Abstract
Background
Men diagnosed with prostate cancer have increased risk for disease progression, cardiovascular events and impairments in quality of life. This pilot study evaluated the feasibility of a randomized walking group intervention to improve quality of life, circulating biomarkers, and morbidity among men with newly diagnosed prostate cancer.
Methods
Men were recruited at Örebro University Hospital, Sweden and randomized to an 11-week walking group intervention (n=21) or usual care (n=20). The intervention included weekly 1-hour walking group sessions and maintenance of 10,000 steps/day. Outcomes were changes in body composition, clinical factors, biomarkers of cardiovascular health, and quality of life between baseline and end of study. Analysis of covariance was used to compare outcomes in each group adjusted for baseline values.
Results
All 41 men randomized completed the 11-week trial. Men assigned to the intervention walked on average 10,644 steps/day, and 92% reported missing two or fewer sessions. Both groups experienced similar weight loss at 11-weeks. Men in the intervention had a significant adjusted mean change in high-density lipoprotein of 0.14 mmol/L (95% CI: 0.01-0.27; p=0.04), and suggestive adjusted mean changes in low-density lipoprotein of -0.22 mmol/L (95% CI: -0.47-0.03; p=0.08) and in systolic blood pressure of -8.5 mm Hg (95% CI: -21.2-4.2; p=0.18), compared to the usual care group.
Conclusions
A walking group intervention among men with recent diagnosis of prostate cancer is feasible and potentially effective in improving cardiovascular health. A larger randomized trial of longer duration is required to elucidate its potential for improvement in longer-term outcomes.
Keywords: exercise, intervention, quality of life, biomarkers, pilot study, prostate cancer
Introduction
Globally, over 11 million men are living with prostate cancer1. Although prostate cancer mortality is the third most common cause of cancer death among men in highly developed regions, the ratio between incidence and mortality is 6:1.2 Among those diagnosed with localized, well-differentiated tumors, most men die from other causes than prostate cancer, primarily from cardiovascular disease and other cancers.3, 4 Prostate cancer survivors frequently experience marked impairments in physical quality of life such as urinary and sexual function.5, 6 Moreover, symptoms such as sleep disruption, depression, fatigue, and anxiety are commonly reported,5-7 and recent data suggest that emotional stress faced by newly diagnosed men may even cause severe health consequences such as cardiovascular mortality and suicide.8, 9 Men with prostate cancer experience significant morbidity from the cancer itself as well as from treatment, and strategies for mitigating these adverse effects have been proposed, including physical activity.7, 10
Physical activity has emerged as an effective strategy for improving both physical and emotional quality of life among cancer survivors diagnosed with several forms of cancer including colon, breast, and prostate cancers.11-13 Observational cohort studies have linked higher levels of physical activity, including brisk walking, to decreased prostate cancer progression as well as to lower overall and prostate cancer-specific mortality.12, 14, 15 However, the implementation of effective strategies to create sustainable behavior change presents unique challenges and requires an evidence-based approach. Findings from a variety of physical activity intervention studies among prostate cancer patients have emerged, indicating benefits in physical fitness and key quality of life areas, including urinary incontinence,16, 17 fatigue,10, 18-20 mental health and depression.19, 21, 22 These studies often include a variety of high-cost, clinically supervised aerobic or resistance training programs that require considerable learning or adjustment efforts from the patients. It is unclear whether these behavior changes are sustainable long-term among prostate cancer patients. Walking represents a low-cost and accessible form of physical activity that may be sustainable throughout a patient's life. Only a handful of studies have as yet used walking as an intervention strategy for improving quality of life among prostate cancer patients, and no randomized study to date has investigated the association between group walking and changes in clinical parameters or biomarkers among prostate cancer patients.
The motivation for this study was to demonstrate feasibility and effectiveness for eventual scale-up to a larger randomized study to investigate long-term prostate cancer outcomes. Specifically, the objectives of this pilot study were to evaluate the feasibility of recruiting and randomizing men with newly diagnosed prostate cancer to a walking group intervention or usual care, adherence to the intervention and feasibility of collecting outcome assessments. Furthermore, we aimed to assess the 11-week effects of this physical activity on measures relevant to prostate cancer patients including clinical factors, such as body composition and blood pressure, physical and emotional quality of life, and cardiovascular biomarkers, such as cholesterol and C-reactive protein. Our overarching premises for this study are that a cancer diagnosis represents a teachable moment when men are amenable to lifestyle change,23 that walking represents a physical activity sustainable through life, and a group environment provides men an opportunity to discuss issues related to their cancer.
Materials and Methods
Study Population
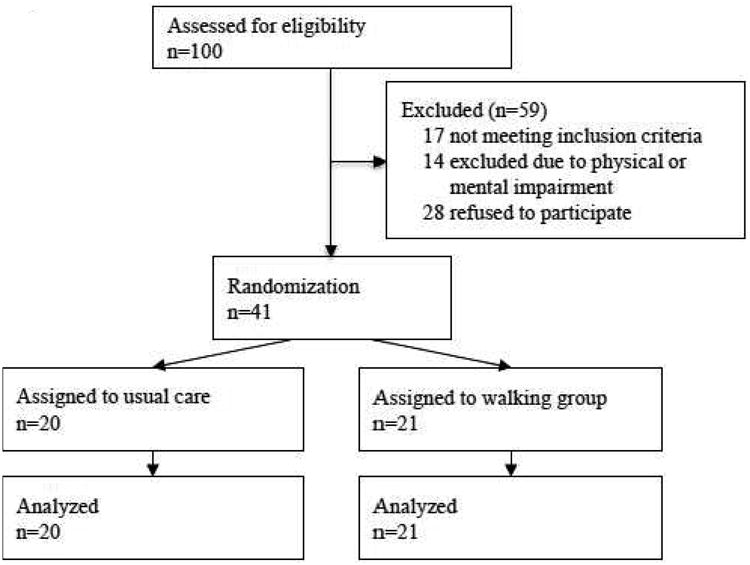
The study base included prostate cancer patients in the catchment area of Örebro University Hospital in Sweden. The hospital serves all patients in the Örebro Healthcare region and thus the study is population-based. Eligible men had a histological diagnosis of prostate cancer without evidence of distant metastases, had completed initial treatment at least one month prior to study enrollment, and had a life expectancy of at least 5 years. Additional eligibility criteria included the ability to speak Swedish and be mentally and physically able to complete the questionnaires and clinical exam and to participate in group walking sessions. The study team reviewed 100 medical records of patients diagnosed between January and December 2009 and contacted those who fulfilled the inclusion criteria (n=83). After contacting each patient to take part in the study, we excluded 14 men deemed unable to participate due to physical or mental impairment and 28 men who refused to participate. The primary reason for refusal to participate was that the men felt they no longer had a cancer after being treated. In total, 41 men were randomized to the study through a random number generator after completing the baseline questionnaire and clinical assessment in March 2010 (Figure 1). This study was approved by the ethics committee at Örebro University Hospital and received an exemption determination by the institutional review board at the Harvard T.H. Chan School of Public Health. Written informed consent was obtained from all participants. This trial was registered with identifier NCT01696539.
Figure 1. Diagram of subject flow through the study.
Questionnaire and Clinical Assessments
Prior to randomization, the men completed an 87-item self-report questionnaire at the Department of Urology assessing demographic information, smoking status, alcohol use, and current physical activity. Men also reported on stress (Perceived Stress Scale-4), sleep quality (Karolinska Sleep Questionnaire), anxiety and depression (Hospital Anxiety and Depression Scale), and urinary, bowel, and sexual function using selected questions from the National Prostate Cancer Registry of Sweden questionnaire.24-27 After completing the questionnaires, the men were seen by a research nurse who conducted a physical examination that included measurement of height (cm) and weight (kg), waist circumference (cm), and blood pressure (systolic, diastolic, mm Hg). A blood specimen was also collected and stored for biomarker studies described below.
At the end of the 11-week intervention, all participants returned to the Örebro University Hospital for group meetings with the urology team conducted separately for the usual care group and for the intervention group. Participants completed a 66-item self-report end-of-study questionnaire, with three additional questions completed only by the intervention arm regarding satisfaction with the walking groups. Participants also completed a final physical examination and provided a second blood specimen. All of the men returned for the follow-up visit and are included in the analysis.
Walking Intervention
In March 2010, all 41 men visited the Department of Urology at the Örebro University Hospital as a group. The urology team (SO Andersson & O Andrén) gave a 10-minute presentation to participants about the study objectives, as well as more general information on prostate cancer. Men had an opportunity to ask questions about both the study and their disease. Men assigned to usual care completed the baseline and follow-up assessments, but received no intervention other than their usual medical care. In the intervention arm, men were assigned to groups of six to eight men, based on the participants' availability for walking on specific times of the day. For the 11-week intervention, the men walked together weekly in their groups for one hour along with a research nurse who answered patients' questions about prostate cancer and led discussions. On other days, the men were instructed to wear pedometers to monitor the number of steps they took on a given day (Timex W-180 US 621 095000). In addition, they recorded their daily number of steps in a diary. The men were encouraged to maintain 10,000 steps per day. The rationale for this was to provide a concrete goal that was simple and easy to remember while encompassing both exercise and regular daily activity. Furthermore, it was expected that participants would need to increase their activity in order to achieve the goal, which has been shown to have health benefits beyond average daily activity.28, 29 During week two of the study, one group was further separated as two participants walked at a slower pace. One man who was visually impaired walked with an assistant in addition to the research nurse.
Outcomes
In both the intervention and usual care group, all outcome variables were measured at baseline and at the end of the 11-week study. Clinical outcomes included body mass index (BMI, kg/m2), calculated using height and weight, waist circumference, and blood pressure. Circulating biomarkers included C-reactive protein (CRP), C-peptide, high and low density lipoprotein (HDL, LDL), testosterone, and sex hormone-binding globulin (SHBG) adjusted for testosterone. These were measured in the Central laboratory at the University of Örebro (CRP, HDL, LDL, testosterone, SHBG) or in the Akademiska laboratory at Uppsala University Hospital (C-peptide).
Physical quality of life outcomes included self-reported problems with bowel, urinary, sexual, and overall function (scale 0 to 10). The outcomes of urinary and bowel function were assessed by asking participants to answer the question, ‘How much do urinary/bowel problems limit your daily activity?’ (with response options ranging from ‘None’ to ‘A lot’). Sexual and overall function were assessed by asking the questions, ‘Do you have problems with your sex life?’ and, ‘How much does your prostate cancer or its treatment limit your daily activity?’, respectively (with response options ranging from ‘None’ to ‘A lot’). Anxiety and depression were assessed using the 14-item Hospital Anxiety and Depression Scale (HADS). Each of the HADS depression and anxiety subscales consists of 7 questions scored 0 to 3. A score ranging from 0 to 21 was obtained for each of the HADS depression and anxiety subscales by summing the scores for each question. Subjects were categorized as anxious/depressive or non-anxious/non-depressive using a cutoff score of 8 for each subscale.25 Psychological stress was assessed using the 4-item Perceived Stress Scale (PSS-4) consisting of 4 questions scored 0 to 4. A score ranging from 0 to 16 was obtained by summing the scores for each question.
Sleep outcomes included the average number of hours slept per night, regular naps (yes/no), and sleep quality dichotomized as high or low. Sleep quality was assessed by asking participants to answer the question ‘How do you think you sleep overall?’ and was categorized as high if participants reported either ‘pretty good’ or ‘very good’ and low if participants reported ‘neither good nor bad,’ ‘pretty bad,’ or ‘very bad.’ Social support from the participant's partner or from others was assessed by asking participants to rate on a scale of 1 to 7 their response to the questions, ‘Can you discuss your emotional problems or worries with your wife/life-partner?’ and ‘Can you discuss your emotional problems or worries with someone other than your wife/life-partner?’ Social support from the participant's partner or from others was categorized into low (1-2), medium (3-5), or high (6-7). A subject was defined as having emotional isolation if he reported discussing none or almost none of his emotional problems with his wife, partner, or others.
Statistical Analysis
Analyses to compare baseline characteristics between groups included descriptive statistics. To evaluate the effect of the intervention, group differences in change over the 11-week study period for each continuous outcome were determined using analysis of covariance adjusted for baseline values. Due to the small number of men exceeding the cutoff for anxiety or depression at baseline, these outcomes were analyzed using a continuous score. We assessed interactions between group assignment and baseline values by entering the cross product of these variables in each model. Wilcoxon rank sum tests were applied to categorical outcomes. Subjects with missing values or implausible values were excluded from the main analyses. In analyses with CRP as the outcome, participants with levels of CRP greater than 10 mg/L at baseline or at the end of follow-up were excluded.
We conducted further analyses to evaluate effects of potential confounding by variables that were imbalanced between groups at baseline. Variables considered to be potential confounders were added to each model to determine change in parameter estimates and statistical significance. Due to the small sample size, we evaluated the effect of adjustment for each potential confounder separately. To account for potential weight loss due to advanced disease, we also examined change in body composition excluding subjects with stage T3 prostate cancer. All tests were two-sided and an α-level of 0.05 was applied to evaluate statistical significance. Data analysis was performed using SAS version 9.4.
Results
Patient Characteristics
As shown in Figure 1, all 20 (100%) men in the usual care group and all 21 (100%) men in the walking intervention group completed the 11-week study. Baseline characteristics of the study participants according to randomized group assignment are presented in Table 1. The mean age of the men was 69 years, 98% were retired, and 83% lived with a spouse or partner. Men in both groups were similar in terms of education level, smoking history, pre-study exercise habits, and Charlson co-morbidity score. Although men in the intervention group were somewhat less likely to have anxiety (5% vs. 30%) at baseline, none of the men in either group exceeded the cutoff for depression. Men in the intervention group were somewhat more likely to live in an urban area (67% vs. 45%), to have ever used snus (33% vs. 15%), and had a lower median daily alcohol intake (9 vs. 16.6 cL/day). Fewer men in the intervention group than in the usual care group were diagnosed with advanced (T3) tumors (10% vs. 30%). Although the frequencies of poorly differentiated (Gleason score 8-10) tumors were similar, more men in the intervention group had Gleason 5-6 tumors compared to the usual care group (57% vs. 45%, respectively). Men in the walking group were somewhat more likely to receive active surveillance (38% vs. 25%) and somewhat less likely to receive radiation (10% vs. 25%) compared to the usual care group. The proportions of men with primary treatment of hormonal therapy and surgery were similar between groups.
Table 1. Baseline characteristics of prostate cancer patients (N=41) randomized to usual care versus the walking group intervention.
| Characteristic | Usual Care n=20 | Walking Group n=21 | ||
|---|---|---|---|---|
|
|
|
|||
| Median | Range | Median | Range | |
| Age, years | 69.0 | 54.5-81.6 | 67.1 | 61.7-81.7 |
| Charlson co-morbidity score | 2 | 2-4 | 2 | 2-6 |
| Alcohol intake, cl/day | 16.6 | 0-50.5 | 9.0 | 0-139 |
| PSA level, ng/mL | 11.0 | 3.2-1295 | 12.0 | 3.3-150 |
| N | % | N | % | |
|
|
||||
| Urban residence | 9 | 45 | 14 | 67 |
| University education | 4 | 20 | 4 | 19 |
| Retired or not working | 19 | 95 | 21 | 100 |
| Co-habitation | 17 | 85 | 17 | 81 |
| Emotional isolation | 1 | 5 | 4 | 19 |
| Anxiety (subscale score ≥8) | 6 | 30 | 1 | 5 |
| Ever smokers | 10 | 50 | 10 | 48 |
| Ever snus use | 3 | 15 | 7 | 33 |
| Brisk walking pace (7 km/hr) | 2 | 10 | 3 | 14 |
| Vigorous activity (≥2 hrs/week) | 14 | 70 | 14 | 67 |
| Stage at diagnosis | ||||
| T1c/T2 | 14 | 70 | 19 | 90 |
| T3 | 6 | 30 | 2 | 10 |
| Gleason Grade | ||||
| 5-6 | 9 | 45 | 12 | 57 |
| 7 | 8 | 40 | 6 | 29 |
| 8-10 | 3 | 15 | 3 | 14 |
| Primary treatment | ||||
| Active surveillance | 5 | 25 | 8 | 38 |
| Surgery | 5 | 25 | 6 | 29 |
| Hormonal therapy | 5 | 25 | 5 | 24 |
| Radiation | 5 | 25 | 2 | 10 |
PSA: prostate-specific antigen
Feasibility of Intervention
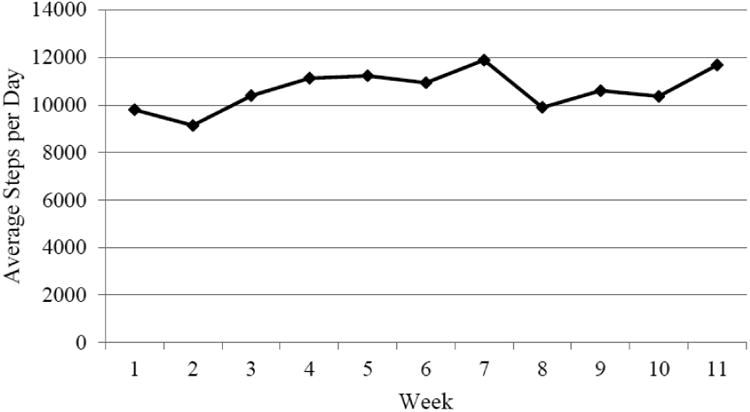
As shown in Figure 2, men assigned to the intervention group were able to adhere to the intervention and walked an average of 10,644 steps per day throughout the 11-week intervention period. Of the 17 men (81%) who provided diary records of their daily steps, 13 men walked within 1,000 steps of the study goal of 10,000 steps per day on average over the 11 weeks. 4 men (19%) walked an average of 5,301 steps per day, and 4 men (19%) did not provide diaries. Information from participants in the post-study questionnaire indicated that they found the intervention rewarding. Of the 13 men who responded to the question regarding their extent of participation in the organized walks, 12 men (93%) reported that they had missed no more than one or two sessions, for reasons such as inconvenient scheduling or lack of time, physical inability, going on vacation, or participating in another type of exercise. No men reported lack of motivation as a reason for absence from the organized walking groups. In an end-of-study discussion group between the research team and study participants, the men randomized to the walking intervention reported that supporting others in their walking group was a motivating factor. Even men who had previously engaged in regular physical activity stated that the walking group helped motivate them to achieve their individual goal of 10,000 steps per day. It is noteworthy that at least one of the walking groups continued to walk together for at least six months after this pilot study ended (personal communication, SO Andersson & O Andrén).
Figure 2. Average number of daily steps among men randomized to the walking intervention during the 11-week study.
Body Composition and Clinical Characteristics
No significant differences between the intervention and usual care groups were observed for change in BMI, waist circumference, or blood pressure over the 11-week study period (Table 2). Subjects in both groups experienced similar weight loss over the study period, with a decrease in waist circumference of 3% and 2% over follow-up in the intervention and usual care groups, respectively. A non-significant decrease in systolic blood pressure was observed in the intervention arm compared to usual care, with men in the walking group having on average an 8.51 (95% CI= -21.23, 4.21) mm Hg decrease over follow-up. Diastolic blood pressure decreased similarly in both groups.
Table 2. Body composition, clinical factors and circulating biomarkers absolute values and change over 11 weeks.
| Baseline Mean | Follow-up Mean | Percent Change (%) | Adjusted Group Difference in Mean Change Over Follow-upa | Interaction | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
||||||
| Usual Care | Walking Group | Usual Care | Walking Group | Usual Care | Walking Group | Mean | 95% CI | P | Pb | |
|
|
|
|
|
|
||||||
| BMI, kg/m2 | 28.0 | 28.1 | 27.7 | 27.7 | -1.1 | -1.4 | -0.08 | (-0.53, 0.36) | 0.70 | 0.32 |
| Waist circumference, cm | 102.0 | 103.8 | 99.9 | 100.6 | -2.0 | -3.0 | -0.87 | (-3.82, 2.08) | 0.55 | 0.38 |
| SBP, mm Hg | 162 | 170 | 163 | 157 | 0.7 | -7.6 | -8.51 | (-21.23, 4.21) | 0.18 | 0.37 |
| DBP, mm Hg | 89 | 93 | 88 | 90 | -1.9 | -3.6 | -0.02 | (-6.32, 6.28) | 0.99 | 0.21 |
| C Reactive Protein, mg/L | 0.9 | 1.1 | 1.5 | 0.7 | 40.5 | -61.0 | -0.97 | (-2.27, 0.33) | 0.14 | 0.07 |
| HDL, mmol/L | 1.4 | 1.5 | 1.4 | 1.6 | -0.3 | 8.6 | 0.14 | (0.01, 0.27) | 0.04 | 0.56 |
| LDL, mmol/L | 3.0 | 2.6 | 2.8 | 2.3 | -5.3 | -10.4 | -0.22 | (-0.47, 0.03) | 0.08 | 0.02 |
| C-peptide, ng/mL | 1.5 | 1.6 | 1.9 | 2.1 | 25.5 | 27.8 | 0.09 | (-0.44, 0.62) | 0.74 | 0.36 |
| Testosterone, nmol/L | 10.8 | 11.5 | 10.7 | 11.6 | -0.7 | 0.6 | 0.21 | (-2.02, 2.44) | 0.85 | 0.46 |
| SHBG, nmol/L, adjusted for testosterone | 27. 5 | 28.3 | 24.5 | 25.6 | -10.7 | -9.4 | 0.59 | (-4.18, 5.36) | 0.80 | 0.32 |
BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; HDL: high-density lipoprotein; LDL: low-density lipoprotein; SHBG: sex hormone-binding globulin; CI: confidence interval.
Between group change based on analysis of covariance adjusted for baseline values.
P-value for interaction between intervention group and baseline value on follow-up value.
Circulating Biomarkers
The adjusted mean change in plasma HDL over the 11-week study period was significantly different between groups, with the intervention group having an increase of 0.14 (95% CI = 0.01, 0.27) mmol/L compared to the usual care group. There was a non-significant improvement in plasma LDL in the intervention group, with an adjusted mean group difference of -0.22 (95% CI = -0.47, 0.03) mmol/L. Additionally, we observed a significant interaction between baseline LDL and group assignment on LDL at follow-up (p=0.02), indicating a greater effect of the intervention on LDL among men with lower levels of LDL at baseline. There was a non-significant adjusted mean change in CRP of -0.97 (95% CI = -2.27, 0.33) ng/mL in the intervention group compared to usual care, with the intervention group experiencing a 61% decrease in CRP over follow-up. No significant differences were observed between the intervention and usual care groups for change in C-peptide, testosterone, or SHBG over the 11-week study period (Table 2).
Quality of Life
Results on the effect of the intervention on physical quality of life, anxiety and depression, psychological stress, sleep, and social support are presented in Table 3. Baseline physical quality of life outcome measures were significantly different between groups, with men in the intervention group reporting lower bowel, urinary, and overall problems compared to the usual care group. There were no statistically significant changes in physical and emotional quality of life over follow-up between the groups (Table 3). Interestingly, men in the intervention group reported a non-statistically significant increase in sexual problems over the study period compared to the usual care group (p=0.06). There was no significant difference between groups in regular napping or hours of sleep per night over the 11-week study period. However, we observed a suggestive improvement in sleep quality in the intervention group, with a 50% decrease in men reporting poor sleep quality in the intervention and no change in the usual care group (p=0.05). Although there was no change in social support observed in the usual care group, 20% of men in the intervention group reported an increase in social support from individuals other than their spouse or partner over the 11-week study period.
Table 3. Physical and emotional quality of life absolute values and change over 11 weeks.
| Baseline Mean | Follow-up Mean | Percent Change | Adjusted Group Difference in Mean Change Over Follow-upa | Interaction Model | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
||||||
| Usual Care | Walking Group | Usual Care | Walking Group | Usual Care | Walking Group | Mean | 95% CI | P | Pb | |
|
|
|
|
|
|
||||||
| Bowelc | 0.8 | 0.1 | 1.1 | 0.3 | 33.3% | 150.0% | -0.38 | (-1.61, 0.85) | 0.53a | 0.45 |
| Urinaryc | 2.0 | 0.5 | 1.9 | 0.4 | -2.8% | -25.0% | -0.78 | (-1.94, 0.37) | 0.18a | 0.67 |
| Sexualc | 5.1 | 4.4 | 3.7 | 5.6 | -28.6% | 25.8% | 2.36 | (-0.07, 4.79) | 0.06a | 0.93 |
| Overallc | 2.1 | 0.4 | 2.1 | 0.6 | -2.6% | 66.6% | -0.82 | (-2.55, 0.91) | 0.34a | 0.60 |
| Anxietyc | 3.6 | 3.5 | 4.0 | 3.7 | 12.5% | 5.0% | -0.27 | (-1.41, 0.87) | 0.63a | 0.93 |
| Depressionc | 3.0 | 3.2 | 3.3 | 3.0 | 9.3% | -5.5% | -0.43 | (-1.55, 0.69) | 0.44a | 0.21 |
| Psychological stressc | 2.6 | 2.5 | 3.6 | 3.1 | 38.3% | 22.4% | -0.48 | (-1.70, 0.73) | 0.42a | 0.36 |
| Hours of sleep | 6.9 | 7.1 | 6.8 | 7.1 | -0.9% | -0.9% | 0.06 | (-0.46, 0.58) | 0.82a | 0.14 |
| Napping | 44.4% | 43.8% | 44.4% | 31.3% | 0% | -22.2% | 0.28d | |||
| Quality of sleep (score ≥ 3)c | 22.2% | 40.0% | 22.2% | 20.0% | 0% | -50% | 0.05d | |||
| Social support from partner | 0.97d | |||||||||
| Low | 6.7% | 21.4% | 6.7% | 21.4% | 0% | 0% | ||||
| Moderate | 20.0% | 14.3% | 20.0% | 21.4% | 0% | 7.1% | ||||
| High | 73.3% | 64.3% | 73.3% | 57.1% | 0% | -7.2% | ||||
| Social support from others | 0.26d | |||||||||
| Low | 70.6% | 93.3% | 70.6% | 73.3% | 0% | -20% | ||||
| Moderate | 17.7% | 6.7% | 17.7% | 13.3% | 0% | 6.7% | ||||
| High | 11.8% | 0% | 11.8% | 13.3% | 0% | 13.3% | ||||
CI: confidence interval;
Based on analysis of covariance adjusted for baseline value;
P-value for interaction term between treatment group and baseline value on follow-up value;
Values for this outcome are worse if higher;
P-value based on Wilcoxon rank sum test.
In further analyses, we evaluated potential confounding by ever snus use, daily alcohol intake, clinical stage, and physical quality of life indicators, including problems with urinary, bowel, sexual, and overall function. The results above did not change substantially after adjustment for these potential confounders (data not shown). The results for the outcomes of BMI and waist circumference did not change substantially after exclusion of men diagnosed with stage T3 prostate cancer (data not shown).
Discussion
This pilot study demonstrates the feasibility of a walking group intervention among men with newly diagnosed prostate cancer. Nearly all men who were randomized completed the 11-week study and found the intervention to be rewarding. The retention rate in this trial was somewhat higher than those observed in other physical activity interventions, particularly those that involved more complex exercise regiments.19, 21, 30, 31 Men in this study tended to be over 68 years of age, retired, and married, which may have promoted retention. A notable finding in this study was that the primary reason for declining to participate was that men felt they no longer had a cancer after being treated. Previous intervention studies were conducted populations receiving a common therapy, such as men treated with androgen deprivation therapy (ADT)19, 32 or radical prostatectomy.33 In this study, participants received a variety of treatments and 24% received hormonal therapy. Differences across studies in type and timing of cancer treatment in relation to the intervention may explain some variability in adherence.
Importantly, our findings show that a walking group is potentially effective in improving cardiovascular health, as indicated by improvements in serum cholesterol in the intervention group. Cardiovascular disease is an important concern among men diagnosed with prostate cancer, as it is the second most common cause of death in this population.4 Few intervention studies have examined changes in circulating biomarkers among men with prostate cancer. Galvao et al. (2014) found a significant improvement in HDL cholesterol among long-term prostate cancer survivors randomized to a 12-month multi-component exercise intervention.32 However, two other physical activity trials among prostate cancer patients found no significant effect on lipids.18, 33 Similar to other studies, we found no significant effect of the intervention on levels of testosterone over follow-up.18, 31, 32 Although the overall group effect for CRP was not significant, our results suggest that a walking group intervention may be more effective in lowering CRP among men with higher levels at baseline. This is consistent with the decrease in CRP observed by Galvao et al. (2010) after a 12-week resistance and aerobic training intervention.18 Additionally, our findings suggest that a walking group intervention is potentially effective in lowering blood pressure. Although it did not reach statistical significance, the adjusted mean change in SBP of -8.5 mmHg in the intervention group compared to usual care represents a clinically significant reduction. Similar intervention studies also did not observe significant effects of the intervention on blood pressure.19, 32
Our results showed no significant effects of the intervention on body composition over the 11-week study period. Intervention studies have reported mixed results regarding changes in body composition among prostate cancer survivors. This may be explained in part by use of ADT in this population, which has been shown to cause gain in fat mass and loss of muscle.34 Resistance training in particular has been associated with increased lean mass and reduced body fat among men receiving ADT and radiotherapy.18, 31 However, the effect of aerobic training on body composition warrants further investigation.
Contrary to the literature, our study did not find a significant effect of the walking group intervention on quality of life. Previous studies have shown improvement in quality of life from supervised and non-supervised exercise interventions among men with prostate cancer receiving ADT, particularly when activity can be sustained in the long-term.18, 19, 30, 31 Two trials in men with prostate cancer showed decreases in depression in the intervention group but these differences did not reach statistical significance.19, 35 As in our study, Culos-Reed et al. (2010) also included a group-based component to increase social support but did not observe a significant effect on quality of life19. The relatively high quality of life reported by participants in this study at baseline may have limited our ability to observe an effect of the 11-week intervention. Indeed, Carmack Taylor et al. (2007) found a benefit of group physical activity only among participants with low psychosocial functioning at baseline.36 Although we saw no significant differences in social support between groups, men in the intervention reported an increase in social support from individuals other than their spouse or partner. The upward trend in report of sexual problems in the intervention group may be due in part to increased awareness or openness towards these problems after discussion with other men in the walking groups. Previous studies also support the effectiveness of aerobic training in decreasing fatigue.19, 30, 31, 35, 37 We observed a non-significant increase in quality of sleep among men in the intervention group but found no significant intervention effects on sleep duration or napping. These findings warrant further investigation into whether physical activity, such as through walking group sessions, may enhance quality of life, social function, and sleep.
This study has several potential limitations. First, the small sample size limited our statistical power to detect significant associations and led to some baseline imbalances between groups. Men at baseline differed significantly on physical quality of life indicators between groups. However, our analyses suggest that baseline imbalances between groups did not substantially bias the results. Secondly, the 11-week study period may have been insufficient to observe longer-term effects of the walking group intervention such as physical and emotional quality of life. Further study is needed to determine the minimal threshold of activity needed to achieve benefits. Lastly, our study population was composed of men with prostate cancer who were primarily over 68 years of age, retired, and had relatively few comorbidities. The feasibility of this intervention may not generalize to populations that differ from ours in age, employment status, comorbidities, or cancer type. Given the effect of ADT on cardiovascular health, the effectiveness and adherence of this intervention may differ among men receiving this treatment compared to other therapies.38 Future studies are needed to determine what specific strategies can be implemented to optimize benefits among men who are treated by ADT.
The main strengths of this study were the randomized design, high retention rate, and high adherence to the intervention. In addition, blood based measures allowed us to identify promising biomarkers associated with activity in this patient population. The weekly group walking sessions were well attended, and on average men in the intervention surpassed the study goal of maintaining 10,000 steps per day as recorded objectively using pedometers. In comparison to previous interventions involving substantial learning and effort, our intervention is low impact and accessible to men of older ages and a broad range of physical functioning. Overall, our findings support the literature that physical activity among men with prostate cancer can improve cardiovascular health and quality of life.
Conclusions
In summary, our study showed that a walking group intervention among men with newly diagnosed prostate cancer is feasible and potentially effective in improving cardiovascular health. A large-scale randomized trial is needed to examine the effect of our intervention on quality of life as well as overall and prostate cancer-specific survival.
Clinical Practice Points.
A variety of physical intervention studies among men diagnosed with prostate cancer indicate benefits in physical fitness and key quality of life areas. These studies often involve high-cost, clinically supervised training programs that pose challenges to long-term sustainability. Walking represents a low-cost and accessible form of physical activity that may be sustainable throughout a patient's life.
Men randomized to the intervention regularly attended walking group sessions and maintained 10,000 steps per day on average. Furthermore, men on the intervention experienced improvements in cholesterol and systolic blood pressure compared to usual care.
A walking group intervention among men diagnosed with prostate cancer is feasible and may be potentially effective in improving cardiovascular health. These findings support the need for a larger randomized trial to examine its potential for improvement in longer-term quality of life and prostate cancer outcomes.
Acknowledgments
C.H. Pernar and S.C. Markt are supported by National Institutes of Health (NIH) training grants (T32 ES 007069, T32 CA 09001). This project was supported in part by funding from the Prostate Cancer Foundation (PCF). L.A. Mucci and J.R. Rider are PCF Young Investigators.
Footnotes
Conflict of Interest: None to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Global, regional, and national incidence,prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Rider JR, Sandin F, Andren O, Wiklund P, Hugosson J, Stattin P. Long-term outcomes among noncuratively treated men according to prostate cancer risk category in a nationwide, population-based study. Eur Urol. 2013;63:88–96. doi: 10.1016/j.eururo.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Epstein MM, Edgren G, Rider JR, Mucci LA, Adami HO. Temporal trends in cause of death among Swedish and US men with prostate cancer. J Natl Cancer Inst. 2012;104:1335–1342. doi: 10.1093/jnci/djs299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358:1250–1261. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 6.Steineck G, Helgesen F, Adolfsson J, et al. Quality of life after radical prostatectomy or watchful waiting. N Engl J Med. 2002;347:790–796. doi: 10.1056/NEJMoa021483. [DOI] [PubMed] [Google Scholar]
- 7.Gomella LG, Johannes J, Trabulsi EJ. Current prostate cancer treatments: effect on quality of life. Urology. 2009;73:S28–35. doi: 10.1016/j.urology.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Fall K, Fang F, Mucci LA, et al. Immediate risk for cardiovascular events and suicide following a prostate cancer diagnosis: prospective cohort study. PLoS Med. 2009;6:e1000197. doi: 10.1371/journal.pmed.1000197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang F, Keating NL, Mucci LA, et al. Immediate risk of suicide and cardiovascular death after a prostate cancer diagnosis: cohort study in the United States. J Natl Cancer Inst. 2010;102:307–314. doi: 10.1093/jnci/djp537. [DOI] [PubMed] [Google Scholar]
- 10.Bourke L, Doll H, Crank H, Daley A, Rosario D, Saxton JM. Lifestyle intervention in men with advanced prostate cancer receiving androgen suppression therapy: a feasibility study. Cancer Epidemiol Biomarkers Prev. 2011;20:647–657. doi: 10.1158/1055-9965.EPI-10-1143. [DOI] [PubMed] [Google Scholar]
- 11.Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293:2479–2486. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 12.Kenfield SA, Stampfer MJ, Giovannucci E, Chan JM. Physical activity and survival after prostate cancer diagnosis in the health professionals follow-up study. J Clin Oncol. 2011;29:726–732. doi: 10.1200/JCO.2010.31.5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meyerhardt JA, Giovannucci EL, Holmes MD, et al. Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol. 2006;24:3527–3534. doi: 10.1200/JCO.2006.06.0855. [DOI] [PubMed] [Google Scholar]
- 14.Richman EL, Kenfield SA, Stampfer MJ, Paciorek A, Carroll PR, Chan JM. Physical activity after diagnosis and risk of prostate cancer progression: data from the cancer of the prostate strategic urologic research endeavor. Cancer Res. 2011;71:3889–3895. doi: 10.1158/0008-5472.CAN-10-3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonn SE, Sjolander A, Lagerros YT, et al. Physical activity and survival among men diagnosed with prostate cancer. Cancer Epidemiol Biomarkers Prev. 2015;24:57–64. doi: 10.1158/1055-9965.EPI-14-0707. [DOI] [PubMed] [Google Scholar]
- 16.Overgard M, Angelsen A, Lydersen S, Morkved S. Does physiotherapist-guided pelvic floor muscle training reduce urinary incontinence after radical prostatectomy? A randomised controlled trial. Eur Urol. 2008;54:438–448. doi: 10.1016/j.eururo.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 17.Centemero A, Rigatti L, Giraudo D, et al. Preoperative pelvic floor muscle exercise for early continence after radical prostatectomy: a randomised controlled study. Eur Urol. 2010;57:1039–1043. doi: 10.1016/j.eururo.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 18.Galvao DA, Taaffe DR, Spry N, Joseph D, Newton RU. Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: a randomized controlled trial. J Clin Oncol. 2010;28:340–347. doi: 10.1200/JCO.2009.23.2488. [DOI] [PubMed] [Google Scholar]
- 19.Culos-Reed SN, Robinson JW, Lau H, et al. Physical activity for men receiving androgen deprivation therapy for prostate cancer: benefits from a 16-week intervention. Support Care Cancer. 2010;18:591–599. doi: 10.1007/s00520-009-0694-3. [DOI] [PubMed] [Google Scholar]
- 20.Gjerset GM, Fossa SD, Dahl AA, Loge JH, Ensby T, Thorsen L. Effects of a 1-week inpatient course including information, physical activity, and group sessions for prostate cancer patients. J Cancer Educ. 2011;26:754–760. doi: 10.1007/s13187-011-0245-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park SW, Kim TN, Nam JK, et al. Recovery of overall exercise ability, quality of life, and continence after 12-week combined exercise intervention in elderly patients who underwent radical prostatectomy: a randomized controlled study. Urology. 2012;80:299–305. doi: 10.1016/j.urology.2011.12.060. [DOI] [PubMed] [Google Scholar]
- 22.Craft LL, Vaniterson EH, Helenowski IB, Rademaker AW, Courneya KS. Exercise effects on depressive symptoms in cancer survivors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2012;21:3–19. doi: 10.1158/1055-9965.EPI-11-0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Demark-Wahnefried W, Aziz NM, Rowland JH, Pinto BM. Riding the crest of the teachable moment: promoting long-term health after the diagnosis of cancer. J Clin Oncol. 2005;23:5814–5830. doi: 10.1200/JCO.2005.01.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 25.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 26.Nordin M, Akerstedt T, Nordin S. Psychometric evaluation and normative data for the Karolinska Sleep Questionnaire. Sleep Biol Rhythms. 2013;11:216–226. [Google Scholar]
- 27.Van Hemelrijck M, Wigertz A, Sandin F, et al. Cohort Profile: the National Prostate Cancer Register of Sweden and Prostate Cancer data Base Sweden 2.0. Int J Epidemiol. 2013;42:956–967. doi: 10.1093/ije/dys068. [DOI] [PubMed] [Google Scholar]
- 28.Ewald B, Attia J, McElduff P. How many steps are enough? Dose-response curves for pedometer steps and multiple health markers in a community-based sample of older Australians. J Phys Act Health. 2014;11:509–518. doi: 10.1123/jpah.2012-0091. [DOI] [PubMed] [Google Scholar]
- 29.Tudor-Locke C, Bassett DR., Jr How many steps/day are enough? Preliminary pedometer indices for public health. Sports medicine. 2004;34:1–8. doi: 10.2165/00007256-200434010-00001. [DOI] [PubMed] [Google Scholar]
- 30.Hansen PA, Dechet CB, Porucznik CA, LaStayo PC. Comparing eccentric resistance exercise in prostate cancer survivors on and off hormone therapy: a pilot study. PM&R. 2009;1:1019–1024. doi: 10.1016/j.pmrj.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 31.Segal RJ, Reid RD, Courneya KS, et al. Randomized controlled trial of resistance or aerobic exercise in men receiving radiation therapy for prostate cancer. J Clin Oncol. 2009;27:344–351. doi: 10.1200/JCO.2007.15.4963. [DOI] [PubMed] [Google Scholar]
- 32.Galvao DA, Spry N, Denham J, et al. A multicentre year-long randomised controlled trial of exercise training targeting physical functioning in men with prostate cancer previously treated with androgen suppression and radiation from TROG 03.04 RADAR. Eur Urol. 2014;65:856–864. doi: 10.1016/j.eururo.2013.09.041. [DOI] [PubMed] [Google Scholar]
- 33.Jones LW, Hornsby WE, Freedland SJ, et al. Effects of nonlinear aerobic training on erectile dysfunction and cardiovascular function following radical prostatectomy for clinically localized prostate cancer. Eur Urol. 2014;65:852–855. doi: 10.1016/j.eururo.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Basaria S, Lieb J, 2nd, Tang AM, et al. Long-term effects of androgen deprivation therapy in prostate cancer patients. Clin Endocrinol (Oxf) 2002;56:779–786. doi: 10.1046/j.1365-2265.2002.01551.x. [DOI] [PubMed] [Google Scholar]
- 35.Monga U, Garber SL, Thornby J, et al. Exercise prevents fatigue and improves quality of life in prostate cancer patients undergoing radiotherapy. Arch Phys Med Rehabil. 2007;88:1416–1422. doi: 10.1016/j.apmr.2007.08.110. [DOI] [PubMed] [Google Scholar]
- 36.Carmack Taylor CL, de Moor C, Basen-Engquist K, et al. Moderator analyses of participants in the Active for Life after cancer trial: implications for physical activity group intervention studies. Ann Behav Med. 2007;33:99–104. doi: 10.1207/s15324796abm3301_11. [DOI] [PubMed] [Google Scholar]
- 37.Windsor PM, Nicol KF, Potter J. A randomized, controlled trial of aerobic exercise for treatment-related fatigue in men receiving radical external beam radiotherapy for localized prostate carcinoma. Cancer. 2004;101:550–557. doi: 10.1002/cncr.20378. [DOI] [PubMed] [Google Scholar]
- 38.Nguyen PL, Alibhai SM, Basaria S, et al. Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur Urol. 2015;67:825–836. doi: 10.1016/j.eururo.2014.07.010. [DOI] [PubMed] [Google Scholar]


