although its components were monitored.
Outcomes
The primary outcome of the trial was survival to hospital discharge. Secondary outcome were survival to discharge with favorable neurological functional status, defined on the modified Rankin scale (ranging from 0, no symptoms, to 6, death) as 3 or less, meaning being able to conduct activities of daily living independently or with minimal assistance,12 and adverse drug-related effects. These were defined as effects previously reported with these medications that occurred within 24 hours of their administration, including anaphylaxis, thrombophlebitis requiring treatment, clinical seizures and bradycardia requiring temporary cardiac pacing. Other prespecified mechanistic outcomes included return of spontaneous circulation (ROSC), survival to hospital admission and responses to treatment (number of shocks and need for ancillary therapies).
Statistics
The previously reported primary analysis trial was powered to detect an absolute improvement of 6% in survival to hospital discharge from amiodarone vs placebo among treatment recipients with OHCA due to initial VF/VT; differences between lidocaine vs placebo and amiodarone vs lidocaine were secondary comparisons.8 While not its main focus, patients with late-occurring VF/VT were included in the trial for both pragmatic and scientific reasons. Pragmatically, the dynamic nature of cardiac rhythms during resuscitation made it infeasible to acutely discriminate early from later occurring VF/VT without giving confusing treatment directives to paramedic providers that might only serve to impede patient care. Based on previous work, survival in this population was expected to be directionally similar to patients with OHCA due to initial VF/VT, but poor regardless of antiarrhythmic drug administration.13, 14 Though recognized as underpowered, this analysis was pre-specified to collect valuable information about the efficacy and safety of therapies that are often given under similar circumstances in clinical practice, which the trial was designed to represent. As such, finding a signal of potential benefit could serve to justify and inform more definitive future studies.
Because of potential imbalances in the characteristics between treatment arms in this population, we conducted multiple logistic regression to evaluate the trial’s main endpoints of survival and neurological outcome at hospital discharge, adjusting for age, sex, arrest etiology (presumed cardiac versus not), arrest location (public versus private), bystander or EMS-witnessed status of the OHCA, provision of bystander CPR, the incident call to EMS arrival interval and by trial site. Multiple imputation analysis (with 20 imputed data sets) was used to address any incomplete covariate data, using the ‘mice’ package in R to minimize the potential bias of estimates compared to analyses limited to only cases with complete data.15, 16, 17 Adjusted complete case analyses were also performed as an added sensitivity analysis of the imputation model. We tested for treatment differences according to the initial rhythm (PEA versus asystole) by adding an interaction term between study arm and initial rhythm. P values were 2-sided with statistical significance defined as an alpha of 0.05. There were no adjustments for multiple comparisons, given the exploratory nature of the analyses.
The trial was approved by institutional review boards of all participating sites, with oversight by the U. S. Food and Drug Administration (FDA) and Health Canada, and monitored by an independent data and safety board appointed by the National Heart, Lung, and Blood Institute (NHLBI). All the authors have read and approved the manuscript.
Results
Patients
Enrollment for the trial began on May 7, 2012 and was completed on October 25, 2015. Of 37,889 patients with non-traumatic OHCA, 7,903 had initial VF/VT (3,026 of these were previously reported randomized recipients of study drug) and 29,986 had an initially non-shockable arrest rhythm, 1,864 of whom subsequently developed VF/VT that was refractory to ≥1 shock(s) (Figure 1). Of these, 1,320 patients were randomized to drug treatment, of whom 1,063 with known treatment assignment remained study-eligible with ongoing episodes of VF/VT at time of treatment and constituted the primary analysis group for this study. In all 389 patients received amiodarone, 358 lidocaine and 316 patients received placebo. Outcome was known in 1,061 (99.8%) and 1,032 (97%) had complete covariate data.
Figure 1.
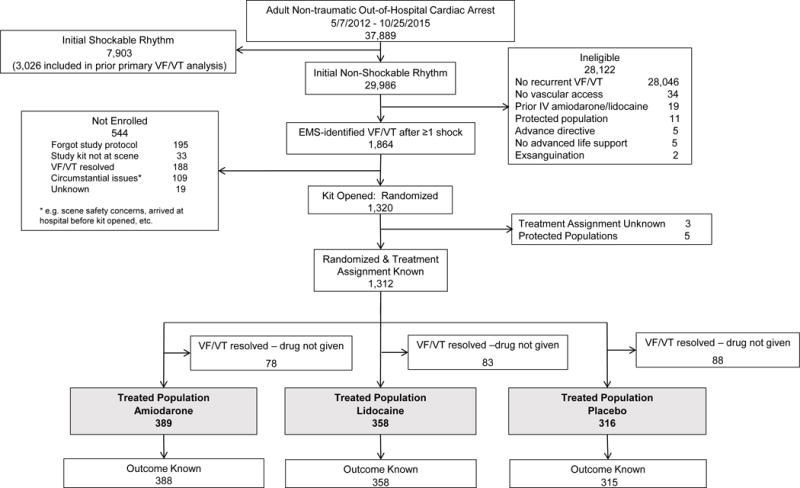
Patient flow in the trial. Out-of-hospital cardiac arrest was defined as the absence of consciousness and pulses that required cardiopulmonary resuscitation (CPR) by Emergency Medical Services (EMS) personnel. The criteria shown in the boxes corresponding to the “Ineligible 28,122” patients and the “Not enrolled 544” patients are listed in hierarchical fashion proceeding from the top to the bottom of each list. Thus patients excluded (or not enrolled) for reasons shown higher on the list may have also met criteria shown lower on the list but were not duplicated in the numbers shown for these lower listed categories. Abbreviations: IV – intravenous; VF/VT – ventricular fibrillation or pulseless ventricular tachycardia
Among the 1063 randomized patients, the initial non-shockable OHCA rhythm was PEA in 400 (38%), asystole in 587 (55%) and not characterized in 76 patients (7%). Patients (mean ± standard deviation (SD)) were 64.5 ± 16.5 years in age, 70% were men, 44.7% had a bystander-witnessed OHCA, 14.7% occurred in a public location, and 46.1% received bystander CPR. From the time of the incident call to EMS arrival (mean ± SD) was 6.1 ± 2.8 minutes; to first EMS shock 20.7 ± 8.3 minutes, and to receipt of study drugs 26.9 ± 8.9 minutes which were administered after 2.2 ± 1.1 shocks. These baseline and resuscitation event characteristics were generally balanced across active drug and placebo treatment arms, except for fewer men and a lower frequency of bystander CPR in the placebo arms among patients with initial PEA group and in the combined group with initially non-shockable-turned-shockable rhythms (Table 1).
Table 1.
Baseline Characteristics of Patients
| Characteristic | Initial Cardiac Arrest Rhythm | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| All non-shockable Initial Rhythms (n=1063*) |
Pulseless Electrical Activity (n=400) |
Asystole (n=587) |
|||||||
| Placebo (n=316) |
Lidocaine (n=358) |
Amiodarone (n=389) |
Placebo (n=119) |
Lidocaine (n=141) |
Amiodarone (n=140) |
Placebo (n=178) |
Lidocaine (n=194) |
Amiodarone (n=215) |
|
| Age (years) mean (SD) | 64.4 (16.1) | 63.8 (16.9) | 65.3 (16.6) | 67.5 (15.7) | 66.8 (16.3) | 68.9 (16.5) | 62.3 (16.6) | 61.5 (17.3) | 62.9 (15.7) |
| Men, % | 65.2%† | 69.3% | 75.1% | 62.2%† | 68.1% | 77.9% | 66.9% | 68.6% | 75.3% |
| Arrest location known, n Public location, % |
316 14.6% |
358 14.2% |
386 15.3% |
119 10.9% |
141 15.6% |
140 16.4% |
178 16.9% |
194 11.9% |
212 12.3% |
| Bystander-witnessed arrest status known, n Bystander-witnessed, % |
313 45.0% |
348 44.8% |
381 44.4% |
118 49.2% |
139 50.4% |
137 48.2% |
176 39.2% |
187 40.6% |
210 40.0% |
| Bystander CPR status known, n Bystander CPR, % |
316 41.1%† | 358 44.4% |
389 51.7% |
119 34.5%† |
141 38.3% |
140 49.3% |
178 43.8% |
194 47.9% |
215 52.1% |
| Resuscitation Events | |||||||||
| Incident Call‡ to 1st EMS recorded, n Incident Call to 1st EMS (min), mean (SD) Median (IQR) |
316 6 (2.8) 5.4 (4.4–7) |
358 6.3 (2.9) 5.7 (4.4–7.4) |
388 6 (2.7) 5.7 (4.3–7.2) |
119 6 (2.6) 5.5 (4.5–7.2) |
141 6.4 (2.9) 6 (4.8–7.4) |
140 5.8 (2.5) 5.4 (4.1–7.1) |
178 5.9 (2.7) 5.3 (4.2–6.9) |
194 6 (3) 5.3 (4.1–7.2) |
215 5.9 (2.7) 5.6 (4.3–7) |
| Incident Call‡ to 1st shock recorded, n Incident call to 1st shock (min), mean (SD) Median (IQR) |
312 21.1 (9.3) 20 (14–26) |
353 20.4 (8) 19 (15–25) |
384 20.5 (8.5) 19 (15–25) |
119 21.3 (10.2) 20 (13–27) |
140 20.5 (8.2) 20 (15–25) |
139 20.4 (8.6) 19 (15–24) |
177 21 (8.6) 20 (15–26) |
192 20.4 (8.1) 19 (15–25) |
214 20.7 (8.4) 19 (15–25) |
| Incident Callठto Study Drug recorded, n Incident Call to Study Drug (min), mean(SD) Median (IQR) |
278 28.1 (9.2) 26 (21–34) |
317 27.1 (8.7) 26 (21–32) |
354 27.3 (8.9) 26 (21–32) |
93 29.1 (8.9) 28 (23–36) |
111 27.8 (9.3) 27 (21–32) |
117 26.8 (8.3) 25 (21–32) |
166 27.3 (9.1) 25 (21–32) |
187 26.3 (8.2) 26 (20–32) |
207 27.6 (8.9) 26 (21–33) |
| EMS shocks before study drug recorded, n EMS shocks before study drug, mean (SD) Median (IQR) | 315 2.1 (0.9) 2 (2–2) |
357 2.2 (1.1) 2 (2–2) |
382 2.2 (1) 2 (2–2) |
119 2 (0.8) 2 (2–2) |
141 2.1 (0.9) 2 (2–2) |
139 2 (0.8) 2 (1–2) |
177 2.1 (0.9) 2 (2–2) |
194 2.2 (1.1) 2 (2–2) |
212 2.3 (1.1) 2 (2–3) |
| Emergency Medical Services CPR | |||||||||
| Compression rate recorded, n Compression rate/min, mean (SD) Median (IQR) |
287 110 (9) 109 (104–114) |
322 110 (10) 109 (103–116) |
359 109 (9) 108 (103–114) |
110 110 (10) 109 (104–113) |
126 109 (10) 108 (101–115) |
129 110 (10) 108 (103–115) |
159 111 (9) 109 (105–116) |
178 110 (11) 109 (103–117) |
202 109 (9) 107 (103–114) |
| Compression depth recorded, n Compression depth (mm) mean (SD) Median (IQR) |
136 51 (10) 51 (45–57) |
157 51 (10) 51 (44–57) |
180 51 (9) 51 (45–57) |
51 51 (11) 50 (44–57) |
62 49 (11) 49 (44–57) |
61 51 (9) 50 (46–56) |
77 51 (10) 51 (46–57) |
89 52 (10) 52 (46–58) |
108 51 (10) 52 (45–58) |
| CPR Fraction recorded, n CPR Fraction, mean %, (SD) Median (IQR) |
266 85% (8) 86 (80–91) |
305 86% (9) 87 (81–92) |
334 86% (8) 87 (81–92) |
107 84% (9) 85 (80–91) |
124 84% (10) 86 (80–91) |
126 84% (9) 86 (78–91) |
148 86% (8) 87 (82–91) |
170 87% (8) 88 (82–92) |
190 86% (8) 87 (83–92) |
| Advanced airway status known, n Successfully placed advanced airway, % |
316 90.5% |
358 92.7% |
389 93.3% |
119 89.1% |
141 94.3% |
140 95.7% |
178 91% |
194 91.8% |
215 91.2% |
In 76 patients, the non-shockable rhythm diagnosis (pulseless electrical activity versus asystole) was not known.
p <0.05 between treatment arms within the described rhythm category
The incident call was defined as the initial contact with the Public Safety Answering Point that served as the emergency call center in each locality, and represented the initial activation of EMS for the OHCA event. The incident call to 1st EMS arrival was defined as the time interval from this call to the first arriving EMS vehicle at the street address of the OHCA event.
Non-Emergency Medical Services-witnessed events
Abbreviations: CPR – cardiopulmonary resuscitation; IQR – interquartile range; SD –standard deviation
Hospital care
No significant differences in subsequent care were observed between treatment arms among patients who survived to hospital admission (Table 2). In the combined group of non-shockable-turned-shockable OHCA, approximately half or more of hospitalized patients received targeted temperature management and early coronary catheterization. Life sustaining therapies were limited or withdrawn in approximately one-third of patients within the first 3 days following their OHCA, and in about half of patients overall.
Table 2.
Post randomization treatments and adverse events
| Characteristic | All Non-shockable Initial Rhythms n=1063 |
Pulseless Electrical Activity n=400 |
Asystole n=587 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Placebo (n=316) |
Lidocaine (n=358) |
Amiodarone (n=389) |
Placebo (n=119) |
Lidocaine (n=141) |
Amiodarone (n=140) |
Placeb/o (n=178) |
Lidocaine (n=194) |
Amiodarone (n=215) |
|
| Recorded number of study drug syringes administered, n | 314 | 354 | 384 | 118 | 139 | 139 | 177 | 192 | 211 |
| 3 syringes, % of patients | 65%* | 51.4% | 59.9% | 61.9%* | 41.7% | 58.3% | 66.1%* | 58.3% | 62.1% |
| 2 syringes, % of patients | 32.8% | 46% | 39.6% | 36.4% | 53.2% | 41% | 31.1% | 40.6% | 37.4% |
| 1 syringe, % of patients | 2.2% | 2.5% | 0.5% | 1.7% | 5% | 0.7% | 2.8% | 1% | 0.5% |
| Recorded number of EMS shocks, n | 315 | 357 | 382 | 119 | 141 | 139 | 177 | 194 | 212 |
| EMS shocks after study drug, mean (SD) | 2.8 (3.0)* | 1.7 (2.1) | 2.2 (2.2) | 2.5 (2.6)* | 1.7 (2.3) | 2.2 (2.3) | 3 (3.0)* | 1.7 (1.9) | 2.1 (2) |
| Median (IQR) | 2 (1–4) | 1 (0–2) | 2 (1–3) | 2 (0–3) | 1 (0–2) | 2 (1–3) | 2 (1–4) | 1 (0–2) | 2 (2–3) |
| Cumulative epinephrine dose recorded, n | 316 | 355 | 383 | 119 | 140 | 139 | 178 | 194 | 210 |
| mg, mean (SD) | 5.5 (2.8) | 5.5 (2.6) | 5.7 (2.3) | 5.7 (2.6) | 5.5 (2.5) | 5.8 (2.3) | 5.6 (2.6) | 5.4 (2.7) | 5.4 (2.0) |
| Median (IQR) | 5 (4–7) | 5 (4–7) | 5 (4–7) | 5 (3–7) | 5 (4–7) | 6 (4–7) | 5 (4–7) | 5 (4–7) | 5 (4–6) |
| Prehospital drugs administered (% of all randomized patients)† | |||||||||
| Epinephrine(%) | 100% | 99.7% | 99.5% | 100% | 93.3% | 100% | 100% | 100% | 99.1% |
| Vasopressin (%) | 5.1% | 3.4% | 4.1% | 4.2% | 2.1% | 6.4% | 6.2% | 4.6% | 3.3% |
| Bicarbonate (%) | 42.4% | 39.9% | 40.9% | 46.2% | 41.1% | 40% | 41% | 38.7% | 40.9% |
| Atropine (%) | 5.7% | 4.5% | 8.7% | 8.4% | 5% | 6.4% | 3.4% | 4.1% | 9.3% |
| Beta blocker (%) | 0.9% | 0 | 0 | 0.8% | 0 | 0 | 0.6% | 0 | 0 |
| Procainamide (%) | 7.9% | 5% | 5.9% | 10.1% | 6.4% | 6.4% | 6.7% | 4.6% | 5.6% |
| Magnesium, n (%) | 9.5%* | 3.9% | 7.2% | 7.6% | 4.3% | 5.7% | 10.1%* | 3.1% | 7.4% |
| Possible drug-related adverse events (% of all randomized patients) | |||||||||
| Thrombophlebitis within 24 hours, n (%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Anaphylaxis within 24 hours, n (%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Clinical seizure activity within 24 hours, n (%) | 10 (3.2%) | 13 (3.6%) | 10 (2.6%) | 5 (4.2%) | 2 (1.4%) | 5 (3.6%) | 4 (2.2%) | 10 (5.2%) | 5 (2.3%) |
| Pacing within 24 hours,‡ n (%) | 7 (2.2%) | 7 (2.0%) | 9 (2.3%) | 1 (0.8%) | 3 (2.1%) | 1 (0.7%) | 3 (1.7%) | 2 (1.0%) | 6 (2.8%) |
| IV/IO complications within 24 hours n (%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Any adverse event within 24 hours, n (%) | 17 (5.4%) | 19 (5.3%) | 19 (4.9%) | 6 (5.0%) | 5 (3.5%) | 6 (4.3%) | 7 (3.9%) | 11 (5.7%) | 11 (5.1%) |
| Hospital Treatments (% of hospitalized patients) | |||||||||
| Patients Admitted to Hospital (n) | 65 | 74 | 64 | 25 | 26 | 21 | 35 | 44 | 35 |
| Targeted temperature management, % | 67.7% | 56.8% | 67.2% | 72.0% | 42.3% | 66.7% | 71.4% | 63.6% | 65.7% |
| Coronary catheterization in first 24 hours, % | 58.5% | 40.5% | 56.2% | 64.0% | 30.8% | 76.2% | 57.1% | 40.9% | 40.0% |
| Implantable defibrillator status known, n | 64 | 74 | 63 | 25 | 26 | 21 | 35 | 44 | 35 |
| Implantable defibrillator placed, % | 6.2% | 2.7% | 9.5% | 8.0% | 7.7% | 0% | 0% | 0% | 5.7% |
| Care limited or withdrawn, % | 60.0% | 51.4% | 50.0% | 48.0% | 46.2% | 47.6% | 71.4% | 52.3% | 54.3% |
| Time of care withdrawal known, n | 63 | 70 | 61 | 24 | 24 | 19 | 35 | 43 | 34 |
| Care limited or withdrawn within 3 days of arrest,§ % | 36.5% | 34.3% | 27.9% | 20.8% | 33.3% | 21.1% | 51.4% | 34.9% | 29.4% |
p <0.05 between treatment arms within the described rhythm category
Whether the listed prehospital drugs were or were not administered was recorded in all randomized patients with the exception of one patient in the lidocaine treatment arm in whom information about epinephrine administration was missing (and whose initial non-shockable rhythm diagnosis (asystole versus pulseless electrical activity) was also not known).
Not initiated before study drug given
If the time of withdrawal of care was not recorded, it was assumed to be >3 days
Abbreviations: EMS – Emergency Medical Services; IO – intraosseous; IV –intravenous; IQR – interquartile range; SD – standard deviation
Outcome
Unadjusted survival to hospital discharge among the 1061 of 1063 study-drug recipients with known outcome was 1.9% in the placebo arm, 3.1% in the lidocaine arm, and 4.1% in the amiodarone arm, and though reflecting one and one-half to two-fold relative differences in outcome were not statistically significant (p=0.24) (Table 2). Patients who survived to hospital discharge had a mean MRS score of 3 ± 2 (median 3), 52% of whom were discharged with MRS ≤ 3 without significant differences between treatment arms (Table 3). Among 1309 patients with known treatment assignment and outcome from the 1312 patients in the “intention-to-treat” population (Figure 1), 19 of 466 of those randomized to amiodarone (4.1%), 15 of 440 to lidocaine (3.4%) and 13 of 403 to placebo (3.2%) survived to hospital discharge, which did not significantly differ between treatment groups. These differences were understandably attenuated by changes in patients’ eligibility for drug treatment after initial randomization.
Table 3.
Unadjusted outcomes
| Characteristic | All Non-shockable Initial Rhythms n=1063 |
Pulseless Electrical Activity n=400 |
Asystole n=587 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Placebo (n=316) |
Lidocaine (n=358) |
Amiodarone (n=389) |
Placebo (n=119) |
Lidocaine (n=141) |
Amiodarone (n=140) |
Placebo (n=178) |
Lidocaine (n=194) |
Amiodarone (n=215) |
|
| Return of Spontaneous Circulation (ROSC) | |||||||||
| Any ROSC, n (%) | 118 (37.3%) | 145 (40.5%) | 124(31.9%)* | 52 (43.7%) | 59 (41.8%) | 43 (30.7%) | 57 (32%) | 77 (39.7%) | 69 (32.1%) |
| ROSC at Emergency Department arrival, n (%) | 59 (18.7%) | 66 (18.4%) | 52 (13.4%) | 24 (20.2%) | 22 (15.6%) | 18 (12.9%) | 30 (16.9%) | 40 (20.6%) | 29 (13.5%) |
| Incident call to first ROSC,† n Mean minutes (SD) Median minutes (IQR) |
117 26.6 (10.5) 26 (20–34) |
144 27.8 (10.5) 27 (21–33) |
124 28.2 (10.1) 27 (21–34) |
52 24.9 (11.8) 25 (18–31) |
59 27.1 (11.3) 27 (19–33) |
43 27.3 (11.3) 25 (20–33) |
56 27.6 (9.0) 27 (21–34) |
76 28.1 (9.5) 26 (21–32) |
69 27.9 (9.5) 27 (21–33) |
| Incident call to resuscitation termination,† n Mean minutes (SD) Median minutes (IQR) |
115 46.9 (10.8) 45 (39–54) |
133 44.1 (11.0) 42 (37–49) |
159 44.4 (10.2) 43 (37–50) |
41 46.0 (11.3) 44 (38–53) |
52 44.4 (11.8) 42 (37–50) |
54 44.4 (10.7) 43 (37–51) |
68 46.8 (10.2) 45 (39–54) |
73 43.7 (9.6) 42 (38–49) |
95 44.2 (9.9) 43 (37–50) |
| Survival outcome | |||||||||
| Known survival outcome, n | 315 | 358 | 388 | 119 | 141 | 140 | 177 | 194 | 215 |
| Survival to Hospital Admission, n (%) | 65 (20.6%) | 74 (20.7%) | 64 (16.5%)* | 25 (21%) | 26 (18.4%) | 21 (15%)* | 35 (19.7%) | 44 (22.7%) | 35 (16.3%)* |
| Survival to Discharge, n (%) | 6 (1.9%) | 11 (3.1%) | 16 (4.1%) | 4 (3.4%) | 6 (4.3%) | 7 (5.0%) | 1 (0.6%) | 4 (2.1%) | 7 (3.3%) |
| Survival with mRS ≤ 3, n (%) | 3 (1.0%) | 6 (1.7%) | 8 (2.1%) | 2 (1.7%) | 4 (2.8%) | 4 (2.9%) | 0 (0.0%) | 1 (0.5%) | 2 (0.9%) |
| Survivors with recorded mRS, n mRS in Survivors mean (SD) Median (IQR) |
6 2.8 (2.1) 3 (1 – 5) |
11 3.0 (1.9) 3 (2 – 5) |
16 3.1 (2.0) 4(2 – 5) |
4 3.3 (2.1) 4 (2 – 5) |
6 2.7 (1.9) 3 (2 – 4) |
7 2.7 (2.0) 3 (2 – 4) |
1 4.0 (0) 4 (4 – 4) |
4 3.5 (2.4) 5 (3 – 5) |
7 4.3 (1.3) 5 (4 – 5) |
p≤ 0.05 within the described rhythm category
Incident call defined as in Table 1.
Abbreviations: IQR – interquartile range; mRS – modified Rankin scale; ROSC – return of spontaneous circulation; SD – standard deviation
Adjusted analyses
In multiple imputation adjusted analyses of the combined group of randomized study-drug treated patients with an initial non-shockable-turned shockable OHCA, the absolute difference in survival to hospital discharge when treated with amiodarone versus placebo was 2.3% (95% confidence interval (CI), −0.3% to 4.8%), p=0.08, and for lidocaine versus placebo was 1.2% (95% CI, −1.1% to 3.6%), p=0.30. Similar relationships were observed for the secondary endpoint of survival with MRS ≤ 3 in each of the case models and rhythm groups (Table 4). Differences between amiodarone and lidocaine in any of these outcomes were not statistically significant (data not shown). Notably, the relationships between treatment arm and outcome were not different according to the initial rhythm (asystole or PEA) (p=0.87 test for interaction). In addition, no interaction was found between treatment arm and whether the initial OHCA rhythm was shockable (VF/VT)8 or became shockable (non-shockable-turned-shockable) during the course of resuscitation on outcome (p=0.84 test for interaction). That is, from a survival perspective, whether the initial rhythm was asystole, PEA, or VF/VT did not significantly alter the response to antiarrhythmic treatment. While not statistically different, survival trends all favored use of either antiarrhythmic agent (Figure 2).
Table 4.
Adjusted outcomes
| Analysis | All Non-Shockable Initial Rhythms | Pulseless Electrical Activity (PEA) | Asystole | ||||
|---|---|---|---|---|---|---|---|
| Overall Sample Size | Amiodarone vs Placebo (95% CI) P |
Lidocaine vs Placebo (95% CI) P |
Amiodarone vs Placebo (95% CI) P |
Lidocaine vs Placebo (95% CI) P |
Amiodarone vs Placebo (95% CI) P |
Lidocaine vs Placebo (95% CI) P |
|
| Survival to Hospital Discharge (Absolute Differences) | |||||||
| Unadjusted Available-Case Analysis | 1061* | 2.2% (−0.3%, 4.7%) 0.08 |
1.2% (−1.2%, 3.5%) 0.33 |
1.6% (−3.2%, 6.5%) 0.51 |
0.9% (−3.8%, 5.6%) 0.71 |
2.7% (0.1%, 5.3%) 0.04 |
1.5% (−0.8%, 3.8%) 0.20 |
| Unadjusted Complete-Case Analysis | 1032† | 2.3% (−0.2%, 4.9%) 0.08 |
0.9% (−1.4%, 3.3%) 0.44 |
1.7% (−3.3%, 6.7%) 0.51 |
0.9% (−3.9%, 5.7%) 0.71 |
2.8% (0.1%, 5.6%) 0.04 |
1.0% (−1.1%, 3.2%) 0.35 |
| Adjusted Analysis Complete-Case Analysis |
1032† | 2.5% (−0.2%, 5.1%) 0.07 |
1.1% (−1.3%, 3.4%) 0.38 |
1.6% (−3.6%, 6.8%) 0.55 |
0.7% (−4.0%, 5.4%) 0.77 |
2.6% (−0.1%, 5.3%) 0.06 |
1.1% (−1.0%, 3.2%) 0.31 |
| Multiple Imputation Adjusted Analysis | 1063 | 2.3% (−0.3%, 4.8%) 0.08 |
1.2% (−1.1%, 3.6%) 0.30 |
1.5% (−3.6%, 6.5%) 0.57 |
0.6% (−3.9%, 5.2%) 0.79 |
2.3% (−0.3%, 4.9%) 0.08 |
1.5% (−0.8%, 3.8%) 0.20 |
| Survival to Hospital Discharge with MRS ≤ 3 (Absolute Differences) | |||||||
| Unadjusted Available-Case Analysis | 1061‡ | 1.1% (−0.7%, 2.9%) 0.22 |
0.7% (−1.0%, 2.4%) 0.41 |
1.2% (−2.4%, 4.8%) 0.52 |
1.2% (−2.5%, 4.8%) 0.53 |
0.9% (−0.4%, 2.2%) 0.16 |
0.5% (−0.5%, 1.5%) 0.32 |
| Unadjusted Complete-Case Analysis | 1032§ | 1.2% (−0.7%, 3.0%) 0.21 |
0.8% (−1.0%, 2.5%) 0.40 |
1.2% (−2.5%, 4.9%) 0.52 |
1.2% (−2.5%, 4.8%) 0.53 |
1.0% (−0.4%, 2.3%) 0.16 |
0.5% (−0.5%, 1.6%) 0.32 |
| Adjusted Analysis Complete-Case Analysis |
1032§ | 1.3% (−0.6%, 3.2%) 0.19 |
0.8% (−1.0%, 2.7%) 0.36 |
0.5% (−3.6%, 4.6%) 0.80 |
0.9% (−2.7%, 4.5%) 0.62 |
1.0% (−0.3%, 2.3%) 0.13 |
0.6% (−0.5%, 1.6%) 0.28 |
| Multiple Imputation Adjusted Analysis | 1063 | 1.2% (−0.6%, 3.0%) 0.20 |
0.8% (−0.9%, 2.5%) 0.37 |
0.5% (−3.5%, 4.4%) 0.81 |
0.9% (−2.6%, 4.4%) 0.62 |
0.9% (−0.3%, 2.1%) 0.13 |
0.5% (−0.5%, 1.5%) 0.29 |
2 patients were excluded due to missing survival status
31 patients were excluded due to missing survival status or missing covariates
2 patients were excluded due to missing MRS
31 patients were excluded due to missing values for MRS, covariates or both
Abbreviations: MRS – modified Rankin scale
Figure 2.
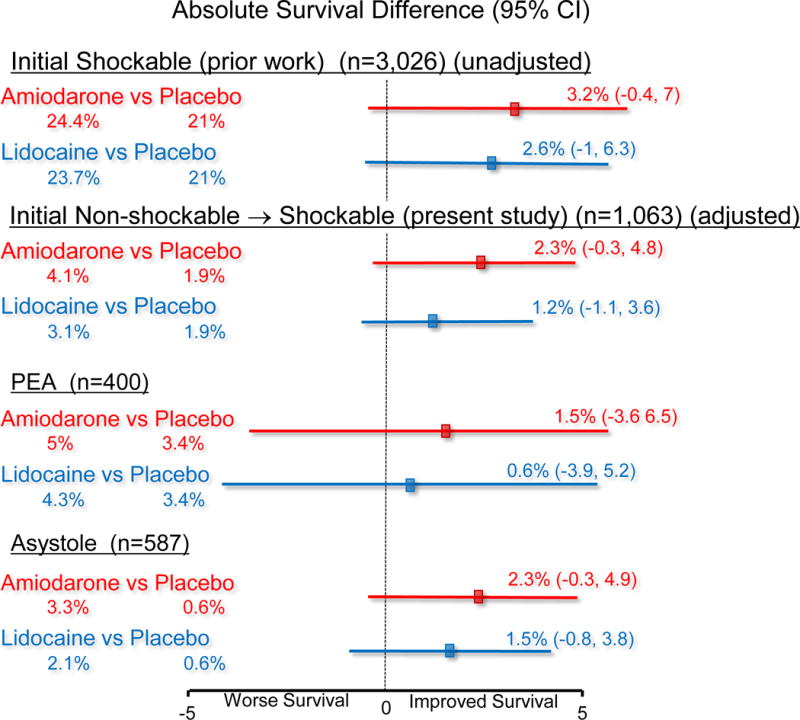
Depiction of absolute differences in survival in the previously published group of patients with cardiac arrest due to initial VF/VT (unadjusted)8 and the present study of patients with non-shockable-turned shockable cardiac arrest (adjusted using multiple imputation analyses). Survival was adjusted for baseline differences in the non-shockable-turned-shockable group, whereas these were balanced and not adjusted in the initial shockable group. PEA = pulseless electrical activity.
Mechanistic outcomes
After randomization, placebo recipients were more likely to require an additional blinded dose of study drug and a greater number of shocks than active drug (amiodarone or lidocaine) treatment arms (p<0.05) (Table 2). As an ancillary antiarrhythmic drug, magnesium was more commonly administered to patients in the placebo than active drug treatment arms in patients with initial asystole and in the combined non-shockable rhythm group (p<0.05). The use of vasopressin was infrequent and along with other resuscitation medications (bicarbonate, atropine, procainamide and beta blockers) did not differ significantly between treatment arms in any of the rhythm groups. Epinephrine was administered to virtually all study patients, who received a mean (± SD) cumulative dose of 5.6 ± 2.6 mg that was similar across treatment arms (Table 2). In the combined group of non-shockable-turned-shockable OHCA, the likelihood of obtaining any ROSC (either transient or sustained) was lower among amiodarone recipients than in patients receiving lidocaine or placebo (31.5% versus 40.5% and 37.3% of patients respectively, p=0.05). No significant differences were seen in the time interval from the incident call to initial (first) ROSC between the treatment groups (Table 3). Similarly, the interval from the incident call to termination of resuscitation efforts among patients who were not transported to hospital averaged (± SD) 45 ± 10.7 minutes, and did not differ significantly between treatment groups or by the presenting non-shockable rhythm. Unadjusted survival to hospital admission was significantly lower in amiodarone than in lidocaine and placebo recipients across each of the initial non-shockable rhythm groups. In the combined group of non-shockable-turned-shockable OHCA, 64 (16.5%) amiodarone recipients versus 74 (20.7%) lidocaine and 65 (20.6%) placebo recipients were admitted alive to hospital (p<0.05) (Table 3).
Adverse drug-related events
Adverse drug-related events, either overall or considered categorically (including thrombophlebitis, anaphylaxis, clinical seizures and need for temporary cardiac pacing within the first 24 hours of treatment) did not differ significantly in frequency between treatment arms in the initial non-shockable-turned-shockable rhythm group (Table 2).
Discussion
In this prospective, randomized trial, we found outcome after non-shockable-turned-shockable OHCA was poor, but not invariably fatal or neurologically devastating. Of the 33 survivors (3.1%), more than half had a favorable functional recovery at hospital discharge (MRS ≤3). Although the differences did not achieve statistical significance, patients treated with amiodarone or lidocaine experienced up to a doubling of survival over placebo, without greater risk of adverse effects or functional disability. These findings were consistent with the trends toward better survival observed after active-drug treatment of patients who presented with an initial OHCA rhythm of VF/VT (Figure 2).8 Taken together these findings may signal a therapeutic benefit from amiodarone and lidocaine when shock-refractory VF/VT arises at any time or from any OHCA rhythm along the course of resuscitation.
Previous studies
Compared to an initial rhythm of VF/VT, non-shockable OHCA due to asystole or PEA carries an ominous prognosis for which no treatment apart from high quality CPR has yet proven to be effective.18, 19, 20, 21 The comorbidities associated with non-shockable cardiac arrest, the lower treatment-responsiveness of the rhythms themselves, as well as the circumstances under which the OHCA occurs may all contribute to this poor outcome. For example, compared to patients presenting with an initial VF/VT rhythm in the main study,8 patients in the current study were older, less likely to present with OHCA in a public setting, to be bystander-witnessed or receive bystander CPR, and had a longer interval from the incident call to EMS arrival, all factors associated with poor survival.22, 23
Overall survival in this study fell in the same range reported by others when non-shockable OHCA evolves to VF/VT.7, 24, 25 Previous studies have variably observed prognosis to be improved, worsened or indifferent to an arrhythmia’s evolution from non-shockable to shockable, depending on the clinical presentation. For example, survival was found to be better after conversion of a non-shockable rhythm to VF/VT when the antecedent rhythm was asystole rather than PEA,26 or if shock was administered sooner upon its occurrence,27 or in context of a history of cardiovascular disease.7 Others found no apparent association between outcome and patient or resuscitation characteristics,28 or suggested that worse outcomes might result from providers placing a greater emphasis on rhythm analysis and shock than on the greater need for uninterrupted CPR in such circumstances.14 Since the use of antiarrhythmic medications was not reported in these studies, whether and how their administration might have contributed to these disparate findings and shaped the outcome of the patients is not known. This issue has been largely unexplored until now.
Two previous randomized clinical trials of OHCA due to shock-refractory VF/VT also included patients in whom antiarrhythmic drugs were administered after conversion of initial asystole or PEA arrest to VF/VT. Unlike our findings, each reported numerically higher rates of hospital admission with amiodarone than with placebo (17% and 12%, respectively among 83 patients with non-shockable-turned-shockable OHCA),13 and with amiodarone than lidocaine (12% and 4%, respectively among 61 patients),29 but had no ultimate survivors in any of the treatment arms. By comparison, the lower rates of any ROSC and of survival to hospital admission with amiodarone than with lidocaine or placebo observed in our study may signal a real effect whereby amiodarone adversely affects return of circulation. Alternatively, the unadjusted relationships may be attributable to other confounding factors or to chance, given the number of comparisons performed. Importantly, while the prospect of harm was not entirely excluded, patients who received amiodarone in this study experienced no obvious worsening of survival to discharge, highlighting the potential challenge of drawing clinical inference from intermediate or surrogate outcomes in resuscitation research.
Because treatment of late-occurring arrhythmias is itself invariably administered late, the resulting delay in restoring circulation may be another factor contributing to poor outcomes in this population.30 In a previous report, survival only improved upon conversion of asystole or PEA to VF/VT when the interval from the incident call to shock was 20 minutes or less.27 In the current study, the time from the incident call to the first shock in the non-shockable-turned-shockable group averaged nearly 21 minutes and nearly 27 minutes transpired before their receipt of study drug. This long interval and its consequence on the patient’s physiology may have diluted a benefit from active drugs that might have occurred with earlier treatment.
Adverse events
Due to their conduction slowing and other rhythm-suppressive properties, it is possible that the administration of antiarrhythmic drugs for VF/VT particularly in patients whose preceding rhythm was asystole or PEA could theoretically lead to greater harm, including a recrudescence or worsening of bradyarrhythmias. Such adverse effects were not appreciated in this study since a significantly greater need for temporary pacing was not seen in the aftermath of drug treatment. Nor were there significant differences in the incidence of other adverse drug-related events across the 3 treatment arms in this non-shockable-turned-shockable OHCA cohort (Table 2). A greater need for temporary cardiac pacing in amiodarone than in lidocaine or placebo recipients was reported previously among patients with OHCA due to initial VF/VT, though the increment was relatively small.8
Limitations
This trial evaluated the risks and benefits of amiodarone and lidocaine (vs placebo) in a high risk population in whom survival was expected to be poor regardless of treatment, and which the study was intended to explore but was not robustly powered to prove clinical effects. We did not observe a statistically significant survival difference between the treatment arms. However, the consistent trends toward improved survival could also be interpreted as potential signals of benefit from active-drug treatment. If amiodarone or lidocaine achieved a true absolute improvement in survival of 2% over placebo as seen in this study, a trial of approximately 3000 patients would be required to establish this benefit with 90% power.
The trial was also not powered to make direct comparisons between the effectiveness of amiodarone and lidocaine. Thus, while point estimates and statistical trends tended to favor a stronger effect from amiodarone than lidocaine on survival, these differences do not necessarily imply the superiority of one drug over the other. Similarly, the absence of significant differences in the incidence of adverse drug-related effects across treatment arms does not completely preclude this possibility, although our findings indicate their overall frequency was low.
Comorbid conditions that might have contributed to OHCA and to its outcome were not assessed in the study population and though a randomized design, we cannot confirm that treatment groups were balanced in all respects. In addition, hospital treatments, though monitored, were not controlled and might have influenced outcomes, though we did not observe differences in prognostic hospital treatments across the groups. The primary endpoint of the trial was survival to hospital discharge, which could be reliably ascertained in virtually all study patients. While arguably more meaningful, 30-day or 1 year survival can be more challenging to obtain, and being less complete potentially more subject to bias. These limitations should be balanced with the strengths of the study: the results were derived from a large population of patients with OHCA who were prospectively randomized in a double-blind trial design, systematically assessed and involved analyses that accounted for important confounders.
Implications
Shock-refractory VF/VT as a primary or secondary event continues to be a frequently encountered arrhythmia during resuscitation. If ineffective, the added cost and needless distraction created by antiarrhythmic drugs like amiodarone and lidocaine argues against their continued use in such patients. However, if effective, improving absolute survival by merely 2% in this patient population means more than 1000 additional lives might be saved each year in North America from non-shockable-turned-shockable OHCA alone, many of whom will be functionally independent or require minimal assistance with daily living.
Conclusions
Outcome from non-shockable-turned shockable OHCA is poor, but not invariably fatal. Though the differences were not statistically significant, point estimates for survival were higher among patients randomized to amiodarone or lidocaine than to placebo, without increased risk of adverse drug-related effects or functional disability. These results are consistent with previously reported trends toward better survival from antiarrhythmic drug treatment when OHCA initially presents as shock-refractory VF/VT. Taken together the findings may signal a clinical benefit from amiodarone or lidocaine when shock-refractory VF/VT arises at any time or from any OHCA rhythm along the course of resuscitation, and invites further investigation.
Clinical Perspective.
What is new?
Out-of-hospital cardiac arrest (OHCA) claims hundreds of thousands of lives each year.
Though historically OHCA commonly presented with ventricular fibrillation or pulseless ventricular tachycardia (VF/VT), it is now more often seen with non-shockable rhythms (asystole, pulseless electrical activity).
These rhythms can evolve to shock-refractory VF/VT during resuscitation in about a quarter of patients, for whom the effectiveness of antiarrhythmic drugs is unknown.
This trial prospectively randomized 1,063 such patients to amiodarone, lidocaine or placebo.
A statistically insignificant trend toward better survival was found in drug than placebo recipients, without increased risk of adverse events or neurological disability.
What are the clinical implications?
OHCA due to non-shockable rhythms is poor but not invariably fatal.
When non-shockable OHCA turns shockable, absolute differences in survival in response to lidocaine or amiodarone as compared with placebo are consistent with the favorable trends in response to these drugs seen among patients in whom OHCA is caused by initial VF/VT.
Taken together, these findings while not definitive, may signal a clinical benefit from antiarrhythmic medications when shock-refractory VF arises at any time and from any OHCA rhythm during the course of resuscitation.
The role of antiarrhythmic drugs in shock-refractory OHCA invites further investigation.
Acknowledgments
Funding Acknowledgement
The Resuscitation Outcomes Consortium was supported by a series of cooperative agreements to nine regional clinical centers (spanning 10 North American communities) and one Data Coordinating Center (5U01 HL077863-University of Washington Data Coordinating Center, HL077866-Medical College of Wisconsin, HL077867-University of Washington, HL077871-University of Pittsburgh, HL077872-St. Michael’s Hospital, HL077873-Oregon Health and Science University, HL077881-University of Alabama at Birmingham, HL077885-Ottawa Hospital Research Institute, HL077887-University of Texas SW Medical Center/Dallas, HL077908-University of California San Diego) from the National Heart, Lung and Blood Institute in partnership with the U.S. Army Medical Research & Material Command, The Canadian Institutes of Health Research (CIHR) - Institute of Circulatory and Respiratory Health, Defence Research and Development Canada, the Heart, Stroke Foundation of Canada and the American Heart Association. Trial drugs and testing of their stability were provided by Baxter Healthcare without cost, who otherwise had no role in the trial. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung and Blood Institute, the National Institutes of Health or other funding organizations.
Footnotes
Disclosures
No conflicts of interest relevant to the topic of discussion by any of the authors.
Clinical Trial Registration: ClinicalTrials.gov number, NCT01401647
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozafarrian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritches M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Hu JHY, Alter HM, Wong SS, Muntner P. Heart disease and stroke statistics – 2017 update: A report from the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berdowski J, Berg RA, Tijssen JG, Koster RW. Global incidences of out-of-hospital cardiac arrest and survival rates. Resuscitation. 2010;81:1479–1487. doi: 10.1016/j.resuscitation.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Cobb LA, Fahrenbruch CE, Olsufka M, Copass MK. Changing incidence of out-of-hospital ventricular fibrillation, 1980–2000. JAMA. 2002;288:3008–3013. doi: 10.1001/jama.288.23.3008. [DOI] [PubMed] [Google Scholar]
- 4.Keller SP, Halperin HR. Cardiac arrest: the changing incidence of ventricular fibrillation. Curr Treat Options Cardiovasc Med. 2015;392 doi: 10.1007/s11936-0150-0392-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herlitz J, Svensson L, Engdahl J, Silfverstolpe J. Characteristics and outcome I out of hospital cardiac arrest when patients are found in a non-shockable rhythm. Resuscitation. 2008;76:31–36. doi: 10.1016/j.resuscitation.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 6.Hallstrom A, Herlitz J, Kajino K, Olasveengen TM. Treatment of asystole and PEA. Resuscitation. 2009;80:975–976. doi: 10.1016/j.resuscitation.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 7.Rajan S, Folke F, Hansen SM, Hansen CM, Kragholm K, Gerds TA, Lippert FK, Karlsson L, Moller S, Kober L, Gislason GH, Torp-Pedersen C, Wissenberg M. Incidence and survival outcome according to heart rhythm during resuscitation attempt in out-of-hospital cardiac arrest patients with presumed cardiac etiology. Resuscitation. 2017;114:157–163. doi: 10.1016/j.resuscitation.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 8.Kudenchuk PJ, Brown SP, Daya M, Rea T, Nichol G, Morrison LJ, Leroux B, Vaillancourt C, Wittwer L, Callaway CW, Christenson J, Egan D, Ornato JP, Weisfeldt ML, Stiell IG, Idris AH, Aufderheide TP, Dunford JV, Colella MR, VIlke GM, Brienza AM, Desvigne-Nickens P, Gray PC, Gray R, Seals N, Straight R, Dorian P. Amiodarone, lidocaine or placebo in out-of-hospital cardiac arrest. N Engl J Med. 2016;374:1711–1722. doi: 10.1056/NEJMoa1514204. [DOI] [PubMed] [Google Scholar]
- 9.Kudenchuk PJ, Brown SP, Daya M, Morrison LJ, Grunau BE, Rea TD, Aufderheidi T, Powell J, Leroux B, Vaillancourt C, Larsen J, Wittwer L, Colella RM, Stephens SW, Gamber M, Egan D, Dorian P. Resuscitation Outcomes Consortium amiodarone lidocaine or placebo study (ROC-ALPS): Rationale and methodology behind an out-of-hospital cardiac arrest antiarryhthmic drug trial. Am Heart J. 2014;167:653–659. doi: 10.1016/j.ahj.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis DP, Garberson LA, Andrusiek DL, Hostler D, Daya M, Pirrallo R, Craig A, Stephens S, Larsen J, Drum AF, Fowler R. A descriptive analysis of Emergency Medical Service systems participating in the Resuscitation Outcomes Consortium (ROC) network. Prehosp Emerg Care. 2007;11:369–382. doi: 10.1080/10903120701537147. [DOI] [PubMed] [Google Scholar]
- 11.Neumar RW, Otto CW, Link MS, Kronick SL, Shuster M, Callaway CW, Kudenchuk PJ, Ornato JP, McNally B, Silvers SM, Passman RS, White RD, Hess EP, Tang W, Davis D, Sinze E, Morrison LJ. Adult advanced cardiovascular life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(Suppl 3):S729–S767. doi: 10.1161/CIRCULATIONAHA.110.970988. [DOI] [PubMed] [Google Scholar]
- 12.Reynolds JC, Frisch A, Rittenberger JC, Callaway CW. Duration of resuscitation efforts and functional outcome after out-of-hospital cardiac arrest: when should we change to novel therapies? Circulation. 2013;128:2488–2494. doi: 10.1161/CIRCULATIONAHA.113.002408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kudenchuk PJ, Cobb LA, Copass MK, Cummins RO, Doherty AM, Fahrenbruch CE, Hallstrom AP, Murray WA, Olsufka M, Walsh T. Amiodarone for resuscitation after out of hospital cardiac arrest due to ventricular fibrillation. New Engl J Med. 1999;341:871–878. doi: 10.1056/NEJM199909163411203. [DOI] [PubMed] [Google Scholar]
- 14.Hallstrom A, Rea TD, Mosseso VN, Jr, Cobb LA, Anton AR, VanOttingham L, Sayre MR, Christenson J. The relationship between shocks and survival in out-of-hospital cardiac arrest patients initially found in PEA or asystole. Resuscitation. 2007;74:418–426. doi: 10.1016/j.resuscitation.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 15.van der Heijden Geert JMG, Donders ART, Stijnen T, Moons KGM. Imputation of missing values is superior to complete case analysis and the missing-indicator method in multivariable diagnostic research. J Clin Epidemiol. 2006;59:1102–1109. doi: 10.1016/j.jclinepi.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 16.Newgard CD, Haukoos JS. Advanced statistics: Missing data in clinical research part 2: multiple imputation. Acad Emerg Med. 2007;14:669–678. doi: 10.1197/j.aem.2006.11.038. [DOI] [PubMed] [Google Scholar]
- 17.Schafer J, Olsen M. Multiple imputation for multivariate missing data problems: A data analyst’s perspective. Multivar Behav Res. 1998;33:545–571. doi: 10.1207/s15327906mbr3304_5. [DOI] [PubMed] [Google Scholar]
- 18.Vayrynen T, Kuisma M, Maata T, Boyd J. Who survives from out of hospital pulseless electrical activity? Resuscitation. 2008;76:207–213. doi: 10.1016/j.resuscitation.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 19.Cummins RO, Graves JR, Larsen MP, Hallstrom AP, Hearne TR, Ciliberti J, Nicola RM, Horan S. Out of hospital transcutaneous pacing by emergency medical technicians in asystolic cardiac arrest. N Engl J Med. 1993;328:1377–1382. doi: 10.1056/NEJM199305133281903. [DOI] [PubMed] [Google Scholar]
- 20.Martin DR, Gavin T, Bianco J, Brown CG, Stueven H, Pepe PE, Cummins RO, Gonzalez, Jastremski M. Initial countershock in the treatment of asystole. Resuscitation. 1993;26:63–68. doi: 10.1016/0300-9572(93)90164-l. [DOI] [PubMed] [Google Scholar]
- 21.Kudenchuk PJ, Redshaw JF, Stubbs BA, Fahrenbruch CE, Dumas F, Phelps R, Blackwood J, Rea TD, Eisenberg MS. Impact of changes in resuscitation practice on survival and neurological outcome after out of hospital cardiac arrest resulting from nonshockable arrhythmias. Circulation. 2012;125:1787–1794. doi: 10.1161/CIRCULATIONAHA.111.064873. [DOI] [PubMed] [Google Scholar]
- 22.Sasson C, Rogers MAM, Dahl J, Kellermann AL. Predictors of survival from out of hospital cardiac arrest. Circ Cardiovasc Qual Outcomes. 2010;3:63–81. doi: 10.1161/CIRCOUTCOMES.109.889576. [DOI] [PubMed] [Google Scholar]
- 23.Herlitz J, Engdahl J, Svensson L, Angquist KA, Young M, Holmberg S. Factors associated with an increased chance of survival among patients suffering from an out of hospital cardiac arrest in a national perspective in Sweden. Am Heart J. 2005;149:61–66. doi: 10.1016/j.ahj.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 24.Wah W, Wai KL, Pek PP, Ho AF, Alsakaf O, Chia MY, Noor JM, Kajino K, DeSouza NN, Ong ME, PAROS Investigators Conversion to shockable rhythms during resuscitation and survival for out of hospital cardiac arrest. Am J Emerg Med. 2017;35:206–213. doi: 10.1016/j.ajem.2016.10.042. [DOI] [PubMed] [Google Scholar]
- 25.Hallstrom A, Herlitz J, Kajino K, Olasveengen TM. Treatment of asystole and PEA. Resuscitation. 2009;80:975–976. doi: 10.1016/j.resuscitation.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 26.Zheng R, Luo S, Liao J, Liu A, Xu J, Xhan H, Liao X, Xiong Y, Idris A. Conversion to shockable rhythms is associated with better outcomes in out of hospital cardiac arrest patients with initial asystole but not in those with pulseless electrical activity. Resuscitaiton. 2016;107:88–93. doi: 10.1016/j.resuscitation.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Goto Y, Maeda T, Nakatsu-Goto Y. Prognostic implications of conversion from nonshockable to shockable rhythms in out of hospital cardiac arrest. Crit Care. 2014(18):528. doi: 10.1186/s13054-014-0528-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomas AJ, Newgard CD, Fu R, Zive DM, Daya MR. Survival in out of hospital cardiac arrests with initial asystole or pulseless electrical activity and subsequent shockable rhythms. Resuscitation. 2013;84:1261–1266. doi: 10.1016/j.resuscitation.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dorian P, Cass D, Schwartz B, Cooper R, Gelaznikas R, Barr A. Amiodarone as compared with lidocaine for shock-resistant ventricular fibrillation. N Engl J Med. 2002;346:884–890. doi: 10.1056/NEJMoa013029. [DOI] [PubMed] [Google Scholar]
- 30.Hallstrom AP, Cobb LA, Swain M, Mensinger K. Predictors of hospital mortality after out of hospital cardiopulmonary resuscitation. Crit Care Med. 1985;13:927–929. doi: 10.1097/00003246-198511000-00019. [DOI] [PubMed] [Google Scholar]


