Abstract
Objective
To estimate sacroiliac joint radiographic (X-SIJ) progression in patients with axial spondyloarthritis (axSpA) and to evaluate the effects of inflammation on MRI (MRI-SIJ) on X-SIJ progression.
Methods
X-SIJ and MRI-SIJ at baseline and after 2 and 5 years in patients with recent onset axSpA from the DESIR cohort were scored by three central readers. Progression was defined as (1) the shift from non-radiographic (nr) to radiographic (r) sacroiliitis (by modified New York (mNY) criteria) or alternative criteria, (2) a change of at least one grade or (3) a change of at least one grade but ignoring a change from grade 0 to 1. The effects of baseline inflammation on MRI-SIJ on 5-year X-SIJ damage (mNY) were tested by generalised estimating equations.
Results
In 416 patients with pairs of baseline and 5-year X-SIJ present, net progression occurred in 5.1% (1), 13.0% (2) and 10.3% (3) respectively, regarding a shift from nr-axSpA to r-axSpA (1), a change of at least one grade (2) or a change of at least one grade but ignoring a change from grade 0 to 1 (3). Baseline MRI-SIJ predicted structural damage after 5 years in human leukocyte antigen-B27 (HLA-B27) positive (OR 5.39 (95% CI 3.25 to 8.94)) and in HLA-B27 negative (OR 2.16 (95% CI 1.04 to 4.51)) patients.
Conclusions
Five-year progression of X-SIJ damage in patients with recent onset axSpA is limited but present beyond measurement error. Baseline MRI-SIJ inflammation drives 5-year radiographic changes.
Keywords: spondyloarthritis, magnetic resonance imaging, epidemiology, outcomes research
Introduction
Axial spondyloarthritis (axSpA) comprises two subcategories based on the presence of structural changes in the sacroiliac joints (SIJs): radiographic (r)-axSpA and non-radiographic (nr)-axSpA. R-axSpA implies the fulfilment of the modified New York criteria (mNY).1 2
Information about the natural course of radiographic sacroiliitis and factors that contribute to it is scarce.3 Prospective cohorts should give resolution, and long-term follow-up of patients with recent onset disease is mandatory to ‘capture’ meaningful progression. Inherently, such studies face the risk of loss to follow-up and attrition bias.
DESIR (acronym in French for outcome of recent onset spondyloarthritis) is a prospective cohort of patients with recent onset axSpA (NCT01648907). With this study, we address the primary objectives of DESIR, formulated as follows: (1) what proportion of patients switches from nr-axSpA to r-axSpA after 5 years?; (2) how sensitive are different outcome measures for radiographic damage of SIJ (X-SIJ) to change?; (3) does inflammation on MRI of the SIJ (MRI-SIJ) lead to structural damage on X-SIJ after 5 years?
Methods
Patients
The DESIR cohort has been previously described.4 Briefly, consecutive patients (aged 18–50 from 25 centres in France) with inflammatory back pain5 6 and a duration ≥3 months but <3 years were included if the treating rheumatologist considered the symptoms suggestive of axSpA (a score ≥5 on a scale from 0 to 10, in which 0 was ‘not suggestive’ and 10 ‘very suggestive’). Between December 2007 and April 2010, 708 patients were included.
The study was conducted according to good clinical practice guidelines and was approved by the appropriate local medical ethical committees. A detailed description of the study protocol is available at the DESIR website (http://www.lacohortedesir.fr/desir-in-english/). The research proposal for this particular analysis was approved by the scientific committee of the DESIR cohort.
Clinical data
By using a standardised case report form (CRF) information was collected with questionnaires, physical examination, ongoing treatments and laboratory tests according to the DESIR protocol. The database used for this analysis was locked in June 2016.
At baseline, age, gender, smoking status, HLA-B27 and duration of axial symptoms had been collected. At baseline, every 6 months during the first 2 years of follow-up, and annually thereafter the following parameters had been collected: Bath Ankylosing Spondylitis Disease Activity Index (BASDAI),7 Bath Ankylosing Spondylitis Functional Index,8 C-reactive protein (CRP), treatment including non-steroidal anti-inflammatory drugs (NSAID) by the Assessment of Spondyloarthritis International Society (ASAS)-NSAID score and tumour necrosis factor inhibitors (TNFi).9
Pelvic radiographs
Pelvic radiographs collected at baseline, 2 years and 5 years of follow-up were evaluated in one session independently by three central readers (MdH, VNC and RvdB). Readers were blinded for time order and clinical information. Each reader evaluated each SIJ according to the mNY grading method (0: normal; 1: suspicious changes; 2: minimal abnormalities; 3: unequivocal abnormalities; and 4: severe abnormalities (complete ankylosis).10
Pelvic MRI
MRI-SIJ collected at baseline, 2 years and 5 years of follow-up were evaluated in one session independently by three central readers (MdH, VNC and MvL). Readers were blinded for time order and clinical information. MRI-SIJ was considered positive if bone marrow oedema (BMO) lesions highly suggestive of SpA were present (either one BMO lesion on ≥2 consecutive slides or several BMO lesions on one slice).11 An MRI-SIJ was considered positive if at least two out of three readers judged positivity. MRI-SIJ and X-SIJ were scored entirely independently.
Sample size calculation
The sample size calculation was based on an estimated prevalence of radiographic damage between 70% and 90% at year 5 irrespective of the baseline status. Moreover, we estimated the prevalence of inflammation on MRI-SIJ at baseline between 30% and 50%.12 13
The number of patients was calculated based on a relative risk of 2–3 to observe radiographic damage at year 5 in case of a baseline MRI-SIJ inflammation. For a 5% bilateral alpha risk, a 90% power, and the different assumptions including an attrition rate between 15% and 20%, the number of required patients ranged from 685 to 768, and 700 was the chosen number.
Statistical analysis
SIJ radiographic progression
The 5-year X-SIJ progression was assessed in patients in whom baseline and year 5 X-SIJ were present (completers’ population). Assessed were: (A) switch from nr-axSpA at baseline to r-axSpA (mNY score) at 5 years; (B) worsening of at least one grade in at least one SIJ; (C) worsening of at least one grade in at least one SIJ, but with a 5-year grade of at least 2 in the worsened joint; and (D) change in the total mNY score (expressed as a continuous variable) with a range from 0 to 8 (4 grades per SIJ).
In order to give sufficient credit to measurement error, we determined the proportion of ‘progressors’ (% of patients with worsening) as well as the proportion of ‘regressors’ (% of patients with improvement). Improvement was defined per outcome measure: (A) switching from r-axSpA at baseline to nr-axSpA at 5 years; (B) reduction of at least one grade in at least one SIJ; and (C) reduction of at least one grade in at least one SIJ with a baseline score of at least 2 in the improved joint. In addition, ‘net’ percentage of progression was defined as the number of ‘progressors’ minus the number of ‘regressors’ (numerator) divided by the total number of the study population (denominator) and was analysed in the entire population and clinically relevant subgroups.
Sensitivity analyses that addressed the impact of missing data were performed in patients with a baseline and at least one postbaseline radiograph available (‘intention-to-follow’ population) using two imputation techniques:(1) last observation carried forward (LOCF) and (2) linear extrapolation (LE).
The continuous SIJ score (total scores of left plus right SIJ (ranging from 0 to 8)) was the mean score of the three readers; for the binary definitions, a change was considered present if at least two out of the three readers agreed.
Effect of baseline MRI-SIJ inflammation on the 5-year X-SIJ damage
The association between baseline MRI-SIJ inflammation and 5-year X-SIJ damage (primary outcome) was analysed by three different models: (1) binomial multivariable generalised estimating equations (GEEs) on the individual readers’ scores (1-level GEE model); (2) ‘traditional’ multivariable logistic regression on the aggregated (two out of three reader consensus scores for MRI and SIJ) X-SIJ progression scores; (3) a true longitudinal (2-level) multivariable GEE with time-lagged autoregressive variables (as in Ramiro et al).14 The logistic regression models were also fit after multiple imputations with chainedequations (MICE) in the ‘intention-to-follow’ population.
Potential baseline confounders for the association of interest were selected based on their clinical relevance (gender, symptom duration, CRP, BASDAI, smoking status and treatment with NSAIDs). Statistical interactions between MRI-SIJ inflammation and baseline variables were excluded first and, if relevant (p<0.15 for the interaction term), the model was fitted per stratum.
Results
Patients and study course
Pelvic radiographs were available for 685 of the 708 patients at baseline. Of the 685 patients with baseline X-SIJ, 519 and 416 patients had X-SIJ, from all readers, after 2 and 5 years, respectively (completer’s population). A postbaseline X-SIJ (either at year 2 or 5) was available for 557 patients (intention to follow population). A baseline MRI-SIJ was available for 679 patients.
Table 1 summarises the baseline characteristics for patients with complete 5-year pelvic radiograph data and those without.
Table 1.
Baseline characteristics according to the availability of complete 5-year radiographic data of the sacroiliac joints
| Characteristics | Status at year 5 | ||
| Completers* | Non-completers | All patients | |
| Number of patients | 417 | 291 | 708 |
| Age (mean, SD) | 34.1 (8.6) | 33.2 (8.6) | 33.7 (8.6) |
| Symptom duration (years), (mean, SD) | 1.5 (0.9) (n=416) | 1.5 (0.8) (n=291) | 1.5 (0.9) (n=707) |
| Male gender (%) | 198 (47.5) | 129 (44.3) | 327 (46.2) |
| HLA-B27 positivity (%) | 267 (64.0) (n=417) | 143 (49.3) (n=290) | 410 (58.0) (n=707) |
| X-SIJ structural damage† (mNY) (%) | 62 (14.9) (n=416) | 29 (10.8) (n=268) | 92 (13.5) (n=684) |
| MRI-SIJ inflammation†‡ (%) | 113 (28.1) (n=402) | 67 (24.2) (n=277) | 180 (26.5) (n=679) |
| Abnormal CRP§ (%) | 126 (31.5) (n=400) | 78 (27.4) (n=285) | 204 (29.8) (n=685) |
| BASDAI (0–10, mean, SD) | 4.34 (1.99) (n=416) | 4.65 (2.01) (n=288) | 4.47 (2.00) (n=704) |
| ASDAS (mean, SD) | 2.6 (1.0) (n=395) | 2.6 (0.9) (n=281) | 2.6 (1.0) (n=676) |
| BASFI (0–10, mean, SD) | 2.92 (2.24) (n=413) | 3.23 (2.32) (n=288) | 3.04 (2.28) (n=701) |
*Patients with both baseline and 5-year X-SIJ available.
†According to the ‘2 out of 3’ definition: agreement of at least two out of the three readers—if two readers disagree and the third reading is missing, the combined score is set as missing (one case for X-SIJ).
‡Presence of bone marrow oedema according to the ASAS criteria at MRI-SIJ.
§≥6 mg/L.
ASDAS, Ankylosing Spondylitis Disease Activity Score; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BASFI, Bath Ankylosing Spondylitis Functional Index; CRP, C reactive protein; mNY, modified New York criteria; MRI-SIJ, MRI of the sacroiliac joints; X-SIJ, radiograph of the sacroiliac joints.
Radiographic progression after 5 years of follow-up
At baseline, the mNY criteria were fulfilled by 62/416 (14.9%; according to two out of three readers) of the patients in the completers’ population. After 5 years, this proportion has increased to 20.0% in the completers’ population and to 18.0% and 17.7% in the ‘intention-to-follow’ population (n=557), after LOCF and LE, respectively. A statistically significant worsening of the mean (SD) SIJ score was found in all scenarios (from 1.41 (1.68) to 1.60 (1.83) (Δ:0.19 (0.55); p<0.001) in the completers’ population and from 1.32 (1.65) to 1.49 (1.81) (Δ:0. 17 (0.59); p<0.001) (LOCF) or from 1.33 (1.65) to 1.50 (1.84) (Δ:0.17 (0.61); p<0.001) (LE) in the ‘intention to follow’ population).
Figure 1 summarises the observed changes in the binary outcome measures in the completers’ population, in terms of ‘% worsened’, ‘% improved’ and ‘net % progression’ (online supplementary figures S1 and S2 provide the same information for the ‘intention-to-follow’ population after LOCF and LE, yielding similar results).
Figure 1.
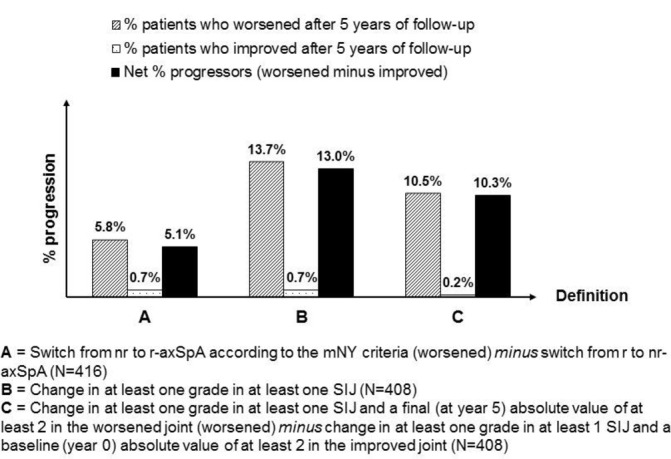
Changes in different binary SIJ-Plain X-ray outcome measures (completers’ population). nr-axSpA, radiographic axial spondyloarthritis; r-axSpA, radiographic axial spondyloarthritis; SIJ, sacroiliac joint.
Effects of MRI-SIJ inflammation on X-SIJ damage
Figure 2 shows the effect of baseline MRI-SIJ inflammation on 5-year SIJ damage according to the mNY criteria, stratified for HLA-B27 (interaction: p=0.033). Baseline MRI-SIJ inflammation was associated with radiographic damage after 5 years in HLA-B27 positive patients (OR 5.39 (95% CI 3.25 to 8.94)) as well as HLA-B27 negative patients (OR 2.16 (95% CI 1.04 to 4.51)). The association between baseline MRI inflammation and 5-year SIJ damage was consistently found, regardless of the analytical method and the definition of SIJ progression (table 2).
Figure 2.

Effect of inflammation on MRI-SIJ on being mNY-positive after 5 years irrespective of baseline mNY status stratified according to the HLA-B27 status at baseline (1-level binomial multivariable GEE). Interaction between inflammation on MRI-SIJ and HLA-B27 at baseline: p=0.033. BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; CRP, creactive protein; GEE, generalised estimating equations; mNY, modified New York criteria; MRI-SIJ, magnetic resonance imaging of the sacroiliac joints; NSAIDs, non-steroidal anti-inflammatory drugs.
Table 2.
Sensitivity analyses: effect of baseline MRI-SIJ inflammation on different SIJ radiographic progression definitions, irrespective of baseline mNY status and using different analytical approaches
| Main effect aOR (95% CI) | HLA-B27 positive aOR (95% CI) | HLA-B27negative aOR (95% CI) | p Value interaction | |
| Outcome: mNY positive | ||||
| Logistic regression* | NA | 9.26(4.32 to 19.86) (n=247) |
3.79 (1.01 to 14.28) (n=143) |
0.106 |
| Logistic regression after MI† | 6.64 (3.67 to 12.00) (n=557) |
NA | NA | NS |
| 1-level GEE‡ | NA | 5.39 (3.25 to 8.94) (n=248) |
2.16 (1.04 to 4.51) (n=143) |
0.033 |
| 2-level GEE (longitudinal)§ | 2.42 (1.01 to 5.78) (n=493) |
NA | NA | NS |
| Outcome: 1-grade progression | ||||
| Logistic regression* | 2.33 (1.21 to 4.49) (n=373) |
NA | NA | NS |
| Logistic regression after MI† | 2.35 (1.13 to 4.86) (n=557) |
NA | NA | NS |
| 1-level GEE‡ | 1.74 (1.05 to 2.88) (n=381) |
NA | NA | NS |
| 2-level GEE (longitudinal)§ | 1.90 (1.16 to 3.13) (n=486) |
NA | NA | NS |
| Outcome: 1-grade progression + follow-up grade≥2 | ||||
| Logistic regression* | 3.45 (1.65 to 7.23) (n=373) |
NA | NA | NS |
| Logistic regression after MI† | 3.47 (1.60 to 7.54) (n=557) |
NA | NA | NS |
| 1-level GEE‡ | 1.82 (1.02 to 3.27) (n=381) |
NA | NA | NS |
| 2-level GEE (longitudinal)§ | 1.87 (1.04 to 3.36) (n=486) |
NA | NA | NS |
*Association between baseline MRI-SIJ inflammation and the X-SIJ score at year 5 with both variables according to the ‘2 out of 3’ definition; N=patients with X-SIJ score available at year 5 and complete data on all covariates at baseline.
†Association between baseline MRI-SIJ inflammation and the X-SIJ score at year 5 both variables according to the ‘2 out of 3’ definition, after multiple imputation; N= patients with X-SIJ available at baseline and in at least one postbaseline visit and complete data on all covariates at baseline.
‡Association between baseline MRI-SIJ inflammation and the X-SIJ score at year 5 incorporating measurements from all readers at baseline for MRI-SIJ and year 5 for the X-SIJ score and taking into account the within-reader correlation; N=patients with at least one baseline MRI-SIJ/5-year X-SIJ pair (ie, at the same time points available) and complete data on all covariates at baseline.
§Longitudinal association between MRI-SIJ inflammation and X-SIJ score (all measurements from all readers for both modalities) over the 5-year follow-up with time-lagged models and first-order autoregression, taking into account the within-reader and within-patient correlation for the repeated measurements; N=patients with at least one X-SIJ/MRI-SIJ pair and complete data on all covariates for the available pairs.
aOR, adjusted OR (adjusted for: symptom duration, gender, CRP, BASDAI, smoking status, treatment with NSAIDs and treatment with TNFi for longitudinal models); BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; CRP, C reactive protein; GEE, generalised estimating equations; MI, multiple imputation; mNY, modified New York criteria; MRI-SIJ, MRI of the sacroiliac joints; NSAIDs, non-steroidal anti-inflammatory drugs; NA, not applicable—the main effect of MRI-SIJ inflammation on the different outcomes is only shown if the interaction with HLA-B27 is not significant (p≥0.15); NS, not significant; otherwise the effect of MRI-SIJ in each strata of HLA-B27 is shown; TNFi, tumour necrosis factor inhibitors.
Radiographic progression across clinically relevant subgroups
Figure 3 shows the ‘net’ progression from nr-axSpA to r-axSpA in different subgroups of patients according to relevant clinical characteristics and the interaction with HLA-B27. HLA-B27-positive nr-axSpA patients with a positive MRI-SIJ and CRP had a likelihood of ‘net’ progression of at least 1-grade of the X-SIJ mNY score that was more than twice as high as r-axSpA patients with similar baseline features (see online supplementary figures S3 and S4).
Figure 3.
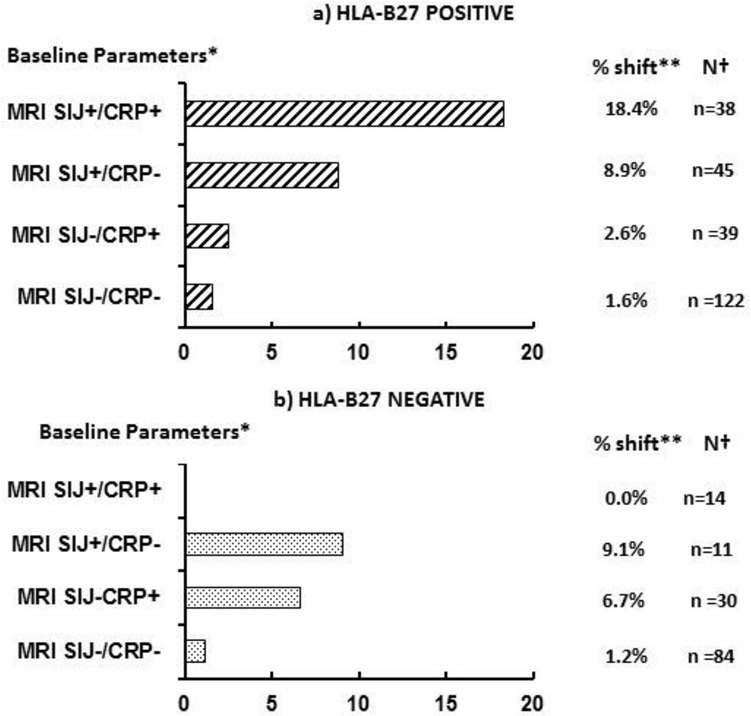
Net progression from nr-axSpA to r-axSpA according to baseline objective inflammatory markers and stratified on HLA-B27 ststus. BMO, bone marrow oedema; CRP, C reactive protein; MRI-SIJ, MRI of the sacroiliac joints; nr-axSpA, non-radiographic axial spondyloarthritis; r-axSpA, radiographic axial spondyloarthritis.
Discussion
The main findings of this 5-year follow-up study can be summarised as follows: (1) 5-year radiographic SIJ progression is statistically significant but of limited magnitude; (2) strategically chosen definitions of radiographic progression may be more sensitive to change over time than the rigid (binary) mNY-based definition; and (3) inflammation on MRI-SIJ is highly predictive of a structural radiographic SIJ progression. Moreover, these data provide meaningful information for the clinician who likes to determine the risk of progression in an individual patient, using baseline parameters such as HLA-B27 positivity, radiographic structural damage, MRI-SIJ inflammation and abnormal CRP.
In order to properly interpret the rate of progression of SIJ damage that we found in this study, two quantities have to be considered: (A) the proportion of patients with radiographic SIJ damage at baseline; and (B) the proportion of patients that change from nr-axSpA to r-axSpA over time.
Observed radiographic SIJ damage in the DESIR cohort (15%) is in accordance with what has been found before, in light of the relatively short duration of the symptoms (between 3 months and 3 years).15–17 These data suggest that structural damage can already be found very early in the disease.
Longitudinal studies that allow a proper evaluation of change from nr-axSpA to r-axSpA are scarce: Sampaio-Barros et al found a 10% progression rate over 2 years in one study18 and a 24% progression rate over 10 years in another study.19 However, only the researchers of the GESPIC cohort realised that a proper progression estimate should aggregate worsening as well as improvement and reported progression in 9% after 2 years.17
The mNY criteria that quantify radiographic damage in SIJ have been proposed several decades ago for classifying a particular patient at a particular point of time. These inherently binary criteria (mNY+ or mNY−) were not intended to evaluate the natural course of the disease. Adaptations thereof may be more sensitive to change and simpler to interpret: our continuous score modification (a score from 0 to 8 based on the ordinal scale of mNY grading) is more sensitive but harder to interpret to the data analyst and the clinician. The statistician will worry about the handling of a semiquantitative variable as if it were a continuous one and will argue the seemingly similar distance between different grades. Moreover, a continuous score is simply the sum of the scores obtained in two SIJs, as if they were independent. A simpler means to express progression to the clinician is to define progression as a change of at least 1 grade in at least one SIJ. This proposal has been used for the first time by the GESPIC researchers.16 Since we felt that a change between grade 0 and grade 1 (and vice versa) is not clinically relevant, we proposed a third definition by ignoring a change from 0 to 1.3 Our study has confirmed that the sensitivity to change of this adjusted definition is better than the one based on the mNY criteria.
The main weakness of these X-SIJ-based definitions is likely the poor interobserver reliability: the assessment of radiographic damage in the SIJ according to the binary mNY criteria is particularly susceptible to measurement error.20 While trained central readers have shown better reliability than single (local) readers, a combined-score by our three central readers (‘2 out of 3’ score) is still fallible in terms of measurement error, as is suggested by the finding of ‘improvement’ of SIJ damage under fully blinded conditions in a significant proportion of patients.
This means that measurement error (ie, scoring variability) must be taken into account when analysing X-SIJ progression. We have addressed this in two ways: first, our analysis was assumption free. We allowed ‘positive change’ as well as ‘negative change’ to occur without labelling this as ‘true progression’ or ‘noise’. We analysed to what extent 5-year SIJ structural damage was driven by baseline inflammation on MRI-SIJ, and we could confirm a positive association: more MRI-inflammation at baseline leads to a higher 5-year SIJ score. In addition, we have used an analytical approach that most efficiently captures all the available information in the model, which adds to precision. In fact, our main analysis (the 1-level GEE) was more precise (narrower CI) than the ‘traditional’ logistic regression.
The other weakness of the X-SIJ outcome measures is the lack of information concerning their external validity and in particular the lack of information related to the impact of the changes in X-SIJ on the patient’s functional disability. In this regard, syndesmophyte development at the spine level might be more relevant.
This cohort study in early axSpA reiterates the importance of BMO on MRI-SIJ as a predisposing factor for developing radiographic sacroiliitis 5 years later.3 20 Of note, HLA-B27 was an effect modifier: patients carrying this genetic (risk) marker had a larger effect of MRI inflammation on radiographic damage than those not carrying this marker. This disparate effect suggests HLA-B27 is a critical factor for the severity of axSpA.21 22
Our data suggest that a proper risk estimation in individual patients is within our scope: an nr-axSpA patient that is HLA-B27-negative has a normal CRP and a negative MRI-SIJ has a likelihood of only 1.2% to progress to r-axSpA. In contrast, this likelihood is 18.4%; if the patient is HLAB27-positive, the CRP is increased and the MRI-SIJ shows BMO.
Further studies are required to better estimate the X-SIJ progression in axSpA and to better understand the role of inflammation on this progression.
annrheumdis-2017-211596supp001.jpg (86KB, jpg)
annrheumdis-2017-211596supp002.jpg (84.5KB, jpg)
annrheumdis-2017-211596supp003.jpg (103.6KB, jpg)
annrheumdis-2017-211596supp004.jpg (116.5KB, jpg)
Acknowledgments
The DESIR cohort was sponsored by the Département de la Recherche Clinique et du Développement de l’Assistance Publique–Hôpitaux de Paris. This study is conducted under the umbrella of the French Society of Rheumatology and INSERM (Institut National de la Santé et de la Recherche Médicale). The database management is performed within the department of epidemiology and biostatistics (Professor Paul Landais, D.I.M., Nîmes, France). An unrestricted grant from Pfizer was allocated for the 10 years of the follow-up of the recruited patients. The authors would like to thank the different regional participating centres: Pr Maxime Dougados (Paris – Cochin B), Pr André Kahan (Paris - Cochin A), Pr Olivier Meyer (Paris - Bichat), Pr Pierre Bourgeois (Paris - La Pitié Salpetrière), Pr Francis Berenbaum (Paris - Saint Antoine), Pr Pascal Claudepierre (Créteil), Pr Maxime Breban (Boulogne Billancourt), Dr Bernadette Saint-Marcoux (Aulnay-sous-Bois), Pr Philippe Goupille (Tours), Pr Jean-Francis Maillefert (Dijon), Dr Xavier Puéchal, Dr Emmanuel Dernis (Le Mans), Pr Daniel Wendling (Besançon), Pr Bernard Combe (Montpellier), Pr Liana Euller-Ziegler (Nice), Pr Philippe Orcel, Dr Pascal Richette (Paris - Lariboisière), Pr Pierre Lafforgue (Marseille), Dr Patrick Boumier (Amiens), Pr Jean-Michel Ristori, Pr Martin Soubrier (Clermont-Ferrand), Dr Nadia Mehsen (Bordeaux), Pr Damien Loeuille (Nancy), Pr René-Marc Flipo (Lille), Pr Alain Saraux (Brest), Pr Corinne Miceli (Le Kremlin Bicêtre), Pr Alain Cantagrel (Toulouse), Pr Olivier Vittecoq (Rouen). The authors would also like to thank URC-CIC Paris Centre for the coordination and monitoring of the study.
Footnotes
Contributors: All authors contributed and finally approved the current manuscript.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: Comitte de Protection des Personnes Ile de France III.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Dougados M, Baeten D. Spondyloarthritis. Lancet 2011;377:2127–37. 10.1016/S0140-6736(11)60071-8 [DOI] [PubMed] [Google Scholar]
- 2.Sieper J, Braun J, Dougados M, et al. Axial spondyloarthritis. Nat Rev Dis Primers 2015;1:15013 10.1038/nrdp.2015.13 [DOI] [PubMed] [Google Scholar]
- 3.Dougados M, Demattei C, van den Berg R, et al. Rate and Predisposing Factors for Sacroiliac Joint Radiographic Progression After a Two-Year Follow-up Period in Recent-Onset Spondyloarthritis. Arthritis Rheumatol 2016;68:1904–13. 10.1002/art.39666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dougados M, Etcheto A, Molto A, et al. DESIR cohort. Clinical presentation of patients suffering from recent onset chronic inflammatory back pain suggestive of spondyloarthritis: The DESIR cohort. Joint Bone Spine 2015;82:345–51. 10.1016/j.jbspin.2015.02.006 [DOI] [PubMed] [Google Scholar]
- 5.Calin A, Porta J, Fries JF, et al. Clinical history as a screening test for ankylosing spondylitis. JAMA 1977;237:2613–4. 10.1001/jama.1977.03270510035017 [DOI] [PubMed] [Google Scholar]
- 6.Rudwaleit M, Metter A, Listing J, et al. Inflammatory back pain in ankylosing spondylitis: a reassessment of the clinical history for application as classification and diagnostic criteria. Arthritis Rheum 2006;54:569–78. 10.1002/art.21619 [DOI] [PubMed] [Google Scholar]
- 7.Garrett S, Jenkinson T, Kennedy LG, et al. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol 1994;21:2286–91. [PubMed] [Google Scholar]
- 8.Calin A, Garrett S, Whitelock H, et al. A new approach to defining functional ability in ankylosing spondylitis: the development of the Bath Ankylosing Spondylitis Functional Index. J Rheumatol 1994;21:2281–5. [PubMed] [Google Scholar]
- 9.Dougados M, Simon P, Braun J, et al. ASAS recommendations for collecting, analysing and reporting NSAID intake in clinical trials/epidemiological studies in axial spondyloarthritis. Ann Rheum Dis 2011;70:249–51. 10.1136/ard.2010.133488 [DOI] [PubMed] [Google Scholar]
- 10.van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 1984;27:361–8. [DOI] [PubMed] [Google Scholar]
- 11.Rudwaleit M, Jurik AG, Hermann KG, et al. Defining active sacroiliitis on magnetic resonance imaging (MRI) for classification of axial spondyloarthritis: a consensual approach by the ASAS/OMERACT MRI group. Ann Rheum Dis 2009;68:1520–7. 10.1136/ard.2009.110767 [DOI] [PubMed] [Google Scholar]
- 12.Mau W, Zeidler H, Mau R, et al. Clinical features and prognosis of patients with possible ankylosing spondylitis. Results of a 10-year followup. J Rheumatol 1988;15:1109–14. [PubMed] [Google Scholar]
- 13.Heuft-Dorenbosch L, Weijers R, Landewé R, et al. Magnetic resonance imaging changes of sacroiliac joints in patients with recent-onset inflammatory back pain: inter-reader reliability and prevalence of abnormalities. Arthritis Res Ther 2006;8:R11 10.1186/ar1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramiro S, van der Heijde D, van Tubergen A, et al. Higher disease activity leads to more structural damage in the spine in ankylosing spondylitis: 12-year longitudinal data from the OASIS cohort. Ann Rheum Dis 2014;73:1455–61. 10.1136/annrheumdis-2014-205178 [DOI] [PubMed] [Google Scholar]
- 15.Poddubnyy D, Brandt H, Vahldiek J, et al. The frequency of non-radiographic axial spondyloarthritis in relation to symptom duration in patients referred because of chronic back pain: results from the Berlin early spondyloarthritis clinic. Ann Rheum Dis 2012;71:1998–2001. 10.1136/annrheumdis-2012-201945 [DOI] [PubMed] [Google Scholar]
- 16.Said-Nahal R, Miceli-Richard C, Berthelot JM, et al. The familial form of spondylarthropathy: a clinical study of 115 multiplex families. Groupe Français d’Etude Génétique des Spondylarthropathies. Arthritis Rheum 2000;43:1356–65. [DOI] [PubMed] [Google Scholar]
- 17.Poddubnyy D, Rudwaleit M, Haibel H, et al. Rates and predictors of radiographic sacroiliitis progression over 2 years in patients with axial spondyloarthritis. Ann Rheum Dis 2011;70:1369–74. 10.1136/ard.2010.145995 [DOI] [PubMed] [Google Scholar]
- 18.Sampaio-Barros PD, Conde RA, Donadi EA, et al. Undifferentiated spondyloarthropathies in Brazilians: importance of HLA-B27 and the B7-CREG alleles in characterization and disease progression. J Rheumatol 2003;30:2632–7. [PubMed] [Google Scholar]
- 19.Sampaio-Barros PD, Bortoluzzo AB, Conde RA, et al. Undifferentiated spondyloarthritis: a longterm followup. J Rheumatol 2010;37:1195–9. 10.3899/jrheum.090625 [DOI] [PubMed] [Google Scholar]
- 20.van den Berg R, Lenczner G, Feydy A, et al. Agreement between clinical practice and trained central reading in reading of sacroiliac joints on plain pelvic radiographs. Results from the DESIR cohort. Arthritis Rheumatol 2014;66:2403–11. 10.1002/art.38738 [DOI] [PubMed] [Google Scholar]
- 21.Chung HY, Machado P, van der Heijde D, et al. HLA-B27 positive patients differ from HLA-B27 negative patients in clinical presentation and imaging: results from the DESIR cohort of patients with recent onset axial spondyloarthritis. Ann Rheum Dis 2011;70:1930–6. 10.1136/ard.2011.152975 [DOI] [PubMed] [Google Scholar]
- 22.Bennett AN, McGonagle D, O’Connor P, et al. Severity of baseline magnetic resonance imaging-evident sacroiliitis and HLA-B27 status in early inflammatory back pain predict radiographically evident ankylosing spondylitis at eight years. Arthritis Rheum 2008;58:3413–8. 10.1002/art.24024 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
annrheumdis-2017-211596supp001.jpg (86KB, jpg)
annrheumdis-2017-211596supp002.jpg (84.5KB, jpg)
annrheumdis-2017-211596supp003.jpg (103.6KB, jpg)
annrheumdis-2017-211596supp004.jpg (116.5KB, jpg)


