Abstract
Objectives
Little is known about the economic burden of influenza‐related hospitalizations in Japan. This study sought to identify the factors that contribute to the total healthcare costs (THCs) associated with hospitalizations due to influenza in the Japanese population.
Study design
A retrospective cross‐sectional database analysis study.
Methods
A structural equation modelling approach was used to analyse a nationwide Japanese hospital claims data. This study included inpatients with at least 1 confirmed diagnosis of influenza and with a hospital stay of at least 2 days, who were admitted between April 2014 and March 2015.
Results
A total of 5261 Japanese inpatients with a diagnosis of influenza were included in the final analysis. The elderly (≥65 years) and the young (≤15 years) comprised more than 85% of patients. The average length of stay (LOS) was 12.5 days, and the mean THC was 5402 US dollars (US$) per hospitalization. One additional hospital day increased the THC by 314 US$. Intensive care unit hospitalizations were linked to higher costs (+4957 US$) compared to regular hospitalizations. The biggest procedure‐related cost drivers, which were also impacted by LOS, were blood transfusions (+6477 US$), tube feedings (+3501 US$) and dialysis (+2992 US$).
Conclusions
In Japan, the economic burden due to influenza‐related hospitalizations for both children and the elderly is considerable and is further impacted by associated comorbidities, diagnostic tests and procedures that prolong the LOS.
Keywords: economic burden, healthcare costs, hospitalizations, Influenza, Japan, structural equation modelling
1. INTRODUCTION
Influenza is a contagious respiratory illness caused by a highly infectious viral pathogen. The illness ranges from mild to severe and can lead to numerous complications such as superimposed infections, exacerbation of cardiovascular conditions and asthma. Most of the fatal cases occur in the elderly over 65 years old1 and in high‐risk populations including children younger than 2 years old,2 pregnant women,3 healthcare workers4 and patients with associated comorbidities such as asthma, chronic lung disease, kidney disorders and blood disorders.5, 6
Annual epidemics of influenza result in approximately 250 000 to 500 000 deaths worldwide.7 Cases of influenza also have a substantial socioeconomic impact in terms of medical care, healthcare utilization (eg increase in consultations, hospitalizations and length of stay [LOS]) and work absenteeism.8 In Europe, influenza is responsible for approximately 10% of sickness‐related workplace absence.9 As influenza is an epidemic disease, it may disturb the healthcare services by acute overloading during the epidemic.
Elderly patients comprise the group with the highest burden of influenza‐related complications. Patients aged ≥85 years are 6 times more likely to be hospitalized and 16 times more likely to die compared with patients aged 65‐69 years.10, 11
The costs associated with influenza and its complications can be substantial. In the United States, a study based on the 2003 population estimated that the annual burden of influenza was 3.1 million hospital days and 31.4 million outpatient visits. From a societal perspective, the total economic burden of influenza (direct costs and indirect costs, including loss of earnings and loss of life) has been estimated to be 87.1 billion US$ annually, with direct costs accounting for more than 10 billion US$, of which 40% is spent on the treatment of patients older than 65 years of age.12
In the United States, the mean total cost of hospitalization for influenza‐related illness for children was 13 159 US$ (39 792 US$ for patients admitted to an intensive care unit (ICU) and 7030 US$ for patients cared for exclusively on the wards). High‐risk patients had a higher mean total cost than low‐risk patients (15 269 vs 9107 US$, respectively).13
In Japan, it is estimated that 5%‐10% of the population develops influenza annually, resulting in approximately 1000 to 2000 deaths from influenza alone and an additional 5000 deaths due to complications such as pneumonia.14 Approximately 20% to 25% of elderly Japanese patients with influenza develop pneumonia, 5% of whom die.14 From 1988 to 1991, 14.0% of all admissions to paediatric hospitals during the winter season in Japan were due to influenza viral infections, while respiratory syncytial virus accounted for 17.5% of admissions.15 Despite these statistics, there is limited information available about the extent of the disease burden due to influenza‐related illness in Japan. Therefore, the aim of this study was to identify factors that impact hospitalization costs for patients with influenza in Japan utilizing a Japanese administrative database.
2. METHODS
2.1. Patient selection
We utilized a commercially available hospital claims databank from Medical Data Vision Co., Ltd (MDV, Tokyo, Japan). This is an administrative database including approximately 4 400 000 patients, which represents approximately 3% of the total Japanese population.16 The MDV database has been used to investigate a wide range of conditions in Japan such as rheumatoid arthritis,17, 18 schizophrenia,19 infectious diseases,20 multiple sclerosis21 and hypertension.22 We considered the inpatient claims from patients who were admitted between 1 April 2014 and 31 March 2015 with at least 1 confirmed diagnosis of influenza [International Classification of Diseases 10th Revision (ICD‐10) codes: J10.1, J11.1 and J11.8] and a minimum hospital stay of 2 days (defined by at least 1 night was spent in the hospital).
2.2. Hospitalization cost calculation
Total healthcare costs (THCs) comprised all costs of healthcare services incurred during each hospitalization. These included basic management fees, examination, procedures and medication. Both Diagnosis Procedure Combination cost (DPC cost, which is a case‐mix reimbursement cost) and total actual heath care cost were calculated. All costs were converted from Japanese yen to US$ based on the average exchange rate during April 2014‐March 2015 (Financial Market Department, Bank of Japan; 1 US$ = 109.33 yen).23
2.3. Statistical analysis
Descriptive analyses were performed on baseline characteristics as well as resource use, LOS and THC. As LOS is usually an important driver of the total hospitalization costs,24, 25 we considered a structural equation modelling (SEM) approach to assess the relationship between the patients’ characteristics, procedures, LOS and hospitalization costs by considering LOS as an intermediate effect. Indeed, SEM is a flexible multivariate statistical framework that can be used to model complex relationships between variables.26 The SEM framework allows evaluating relationships among variables by combining the strengths of factor analysis and multiple regression in a single model that can be tested statistically.27 More specifically, in this study, a path analysis was conducted, which is a special case of the SEM framework that allows an exploration of the causal links (direct and indirect effects) between exogenous variables and 1 or more endogenous variables. In this framework, the total effects of a covariate on the main dependent variable can be decomposed into 2 categories of effects: (i) the indirect effects, consisting of the effect of the covariate on 1 or more intermediary endogenous variables, which in turn translates into an effect on the main variable; and (ii) the direct effect, which is the remaining effect of the covariate on the main variable while controlling for their indirect effects.28 In our case, the main endogenous variable of interest in the analysis was the total hospitalization cost expressed in Japanese yen, while we assumed that independent variables would have both a direct effect on total hospitalization costs and indirect effects through the LOS. Figure 1 depicts the underlying path diagram showing the relationship between each variable. We also conducted subgroup analyses of the children (≤15 years), the elderly (≥65 years) populations and the infants and toddlers (children ≤2 years old), 3 groups that are particularly susceptible to influenza complications and hospitalization. Statistical analyses were performed using stata 15.0.29
Figure 1.
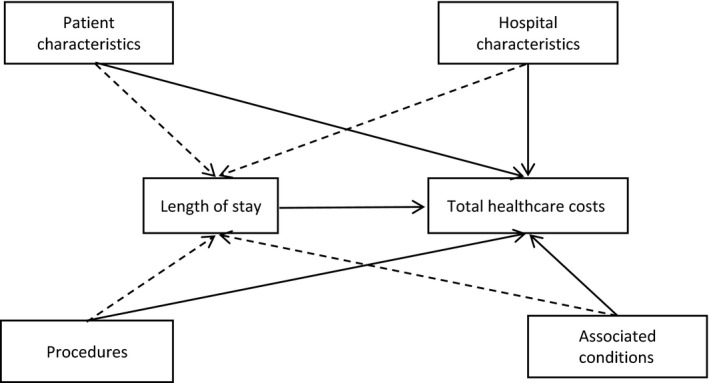
Path showing the relationship between each variable using structural equation modelling. Patient characteristics: age, gender, origin of patients and main diagnosis. Hospital characteristics: hospital type (regular, ER, ICU). Procedures: blood transfusion, cardiac catheterization, dialysis, mechanical ventilator, oxygen therapy, tube feeding, biochemical testing, bronchoscopy, chest X‐ray, echocardiography, CT scan, immunology test, sputum test and oxygen saturation test. Associated conditions: congestive heart failure, atrial fibrillation, acute respiratory failure, pneumonia, asthma, COPD, chronic renal failure, diabetes mellitus, disease involving the immune mechanism, Parkinson's disease, ischaemic heart disease and malignant neoplasm (cancer). Indirect effect: dashed line. Direct effect: continuous line. ER, emergency; ICU, intensive care unit; CT scan, computerized axial tomography scan; COPD, chronic obstructive pulmonary disease
3. RESULTS
A total of 5261 Japanese inpatients with influenza were included in the final analysis. We excluded 15 rehospitalized admissions due to the limited number of patients (Figure 2).
Figure 2.
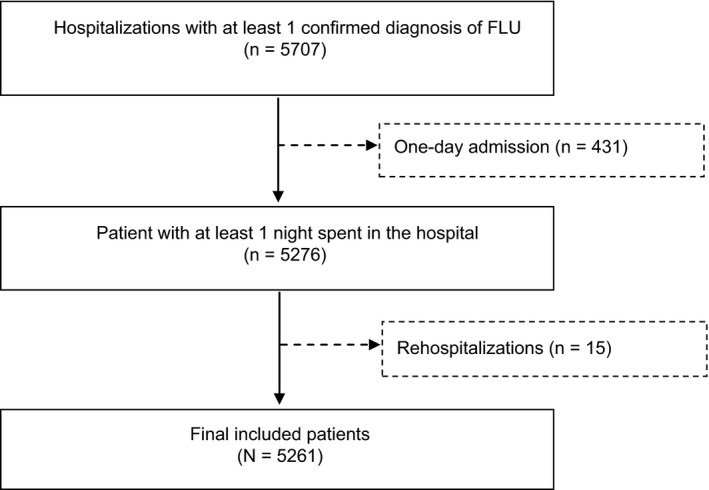
Patient selection
Table 1 shows patient baseline characteristics for all patients and each subgroup. The elderly (≥65 years) and children (≤15 years) were 61.8% and 26.1% of the patients, respectively. Overall, the average length of hospital stay was 12.5 days, and the mean THC was 5402 US$. 4.5% of the patients were admitted to an ICU, and 4.7% of the patients died in the hospital. The most prominent comorbidities were diabetes (14.9%), congestive heart failure (13.1%) and pneumonia (13.1%). A computerized tomography (CT) scan was used as a diagnostic aid in 49.9% of patients, 44.0% of patients received oxygen therapy, approximately 5.5% of patients received a blood transfusion during their hospitalization, 5.6% received tube feeding and 4.1% required mechanical ventilation.
Table 1.
Characteristics of included influenza patients
| Characteristics N (%) | Total N (%) | Children (≤15 y) N (%) | Adults (16‐64 y) N (%) | Elderly (≥65 y and older) N (%) | Subgroup: Infants and toddlers (≤2 y) N (%) |
|---|---|---|---|---|---|
| Number of patients | 5261 | 1375 (26) | 637 (12) | 3249 (62) | 654 (12) |
| Demographics | |||||
| Gender | |||||
| Female | 2559 (49) | 567 (41) | 303 (47) | 1689 (52) | 276 (42) |
| Age | |||||
|
Mean ± SD (median [Q1; Q3]) |
57.5 ± 34.9 (75 [12; 85]) |
4.0 ± 3.8 (3 [1; 7]) |
45.6 ± 14.6 (49 [35; 59]) |
82.5 ± 8.0 (83 [77; 88]) |
0.8 ± 0.8 (1 [0; 2]) |
| Hospitalization characteristics | |||||
| Influenza as diagnosis incurring most resources | 1867 (35) | 884 (64) | 130 (20) | 853 (26) | 436 (67) |
| Influenza as primary medical diagnosis | 2343 (44) | 924 (67) | 169 (26) | 1250 (38) | 445 (68) |
| Nature of hospitalization | |||||
| Regular | 3033 (58) | 1196 (87) | 385 (61) | 1452 (45) | 566 (87) |
| Emergency | 1990 (38) | 161 (12) | 205 (32) | 1624 (50) | 79 (12) |
| ICU | 238 (4) | 18 (1) | 47 (7) | 173 (5) | 9 (1) |
| Origin of patient before hospitalization | |||||
| Hospitalized from home | 4708 (89) | 1355 (98) | 612 (96) | 2714 (84) | 643 (98) |
| Transfer | 91 (2) | 10 (1) | 8 (1) | 73 (2) | 6 (1) |
| Nursing home or welfare facilities | 436 (8) | 0 (0) | 11 (2) | 425 (13) | 0 (0) |
| Missing | 26 (1) | 10 (1) | 6 (1) | 10 (1) | 5 (1) |
| Destination/outcome after discharge | |||||
| Home | 4208 (80) | 1357 (99) | 595 (93) | 2256 (70) | 647 (99) |
| Transfer | 349 (7) | 5 (0) | 18 (3) | 326 (10) | 2 (0) |
| Long‐term care facilities | 414 (8) | 0 (0) | 12 (2) | 402 (12) | 0 (0) |
| Death | 248 (5) | 1 (0) | 8 (1) | 239 (7) | 1 (0) |
| Missing | 42 (0) | 12 (1) | 4 (1) | 26 (1) | 4 (1) |
| Associated conditions | |||||
| Congestive heart failure | 690 (13) | 9 (1) | 41 (6) | 640 (80) | 7 (1) |
| Atrial fibrillation | 305 (6) | 0 (0) | 8 (1) | 297 (9) | 0 (0) |
| Acute respiratory failure | 535 (10) | 67 (5) | 40 (6) | 428 (13) | 29 (4) |
| Acute renal failure | 65 (1) | 3 (0) | 14 (2) | 48 (1) | 1 (0) |
| Pneumonia | 689 (13) | 114 (8) | 43 (7) | 532 (16) | 59 (9) |
| Asthma | 562 (11) | 265 (19) | 59 (9) | 238 (7) | 121 (10) |
| COPD | 285 (5) | 1 (0) | 21 (3) | 263 (8) | 0 (0) |
| Chronic renal failure | 196 (4) | 1 (0) | 26 (4) | 169 (5) | 1 (0) |
| Diabetes mellitus | 785 (15) | 0 (0) | 97 (15) | 688 (21) | 0 (0) |
| Disease involving the immune mechanism | 11 (0) | 3 (0) | 4 (1) | 4 (0) | 0 (0) |
| Parkinson's disease | 82 (2) | 2 (0) | 3 (0) | 77 (2) | 0 (0) |
| Ischaemic heart disease | 405 (8) | 1 (0) | 33 (5) | 371 (11) | 0 (0) |
| Malignant neoplasm (cancer) | 503 (10) | 7 (1) | 78 (12) | 418 (13) | 1 (0) |
| Procedures (patients with at least 1 procedure charged) | |||||
| Surgery and interventions | |||||
| Blood transfusion | 288 (6) | 10 (1) | 40 (6) | 238 (7) | 4 (1) |
| Cardiac catheterization | 943 (18) | 12 (1) | 78 (12) | 853 (26) | 4 (1) |
| Dialysis | 73 (1) | 0 (0) | 18 (3) | 55 (2) | 0 (0) |
| Mechanical ventilation | 217 (4) | 22 (2) | 28 (4) | 167 (5) | 11 (2) |
| Oxygen therapy | 2317 (44) | 227 (16) | 201 (31) | 1888 (58) | 107 (16) |
| Tube feeding | 296 (6) | 18 (1) | 20 (3) | 258 (8) | 6 (1) |
| Other surgery procedures and anaesthesia | 839 (16) | 53 (4) | 159 (25) | 627 (19) | 19 (3) |
| Tests/imaging | |||||
| Biochemical testing | 5092 (97) | 1277 (93) | 604 (95) | 3210 (99) | 603 (92) |
| Bronchoscopy/pulmonary function test | 142 (3) | 9 (1) | 28 (4) | 105 (3) | 2 (0) |
| Chest X‐ray | 4598 (87) | 919 (67) | 540 (85) | 3139 (97) | 418 (91) |
| Colour Doppler ultrasound/echocardiography | 1174 (22) | 76 (6) | 150 (24) | 948 (29) | 29 (4) |
| Computerized tomography scan | 2597 (49) | 235 (17) | 288 (45) | 2074 (64) | 81 (12) |
| Immunology test | 4690 (89) | 996 (72) | 575 (90) | 3119 (96) | 481 (73) |
| Oxygen saturation test | 2551 (48) | 384 (28) | 223 (35) | 1944 (60) | 186 (28) |
| Sputum test | 3046 (58) | 687 (50) | 313 (49) | 2046 (63) | 328 (58) |
| Length of stay | |||||
| LOS | |||||
|
Mean ± SD (median [Q1; Q3]) |
12.5 ± 12.7 (8 [4; 17]) |
4.3 ± 5.4 (3 [2; 5]) |
11.3 ± 11.0 (7 [4; 14]) |
16.1 ± 13.5 (12 [6; 22]) |
4.2 ± 5.1 (3 [2; 5]) |
| Total costs in USD | |||||
| 1. Total costs (sum of costs of all procedures) | |||||
| Mean ± SD | 5402 ± 5597 | 2619 ± 3500 | 5572 ± 6146 | 6546 ± 5793 | 2538 ± 3629 |
| Median [Q1; Q3] | 3409 [2036; 6638] | 1937 [1570; 2546] | 3491 [2120; 6687] | 4669 [2793; 8224] | 1935 [1607; 2441] |
| 2. Total cost of DPC | |||||
| Mean ± SD | 4582 ± 5075 | 2265 ± 3144 | 4770 ± 5675 | 5526 ± 5296 | 2205 ± 3198 |
| Median [Q1; Q3] | 2718 [1635; 5566] | 1679 [1363; 2185] | 2744 [1536; 5817] | 3821 [2124; 6900] | 1686 [1393; 2124] |
DPC, Diagnosis Procedure Combination; SD, standard deviation; ICU, intensive care unit; COPD, chronic obstructive pulmonary disease; LOS, length of stay; ¥, Japanese yen; USD, US$.
Exchange rate: 1 USD = 109.33 Japanese yen.
The results of the SEM method are reported in Table 2.
Table 2.
Direct, indirect and total effects of the factors on THC using a structure equation model
| Variable | Direct effect (USDa) | Indirect effect (USDa) | Total effects (USDa) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| →THC | →LOS→THC | →THC + (→LOS→THC) | |||||||
| Coeff. | 95% CI | Coeff. | 95% CI | Coeff. | 95% CI | ||||
| LOS (day) | 314 | 297 | 330 | 314 | 297 | 330 | |||
| Gender (reference: male) | |||||||||
| Female | −176 | −307 | −45 | 259 | 94 | 424 | 82 | −125 | 291 |
| Age (reference: 16‐64 y) | |||||||||
| ≤15 y | 457 | 196 | 719 | −484 | −753 | −216 | −26 | −429 | 375 |
| 16‐64 y | Reference | Reference | Reference | ||||||
| 65 y and older | −473 | −764 | −182 | 854 | 580 | 1127 | 381 | −33 | 795 |
| Hospitalization characteristics | |||||||||
| Influenza as primary medical diagnosis | −415 | −543 | −287 | −1579 | −1767 | −1390 | −1994 | −2195 | −1793 |
| Nature of hospitalization | |||||||||
| Regular | Reference | Reference | Reference | ||||||
| Emergency | 459 | 326 | 593 | −261 | −462 | −61 | 197 | −33 | 429 |
| ICU | 4769 | 3915 | 5623 | 188 | −384 | 762 | 4957 | 3832 | 6083 |
| Patient origin | |||||||||
| From home | Reference | Reference | Reference | ||||||
| Transfer | −330 | −906 | 246 | 763 | −120 | 1647 | 433 | −423 | 1290 |
| Nursing home or welfare facilities | −685 | −898 | −472 | 209 | −151 | 569 | −476 | −853 | −98 |
| Associated conditions | |||||||||
| Congestive heart failure | −139 | −446 | 167 | 218 | −108 | 544 | 78 | −347 | 505 |
| Atrial fibrillation | −246 | −651 | 157 | 28 | −407 | 464 | −218 | −758 | 322 |
| Acute respiratory failure | −64 | −315 | 186 | −314 | −639 | 10 | −379 | −769 | 11 |
| Acute renal failure | 1001 | −713 | 2716 | −881 | −1777 | 13 | 119 | −1849 | 2088 |
| Pneumonia | −405 | −597 | −213 | −41 | −306 | 223 | −446 | −748 | −144 |
| Asthma | −177 | −406 | 52 | 97 | −148 | 343 | −79 | −428 | 269 |
| COPD | −330 | −590 | −71 | 288 | −143 | 720 | −42 | −500 | 414 |
| Chronic renal failure | −1042 | −1728 | −357 | 141 | −495 | 779 | −900 | −1782 | −19 |
| Diabetes mellitus | 141 | −114 | 398 | 110 | −161 | 383 | 252 | −111 | 617 |
| Disease involving the immune mechanism | 1499 | −2046 | 5046 | −1379 | −2709 | −49 | 120 | −4389 | 4630 |
| Parkinson's disease | −128 | −533 | 277 | 1755 | 799 | 2710 | 1626 | 680 | 2573 |
| Ischaemic heart disease | 516 | 99 | 934 | 334 | −50 | 720 | 851 | 339 | 1363 |
| Malignant neoplasm (cancer) | 464 | 124 | 804 | 997 | 597 | 1397 | 1462 | 904 | 2019 |
| Procedures (patients with at least 1 procedure charged) | |||||||||
| Surgery and interventions | |||||||||
| Blood transfusion | 3557 | 2846 | 4268 | 2919 | 2231 | 3608 | 6477 | 5379 | 7575 |
| Cardiac catheterization | 24 | −242 | 292 | 1744 | 1388 | 2101 | 1769 | 1348 | 2191 |
| Dialysis | 2453 | 1109 | 3797 | 539 | −483 | 1561 | 2992 | 1311 | 4673 |
| Mechanical ventilation | 2435 | 1618 | 3252 | −718 | −1355 | −80 | 1717 | 678 | 2756 |
| Oxygen therapy | 301 | 83 | 519 | 199 | −68 | 468 | 501 | 149 | 853 |
| Tube feeding | 881 | 240 | 1522 | 2619 | 2028 | 3210 | 3501 | 2639 | 4362 |
| Tests/imaging | |||||||||
| Biochemical testing | 56 | −178 | 291 | −304 | −619 | 9 | −248 | −656 | 160 |
| Bronchoscopy/pulmonary function test | 2032 | 1045 | 3020 | 1449 | 717 | 2181 | 3482 | 2218 | 4746 |
| Chest X‐ray | 282 | 114 | 450 | 109 | −90 | 309 | 391 | 117 | 666 |
| Colour Doppler ultrasound/echocardiography | 538 | 338 | 739 | 972 | 690 | 1254 | 1511 | 1174 | 1848 |
| Computerized tomography | 19 | −130 | 170 | 588 | 392 | 785 | 608 | 362 | 853 |
| Immunology test | −64 | −261 | 132 | 420 | 218 | 622 | 355 | 31 | 680 |
| Oxygen saturation test | −67 | −262 | 127 | 235 | −2 | 474 | 168 | −137 | 474 |
| Sputum test | −90 | −242 | 60 | 529 | 358 | 700 | 438 | 207 | 669 |
Statistical significance at P‐value < 0.05 in bold. Coeff., unstandardized coefficient; USD, US$; LOS, length of stay; THC, total healthcare cost; COPD, chronic obstructive pulmonary disease; ICU, intensive care unit.
Exchange rate: 1 USD = 109.33 Japanese yen.
Results of the SEM analysis showed that hospitalizations where influenza was the primary diagnosis were 1994 US$ less costly than those with another medical diagnosis. One additional hospital day increased the THC by 314 US$. Not surprisingly, ICU stays were significantly more costly (+4957 US$) than regular stays. Among comorbidities, ischaemic heart disease, malignant neoplasm and Parkinson's disease significantly increased the THC by 851 US$, 1462 US$ and 1626 US$, respectively.
Overall, patients who were transferred from other hospitals incurred higher total costs; however, the opposite was found for toddlers under the age of 2. Patients who were referred from nursing home or welfare facilities are less costly than those who were hospitalized from home.
The majority of additional procedures were significantly associated with higher THC both directly and due to an increase in the LOS. Among surgeries and interventions, the largest cost drivers were blood transfusions (+6477 US$), tube feedings (+3501 US$) and dialysis (+2992 US$). Bronchoscopy and echocardiography were the imaging procedures that increased the THC most significantly (+3482 and +1511 US$, respectively). Overall, the effects on DPC costs compared with total costs were similar (Data S1).
Subgroup analyses of children (≤15 years) (Table 3), the elderly (≥65 years) (Table 4) and the infants and toddlers (≤2 years old) (Table 5) showed similar results, although the magnitude of the effect was higher in children for most of the surgeries and interventions.
Table 3.
Direct, indirect and total effects of the factors on THC in children (≤15 y old) using a structure equation model
| Variable | Direct effect (USDa) | Indirect effect (USDa) | Total effects (USDa) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| →THC | →LOS→THC | →THC + (→LOS→THC) | |||||||
| Coeff. | 95% CI | Coeff. | 95% CI | Coeff. | 95% CI | ||||
| LOS (day) | 549 | 485 | 612 | 549 | 485 | 612 | |||
| Gender (reference: male) | |||||||||
| Female | −44 | −140 | 52 | 1 | −240 | 243 | −42 | −292 | 207 |
| Hospitalization characteristics | |||||||||
| Influenza as primary medical diagnosis | 81 | −37 | 199 | −980 | −1295 | −665 | −899 | −1208 | −589 |
| Nature of hospitalization | |||||||||
| Regular | Reference | Reference | Reference | ||||||
| Emergency | 336 | 230 | 443 | −73 | −517 | 370 | 263 | −165 | 692 |
| ICU | 2688 | 979 | 4398 | 140 | −5917 | 6198 | 2829 | −4505 | 10 164 |
| Patient origin | |||||||||
| From home | Reference | Reference | Reference | ||||||
| Transfer | −203 | −581 | 173 | −126 | −731 | 478 | −330 | −791 | 130 |
| Nursing home or welfare facilities | Omitted | Omitted | Omitted | ||||||
| Procedures (patients with at least 1 procedure charged) | |||||||||
| Surgery and interventions | |||||||||
| Blood transfusion | 3865 | 37 | 7692 | 13 070 | 2433 | 23 707 | 16 935 | 3624 | 30 246 |
| Cardiac catheterization | 764 | −2491 | 4020 | 5278 | −4391 | 14 948 | 6043 | −5899 | 17 986 |
| Mechanical ventilation | 848 | −320 | 2018 | 2003 | −1088 | 5094 | 2851 | −973 | 6677 |
| Oxygen therapy | −104 | −267 | 59 | 860 | 373 | 1347 | 756 | 259 | 1254 |
| Tube feeding | 914 | −453 | 2282 | −2856 | −7346 | 1634 | −1941 | −7233 | 3350 |
| Tests/imaging | |||||||||
| Biochemical testing | 145 | −37 | 329 | −348 | −718 | 22 | −202 | −552 | 147 |
| Bronchoscopy/pulmonary function test | 755 | −1534 | 3046 | −378 | −2282 | 1526 | 377 | −3249 | 4005 |
| Chest X‐ray | −9 | −137 | 117 | 306 | 22 | 589 | 296 | 16 | 576 |
| Colour Doppler ultrasound/echocardiography | 391 | −116 | 899 | 2068 | 770 | 3366 | 2460 | 998 | 3922 |
| Computerized tomography | 323 | 148 | 498 | 61 | −314 | 436 | 384 | −14 | 784 |
| Immunology test | 45 | −80 | 171 | 40 | −291 | 371 | 85 | −249 | 420 |
| Oxygen saturation test | 88 | −25 | 202 | −179 | −379 | 20 | −91 | −302 | 120 |
| Sputum test | 1 | −88 | 91 | 319 | 49 | 588 | 320 | 52 | 588 |
Statistical significance at P‐value < 0.05 in bold. Coeff., unstandardized coefficient; USD, US$; LOS, length of stay; THC, total healthcare cost.
Exchange rate: 1 USD = 109.33 Japanese yen.
Table 4.
Direct, indirect and total effects of the factors on THC in elderly patients (≥65 y old) using a structure equation model
| Variable | Direct effect (USDa) | Indirect effect (USDa) | Total effects (USDa) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| →THC | →LOS→THC | →THC + (→LOS→THC) | |||||||
| Coeff. | 95% CI | Coeff. | 95% CI | Coeff. | 95% CI | ||||
| LOS (day) | 293 | 279 | 307 | 293 | 279 | 307 | |||
| Gender (reference: male) | |||||||||
| Female | −201 | −385 | −17 | 471 | 238 | 703 | 269 | −28 | 567 |
| Hospitalization characteristics | |||||||||
| Influenza as primary medical diagnosis | −526 | −690 | −361 | −1920 | −2160 | −1680 | −2446 | −2716 | −2176 |
| Nature of hospitalization | |||||||||
| Regular | Reference | Reference | Reference | ||||||
| Emergency | 443 | 283 | 603 | −265 | −556 | 673 | 177 | −109 | 464 |
| ICU | 4943 | 3966 | 5920 | 58 | −556 | 673 | 5002 | 3732 | 6272 |
| Patient origin | |||||||||
| From home | Reference | Reference | Reference | ||||||
| Transfer | −332 | −929 | 265 | 815 | −179 | 1810 | 482 | −515 | 1481 |
| Nursing home or welfare facilities | −662 | −875 | −448 | 124 | −221 | 470 | −537 | −922 | −152 |
| Associated conditions | |||||||||
| Congestive heart failure | −216 | −514 | 81 | 156 | −171 | 484 | −60 | −493 | 373 |
| Atrial fibrillation | −198 | −589 | 192 | 48 | −368 | 465 | −150 | −687 | 387 |
| Acute respiratory failure | −33 | −289 | 222 | −345 | −712 | 22 | −379 | −799 | 41 |
| Acute renal failure | 1093 | −958 | 3145 | −715 | −1679 | 248 | 377 | −1967 | 2723 |
| Pneumonia | −406 | −645 | −168 | −41 | −358 | 274 | −448 | −824 | −72 |
| Asthma | −48 | −518 | 422 | 26 | −423 | 475 | −21 | −699 | 656 |
| COPD | −316 | −601 | −30 | 287 | −159 | 733 | −28 | −542 | 484 |
| Chronic renal failure | −1009 | −1516 | −502 | 58 | −595 | 713 | −950 | −1693 | −207 |
| Diabetes mellitus | 182 | −82 | 448 | 73 | −207 | 355 | 256 | −132 | 645 |
| Disease involving the immune mechanism | 5115 | −3837 | 14 067 | −874 | −3519 | 1770 | 4240 | −7000 | 15 481 |
| Parkinson's disease | 11 | −386 | 409 | 1574 | 643 | 2505 | 1586 | 585 | 2586 |
| Ischaemic heart disease | 271 | −132 | 675 | 336 | −53 | 726 | 608 | 82 | 1134 |
| Malignant neoplasm (cancer) | 259 | −83 | 603 | 723 | 333 | 1112 | 982 | 453 | 1512 |
| Procedures (patients with at least 1 procedure charged) | |||||||||
| Surgery and interventions | |||||||||
| Blood transfusion | 3354 | 2608 | 4099 | 2583 | 1936 | 3231 | 5938 | 4882 | 6993 |
| Cardiac catheterization | 70 | −196 | 338 | 1516 | 1171 | 1862 | 1587 | 1167 | 2008 |
| Dialysis | 2123 | 1232 | 3014 | 659 | −524 | 1842 | 2782 | 1288 | 4276 |
| Mechanical ventilation | 2519 | 1590 | 3448 | −630 | −1316 | 55 | 1888 | 741 | 3035 |
| Oxygen therapy | 241 | −53 | 536 | 150 | −204 | 505 | 392 | −86 | 870 |
| Tube feeding | 1014 | 366 | 1662 | 2563 | 1956 | 3169 | 3578 | 2691 | 4464 |
| Tests/imaging | |||||||||
| Biochemical testing | 114 | −357 | 586 | −460 | −1313 | 392 | −345 | −1318 | 627 |
| Bronchoscopy/pulmonary function test | 2222 | 1123 | 3322 | 1636 | 766 | 2506 | 3859 | 2407 | 5311 |
| Chest X‐ray | 248 | −124 | 621 | 733 | 249 | 1218 | 982 | 374 | 1590 |
| Colour Doppler ultrasound/echocardiography | 553 | 338 | 768 | 753 | 455 | 1051 | 1306 | 947 | 1665 |
| Computerized tomography | −20 | −213 | 172 | 533 | 292 | 774 | 513 | 204 | 821 |
| Immunology test | 37 | −324 | 399 | 1166 | 711 | 1620 | 1203 | 619 | 1788 |
| Oxygen saturation test | −51 | −334 | 231 | 258 | −83 | 600 | 207 | −246 | 660 |
| Sputum test | −212 | −431 | 6 | 474 | 241 | 708 | 262 | −58 | 582 |
Statistical significance at P‐value < 0.05 in bold. Coeff., unstandardized coefficient; USD, US$; LOS, length of stay; THC, total healthcare cost; COPD, chronic obstructive pulmonary disease.
Exchange rate: 1 USD = 109.33 Japanese yen.
Table 5.
Direct, indirect and total effects of the factors on THC in infants and toddlers (≤2 y old) using a structure equation model
| Variable | Direct effect (USDa) | Indirect effect (USDa) | Total effects (USDa) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| →THC | →LOS→THC | →THC + (→LOS→THC) | |||||||
| Coeff. | 95% CI | Coeff. | 95% CI | Coeff. | 95% CI | ||||
| LOS (day) | 547 | 489 | 604 | 547 | 489 | 604 | |||
| Gender (reference: male) | |||||||||
| Female | 34 | −48 | 117 | 54 | −136 | 245 | 88 | −95 | 272 |
| Hospitalization characteristics | |||||||||
| Influenza as primary medical diagnosis | 8 | −114 | 131 | −675 | −940 | −410 | −667 | −899 | −434 |
| Nature of hospitalization | |||||||||
| Regular | Reference | Reference | Reference | ||||||
| Emergency | 393 | 286 | 501 | −124 | −395 | 147 | 269 | 1 | 537 |
| ICU | 2229 | 525 | 3922 | 777 | −1659 | 3213 | 3006 | −605 | 6618 |
| Patient origin | |||||||||
| From home | Reference | Reference | Reference | ||||||
| Transfer | −424 | −969 | 121 | 31 | −621 | 685 | −392 | −994 | 209 |
| Nursing home or welfare facilities | Omitted | Omitted | Omitted | ||||||
| Procedures (patients with at least 1 procedure charged) | |||||||||
| Surgery and interventions | |||||||||
| Blood transfusion | 5714 | −80 | 11 509 | 21 181 | 8702 | 33 659 | 26 895 | 10 267 | 43 523 |
| Cardiac catheterization | 2940 | −1132 | 7012 | 15 770 | 7533 | 24 007 | 18 710 | 7647 | 29 773 |
| Mechanical ventilation | 641 | −594 | 1877 | 1178 | −131 | 2488 | 1819 | 163 | 3476 |
| Oxygen therapy | −119 | −321 | 81 | 1226 | 636 | 1817 | 1106 | 523 | 1689 |
| Tube feeding | 262 | −3626 | 4152 | −12 563 | −25 823 | 696 | −12 300 | −29 057 | 4456 |
| Tests/Imaging | |||||||||
| Biochemical testing | 207 | 30 | 384 | −205 | −572 | 161 | −1 | −304 | 307 |
| Bronchoscopy/pulmonary function test | 4844 | −3196 | 12 886 | 3309 | −1341 | 7960 | 8154 | −3524 | 19 832 |
| Chest X‐ray | 50 | −36 | 138 | 351 | 127 | 574 | 402 | 194 | 609 |
| Colour Doppler ultrasound/echocardiography | 605 | 134 | 1076 | 2343 | 964 | 3722 | 2949 | 1428 | 4470 |
| Computerized tomography | 114 | −114 | 343 | −540 | −887 | −194 | −426 | −854 | 1 |
| Immunology test | 7 | −83 | 98 | 99 | −93 | 293 | 107 | −261 | 276 |
| Oxygen saturation test | 86 | −63 | 236 | −208 | −458 | 41 | −121 | −391 | 147 |
| Sputum test | −80 | −172 | 11 | −26 | −256 | 203 | −106 | −322 | 119 |
Statistical significance at P‐value < 0.05 in bold. Coeff., unstandardized coefficient; USD, US$; LOS, length of stay; THC, total healthcare cost.
Exchange rate: 1 USD = 109.33 Japanese yen.
4. DISCUSSION
Using an administrative database of hospitalized Japanese patients with influenza, we found that influenza‐related hospitalizations mostly consisted of elderly and young patients, confirming that these 2 age groups are at high risk of influenza complications.
4.1. Impact of comorbidities on THC
It is not surprising that healthcare costs significantly increase when influenza strikes in association with other medical disorders. Our data revealed that Parkinson's disease had the highest impact on cost although it represented only 1.6% of the population, followed by cancer and ischaemic heart disease, which were 9.6% and 7.7% of the cases, respectively. The increased healthcare cost is most likely a reflection of the high incidence of influenza‐related complications that occur with these comorbidities.
Despite the low incidence of neurologic disorders associated with influenza viral infection, patients have a high risk of developing complications.30, 31 In addition, Parkinson's disease has been reported to be a clinical manifestation of influenza,32 and parkinsonian‐like symptoms such as tremors have also been described in severe influenza cases.33 Of note, influenza A is one of several viruses that have been implicated in the pathogenesis of Parkinson's disease.34, 35 Although a causal link has been difficult to establish in humans,31, 34, 36 a reduction in neuropsychiatric reactions in influenza patients treated with the antiviral oseltamivir suggests that the influenza virus may play a role in the pathogenesis of certain neurologic symptoms.37
Cancer patients are susceptible to infections such as influenza because of either treatment‐associated immunosuppression or the type of malignancy.38 These patients are also at high risk of developing influenza‐related complications. A German study that included 203 patients who had influenza along with haematologic and solid tumours reported a high rate of pneumonia and bacterial or fungal superinfections.39 Influenza also appears to have a detrimental impact on the outcome of cancer treatment by delaying the initiation of anticancer therapy.40
Chronic heart disease is one of the highest predictors of influenza‐related hospitalizations and complications.41 Epidemiologic studies have long reported an association between influenza epidemics and cardiovascular disease (CVD). For instance, acute myocardial infarctions (AMI) have their highest incidence in the winter months and are often preceded by an upper respiratory tract infection.42 In addition to the influenza‐related increase in hospitalizations for CVD, influenza is also linked to both increases in AMI43 and AMI‐related deaths.44, 45 Influenza infection has also been associated with damage to the heart muscle leading to cardiomyopathy and myocarditis.46 Taken together, these observations are consistent with our findings that patients with heart disease comprised a significant share of influenza‐related hospitalizations, and heart disease was an important driver of the increase in THC.
4.2. Role of patient origin
It was found that patients who were referred from nursing home or welfare facilities incurred less cost than those who were hospitalized from home. One possible interpretation of this interesting finding is that institutions such as nursing homes or welfare facilities do monitor their clients well and send them to the hospital even in case of a mild form of the disease. Elderly who live at home, on the other hand, might miss the right timing to seek medical advice.
4.3. Impact of procedures and ER and ICU admissions on THC
The most significant cost drivers among procedures were blood transfusions and tube feedings, which increased the THC by 380 000 Japanese yen (approximately 3450 US$).
Our findings of a high cost burden associated with ICU or ER admissions when compared with routine hospitalizations are consistent with other reports. In European countries, for instance, the daily cost of ICU admissions ranged from €1168 to €2025 (1240 to 2150 US$),47 while in the United States the estimated additional cost was 2190 US$ per day.48 These statistics underscore the importance of avoiding ICU or ER admissions whenever possible.
4.4. A role of vaccinations and antiviral treatment
The potential policy implication of our findings is that vaccination programmes should be promoted to avoid influenza‐related hospitalizations. From 1977 to 1987, there was already a vaccination programme for Japanese schoolchildren that achieved between 50% and 85% annual coverage in children aged 3‐15 years. It was shown that this vaccination programme was associated with a decrease in the overall number of influenza‐related excess deaths and that excess deaths increased once the programme was discontinued.49 Furthermore, because the vaccination of schoolchildren can reduce influenza‐related morbidity and mortality among non‐immunized contacts as well as the elderly, it was estimated that the vaccination programme could also save 1000 elderly lives per year.50
For those patients still requiring hospitalization, medical treatment may be an option to reduce hospital LOS and healthcare costs. A recent study in the United States that included 1 557 437 cases of influenza from 4 influenza seasons found an overall 11% reduction in the risk of complications in oseltamivir‐treated patients (an 81% reduction in those treated <2 days after the diagnosis).31 Antiviral treatment also decreased the risk of hospitalizations and emergency room visits by 29% and 24%, respectively. A recent cost‐effectiveness analysis in the Japanese healthcare context, for instance, demonstrated that treatment with oseltamivir was highly cost‐effective with an incremental cost‐effectiveness ratio (ICER) of 398 571 Japanese yen (3645 US$) per quality‐adjusted life year from a health insurance perspective. With the inclusion of productivity costs, the ICER for oseltamivir turned negative, meaning that medical treatment with oseltamivir was both cost‐saving and more effective.51
4.5. Limitations
There are several limitations to our study. First, this analysis is based on a 1‐year database. Thus, we could not capture the potential changes due to prescribing behaviour changes and the change of treatment guideline over time. Second, due to the limitations of the database, potentially useful information that might explain costs was lacking. For instance, we could not retrieve hospital ID numbers, which could have been used to identify heterogeneity between hospitals as well as patient characteristics such as region, social and professional status and clinical severity of their disease. Nevertheless, our analysis examined all available patient characteristics (such as age, gender and relevant comorbidities) that could be retrieved from the database. Third, bias may have resulted from the current DPC system that allows hospitals to choose the diagnosis that is incurring the most medical resource utilization as the main diagnosis. In general, patients with comorbidities will receive a higher reimbursement if hospitals choose comorbidities as the primary diagnosis. Finally, a major limitation of this study is that influenza‐related hospitalizations can be difficult to identify because influenza is not always detected as the primary cause of the hospitalization, especially in severe cases.10 As a result, this study may underestimate the true burden of influenza as well as the cost of influenza‐related hospitalizations because of the coding incentive. Only hospitalizations with influenza diagnosis, which are less costly, were included.10
ETHICAL APPROVAL
The study was in line with the guidelines provided by Johnson & Johnson and was approved by the Janssen Approval Committee.
CONFLICT OF INTEREST
JM, KH and RS are affiliated with Janssen Pharmaceutical KK, a pharmaceutical company. SF and AJ are employees of Creativ‐Ceutical, which received funding from Janssen Pharmaceutical KK to perform the study.
Supporting information
ACKNOWLEDGEMENTS
We thank Margueritte Mabry White M.D. for editing and proofreading the manuscript.
Sruamsiri R, Ferchichi S, Jamotte A, Toumi M, Kubo H, Mahlich J. Impact of patient characteristics and treatment procedures on hospitalization cost and length of stay in Japanese patients with influenza: A structural equation modelling approach. Influenza Other Respi Viruses. 2017;11:543–555. https://doi.org/10.1111/irv.12505
Funding information
This work was supported by Janssen Pharmaceutical KK.
REFERENCES
- 1. Ambrose CS, Levin MJ. The rationale for quadrivalent influenza vaccines. Hum Vaccin Immunother. 2012;8:81‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Paget WJ, Balderston C, Casas I, et al. Assessing the burden of paediatric influenza in Europe: the European Paediatric Influenza Analysis (EPIA) project. Eur J Pediatr. 2010;169:997‐1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mak TK, Mangtani P, Leese J, Watson JM, Pfeifer D. Influenza vaccination in pregnancy: current evidence and selected national policies. Lancet Infect Dis. 2008;8:44‐52. [DOI] [PubMed] [Google Scholar]
- 4. Kuster SP, Shah PS, Coleman BL, et al. Incidence of influenza in healthy adults and healthcare workers: a systematic review and meta‐analysis. PLoS One. 2011;6:e26239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention . People at High Risk of Developing Flu–Related Complications: Centers for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases (NCIRD); 2017. [cited 2017 3 March]. Available from: https://www.cdc.gov/flu/about/disease/high_risk.htm
- 6. Nokleby H, Nicoll A. Risk groups and other target groups — preliminary ECDC guidance for developing influenza vaccination recommendations for the season 2010–11. Euro Surveill. 2010;15:pii: 19525. [PubMed] [Google Scholar]
- 7. Alden DL, Merz MY, Akashi J. Young adult preferences for physician decision‐making style in Japan and the United States. Asia Pac J Public Health. 2012;24:173‐184. [DOI] [PubMed] [Google Scholar]
- 8. Simmerman JM, Uyeki TM. The burden of influenza in East and South‐East Asia: a review of the English language literature. Influenza Other Respir Viruses. 2008;2:81‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Keech M, Scott AJ, Ryan PJ. The impact of influenza and influenza‐like illness on productivity and healthcare resource utilization in a working population. Occup Med (Lond). 1998;48:85‐90. [DOI] [PubMed] [Google Scholar]
- 10. Thompson WW, Comanor L, Shay DK. Epidemiology of seasonal influenza: use of surveillance data and statistical models to estimate the burden of disease. J Infect Dis. 2006;194(Suppl 2):S82‐S91. [DOI] [PubMed] [Google Scholar]
- 11. Rothberg MB, Haessler SD, Brown RB. Complications of viral influenza. Am J Med. 2008;121:258‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Molinari NA, Ortega‐Sanchez IR, Messonnier ML, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007;25:5086‐5096. [DOI] [PubMed] [Google Scholar]
- 13. Keren R, Zaoutis TE, Saddlemire S, Luan XQ, Coffin SE. Direct medical cost of influenza‐related hospitalizations in children. Pediatrics. 2006;118:e1321‐e1327. [DOI] [PubMed] [Google Scholar]
- 14. Kashiwagi S, Ikematsu H, Hayashi J, Nomura H, Kajiyama W, Kaji M. An outbreak of influenza A (H3N2) in a hospital for the elderly with emphasis on pulmonary complications. Jpn J Med. 1988;27:177‐182. [DOI] [PubMed] [Google Scholar]
- 15. Sugaya N, Mitamura K, Nirasawa M, Takahashi K. The impact of winter epidemics of influenza and respiratory syncytial virus on paediatric admissions to an urban general hospital. J Med Virol. 2000;60:102‐106. [PubMed] [Google Scholar]
- 16. Saokaew S, Sugimoto T, Kamae I, Pratoomsoot C, Chaiyakunapruk N. Healthcare databases in Thailand and Japan: potential sources for health technology assessment research. PLoS One. 2015;10:e0141993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mahlich J, Sruamsiri R. Treatment patterns of rheumatoid arthritis in Japanese hospitals and predictors of the initiation of biologic agents. Curr Med Res Opin. 2017;33:101‐107. [DOI] [PubMed] [Google Scholar]
- 18. Mahlich J, Sruamsiri R. Persistence with biologic agents for the treatment of rheumatoid arthritis in Japan. Patient Prefer Adherence. 2016;10:1509‐1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cheung S, Hamuro Y, Mahlich J, Nakahara T, Sruamsiri R, Tsukazawa S. Drug utilization of Japanese patients diagnosed with schizophrenia: an administrative database analysis. Clin Drug Invest. 2017;37:559‐569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Udagawa Y, Ohno S, Nakagawa S, Sugimoto K, Mochizuki J. Using clinical databases to verify the impact of regulatory agency alerts in Japan: hepatitis B testing behavior after an alert regarding risk of viral reactivation. Drugs Real World Outcomes. 2015;2:227‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ogino M, Kawachi I, Otake K, et al. Current treatment status and medical cost for multiple sclerosis based on analysis of a Japanese claims database. Clin Exp Neuroimmunol. 2016;7:158‐167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hashikata H, Harada KH, Kagimura T, Nakamura M, Koizumi A. Usefulness of a large automated health records database in pharmacoepidemiology. Environ Health Prev Med. 2011;16:313‐319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Japan Bo. Exchange rate 2016 [cited 2017 09 March]. Available from: http://www.boj.or.jp/en/statistics/outline/notice_2015/not151120a.htm/
- 24. Cupurdija V, Lazic Z, Petrovic M, et al. Community‐acquired pneumonia: economics of inpatient medical care vis‐à‐vis clinical severity. J Bras Pneumol. 2015;41:48‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang J, Jit M, Leung KS, et al. The economic burden of influenza‐associated outpatient visits and hospitalizations in China: a retrospective survey. Infect Dis Poverty. 2015;4:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stein CM, Morris NJ, Nock NL. Structural equation modeling. Methods Mol Biol. 2012;850:495‐512. [DOI] [PubMed] [Google Scholar]
- 27. Hays RD, Revicki D, Coyne KS. Application of structural equation modeling to health outcomes research. Eval Health Prof. 2005;28:295‐309. [DOI] [PubMed] [Google Scholar]
- 28. Cella D, Nichol MB, Eton D, Nelson JB, Mulani P. Estimating clinically meaningful changes for the Functional Assessment of Cancer Therapy–Prostate: results from a clinical trial of patients with metastatic hormone‐refractory prostate cancer. Value Health. 2009;12:124‐129. [DOI] [PubMed] [Google Scholar]
- 29. Chan CM, Ahmad WA. Differences in physician attitudes towards patient‐centredness: across four medical specialties. Int J Clin Pract. 2012;66:16‐20. [DOI] [PubMed] [Google Scholar]
- 30. Centers for Disease Control and Prevention (CDC) . Seasonal influenza (flu) – People at High Risk of Developing Flu‐Related Complications; 2011.
- 31. Spagnuolo PJ, Zhang M, Xu Y, et al. Effects of antiviral treatment on influenza‐related complications over four influenza seasons: 2006–2010. Curr Med Res Opin. 2016;32:1399‐1407. [DOI] [PubMed] [Google Scholar]
- 32. Henry J, Smeyne RJ, Jang H, Miller B, Okun MS. Parkinsonism and neurological manifestations of influenza throughout the 20th and 21st centuries. Parkinsonism Relat Disord. 2010;16:566‐571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Toovey S, Jick SS, Meier CR. Parkinson's disease or Parkinson symptoms following seasonal influenza. Influenza Other Respir Viruses. 2011;5:328‐333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jang H, Boltz DA, Webster RG, Smeyne RJ. Viral parkinsonism. Biochim Biophys Acta. 2009;1792:714‐721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jang H, Boltz D, Sturm‐Ramirez K, et al. Highly pathogenic H5N1 influenza virus can enter the central nervous system and induce neuroinflammation and neurodegeneration. Proc Natl Acad Sci USA. 2009;106:14063‐14068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McCall S, Henry JM, Reid AH, Taubenberger JK. Influenza RNA not detected in archival brain tissues from acute encephalitis lethargica cases or in postencephalitic Parkinson cases. J Neuropathol Exp Neurol. 2001;60:696‐704. [DOI] [PubMed] [Google Scholar]
- 37. Smith JR, Sacks S. Incidence of neuropsychiatric adverse events in influenza patients treated with oseltamivir or no antiviral treatment. Int J Clin Pract. 2009;63:596‐605. [DOI] [PubMed] [Google Scholar]
- 38. Borella L, Webster RG. The immunosuppressive effects of long‐term combination chemotherapy in children with acute leukemia in remission. Cancer Res. 1971;31:420‐426. [PubMed] [Google Scholar]
- 39. Hermann B, Lehners N, Brodhun M, et al. Influenza virus infections in patients with malignancies – characteristics and outcome of the season 2014/15. A survey conducted by the Infectious Diseases Working Party (AGIHO) of the German Society of Haematology and Medical Oncology (DGHO). Eur J Clin Microbiol Infect Dis. 2017;36:565‐573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shehata MA, Karim NA. Influenza vaccination in cancer patients undergoing systemic therapy. Clin Med Insights Oncol. 2014;8:57‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Irwin DE, Weatherby LB, Huang WY, Rosenberg DM, Cook SF, Walker AM. Impact of patient characteristics on the risk of influenza/ILI‐related complications. BMC Health Serv Res. 2001;1:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Madjid M, Aboshady I, Awan I, Litovsky S, Casscells SW. Influenza and cardiovascular disease: is there a causal relationship? Tex Heart Inst J. 2004;31:4‐13. [PMC free article] [PubMed] [Google Scholar]
- 43. Macintyre CR, Heywood AE, Kovoor P, et al. Ischaemic heart disease, influenza and influenza vaccination: a prospective case control study. Heart. 2013;99:1843‐1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tillett HE, Smith JW, Gooch CD. Excess deaths attributable to influenza in England and Wales: age at death and certified cause. Int J Epidemiol. 1983;12:344‐352. [DOI] [PubMed] [Google Scholar]
- 45. Warren‐Gash C, Bhaskaran K, Hayward A, et al. Circulating influenza virus, climatic factors, and acute myocardial infarction: a time series study in England and Wales and Hong Kong. J Infect Dis. 2011;203:1710‐1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ruf BR, Szucs T. Reducing the burden of influenza‐associated complications with antiviral therapy. Infection. 2009;37:186‐196. [DOI] [PubMed] [Google Scholar]
- 47. Tan SS, Bakker J, Hoogendoorn ME, et al. Direct cost analysis of intensive care unit stay in four European countries: applying a standardized costing methodology. Value Health. 2012;15:81‐86. [DOI] [PubMed] [Google Scholar]
- 48. Dasta JF, McLaughlin TP, Mody SH, Piech CT. Daily cost of an intensive care unit day: the contribution of mechanical ventilation. Crit Care Med. 2005;33:1266‐1271. [DOI] [PubMed] [Google Scholar]
- 49. Reichert TA, Sugaya N, Fedson DS, Glezen WP, Simonsen L, Tashiro M. The Japanese experience with vaccinating schoolchildren against influenza. N Engl J Med. 2001;344:889‐896. [DOI] [PubMed] [Google Scholar]
- 50. Charu V, Viboud C, Simonsen L, et al. Influenza‐related mortality trends in Japanese and American seniors: evidence for the indirect mortality benefits of vaccinating schoolchildren. PLoS One. 2011;6:e26282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nagase H, Moriwaki K, Kamae M, Yanagisawa S, Kamae I. Cost‐effectiveness analysis of oseltamivir for influenza treatment considering the virus emerging resistant to the drug in Japan. Value Health. 2009;12(Suppl 3):S62‐S65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


