Abstract
We sought to identify brain activation differences in conduct-problem youth with limited prosocial emotions (LPE) compared to conduct-problem youth without LPE and community adolescents, and to test associations between brain activation and severity of callous-unemotional traits. We utilized a novel task, which asks subjects to repeatedly decide whether to accept offers where they will benefit but a beneficent other will be harmed. Behavior on this task has been previously associated with levels of prosocial emotions and severity of callous-unemotional traits, and is related to empathic concern. During fMRI acquisition, 66 male adolescents (21 conduct-problem patients with LPE, 21 without, and 24 typically-developing controls) played this novel game. Within typically-developing controls, we identified a network engaged during decision involving bilateral insula, and inferior parietal and medial frontal cortexes, among other regions. Group comparisons using non-parametric (distribution-free) permutation tests demonstrated LPE patients had lower activation estimates than typically-developing adolescents in right anterior insula. Additional significant group differences emerged with our a priori parametric cluster-wise inference threshold. These results suggest measurable functional brain activation differences in conduct-problem adolescents with LPE compared to typically-developing adolescents. Such differences may underscore differential treatment needs for conduct-problem males with and without LPE.
Keywords: callous, antisocial, drug abuse, prosocial, imaging
1. INTRODUCTION
Conduct disorder affects approximately 5% of U.S. adolescents and is associated with a number of negative outcomes (Coker et al., 2014). But conduct disorder is a heterogeneous phenotype. About 40% of conduct-disordered youth continue to exhibit clinically-meaningful levels of antisocial behavior into adulthood, while others apparently remit from high levels of antisocial behavior problems, having an adolescence-limited phenotype (Moffitt, 1993; Steiner and Dunne, 1997). Those with chronic antisocial trajectories account for a disproportionately large amount (about 50%) of all violent behaviors (Dodge and Pettit, 2003). In DSM-5, youths with conduct disorder may be categorized based on the presence or absence of “limited prosocial emotions” (LPE), which is characterized by high levels of callous-unemotional traits and is defined by four criteria: lack of remorse or guilt, callous lack of empathy, unconcerned about performance, and shallow or deficient affect. However, the LPE specifier is a relatively new construct and therefore most published work to date has used a related but distinct phenotype, high levels of callous-unemotional traits (i.e. a dimensional severity score (e.g.,(Sebastian et al., 2012)) or a median split to categorize individuals with high and low levels of callous-unemotional traits (e.g., (Hwang et al., 2016)). High levels of callous-unemotional traits are stable through adolescence, predict persistent antisocial behavior problems, are associated with greater violence and aggression, and predict poorer outcomes to standard treatment (Frick et al., 2014). Conduct-disordered youth with and without high levels of callous-unemotional traits may have different biological mechanisms that underlie their problem behaviors (Blair et al., 2014) and more research is needed to identify those biological differences. In addition, more research is needed comparing results from these related but distinct phenotypes: LPE and high levels of callous-unemotional traits.
The growing research literature on the neuroscience of callous-unemotional traits and antisocial behavior problems in adolescence has implicated multiple areas of impairment including poor emotional empathy, exaggerated threat responsivity, impaired reinforcement-based decision making and punishment processing, and impaired response inhibition (Blair et al., 2016; Byrd et al., 2014). But problems of empathizing may be particularly relevant to callous-unemotional traits (e.g., see Figure 1 in (Blair et al., 2016)) and such deficits in emotional empathy may impact the development of conditioned associations between one’s harmful behaviors and others’ negative reactions to those behaviors (Blair et al., 2014). Empathy, broadly defined, may be studied in various ways in the MRI environment. To date this adolescent literature has tested brain activation while: viewing pictures of painful injuries and asking subjects to imagine the injury is occurring to “Yourself” vs. “Someone Else” (Marsh et al., 2013); viewing fearful or other emotional facial expressions (Viding et al., 2012); and choosing endings to cartoon scenarios where the cartoons require understanding the internal states and perspectives of the characters to choose correct endings and test cognitive and emotional theory of mind (Sebastian et al., 2012). Some work has also shown that the emotional deficits seen in youth with psychopathic traits while viewing fearful facial expressions are not secondary to group differences in top-down attentional control (White et al., 2012). The developmental consequences of such deficits in emotional empathy can be in turn studied through examining care-based moral development (Blair et al., 2016) and moral determinations (Harenski et al., 2014). Together these studies provide very important advances in our understanding of callous-unemotional traits and demonstrate that differences in activation, especially of the amygdala (Marsh et al., 2008; Sebastian et al., 2012) and anterior insula (Blair et al., 2016), may be particularly important to those differences in emotional empathy seen among youth with high levels of callous unemotional traits.
Conduct-disordered individuals often engage in behaviors that violate the rights of others or social norms, and in turn, such behaviors often cause substantive familial disruption (Dodge and Pettit, 2003); thus many of the problems caused by these youth result from decision making in a self-versus-others (Self:Other) context. In this study, we aimed not at passive viewing of images or scenarios in the Magnetic Resonance Imaging (MRI) environment, but toward studying active decision-making about actual behaviors. Some studies have utilized advanced modeling in reinforcement learning paradigm (White et al., 2014b) and passive avoidance tasks (White et al., 2016a) to better understand decision making in this population. Here we aim to build on this growing literature by studying decision making, but within a Self:Other context. Prior work has utilized versions of the Ultimatum Game to study decisions to punish the unfair behavior of others (e.g., (White et al., 2014a)). Van den Bos and colleagues (van den Bos et al., 2014) compared antisocial youth and controls while playing the Ultimatum Game and demonstrated differences in activation of the right inferior frontal gyrus and right temporal parietal junction, though these findings were not significantly associated with levels of callousness within delinquent youth. White and colleagues (White et al., 2016b) extended this work by examining the tendency to retaliate in response to perceived unfairness of others. The study benefitted from utilizing samples of antisocial youth with and without high levels of callous-unemotional traits but the findings regarding retaliatory behavior were specific to conduct disorder with low levels of callous-unemotional traits. With the Ultimatum Game, we can examine how much people are willing to give up to punish a bad actor (i.e., costly punishment). In the game utilized in this study, the Altruism-Antisocial (AlAn’s) game, we examine a related but distinctly different construct: how much are people willing to refrain from taking for themselves in order to help a good actor (i.e., what we have previously called costly helping; Sakai et al., 2016a). Our prior publications examining this game out of the MRI environment support that individuals with high levels of callousness tend to both take more for themselves and leave less for the beneficent other, compared to comparison participants (Sakai et al., 2012, 2016a). Thus, while results examining costly punishment to date appear to be more specific to conduct disorder and may evoke systems more related to acute threat response (Blair et al., 2016), this early work with the AlAn’s game raises the possibility that differences in costly helping are more strongly related to levels of callousness and in turn, may be related more to paradigms relevant to care-based moral decision making and emotional empathy. The AlAn’s game was designed to examine decision-making with MRI.
One challenge in studying youth with conduct problems and LPE is the very common co-morbidity in this population. For example, more than half of conduct-disordered adolescents may have a co-occurring substance use disorder (Coker et al., 2014). Co-morbid ADHD and depression are also common (Sakai et al., 2016b). One approach is to study subjects without co-morbid disorders, selecting those youths with only conduct disorder and LPE. However, among conduct-disordered youths, greater co-morbidity predicts persistent antisocial behavior problems (Moffitt et al., 2001; Myers et al., 1998). Thus, excluding co-morbidity would bias results towards atypical, less severely affected samples (Krueger, 1999). An alternate strategy is to utilize other conduct-problem youths (i.e., with serious antisocial behavior problems but scoring at about average levels for callous-unemotional traits) as a control (Hwang et al., 2016). We have previously demonstrated that patients with conduct disorder with and without LPE have similar patterns of co-morbidity in terms of prevalence of depression, ADHD, and specific substance use disorders when recruited in the same manner as done in this study (Sakai et al., 2016b). A small set of work in detained adolescents has shown similar results (Colins and Vermeiren, 2013; Van Damme et al., 2016). Therefore, including both typically-developing adolescents and youth with serious conduct problems without high levels of callous-unemotional traits as comparison groups, as in prior important work (Viding et al., 2012; White et al., 2016b; Hwang et al., 2016), may provide a useful strategy to reduce confounds driven by some co-morbid disorders. But such an approach will not control for all confounds. For example, adolescent patients may differ in conduct disorder severity (e.g., symptom count; (Sakai et al., 2016b). Thus controlling for such differences in analyses may still be required.
Sex differences present a second complication in studying youth with conduct disorder and high levels of callous-unemotional traits. Conduct disorder is more common in males than females but when considering persistent antisocial behavior problems, these sex differences become quite pronounced (Eme, 2007). Similarly, males on average have higher levels of callous-unemotional traits than females (Essau et al., 2006). Therefore, this study describes a male-only sample to eliminate potential confounds of sex.
We present here for the first time results from a novel decision-making task, the AlAn’s game, in the MRI environment with three groups of male adolescents: typically-developing youths, patients with conduct problems without LPE, and patients with conduct problems with LPE. Participants underwent fMRI neuroimaging as they repeatedly decided whether or not to take actions that benefitted themselves but harmed a beneficent other. In the application for the NIH grant funding this work, we hypothesized that adolescents with serious conduct problems and LPE would differ from the two other groups in activity of the “paralimbic system,” including orbitofrontal, anterior cingulate, insula, amygdala, and superior temporal/angular gyrus. In addition, our approach allows examining the relationship between severity of callous-unemotional traits (dimensional measure) and brain activation, and secondarily also allows examining how results change when dividing patients into high and low callous-unemotional traits by median split, in comparison to our pre-hoc approach (i.e. dividing groups based on the LPE specifier).
2. METHODS
The study protocol and consents were approved by the Colorado Multiple Institutional Review Board. All adolescents under age 18 provided written assent and their parent’s written consent. Youth who were 18 years of age provided written consent to study participation.
2.1 Sample Description
Typically-developing adolescents with neither conduct disorder nor LPE specifier were recruited via online and printed advertisements. Community youths were excluded for prior convictions (minor traffic or curfew violations were permitted) or a history of substance-related school expulsion or substance-related treatment. Youth with conduct problems were drawn from patients at a university-based treatment program for adolescents with serious antisocial and substance problems and all had at least one non-nicotine DSM-IV substance use disorder. General inclusion criteria were: ages 15–18 years, male, estimated IQ ≥80, self-reported abstinence ≥30 days from drugs/alcohol (confirmed in past week by research-staff collected negative urine drug and saliva alcohol tests and review of all clinical urine drug screens in past 30 days), English proficiency judged adequate for valid assent, right handed, and participant and all first degree relatives have neither volunteered or worked for the Red Cross nor received assistance from that agency (note: this exclusion is related to the behavioral paradigm described in Section 2.2). Exclusion criteria were: history of head injury with loss of consciousness ≥15 minutes, history of neurosurgical procedures, physical illness which would prevent participation or that has a well-documented effect on brain morphometry, red-green color blindness, psychotic, bipolar or anxiety disorder as indicated by assessment and confirmed by clinician interview [JTS], current dangerousness or intoxication, and current experience of caffeine/nicotine withdrawal with cessation of use. Adolescents were instructed to refrain from caffeine and nicotine use for 12 hours prior to scanning. Standard MRI exclusion criteria were also employed (Sakai et al., 2016a).
Seventy one adolescents were enrolled and completed all study procedures. Excluded from MRI analyses were two patients with LPE (excessive motion), one patient without LPE (excessive motion) and two typically-developing youths (data loss on the server). Table 1 shows demographic and diagnostic information for our 3 adolescent groups. As expected, typically-developing adolescents had significantly lower scores than conduct-problem youth with LPE on levels of callousness and ADHD severity. Conduct-problem adolescents without LPE, compared to those with LPE, had significantly lower ICU total scores (p<0.001) and were about 0.6 years older than those with LPE (p=0.04). In agreement with our predictions and prior work (Sakai et al., 2016b), these two patient groups were similar in their diagnostic profiles and did not significantly differ in cannabis, tobacco, alcohol or cocaine use disorder, conduct disorder diagnosis, whole-life major depression prevalence, or ADHD symptom severity.
Table 1.
Male Adolescent Conduct-Problem Patients Meeting the Limited Prosocial Emotions (LPE) Specifier and 2 Comparison groups (Male Adolescent Patients without LPE and Typically Developing Male Adolescents). Note: highlighted cells provide an easy visual cue indicating that group differed from patients with LPE at p<0.05.
| Group | Analyses | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Conduct problems with LPE (n=21) | Conduct problems without LPE (n=21) | Typically developing controls (n=24) | Three group comparison | Post-hoca | ||
| Demographics/Cognitive | ||||||
| Mean (SEM) | Age in years (SEM) | 16.6 (0.16) | 17.2 (0.18) | 16.5 (0.16) | p=0.009 | 1,3 |
| n (%) | Race – number (percent Caucasian) | 14 (66.7%) | 16 (76.2%) | 21 (87.5%) | LRT p=0.24 | |
| n (%) | Hispanic ethnicity (yes) | 9 (42.9%) | 7 (33.3%) | 6 (25.0%) | p=0.45 | |
| Mean (SEM) | Parental SES (SEM)b | 49.0 (3.68) | 44.9 (2.38) | 40.1 (2.95) | p=0.17 | |
| Mean (SEM) | Estimated IQ (SEM)c | 100.5 (1.74) | 100.5 (2.23) | 106.1 (2.14) | p=0.09 | |
| Clinical Measures | ||||||
| Substance abuse or dependence n (%) | Cannabis abuse or dependenced | 21 (100%) | 20 (95.2%) | 0 (0%) | p<0.001 | 1,2 |
| Tobacco dependenced | 14 (66.7%) | 11 (52.4%) | 0 (0%) | p<0.001 | 1,2 | |
| Alcohol abuse or dependenced | 17 (81.0%) | 13 (61.9%) | 0 (0%) | p<0.001 | 1,2 | |
| Cocaine abuse or dependenced | 7 (33.3%) | 7 (33.3%) | 0 (0%) | LR p=0.001 | FE 1,2 | |
| Conduct Disorder, callousness, ADHD, and depression n (%) or Mean (SEM) | Whole life Conduct Disorder diagnosise | 21 (100%) | 19 (90.5%) | 0 (0%) | p<0.001 | 1,2 |
| Conduct disorder symptom count (whole life) | 6.7 (0.57) | 5.0 (0.54) | 0.4 (0.12) | p<0.001 | 1,2 | |
| Anger/Irritability Symptom Phenotype of ODDf | 1 (4.8%) | 3 (14.3%) | 0 (0%) | LRT p=0.09 | ||
| ICU total scoreg | 30.9 (1.34) | 20.7 (1.09) | 18.2 (1.39) | p<0.001 | 2,3 | |
| ADHD severityh | 71.3 (4.24) | 78.0 (4.12) | 56.9 (2.44) | KW p=0.002 | MW 1,2 | |
| Whole life Major Depressione | 5 (23.8%) | 4 (19.0%) | 1 (4.2%) | LRT p=0.12 | ||
| Medications | ||||||
| n (%) | Prescribed medications for ADHD | 2 (9.5%) | 3 (14.3%) | 1 (4.2%) | LRT p=0.48 | |
| n (%) | Prescribed medications for other mental health conditions | 6 (28.6%) | 5 (23.8%) | 1 (4.2%) | LRT p=0.05 | 2 |
| n (%) | Other Prescribed Medications | 5 (23.8%) | 3 (14.3%) | 1 (4.2%) | LRT p=0.14 | |
| Empathy Scoresi | ||||||
| Mean (SEM) | Empathic Concern | 15.7 (1.19) | 17.8 (0.43) | 19.0 (0.86) | p=0.04 | |
| Mean (SEM) | Perspective Taking | 14.8 (1.04) | 15.5 (1.26) | 17.7 (1.03) | p=0.16 | |
| Mean (SEM) | Personal Distress | 9.2 (0.79) | 9.6 (0.79) | 9.5 (0.86) | p=0.93 | |
| Mean (SEM) | Fantasy | 12.8 (1.17) | 12.9 (0.97) | 15.0 (1.00) | p=0.24 | |
KW = Kruskal-Wallis Test. MW = Mann-Whitney U Test. LRT indicates that Likelihood Ratio was utilized instead of the
Pearson Chi Square test because expected cell counts were small. FE indicates Fisher’s Exact Test. SEM indicates standard error of the mean.
Note that 1=Controls vs. patients without LPE significant (p<0.05); 2=Controls vs. patients with LPE significant; 3=Patients with LPE vs. patients without LPE. Post hoc 2 group comparisons were either completed with Tukey HSD (for approximately normally distributed variables), the Games-Howell post-hoc test (when equality of variances could not be assumed) or the Mann- Whitney U tests (when variables were not approximately normally distributed in this sample).
parents of 3 patients (2 with LPE and 1 without LPE) did not complete this measure.
Estimated IQ assessed by the vocabulary and matrix reasoning subtests of the Wechsler Abbreviated Scale of Intelligence.
Assessed by the Composite International Diagnostic Interview – Substance Abuse Module; DSM-IV criteria.
Assessed by the Diagnostic Interview Schedule for Children (and for lifetime conduct disorder, supplemental conduct disorder questions).
The anger/irritability symptom phenotype for ODD (oppositional defiant disorder) was calculated using past-year ODD symptoms from the Diagnostic Interview Schedule for Children and required endorsement of at least four ODD symptoms including (1) often loses temper, (2) is often touchy or easily annoyed by others, and (3) is often angry and resentful.
Total score from Inventory of Callous Unemotional Traits.
Diagnostic and Statistical Manual-oriented attention-deficit hyperactivity problems raw score from the Youth Self Report.
Measured using the Interpersonal Reactivity Index, appropriately reverse scoring items and summing using the 0–4 scoring system. Note that data were not available for this measure for 1 typically developing youth and 1 patient with LPE.
2.2 Measures
Measures included the Inventory of Callous Unemotional traits (ICU; (Frick, 2004)), the Diagnostic Interview Schedule for Children (Shaffer et al., 2000), the Composite International Diagnostic Interview - Substance Abuse Module (Robins et al., 1988), the Vocabulary and Matrix Reasoning subtests of the Wechsler Abbreviated Intelligence Scale (Wechsler, 1999), the Interpersonal Reactivity Index (Davis, 1980) and the Youth Self Report (Achenbach, 1991). Patients were divided into those with and without the DSM-5 LPE specifier using four questions from the ICU as previously described (Frick and Moffitt, accessed May 8, 2012) and following our prior procedures (Sakai et al., 2012; Sakai et al., 2016a). Specifically, we utilized ICU questions 3, 5, 6 and 8 and scored them on a 0–3 scale (note: with reverse scoring for positively worded items, 3, 5 and 8). Scores of 2 or 3 (indicating greater callousness) were counted as endorsed and at least 2 of the 4 questions had to be endorsed to meet the LPE specifier.
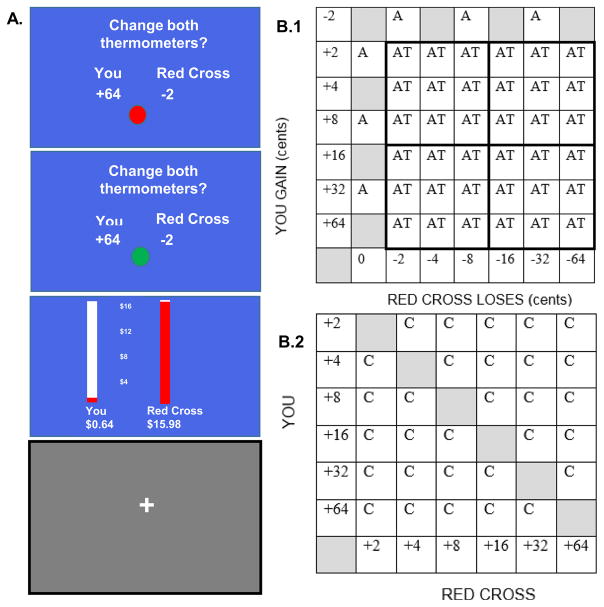
The Altruism-Antisocial (AlAn’s) Game: AlAn’s game asks subjects to accept or reject a series of offers (termed Active Trials) in which they will benefit but a beneficent other will be harmed (a real donation to the Red Cross will be reduced). For detailed descriptions of the AlAn’s game please see Figure 1 and our prior publications (Sakai et al., 2012; Sakai et al., 2016a). Data on the behavioral outcomes while playing the game have been previously described for this sample and showed that the three groups differed significantly in the amount of costly helping (number of times not accepting Active Trials), money taken for self, and the amount of money left in the Red Cross donation. Patients with LPE took the most money, leaving the least money in the donation (Sakai et al., 2016a). Supplemental Figure 1 shows the percent of offers accepted for each Active Trial by group. AlAn’s game behavior has been previously related to level of callous-unemotional traits (Sakai et al., 2016a) and is related to both empathic concern and perspective taking (see Supplemental Figure 1, panel D).
Figure 1.
The AlAn s Game. Panel A. Subjects start with no money and the Red Cross donation begins at $16. While subjects play the game, they are presented with a series of offers where they are asked, Change both thermometers? (Top Blue Screen). In this offer the subject will get 64 cents and the Red Cross donation will go down by 2 cents (5 seconds deliberation period). When the ball turns from red to green (Middle Blue Screen) subjects have 1 second to accept or reject the offer. Then the thermometers (Bottom Blue Screen) show how much money the subject has and the current value of the Red Cross donation (4 seconds). Then a jittered Gray Fixation Screen appears. The dollar amounts for each individual choice are small (range 2–64 cents) but by accepting all Active Trials, subjects would earn $15.12 and the Red Cross donation, which starts at $16, would be reduced to 88 cents. Panel B.1. Shows the matrix of offers (amount of money participant gains vs. amount of loss to the Red Cross). Decision Trials are indicated by AT or Active Trials. A Trials assure attention to game content and are not discussed here. Panel B.2. C indicates Calculation Trials. In these trials subjects are asked, Is the You number bigger? If the You number is bigger than the Red Cross number, subjects should indicate yes. Calculation Trials present similar visual cues to Active Trials, require left and right button presses, require assessment of relative values similar to those found in the Active Trials, but are devoid of deliberations about self-benefit and other-harm. Calculation Trials therefore serve as our baseline. Note: this figure utilizes components from previously published descriptions of the AlAn s game protected under the Creative Commons Attribution license (Sakai et al., 2012 and 2016a; http://journals.plos.org/plosone/s/content-license).
2.3 fMRI Data Acquisition
We obtained functional brain images with Blood Oxygenation Level Dependent (BOLD) contrast using a T2*-weighted gradient-echo echo-planar imaging (EPI) technique over a 64x64 matrix (TE/TR/TI (in milliseconds): 26/2000/70; Flip angle: 70 degree; FOV: 220X220 mm2 in axial acquisition. See Supplemental Materials for details on acquisition (section S.1) and fMRI pre-processing procedures (section S.2).
2.4 Single Subject Level Analysis
We fit the BOLD time series data for each voxel using a general linear model (GLM) for each subject individually. We specified onsets of AlAn’s trial types (Active, Calculation, Attention; see Figure 1) and each trial outcome and then estimated the GLM model specifying the contrast of interest (Active Trials minus Calculation Trials). Each regressor was convolved with a canonical hemodynamic response function. The estimated BOLD signal change for this contrast for each voxel at the subject level is utilized in subsequent group-level and regression analyses (described next) and is hereafter termed ΔBOLD(Active Trial – Calculation).
2.5 Group Level Analyses
All analyses were whole-brain, controlled for age, IQ and motion (refrms; see Supplemental Materials for definition) and utilized a voxel level threshold of 0.005 cluster-corrected at family-wise error (FWE) p<0.05 (23 voxel-level threshold (voxel=3mm3)). Within typically-developing youths we evaluated whether average ΔBOLD(Active – Trial Calculation) differed significantly from zero at each voxel to identify the network engaged by decisions in the game. Next we compared the three groups, testing for differences in ΔBOLD(Active – Trial Calculation). Supplementary analyses evaluated for differences in ΔBOLD(Active – Trial Calculation) between all possible two-group comparisons with whole brain analyses.
Secondary Group level analyses re-analyzing these data (1) while controlling for conduct disorder symptom count in the patient two-group comparison, (2) while grouping patients by a median split of the total score from the ICU and repeating the three-group and two-group comparisons described above, and (3) while excluding subjects on prescribed medications are described in Supplementary Materials Section S.3.
2.6 Regression Analyses
Our method for categorizing conduct-problem youth into those with and without LPE, yielded groups that significantly differed in mean severity of callous-unemotional traits (see Table 1). However, examination of group distributions showed that some youth with LPE scored relatively low in ICU total score, while some youths without LPE scored high on this measure (see Supplemental Figure 2). Accordingly, we also completed across-group whole-brain regression analyses examining the relationship between ICU total score and brain activation, while covarying age, IQ, motion, and conduct disorder symptom counts.
To control for co-morbidity we extracted using MarsBaR the average beta estimates for each significant cluster from whole-brain regressions for each subject. We regressed ICU total score (dependent variable) on each cluster while covarying age, IQ, conduct disorder symptom count, ADHD severity, number of substance use disorder diagnoses, and whole-life major depression to determine if co-morbidity explained the observed associations (see Supplemental Materials S.4).
We completed exploratory regressions using behavioral measures from the AlAn’s game to test whether specific clusters (identified as significant in our three-group and/or whole-brain regression analyses) and their interactions predicted AlAn’s game behavior (see Supplemental Materials section S.5).
Finally, in the analyses described above, we utilize voxel level threshold of 0.005 cluster corrected at FWE p<0.05 (23 voxel-level threshold (voxel=3mm3)), as specified in the procedures of our funded-grant application (DA031761) and for which our study was powered. But very recently Eklund et al., (Eklund et al., 2016) have demonstrated that parametric cluster-wise extent thresholding may lead to type I errors above the expected 5% threshold, especially with a small ad-hoc cluster inference, but even for other approaches to cluster inference like those utilized here. Eklund and colleagues’ (Eklund et al., 2016) work supports that a non-parametric (distribution-free) permutation method provides results in-line with the expected alpha. Therefore, to provide additional information, we reran our main analyses using this more conservative method (Nichols and Holmes, 2002) smoothing with 6 mm full-width-half-maximum (FWHM) Gaussian filter, utilizing a going-in p=0.001 and 10,000 permutations.
3. RESULTS
3.1 Network Engaged by Decision
Within typically-developing adolescents the contrast ΔBOLD(Active – Trial Calculation) demonstrated prominent activation in medial frontal regions, bilateral inferior frontal gyrus and insula, thalamus, midbrain, caudate, precuneus and right inferior parietal regions (see Supplemental Figure 3, Supplemental Table 1.A).
3.2 Group Comparisons
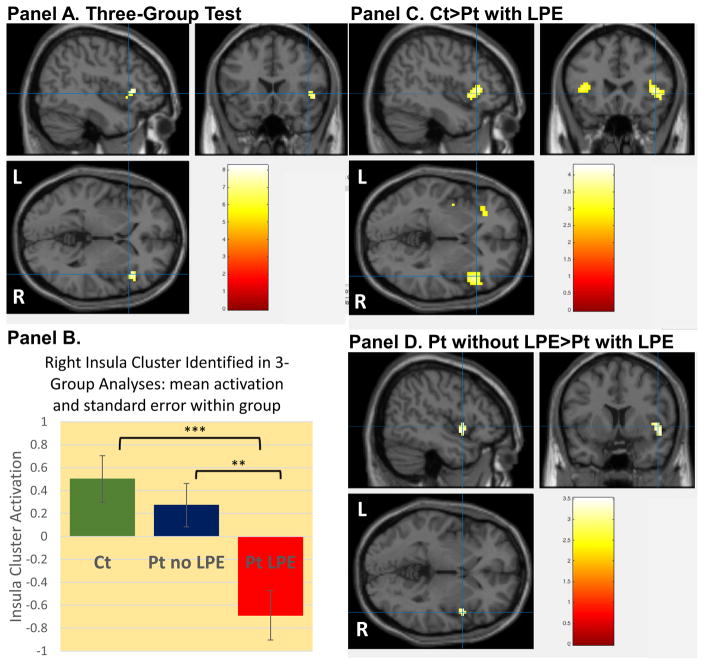
Three-group whole-brain comparisons in ΔBOLD(Active – Trial Calculation) demonstrated a significant activation difference between groups during decision in the right insula, inferior frontal and superior temporal gyrus (see Figure 2, panel A, Table 2.A). This significant cluster was identified utilizing MarsBaR and post-hoc three group comparisons were completed in SPSS (F=9.5; p<0.001; see Figure 2, panel B). Supplemental Table 2 shows that whole-brain two-group comparisons confirmed a pattern where patients with LPE had hypoactivity in this region and also that controls had significantly greater activation than patients with LPE in left insula. Secondary group level analyses (controlling for conduct disorder severity in patient two-group analyses, dividing patients by median split from ICU total score and excluding subjects on prescribed medications) are described in Supplemental Materials S.3.
Figure 2.
Testing for group differences during decision (Active Trials minus Calculation Trials). Panel A. Whole-brain three-group test for activation differences demonstrating a cluster in right insula, inferior frontal gyrus and superior temporal gyrus. [Cluster is referred to as insula cluster in this figure and the manuscript results and discussion sections; crosshair x,y,z coordinates 44.6, 18.7 and 0.6, respectively]. Panel B. Cluster from three-group analyses was identified for each subject (using MarsBaR) and within group means and standard errors are presented. Three group ANOVA was significant (p<0.001) as expected. Two group comparisons demonstrated that patients with LPE differed significantly from (1) typically-developing controls (p<0.001) and (2) patients without LPE (p=0.005) using post-hoc Tukey s tests. Panel C. Whole-brain two-group analyses testing for differences typically-developing controls > patients with limited prosocial emotions (LPE), showing activation differences in the bilateral insula and inferior frontal gyrus (comparison of patients with LPE > typically-developing controls showed no significant differences; x,y,z coordinates 45, 23.4, −0.03, respectively). Panel D. Whole-brain two-group analyses testing for differences patients without LPE > patients with LPE showing activation differences in right insula and superior temporal gyrus (comparison of patients with LPE > patients without LPE showed no significant differences; x,y,z coordinates 48.6, 8.04, −0.70, respectively). Note: two-group comparisons of typically-developing controls and patients without LPE showed no significant differences. Detailed results of two-group analyses are available in Supplemental Table 2.
Table 2.A.
Regions showing significant activation differences among typically developing adolescents, patients with LPE and patients without LPE during decision-making (Three-Group Whole-Brain Comparisons).
| A. Comparing Typically Developing Adolescents, Patients with Conduct Problems Without LPE and Patients with Conduct Problems and LPE | |||||||
|---|---|---|---|---|---|---|---|
| CLUSTER NUMBER | NUMBER OF VOXELS | STRUCTURE | SIDE/BA | X | Y | Z | F statistic |
| 1 | 37 | Insula (aal) | R | 45 | 20 | −1 | 6.14 |
| Superior temporal gyrus | R; 22 | 51 | 14 | −4 | 6.02 | ||
| Inferior frontal gyrus | R; 13, 38, 45, 47 | 45 | 23 | 2 | 8.23 | ||
3.3 Whole-Brain Regression Analyses
Regression analyses testing the association of ΔBOLD(Active – Trial Calculation) with severity of callous-unemotional traits, while covarying age, IQ, motion, and conduct disorder symptom counts as has been done in prior studies (Sebastian et al., 2012) demonstrated a negative association with a left inferior parietal lobule cluster and a positive association with a posterior cingulate cluster (see Table 2.B). Regressions controlling for comorbidity are presented in Supplementary Materials S.4 and exploratory regressions with game behavior are presented in Supplemental Materials S.5.
Table 2.B.
Associations Between Severity of Callous Unemotional Traits and Brain Activation During Decision. Whole-brain regression analyses examining the relationship between brain activation and total score from the Inventory of Callous and Unemotional traits while controlling for age, IQ, frame displacement and conduct disorder symptom count.
| B.i Clusters of activation negatively associated with severity of callous-unemotional traits | |||||||
|---|---|---|---|---|---|---|---|
| CLUSTER NUMBER | NUMBER OF VOXELS | STRUCTURE | SIDE/BA | X | Y | Z | t |
| 1. | 33 | Inferior parietal lobule | L; BA 40 | −57 | −34 | 47 | −3.61 |
| Postcentral gyrus | L; BA 2 | −57 | −31 | 44 | −3.00 | ||
| B.ii. Clusters of activation positively associated with severity of callous-unemotional traits | |||||||
| 1. | 37 | Posterior cingulate | L; BA 30, 31 | −9 | −64 | 8 | 3.31 |
| Lingual gyrus | L; BA 18, 19 | −12 | −58 | 2 | 3.40 | ||
| Cuneus | L; 30 | −9 | −61 | 5 | 2.95 | ||
aal=Automated Anatomical Labeling system (see Supplemental Materials Section S.6).
3.4 Results Utilizing More Conservative Permutation Analyses
All primary analyses were re-run utilizing non-parametric permutation tests. The network engaged within typically-developing adolescents the contrast ΔBOLD(Active Trial – Calculation) remained generally consistent with that shown in Supplemental Figure 3 (see Supplemental Figure 6 and Supplemental Table 1.B). Between-group tests confirmed right anterior insula differences utilizing the contrast typically-developing adolescents>patients with LPE (see Supplemental Figure 7). Regression and other between-group permutation analyses did not demonstrate significant results.
4. DISCUSSION
Within typically-developing adolescents, we identify a network engaged during decision. The network is remarkably similar to prior studies of alternative approaches to presenting Self:Other consideration in normative populations (Hein et al., 2016; Sommer et al., 2014). However, our main aim was to identify regions in this network associated with high levels of callous-unemotional traits. Our three-group analyses demonstrate activation differences in the right anterior insula. Those with LPE had significantly less activation in this region compared to both typically-developing youths and conduct-problem youths without LPE. This latter group is useful in controlling for many co-morbidities seen in conduct-problem youth with LPE (see Table 1) and it is reassuring that our insula finding remained while also controlling for conduct disorder severity in comparing our two patient groups (Supplemental Figure 4.A). Our secondary analyses dividing patients by median split on ICU total scores, rather than LPE, similarly show this insula cluster; typically developing youth had more activation in insula during decision compared to patients with high levels of callous-unemotional traits (Figure 2 and Supplemental Figure 4.B). However, the results using the median split compared to the LPE in this sample provided less consistent findings. Our secondary analyses also support that this right insula cluster finding in our three-group analyses was robust to excluding medicated subjects (Supplemental Figure 5).
The anterior insula is an agranular heteromodal association cortex involved in multiple processes, including self-related and empathetic feelings, bodily awareness, response inhibition, salience (both interoceptive and exteroceptive), and assessment of risk and uncertainty (Hwang et al., 2016; Singer et al., 2009; Uddin, 2015). Mounting work has examined the anterior insula’s role in decision-making, especially risky decision-making. For example, while making decisions involving risk, anterior insula appears critical to loss prediction, is associated with risk-averse choices (Kuhnen and Knutson, 2005), and is important to evaluation of wrong choices (e.g., not taking a risk when the outcome would have been beneficial and taking a risk when the outcome results in a loss; (Liu et al., 2007). White and colleagues (2016a) have examined reinforcement-based decisions using computational modeling of performance on a passive avoidance task. They report three relevant findings: that conduct problem severity, but not level of callousness, was related to poorer performance on this task; that severity of conduct problems was associated with activity in the anterior insula when considering expected value during avoidance responses; and that the relationship between conduct problem severity and task performance was mediated by representations of expected value in anterior insula. This anterior insula finding was specific to conduct problems and was not related to severity of callous unemotional traits.
Our result in the anterior insula, on the contrary, is specific to LPE, and not conduct problems, which at first glance appears to contradict the work of (White et al., 2016a). We propose that the AlAn’s game may require different decision making processes, such as determinations about equity and fairness and processes related to care-based moral decision making, rather than reinforcement-based decision making. As mentioned in Section 2.2 behavior on the AlAn’s game is significantly related to measures of empathic concern and perspective taking. Unlike the passive avoidance task utilized by White and colleagues (White et al., 2016a), the AlAn’s game does not require any assessment of risk, prediction of outcome probabilities, or learning. Instead the game clearly informs subjects what will happen and subjects must simply weigh the importance of the relative magnitude of self-benefit to other-harm. The anterior insula has been implicated in many processes beyond reinforcement-based decision making. For example, anterior insula is engaged when passively viewing harm to others (Michalska et al., 2016) and plays a critical role in vicariously feeling another’s experience (Lockwood, 2016), is activated during determinations about equity and fairness (Hsu et al., 2008) and is related to affective empathy and moral disgust (Lockwood, 2016; Tusche et al., 2016). It is of course well documented that youth with high levels of callous-unemotional traits exhibit low levels of affective empathy (Lui et al., 2016). Other work in adults supports that anterior insula encodes anticipatory feelings of guilt when deciding how to act during scenarios about moral transgressions (Seara-Cardoso et al., 2016). Such work may be particularly relevant to the current paradigm, and would imply that patients with LPE may experience less anticipatory guilt prior to accepting trials while playing the AlAn’s game. However, it is important to note that we did not specifically measure self-reported guilt in this study and therefore, it is not possible to confirm this hypothesis. Recent work has also shown that as subjects repeatedly behave dishonestly, particularly during a self-serving other-harming condition, insula activation decreases, suggesting adaptation (see Supplemental Table 2 in (Garrett et al., 2016). Such work has been interpreted to mean that repeated moral transgressions may dampen emotional reactivity to such acts over time. Thus our anterior insula cortex finding may reflect deficits in affective empathy or equity/fairness assessments or diminished anticipatory guilt in LPE youths. In some models the anterior insula is subdivided into: (1) dorsal anterior insula (associated with cognitive processes, such as task switching, inhibition, and error processing), and (2) ventral anterior insula (associated with empathy and affective processing, such as perception of another’s affect) (Uddin, 2015). Results from two-group analyses in Figure 2 supports that the comparison [typically-developing adolescents > patients with LPE] reveals differences in both dorsal and ventral anterior insula, while our comparison [patients without LPE > patients with LPE] shows differences mainly in ventral anterior insula. This suggests that differences in dorsal anterior insula may reflect cognitive deficits associated with the disorder phenotype independent of LPE and the LPE phenotype may be related more specifically with a deficit in higher-order affective and empathetic processing.
Because the LPE specifier results in groups with overlapping distributions of callousness severity (see Supplemental Figure 2), we also completed across-group whole-brain regression analyses examining the relationship between ICU total score and brain activation. Those analyses yielded a region in the left inferior parietal lobule that was negatively related to severity of callousness (Table 2.B). This finding unfortunately did not hold when more conservative non-parametric tests were utilized and requires future replication. The left inferior parietal lobe has been previously implicated in language processing, attention control, perspective taking and social cognition, and, for dorsal portions, in more general cognitive processing (Blair et al., 2016; Bzdok et al., 2016; Tusche et al., 2016). Consistent with this finding, high levels of callousness have also been associated with deficits in taking another’s perspective (Lui et al., 2016). Our inferior parietal cluster, extracted from our regression analyses, was significantly associated with behavior on the AlAn’s game (Supplemental Table 4). This suggests that inferior parietal lobule is involved in restraint, promoting rejection of Active Trials.
Our whole-brain regression analyses also identified a paralimbic structure (Kiehl, 2006), the posterior cingulate, which was positively associated with severity of callous-unemotional traits (Table 2.B). This finding unfortunately did not hold when non-parametric tests were utilized and requires future replication. Although the role of posterior cingulate remains debated, including some work implicating posterior cingulate in reward related processes (e.g., (Clithero and Rangel, 2014; McClure et al., 2004)), recent models suggest that this region may help to control balance between internally and externally focused attention, and may be particularly active during periods of internally-directed thought (Leech and Sharp, 2014). Other models have suggested that posterior cingulate and precuneus may serve as an important hub for “transmitting socially significant information” between regions (Zaki et al., 2007). Although our posterior cingulate and insula clusters were not directly associated with game behavior, their interaction significantly predicted behavior during decisions about high other-harm, low self-benefit trials (see post-hoc analyses in Supplemental Table 4). Among individuals who have relatively high posterior cingulate activity, perhaps indicating an internally-directed self-focused attentional state, insula activity is positively, though not significantly, related to taking high other-harm, low self-benefit trials. But among those with relatively low posterior cingulate activity, perhaps indicating externally-focused other-directed attention, insula activity is significantly, and negatively, related to taking high other-harm low self-benefit trials. Thus, our results suggest that the effects of insula activity on behavior may be moderated by internally vs. externally focused attentional states.
Our results must be viewed within the context of several limitations. First, we recruited a male-only sample. Results should not be extrapolated to females and future studies should evaluate sex differences. Second, we relied on self-reported callous-unemotional traits. While this is consistent with several prior studies (e.g, Fanti et al., 2013; Feilhauer et al., 2012) and the levels of callousness in our study (see Table 1) are similar to several prior studies of normative (Roose et al., 2010; Fanti et al., 2013; Feilhauer et al., 2012) and high risk (Fanti et al., 2013; Feilhauer et al., 2012) samples, many other studies have utilized combinations of self-, parent- and teacher-reports (e.g., Roose et al., 2010). This multi-informant approach can lead to higher callous-unemotional trait scores (Roose et al., 2010). Third, we recruited a narrow age band (15–18 years) and results may not apply to younger children or adults. However, this narrow age-band is also a strength, as it may prevent potential confounds, given the large developmental effects in the adolescent years. Similarly, our exclusion criteria help to reduce potential confounds from multiple domains. But such an approach may hamper the generalizability of our results, for example by excluding those with anxiety disorders. In addition, our paradigm allows studying Self:Other decision making in the MRI environment, but it is important to note that the Other used here is a charity. It is possible that utilizing another player, or a less abstract other, would affect subjects’ behavior. Reasons for utilizing this Other have been previously outlined (Sakai et al., 2012). In addition, our findings appear specific to having LPE and not conduct problems without LPE. Additional studies will likely be needed to better define findings specific to, and common across, these related phenotypes. Finally, it is important to note that while our anterior insula result remains significant using more stringent permutation tests (comparison of typically-developing adolescents > patients with LPE), the overall study findings are more modest utilizing this more stringent threshold. Future studies with larger sample sizes will be needed to confirm our results using these more stringent tests. However, there are a few reasons why the important work of Eklund and colleagues (Eklund et al., 2016) may not perfectly apply to this study. First, they utilized resting state data, as opposed to data from a rapid event design like ours. Second, they utilized only community samples. Patient samples may include more variability in activation levels, and using permutation methods with such variability might push thresholds to be more extreme, potentially increasing risk of type II errors.
Conclusions and Future Directions
We demonstrate three brain regions where activation during Self:Other decisions is related to callousness among conduct-problem youths. Our game offers a unique opportunity to explore how brain regions may be related to actual behaviors, perhaps bringing us one step closer to understanding not only how youth with LPE differ in brain activity, but how those differences in brain activity may lead to maladaptive behaviors. Inferior parietal lobule is easily accessible to brain stimulation techniques, offering one avenue to test the effects of experimental manipulation of this region on game behavior. Anterior insula activation discriminated groups in a pattern suggesting youth with LPE have hypoactivity in this region. However, this region’s relationship with behavior appears more complex, with posterior-cingulate activation perhaps moderating insula activation effects.
Supplementary Material
Highlights.
Conduct problem(CP) patients with/without limited prosocial emotions(LPE), controls
Imaged playing a novel game requiring decisions about self-benefit and other-harm
Network engaged: medial frontal, accumbens, bilateral insula, among other areas
CP with LPE had less activation in right anterior insula during decision making
Acknowledgments
Funding: Research support from National Institute on Drug Abuse (NIDA) grant DA031761, the Kane Family Foundation and the Hewit Family Foundation. Dr. Mikulich-Gilbertson’s effort is also supported by DA034604. The funders had no role in: the study design; the collection, analyses or interpretation of the data; the writing of the report; or in the decision to submit this manuscript for publication.
Footnotes
Disclosures: Dr. Crowley served on the National Advisory Council of the National Institute on Drug Abuse, and on a Task Force of the American Psychiatric Association for drafting the Diagnostic and Statistical Manual of Mental Disorders, Edition 5, receiving travel reimbursement from those organizations. Dr. Sakai received reimbursement in 2012 for completing a policy review for the WellPoint Office of Medical Policy & Technology Assessment (OMPTA), WellPoint, Inc., Thousand Oaks, CA. He also served as a board member of the ARTS (Addiction Research and Treatment Services) Foundation until the summer of 2015. All other authors report no competing interests.
Portions of this work were presented at the 2016 meeting of the College on Problems of Drug Dependence.
References
- Achenbach T. Manual for the Youth Self-Report and 1991 Profile. University of Vermont, Department of Psychiatry; Burlington VT: 1991. [Google Scholar]
- Blair RJ. The neurobiology of psychopathic traits in youths. 2013 doi: 10.1038/nrn3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJ, Leibenluft E, Pine DS. Conduct disorder and callous-unemotional traits in youth. N Engl J Med. 2014;371:2207–2216. doi: 10.1056/NEJMra1315612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJ, Veroude K, Buitelaar JK. Neuro-cognitive system dysfunction and symptom sets: A review of fMRI studies in youth with conduct problems. Neurosci Biobehav Rev. 2016 doi: 10.1016/j.neubiorev.2016.10.022. [DOI] [PubMed] [Google Scholar]
- Byrd AL, Loeber R, Pardini DA. Antisocial behavior, psychopathic features and abnormalities in reward and punishment processing in youth. Clin Child Fam Psychol Rev. 2014;17:125–156. doi: 10.1007/s10567-013-0159-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzdok D, Hartwigsen G, Reid A, Laird AR, Fox PT, Eickhoff SB. Left inferior parietal lobe engagement in social cognition and language. Neurosci Biobehav Rev. 2016 doi: 10.1016/j.neubiorev.2016.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clithero JA, Rangel A. Informatic parcellation of the network involved in the computation of subjective value. Soc Cogn Affect Neurosci. 2014;9:1289–1302. doi: 10.1093/scan/nst106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coker KL, Smith PH, Westphal A, Zonana HV, McKee SA. Crime and psychiatric disorders among youth in the US population: an analysis of the National Comorbidity Survey-Adolescent Supplement. J Am Acad Child Adolesc Psychiatry. 2014;53:888–898. 898 e881–882. doi: 10.1016/j.jaac.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colins OF, Vermeiren RR. The usefulness of DSM-IV and DSM-5 conduct disorder subtyping in detained adolescents. J Nerv Ment Dis. 2013;201:736–743. doi: 10.1097/NMD.0b013e3182a20e94. [DOI] [PubMed] [Google Scholar]
- Davis MH. A multidimensional approach to individual differences in empathy. JSAS Catalog of Selected Documents in Psychology. 1980:85. [Google Scholar]
- Dodge KA, Pettit GS. A biopsychosocial model of the development of chronic conduct problems in adolescence. Dev Psychol. 2003;39:349–371. doi: 10.1037//0012-1649.39.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci U S A. 2016;113:7900–7905. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eme RF. Sex differences in child-onset, life-course-persistent conduct disorder. A review of biological influences. Clin Psychol Rev. 2007;27:607–627. doi: 10.1016/j.cpr.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Essau CA, Sasagawa S, Frick PJ. Callous-unemotional traits in a community sample of adolescents. Assessment. 2006;13:454–469. doi: 10.1177/1073191106287354. [DOI] [PubMed] [Google Scholar]
- Frick PJ. The Inventory of Callous-Unemotional traits. University of Louisiana; New Orleans, AL: 2004. [Google Scholar]
- Frick PJ, Moffitt T. [accessed May 8, 2012];A Proposal to the DSM-V Childhood Disorders and the ADHD and Disruptive Behavior Disorders work Groups to Include a Specifier to the Diagnosis of Conduct Disorder Based on the Presence of Callous-Unemotional Traits. American Psychiatric Association [Google Scholar]
- Frick PJ, Ray JV, Thornton LC, Kahn RE. Annual research review: A developmental psychopathology approach to understanding callous-unemotional traits in children and adolescents with serious conduct problems. J Child Psychol Psychiatry. 2014;55:532–548. doi: 10.1111/jcpp.12152. [DOI] [PubMed] [Google Scholar]
- Garrett N, Lazzaro SC, Ariely D, Sharot T. The brain adapts to dishonesty. Nat Neurosci. 2016;19:1727–1732. doi: 10.1038/nn.4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harenski CL, Harenski KA, Kiehl KA. Neural processing of moral violations among incarcerated adolescents with psychopathic traits. Dev Cogn Neurosci. 2014;10:181–189. doi: 10.1016/j.dcn.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein G, Morishima Y, Leiberg S, Sul S, Fehr E. The brain's functional network architecture reveals human motives. Science. 2016;351:1074–1078. doi: 10.1126/science.aac7992. [DOI] [PubMed] [Google Scholar]
- Hsu M, Anen C, Quartz SR. The right and the good: distributive justice and neural encoding of equity and efficiency. Science. 2008;320:1092–1095. doi: 10.1126/science.1153651. [DOI] [PubMed] [Google Scholar]
- Hwang S, Nolan ZT, White SF, Williams WC, Sinclair S, Blair RJ. Dual neurocircuitry dysfunctions in disruptive behavior disorders: emotional responding and response inhibition. Psychol Med. 2016;46:1485–1496. doi: 10.1017/S0033291716000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehl KA. A cognitive neuroscience perspective on psychopathy: evidence for paralimbic system dysfunction. Psychiatry Res. 2006;142:107–128. doi: 10.1016/j.psychres.2005.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF. The structure of common mental disorders. Arch Gen Psychiatry. 1999;56:921–926. doi: 10.1001/archpsyc.56.10.921. [DOI] [PubMed] [Google Scholar]
- Kuhnen CM, Knutson B. The neural basis of financial risk taking. Neuron. 2005;47:763–770. doi: 10.1016/j.neuron.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain. 2014;137:12–32. doi: 10.1093/brain/awt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Powell DK, Wang H, Gold BT, Corbly CR, Joseph JE. Functional dissociation in frontal and striatal areas for processing of positive and negative reward information. J Neurosci. 2007;27:4587–4597. doi: 10.1523/JNEUROSCI.5227-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood PL. The anatomy of empathy: Vicarious experience and disorders of social cognition. Behav Brain Res. 2016 doi: 10.1016/j.bbr.2016.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui JH, Barry CT, Sacco DF. Callous-unemotional traits and empathy deficits: Mediating effects of affective perspective-taking and facial emotion recognition. Cogn Emot. 2016;30:1049–1062. doi: 10.1080/02699931.2015.1047327. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Fowler KA, Adalio CJ, Jurkowitz IT, Schechter JC, Pine DS, Decety J, Blair RJ. Empathic responsiveness in amygdala and anterior cingulate cortex in youths with psychopathic traits. J Child Psychol Psychiatry. 2013;54:900–910. doi: 10.1111/jcpp.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Mitchell DG, Reid ME, Sims C, Kosson DS, Towbin KE, Leibenluft E, Pine DS, Blair RJ. Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. Am J Psychiatry. 2008;165:712–720. doi: 10.1176/appi.ajp.2007.07071145. [DOI] [PubMed] [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- Michalska KJ, Zeffiro TA, Decety J. Brain response to viewing others being harmed in children with conduct disorder symptoms. J Child Psychol Psychiatry. 2016;57:510–519. doi: 10.1111/jcpp.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt TE. Adolescence-limited and life-course-persistent antisocial behavior: a developmental taxonomy. Psychol Rev. 1993;100:674–701. [PubMed] [Google Scholar]
- Moffitt TE, Caspi K, Rutter M, Silva PA. Sex differences in antisocial behaviour: conduct disorder, delinquency, and violence in the Dunedin Longitudinal Study. Cambridge Univeristy Press; Cambridge, UK: 2001. [Google Scholar]
- Myers MG, Stewart DG, Brown SA. Progression from conduct disorder to antisocial personality disorder following treatment for adolescent substance abuse. Am J Psychiatry. 1998;155:479–485. doi: 10.1176/ajp.155.4.479. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins LN, Wing J, Wittchen HU, Helzer JE, Babor TF, Burke J, Farmer A, Jablenski A, Pickens R, Regier DA, et al. The Composite International Diagnostic Interview. An epidemiologic Instrument suitable for use in conjunction with different diagnostic systems and in different cultures. Arch Gen Psychiatry. 1988;45:1069–1077. doi: 10.1001/archpsyc.1988.01800360017003. [DOI] [PubMed] [Google Scholar]
- Sakai JT, Dalwani MS, Gelhorn HL, Mikulich-Gilbertson SK, Crowley TJ. A behavioral test of accepting benefits that cost others: associations with conduct problems and callous-unemotionality. PLoS One. 2012;7:e36158. doi: 10.1371/journal.pone.0036158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai JT, Dalwani MS, Mikulich-Gilbertson SK, McWilliams SK, Raymond KM, Crowley TJ. A Behavioral Measure of Costly Helping: Replicating and Extending the Association with Callous Unemotional Traits in Male Adolescents. PLoS One. 2016a;11:e0151678. doi: 10.1371/journal.pone.0151678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai JT, Mikulich-Gilbertson SK, Young SE, Rhee SH, SKM, Dunn R, Salomonsen-Sautel S, Thurstone C, Hopfer CJ. Adolescent Male Conduct-Disordered Patients in Substance Use Disorder Treatment: Examining the “Limited Prosocial Emotions” Specifier. Journal of Child & Adolescent Substance Abuse. 2016b doi: 10.1080/1067828X.2016.1175983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seara-Cardoso A, Sebastian CL, McCrory E, Foulkes L, Buon M, Roiser JP, Viding E. Anticipation of guilt for everyday moral transgressions: The role of the anterior insula and the influence of interpersonal psychopathic traits. Sci Rep. 2016;6:36273. doi: 10.1038/srep36273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian CL, McCrory EJ, Cecil CA, Lockwood PL, De Brito SA, Fontaine NM, Viding E. Neural responses to affective and cognitive theory of mind in children with conduct problems and varying levels of callous-unemotional traits. Arch Gen Psychiatry. 2012;69:814–822. doi: 10.1001/archgenpsychiatry.2011.2070. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends Cogn Sci. 2009;13:334–340. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Sommer M, Meinhardt J, Rothmayr C, Dohnel K, Hajak G, Rupprecht R, Sodian B. Me or you? Neural correlates of moral reasoning in everyday conflict situations in adolescents and adults. Soc Neurosci. 2014;9:452–470. doi: 10.1080/17470919.2014.933714. [DOI] [PubMed] [Google Scholar]
- Steiner H, Dunne JE. Summary of the practice parameters for the assessment and treatment of children and adolescents with conduct disorder. J Am Acad Child Adolesc Psychiatry. 1997;36:1482–1485. doi: 10.1097/00004583-199710000-00037. [DOI] [PubMed] [Google Scholar]
- Tusche A, Bockler A, Kanske P, Trautwein FM, Singer T. Decoding the Charitable Brain: Empathy, Perspective Taking, and Attention Shifts Differentially Predict Altruistic Giving. J Neurosci. 2016;36:4719–4732. doi: 10.1523/JNEUROSCI.3392-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ. Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci. 2015;16:55–61. doi: 10.1038/nrn3857. [DOI] [PubMed] [Google Scholar]
- Van Damme L, Colins O, Vaandering EW. The Limited Prosocial Emotions Specifier for Conduct Disorder Among Detained Girls A Multi-Informant Approach. Criminal Justice and Behavior. 2016;43:778–792. [Google Scholar]
- van den Bos W, Vahl P, Guroglu B, van Nunspeet F, Colins O, Markus M, Rombouts SA, van der Wee N, Vermeiren R, Crone EA. Neural correlates of social decision-making in severely antisocial adolescents. Soc Cogn Affect Neurosci. 2014;9:2059–2066. doi: 10.1093/scan/nsu003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viding E, Sebastian CL, Dadds MR, Lockwood PL, Cecil CA, De Brito SA, McCrory EJ. Amygdala response to preattentive masked fear in children with conduct problems: the role of callous-unemotional traits. Am J Psychiatry. 2012;169:1109–1116. doi: 10.1176/appi.ajp.2012.12020191. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler abbreviated intelligence scale. The Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- White SF, Brislin SJ, Sinclair S, Blair JR. Punishing unfairness: rewarding or the organization of a reactively aggressive response? Hum Brain Mapp. 2014a;35:2137–2147. doi: 10.1002/hbm.22316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SF, Fowler KA, Sinclair S, Schechter JC, Majestic CM, Pine DS, Blair RJ. Disrupted expected value signaling in youth with disruptive behavior disorders to environmental reinforcers. J Am Acad Child Adolesc Psychiatry. 2014b;53:579–588. e579. doi: 10.1016/j.jaac.2013.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SF, Marsh AA, Fowler KA, Schechter JC, Adalio C, Pope K, Sinclair S, Pine DS, Blair RJ. Reduced amygdala response in youths with disruptive behavior disorders and psychopathic traits: decreased emotional response versus increased top-down attention to nonemotional features. Am J Psychiatry. 2012;169:750–758. doi: 10.1176/appi.ajp.2012.11081270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SF, Tyler P, Erway A, Botkin M, Kolli V, Meffert H, Pope K, Blair J. Dysfunctional representation of expected value is associated with reinforcement-based decision-making deficits in adolescents with conduct problems. J Child Psychol Psychiatry. 2016a;57:938–946. doi: 10.1111/jcpp.12557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SF, VanTieghem M, Brislin SJ, Sypher I, Sinclair S, Pine DS, Hwang S, Blair RJ. Neural Correlates of the Propensity for Retaliatory Behavior in Youths With Disruptive Behavior Disorders. Am J Psychiatry. 2016b;173:282–290. doi: 10.1176/appi.ajp.2015.15020250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J, Ochsner KN, Hanelin J, Wager TD, Mackey SC. Different circuits for different pain: patterns of functional connectivity reveal distinct networks for processing pain in self and others. Soc Neurosci. 2007;2:276–291. doi: 10.1080/17470910701401973. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.