Abstract
Despite improvements that occurred in the last decades in the acute myeloid leukemia (AML) treatment, clinical results are still unsatisfactory. More effective therapies are required, and innovative approaches are ongoing, including the discovery of novel antileukemia natural compounds. Several studies have described the activity of extracts from mushrooms which produce compounds that exhibited immunological and antitumor activities. The latter has been demonstrated to be promoted in vitro by mushroom polysaccharides via induction of apoptosis. However, the antileukemia activity of these compounds on primary cells is still not reported. In the present study, we examined the in vitro effects of Tramesan (TR), a bioactive compound extracted from Trametes versicolor, on leukemic cell lines and primary cells. Our results demonstrated that TR induced a marked growth inhibition of leukemic cell lines and primary cells from AML patients. The antiproliferative effects of TR were associated in primary AML cells with a significant increase of apoptosis. No significant cytotoxic effects were observed in normal peripheral blood mononuclear cells (MNC) from healthy donors. Our data demonstrated a cytotoxic activity of TR on leukemia cells prompting further translational applications. Ongoing studies are elucidating the molecular mechanisms underlying its antileukemic activity.
1. Introduction
Acute leukemia is a disorder of the hematopoietic system characterized by clonal proliferation of variably differentiated myeloid or lymphoid precursors [1]. These hematological disorders show a high level of genetic complexity, which is the major explanation of the high rate of chemotherapy failure and of the result stagnation over the last decades [1]. Therefore, efforts have been made to identify novel therapeutic approaches aimed at overcoming drug resistance and exploring novel approaches based on chemo-free strategies. The successful example of the acute promyelocytic leukemia, nowadays treated in the majority of the cases without chemotherapy by regimen based on the combined use of retinoic acid and arsenic trioxide [2], is worth mentioning. On this basis, many attempts are ongoing to identify other similar approaches. In fact, besides targeted therapies active on leukemia-specific aberrant molecular alterations [3], an increasing number of studies are directed toward the drug discovery attempt, including the evaluation of natural compounds on different pathologies [4–6].
Mushrooms are rich sources of natural compounds used as biological response modifiers for a long time in oriental medicine and nowadays also in Western countries [7–9]. Mushrooms have emerged as a rich font of antioxidant, immunomodulating, anti-inflammatory, antimicrobial, and anticancer [10] compounds. Among different extracts from mushrooms, polysaccharides are important bioactive molecules and known as potent inhibitors of proliferation and apoptosis inducers in in vitro experimental models. In particular, the antitumor activity of PSK, a protein-bound polysaccharide obtained from the fungus Trametes versicolor (T. versicolor), has been documented in in vitro and in vivo experimental models [11]. Efficacy of the adjuvant immunotherapy with PSK was also demonstrated in human clinical trials [12–14]. Moreover, the combination of PSK with chemotherapy or radiotherapy has been shown to increase the efficacy of the latter in solid cancer treatments [7].
To date, few studies have reported the antineoplastic activity of PSK in hematologic models [11, 15–17]. In leukemia cell line, it was demonstrated that PSK exerted an antiproliferative and proapoptotic activity in HL-60 myeloid cell line, via activation of the mitochondrial and the p38 MAPK signaling cascades [16, 17].
Previous studies from our group have demonstrated that nonpurified extracts from the filtrates of L. edodes and T. versicolor inhibited mycotoxin synthesis in different fungi [18–20]. The mechanism of inhibition seems related to the promotion of fungal antioxidant activity on counteracting redox unbalance. It is now established that mycotoxigenic fungi are able to vehiculate the unscavenged ROS toward toxin synthesis.
Our group has patented a polysaccharidic fraction, Tramesan (TR), isolated from the liquid culture of the edible basidiomycete T. versicolor (Patent number RM2012A000573), able to significantly inhibit the synthesis of carcinogenic mycotoxins produced from different fungi (such as Aspergillus flavus, A. parasiticus, and Fusarium), by promoting an antioxidant activity [18, 20, 21].
The aim of this study was to investigate the activity of TR on leukemia cells by assessing proliferation inhibition and apoptosis induction on leukemia cell lines and on primary acute myeloid leukemia (AML) cells.
2. Materials and Methods
2.1. Fungal Strain and Growth Culture Conditions
The basidiomycete Trametes versicolor CF 117 was supplied from the mushroom collection of the laboratory of Mycology and Plant Pathology directed from Professor Corrado Fanelli (Sapienza University of Rome). The isolates were kept in potato dextrose agar (PDA) medium at 4°C, and the cultures have been renewed every 30 days.
2.2. Fungal Growth Substrate
T. versicolor was grown for 7 days in potato dextrose broth (PDB) and incubated at 25°C. The liquid culture was homogenized, in sterile condition in a Waring blender 8012. After homogenization, 5% (v/v) of the fungal culture was subsequently inoculated in 500 mL of PDB in 1 L Erlenmeyer flask and the T. versicolor cultures were incubated for 14 days at 25°C under shaken conditions (100 rpm). The mycelia were then separated from the culture filtrates by subsequent filtrations with different size filters (Whatman) to eliminate all the mycelia. The obtained culture filtrate was lyophilized and utilized for subsequent analyses.
2.3. Exopolysaccharide Extraction and Purification
The lyophilized T. versicolor culture filtrate (1 g) was dissolved in 30 mL of ultrapure H2O and filtrated to separate the insoluble from the soluble part. The sample was then incubated at 4°C for 2 h, and at the same time, a solution of absolute ethanol was cooled at 4°C. The cooled culture filtrate was added slowly to cooled ethanol, mixing with a glass rod. The precipitate was recovered by centrifugation at 4000 rpm for 20 min at 4°C. The recovered pellet was resuspended in 4 mL of ultrapure H2O and 4 mL of 20 mM phosphate buffer pH 7.5 to achieve the optimal conditions of ionic strength and pH for pronase E (Sigma-Aldrich) activity. The proteolysis was carried out at 37°C overnight. The elimination of salts and amino acids was performed through dialysis versus H2O, utilizing membrane of 12000 Da cutoff. The dialyzed samples were lyophilized and subjected to total carbohydrate determination by Yemm and Willis [22].
To separate, on the basis of their molecular weight, the exopolysaccharides precipitated with EtOH and present in T. versicolor culture filtrates, a size exclusion chromatography was performed. The mobile phase was NaNO3 0.05 M degassed for about 30 min and filtered twice with Millipore 0.45 μm filters. The Sephacryl S-300 (1.6 id × 90 cm) column was equilibrated with the eluent for about 30 h, with a flux of about 6 mL/h. The gel performances of the column allow to discriminate polysaccharides with a molecular weight between 2 and 400 kDa. About 40 mg of the sample was dissolved in 1.9 mL of 50 mM NaNO3, the solution was centrifuged for 10 min at 13000 rpm, and subsequently it was loaded in a column. The column was connected to a refractive index detector. Each fraction was collected every 20 min during 30 h.
The chromatographic fraction containing only polysaccharides (TR) was used for biological activity assays.
The polysaccharide TR, isolated and purified was patented from our group (Patent number RM2012A00057).
2.4. Cell Lines and Primary Samples
Human myeloid (OCI-AML3) and lymphoid (Jurkat) cell lines were maintained in RPMI 1640 medium containing 10% heat-inactivated fetal calf serum (FCS), 1 mM L-glutamine, and 50 μg/mL penicillin/streptomycin (Gibco, Milan), in a humidified atmosphere containing 5% CO2 at 37°C. Cell lines were harvested in log-phase growth for all experiments, washed, and cultured to the appropriate concentration in RPMI 10% FCS ± TR at scalar concentrations (0.5–2 mg/mL).
Peripheral blood (PB) samples and bone marrow (BM) aspirate samples were obtained from normal donors and from 4 AML patients, respectively, all referred to our Hematology at Sapienza University of Rome, Italy. Written informed consent for in vitro studies was obtained from donors and patients in accordance with regulations and protocols sanctioned by the Human Subjects Committee of Helsinki and were approved by the Sapienza Institutional Review Board (protocol number 158/10 signed on February 18, 2010). MNC obtained from the PB of a normal volunteer donor and blast cells from the BM of AML patients were separated by layering on Ficoll-Hypaque density gradient (Lymphoprep; 1.007 g/mL). Cells used for in vitro studies were resuspended at a concentration of 1.0 × 106/mL, in RPMI 10% FCS ± TR at scalar concentrations (0.5–2 mg/mL). In particular, the circulating mononuclear cells obtained from healthy donors were placed in liquid culture ± scalar concentrations of TR in the absence and in the presence of a proliferative stimulus (PHA).
2.5. Cell Cycle and Apoptosis Analysis
Cell cycle distribution changes were evaluated using the Acridine Orange (AO) technique as previously described [23]. The percentage of cells in G0, G1, S, and G2 M was determined by measuring simultaneously the DNA and RNA total cellular content. The percentage of apoptotic cells was measured based on the decreased stainability of apoptotic elements in DNA green fluorescence (sub-G0/1 peak on DNA frequency histograms) coupled with a higher RNA red fluorescence (which is common to chromatin condensation); cell debris was excluded from the analysis on the basis of their forward light scatter properties. Cell cycle distribution was analyzed using the ModFit LT software (Verity Software House, Topsham, ME).
Induction of apoptosis was also assessed by measuring Annexin V binding to externalized phosphatidylserine, as previously described [24]. Briefly, cells were washed twice with PBS and resuspended in binding buffer (10 mM Hepes/NaOH pH 7.4, 140 mM NaCl, 2.5 mM CaCl2, Sigma Chemical Co.). FITC-conjugated Annexin V (Roche Diagnostic Corp., Indianapolis, Indiana, USA) was added at a final concentration of 1 μg/mL. The mixture was incubated at room temperature for 15 min in the dark prior to flow cytometric analysis. Membrane integrity was simultaneously assessed by propidium iodide (PI, 0.25 μg/mL) exclusion.
2.6. Analysis of Intracellular ROS Levels
The generation of ROS was measured by using DCFH-DA, a ROS-sensitive fluorescent probe. Briefly, OCI-AML3 cells were cultured for 24 h with different concentrations of TR. After exposure, cells were incubated with 10 mM DCFH-DA for 30 min at 37°C, then washed, resuspended in PBS, and immediately analyzed by flow cytometry. Results were expressed as mean fluorescence intensity (MFI) relative to that of control.
2.7. Statistical Analysis
The two-sided Student t-test was used to evaluate the significance of differences between groups. Results were expressed as the mean ± standard deviation (SD). Values of p < 0.05 were considered significant from a statistical point of view.
3. Results
3.1. Effects of TR on Leukemia Cell Lines
We started to evaluate the effects of TR on human myeloid (OCI-AML3) and lymphoid (Jurkat) leukemia cell lines exposed to scalar concentrations of TR (0.5–2 mg/mL). Cell aliquots were harvested at 24, 48, and 72 hours and analyzed for cell counts (trypan blue), cell cycle (flow cytometric analysis by AO), and apoptosis (flow cytometry assessment with AO, Annexin V).
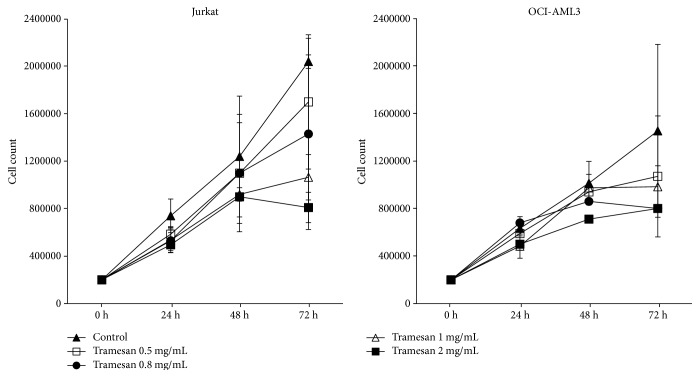
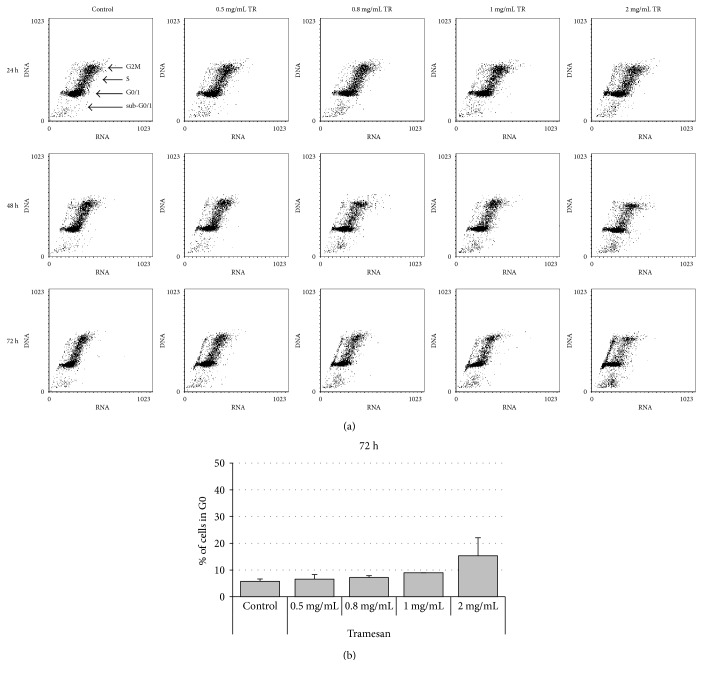
Exposure to TR induced a dose- and time-dependent reduction in cell count in all hematopoietic cell lines studied (Figure 1). In fact, the effects were markedly evident at a dose of 1 mg/mL after 48 hours of liquid culture. The prolonged exposure to TR and increasing scalar concentrations of the molecule potentiated the effectiveness (Figure 1). Detection of cell cycle changes, measured at the same time points, however, showed that on leukemia cell lines TR was unable to affect cell cycle distribution and apoptosis. Only on Jurkat cell line it was observed, after 72 h of culture, a mild, not significant, dose-dependent increase of cells in G0 phase associated with a decrease of G1 and S compartment and with a nonsignificant increase of sub-G0/1 (Figure 2).
Figure 1.
TR induces a dose-dependent reduction in cell count in hematopoietic cell lines. Jurkat and OCI-AML3 cells were exposed to increasing concentrations of TR for the indicated time points. Cell counts and viability were then assessed by trypan blue exclusion counting. Results are expressed as the average ± SD of seven independent experiments.
Figure 2.
TR induces a dose-dependent increase of cells in G0 phase of cell cycle in hematopoietic cell lines. Jurkat cells were exposed to increasing concentrations of TR. Cell cycle changes were then evaluated by the AO technique. Results showed are representative of 7 independent experiments (a). Percentages of cells in G0 phase after 72 h of TR exposure are shown in (b).
Analysis of intracellular ROS levels in cells stained with DCFH-DA depicts (Figure 3) that TR induced accumulation of ROS as demonstrated by an increase of DCFH-DA MFI in OCI-AML3 treated with TR as compared to control: from MFI of 0.46 × 106 (control) to MFI of 2.94 × 106, 2.61 × 106 and, 2.98 × 106 in the presence of 0.5, 1, and 2 mg/mL TR, respectively.
Figure 3.
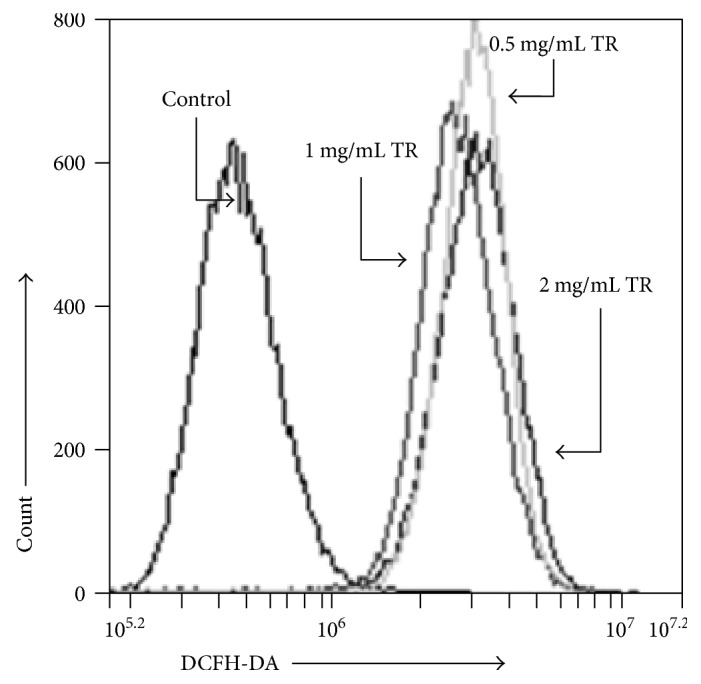
TR induced accumulation of ROS in hematopoietic cells line. Cells were exposed to increasing concentrations of TR. After 24 h, cells were stained with DCFH-DA for intracellular ROS level analysis.
3.2. Effects of TR on Primary Cells from AML Patients
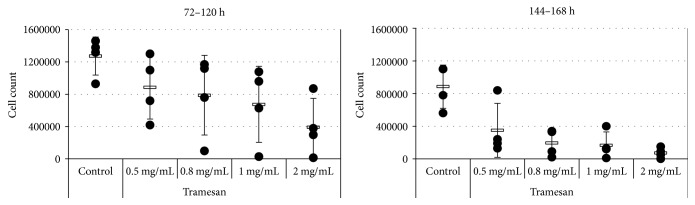
We then examined the biological effects induced in vitro by TR on primary cells from 4 patients with newly diagnosed AML. Our results indicate that TR induced reduction in cell count and significant cytotoxic effects in a dose- and time-dependent fashion in all (4/4) samples studied. After 72–120 hours of culture, TR was able to exert a significant reduction in cell counts from 1272500 ± 235425 cells/mL, in the control condition, to 885000 ± 392386 (p = 0.06), 787500 ± 493381 (p = 0.04), 675000 ± 470213 (p = 0.02), and 391750 ± 356435 (p = 0.01) cells/mL in the presence of 0.5, 0.8, 1, and 2 mg/mL of TR, respectively (Figure 4).
Figure 4.
TR induces a dose- and time-dependent reduction in cell count in primary cells from AML patients. AML primary cells were exposed to the indicated concentrations of TR. Cell counts and viability were assessed for the individual sample after 72–120 h and 144–168 h by trypan blue exclusion.
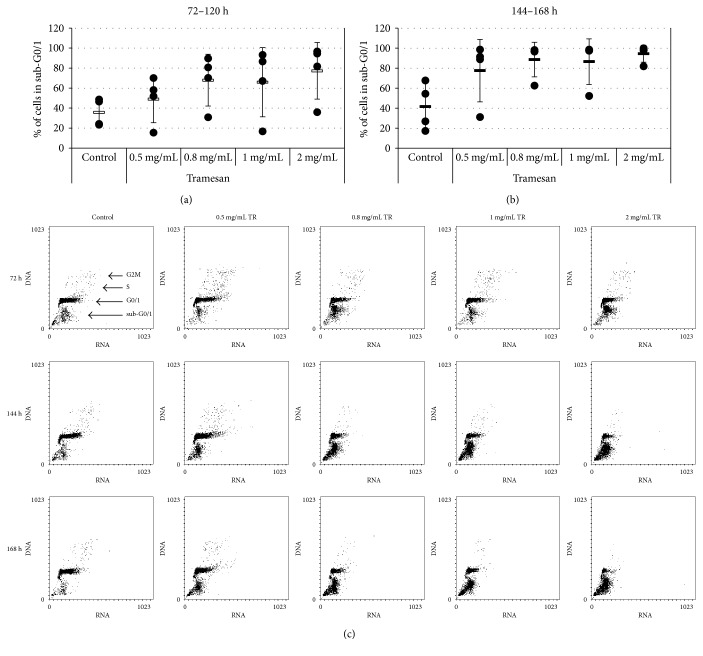
Notably, in primary AML samples, TR significantly increased apoptosis levels (sub-G0/1) from 35.7 ± 13.8%, in the control conditions, to 48.9% ± 23.5, 67.9% ± 25.9, 65.9% ± 34.6, and 77.2% ± 28.3 (p = 0.04) in the presence of 0.5, 0.8, 1, and 2 mg/mL of TR, respectively (Figure 5(a)). This effect was, however, even more marked at a prolonged exposure to the molecule (144–168 hours), reaching the statistical significance already at the lowest dose: from 41.6% ± 23.5, in the control condition, to 77.5% ± 31.1 (p = 0.04), 88.6% ± 17.3 (p = 0.01), 86.5% ± 22.8 (p = 0.01), and 94.5% ± 8.4 (p = 0.01), with 0.5, 0.8, 1, and 2 mg/mL of TR, respectively (Figures 5(b) and 5(c)).
Figure 5.
TR induces a dose- and time-dependent apoptosis in primary cells from AML. AML primary cells were exposed to the indicated concentrations of TR. Apoptosis induction was then evaluated by the AO technique. Percentages of sub-G0/1 cells are shown for the individual sample after 72–120 h (a) and 144–168 h (b) exposure to scalar concentrations of TR. A representative experiment is shown (c).
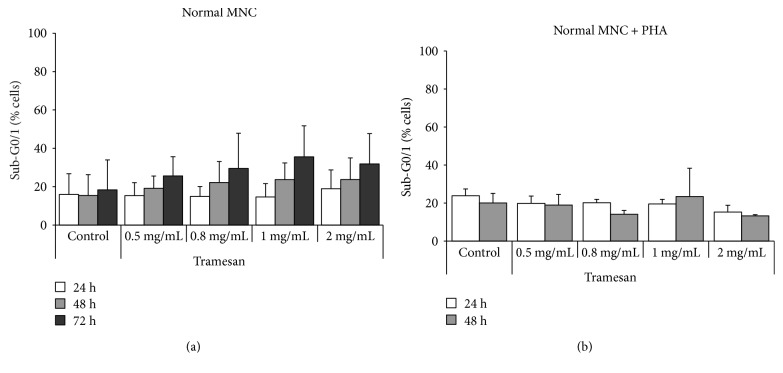
3.3. Effects of TR on Normal Cells
In order to analyze the activity of the compound on normal hematopoietic cells, we investigated the effects of TR on mononuclear cells obtained from PB of normal donors. The MNC were then exposed in liquid culture ± TR at scalar concentrations (0.5, 0.8, 1, and 2 mg/mL), in the absence and in the presence of a proliferative stimulus (PHA). The results obtained showed that TR was able to induce only a moderate, not statistically significant, proapoptotic activity, regardless of the dose: after 72 hours, apoptosis levels were 18.3% ± 15.6, in the control condition, and 25.65% ± 10.0, 29.5% ± 18.4, 35.5% ± 16.2, and 31.8% ± 15.8, with 0.5, 0.8, 1, and 2 mg/mL of TR, respectively (Figure 6(a)). These effects were not enhanced by the pretreatment with PHA: at 48 hours, apoptosis levels were 20.1% ± 5.0, in the control condition, and 18.9% ± 5.6, 14.1% ± 2.6, 23.4% ± 14.9, and 13.3% ± 1.0, with 0.5, 0.8, 1, and 2 mg/mL of TR, respectively (Figure 6(b)).
Figure 6.
TR does not exert proapoptotic activity on normal MNC. Analysis of the levels of apoptosis (% sub-G0/1) in nonstimulated lympho-monocytes (a) and in those stimulated with PHA (b) and subsequently exposed to scalar concentrations of TR.
4. Discussion
The present study demonstrated that TR, a polysaccharide extract from T. versicolor, significantly suppresses cell growth of human leukemia cell lines from different ontogenesis and exerts a cytotoxic activity on primary cells from AML patients.
T. versicolor is one of the most commonly used medicinal mushrooms, and several extracts have already been investigated for their antineoplastic effects. The cytotoxic activity of PSP and PSK extract from T. versicolor has been reported in myeloid and lymphoid leukemia cell line [25, 26]. It was also demonstrated that an ethanol-water extract from wild T. versicolor exerted in vitro antiproliferative and cytotoxic effects against leukemia and lymphoma cell lines [26]. Moreover, the antioxidant effects of different molecular mass enzymatic hydrolysates from PSPs were described [27]. In addition, results from a phase 1 clinical trial proved that an orally administered preparation from the T. versicolor may improve immune response in women with breast cancer after standard chemotherapy and radiotherapy [14].
We demonstrated, for the first time at our knowledge, the in vitro cytotoxic activity of a polysaccharidic fraction purified from T. versicolor on primary AML cells, as shown by a time- and dose-dependent induction of apoptosis.
One of the fundamental hallmarks of cancer cells is their capacity to proliferate persistently, eluding cell cycle checkpoint controls and growth inhibitory signals [28, 29]. Moreover, the acquired resistance toward apoptosis represents another key hallmark of cancer. Hence, molecules capable to block the uncontrolled proliferation and/or to induce to cell death have been evermore considered very promising for therapeutic applications in cancers. Previous reports demonstrated that PSK induces in vitro cytotoxic activity in tumor cell lines, via arrest of cell cycle and induction of apoptosis [11, 15].
Data presented in our study demonstrated that TR causes in leukemia models a dose-dependent increase of cells in G0 phase and a decrease in both G1 and S phases. Nevertheless, lack of statistical significance argues in favor of a deceleration of cell cycle rather than a cell cycle arrest and apoptosis induction at these concentrations. The preclinical relevance of TR was showed on primary samples obtained from de novo AML patients. TR was indeed able to decrease the leukemic blast viability by significantly inducing apoptosis in all AML samples tested. By contrast, no significant cytotoxicity was observed on resting and activated normal MNC thus suggesting the existence of a therapeutic window in leukemia setting.
The direct antiproliferative effect of several fungal polysaccharides on cancer cells, caused by cell cycle arrest and apoptosis induction, has been also reported associated with oxidative stress [30, 31]. Here, in fact, we documented ROS accumulation in leukemia cell line. It was widely described that oxidants can contribute to cancer development by promoting cell mutation and growth [32]. Nevertheless, it was also reported that excessive oxidative stress can slow proliferation and induce cell death [32]. TR-induced cytotoxicity observed in primary AML samples could then be due to oxidative stress-induced cell death.
Although the literature seems to suggest the involvement of Toll-like receptors or of other cell surface receptors in the increased oxidative stress induced by fungal polysaccharides [33, 34], the exact mechanism remains to be clarified.
In conclusion, the cytotoxic preclinical activity of TR on primary leukemia cells, as compared to normal MNC obtained from healthy cells, makes TR an interesting bioactive compound among other antileukemic treatments and prompts further investigation aiming to elucidate its mechanism of action for a translational application.
Acknowledgments
The authors thank RomaAIL (Associazione Italiana contro le Leucemie-linfomi e Mieloma) for providing laboratory space. This work was supported by grants from Sapienza University of Rome (C26A10SW9X and C26A14CPRB) and Fondazione Frisiani-Santini.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Arber D. A., Orazi A., Hasserjian R., et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- 2.Cicconi L., Lo-Coco F. Current management of newly diagnosed acute promyelocytic leukemia. Annals of Oncology. 2016;27(8):1474–1481. doi: 10.1093/annonc/mdw171. [DOI] [PubMed] [Google Scholar]
- 3.Shafer D., Grant S. Update on rational targeted therapy in AML. Blood Reviews. 2016;30(4):275–283. doi: 10.1016/j.blre.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cragg G. M., Newman D. J. Plants as a source of anti-cancer agents. Journal of Ethnopharmacology. 2005;100(1-2):72–79. doi: 10.1016/j.jep.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 5.Nobili S., Lippi D., Witort E., et al. Natural compounds for cancer treatment and prevention. Pharmacological Research. 2009;59(6):365–378. doi: 10.1016/j.phrs.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 6.Siveen K. S., Uddin S., Mohammad R. M. Targeting acute myeloid leukemia stem cell signalling by natural products. Molecular Cancer. 2017;16(1):p. 13. doi: 10.1186/s12943-016-0571-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guggenheim A. G., Wright K. M., Zwickey H. L. Immune modulation from five major mushrooms: application to integrative oncology. Integrative Medicine. 2014;13(1):32–44. [PMC free article] [PubMed] [Google Scholar]
- 8.Paterson R. R., Lima N. Biomedical effects of mushrooms with emphasis on pure compounds. Biomedical Journal. 2014;37(6):357–368. doi: 10.4103/2319-4170.143502. [DOI] [PubMed] [Google Scholar]
- 9.Bisen P. S., Baghel R. K., Sanodiya B. S., Thakur G. S., Prasad G. B. Lentinus edodes: a macrofungus with pharmacological activities. Current Medicinal Chemistry. 2010;17(22):2419–2430. doi: 10.2174/092986710791698495. [DOI] [PubMed] [Google Scholar]
- 10.Patel S., Goyal A. Recent developments in mushrooms as anti-cancer therapeutics: a review. 3 Biotech. 2012;2(1):1–15. doi: 10.1007/s13205-011-0036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiménez-Medina E., Berruguilla E., Romero I., et al. The immunomodulator PSK induces in vitro cytotoxic activity in tumour cell lines via arrest of cell cycle and induction of apoptosis. BMC Cancer. 2008;8(78) doi: 10.1186/1471-2407-8-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakamoto J., Morita S., Oba K., et al. Efficacy of adjuvant immunochemotherapy with polysaccharide K for patients with curatively resected colorectal cancer: a meta-analysis of centrally randomized controlled clinical trials. Cancer Immunology, Immunotherapy. 2006;55(4):404–411. doi: 10.1007/s00262-005-0054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ueda Y., Fujimura T., Kinami S., et al. A randomized phase III trial of postoperative adjuvant therapy with S-1 alone versus S-1 plus PSK for stage II/IIIA gastric cancer: Hokuriku-Kinki Immunochemo-Therapy Study Group-Gastric Cancer (HKIT-GC) Japanese Journal of Clinical Oncology. 2006;36(8):519–522. doi: 10.1093/jjco/hyl048. [DOI] [PubMed] [Google Scholar]
- 14.Torkelson C. J., Sweet E., Martzen M. R., et al. Phase 1 clinical trial of Trametes versicolor in women with breast cancer. ISRN Oncology. 2012;2012:7. doi: 10.5402/2012/251632.251632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hui K. P., Sit W. H., Wan J. M. Induction of S phase cell arrest and caspase activation by polysaccharide peptide isolated from Coriolus versicolor enhanced the cell cycle dependent activity and apoptotic cell death of doxorubicin and etoposide, but not cytarabine in HL-60 cells. Oncology Reports. 2005;14(1):145–155. doi: 10.3892/or.14.1.145. [DOI] [PubMed] [Google Scholar]
- 16.Hirahara N., Fujioka M., Edamatsu T., et al. Protein-bound polysaccharide-K (PSK) induces apoptosis and inhibits proliferation of promyelomonocytic leukemia HL-60 cells. Anticancer Research. 2011;31(9):2733–2738. [PubMed] [Google Scholar]
- 17.Hirahara N., Edamatsu T., Fujieda A., Fujioka M., Wada T., Tajima Y. Protein-bound polysaccharide-K induces apoptosis via mitochondria and p38 mitogen-activated protein kinase-dependent pathways in HL-60 promyelomonocytic leukemia cells. Oncology Reports. 2013;30(1):99–104. doi: 10.3892/or.2013.2412. [DOI] [PubMed] [Google Scholar]
- 18.Reverberi M., Fabbri A. A., Zjalic S., Ricelli A., Punelli F., Fanelli C. Antioxidant enzymes stimulation in Aspergillus parasiticus by Lentinula edodes inhibits aflatoxin production. Applied Microbiology and Biotechnology. 2005;69(2):207–215. doi: 10.1007/s00253-005-1979-1. [DOI] [PubMed] [Google Scholar]
- 19.Reverberi M., Zjalic S., Ricelli A., et al. Modulation of antioxidant defense in Aspergillus parasiticus is involved in aflatoxin biosynthesis: a role for the ApyapA gene. Eukaryotic Cell. 2008;7(6):988–1000. doi: 10.1128/ec.00228-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scarpari M., Bello C., Pietricola C., et al. Aflatoxin control in maize by Trametes versicolor. Toxins. 2014;6(12):3426–3437. doi: 10.3390/toxins6123426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zjalic S., Fabbri A. A., Reverberi M., Ricelli A., Punelli F., Fanelli C. Use of Trametes versicolor in control of aflatoxins produced by Aspergillus parasiticus. International Journal of Medicinal Mushrooms. 2007;9(3&4):236–237. [Google Scholar]
- 22.Yemm E. W., Willis A. J. The estimation of carbohydrates in plant extracts by anthrone. Biochemical Journal. 1954;57(3):508–514. doi: 10.1042/bj0570508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tafuri A., Andreeff M. Kinetic rationale for cytokine induced recruitment of myeloblastic leukemia followed by cycle-specific chemotherapy in vitro. Leukemia. 1990;4(12):826–834. [PubMed] [Google Scholar]
- 24.Ricciardi M. R., Petrucci M. T., Gregorj C., et al. Reduced susceptibility to apoptosis correlates with kinetic quiescence in disease progression of chronic lymphocytic leukaemia. British Journal of Haematology. 2001;113(2):391–399. doi: 10.1046/j.1365-2141.2001.02708.x. [DOI] [PubMed] [Google Scholar]
- 25.Dong Y., Yang M. M., Kwan C. Y. In vitro inhibition of proliferation of HL-60 cells by tetrandrine and Coriolus versicolor peptide derived from Chinese medicinal herbs. Life Sciences. 1997;60(8):135–140. doi: 10.1016/s0024-3205(96)00695-9. [DOI] [PubMed] [Google Scholar]
- 26.Lau C. B., Ho C. Y., Kim C. F., et al. Cytotoxic activities of Coriolus versicolor (Yunzhi) extract on human leukemia and lymphoma cells by induction of apoptosis. Life Sciences. 2004;75(7):797–808. doi: 10.1016/j.lfs.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Jhan M. H., Yeh C. H., Tsai C. C., Kao C. T., Chang C. K., Hsieh C. W. Enhancing the antioxidant ability of Trametes versicolor polysaccharopeptides by an enzymatic hydrolysis process. Molecules. 2016;21(9):p. 1215. doi: 10.3390/molecules21091215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanahan D., Weinberg R. A. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 29.Otto T., Sicinski P. Cell cycle proteins as promising targets in cancer therapy. Nature Reviews Cancer. 2017;17(2):93–115. doi: 10.1038/nrc.2016.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsieh T. C., Wu J. M. Suppression of proliferation and oxidative stress by extracts of Ganoderma lucidum in the ovarian cancer cell line OVCAR-3. International Journal of Molecular Medicine. 2011;28(6):1065–1069. doi: 10.3892/ijmm.2011.788. [DOI] [PubMed] [Google Scholar]
- 31.Queiroz E. A., Fortes Z. B., da Cunha M. A., Barbosa A. M., Khaper N., Dekker R. F. Antiproliferative and pro-apoptotic effects of three fungal exocellular β-glucans in MCF-7 breast cancer cells is mediated by oxidative stress, AMP-activated protein kinase (AMPK) and the Forkhead transcription factor, FOXO3a. The International Journal of Biochemistry & Cell Biology. 2015;67:14–24. doi: 10.1016/j.biocel.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 32.Schieber M., Chandel N. S. ROS function in redox signaling and oxidative stress. Current Biology. 2014;24(10):R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chan G. C. F., Chan W. K., Sze D. M. Y. The effects of β-glucan on human immune and cancer cells. Journal of Hematology & Oncology. 2009;2:p. 25. doi: 10.1186/1756-8722-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Batbayar S., Lee D. H., Kim H. W. Immunomodulation of fungal β-glucan in host defense signaling by Dectin-1. Biomolecules & Therapeutics. 2012;20(5):433–445. doi: 10.4062/biomolther.2012.20.5.433. [DOI] [PMC free article] [PubMed] [Google Scholar]