Abstract
Over the last two decades, the outcomes for patients with multiple myeloma, a plasma cell malignancy, have dramatically improved. The development of the immunomodulatory drugs (IMiDs) which include thalidomide, lenalidomide, and pomalidomide, has contributed significantly to these improved outcomes. While thalidomide is now less commonly prescribed, lenalidomide is widely used in the treatment of newly diagnosed transplant-eligible and transplant-ineligible patients, in the maintenance setting post-transplant and in the relapsed/refractory setting, while pomalidomide is currently utilized in the relapsed/refractory setting. The IMiDs have been reported to have a multitude of activities, including anti-angiogenic, cytotoxic, and immunomodulatory, however, the more recent discoveries that the IMiDs bind to cereblon and thus regulate the ubiquitination of key transcription factors including IKZF1 and IKZF3, have provided greater insight into their mechanism of action. Here the clinical efficacy of these agents in myeloma is reviewed as well as discussion of structure-function relationship, the molecular mechanisms of action, and the association of IMiDs with second primary malignancies and thrombosis.
1. Multiple myeloma
Multiple myeloma is a plasma cell malignancy characterized by the production of monoclonal protein, anemia, and disordered bone remodeling with lytic bone disease. Until the 2000’s, there were very limited treatment options for myeloma, primarily consisting of corticosteroids, melphalan, the VAD regimen (vincristine, doxorubicin, dexamethasone), and autologous stem cell transplant. Median survival during that era was 2–3 years. With the advent of the immunomodulatory drugs (IMiDs) and the proteasome inhibitors (PIs) in the 2000’s the outcomes of patients are now significantly improving, with many patients living with their disease for more than 10 years. IMiDs are now widely used as induction therapy for both transplant eligible and ineligible patients, in the post-transplant maintenance setting, and for relapsed/refractory disease. Presently, the novel agents in the myeloma armamentarium consist of not only multiple IMiDs and PI’s, but also a histone deacetylase inhibitor (HDAC) as well as two monoclonal antibodies.
2. Thalidomide
The first-in-class IMiD, thalidomide, has had a complicated history. It was initially synthesized in the 1950’s and was noted to be “virtually non-toxic” to mice, and as a consequence, thought to be nontoxic to humans as well. Its first therapeutic use was in Europe and Canada where it could be obtained without a prescription and where it was primarily used as a sleep aid and as an anti-emetic during pregnancy (Figure 1). Its use was not approved in the United States due to concerns by the FDA over the safety of the drug. In 1961, a marked increase in occurrence of infants born with phocomelia (“seal extremities”) was noted in Germany and Australia [1, 2]. Other malformations were also noted including other limb and bone abnormalities such as amelia, syndactyly, and underdeveloped long bones as well as atresia of the esophagus, duodenum, and anus, cardiac abnormalities, and aplasia of the gallbladder and appendix [1–4]. Subsequent investigations revealed that the mothers of these newborns had used thalidomide. The most critical period for exposure was found to be 20–34 days post-fertilization [5]. Unfortunately, around 10,000 infants were born with malformations by the time thalidomide was withdrawn from the market and it was estimated that up to 40% of affected infants died within one year. Subsequent testing in other species has demonstrated marked differences in interspecies sensitivity with respect to the teratogenic effects, with rabbits and primates being very sensitive [6].
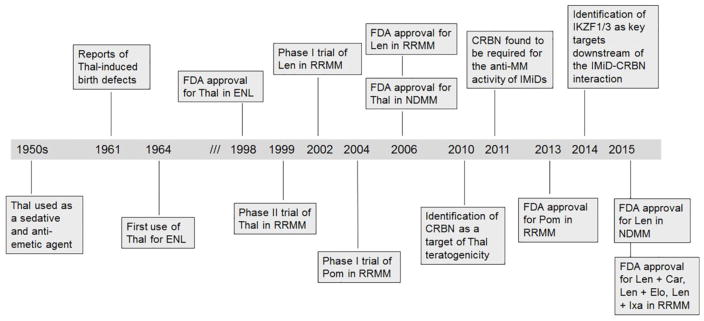
Figure 1.
Key events in the development of the IMiDs for the treatment of myeloma. Abbreviations: Car, carfilzomib; Elo, elotuzumab; ENL, erythema nodosum leprosum; Ixa, ixazomib; Len, lenalidomide; MM, multiple myeloma; NDMM, newly diagnosed multiple myeloma; Pom, pomalidomide; RRMM, relapsed/refractory multiple myeloma; Thal, thalidomide.
The next stage in the evolution of thalidomide occurred following the observation that thalidomide had activity in patients with reactive lepromatous leprosy [7]. In 1975 the FDA allowed thalidomide to be used in the treatment of leprosy through a compassionate use program. The FDA approved the use of thalidomide for the treatment of leprosy in 1998. Beneficial effects with thalidomide have also been observed in other inflammatory dermatoses including, but not limited to, cutaneous lupus erythematosus, recurrent erythema multiform, recurrent aphthous ulcers in HIV patients, Behçet disease, cutaneous sarcoidosis, and pyoderma granulosum [8] as well as chronic graft vs host disease [9].
In the 1990’s five myeloma patients with end-stage disease were given thalidomide through a compassionate-use protocol [10]. One patient experienced a significant response despite being refractory to prior therapies. This observation prompted a phase II study in 84 patients with refractory disease [10]. The starting dose was 200 mg nightly and was escalated to 800 mg. Twenty-nine percent of patients achieved at least a 50% reduction in their paraprotein, including 2 patients who achieved a complete response (CR). The most common adverse events included constipation, weakness/fatigue, somnolence, numbness/tingling, dizziness, rash, mood changes/depression, incoordination, tremors, and edema. Subsequent studies focused on the combination of thalidomide with dexamethasone in both relapsed/refractory [11, 12] and newly diagnosed patients [13–15] and this quickly became the standard of care for newly diagnosed patients with FDA approval for this indication in 2006. Eight randomized studies investigated the use of thalidomide following ASCT (for review, see [16]). These studies varied with respect to thalidomide dosing and inclusion of corticosteroids. While all demonstrated a progression-free survival (PFS) benefit there was not a consistent overall survival (OS) benefit. Prolonged use of thalidomide in the post-transplant setting was generally limited to one year due to toxicities, particularly peripheral neuropathy.
Thalidomide has been studied in combination with multiple different agents, in both the upfront and relapsed/refractory setting, including with low dose melphalan [17], oral cyclophosphamide [18–21], liposomal doxorubicin-containing regimens [22–25], bortezomib [26–29], carfilzomib [30, 31], elotuzumab [32], as well as more intensive chemotherapy regimens such as D-PACE (dexamethasone, cisplatin, doxorubicin, cyclophosphamide, etoposide) [33] and hyperfractionated cyclophosphamide [34]. Notable phase III trials which demonstrated improved outcomes with thalidomide-containing triplets include thalidomide with oral melphalan and prednisone (versus melphalan + prednisone) [35, 36] and thalidomide with bortezomib and dexamethasone (versus thalidomide + dexamethasone) [37].
3. Lenalidomide
In 2006 lenalidomide, the second member of the IMiD class, was approved for use in combination with dexamethasone for the treatment of relapsed/refractory myeloma. In 2015, this combination was approved for the treatment of newly diagnosed multiple myeloma. The initial phase I study determined the maximal tolerated dose to be 25 mg, demonstrated a lack of typical thalidomide side effects such as somnolence, constipation, or neuropathy, and showed activity in patients who had received prior thalidomide [38]. Subsequent studies revealed overall response rates of 48–61% and 46–57% response rates in patients previously treated with thalidomide [39–41]. The most common toxicities were hematological in nature, including neutropenia (grade 3/4 25–40%), anemia (grade 3/4 9–26%), thrombocytopenia (grade 3/4 11–15%). In the newly diagnosed setting, lenalidomide with dexamethasone was found to have an overall response rates of 68–91% [42, 43] and this regimen supplanted thalidomide/dexamethasone as one of the most commonly used induction regimens in the United States. A study comparing “high-dose” dexamethasone (40 mg days 1–4, 9–12, 17–20 of a 28-day cycle) to “low-dose” dexamethasone (40 mg days 1, 8, 15, 22 of a 28-day cycle) in combination with lenalidomide revealed a better one-year overall survival rate with an improved side effect profile for the low-dose weekly dexamethasone [43]. This weekly dosing of dexamethasone is now routinely used in many lenalidomide-based regimens. More recently, the triplet combination of lenalidomide, bortezomib, and dexamethasone [44] has become a standard of care of newly diagnosed patients. In the post-transplant maintenance setting, lenalidomide is widely used. Several randomized phase III studies have been performed, all of which have demonstrated significant PFS benefit with lenalidomide [45–47]. One of the studies, CALGB 100104, revealed a significant OS benefit [45] and a recent meta-analysis of the three studies confirmed an OS benefit [48].
As with thalidomide, lenalidomide has been studied in combination with multiple different agents including alkylating agents (low-dose cyclophosphamide [49–51], bendamustine [52, 53], melphalan [54]), liposomal doxorubicin [55, 56], proteasome inhibitors (bortezomib [57, 58], carfilzomib [59–61], ixazomib [62]), HDAC inhibitors (panobinostat [63], ricolinostat [64]), and monoclonal antibodies (elotuzumab [65], daratumumab [66], pembrolizumab [67]). Several recent phase III studies have been reported in which lenalidomide/dexamethasone was compared with triplet regimens in the relapsed/refractory setting [68–71]. The results of these studies are summarized in table 2. Of note, these studies consisted of lenalidomide-sensitive patients, with the majority being lenalidomide-naïve.
Table 2.
Summary of randomized phase III trials comparing lenalidomide/dexamethasone to lenalidomide/dexamethasone/novel agent in relapsed/refractory myeloma
| Novel Agent | Patients with prior lenalidomide exposure (%) | Number of prior therapies | High-risk cytogenetics (%) | Response rates (%) (triplet vs doublet) | PFS (mos) (triplet vs doublet) | OS (triplet vs doublet) |
|---|---|---|---|---|---|---|
| Carfilzomib [68] | 20 | 1–3 | 12–13 | 87 vs 67 (p<0.001) | 26.3 vs 17.6 (p=0.0001); hazard ratio 0.69 (p=0.0001) | 24 mos: 73% vs 65% (p=0.04) |
| Daratumumab [71] | 18 | >1 | 15–17 | 93 vs 64 (p<0.001) | Not reached vs 18.4; hazard ratio 0.37 (p<0.001) | Median OS: not reached vs 20.3 mos (p=0.0534) |
| Elotuzumab [69] | 6 | 1–3 | 41–42 | 79 vs 66 (p<0.001) | 19.4 vs 14.9 (p=0.014); hazard ratio 0.7 (p<0.001) | Median OS: 43.7 vs 39.6 mos (p=0.026) |
| Ixazomib [70] | 12 | 1–3 | 17–21 | 78 vs 72 (p=0.04) | 20.6 vs 14.7 (p=0.012); hazard ratio 0.74 (p=0.01) | Median OS not reached in either arm |
4. Pomalidomide
The third member of the IMiD class, pomalidomide, was approved for the treatment of relapsed/refractory myeloma in 2013 for patients who had received at least two prior regimens including lenalidomide and bortezomib. Thus far pomalidomide has not been studied in the upfront setting or in the post-transplant setting for myeloma. The primary toxicities with pomalidomide are hematologic in nature (50–60% grade 3–4 myelosuppression, 25–33% anemia, and 24–32% thrombocytopenia) while the most common grade 3–4 non-hematological adverse events are pneumonia (~11%) and fatigue (~6%) [72–74]. The rates of grade 3–4 peripheral neuropathy have been quite low (0–3%) [73–75]. In one phase II study, the combination of pomalidomide and dexamethasone (pomalidomide dosed 2 mg daily) had a 63% overall response rate including 40% ORR in lenalidomide-refractory patients and 37% ORR in thalidomide-refractory patients [74]. In another study which included only lenalidomide-refractory patients, the overall response rate was 47% [76]. An alternative dosing strategy of 4 mg daily for 21 out of 28 days was also explored and the ORR was 35–42% [72, 77]. The latter dosing schedule was approved by the FDA.
Pomalidomide has been used in combination with the other typical classes of myeloma drugs including proteasome inhibitor (bortezomib [78, 79], carfilzomib [80], ixazomib [81, 82]), alkylating agents (cyclophosphamide [83, 84] ), and monoclonal antibodies (daratumumab [85], pembrolizumab [86]).
5. Structure-function relationship
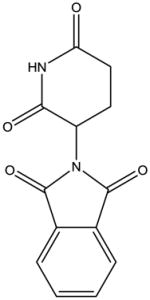
From a chemical perspective, the IMiDs are small molecules which share common phthalimide and glutarimide moieties with differences found only in the glutarimide portion (Table 1). Although the chemical structures are quite similar, the clinically used IMiDs (thalidomide, lenalidomide, pomalidomide) differ with respect to several pharmacological properties, including half-life, metabolism, clearance, and side-effect profile (Table 1). Thalidomide is not a substrate for the hepatic CYP 450 system but has been shown to undergo non-enzymatic hydrolytic cleavage at physiological pH, resulting in the generation of up to 50 different metabolites [87]. Interestingly, the (R)- and (S)-enantiomers have different clearances, resulting in higher blood concentrations of the (R)-enantiomer, although interconversion between the enantiomers in vivo has been noted [88, 89]. Lenalidomide undergoes minimal metabolism and is primarily excreted in the urine unchanged, while pomalidomide is a substrate for CYP1A2 (major), CYP2C19 (minor), CYP2D6 (minor), CYP3A4 (major) and is thus at risk for drug-drug interactions [90, 91].
Table 1.
Pharmacological properties of the IMiDs.
| Thalidomide | Lenalidomide | Pomalidomide | |
|---|---|---|---|
| Structure |
|
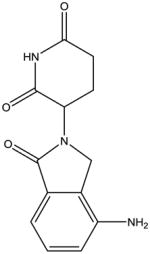
|
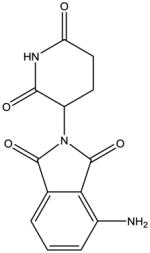
|
| Daily dosage (mg) | 50–200 | 2.5–25 | 1–4 |
| Half-life (hrs) | 5.5–7.3 | 3–5 (3-fold increase with moderate/severe renal impairment) | 7.5–9.5 |
| Renal dosing | No adjustments needed | Adjustments recommended for CrCl <60 mL/min | Adjustments recommended for patients requiring dialysis (at least 25% reduction) |
| Liver metabolism | Minimal | Minimal | CYP1A2 (major), CYP2C19 (minor), CYP2D6 (minor), CYP3A4 (major) |
| Excretion | ~90% in the urine (<4% as unchanged drug) | ~80% in the urine as unchanged drug | ~70% in the urine (2% as unchanged drug) |
| Side effects | Sedation, constipation, neuropathy, muscle weakness | Myelosuppression, fatigue, diarrhea/constipation, muscle cramps | Myelosuppression, fatigue, diarrhea/constipation |
| Non-myeloma FDA-labeled indications | Erythema nodosum leprosum | Del 5q myelodysplastic syndrome Mantle cell lymphoma |
|
| Non-FDA-labeled indications | Behcet’s syndrome Chronic graft vs host disease Lupus erythematosus Systemic mastocytosis Waldenstrom macroglobulinemia |
Chronic lymphoid leukemia Light chain amyloidosis Myelofibrosis Waldenstrom macroglobulinemia Non-germinal center diffuse large B cell lymphoma |
Light chain amyloidosis Myelofibrosis Kaposi sarcoma |
Prior to beginning to understand the molecular mechanisms of the IMiDs, it was appreciated that this drug class displayed a wide range of biological activities, many of which appeared to be relevant from an anti-myeloma perspective. In 1994, thalidomide was found to have anti-angiogenic properties associated with the inhibition of basic fibroblast growth factor [92]. While thalidomide displays little activity in cytotoxicity assays, both lenalidomide and pomalidomide have been shown to induce MM cell death [93]. These cytotoxic effects have been attributed to a variety of mechanisms, including inhibition of NFkB, decrease in IRF-4 production as a consequence of downregulation of C/EBPβ, activation of caspases, increased expression of pro-apoptotic factors and decreased expression of anti-apoptotic factors, and disruption of the PI3K/Akt pathway [93–96]. The ability of IMiDs to disrupt the myeloma cell-bone marrow stromal cell interaction was attributed to a decrease in expression of cell surface adhesion molecules and decreased IL-6 production [97]. There is a very complex relationship between myeloma cells, osteoclasts, osteoblasts, stromal cells and other members of the bone marrow microenvironment. Lenalidomide has been shown to downregulate osteoclastogenic hyperactivity and to inhibit secretion of osteoclastogenic factors such as MIP-1a, BAFF, APRIL, and RANK-L [98, 99].
Even more complex, were the myriad observations reported involving the immunomodulatory effects. Lenalidomide was noted to be significantly more potent than thalidomide in decreasing TNF-α, IL-1β, IL-6, and IL-12 production and in increasing IL-2 and IFN-γ synthesis [100]. Le Blanc et al. reported that IMiDs co-stimulate T cells via the B7-CD28 pathway [101]. IMiDs were also noted to increase T cell priming and to enhance tumor antigen uptake by dendritic cells with increased efficacy of antigen presentation [102]. IMiDs have been shown to increase and enhance the activity of both NK and NK T cells [103, 104] as well as inhibit the proliferation and function of T regulatory cells [105]. IMiDs decrease IL-2, IFNγ, and SOCS1 expression in CD4 T cells, CD8 T cells, NK T cells, and NK cells in the peripheral blood and bone marrow of myeloma patients [106]. A central mechanism of action underlying these effects was not readily apparent.
6. Cereblon-binding agents
In 2010, Ito et al., identified cereblon (CRBN) as the primary target of thalidomide teratogenicity [107], proving to be a major breakthrough for this field. CRBN is ubiquitously expressed and forms a complex with three other proteins (CUL4, DDB1, and Roc1) to produce the cullin-4 RING E3 ligase (CRL4) complex which has E3 ubiquitin ligase activity [107–109]. The importance of CRBN in mediating the anti-myeloma effects of IMiDs was demonstrated by studies in which knockdown of CRBN decreased myeloma cell viability and conferred resistance to lenalidomide and pomalidomide [110]. IMiDs have also been shown to stabilize CRBN and inhibit its own ubiquitination, thus leading to an increase in CRL4-mediated degradation of target proteins [111]. CRBN has also recently been shown to have a ubiquitin-independent function related to chaperone-like activity which facilitates the formation and activation of the CD147-MCT1 transmembrane complex [112]. IMiDs compete with CD147 and MCT1 for CRBN binding, leading to destabilization of the CD147-MCT1 complex, which in turn contributes to the anti-tumor and teratogenic effects [112].
In 2014, several groups identified Ikaros (IKZF1) and Aiolos (IKZF3) as key CRBN-interacting proteins [113–115]. IKZF1 and IKZF3 were known to be zinc finger transcription factors involved in B and T cell development [116]. In addition, IKZF3 is important for the development of long-lived plasma cells [117]. Following binding of an IMiD to CRBN, there is enhanced affinity of CRBN for IKZF1 and IKZF3, with subsequent ubiquitination and degradation of these transcription factors [113–115]. This in turn leads to changes in gene transcription including decreased expression of IRF4 and increased expression of IL-2.
With the identification of CRBN as an IMiD-binding protein, studies were performed to determine whether either CRBN mRNA or protein levels could be used as a predictive biomarker. Gene expression profiling studies failed to show differences in CRBN expression levels between normal or malignant plasma cells [118] and real time PCR studies of bone marrow mononuclear cells did not show differences in CRBN expression [119]. However, these studies did suggest a correlation between CRBN expression and response to IMiD/dexamethasone therapy [118, 119]. It should be noted that the interpretation of CRBN expression studies is complicated by multiple factors: 1) the presence of alternative splice variants of CRBN mRNA, 2) CRBN gene expression and CRBN protein levels do not appear to correlate with each other, and 3) the CRBN gene is located on chromosome 3, and chromosome 3 trisomy is a common feature of hyperdiploidy [120]. Thus currently there is insufficient evidence to support the use CRBN expression as predictive biomarker. While very low IKZF1 expression has been reported to be associated with lack of responsiveness to IMiD therapy in refractory patients treated with pomalidomide and dexamethasone [113], further validation of IKZF1 expression as a biomarker is also needed.
X-ray crystallography studies have provided additional insight into how IMiDs interact with CRBN. A crystal structure of human CRBN in complex with DDB1 and lenalidomide revealed that the IMiD-binding site consists of a shallow hydrophobic pocket on the surface of CRBN in which there are three tryptophan residues which interact with the glutarimide ring [121]. Interestingly, in studies involving a resistant cell line, re-expression of wildtype human CRBN restored drug sensitivity, however, expression of either human CRBN with mutations in the key tryptophans or wildtype mouse CRBN could not restore sensitivity [121]. The latter is noteworthy considering prior data which demonstrated that mice are resistant to IMiD-induced teratogenicity. Additional x-ray crystallography studies revealed that thalidomide, lenalidomide, and pomalidomide have almost identical binding modes and affinities for CRBN [122]. Despite this, lenalidomide and pomalidomide more efficiently target IKZF1 and IKZF3 for degradation [114, 115, 120]. This difference is thought to be due to the solvent-exposed C4 aniline functionality shared by lenalidomide and pomalidomide, as adding small functional groups to C4 of thalidomide improved IKZF1 degradation [122]. Consistent with prior in vitro experiments [123], the crystallography studies also revealed that the IMiD-binding pocket favors the (S)-enantiomer over the (R)-enantiomer [122]. Finally, these studies also confirmed the inherent complexity of the effects of IMiDs by demonstrating that IMiDs can block endogenous substrates such as MEIS2 from binding the CUL4-RBX1-DDB1-CRBN complex while IKZF1 or IKZF3 are being recruited, thus revealing that IMiDs can both upregulate and downregulate ubiquitination [122].
While it is clear that IKZF1 and IKZF3 are important players in mediating the effects of IMiDs, it should also be noted that in work by Zhu et al., 46 different CRBN-binding proteins were identified as being decreased following lenalidomide treatment [113]. Notably, gene expression profiling studies have revealed that lenalidomide treatment induced at least a two-fold change in expression of 1200 genes (600 upregulated and 600 downregulated) in a myeloma cell line [110]. In contrast, lenalidomide treatment of cells made resistance to the drug by knocking down CRBN expression results in the upregulation of 150 genes and downregulation of 30 genes, suggesting the existence of non-CRBN-mediated pathways [118].
There remain a number of unanswered questions regarding the current understanding of the IMiD-CRBN-IKZF1/3 axis and its relationship to clinical observations, including the synergy of IMiDs and PIs, the different side effect profiles and resistance patterns of the IMiDs. In vitro studies have demonstrated that pre-treatment with the PI MG132 prevents lenalidomide-induced downregulation of IKZF1/3 [115, 120], consistent with the dependence of IMiDs on proteasomal activity. However, clinically it is established that the combination of an IMiD and a PI (along with a corticosteroid) induce better responses than doublet therapy and can at least partially overcome the adverse outcomes associated with high-risk cytogenetic features [30, 59, 68, 70, 80]. It remains to be determined whether this seeming paradox can be explained by selective activity of the clinically used PIs or whether there are other factors at play. With respect IMiD cross-resistance, patients who have previously been exposed to thalidomide have a lower overall response rate to lenalidomide/dexamethasone than thalidomide-naïve patients [124] and approximately 30–47% of patients who are refractory to lenalidomide respond to pomalidomide/dexamethasone [76, 125]. There are some data which suggest that resistance to lenalidomide can be overcome by concurrent administration of thalidomide and lenalidomide [126]. In aggregate these findings would suggest that these drugs interact with CRBN in different ways leading to differential downstream effects and/or that there are other relevant targets.
7. IMiDs and monoclonal antibodies
The immune-modulating properties of the IMiDs include the potentiation of monoclonal antibody therapy. This was first noted in a mouse model of lymphoma where synergistic activity was observed between the anti-CD20 antibody rituximab and lenalidomide or pomalidomide [127]. In vivo depletion of NK cells abrogated this effect [127]. One report showed that lenalidomide enhances NK cell and monocyte-mediated antibody-dependent cellular cytotoxicity (ADCC) of rituximab-treated CD20-positive lymphoma cell line cells [128]. Lenalidomide in combination with SGN-40, an anti-CD40 antibody, enhanced direct apoptosis and ADCC against primary CLL cells, presumably as a consequence of lenalidomide-induced upregulation of CD40 expression on CLL cells and activation of NK cells [129]. Subsequently, the clinical efficacy of lenalidomide and rituximab has been demonstrated in both indolent and aggressive B-cell lymphomas as well as CLL [130–136]. In some cases, lenalidomide appears to overcome rituximab resistance [137, 138].
Lenalidomide appears to augment NK cell activity via a variety of mechanisms. Lagrue et al. reported that lenalidomide lowers the threshold for NK cell activation and increases the amount of IFNγ production in stimulated cells [139]. Examination of immune synapses revealed that lenalidomide increases the area of the actin mesh which can be penetrable to vesicles containing IFNγ [139]. Of note, pomalidomide had previously been demonstrated to reorganize the actin cytoskeleton via modulation of Rho GTPase activity [140]. Fionda et al., reported that IMiDs enhance the expression of NK cell activating receptor ligands MICA and PVR/CD155 in malignant plasma cells, thereby enhancing the recognition of the plasma cells by the NK cells [141]. This mechanism is dependent on IMiD-induced degradation of IKZF-1/IKZF-3 and IRF4 [141]. Interestingly, the ability of lenalidomide to stimulate NK cell activity has been reported to be diminished by concurrent dexamethasone treatment [142]. Due to its ability to stimulate cytokine production and enhance ADCC activity, lenalidomide has been studied in combination with cetuximab for colorectal and head and neck cancer [143, 144].
In myeloma, lenalidomide has been combined with a number of monoclonal antibodies, including elotuzumab, daratumumab, isatuximab, pembrolizumab, and an anti-KIR antibody [145]. Elotuzumab is a monoclonal antibody targeting SLAMF-7 (CS1), a cell surface glycoprotein which is expressed on plasma cells and to a lesser extent NK cells and CD8+ T cells. Preclinical studies demonstrated that elotuzumab enhances NK cell ADCC against myeloma cells [146, 147]. Although elotuzumab was subsequently determined to lack activity as a single agent in relapsed/refractory myeloma [148], it did show efficacy in combination with lenalidomide and was recently FDA-approved for relapsed/refractory disease [65, 69]. Daratumumab is an anti-CD38 monoclonal antibody which induces myeloma cell death via a variety of mechanisms including ADCC, antibody-dependent cellular phagocytosis, apoptosis, and inhibition of CD38 enzymatic activity [149, 150]. While daratumumab has impressive single agent activity in heavily treated relapsed/refractory patients (overall response rates of 29–36%) [151, 152], recent studies have demonstrated even better activity when combined with either lenalidomide [66, 153] or pomalidomide [85]. Of note, daratumumab has recently been demonstrated to have its own immune modulatory effects related to depletion of CD38-positive regulatory T cells and an increase in T-helper cells, cytotoxic T cells, and T-cell receptor clonality [154] and further studies are needed to understand how these effects are modulated by co-treatment with an IMiD. Isatuximab is another anti-CD38 monoclonal antibody [155, 156] with single agent activity (ORR ~30%) and also has activity when used in combination with lenalidomide (overall response rate of 50%) [157]. There has also been interest in combining IMiDs with checkpoint inhibitor therapy, including pembrolizumab, an anti-PD-1 antibody. While single agent activity in myeloma has not been reported, studies have been performed combining this agent with either lenalidomide [67] or pomalidomide [86] and have shown impressive activity in patients with heavily treated disease.
8. IMiDs and thrombosis
Although patients with myeloma have an increased risk for both venous and arterial thrombotic events [158, 159], this risk is further increased by the IMiD therapy. While the incidence of venous thromboembolic events (VTE) was generally less than 5% in studies using thalidomide as monotherapy, the addition of dexamethasone significantly increased the risk (8–26%) (reviewed in [160]). This risk was even greater when thalidomide was added to traditional chemotherapy agents, particularly anthracyclines (6–58%) (reviewed in [160]). In initial studies involving lenalidomide with dexamethasone without the use of thromboprophylaxis, the rates of VTE were 8–75% ((reviewed in [160]). Subsequently, studies began to routinely incorporate thromboprophylaxis, including aspirin [54, 161–165]. In a phase III study involving previously untreated patients receiving thalidomide-containing regimens, patients were randomized to receive aspirin (100 mg/day), low-dose warfarin (1.25 mg/day), or enoxaparin (40 mg/day) [166]. Serious thromboembolic events, acute cardiovascular events or sudden death in the first six months were observed in 6.5% of patients, with no statistically significant differences between the treatment groups. Another phase III study compared low-dose aspirin to low-molecular weight heparin (LMWH) in newly diagnosed patients receiving lenalidomide/dexamethasone induction and melphalan/prednisone/lenalidomide consolidation [163]. The incidence of VTE was 2.27% with aspirin and 1.20% with LMWH. There is limited data for VTE risk associated with pomalidomide as almost all studies have included thromboprophylaxis, however a phase I study of pomalidomide in patients with relapsed/refractory disease, 4/24 patients (17%) developed VTE [167]. Aspirin has now become the standard of care for patients receiving IMiD therapy without other risk factors for VTE, while for those patients at higher risk, LMWH or full-dose warfarin therapy is recommended [168].
Proposed mechanisms underlying the IMiDs pro-thrombotic effects include changes in thrombomodulin levels, transient elevations in factor VIII and von Willebrand factor, and protective effects on endothelial cell PAR-1 expression following exposure to cytotoxic agents [169–171]. Interestingly, there are data which suggest that the addition of bortezomib to IMiD therapy lowers the risk of thrombosis [172]. The mechanisms underlying these observations are as yet fully identified, however, bortezomib has been shown to have an inhibitory effect on platelet aggregation [173].
9. IMiDs and second primary malignancies
In 2010, two large randomized studies investigating lenalidomide as maintenance therapy post-ASCT reported an increased rate of second primary malignancies (SPMs) in patients receiving lenalidomide compared with placebo. Attal et al. [174] reported a SPM incidence of 2.6% in the lenalidomide group vs. 0.04% in the placebo group while McCarthy et al. [175] reported incidences of 2.6% vs. 1.7%, respectively. In addition, a non-transplant trial conducted by Palumbo et al. [176] reported an incidence of 8% in the arm which contained lenalidomide in both induction and maintenance as compared with 6% in the arm which contained lenalidomide in the induction phase only and 3% in the arm which did not contain lenalidomide. With longer follow-up, the CALGB 100104 study reported that the cumulative incidence risk of SPM was greater in the lenalidomide arm than in the placebo arm (p=0.005) with a total of 14 (6.1%) hematological malignancies and 11 (4.8%) solid tumors in the lenalidomide arm compared with 3 (1.3%) and 5 (2.2%) in the placebo arm [177]. The most recent update of the Attal study reported a total of 20 (6.6%) hematological malignancies and 24 (7.8%) solid tumors in 35 patients in the lenalidomide arm and 6 (1.9%) hematological malignancies and 11 (4.8%) solid tumors in 20 patients in the placebo arm [178]. While the majority of the hematologic SPMs noted in the CALGB 100104 and IFM 2005–02 studies were MDS/AML, there have also been B-cell acute lymphoblastic leukemia (ALL) cases, including on 5 on CALGB 100104 and 3 on IFM 2005–02 [177, 178].
Determining the extent to which factors such as IMiD therapy, alkylator therapy, transplant, or the underlying myeloma contribute to the SPM risk is an area of active investigation. Multiple studies have observed an increased risk of hematological malignancies in patients with myeloma, pre-dating the era of novel agents. In a retrospective cohort study in Asian patients, the incidences of SPM in 3970 newly diagnosed myeloma patients and 15880 patients without myeloma were compared and although the overall incidence of SPM in myeloma patients was not statistically significantly different from that of the control group, the incidence of hematological malignancies was 11-fold greater [179]. A SEER database analysis of myeloma cases between 1973–2008 showed an overall lower risk of breast, prostate, and colon cancers but a higher risk of hematological malignancies (particularly AML) [180]. A Swedish cancer registry study demonstrated an 11-fold increase in the incidence of AML/MDS in myeloma patients [181]. Of note, an 8-fold increase in the incidence of AML/MDS was observed in MGUS patients who would not have received chemotherapy. These studies suggest that patients with plasma cell disorders have a predisposition to myeloid disorders, possibly due to an intrinsic defect in the hematopoietic system.
Several studies have demonstrated that ASCT is associated with an increased risk of SPM. A retrospective cohort study examined the risk of SPM after ASCT for myeloma and found an overall cumulative incidence of 5.3% at 5 years and 11.2% at 10 years (excluding non-melanoma skin cancers) [182]. In an analysis of 4161 myeloma patients in the Center for International Blood and Marrow Transplant Research database who underwent ASCT between 1990–2010 (prior to the routine use of lenalidomide maintenance), an increased incidence of AML and melanoma were observed (observed/expected ratios of 5.19 (p=0.0004) and 3.58 (p<0.0001), respectively) [183].
An association between prolonged use of melphalan for the treatment of myeloma and the development of acute myeloid leukemia (AML) was first noted in the 1970’s [184–186]. More recently conducted studies involving IMiDs and melphalan have been reported the incidence of SPMs. The ECOG E1A06 study randomized newly diagnosed transplant ineligible patients to MPT (melphalan, prednisone, thalidomide) vs MPR (melphalan, prednisone, lenalidomide). The incidence rate (per 100 person-years) was 3.46 in the MPT arm and 2.01 in the MPR arm with 10 hematologic SPMs in the MPT arm and 4 in the MPR arm [164]. The higher hematologic SPM rate in the thalidomide arm was attributed to the MPT regimen having a higher melphalan dose (9 mg/m2) than the MPR regimen (5 mg/m2).
HOVON87/NMSG18 also compared MPT to MPR in newly diagnosed transplant ineligible patients [187]. The numbers of patients with SPMs were not significantly different between the two groups (28 in the MPT arm and 39 in the MPR arm (p=0.37)), and when non-melanoma skin cancers were excluded, the incidence rates were 2.9 (MPT) and 2.1 (MPR) per 100 patient-years (p=0.34). In the FIRST trial, MPT was compared with lenalidomide/dexamethasone (continuous vs 18 cycles) in newly diagnosed transplant-ineligible patients. The incidence of SPMs was 3% in patients on continuous lenalidomide/dexamethasone, 6% in the 18 cycle group, and 5% in the MPT group [188]. There were more hematologic cancers in the MPT arm (12 cases, 2%) than in the lenalidomide/dexamethasone arms (2 cases (<1%) in each arm).
In a pooled analysis of 2459 newly diagnosed patients from 9 European Myeloma network trials, the cumulative incidence of SPM at 3 years was 2.0% for patients who had received lenalidomide and alkylator therapy and 1.1% for those who had not received lenalidomide [189]. Overall, however, the incidence of SPMs was lower than expected and the cumulative incidence of death from myeloma was lower in the group which received lenalidomide (13.8% vs. 26.1%). Another pooled analysis of 11 clinical trials involving lenalidomide and relapsed/refractory myeloma showed an overall incidence rate of SPM of 3.62, but when non-invasive skin cancers were excluded this rate dropped to 2.08 and was comparable to the expected rate of older adults using SEER data [190]. An analysis of 703 patients from the MM-009 and MM-010 phase III trials revealed an SPM incidence rate of 3.98 in the lenalidomide-dexamethasone arms vs. 1.88 in the placebo/dexamethasone arms [190]. However, when non-melanoma skin cancers were excluded, there was not a significant difference in rates between the treatment arms or compared to the expected age-specific incidence rates [190]. An analysis of the Arkansas TT2 (+/− thalidomide) and TT3A/B (TT3A included thalidomide as part of the maintenance regimen, TT3B included lenalidomide as part of the maintenance regimen) trials found no difference in the development of SPMs between the two TT3 trials. In the TT2 arms a trend towards an increased risk of solid tumor SPMs was noted in the thalidomide arm (p=0.31) as well as a trend towards a decreased risk of hematologic malignancies[191].
A meta-analysis of more than 3000 patients from seven trials investigating lenalidomide in newly diagnosed patients showed a cumulative 5-year incidence of 6.9% in patients who received lenalidomide vs. 4.8% in patients who had not (p=0.037) [192]. The increased risk associated with lenalidomide was due to hematological malignancies (3.1% vs. 1.4%, p=0.029) and not solid tumors. Exposure to lenalidomide and oral melphalan was associated with an increased risk of hematological SPMs while exposure to lenalidomide and intravenous melphalan, cyclophosphamide, or dexamethasone were not. This study also noted that the cumulative incidences of death due to myeloma or treatment-related events were higher than those due to SPM. An analysis of patients who had had long-term lenalidomide exposure in the context of the BiRD (clarithromycin, lenalidomide, dexamethasone) regimen showed that SPM development was not associated with age, ASCT history, or length of lenalidomide therapy [193]. Furthermore, the incidence of SPM was not significantly different than what was expected based on SEER data (2.85 vs 2.1 per 100 person-years). In a retrospective cohort study of myelodysplastic syndrome (MDS) patients, lenalidomide was not associated with an increased risk of SPM or transformation to AML [194]. Finally, a recent report of lenalidomide with rituximab and bendamustine as first line therapy for elderly mantle cell lymphoma patients showed a higher number of SPMs than expected, with 9 SPMs in 8 patients (16%) including 2 hematologic and 5 solid tumors [195].
In aggregate these studies demonstrate an increased risk of SPM with lenalidomide, primarily in the post-transplant maintenance setting in a patient population which has an inherent risk of SPM due to the underlying myeloma as well as the transplant and melphalan exposure. This increased risk with lenalidomide is small and generally appears to be out-weighed by the benefit of lenalidomide on overall survival. There is significantly less evidence for an association between thalidomide and SPMs and data are lacking for pomalidomide. Further research is required in order to determine the molecular mechanisms underlying the association between lenalidomide and SPMs. It is interesting to note that loss of IKZF1 has been linked to high-risk B-ALL [196, 197], and whether IMiD-induced changes in IKZF1 might contribute to development of B-ALL remains to be determined.
10. Summary
The IMiDs have made a profound difference in the treatment of multiple myeloma and contributed significantly to the understanding of the pathophysiology of the disease. IMiDs remain the backbone of therapy for both newly diagnosed and relapsed disease and are continuing to be incorporated with new therapeutic strategies, many of which themselves have immune modulating activity. Clinical experience with the IMiDs in myeloma over the past several decades has led to a refinement in steroid dosing, the incorporation of thrombotic prophylaxis, a deeper awareness of the risk of SPMs in this patient population and an appreciation for the synergy between this class of drugs and other key therapies, including PIs and monoclonal antibodies. There is clearly further work to be done in order to fully understand the mechanisms by which IMiDs act in myeloma, to better guide treatment decisions, to understand resistance patterns, and to allow for further drug development targeting these pathways.
Key Points.
The immunomodulatory drugs (IMiDs), including thalidomide, lenalidomide, and pomalidomide, have contributed to the marked improvement in outcomes for patients with multiple myeloma.
IMiDs have pleiotropic effects on myeloma cells and other immune cells.
Footnotes
Compliance with Ethical Standards:
Funding None
Conflict of Interest S.A.H. has served on advisory committees for Celgene, Takeda, and Amgen and has received consulting fees from Celgene; P.L.M. has received honoraria from Bristol-Myers Squibb, Celgene, Sanofi-aventis, Takeda, Binding Site, research funding from Celgene, and has served on advisory committees/review panels/board membership for Bristol-Myers Squibb, Celgene, Sanofi-Aventis, Takeda, Binding Site, and Karyopharm.
References
- 1.Lenz W. Thalidomide and congenital abnormalities. Lancet Haematol. 1962;1:45. [Google Scholar]
- 2.McBride WG. Thalidomide and congenital abnormalities. Lancet. 1961;2:1358. [Google Scholar]
- 3.Mellin GW, Katzenstein M. The saga of thalidomide. Neuropathy to embryopathy, with case reports of congenital anomalies. N Engl J Med. 1962 Dec 6;267:1184–92. doi: 10.1056/NEJM196212062672305. contd. [DOI] [PubMed] [Google Scholar]
- 4.Mellin GW, Katzenstein M. The saga of thalidomide. Neuropathy to embryopathy, with case reports of congenital anomalies. N Engl J Med. 1962 Dec 13;267:1238–44. doi: 10.1056/NEJM196212132672407. concl. [DOI] [PubMed] [Google Scholar]
- 5.Kim JH, Scialli AR. Thalidomide: the tragedy of birth defects and the effective treatment of disease. Toxicol Sci. 2011 Jul;122(1):1–6. doi: 10.1093/toxsci/kfr088. [DOI] [PubMed] [Google Scholar]
- 6.Newman LM, Johnson EM, Staples RE. Assessment of the effectiveness of animal developmental toxicity testing for human safety. Reprod Toxicol. 1993 Jul-Aug;7(4):359–90. doi: 10.1016/0890-6238(93)90025-3. [DOI] [PubMed] [Google Scholar]
- 7.Sheskin J. Thalidomide in the Treatment of Lepra Reactions. Clin Pharmacol Ther. 1965 May-Jun;6:303–6. doi: 10.1002/cpt196563303. [DOI] [PubMed] [Google Scholar]
- 8.Faver IR, Guerra SG, Su WP, el-Azhary R. Thalidomide for dermatology: a review of clinical uses and adverse effects. Int J Dermatol. 2005 Jan;44(1):61–7. doi: 10.1111/j.1365-4632.2004.02445.x. [DOI] [PubMed] [Google Scholar]
- 9.Koc S, Leisenring W, Flowers ME, Anasetti C, Deeg HJ, Nash RA, et al. Thalidomide for treatment of patients with chronic graft-versus-host disease. Blood. 2000 Dec 1;96(12):3995–6. [PubMed] [Google Scholar]
- 10.Singhal S, Mehta J, Desikan R, Ayers D, Roberson P, Eddlemon P, et al. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med. 1999 Nov 18;341(21):1565–71. doi: 10.1056/NEJM199911183412102. [DOI] [PubMed] [Google Scholar]
- 11.Palumbo A, Giaccone L, Bertola A, Pregno P, Bringhen S, Rus C, et al. Low-dose thalidomide plus dexamethasone is an effective salvage therapy for advanced myeloma. Haematologica. 2001 Apr;86(4):399–403. [PubMed] [Google Scholar]
- 12.Dimopoulos MA, Zervas K, Kouvatseas G, Galani E, Grigoraki V, Kiamouris C, et al. Thalidomide and dexamethasone combination for refractory multiple myeloma. Ann Oncol. 2001 Jul;12(7):991–5. doi: 10.1023/a:1011132808904. [DOI] [PubMed] [Google Scholar]
- 13.Rajkumar SV, Hayman S, Gertz MA, Dispenzieri A, Lacy MQ, Greipp PR, et al. Combination therapy with thalidomide plus dexamethasone for newly diagnosed myeloma. J Clin Oncol. 2002 Nov 1;20(21):4319–23. doi: 10.1200/JCO.2002.02.116. [DOI] [PubMed] [Google Scholar]
- 14.Weber D, Rankin K, Gavino M, Delasalle K, Alexanian R. Thalidomide alone or with dexamethasone for previously untreated multiple myeloma. J Clin Oncol. 2003 Jan 1;21(1):16–9. doi: 10.1200/JCO.2003.03.139. [DOI] [PubMed] [Google Scholar]
- 15.Cavo M, Zamagni E, Tosi P, Cellini C, Cangini D, Tacchetti P, et al. First-line therapy with thalidomide and dexamethasone in preparation for autologous stem cell transplantation for multiple myeloma. Haematologica. 2004 Jul;89(7):826–31. [PubMed] [Google Scholar]
- 16.McCarthy PL, Palumbo A. Maintenance therapy for multiple myeloma. Hematol Oncol Clin North Am. 2014 Oct;28(5):839–59. doi: 10.1016/j.hoc.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Palumbo A, Avonto I, Bruno B, Ambrosini MT, Bringhen S, Cavallo F, et al. Intravenous melphalan, thalidomide and prednisone in refractory and relapsed multiple myeloma. Eur J Haematol. 2006 Apr;76(4):273–7. doi: 10.1111/j.1600-0609.2005.00610.x. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Sanz R, Gonzalez-Porras JR, Hernandez JM, Polo-Zarzuela M, Sureda A, Barrenetxea C, et al. The oral combination of thalidomide, cyclophosphamide and dexamethasone (ThaCyDex) is effective in relapsed/refractory multiple myeloma. Leukemia. 2004 Apr;18(4):856–63. doi: 10.1038/sj.leu.2403322. [DOI] [PubMed] [Google Scholar]
- 19.Dimopoulos MA, Hamilos G, Zomas A, Gika D, Efstathiou E, Grigoraki V, et al. Pulsed cyclophosphamide, thalidomide and dexamethasone: an oral regimen for previously treated patients with multiple myeloma. Hematol J. 2004;5(2):112–7. doi: 10.1038/sj.thj.6200326. [DOI] [PubMed] [Google Scholar]
- 20.Hovenga S, Daenen SM, de Wolf JT, van Imhoff GW, Kluin-Nelemans HC, Sluiter WJ, et al. Combined thalidomide and cyclophosphamide treatment for refractory or relapsed multiple myeloma patients: a prospective phase II study. Ann Hematol. 2005 May;84(5):311–6. doi: 10.1007/s00277-004-0981-5. [DOI] [PubMed] [Google Scholar]
- 21.Kyriakou C, Thomson K, D’Sa S, Flory A, Hanslip J, Goldstone AH, et al. Low-dose thalidomide in combination with oral weekly cyclophosphamide and pulsed dexamethasone is a well tolerated and effective regimen in patients with relapsed and refractory multiple myeloma. Br J Haematol. 2005 Jun;129(6):763–70. doi: 10.1111/j.1365-2141.2005.05521.x. [DOI] [PubMed] [Google Scholar]
- 22.Offidani M, Corvatta L, Marconi M, Visani G, Alesiani F, Brunori M, et al. Low-dose thalidomide with pegylated liposomal doxorubicin and high-dose dexamethasone for relapsed/refractory multiple myeloma: a prospective, multicenter, phase II study. Haematologica. 2006 Jan;91(1):133–6. [PubMed] [Google Scholar]
- 23.Offidani M, Corvatta L, Piersantelli MN, Visani G, Alesiani F, Brunori M, et al. Thalidomide, dexamethasone, and pegylated liposomal doxorubicin (ThaDD) for patients older than 65 years with newly diagnosed multiple myeloma. Blood. 2006 Oct 1;108(7):2159–64. doi: 10.1182/blood-2006-03-013086. [DOI] [PubMed] [Google Scholar]
- 24.Zervas K, Dimopoulos MA, Hatzicharissi E, Anagnostopoulos A, Papaioannou M, Mitsouli C, et al. Primary treatment of multiple myeloma with thalidomide, vincristine, liposomal doxorubicin and dexamethasone (T-VAD doxil): a phase II multicenter study. Ann Oncol. 2004 Jan;15(1):134–8. doi: 10.1093/annonc/mdh026. [DOI] [PubMed] [Google Scholar]
- 25.Hussein MA, Baz R, Srkalovic G, Agrawal N, Suppiah R, Hsi E, et al. Phase 2 study of pegylated liposomal doxorubicin, vincristine, decreased-frequency dexamethasone, and thalidomide in newly diagnosed and relapsed-refractory multiple myeloma. Mayo Clin Proc. 2006 Jul;81(7):889–95. doi: 10.4065/81.7.889. [DOI] [PubMed] [Google Scholar]
- 26.Ciolli S, Leoni F, Gigli F, Rigacci L, Bosi A. Low dose Velcade, thalidomide and dexamethasone (LD-VTD): an effective regimen for relapsed and refractory multiple myeloma patients. Leuk Lymphoma. 2006 Jan;47(1):171–3. doi: 10.1080/10428190500272721. [DOI] [PubMed] [Google Scholar]
- 27.Palumbo A, Ambrosini MT, Benevolo G, Pregno P, Pescosta N, Callea V, et al. Bortezomib, melphalan, prednisone, and thalidomide for relapsed multiple myeloma. Blood. 2007 Apr 1;109(7):2767–72. doi: 10.1182/blood-2006-08-042275. [DOI] [PubMed] [Google Scholar]
- 28.Wang M, Giralt S, Delasalle K, Handy B, Alexanian R. Bortezomib in combination with thalidomide-dexamethasone for previously untreated multiple myeloma. Hematology. 2007 Jun;12(3):235–9. doi: 10.1080/10245330701214236. [DOI] [PubMed] [Google Scholar]
- 29.Pineda-Roman M, Zangari M, van Rhee F, Anaissie E, Szymonifka J, Hoering A, et al. VTD combination therapy with bortezomib-thalidomide-dexamethasone is highly effective in advanced and refractory multiple myeloma. Leukemia. 2008 Jul;22(7):1419–27. doi: 10.1038/leu.2008.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sonneveld P, Asselbergs E, Zweegman S, van der Holt B, Kersten MJ, Vellenga E, et al. Phase 2 study of carfilzomib, thalidomide, and dexamethasone as induction/consolidation therapy for newly diagnosed multiple myeloma. Blood. 2015 Jan 15;125(3):449–56. doi: 10.1182/blood-2014-05-576256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mikhael JR, Reeder CB, Libby EN, Costa LJ, Bergsagel PL, Buadi F, et al. Phase Ib/II trial of CYKLONE (cyclophosphamide, carfilzomib, thalidomide and dexamethasone) for newly diagnosed myeloma. Br J Haematol. 2015 Apr;169(2):219–27. doi: 10.1111/bjh.13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mateos MV, Granell M, Oriol A, Martinez-Lopez J, Blade J, Hernandez MT, et al. Elotuzumab in combination with thalidomide and low-dose dexamethasone: a phase 2 single-arm safety study in patients with relapsed/refractory multiple myeloma. Br J Haematol. 2016 Jul 19; doi: 10.1111/bjh.14263. [DOI] [PubMed] [Google Scholar]
- 33.Lee CK, Barlogie B, Munshi N, Zangari M, Fassas A, Jacobson J, et al. DTPACE: an effective, novel combination chemotherapy with thalidomide for previously treated patients with myeloma. J Clin Oncol. 2003 Jul 15;21(14):2732–9. doi: 10.1200/JCO.2003.01.055. [DOI] [PubMed] [Google Scholar]
- 34.Kropff MH, Lang N, Bisping G, Domine N, Innig G, Hentrich M, et al. Hyperfractionated cyclophosphamide in combination with pulsed dexamethasone and thalidomide (HyperCDT) in primary refractory or relapsed multiple myeloma. Br J Haematol. 2003 Aug;122(4):607–16. doi: 10.1046/j.1365-2141.2003.04473.x. [DOI] [PubMed] [Google Scholar]
- 35.Facon T, Mary JY, Hulin C, Benboubker L, Attal M, Pegourie B, et al. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99–06): a randomised trial. Lancet. 2007 Oct 6;370(9594):1209–18. doi: 10.1016/S0140-6736(07)61537-2. [DOI] [PubMed] [Google Scholar]
- 36.Palumbo A, Bringhen S, Caravita T, Merla E, Capparella V, Callea V, et al. Oral melphalan and prednisone chemotherapy plus thalidomide compared with melphalan and prednisone alone in elderly patients with multiple myeloma: randomised controlled trial. Lancet. 2006 Mar 11;367(9513):825–31. doi: 10.1016/S0140-6736(06)68338-4. [DOI] [PubMed] [Google Scholar]
- 37.Cavo M, Pantani L, Petrucci MT, Patriarca F, Zamagni E, Donnarumma D, et al. Bortezomib-thalidomide-dexamethasone is superior to thalidomide-dexamethasone as consolidation therapy after autologous hematopoietic stem cell transplantation in patients with newly diagnosed multiple myeloma. Blood. 2012 Jul 5;120(1):9–19. doi: 10.1182/blood-2012-02-408898. [DOI] [PubMed] [Google Scholar]
- 38.Richardson PG, Schlossman RL, Weller E, Hideshima T, Mitsiades C, Davies F, et al. Immunomodulatory drug CC-5013 overcomes drug resistance and is well tolerated in patients with relapsed multiple myeloma. Blood. 2002 Nov 1;100(9):3063–7. doi: 10.1182/blood-2002-03-0996. [DOI] [PubMed] [Google Scholar]
- 39.Weber DM, Chen C, Niesvizky R, Wang M, Belch A, Stadtmauer EA, et al. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med. 2007 Nov 22;357(21):2133–42. doi: 10.1056/NEJMoa070596. [DOI] [PubMed] [Google Scholar]
- 40.Hou J, Du X, Jin J, Cai Z, Chen F, Zhou DB, et al. A multicenter, open-label, phase 2 study of lenalidomide plus low-dose dexamethasone in Chinese patients with relapsed/refractory multiple myeloma: the MM-021 trial. J Hematol Oncol. 2013;6:41. doi: 10.1186/1756-8722-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dimopoulos M, Spencer A, Attal M, Prince HM, Harousseau JL, Dmoszynska A, et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007 Nov 22;357(21):2123–32. doi: 10.1056/NEJMoa070594. [DOI] [PubMed] [Google Scholar]
- 42.Rajkumar SV, Hayman SR, Lacy MQ, Dispenzieri A, Geyer SM, Kabat B, et al. Combination therapy with lenalidomide plus dexamethasone (Rev/Dex) for newly diagnosed myeloma. Blood. 2005 Dec 15;106(13):4050–3. doi: 10.1182/blood-2005-07-2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rajkumar SV, Jacobus S, Callander NS, Fonseca R, Vesole DH, Williams ME, et al. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. Lancet Oncol. 2010 Jan;11(1):29–37. doi: 10.1016/S1470-2045(09)70284-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Richardson PG, Weller E, Lonial S, Jakubowiak AJ, Jagannath S, Raje NS, et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood. 2010 Aug 5;116(5):679–86. doi: 10.1182/blood-2010-02-268862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCarthy PL, Owzar K, Hofmeister CC, Hurd DD, Hassoun H, Richardson PG, et al. Lenalidomide after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012 May 10;366(19):1770–81. doi: 10.1056/NEJMoa1114083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Attal M, Lauwers-Cances V, Marit G, Caillot D, Moreau P, Facon T, et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012 May 10;366(19):1782–91. doi: 10.1056/NEJMoa1114138. [DOI] [PubMed] [Google Scholar]
- 47.Palumbo A, Cavallo F, Gay F, Di Raimondo F, Ben Yehuda D, Petrucci MT, et al. Autologous transplantation and maintenance therapy in multiple myeloma. N Engl J Med. 2014 Sep 4;371(10):895–905. doi: 10.1056/NEJMoa1402888. [DOI] [PubMed] [Google Scholar]
- 48.Attal M, Palumbo A, Holstein SA, Lauwers-Cances V, Petrucci MT, Richardson P, et al. Lenalidomide (LEN) maintenance (MNTC) after high-dose melphalan and autologous stem cell transplant (ASCT) in multiple myeloma (MM): A meta-analysis (MA) of overall survival (OS) ASCO. 2016 abstr 8001. [Google Scholar]
- 49.Nijhof IS, Franssen LE, Levin MD, Bos GM, Broijl A, Klein SK, et al. Phase 1/2 study of lenalidomide combined with low-dose cyclophosphamide and prednisone in lenalidomide-refractory multiple myeloma. Blood. 2016 Sep 19; doi: 10.1182/blood-2016-07-729236. [DOI] [PubMed] [Google Scholar]
- 50.Schey SA, Morgan GJ, Ramasamy K, Hazel B, Ladon D, Corderoy S, et al. The addition of cyclophosphamide to lenalidomide and dexamethasone in multiply relapsed/refractory myeloma patients; a phase I/II study. Br J Haematol. 2010 Aug;150(3):326–33. doi: 10.1111/j.1365-2141.2010.08250.x. [DOI] [PubMed] [Google Scholar]
- 51.Kumar SK, Lacy MQ, Hayman SR, Stewart K, Buadi FK, Allred J, et al. Lenalidomide, cyclophosphamide and dexamethasone (CRd) for newly diagnosed multiple myeloma: results from a phase 2 trial. Am J Hematol. 2011 Aug;86(8):640–5. doi: 10.1002/ajh.22053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lentzsch S, O’Sullivan A, Kennedy RC, Abbas M, Dai L, Pregja SL, et al. Combination of bendamustine, lenalidomide, and dexamethasone (BLD) in patients with relapsed or refractory multiple myeloma is feasible and highly effective: results of phase 1/2 open-label, dose escalation study. Blood. 2012 May 17;119(20):4608–13. doi: 10.1182/blood-2011-12-395715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kumar SK, Krishnan A, LaPlant B, Laumann K, Roy V, Zimmerman T, et al. Bendamustine, lenalidomide, and dexamethasone (BRD) is highly effective with durable responses in relapsed multiple myeloma. Am J Hematol. 2015 Dec;90(12):1106–10. doi: 10.1002/ajh.24181. [DOI] [PubMed] [Google Scholar]
- 54.Palumbo A, Falco P, Corradini P, Falcone A, Di Raimondo F, Giuliani N, et al. Melphalan, prednisone, and lenalidomide treatment for newly diagnosed myeloma: a report from the GIMEMA--Italian Multiple Myeloma Network. J Clin Oncol. 2007 Oct 1;25(28):4459–65. doi: 10.1200/JCO.2007.12.3463. [DOI] [PubMed] [Google Scholar]
- 55.Baz R, Walker E, Karam MA, Choueiri TK, Jawde RA, Bruening K, et al. Lenalidomide and pegylated liposomal doxorubicin-based chemotherapy for relapsed or refractory multiple myeloma: safety and efficacy. Ann Oncol. 2006 Dec;17(12):1766–71. doi: 10.1093/annonc/mdl313. [DOI] [PubMed] [Google Scholar]
- 56.Baz RC, Shain KH, Hussein MA, Lee JH, Sullivan DM, Oliver EF, et al. Phase II study of pegylated liposomal doxorubicin, low-dose dexamethasone, and lenalidomide in patients with newly diagnosed multiple myeloma. Am J Hematol. 2014 Jan;89(1):62–7. doi: 10.1002/ajh.23587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Richardson PG, Weller E, Jagannath S, Avigan DE, Alsina M, Schlossman RL, et al. Multicenter, phase I, dose-escalation trial of lenalidomide plus bortezomib for relapsed and relapsed/refractory multiple myeloma. J Clin Oncol. 2009 Dec 1;27(34):5713–9. doi: 10.1200/JCO.2009.22.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Richardson PG, Xie W, Jagannath S, Jakubowiak A, Lonial S, Raje NS, et al. A phase 2 trial of lenalidomide, bortezomib, and dexamethasone in patients with relapsed and relapsed/refractory myeloma. Blood. 2014 Mar 6;123(10):1461–9. doi: 10.1182/blood-2013-07-517276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jakubowiak AJ, Dytfeld D, Griffith KA, Lebovic D, Vesole DH, Jagannath S, et al. A phase 1/2 study of carfilzomib in combination with lenalidomide and low-dose dexamethasone as a frontline treatment for multiple myeloma. Blood. 2012 Aug 30;120(9):1801–9. doi: 10.1182/blood-2012-04-422683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Niesvizky R, Martin TG, 3rd, Bensinger WI, Alsina M, Siegel DS, Kunkel LA, et al. Phase Ib dose-escalation study (PX-171–006) of carfilzomib, lenalidomide, and low-dose dexamethasone in relapsed or progressive multiple myeloma. Clin Cancer Res. 2013 Apr 15;19(8):2248–56. doi: 10.1158/1078-0432.CCR-12-3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang M, Martin T, Bensinger W, Alsina M, Siegel DS, Kavalerchik E, et al. Phase 2 dose-expansion study (PX-171–006) of carfilzomib, lenalidomide, and low-dose dexamethasone in relapsed or progressive multiple myeloma. Blood. 2013 Oct 31;122(18):3122–8. doi: 10.1182/blood-2013-07-511170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kumar SK, Berdeja JG, Niesvizky R, Lonial S, Laubach JP, Hamadani M, et al. Safety and tolerability of ixazomib, an oral proteasome inhibitor, in combination with lenalidomide and dexamethasone in patients with previously untreated multiple myeloma: an open-label phase 1/2 study. Lancet Oncol. 2014 Dec;15(13):1503–12. doi: 10.1016/S1470-2045(14)71125-8. [DOI] [PubMed] [Google Scholar]
- 63.Chari A, Cho HJ, Leng S, Dhadwal A, Morgan G, La L, et al. A Phase II Study of Panobinostat with Lenalidomide and Weekly Dexamethasone in Myeloma. Blood. 2015;126(23):4226. [Google Scholar]
- 64.Yee AJ, Bensinger WI, Supko JG, Voorhees PM, Berdeja JG, Richardson PG, et al. Ricolinostat plus lenalidomide, and dexamethasone in relapsed or refractory multiple myeloma: a multicentre phase 1b trial. Lancet Oncol. 2016 Sep 16; doi: 10.1016/S1470-2045(16)30375-8. [DOI] [PubMed] [Google Scholar]
- 65.Lonial S, Vij R, Harousseau JL, Facon T, Moreau P, Mazumder A, et al. Elotuzumab in combination with lenalidomide and low-dose dexamethasone in relapsed or refractory multiple myeloma. J Clin Oncol. 2012 Jun 1;30(16):1953–9. doi: 10.1200/JCO.2011.37.2649. [DOI] [PubMed] [Google Scholar]
- 66.Plesner T, Arkenau H-T, Gimsing P, Krejcik J, Lemech C, Minnema MC, et al. Phase 1/2 study of daratumumab, lenalidomide, and dexamethasone for relapsed multiple myeloma. Blood. 2016;128(14):1821–8. doi: 10.1182/blood-2016-07-726729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.San Miguel J, Mateos M-V, Shah JJ, Ocio EM, Rodriguez-Otero P, Reece D, et al. Pembrolizumab in Combination with Lenalidomide and Low-Dose Dexamethasone for Relapsed/Refractory Multiple Myeloma (RRMM): Keynote-023. Blood. 2015;126(23):505. [Google Scholar]
- 68.Stewart AK, Rajkumar SV, Dimopoulos MA, Masszi T, Spicka I, Oriol A, et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015 Jan 8;372(2):142–52. doi: 10.1056/NEJMoa1411321. [DOI] [PubMed] [Google Scholar]
- 69.Lonial S, Dimopoulos M, Palumbo A, White D, Grosicki S, Spicka I, et al. Elotuzumab Therapy for Relapsed or Refractory Multiple Myeloma. N Engl J Med. 2015 Aug 13;373(7):621–31. doi: 10.1056/NEJMoa1505654. [DOI] [PubMed] [Google Scholar]
- 70.Moreau P, Masszi T, Grzasko N, Bahlis NJ, Hansson M, Pour L, et al. Oral Ixazomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. N Engl J Med. 2016 Apr 28;374(17):1621–34. doi: 10.1056/NEJMoa1516282. [DOI] [PubMed] [Google Scholar]
- 71.Dimopoulos MA, Oriol A, Nahi H, San-Miguel J, Bahlis NJ, Usmani SZ, et al. Daratumumab, Lenalidomide, and Dexamethasone for Multiple Myeloma. N Engl J Med. 2016 Oct 6;375(14):1319–31. doi: 10.1056/NEJMoa1607751. [DOI] [PubMed] [Google Scholar]
- 72.Richardson PG, Siegel D, Baz R, Kelley SL, Munshi NC, Laubach J, et al. Phase 1 study of pomalidomide MTD, safety, and efficacy in patients with refractory multiple myeloma who have received lenalidomide and bortezomib. Blood. 2013 Mar 14;121(11):1961–7. doi: 10.1182/blood-2012-08-450742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dimopoulos MA, Palumbo A, Corradini P, Cavo M, Delforge M, Di Raimondo F, et al. Safety and efficacy of pomalidomide plus low-dose dexamethasone in STRATUS (MM-010): a phase 3b study in refractory multiple myeloma. Blood. 2016 Jul 28;128(4):497–503. doi: 10.1182/blood-2016-02-700872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lacy MQ, Hayman SR, Gertz MA, Dispenzieri A, Buadi F, Kumar S, et al. Pomalidomide (CC4047) plus low-dose dexamethasone as therapy for relapsed multiple myeloma. J Clin Oncol. 2009 Oct 20;27(30):5008–14. doi: 10.1200/JCO.2009.23.6802. [DOI] [PubMed] [Google Scholar]
- 75.Richardson PG, Siegel DS, Vij R, Hofmeister CC, Baz R, Jagannath S, et al. Pomalidomide alone or in combination with low-dose dexamethasone in relapsed and refractory multiple myeloma: a randomized phase 2 study. Blood. 2014 Mar 20;123(12):1826–32. doi: 10.1182/blood-2013-11-538835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lacy MQ, Hayman SR, Gertz MA, Short KD, Dispenzieri A, Kumar S, et al. Pomalidomide (CC4047) plus low dose dexamethasone (Pom/dex) is active and well tolerated in lenalidomide refractory multiple myeloma (MM) Leukemia. 2010 Nov;24(11):1934–9. doi: 10.1038/leu.2010.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Leleu X, Attal M, Arnulf B, Moreau P, Traulle C, Marit G, et al. Pomalidomide plus low-dose dexamethasone is active and well tolerated in bortezomib and lenalidomide-refractory multiple myeloma: Intergroupe Francophone du Myelome 2009–02. Blood. 2013 Mar 14;121(11):1968–75. doi: 10.1182/blood-2012-09-452375. [DOI] [PubMed] [Google Scholar]
- 78.Lacy MQ, LaPlant BR, Laumann KM, Kumar S, Gertz MA, Hayman SR, et al. Pomalidomide, Bortezomib and Dexamethasone (PVD) for Patients with Relapsed Lenalidomide Refractory Multiple Myeloma (MM) Blood. 2014;124(21):304. doi: 10.1182/blood-2017-05-782961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Richardson PG, Hofmeister C, Raje NS, Siegel D, Lonial S, Laubach JP, et al. A Phase 1, Multicenter Study of Pomalidomide, Bortezomib, and Low-Dose Dexamethasone in Patients with Proteasome Inhibitor Exposed and Lenalidomide-Refractory Myeloma (Trial MM-005) Blood. 2015;126(23):3036. [Google Scholar]
- 80.Shah JJ, Stadtmauer EA, Abonour R, Cohen AD, Bensinger WI, Gasparetto C, et al. Carfilzomib, pomalidomide, and dexamethasone for relapsed or refractory myeloma. Blood. 2015 Nov 12;126(20):2284–90. doi: 10.1182/blood-2015-05-643320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Voorhees PM, Mulkey F, Hassoun H, Paba-Prada CE, Efebera YA, Hoke E, et al. Alliance A061202. a Phase I/II Study of Pomalidomide, Dexamethasone and Ixazomib Versus Pomalidomide and Dexamethasone for Patients with Multiple Myeloma Refractory to Lenalidomide and Proteasome Inhibitor Based Therapy: Phase I Results. Blood. 2015;126(23):375. [Google Scholar]
- 82.Krishnan A, Kapoor P, Palmer J, Kumar S, Lonial S, Htut M, et al. A phase I/II study of ixazomib (Ix) pomalidomide (POM) dexamethasone (DEX) in relapsed refractory (R/R) multple myeloma: Initial results. J Clin Oncol. 2016;34(suppl) abstr 8008. [Google Scholar]
- 83.Larocca A, Montefusco V, Bringhen S, Rossi D, Crippa C, Mina R, et al. Pomalidomide, cyclophosphamide, and prednisone for relapsed/refractory multiple myeloma: a multicenter phase 1/2 open-label study. Blood. 2013 Oct 17;122(16):2799–806. doi: 10.1182/blood-2013-03-488676. [DOI] [PubMed] [Google Scholar]
- 84.Baz RC, Martin TG, 3rd, Lin HY, Zhao X, Shain KH, Cho HJ, et al. Randomized multicenter phase 2 study of pomalidomide, cyclophosphamide, and dexamethasone in relapsed refractory myeloma. Blood. 2016 May 26;127(21):2561–8. doi: 10.1182/blood-2015-11-682518. [DOI] [PubMed] [Google Scholar]
- 85.Chari A, Lonial S, Suvannasankha A, Fay JW, Arnulf B, Ifthikharuddin JJ, et al. Open-Label, Multicenter, Phase 1b Study of Daratumumab in Combination with Pomalidomide and Dexamethasone in Patients with at Least 2 Lines of Prior Therapy and Relapsed or Relapsed and Refractory Multiple Myeloma. Blood. 2015;126(23):508. [Google Scholar]
- 86.Badros AZ, Kocoglu MH, Ma N, Rapoport AP, Lederer E, Philip S, et al. A Phase II Study of Anti PD-1 Antibody Pembrolizumab, Pomalidomide and Dexamethasone in Patients with Relapsed/Refractory Multiple Myeloma (RRMM) Blood. 2015;126(23):506. [Google Scholar]
- 87.Lepper ER, Smith NF, Cox MC, Scripture CD, Figg WD. Thalidomide metabolism and hydrolysis: mechanisms and implications. Curr Drug Metab. 2006 Aug;7(6):677–85. doi: 10.2174/138920006778017777. [DOI] [PubMed] [Google Scholar]
- 88.Eriksson T, Bjorkman S, Roth B, Fyge A, Hoglund P. Stereospecific determination, chiral inversion in vitro and pharmacokinetics in humans of the enantiomers of thalidomide. Chirality. 1995;7(1):44–52. doi: 10.1002/chir.530070109. [DOI] [PubMed] [Google Scholar]
- 89.Eriksson T, Bjorkman S, Hoglund P. Clinical pharmacology of thalidomide. Eur J Clin Pharmacol. 2001 Aug;57(5):365–76. doi: 10.1007/s002280100320. [DOI] [PubMed] [Google Scholar]
- 90.Hoffmann M, Kasserra C, Reyes J, Schafer P, Kosek J, Capone L, et al. Absorption, metabolism and excretion of [14C]pomalidomide in humans following oral administration. Cancer Chemother Pharmacol. 2013 Feb;71(2):489–501. doi: 10.1007/s00280-012-2040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen N, Wen L, Lau H, Surapaneni S, Kumar G. Pharmacokinetics, metabolism and excretion of [(14)C]-lenalidomide following oral administration in healthy male subjects. Cancer Chemother Pharmacol. 2012 Mar;69(3):789–97. doi: 10.1007/s00280-011-1760-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.D’Amato RJ, Loughnan MS, Flynn E, Folkman J. Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):4082–5. doi: 10.1073/pnas.91.9.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hideshima T, Chauhan D, Shima Y, Raje N, Davies FE, Tai YT, et al. Thalidomide and its analogs overcome drug resistance of human multiple myeloma cells to conventional therapy. Blood. 2000 Nov 1;96(9):2943–50. [PubMed] [Google Scholar]
- 94.Li S, Pal R, Monaghan SA, Schafer P, Ouyang H, Mapara M, et al. IMiD immunomodulatory compounds block C/EBP{beta} translation through eIF4E down-regulation resulting in inhibition of MM. Blood. 2011 May 12;117(19):5157–65. doi: 10.1182/blood-2010-10-314278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mitsiades N, Mitsiades CS, Poulaki V, Chauhan D, Richardson PG, Hideshima T, et al. Apoptotic signaling induced by immunomodulatory thalidomide analogs in human multiple myeloma cells: therapeutic implications. Blood. 2002 Jun 15;99(12):4525–30. doi: 10.1182/blood.v99.12.4525. [DOI] [PubMed] [Google Scholar]
- 96.Raje N, Kumar S, Hideshima T, Ishitsuka K, Chauhan D, Mitsiades C, et al. Combination of the mTOR inhibitor rapamycin and CC-5013 has synergistic activity in multiple myeloma. Blood. 2004 Dec 15;104(13):4188–93. doi: 10.1182/blood-2004-06-2281. [DOI] [PubMed] [Google Scholar]
- 97.Gupta D, Treon SP, Shima Y, Hideshima T, Podar K, Tai YT, et al. Adherence of multiple myeloma cells to bone marrow stromal cells upregulates vascular endothelial growth factor secretion: therapeutic applications. Leukemia. 2001 Dec;15(12):1950–61. doi: 10.1038/sj.leu.2402295. [DOI] [PubMed] [Google Scholar]
- 98.Breitkreutz I, Raab MS, Vallet S, Hideshima T, Raje N, Mitsiades C, et al. Lenalidomide inhibits osteoclastogenesis, survival factors and bone-remodeling markers in multiple myeloma. Leukemia. 2008 Oct;22(10):1925–32. doi: 10.1038/leu.2008.174. [DOI] [PubMed] [Google Scholar]
- 99.Bolzoni M, Storti P, Bonomini S, Todoerti K, Guasco D, Toscani D, et al. Immunomodulatory drugs lenalidomide and pomalidomide inhibit multiple myeloma-induced osteoclast formation and the RANKL/OPG ratio in the myeloma microenvironment targeting the expression of adhesion molecules. Exp Hematol. 2013 Apr;41(4):387–97.e1. doi: 10.1016/j.exphem.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 100.Corral LG, Haslett PA, Muller GW, Chen R, Wong LM, Ocampo CJ, et al. Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-alpha. J Immunol. 1999 Jul 1;163(1):380–6. [PubMed] [Google Scholar]
- 101.LeBlanc R, Hideshima T, Catley LP, Shringarpure R, Burger R, Mitsiades N, et al. Immunomodulatory drug costimulates T cells via the B7-CD28 pathway. Blood. 2004 Mar 1;103(5):1787–90. doi: 10.1182/blood-2003-02-0361. [DOI] [PubMed] [Google Scholar]
- 102.Henry JY, Labarthe MC, Meyer B, Dasgupta P, Dalgleish AG, Galustian C. Enhanced cross-priming of naive CD8+ T cells by dendritic cells treated by the IMiDs(R) immunomodulatory compounds lenalidomide and pomalidomide. Immunology. 2013 Jul;139(3):377–85. doi: 10.1111/imm.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chang DH, Liu N, Klimek V, Hassoun H, Mazumder A, Nimer SD, et al. Enhancement of ligand-dependent activation of human natural killer T cells by lenalidomide: therapeutic implications. Blood. 2006 Jul 15;108(2):618–21. doi: 10.1182/blood-2005-10-4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhu D, Corral LG, Fleming YW, Stein B. Immunomodulatory drugs Revlimid (lenalidomide) and CC-4047 induce apoptosis of both hematological and solid tumor cells through NK cell activation. Cancer Immunol Immunother. 2008 Dec;57(12):1849–59. doi: 10.1007/s00262-008-0512-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Galustian C, Meyer B, Labarthe MC, Dredge K, Klaschka D, Henry J, et al. The anti-cancer agents lenalidomide and pomalidomide inhibit the proliferation and function of T regulatory cells. Cancer Immunol Immunother. 2009 Jul;58(7):1033–45. doi: 10.1007/s00262-008-0620-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gorgun G, Calabrese E, Soydan E, Hideshima T, Perrone G, Bandi M, et al. Immunomodulatory effects of lenalidomide and pomalidomide on interaction of tumor and bone marrow accessory cells in multiple myeloma. Blood. 2010 Oct 28;116(17):3227–37. doi: 10.1182/blood-2010-04-279893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ito T, Ando H, Suzuki T, Ogura T, Hotta K, Imamura Y, et al. Identification of a primary target of thalidomide teratogenicity. Science. 2010 Mar 12;327(5971):1345–50. doi: 10.1126/science.1177319. [DOI] [PubMed] [Google Scholar]
- 108.Xin W, Xiaohua N, Peilin C, Xin C, Yaqiong S, Qihan W. Primary function analysis of human mental retardation related gene CRBN. Mol Biol Rep. 2008 Jun;35(2):251–6. doi: 10.1007/s11033-007-9077-3. [DOI] [PubMed] [Google Scholar]
- 109.Lee J, Zhou P. DCAFs, the missing link of the CUL4-DDB1 ubiquitin ligase. Mol Cell. 2007 Jun 22;26(6):775–80. doi: 10.1016/j.molcel.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 110.Zhu YX, Braggio E, Shi CX, Bruins LA, Schmidt JE, Van Wier S, et al. Cereblon expression is required for the antimyeloma activity of lenalidomide and pomalidomide. Blood. 2011 Nov 3;118(18):4771–9. doi: 10.1182/blood-2011-05-356063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu Y, Huang X, He X, Zhou Y, Jiang X, Chen-Kiang S, et al. A novel effect of thalidomide and its analogs: suppression of cereblon ubiquitination enhances ubiquitin ligase function. Faseb j. 2015 Dec;29(12):4829–39. doi: 10.1096/fj.15-274050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Eichner R, Heider M, Fernandez-Saiz V, van Bebber F, Garz AK, Lemeer S, et al. Immunomodulatory drugs disrupt the cereblon-CD147-MCT1 axis to exert antitumor activity and teratogenicity. Nat Med. 2016 Jul;22(7):735–43. doi: 10.1038/nm.4128. [DOI] [PubMed] [Google Scholar]
- 113.Zhu YX, Braggio E, Shi CX, Kortuem KM, Bruins LA, Schmidt JE, et al. Identification of cereblon-binding proteins and relationship with response and survival after IMiDs in multiple myeloma. Blood. 2014 Jul 24;124(4):536–45. doi: 10.1182/blood-2014-02-557819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kronke J, Udeshi ND, Narla A, Grauman P, Hurst SN, McConkey M, et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science. 2014 Jan 17;343(6168):301–5. doi: 10.1126/science.1244851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lu G, Middleton RE, Sun H, Naniong M, Ott CJ, Mitsiades CS, et al. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science. 2014 Jan 17;343(6168):305–9. doi: 10.1126/science.1244917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Morgan B, Sun L, Avitahl N, Andrikopoulos K, Ikeda T, Gonzales E, et al. Aiolos, a lymphoid restricted transcription factor that interacts with Ikaros to regulate lymphocyte differentiation. Embo j. 1997 Apr 15;16(8):2004–13. doi: 10.1093/emboj/16.8.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cortes M, Georgopoulos K. Aiolos is required for the generation of high affinity bone marrow plasma cells responsible for long-term immunity. J Exp Med. 2004 Jan 19;199(2):209–19. doi: 10.1084/jem.20031571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schuster SR, Kortuem KM, Zhu YX, Braggio E, Shi CX, Bruins LA, et al. The clinical significance of cereblon expression in multiple myeloma. Leuk Res. 2014 Jan;38(1):23–8. doi: 10.1016/j.leukres.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Heintel D, Rocci A, Ludwig H, Bolomsky A, Caltagirone S, Schreder M, et al. High expression of cereblon (CRBN) is associated with improved clinical response in patients with multiple myeloma treated with lenalidomide and dexamethasone. Br J Haematol. 2013 Jun;161(5):695–700. doi: 10.1111/bjh.12338. [DOI] [PubMed] [Google Scholar]
- 120.Gandhi AK, Kang J, Havens CG, Conklin T, Ning Y, Wu L, et al. Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T cell repressors Ikaros and Aiolos via modulation of the E3 ubiquitin ligase complex CRL4(CRBN.) Br J Haematol. 2014 Mar;164(6):811–21. doi: 10.1111/bjh.12708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chamberlain PP, Lopez-Girona A, Miller K, Carmel G, Pagarigan B, Chie-Leon B, et al. Structure of the human Cereblon-DDB1-lenalidomide complex reveals basis for responsiveness to thalidomide analogs. Nat Struct Mol Biol. 2014 Sep;21(9):803–9. doi: 10.1038/nsmb.2874. [DOI] [PubMed] [Google Scholar]
- 122.Fischer ES, Bohm K, Lydeard JR, Yang H, Stadler MB, Cavadini S, et al. Structure of the DDB1-CRBN E3 ubiquitin ligase in complex with thalidomide. Nature. 2014 Aug 7;512(7512):49–53. doi: 10.1038/nature13527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lopez-Girona A, Mendy D, Ito T, Miller K, Gandhi AK, Kang J, et al. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia. 2012 Nov;26(11):2326–35. doi: 10.1038/leu.2012.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang M, Dimopoulos MA, Chen C, Cibeira MT, Attal M, Spencer A, et al. Lenalidomide plus dexamethasone is more effective than dexamethasone alone in patients with relapsed or refractory multiple myeloma regardless of prior thalidomide exposure. Blood. 2008 Dec 1;112(12):4445–51. doi: 10.1182/blood-2008-02-141614. [DOI] [PubMed] [Google Scholar]
- 125.San Miguel JF, Weisel KC, Song KW, Delforge M, Karlin L, Goldschmidt H, et al. Impact of prior treatment and depth of response on survival in MM-003, a randomized phase 3 study comparing pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone in relapsed/refractory multiple myeloma. Haematologica. 2015 Oct;100(10):1334–9. doi: 10.3324/haematol.2015.125864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shah JJ, Orlowski RZ, Thomas SK, Alexanian R, Wang M, Qazilbash MH, et al. Final Results of a Phase I/II Trial of the Combination of Concurrent Lenalidomide, Thalidomide and Dexamethasone in Patients with Relapsed and/or Refractory Myeloma. Blood. 2012;120(21):75. [Google Scholar]
- 127.Hernandez-Ilizaliturri FJ, Reddy N, Holkova B, Ottman E, Czuczman MS. Immunomodulatory drug CC-5013 or CC-4047 and rituximab enhance antitumor activity in a severe combined immunodeficient mouse lymphoma model. Clin Cancer Res. 2005 Aug 15;11(16):5984–92. doi: 10.1158/1078-0432.CCR-05-0577. [DOI] [PubMed] [Google Scholar]
- 128.Wu L, Adams M, Carter T, Chen R, Muller G, Stirling D, et al. lenalidomide enhances natural killer cell and monocyte-mediated antibody-dependent cellular cytotoxicity of rituximab-treated CD20+ tumor cells. Clin Cancer Res. 2008 Jul 15;14(14):4650–7. doi: 10.1158/1078-0432.CCR-07-4405. [DOI] [PubMed] [Google Scholar]
- 129.Lapalombella R, Gowda A, Joshi T, Mehter N, Cheney C, Lehman A, et al. The humanized CD40 antibody SGN-40 demonstrates pre-clinical activity that is enhanced by lenalidomide in chronic lymphocytic leukaemia. Br J Haematol. 2009 Mar;144(6):848–55. doi: 10.1111/j.1365-2141.2008.07548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zinzani PL, Pellegrini C, Gandolfi L, Stefoni V, Quirini F, Derenzini E, et al. Combination of lenalidomide and rituximab in elderly patients with relapsed or refractory diffuse large B-cell lymphoma: a phase 2 trial. Clin Lymphoma Myeloma Leuk. 2011 Dec;11(6):462–6. doi: 10.1016/j.clml.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 131.Nowakowski GS, LaPlant B, Habermann TM, Rivera CE, Macon WR, Inwards DJ, et al. Lenalidomide can be safely combined with R-CHOP (R2CHOP) in the initial chemotherapy for aggressive B-cell lymphomas: phase I study. Leukemia. 2011 Dec;25(12):1877–81. doi: 10.1038/leu.2011.165. [DOI] [PubMed] [Google Scholar]
- 132.Wang M, Fayad L, Wagner-Bartak N, Zhang L, Hagemeister F, Neelapu SS, et al. Lenalidomide in combination with rituximab for patients with relapsed or refractory mantle-cell lymphoma: a phase 1/2 clinical trial. Lancet Oncol. 2012 Jul;13(7):716–23. doi: 10.1016/S1470-2045(12)70200-0. [DOI] [PubMed] [Google Scholar]
- 133.Badoux XC, Keating MJ, Wen S, Wierda WG, O’Brien SM, Faderl S, et al. Phase II study of lenalidomide and rituximab as salvage therapy for patients with relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol. 2013 Feb 10;31(5):584–91. doi: 10.1200/JCO.2012.42.8623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang M, Fowler N, Wagner-Bartak N, Feng L, Romaguera J, Neelapu SS, et al. Oral lenalidomide with rituximab in relapsed or refractory diffuse large cell, follicular and transformed lymphoma: a phase II clinical trial. Leukemia. 2013 Sep;27(9):1902–9. doi: 10.1038/leu.2013.95. [DOI] [PubMed] [Google Scholar]
- 135.Tuscano JM, Dutia M, Chee K, Brunson A, Reed-Pease C, Abedi M, et al. Lenalidomide plus rituximab can produce durable clinical responses in patients with relapsed or refractory, indolent non-Hodgkin lymphoma. Br J Haematol. 2014 May;165(3):375–81. doi: 10.1111/bjh.12755. [DOI] [PubMed] [Google Scholar]
- 136.Ruan J, Martin P, Shah B, Schuster SJ, Smith SM, Furman RR, et al. Lenalidomide plus Rituximab as Initial Treatment for Mantle-Cell Lymphoma. N Engl J Med. 2015 Nov 5;373(19):1835–44. doi: 10.1056/NEJMoa1505237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chong EA, Ahmadi T, Aqui NA, Svoboda J, Nasta SD, Mato AR, et al. Combination of Lenalidomide and Rituximab Overcomes Rituximab Resistance in Patients with Indolent B-cell and Mantle Cell Lymphomas. Clin Cancer Res. 2015 Apr 15;21(8):1835–42. doi: 10.1158/1078-0432.CCR-14-2221. [DOI] [PubMed] [Google Scholar]
- 138.Ahmadi T, Chong EA, Gordon A, Aqui NA, Nasta SD, Svoboda J, et al. Combined lenalidomide, low-dose dexamethasone, and rituximab achieves durable responses in rituximab-resistant indolent and mantle cell lymphomas. Cancer. 2014 Jan 15;120(2):222–8. doi: 10.1002/cncr.28405. [DOI] [PubMed] [Google Scholar]
- 139.Lagrue K, Carisey A, Morgan DJ, Chopra R, Davis DM. Lenalidomide augments actin remodeling and lowers NK-cell activation thresholds. Blood. 2015 Jul 2;126(1):50–60. doi: 10.1182/blood-2015-01-625004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Xu Y, Li J, Ferguson GD, Mercurio F, Khambatta G, Morrison L, et al. Immunomodulatory drugs reorganize cytoskeleton by modulating Rho GTPases. Blood. 2009 Jul 9;114(2):338–45. doi: 10.1182/blood-2009-02-200543. [DOI] [PubMed] [Google Scholar]
- 141.Fionda C, Abruzzese MP, Zingoni A, Cecere F, Vulpis E, Peruzzi G, et al. The IMiDs targets IKZF-1/3 and IRF4 as novel negative regulators of NK cell-activating ligands expression in multiple myeloma. Oncotarget. 2015 Sep 15;6(27):23609–30. doi: 10.18632/oncotarget.4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hsu AK, Quach H, Tai T, Prince HM, Harrison SJ, Trapani JA, et al. The immunostimulatory effect of lenalidomide on NK-cell function is profoundly inhibited by concurrent dexamethasone therapy. Blood. 2011 Feb 3;117(5):1605–13. doi: 10.1182/blood-2010-04-278432. [DOI] [PubMed] [Google Scholar]
- 143.Bertino EM, McMichael EL, Mo X, Trikha P, Davis M, Paul B, et al. A Phase I Trial to Evaluate Antibody-Dependent Cellular Cytotoxicity of Cetuximab and Lenalidomide in Advanced Colorectal and Head and Neck Cancer. Mol Cancer Ther. 2016 Sep;15(9):2244–50. doi: 10.1158/1535-7163.MCT-15-0879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gandhi AK, Shi T, Li M, Jungnelius U, Romano A, Tabernero J, et al. Immunomodulatory effects in a phase II study of lenalidomide combined with cetuximab in refractory KRAS-mutant metastatic colorectal cancer patients. PLoS One. 2013;8(11):e80437. doi: 10.1371/journal.pone.0080437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Benson DM, Jr, Cohen AD, Jagannath S, Munshi NC, Spitzer G, Hofmeister CC, et al. A Phase I Trial of the Anti-KIR Antibody IPH2101 and Lenalidomide in Patients with Relapsed/Refractory Multiple Myeloma. Clin Cancer Res. 2015 Sep 15;21(18):4055–61. doi: 10.1158/1078-0432.CCR-15-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tai YT, Dillon M, Song W, Leiba M, Li XF, Burger P, et al. Anti-CS1 humanized monoclonal antibody HuLuc63 inhibits myeloma cell adhesion and induces antibody-dependent cellular cytotoxicity in the bone marrow milieu. Blood. 2008 Aug 15;112(4):1329–37. doi: 10.1182/blood-2007-08-107292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hsi ED, Steinle R, Balasa B, Szmania S, Draksharapu A, Shum BP, et al. CS1, a potential new therapeutic antibody target for the treatment of multiple myeloma. Clin Cancer Res. 2008 May 1;14(9):2775–84. doi: 10.1158/1078-0432.CCR-07-4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zonder JA, Mohrbacher AF, Singhal S, van Rhee F, Bensinger WI, Ding H, et al. A phase 1, multicenter, open-label, dose escalation study of elotuzumab in patients with advanced multiple myeloma. Blood. 2012 Jul 19;120(3):552–9. doi: 10.1182/blood-2011-06-360552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.de Weers M, Tai YT, van der Veer MS, Bakker JM, Vink T, Jacobs DC, et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol. 2011 Feb 1;186(3):1840–8. doi: 10.4049/jimmunol.1003032. [DOI] [PubMed] [Google Scholar]
- 150.Overdijk MB, Verploegen S, Bogels M, van Egmond M, Lammerts van Bueren JJ, Mutis T, et al. Antibody-mediated phagocytosis contributes to the anti-tumor activity of the therapeutic antibody daratumumab in lymphoma and multiple myeloma. MAbs. 2015;7(2):311–21. doi: 10.1080/19420862.2015.1007813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lokhorst HM, Plesner T, Laubach JP, Nahi H, Gimsing P, Hansson M, et al. Targeting CD38 with Daratumumab Monotherapy in Multiple Myeloma. N Engl J Med. 2015 Sep 24;373(13):1207–19. doi: 10.1056/NEJMoa1506348. [DOI] [PubMed] [Google Scholar]
- 152.Lonial S, Weiss BM, Usmani SZ, Singhal S, Chari A, Bahlis NJ, et al. Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): an open-label, randomised, phase 2 trial. Lancet. 2016 Apr 9;387(10027):1551–60. doi: 10.1016/S0140-6736(15)01120-4. [DOI] [PubMed] [Google Scholar]
- 153.Dimopoulos MA, Oriol A, Nahi H, San Miguel J, Bahlis NJ, Usmani SZ, et al. Daratumumab, lenaliomide, and dexamethasone in multiple myeloma. N Engl J Med. 2016;375:1319–31. doi: 10.1056/NEJMoa1607751. [DOI] [PubMed] [Google Scholar]
- 154.Krejcik J, Casneuf T, Nijhof IS, Verbist B, Bald J, Plesner T, et al. Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood. 2016 Jul 21;128(3):384–94. doi: 10.1182/blood-2015-12-687749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Martin T, Richter J, Vij R, Cole C, Atanackovic D, Zonder J, et al. A Dose Finding Phase II Trial of Isatuximab (SAR650984, Anti-CD38 mAb) As a Single Agent in Relapsed/Refractory Multiple Myeloma. Blood. 2015;126(23):509. [Google Scholar]
- 156.Richter JR, Martin TG, Vij R, Cole C, Atanackovic D, Zonder JA, et al. Updated data from a phase II dose finding trial of single agent isatuximab (SAR650984, anti-CD38 mAB) in relapsed/refractory multiple myeloma (RRMM) J Clin Oncol. 2016;34(suppl) abstr 8005. [Google Scholar]
- 157.Vij R, Lendvai N, Martin TG, Baz RC, Campana F, Mazuir F, et al. A phase Ib dose escalation trial of isatuximab (SAR650984, anti-CD38 mAb) plus lenalidomide and dexamethasone (Len/Dex) in a relapsed/refractory multiple myeloma (RRMM): Interime results from two new dose cohorts. J Clin Oncol. 2016;34(suppl) abstr 8009. [Google Scholar]
- 158.Kristinsson SY, Pfeiffer RM, Bjorkholm M, Goldin LR, Schulman S, Blimark C, et al. Arterial and venous thrombosis in monoclonal gammopathy of undetermined significance and multiple myeloma: a population-based study. Blood. 2010 Jun 17;115(24):4991–8. doi: 10.1182/blood-2009-11-252072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Srkalovic G, Cameron MG, Rybicki L, Deitcher SR, Kattke-Marchant K, Hussein MA. Monoclonal gammopathy of undetermined significance and multiple myeloma are associated with an increased incidence of venothromboembolic disease. Cancer. 2004 Aug 1;101(3):558–66. doi: 10.1002/cncr.20405. [DOI] [PubMed] [Google Scholar]
- 160.Musallam KM, Dahdaleh FS, Shamseddine AI, Taher AT. Incidence and prophylaxis of venous thromboembolic events in multiple myeloma patients receiving immunomodulatory therapy. Thromb Res. 2009 Mar;123(5):679–86. doi: 10.1016/j.thromres.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 161.Baz R, Li L, Kottke-Marchant K, Srkalovic G, McGowan B, Yiannaki E, et al. The role of aspirin in the prevention of thrombotic complications of thalidomide and anthracycline-based chemotherapy for multiple myeloma. Mayo Clin Proc. 2005 Dec;80(12):1568–74. doi: 10.4065/80.12.1568. [DOI] [PubMed] [Google Scholar]
- 162.Zonder JA, Crowley J, Hussein MA, Bolejack V, Moore DF, Sr, Whittenberger BF, et al. Lenalidomide and high-dose dexamethasone compared with dexamethasone as initial therapy for multiple myeloma: a randomized Southwest Oncology Group trial (S0232) Blood. 2010 Dec 23;116(26):5838–41. doi: 10.1182/blood-2010-08-303487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Larocca A, Cavallo F, Bringhen S, Di Raimondo F, Falanga A, Evangelista A, et al. Aspirin or enoxaparin thromboprophylaxis for patients with newly diagnosed multiple myeloma treated with lenalidomide. Blood. 2012 Jan 26;119(4):933–9. doi: 10.1182/blood-2011-03-344333. quiz 1093. [DOI] [PubMed] [Google Scholar]
- 164.Stewart AK, Jacobus S, Fonseca R, Weiss M, Callander NS, Chanan-Khan AA, et al. Melphalan, prednisone, and thalidomide vs melphalan, prednisone, and lenalidomide (ECOG E1A06) in untreated multiple myeloma. Blood. 2015 Sep 10;126(11):1294–301. doi: 10.1182/blood-2014-12-613927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Palumbo A, Hajek R, Delforge M, Kropff M, Petrucci MT, Catalano J, et al. Continuous lenalidomide treatment for newly diagnosed multiple myeloma. N Engl J Med. 2012 May 10;366(19):1759–69. doi: 10.1056/NEJMoa1112704. [DOI] [PubMed] [Google Scholar]
- 166.Palumbo A, Cavo M, Bringhen S, Zamagni E, Romano A, Patriarca F, et al. Aspirin, warfarin, or enoxaparin thromboprophylaxis in patients with multiple myeloma treated with thalidomide: a phase III, open-label, randomized trial. J Clin Oncol. 2011 Mar 10;29(8):986–93. doi: 10.1200/JCO.2010.31.6844. [DOI] [PubMed] [Google Scholar]
- 167.Schey SA, Fields P, Bartlett JB, Clarke IA, Ashan G, Knight RD, et al. Phase I study of an immunomodulatory thalidomide analog, CC-4047, in relapsed or refractory multiple myeloma. J Clin Oncol. 2004 Aug 15;22(16):3269–76. doi: 10.1200/JCO.2004.10.052. [DOI] [PubMed] [Google Scholar]
- 168.Palumbo A, Rajkumar SV, Dimopoulos MA, Richardson PG, San Miguel J, Barlogie B, et al. Prevention of thalidomide- and lenalidomide-associated thrombosis in myeloma. Leukemia. 2008 Feb;22(2):414–23. doi: 10.1038/sj.leu.2405062. [DOI] [PubMed] [Google Scholar]
- 169.Corso A, Lorenzi A, Terulla V, Airo F, Varettoni M, Mangiacavalli S, et al. Modification of thrombomodulin plasma levels in refractory myeloma patients during treatment with thalidomide and dexamethasone. Ann Hematol. 2004 Sep;83(9):588–91. doi: 10.1007/s00277-004-0891-6. [DOI] [PubMed] [Google Scholar]
- 170.Kaushal V, Kaushal GP, Melkaveri SN, Mehta P. Thalidomide protects endothelial cells from doxorubicin-induced apoptosis but alters cell morphology. J Thromb Haemost. 2004 Feb;2(2):327–34. doi: 10.1046/j.1538-7933.2003.00573.x. [DOI] [PubMed] [Google Scholar]
- 171.Ward CM, Yen T, Harvie R, Pavlakis N. Elevated levels of factor VIII and von Willebrand factor after thalidomide treatment for malignancy: relationship to thromboembolic events. Hematol J. 2003;4(SI) abstr 265. [Google Scholar]
- 172.Zangari M, Fink L, Zhan F, Tricot G. Low venous thromboembolic risk with bortezomib in multiple myeloma and potential protective effect with thalidomide/lenalidomide-based therapy: review of data from phase 3 trials and studies of novel combination regimens. Clin Lymphoma Myeloma Leuk. 2011 Apr;11(2):228–36. doi: 10.1016/j.clml.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 173.Avcu F, Ural AU, Cetin T, Nevruz O. Effects of bortezomib on platelet aggregation and ATP release in human platelets, in vitro. Thromb Res. 2008;121(4):567–71. doi: 10.1016/j.thromres.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 174.Attal M, Lauwers Vc, Marit G, Caillot D, Facon T, Hulin C, et al. Maintenance Treatment with Lenalidomide After Transplantation for MYELOMA: Final Analysis of the IFM 2005-02. ASH Annual Meeting Abstracts; 2010 November 19; 2010. p. 310. [Google Scholar]
- 175.McCarthy PL, Owzar K, Anderson KC, Hofmeister CC, Hurd DD, Hassoun H, et al. Phase III Intergroup Study of Lenalidomide Versus Placebo Maintenance Therapy Following Single Autologous Hematopoietic Stem Cell Transplantation (AHSCT) for Multiple Myeloma: CALGB 100104. ASH Annual Meeting Abstracts; 2010 November 19; 2010. p. 37. [Google Scholar]
- 176.Palumbo A, Delforge M, Catalano J, Hajek R, Kropff M, Petrucci MT, et al. A Phase 3 Study Evaluating the Efficacy and Safety of Lenalidomide Combined with Melphalan and Prednisone In Patients >= 65 Years with Newly Diagnosed Multiple Myeloma (NDMM): Continuous Use of Lenalidomide Vs Fixed-Duration Regimens. ASH Annual Meeting Abstracts; 2010 November 19; 2010. p. 622. [Google Scholar]
- 177.Holstein SA, Owzar K, Richardson PG, Jiang C, Hofmeister CC, Hassoun H, et al. Updated analysis of CALGB/ECOG/BMT CTN 100104: Lenalidomide (Len) vs. placebo (PBO) maintenance therapy after single autologous stem cell transplant (ASCT)for multiple myeloma (MM) J Clin Oncol. 2015;33(suppl) abstr 8523. [Google Scholar]
- 178.Lauwers-Cances V, Marit G, Caillot D, Facon T, Hulin C, Moreau P, et al. Lenalidomide Maintenance After Stem-Cell Transplantation For Multiple Myeloma: Follow-Up Analysis Of The IFM 2005-02 Trial. Blood. 2013;122(21):406. [Google Scholar]
- 179.Tzeng HE, Lin CL, Tsai CH, Tang CH, Hwang WL, Cheng YW, et al. Time trend of multiple myeloma and associated secondary primary malignancies in Asian patients: a Taiwan population-based study. PLoS One. 2013;8(7):e68041. doi: 10.1371/journal.pone.0068041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Razavi P, Rand KA, Cozen W, Chanan-Khan A, Usmani S, Ailawadhi S. Patterns of second primary malignancy risk in multiple myeloma patients before and after the introduction of novel therapeutics. Blood Cancer J. 2013;3:e121. doi: 10.1038/bcj.2013.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mailankody S, Pfeiffer RM, Kristinsson SY, Korde N, Bjorkholm M, Goldin LR, et al. Risk of acute myeloid leukemia and myelodysplastic syndromes after multiple myeloma and its precursor disease (MGUS) Blood. 2011 Oct 13;118(15):4086–92. doi: 10.1182/blood-2011-05-355743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Krishnan AY, Mei M, Sun CL, Thomas SH, Teh JB, Kang T, et al. Second primary malignancies after autologous hematopoietic cell transplantation for multiple myeloma. Biol Blood Marrow Transplant. 2013 Feb;19(2):260–5. doi: 10.1016/j.bbmt.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Mahindra A, Raval G, Mehta P, Brazauskas R, Zhang MJ, Zhong X, et al. New cancers after autotransplantations for multiple myeloma. Biol Blood Marrow Transplant. 2015 Apr;21(4):738–45. doi: 10.1016/j.bbmt.2014.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kyle RA, Pierre RV, Bayrd ED. Multiple myeloma and acute myelomonocytic leukemia. N Engl J Med. 1970 Nov 19;283(21):1121–5. doi: 10.1056/NEJM197011192832101. [DOI] [PubMed] [Google Scholar]
- 185.Bergsagel DE, Bailey AJ, Langley GR, MacDonald RN, White DF, Miller AB. The chemotherapy on plasma-cell myeloma and the incidence of acute leukemia. N Engl J Med. 1979 Oct 4;301(14):743–8. doi: 10.1056/NEJM197910043011402. [DOI] [PubMed] [Google Scholar]
- 186.Gonzalez F, Trujillo JM, Alexanian R. Acute leukemia in multiple myeloma. Ann Intern Med. 1977 Apr;86(4):440–3. doi: 10.7326/0003-4819-86-4-440. [DOI] [PubMed] [Google Scholar]
- 187.Zweegman S, van der Holt B, Mellqvist UH, Salomo M, Bos GM, Levin MD, et al. Melphalan, prednisone, and lenalidomide versus melphalan, prednisone, and thalidomide in untreated multiple myeloma. Blood. 2016 Mar 3;127(9):1109–16. doi: 10.1182/blood-2015-11-679415. [DOI] [PubMed] [Google Scholar]
- 188.Benboubker L, Dimopoulos MA, Dispenzieri A, Catalano J, Belch AR, Cavo M, et al. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. N Engl J Med. 2014 Sep 4;371(10):906–17. doi: 10.1056/NEJMoa1402551. [DOI] [PubMed] [Google Scholar]
- 189.Palumbo A, Larocca A, Zweegman S, Lupparelli G, Siniscalchi A, Musto P, et al. Second Primary Malignancies in Newly Diagnosed Multiple Myeloma Patients Treated with Lenalidomide: Analysis of Pooled Data in 2459 Patients. ASH Annual Meeting Abstracts; 2011 November 18; 2011. p. 996. [Google Scholar]
- 190.Dimopoulos MA, Richardson PG, Brandenburg N, Yu Z, Weber DM, Niesvizky R, et al. A review of second primary malignancy in patients with relapsed or refractory multiple myeloma treated with lenalidomide. Blood. 2012;119(12):2764–7. doi: 10.1182/blood-2011-08-373514. [DOI] [PubMed] [Google Scholar]
- 191.Usmani SZ, Sexton R, Hoering A, Heuck CJ, Nair B, Waheed S, et al. Second malignancies in total therapy 2 and 3 for newly diagnosed multiple myeloma: influence of thalidomide and lenalidomide during maintenance. Blood. 2012 Aug 23;120(8):1597–600. doi: 10.1182/blood-2012-04-421883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Palumbo A, Bringhen S, Kumar SK, Lupparelli G, Usmani S, Waage A, et al. Second primary malignancies with lenalidomide therapy for newly diagnosed myeloma: a meta-analysis of individual patient data. Lancet Oncol. 2014 Mar;15(3):333–42. doi: 10.1016/S1470-2045(13)70609-0. [DOI] [PubMed] [Google Scholar]
- 193.Rossi A, Mark T, Jayabalan D, Christos P, Zafar F, Pekle K, et al. BiRd (clarithromycin, lenalidomide, dexamethasone): an update on long-term lenalidomide therapy in previously untreated patients with multiple myeloma. Blood. 2013 Mar 14;121(11):1982–5. doi: 10.1182/blood-2012-08-448563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Rollison DE, Shain KH, Lee JH, Hampras SS, Fulp W, Fisher K, et al. Subsequent primary malignancies and acute myelogenous leukemia transformation among myelodysplastic syndrome patients treated with or without lenalidomide. Cancer Med. 2016 Jul;5(7):1694–701. doi: 10.1002/cam4.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Albertsson-Lindblad A, Kolstad A, Laurell A, Räty R, Grønbæk K, Sundberg J, et al. Lenalidomide-bendamustine-rituximab in patients older than 65 years with untreated mantle cell lymphoma. Blood. 2016;128(14):1814–20. doi: 10.1182/blood-2016-03-704023. [DOI] [PubMed] [Google Scholar]
- 196.Mullighan CG, Su X, Zhang J, Radtke I, Phillips LA, Miller CB, et al. Deletion of IKZF1 and prognosis in acute lymphoblastic leukemia. N Engl J Med. 2009 Jan 29;360(5):470–80. doi: 10.1056/NEJMoa0808253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Olsson L, Johansson B. Ikaros and leukaemia. Br J Haematol. 2015 May;169(4):479–91. doi: 10.1111/bjh.13342. [DOI] [PubMed] [Google Scholar]