Abstract
Fibroblast growth factor 21 (FGF21), a stress-induced hormone in the liver, has been shown the protective functions in pathological conditions. The study investigated the association of circulating FGF21 with hepatitis B virus (HBV) infection and its related diseases. Serum FGF21 levels were measured in 33 acute hepatitis B (AHB), 75 chronic hepatitis B (CHB) and 66 CHB patients with advanced liver diseases including liver cirrhosis, acute-on-chronic liver failure (ALCF) and hepatocellular carcinoma (HCC) together with 200 age- and BMI-matched healthy controls. FGF21 levels were significantly increased in AHB patients and rapidly returned to normal levels after treatment. FGF21 levels reflected the degree of liver injury caused by AHB. However, serum FGF21 levels were decreased in CHB patients especially in those who developed cirrhosis and were associated with hepatic protein synthesis capacity. Serum FGF21 in CHB patients were increased with the occurrence of ACLF. Notably, in CHB patients who developed HCC, serum FGF21 exhibited a dramatic increase, which may provide important information on monitoring tumorigenesis in CHB patients. In conclusion, we revealed the diverse changes of circulating FGF21 in HBV-related diseases. FGF21 may be a useful biomarker in monitoring the tumorigenesis in patients with CHB.
Introduction
Hepatitis B virus (HBV) is the most common hepatitis virus with more than two billion people have been infected worldwide, and it is estimated that 50 million new cases are added every year and 240 million people are chronically infected with hepatitis B, most in Asia and Africa1,2. Chronic hepatitis B (CHB) infection may progress to liver cirrhosis and liver failure, and is the major cause of hepatocellular carcinoma (HCC)3. Monitoring the disease progression of HBV infection has been a major clinical concern for the early treatment of associated liver disease and lowering morbidity and mortality. Traditional biomarkers like alanine aminotransferase (ALT) have been widely used for detecting liver injury4. When liver injury occurs, the enzymes that are aggregated in the cytosol of the hepatocytes are released into circulation and cause an elevation in serum concentration5. Notably, hepatokines that response to liver stress may reflect liver status, and provide a noninvasive and easy-to-perform means of monitoring therapeutic effect and progression in HBV-related diseases.
Fibroblast growth factor 21 (FGF21) is an important metabolic regulator that is predominantly produced by the liver6,7. Despite its pleiotropic metabolic effects on maintaining glucose and lipid homeostasis, growing evidence from both animal and clinical studies has demonstrated that FGF21 is involved in multiple liver stimuli. FGF21 expression is significantly induced in hepatocytes in response to carcinogenic transformation in mice undergoing chemical and genetic-induced hepatocarcinogenesis8. Acetaminophen overdose causes a drastic increase in both circulating levels and hepatic expression of FGF21 in mice, which in turn acts as a feedback signal to protect mice from acetaminophen-induced hepatotoxicity by enhancing the antioxidant capacity9. In humans, serum FGF21 levels are elevated in the early phase of patients with nonalcoholic fatty liver disease (NAFLD) and positively correlates with gamma-glutamyl transpeptidase (GGT)10. Moreover, in patients with liver transplantation, serum FGF21 levels rise sharply and reach the peak as early as two hours after reperfusion, whereas the peak of serum ALT levels appears at 24 hours after reperfusion, suggesting a superior sensitivity of serum FGF21 compared to the currently used biomarkers for detection of hepatic ischemia/reperfusion injury11.
FGF21 may reflect functional status of the liver, but the role of FGF21 in hepatitis viral infection is still unclear. A previous study performed in 75 chronic hepatitis C patients showed that serum FGF21 were significantly higher in those subjects compared with those in healthy subjects12. However, another study demonstrated that the elevation of hepatitis C virus RNA was accompanied by a decreased expression of FGF21 mRNA in the liver13. Chronic HBV infection may progress to HBV-related liver diseases including cirrhosis, acute-on-chronic liver failure (ACLF) and HCC3, but the association between hepatic FGF21 expression and the progression of those diseases is largely unknown. The objective of the study was to investigate whether serum FGF21 levels are altered in HBV-infected patients and to explore the association of serum FGF21 levels with HBV-related diseases.
Results
Serum FGF21 levels were increased in AHB patients but decreased in CHB patients
The clinical and laboratory characteristics of the patients with AHB and CHB are summarized in Table 1. Compared with controls, patients with AHB and CHB had similar age and BMI. Biochemical indicators of liver injury were significantly higher in AHB patients than those in controls, including ALT, aspartate aminotransferase (AST), GGT, alkaline phosphatase (ALP), total bilirubin (TBil), total bile acid (TBA) and c-reactive protein (CRP) (all P < 0.001). Serum FGF21 levels were found significantly higher in AHB patients (243.3 pg/ml [189.6–451.7]) than those in control subjects (198.4 pg/ml [152.8–272.2]) (P < 0.001). After antiviral and supportive treatment for 11.8 ± 1.7 days, serum FGF21 levels in AHB patients were significantly decreased (112.8 pg/ml [83.2–186.3], P = 0.001) together with the indicators of liver injury.
Table 1.
Anthropometric and biochemical parameters in control, AHB, AHB after treatment and CHB groups.
| Variables | Control (n = 200) | AHB (n = 33) | AHB after treatment (n = 12) | CHB (n = 75) |
|---|---|---|---|---|
| Age (year) | 40.4 ± 9.5 | 44.7 ± 14.9* | 35.9 ± 11.8 | 35.9 ± 11.7*# |
| Male (n, %) | 67 (34%) | 27 (82%) | 8 (67%) | 55 (73%) |
| BMI (kg/m2) | 22.1 ± 2.2 | 22.0 ± 2.2 | 20.2 ± 2.4*,# | 22.3 ± 2.8 |
| AST (U/L)† | 18.0 (16.0, 21.0) | 690.0 (351.5, 845.0)* | 54.0 (26.3, 120.5)*,# | 157.0 (79.0, 315.0)*,# |
| ALT (U/L)† | 14.0 (10.0, 18.0) | 1548.0 (1205.0, 2141.5)* | 299.5 (58.3, 422.8)*,# | 320.0 (161.0, 754.0)*,# |
| GGT (U/L)† | 16.0 (13.0, 20.0) | 175.0 (136.0, 307.5)* | 118.0 (83.0, 224.3)* | 84.0 (50.0, 166.0)*,# |
| ALP (U/L)† | 64.0 (53.0, 76.0) | 144.0 (115.0, 175.0)* | 84.0 (79.0, 111.3)*,# | 86.0 (66.0, 111.0)*,# |
| TBil (μmol/L)† | 11.2 (8.5, 14.6) | 74.4 (36.9, 145.4)* | 21.3 (16.2, 26.4)*,# | 18.5 (13.3, 41.5)*,# |
| TBA (μmol/L)† | 3.3 (2.3, 4.8) | 109.8 (30.8, 212.1)* | 7.7 (4.9, 19.6)*,# | 16.2 (9.1, 53.9)*,# |
| CRP (mg/L)† | 0.4 (0.2, 0.9) | 5.7 (1.9, 15.4)* | / | 2.0 (1.6, 4.4)*,# |
| Cholinesterase (U/L)† | 330.0 (290.3, 370.8) | 255.0 (226.5, 299.5)* | 264.0 (207.8, 316.0)* | 268.0 (186.0, 320.0)* |
| A/G† | 1.88 (1.72, 2.05) | 1.63 (1.51, 1.78)* | 1.81 (1.59, 2.08) | 1.60 (1.40, 1.80)* |
| Albumin (g/L)† | 48.0 (46.0, 49.0) | 41.0 (38.5, 42.5)* | 43.0 (38.5, 45.0)* | 42.0 (38.0, 44.0)* |
| Total protein (g/L)† | 73.0 (70.3, 76.0) | 64.0 (61.0, 68.5)* | 65.0 (62.3, 70.3)* | 67.0 (62.0, 71.0)* |
| TC (mmol/L)† | 4.4 (3.9, 5.0) | 3.5 (3.2, 4.2)* | / | 4.0 (3.7, 4.5)*,# |
| Triglyceride (mmol/L)† | 1.0 (0.7, 1.4) | 1.7 (1.1, 2.4)* | / | 1.1 (0.8, 1.4)# |
| HDL-C (μmol/L)† | 1.4 (1.2, 1.6) | 0.5 (0.3, 0.8)* | / | 1.2 (0.6, 1.4)*,# |
| LDL-C (μmol/L)† | 2.7 (2.3, 3.3) | 1.9 (1.5, 2.5)* | / | 2.2 (1.7, 2.7)* |
| LgHBV-DNA (IU/ml)† | / | 3.7 (1.0, 5.6) | 1.0 (1.0, 2.0)# | 6.8 (5.4, 7.6)# |
| FGF21 (pg/ml)† | 198.4 (152.8, 272.2) | 243.3 (189.6, 451.7)* | 112.8 (83.2, 186.3)*,# | 142.8 (82.0, 265.3)*,# |
*P value < 0.05 vs. control. #P value < 0.05 vs. AHB. †Log-transformed before analysis.
CRP and lipid profiles in the group of AHB after treatment were not measured.
A/G, albumin/globulin; AHB, acute hepatitis B; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CRP, c-reactive protein; CHB, chronic hepatitis B; FGF21, fibroblast growth factor 21; GGT, gamma-glutamyl transpeptidase; HBV, hepatitis B virus; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TBA, total bile acid; TBil, total bilirubin; TC, total Cholesterol.
In patients with CHB, liver synthetic function was impaired, reflected by lower albumin, total protein, and cholinesterase (all P < 0.001). Unlike in AHB patients, serum FGF21 levels in CHB patients (142.8 pg/ml [82.0–265.3]) were significantly lower than those in controls (P < 0.001). (Fig. 1).
Figure 1.
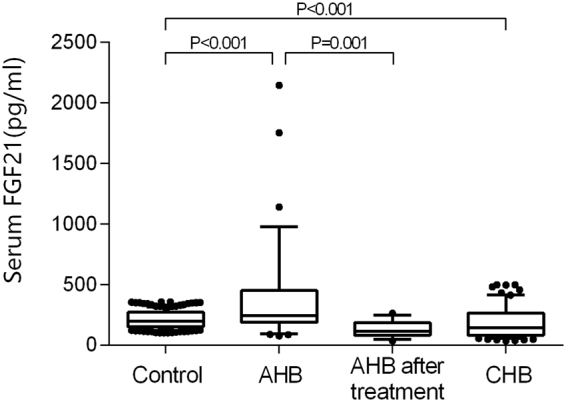
Serum FGF21 concentrations in controls and patients with AHB or CHB. Data are shown as box-and-whisker plots. The horizontal line in the middle of each box indicates the median value. The top and bottom borders of the boxes represent the 75th and 25th percentiles, respectively; the whisker represents the 10th and 90th percentiles, respectively; and the dots represent the outliers.
Serum FGF21 levels further decreased in HBV-related liver cirrhosis but increased in CHB patients developed ACLF or HCC
We next investigated the changes of serum FGF21 in chronic HBV infected patients with advanced liver diseases. Their clinical and laboratory characteristics are summarized in Table 2. Compared with CHB patients, patients who developed HBV-related liver cirrhosis had lower AST, ALT, GGT, and HBV-DNA levels. Liver synthetic function was even worse in cirrhotic patients reflected by lower serum albumin and cholinesterase than CHB patients (all P < 0.05). Serum FGF21 levels were significantly lower in cirrhotic patients (90.2 pg/ml [74.5–142.9]) compared with those in CHB patients (P < 0.001). CHB patients who developed ACLF had significantly higher indicators of inflammation and liver injury (CRP, AST, ALP, TBil, TBA, all P < 0.05). In those subjects with ACLF, serum FGF21 levels (407.1 pg/ml [141.8–640.0]) were significantly higher than those in CHB patients (P = 0.001). In CHB patients who developed HCC, the overall levels of serum α-fetoprotein had a trend to increase, but it was not statistically significant in our study (P = 0.287). In fact, 38% of these HCC patients showed normal α-fetoprotein levels. Notably, serum FGF21 levels in those patients exhibited a dramatic increase (398.6 pg/ml [244.0–827.6]) compared with CHB patients (P < 0.001) (Fig. 2).
Table 2.
Anthropometric and biochemical parameters among CHB patients and CHB patients with advanced liver diseases.
| Variables | CHB (n = 75) | CHB-Cirrhosis (n = 40) | CHB-ACLF (n = 17) | CHB-HCC (n = 9) |
|---|---|---|---|---|
| Age (year) | 35.9 ± 11.7 | 47.6 ± 15.6* | 41.4 ± 15.0 | 56.7 ± 8.7* |
| Male (n, %) | 55 (73%) | 29 (73%) | 12 (71%) | 7 (78%) |
| BMI (kg/m2) | 22.3 ± 2.8 | 22.5 ± 2.4 | 23.1 ± 2.7 | 24.1 ± 2.8 |
| AST (U/L)† | 157.0 (79.0, 315.0) | 50.5 (38.3, 138.0)* | 405.0 (173.0, 879.0)* | 65.0 (34.5, 117.5)* |
| ALT (U/L)† | 320.0 (161.0, 754.0) | 67.5 (33.0, 177.8)* | 692.0 (268.0, 968.5) | 57.0 (37.5, 122.5)* |
| GGT (U/L)† | 84.0 (50.0, 166.0) | 48.5 (25.8, 121.5)* | 103.0 (59.5, 139.0) | 147.0 (73.5, 232.5) |
| ALP (U/L)† | 86.0 (66.0, 111.0) | 92.0 (76.5, 126.5) | 106.0 (91.5, 151.0)* | 123.0 (92.5, 228.5)* |
| TBil (μmol/L)† | 18.5 (13.3, 41.5) | 24.6 (15.1, 48.3) | 197.6 (98.3, 294.2)* | 19.9 (9.7, 79.7) |
| TBA (μmol/L)† | 16.2 (9.1, 53.9) | 29.5 (10.8, 64.3) | 169.1 (109.5, 208.2)* | 6.9 (4.0, 86.6) |
| CRP (mg/L)† | 2.0 (1.6, 4.4) | 0.7 (0.3, 7.9) | 9.6 (4.9, 16.2)* | 9.7 (2.5, 11.4) |
| Cholinesterase (U/L)† | 268.0 (186.0, 320.0) | 164.0 (98.0, 251.5)* | 140.0 (119.5, 173.5)* | 208.0 (112.0, 353.0) |
| A/G† | 1.60 (1.40, 1.80) | 1.30 (0.88, 1.50)* | 1.41 (1.12, 1.64)* | 1.74 (1.22, 1.91) |
| Albumin (g/L)† | 42.0 (38.0, 44.0) | 35.0 (31.0, 40.3)* | 34.0 (31.5, 38.5)* | 42.0 (31.5, 45.0) |
| Total protein (g/L)† | 67.0 (62.0, 71.0) | 67.0 (62.5, 71.0) | 61.0 (58.0, 65.0)* | 66.0 (60.5, 72.5) |
| TC (mmol/L)† | 4.0 (3.7, 4.5) | 3.8 (3.2, 4.3) | 2.6 (2.0, 3.3)* | 4.2 (3.6, 5.6) |
| Triglyceride (mmol/L)† | 1.1 (0.8, 1.4) | 0.9 (0.8, 1.3) | 1.4 (0.9, 1.9)* | 1.1 (0.7, 1.2) |
| HDL-C (μmol/L)† | 1.2 (0.6, 1.4) | 1.3 (1.0, 1.4) | 0.2 (0.1, 0.3)* | 1.1 (0.2, 1.4) |
| LDL-C (μmol/L)† | 2.2 (1.7, 2.7) | 2.0 (1.8, 2.2) | 1.2 (0.8, 1.7)* | 3.2 (2.0, 3.5) |
| LgHBV-DNA (IU/ml)† | 6.8 (5.4, 7.6) | 4.2 (2.7, 5.7)* | 6.5 (3.8, 7.6) | 3.3 (1.0, 5.6)* |
| α-fetoprotein (μg/L)† | 11.6 (5.2, 25.5) | 14.5 (4.2, 46.0) | 37.5 (18.7, 201.3)* | 18.3 (4.8, 105.7) |
| FGF21 (pg/ml)† | 142.8 (82.0, 265.3) | 90.2 (74.5, 142.9)* | 407.1 (141.8, 640.0)* | 398.6 (224.0, 827.6)* |
*P value < 0.05 vs. CHB †Log-transformed before analysis.
ACLF, acute-on-chronic liver failure; A/G, albumin/globulin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CRP, c-reactive protein; CHB, chronic hepatitis B; FGF21, fibroblast growth factor 21; GGT, gamma-glutamyl transpeptidase; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TBA, total bile acid; TBil, total bilirubin; TC, total Cholesterol.
Figure 2.
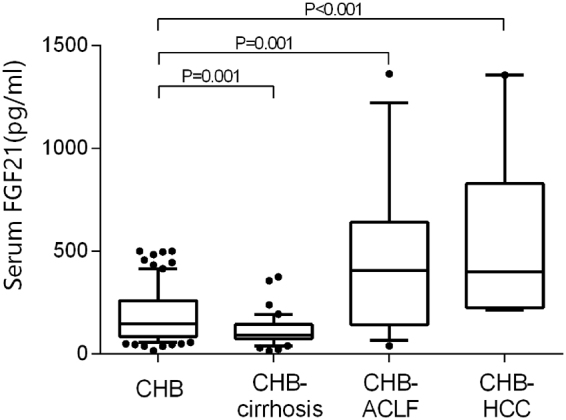
Serum FGF21 levels in patients with advanced liver diseases. Data are shown as box-and-whisker plots. The horizontal line in the middle of each box indicates the median value. The top and bottom borders of the boxes represent the 75th and 25th percentiles, respectively; the whisker represents the 10th and 90th percentiles, respectively; and the dots represent the outliers.
Serum FGF21 levels were positively correlated with the degree of acute liver injury and liver synthetic function
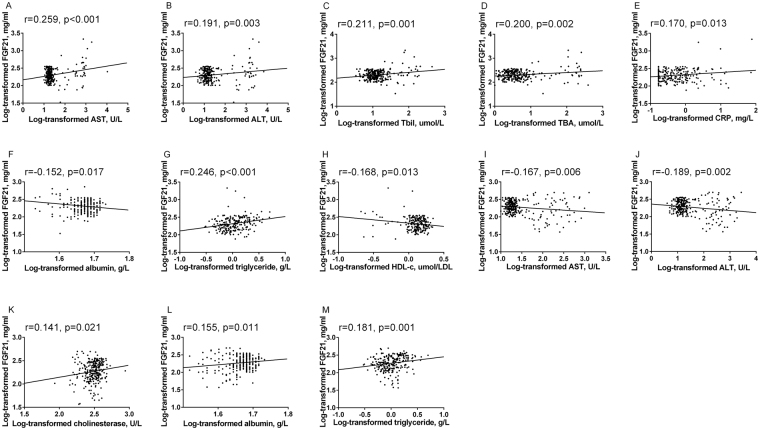
Correlations between serum FGF21 levels and other biochemical indexes in AHB and CHB patients are shown in Table S1, and those with statistical significance are presented in dot plots (Fig. 3). In AHB patients, after adjustment for age and BMI, serum FGF21 levels were positively correlated with the elevation of AST, ALT, TBil, TBA and CRP (both P < 0.05). In CHB patients, serum FGF21 levels were positively associated with indicators of liver function including cholinesterase and albumin (both P < 0.05).
Figure 3.
Relationship between serum FGF21 levels with other parameters in patients with AHB (A–H) and CHB (I–M).
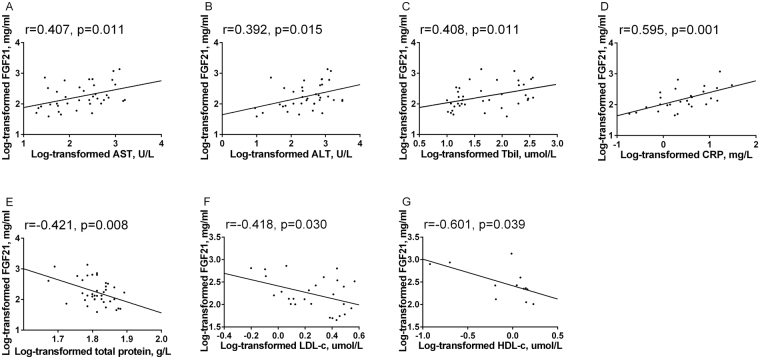
Correlations between serum FGF21 levels and other biochemical indexes in CHB patients with advanced liver diseases are shown in Table S2, and those with statistical significance are presented in dot plots (Fig. 4). In patients developed ACLF, serum FGF21 levels were positively correlated with indexes reflecting liver injury and inflammation including AST, ALT, TBil and CRP (all P < 0.05) after the adjustment for age and BMI. However, no significance was found between FGF21 and serum markers for liver fibrosis such as procollagen III n-terminal propeptide (P3NP), laminin, collagen type IV and hyaluronic acid in cirrhotic patients. Moreover, serum FGF21 levels were not correlated with α-fetoprotein in CHB patients who developed HCC. Notably, no significant associations were found between serum FGF21 levels and HBV-DNA load in each group no matter patients with AHB or CHB.
Figure 4.
Relationship between serum FGF21 levels with other parameters in patients with CHB-ACLF (A–F) and CHB-HCC (G).
Predictive value of FGF21 for HCC in CHB patients
Serum FGF21 showed abnormal elevation in CHB patient who developed HCC, suggested that FGF21 elevation may provide information on HCC onset. To assess the predictive value of FGF21 for HCC, we generated the area under the receiver-operating characteristic (ROC) curve in all CHB patients except for those with ACLF. The result is shown in Fig. 5. For FGF21, the area under the curve (AUC) was 0.892 (95% CI 0.811–0.973, P < 0.001 compared with reference line). The AUC of FGF21 was larger than α-fetoprotein (0.598, 95%CI [0.388–0.807]), P = 0.01. The sensitivity, specificity, positive likelihood ratio, and negative likelihood ratio for predicting HCC with serum FGF21 are shown in Table 3.
Figure 5.
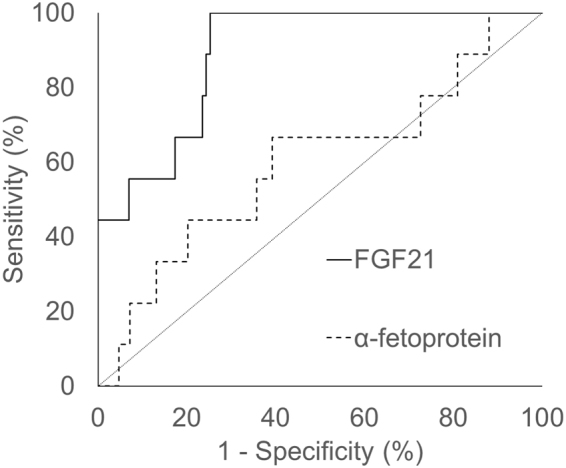
ROC curves for predicting HCC in CHB patients. FGF21, AUC = 0.892 (95% CI, 0.811–0.973); α-fetoprotein, AUC = 0.598 (95% CI, 0.388–0.807); FGF21 vs. α-fetoprotein, p = 0.01.
Table 3.
Sensitivity, specificity, positive likelihood ratio, and negative likelihood ratio for HCC prediction using serum FGF21 in CHB patients.
| Cut-off of Serum FGF21 (pg/ml) | Sensitivity (%) | Specificity (%) | Youden index | Positive likelihood ratio | Negative likelihood ratio |
|---|---|---|---|---|---|
| 200 | 100.0 | 74.8 | 1.748 | 3.968 | 0.000 |
| 225 | 77.8 | 76.5 | 1.543 | 3.311 | 0.290 |
| 250 | 66.7 | 80.9 | 1.476 | 3.492 | 0.412 |
| 275 | 55.6 | 82.6 | 1.382 | 3.195 | 0.538 |
| 300 | 55.6 | 84.3 | 1.399 | 3.541 | 0.527 |
| 325 | 55.6 | 86.1 | 1.417 | 4.000 | 0.516 |
| 350 | 55.6 | 89.6 | 1.452 | 5.346 | 0.496 |
| 375 | 55.6 | 93.0 | 1.486 | 7.943 | 0.477 |
| 400 | 44.4 | 93.0 | 1.374 | 6.343 | 0.598 |
Those result suggested that the increase of serum FGF21 was closely related to the active liver damage reflected by elevation of serum aminotransferases and CRP levels. Meanwhile, the decrease of serum FGF21 in patients with chronic HBV infection was mainly associated with the impaired liver synthetic function. Elevation of serum FGF21 in CHB patients may provide valuable information in the onset of HCC.
Discussion
The study demonstrated for the first time the association of serum FGF21 levels with HBV infection. Serum FGF21 levels were significantly increased in AHB patients and then decreased rapidly to normal after antiviral and supportive treatment. To the contrary, serum FGF21 exhibited a marked decrease in patients with CHB, especially in cirrhotic patients and were related with liver synthetic function. Notably, with the occurrence of ACLF or HCC, serum FGF21 levels were increased dramatically in CHB patients. Serum FGF21 levels were found to reflect the degree of liver injury and impairment of synthetic function caused by HBV infection.
FGF21 is a stress-induced hormone in the liver. In our study, serum FGF21 levels were significantly higher in AHB patients and positively correlated with the elevation of serum aminotransferases and CRP, suggesting the consistency between serum FGF21 levels and the extent of liver damage. The release of mitochondrial enzymes from the liver is considered for evidence of hepatic necrosis14. In our study, FGF21 had a stronger association with AST, which might suggest that FGF21 regulated by mitochondrial stress and hepatic necrosis. After antiviral and supportive therapy, FGF21 decreased dramatically before the normalization of serum aminotransferases. These results suggest that FGF21 might be a quick detector reflecting conditions of liver stress and inflammation in AHB patients. ACLF is a serious consequence of the acute exacerbation of CHB in China15. Serum FGF21 levels in ACLF patients also showed similar changes as in AHB patients. Serum FGF21 levels in ACLF patients were increased remarkably and were positively correlated with CRP, aminotransferases, and other indicators of liver injury. These findings suggest that the severe endoplasmic reticulum (ER) stress and injury of hepatocytes in the progression of ACLF16 might lead to the increase of hepatic FGF21 expression.
HBV can interrupt protein folding and impair lipid metabolism in the host cell, which will initiate ER stress and induce oxidative stress17–19. Several studies have demonstrated that ER stress upregulates the expression of FGF21 in the liver20–23. IRE1α/XBP1 and PERK/eIF2alpha/ATF4 pathway of the unfolded protein response (UPR) can directly induce the expression of FGF21 by interacting with the response elements for XBP1 and ATF4 in the FGF21 promoter region20–22. FGF21 is also the target gene for C/EBP homologous protein (CHOP) as a downstream factor of ER stress23 and elevated FGF21 acts, in turn, to suppress the eIF2α-ATF4-CHOP signaling and alleviate ER stress-induced hepatic damage20. Besides ER stress, oxidative stress could also cause a dramatic elevation of both serum levels and hepatic expression of FGF219. Increased FGF21 presents as a compensatory response for the liver to enhance antioxidant capacity and protects against hepatotoxicity9. Therefore, the elevation of FGF21 may partly be explained by ER stress and oxidative stress that participates in the progression of HBV infection and may exert a beneficial effect in counteracting stress-induced hepatic damage.
However, unlike in patients with acute liver injury, we noted that serum FGF21 levels were lower in CHB patients, although their serum aminotransferases and CRP were still higher than healthy subjects. In previous studies, the decrease of serum FGF21 was also observed under conditions of severe NAFLD and autoimmune diabetes, which resulted from impaired cell function caused by lipotoxicity, hepatic inflammation or autoimmune antibodies24,25. In our study, the drop of serum FGF21 was positively correlated with the decrease of cholinesterase and albumin in CHB patients, indicating a possible correlation between serum FGF21 and liver synthetic function. As the liver is the major source of FGF21, the impaired liver function may explain the decrease of FGF21 in CHB patients. In CHB patients who developed HBV-related liver cirrhosis, even lower serum FGF21 could be explained by worse liver function due to liver fibrosis26. Taken together, these observations support the idea that the decrease of FGF21 in CHB patients was mainly due to the poor synthetic function of the liver. However, FGF21 no longer responded to the prolonged ER stress in CHB patients. The underlying mechanism needs to be further studied.
Our result showed that serum FGF21 significantly increased in CHB patients who developed HCC, indicating that FGF21 could be induced by tumorigenesis in human. Previous study on human hepatic tissues demonstrates a high level of FGF21 expression in low-grade HCC foci area of well-differentiated cells, and also in tumor-adjacent and phenotypically normal liver area. Whereas the lost expression of FGF21 is found in high-grade HCC foci area of poorly differentiated tumor cells8. It has been reported that P53, an important tumor suppressor, negatively regulates the expression of FGF21 through binding to its promoter and mutant p53 leads to a drastic elevation of hepatic FGF21 in genetic-induced hepatocarcinogenesis mice model8. The elevation of FGF21 expression in hepatocyte may be a protective response, as evidence showed that forced expression of hepatic FGF21 in transgenic mice delays the initiation of chemical-induced hepatocarcinogenesis27, and loss of FGF21 plays an important role in HCC carcinogenetic transformation28.
Serum α-fetoprotein is the most widely used tumor marker for detecting HCC. However, elevated serum α-fetoprotein is only observed in 60–70% of HCC patients. Furthermore, nonspecific elevation of α-fetoprotein has been found in 15–58% of CHB patients and 11–47% of cirrhotic patients29. In our study, the abnormal elevation of serum FGF21 in HCC was more obvious, because the patients with CHB or liver cirrhosis had lower serum FGF21 levels. All the 9 patients with HCC had an FGF21 level over 200 pg/ml, whereas the median serum FGF21 levels in CHB and CHB-cirrhosis were 142.8 and 90.2 pg/ml, respectively. Although patients with ACLF also showed a significant elevation in serum FGF21, they had an obvious liver injury at the same time. Therefore, monitoring the elevation of serum FGF21 levels in CHB patients may provide valuable information about tumorigenesis, especially in those without obvious clinical signs of acute liver injury.
There are several limitations in our study. The first one is the lack of dynamic change of serum FGF21 since we only looked at one time point in each subject. Although we enrolled a subgroup of AHB patients after treatment, it is still unclear when is the turning point of serum FGF21 from a high level to low level. Serum FGF21 could change rapidly during acute liver injury according to our previous findings9. Secondly, the sample size of patients with HBV-related liver diseases was relatively small, especially for patients with HCC, which limited the further analysis to find out a diagnostic cutoff of serum FGF21 level. Thirdly, the age and BMI were not matched in disease groups, which may have some impact on the results. Besides, our result showed that, rather than a specific response to HBV infection, change of circulating FGF21 was more likely to be an indirect consequence induced by liver stress.
In conclusion, we present first clinical evidence revealing the diverse changes of serum FGF21 levels in HBV infected patients under different clinical conditions. Serum FGF21 levels were positively associated with serum aminotransferases and could reflect the degree of liver injury caused by HBV infection. However, serum FGF21 levels were decreased in CHB patients, especially in patients with HBV-related liver cirrhosis, due to impaired hepatic protein synthesis capacity. The abnormal elevation of FGF21 in CHB who developed HCC indicated the potential role of FGF21 as a biomarker in monitoring the tumorigenesis in CHB patients. Further studies are needed to clarify the cause-and-effect relationship between serum FGF21 and the progression HBV-related disease. More participants with advanced liver diseases should be involved in the study to make the results more reliable.
Methods
Subjects and study design
The study was cross-sectional designed. From October 2013 to June 2015, 186 HBV infected patients who were hospitalized in the Department of Infectious Diseases were observed. The number of cases in the hospital during the study period determined the sample size. Among all subjects, 33 had AHB, 75 had CHB, 40 had CHB-related liver cirrhosis, 17 had CHB-related ACLF, and 9 had CHB-related HCC. All subjects were interviewed to obtain their history of hepatitis, diabetes mellitus, hypertension, and cardiovascular diseases. Each patient was submitted to abdominal ultrasound or computed tomography (CT) scan. Fasting serum samples were drawn in the first morning after hospitalization. In a subgroup of AHB, the above examinations were conducted, and serum samples were drawn in these patients after receiving antiviral and supportive treatment for 11.8 ± 1.7 days. We also included age- and BMI-matched 200 healthy controls, who were recruited from our community-based epidemiological survey. Subjects with diabetes, obesity (BMI over 28) and cardiovascular diseases were not included due to their association with FGF21. The control subjects had no past or current viral hepatitis infection or other liver diseases. The study was approved by the ethics committee of the Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, following the principles of the declaration of Helsinki. Written informed consent was obtained from all subjects. Authors removed personal identifier of the participants to protect confidential information.
Diagnostic criteria
AHB was defined as patients with immunoglobulin M antibodies to the hepatitis B core antigen (IgM anti-HBc) and clinical signs or symptoms suggestive of acute hepatitis without a history of hepatitis B virus infection before the episode, and with the loss of hepatitis B surface antigen within six months after onset of acute hepatitis. CHB was defined as patients with positive HBsAg for more than six months, serum HBV DNA > 2000 IU/ml, ALT > 40U/L and excluding cirrhosis, liver tumor, and ACLF. CHB-related liver cirrhosis was defined as CHB patients with liver cirrhosis identified by ultrasound or CT. CHB-related ACLF was defined as CHB patients with acute hepatic insult manifesting as jaundice (serum bilirubin ≥ 85 μmol/L) and coagulopathy (international normalized ratio > 1.5), complicated within four weeks by ascites and/or encephalopathy30. CHB-related HCC was defined as CHB patients with space-occupying lesion in the liver discovered by ultrasound or CT.
Anthropometric and biochemical measurements
BMI was calculated as the measured body weight in kilograms divided by the square of the measured height in meters. Biochemical indexes were measured on a Hitachi 7600 analyzer (Hitachi, Tokyo, Japan). Serum levels of total cholesterol (TC), triglyceride, high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) were determined enzymatically. ALT and AST were measured by ultra violet method. GGT was measured by the Szasz-Persijn method. HBV serologic markers were determined with a chemiluminescent microparticle immunoassay using the Abbott Architect immunoassay system (Abbott Laboratories, Abbott Park, IL, USA). The HBV DNA levels were measured by polymerase chain reaction using the Cobas Amplicor HBV monitor test (Roche Diagnostics, Mannheim, Germany), with a lower limit of quantification at 57 IU/ml. Concentrations of FGF21 in serum were quantified using the enzyme-linked immunosorbent assay kits (Antibody and Immunoassay services, The University of Hong Kong).
Statistical analysis
All analysis was performed using SPSS 22 for Windows. Normality was determined by Kolmogorov-Smirnov test31. Normally distributed data were expressed as mean ± SD. Data that were not normally distributed were logarithmically transformed before analysis and expressed as median with interquartile range. The Student’s unpaired t-test was used for comparison between two groups. Correlations between parameters were determined by Pearson’s correlations and partial correlation. One-way ANOVA was used as appropriate for comparisons between groups, and multiple testing was corrected using Bonferroni correction. The accuracy of HCC prediction by serum FGF21 was evaluated using the area under the ROC curve. AUC was presented with 95% CI. In all statistical tests, P values less than 0.05 were considered significant.
Data availability
The data are available from the corresponding author on reasonable request.
Electronic supplementary material
Acknowledgements
This work was financially supported by National Health and Medical Research Council of Australia (NHMRC)/National Natural Science Foundation of China (NSFC) Joint Research Program (81561128016) and National Natural Science Foundation major international (regional) joint research project (81220108006) to W.J., Young Scientists Fund of National Natural Science Foundation (81200292), Hong Kong Scholars Program (XJ2013035) and Chenguang Program supported by Shanghai Education Development Foundation and Shanghai Municipal Education Commission (13CG11) to H.L.
Author Contributions
L.W. and H.L. did data analysis and wrote the manuscript, L.W., and Q.P. contributed to the acquisition of data. G.W, J.Z. and L.Q. assisted in statistical analysis. Q.F, L.Z. and Y.W. contributed to data interpretation. W.J, G.Z. and G.L. critically reviewed the manuscript. W.J. was in charge of the overall conception and design of the study. All authors approved the final version, including those in the authorship list.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Liang Wu and Qingchun Pan contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-16312-6.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Huating Li, Email: huarting99@hotmail.com.
Weiping Jia, Email: wpjia@sjtu.edu.cn.
References
- 1.Lavanchy D. Worldwide epidemiology of HBV infection, disease burden, and vaccine prevention. J Clin Virol. 2005;34(Suppl 1):S1–3. doi: 10.1016/S1386-6532(05)00384-7. [DOI] [PubMed] [Google Scholar]
- 2.Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012;30:2212–2219. doi: 10.1016/j.vaccine.2011.12.116. [DOI] [PubMed] [Google Scholar]
- 3.Yim HJ, Lok AS. Natural history of chronic hepatitis B virus infection: what we knew in 1981 and what we know in 2005. Hepatology. 2006;43:S173–181. doi: 10.1002/hep.20956. [DOI] [PubMed] [Google Scholar]
- 4.Kim WR, Flamm SL, Di Bisceglie AM, Bodenheimer HC. Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology (Baltimore, Md.) 2008;47:1363–1370. doi: 10.1002/hep.22109. [DOI] [PubMed] [Google Scholar]
- 5.Sherman KE. Alanine aminotransferase in clinical practice. A review. Arch Intern Med. 1991;151:260–265. doi: 10.1001/archinte.1991.00400020036008. [DOI] [PubMed] [Google Scholar]
- 6.Ding X, et al. betaKlotho is required for fibroblast growth factor 21 effects on growth and metabolism. Cell metabolism. 2012;16:387–393. doi: 10.1016/j.cmet.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Markan KR, et al. Circulating FGF21 is liver derived and enhances glucose uptake during refeeding and overfeeding. Diabetes. 2014;63:4057–4063. doi: 10.2337/db14-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang C, et al. Activation of Liver FGF21 in hepatocarcinogenesis and during hepatic stress. BMC Gastroenterol. 2013;13:67. doi: 10.1186/1471-230X-13-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ye D, et al. Fibroblast growth factor 21 protects against acetaminophen-induced hepatotoxicity by potentiating peroxisome proliferator-activated receptor coactivator protein-1alpha-mediated antioxidant capacity in mice. Hepatology (Baltimore, Md.) 2014;60:977–989. doi: 10.1002/hep.27060. [DOI] [PubMed] [Google Scholar]
- 10.Li H, et al. High serum level of fibroblast growth factor 21 is an independent predictor of non-alcoholic fatty liver disease: a 3-year prospective study in China. Journal of hepatology. 2013;58:557–563. doi: 10.1016/j.jhep.2012.10.029. [DOI] [PubMed] [Google Scholar]
- 11.Ye D, et al. Circulating Fibroblast Growth Factor 21 Is A Sensitive Biomarker for Severe Ischemia/reperfusion Injury in Patients with Liver Transplantation. Scientific reports. 2016;6:19776. doi: 10.1038/srep19776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kukla M, et al. Serum FGF21 and RBP4 levels in patients with chronic hepatitis C. Scand J Gastroenterol. 2012;47:1037–1047. doi: 10.3109/00365521.2012.694901. [DOI] [PubMed] [Google Scholar]
- 13.Wu C, et al. Alteration of hepatic nuclear receptor-mediated signaling pathways in hepatitis C virus patients with and without a history of alcohol drinking. Hepatology (Baltimore, Md.) 2011;54:1966–1974. doi: 10.1002/hep.24645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frederiks WM, Vogels IM, Fronik GM. Plasma ornithine carbamyl transferase level as an indicator of ischaemic injury of rat liver. Cell biochemistry and function. 1984;2:217–220. doi: 10.1002/cbf.290020407. [DOI] [PubMed] [Google Scholar]
- 15.Lok AS, Lai CL. Acute exacerbations in Chinese patients with chronic hepatitis B virus (HBV) infection. Incidence, predisposing factors and etiology. Journal of hepatology. 1990;10:29–34. doi: 10.1016/0168-8278(90)90069-4. [DOI] [PubMed] [Google Scholar]
- 16.Ren F, et al. The dysregulation of endoplasmic reticulum stress response in acute-on-chronic liver failure patients caused by acute exacerbation of chronic hepatitis B. Journal of viral hepatitis. 2016;23:23–31. doi: 10.1111/jvh.12438. [DOI] [PubMed] [Google Scholar]
- 17.Li B, et al. Hepatitis B virus X protein (HBx) activates ATF6 and IRE1-XBP1 pathways of unfolded protein response. Virus research. 2007;124:44–49. doi: 10.1016/j.virusres.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 18.Wang HC, Huang W, Lai MD, Su IJ. Hepatitis B virus pre-S mutants, endoplasmic reticulum stress and hepatocarcinogenesis. Cancer Sci. 2006;97:683–688. doi: 10.1111/j.1349-7006.2006.00235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang F, et al. Expression of hepatitis B virus proteins in transgenic mice alters lipid metabolism and induces oxidative stress in the liver. Journal of hepatology. 2008;48:12–19. doi: 10.1016/j.jhep.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 20.Jiang S, et al. Fibroblast growth factor 21 is regulated by the IRE1alpha-XBP1 branch of the unfolded protein response and counteracts endoplasmic reticulum stress-induced hepatic steatosis. The Journal of biological chemistry. 2014;289:29751–29765. doi: 10.1074/jbc.M114.565960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schaap FG, Kremer AE, Lamers WH, Jansen PL, Gaemers IC. Fibroblast growth factor 21 is induced by endoplasmic reticulum stress. Biochimie. 2013;95:692–699. doi: 10.1016/j.biochi.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 22.Maruyama R, Shimizu M, Li J, Inoue J, Sato R. Fibroblast growth factor 21 induction by activating transcription factor 4 is regulated through three amino acid response elements in its promoter region. Bioscience, biotechnology, and biochemistry. 2016;80:929–934. doi: 10.1080/09168451.2015.1135045. [DOI] [PubMed] [Google Scholar]
- 23.Wan XS, et al. ATF4- and CHOP-dependent induction of FGF21 through endoplasmic reticulum stress. BioMed research international. 2014;2014:807874. doi: 10.1155/2014/807874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yan H, et al. Circulating fibroblast growth factor 21 levels are closely associated with hepatic fat content: a cross-sectional study. PLoS One. 2011;6:e24895. doi: 10.1371/journal.pone.0024895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao Y, et al. Distinct changes in serum fibroblast growth factor 21 levels in different subtypes of diabetes. The Journal of clinical endocrinology and metabolism. 2012;97:E54–58. doi: 10.1210/jc.2011-1930. [DOI] [PubMed] [Google Scholar]
- 26.EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. Journal of hepatology53, 397–417, 10.1016/j.jhep.2010.05.004 (2010). [DOI] [PubMed]
- 27.Huang X, et al. Forced expression of hepatocyte-specific fibroblast growth factor 21 delays initiation of chemically induced hepatocarcinogenesis. Molecular carcinogenesis. 2006;45:934–942. doi: 10.1002/mc.20241. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Q, et al. Loss of FGF21 in diabetic mouse during hepatocellular carcinogenetic transformation. Am J Cancer Res. 2015;5:1762–1774. [PMC free article] [PubMed] [Google Scholar]
- 29.Taketa K. Alpha-fetoprotein: reevaluation in hepatology. Hepatology. 1990;12:1420–1432. doi: 10.1002/hep.1840120625. [DOI] [PubMed] [Google Scholar]
- 30.Sarin SK, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the study of the liver (APASL) Hepatol Int. 2009;3:269–282. doi: 10.1007/s12072-008-9106-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elliott, A. C. & Woodward, W. A. Statistical analysis quick reference guidebook: With SPSS examples. (Sage, 2007).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are available from the corresponding author on reasonable request.