Summary
Producing the neuronal diversity required to adequately discriminate all elements of somatosensation is a complex task during organogenesis. The mechanisms guiding this process during dorsal root ganglion (DRG) sensory neuron specification remain poorly understood. Here we show that the p75 neurotrophin receptor interacts with Ret and its GFRα co-receptor upon stimulation with glial cell line-derived neurotrophic factor (GDNF). Furthermore, we demonstrate that p75 is required for GDNF-mediated Ret activation, survival, and cell surface localization of Ret in DRG neurons. In mice in which p75 is deleted specifically within sensory neurons beginning at E12.5, we observe that approximately 20% of neurons are lost between P14 and adulthood, and these losses selectively occur within a subpopulation of Ret+ nonpeptidergic nociceptors, with neurons expressing low levels of Ret impacted most heavily. These results suggest that p75 is required for the development of the nonpeptidergic nociceptor lineage by fine-tuning Ret-mediated trophic support.
Graphical abstract
Introduction
The generation of the diverse array of sensory neurons necessary for discriminating all aspects of somatosensation is critical for animals to interact and respond to their environment. Sensory neurons in the dorsal root ganglia (DRG) innervate the peripheral tissues of the body below the neck and communicate sensory information to higher order neurons within the central nervous system. DRG neurons are greatly diversified with respect to their sensory functions, which is mirrored by their unique morphological, physiological, and molecular characteristics. Large, medium, and small diameter neurons carry proprioceptive stimuli (proprioceptors), tactile stimuli (mechanoreceptors) and nociceptive stimuli (nociceptors), respectively. Each of these morphologically distinct groups can be further subdivided based on their expression of different neurotrophic factor receptors, G protein coupled receptors, ion channels and transcription factors. Using these morphological and molecular properties, combined together with electrophysiological properties as well as distinctive central and peripheral innervation patterns, sensory neurons have been categorized into multiple subpopulations (Liu and Ma, 2011).
In adult mice, nociceptors can be divided into two major populations. One population, the peptidergic nociceptors, expresses neuropeptides such as calcitonin gene-related peptide (CGRP) and substance P (SP), and the nerve growth factor (NGF) receptor, TrkA. CGRP- or SP-negative nonpeptidergic nociceptors, in contrast, express the tyrosine kinase Ret and the GFRα co-receptors for the GDNF family ligands. Interestingly, the Ret+ nociceptive population of neurons emerges from TrkA-expressing neurons in early postnatal development, and the deletion of TrkA results in the complete loss of Ret-expressing unmyelinated neurons (Molliver et al., 1997; Silos-Santiago et al., 1995). This Ret-expressing population of nociceptors further differentiates into three subclasses of neurons that express specific Mas-related G protein-coupled receptors (Mrgpr), namely, neurons that express MrgprA3, MrgprB4 or MrgprD (Dong et al., 2001; Liu et al., 2008). Recent physiologic studies suggest that these three classes of neurons are responsive to pruritogens, the gentle stroking of hair, and noxious mechanical stimulation, respectively (Han et al., 2013; Liu et al., 2012; Liu et al., 2007; Zylka et al., 2005). One central question in developmental biology is how such neuronal diversity is developed and maintained throughout life.
The development of sensory neurons in the DRG is regulated by the interplay between neurotrophic factors and various transcription factors (Marmigere and Ernfors, 2007). Recent studies indicate that the expression of transcription factors such as Runx1, Runx3, Er81, Shox2, and MafA play significant roles in the diversification of DRG neurons (Bourane et al., 2009; Chen et al., 2006; Inoue et al., 2007; Kramer et al., 2006; Scott et al., 2011). Neurotrophin family members have been shown to support the survival of various types of DRG neurons during development. Previous loss–of–function studies of neurotrophin family members Nerve Growth Factor (NGF), Brain Derived Neurotrophic Factor (BDNF), Neurotrophin 3 (NT3) and their respective high-affinity receptors TrkA, TrkB and TrkC, demonstrated that different sensory neuron subtypes require different neurotrophins for their survival. For example, in Ngf−/− and TrkA−/− mice, nociceptors are lost (Crowley et al., 1994; Smeyne et al., 1994), while Nt3−/− and TrkC−/− mice lose proprioceptors (Ernfors et al., 1994; Tessarollo et al., 1994), and TrkB−/− and Bdnf−/− mice lose mechanoreceptors (Jones et al., 1994; Klein et al., 1993).
The GDNF family ligands (GFLs) consist of glial cell line-derived neurotrophic factor (GDNF), neurturin (NRTN), artemin (ARTN) and persephin (PSPN). They bind to one of four GDNF family co-receptors (GFRαs) to initiate their signaling, GFRα1-GFRα4. GDNF preferentially binds to GFRα1, neurturin to GFRα2, artemin to GFRα3 and persephin to GFRα4. GFRαs are glycerophosphtidylinositol (GPI) linked proteins that do not have an intracellular domain and, after binding to GFLs, they bind to and activate the tyrosine kinase Ret and initiate downstream signaling. Ret has two unique C-terminal isoforms, Ret9 and Ret51, each with unique signaling properties and functions (Airaksinen and Saarma, 2002). In vitro studies have demonstrated that GFLs promote the survival and axonal growth of various DRG neurons including nociceptive neurons (Molliver et al., 1997). In vivo loss–of–function studies on GFLs and GFRαs have shown that they are necessary for the survival of DRG neurons, their proper peripheral projections and cell body size (Ernsberger, 2008; Lindfors et al., 2006). The analysis of three different conditional Ret mutant mice demonstrated that Ret signaling is important for several aspects of DRG neuron development such as cell survival, central and peripheral neuronal projection and expression of phenotypical markers (Franck et al., 2011; Golden et al., 2010; Luo et al., 2007).
The p75 neurotrophin receptor (p75) is a member of the tumor necrosis receptor super family. p75 binds members of the Trk receptor family and modulates the cell survival functions of neurotrophins (Ceni et al., 2014). p75 is highly expressed in migrating neural crest cells and most sensory neurons during development. p75 null mice lose approximately 50% of DRG neurons across different sensory neuron subtypes, but the mechanism of these deficits is unclear in part due to expression of p75 in multiple cell types in the peripheral sensory system including neurons, Schwann cells and targets (Lee et al., 1992). One hypothesis is that neuronal loss is due to impairment in neurotrophin signaling. Indeed, in vitro experiments have shown that sensory neurons isolated from p75 null mutant mice are 2–3 fold less sensitive to the survival promoting effect of NGF treatment (Davies et al., 1993; Lee et al., 1994). In addition, in vitro experiments suggest that p75 prolongs the activation of TrkA receptors by preventing poly-ubiquitination and degradation of TrkA (Makkerh et al., 2005). These results argue that p75 can serve as a modulator of the neuronal response to neurotrophins. Although the role of p75 for modulating neurotrophin signaling is clearly established, whether the DRG neuron loss in p75 null mutant mice is due to a lack of neurotrophin signaling still remains to be determined.
Here we show that p75 is co-localized with GFRα1, GFRα2 and Ret in a subset of DRG neurons. p75 forms a complex with GFRα1 and Ret in vitro and in vivo. Removal of p75 from neurons reduced the survival effect of GDNF in vitro and impacted a subset of nonpeptidergic nociceptors in vivo, especially those that express low levels of Ret. Surprisingly, p75 deletion did not affect TrkA+ or TrkB+ populations of DRG neurons. p75, therefore, serves to augment GFL signaling and maintains the balance of cell specification and diversity during DRG neuron development.
Results
p75 is expressed in a subset of Ret expressing nociceptive neurons
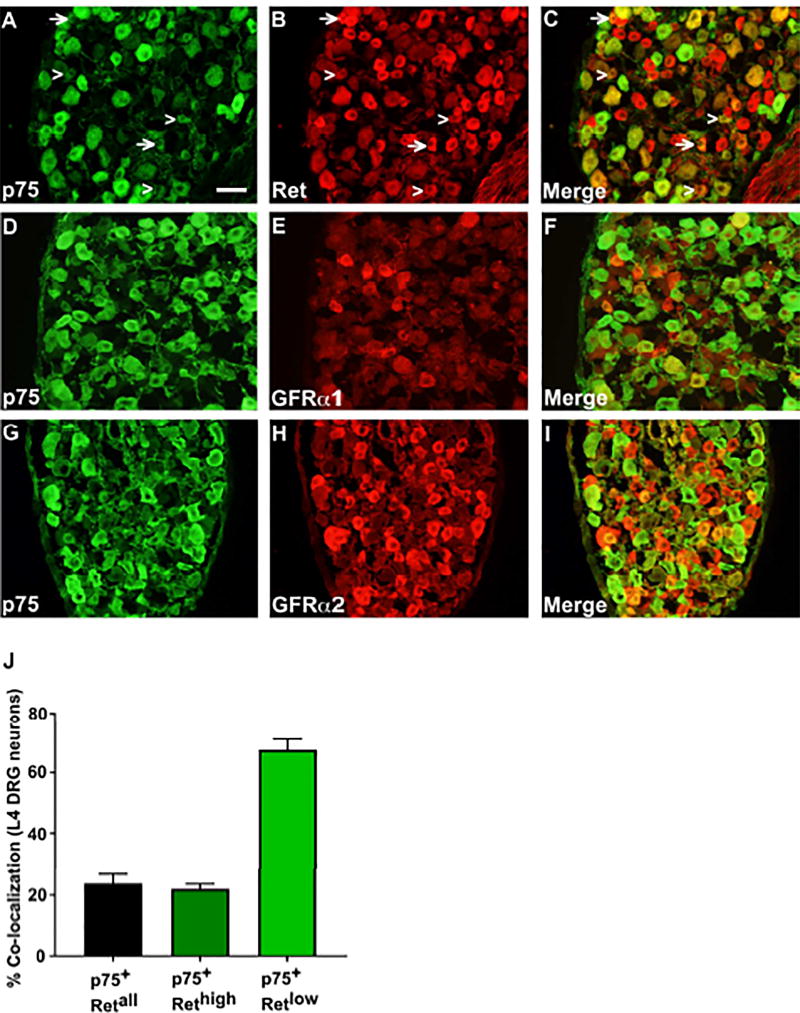
Ret expressing cells in the DRG encompass several neuronal populations mediating different sensory modalities. Previous studies have shown that small diameter nonpeptidergic nociceptors which mediate pain sensation up-regulate Ret expression and down-regulate TrkA expression postnatally. This process is not complete until 2–3 weeks after birth (Molliver et al., 1997). p75 is expressed in a variety of DRG neurons subclasses, and germline deletion of p75 results in a 50% loss of DRG neurons across different neuronal types (Murray et al., 1999). To determine whether the Ret-expressing nonpeptidergic nociceptive neurons express p75, immunostaining was performed on postnatal day 14 (P14) L4 DRG sections with antibodies against p75, Ret, GFRα1 and GFRα2. As shown in Figure 1B, 1E and 1H, small diameter nonpeptidergic nociceptors are Ret+ and most of them are also immunopositive for GFRα1 or GFRα2. p75 expression overlapped with some small diameter neurons that are immunopositive for Ret, GFRα1 or GFRα2 (Figures 1C, 1F, and 1I). When we counted neurons that were immunopositive for both p75 and Ret, we found that ~23.8% of small diameter Ret+ cells are also p75+ (Figure 1J). Previous studies have also shown that Ret+ nociceptive neurons have different levels of Ret expression and can be divided into groups that express a high level of Ret (Rethigh neurons) and a low level of Ret (Retlow neurons), each group with their own characteristic markers (Zylka et al., 2003). When we investigated the co-localization of p75 with Ret in small diameter Rethigh (as indicated by arrows in Figure 1A–C) and Retlow neurons (as indicated by arrowheads in Figure 1A–C), we found that among Rethigh neurons, only 22.1% of them co-express p75, while 67.6% of Retlow neurons express p75 (Figure 1J). The specificity of Ret staining was confirmed by staining sensory neurons taken from tamoxifen (TMX)-treated Rosa26LSL-tdTomato/+; Ret-Cre/ERT2 mice (Figure S1). These results suggest that p75 is in position to regulate the development of a subset of Ret+ nociceptive neurons, and its role may be more significant in Retlow sensory neurons.
Figure 1. p75 is co-expressed with GFRα1, GFRα2 and Ret in DRG neurons.
Immunofluorescence staining of p14 DRG sections with anti-p75 (panel A, D, G), anti-Ret (panel B), anti-GFRα1 (panel E), or anti-GFRα2 (panel H) antibodies. The quantitation of co-localization is presented in panel J. Arrows in panel A–C indicate examples of Rethigh cells and arrowheads indicate examples of Retlow cells. Scale bar, 100µm
p75 is a component of the GFRα/Ret receptor complex
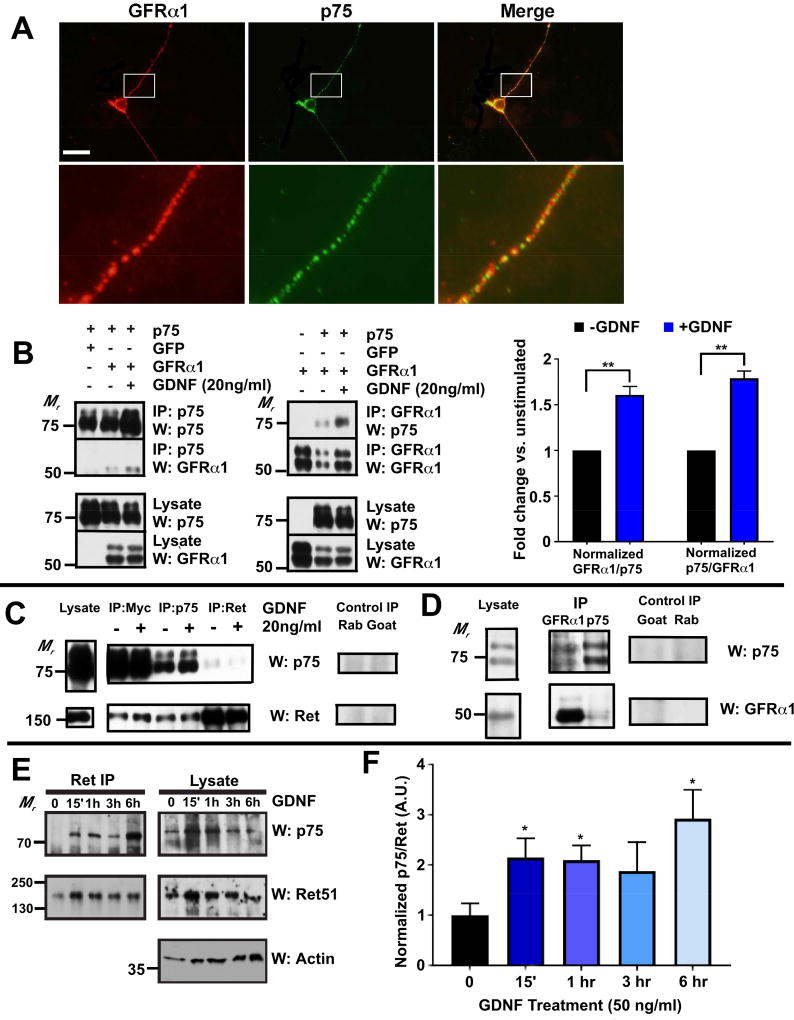
To investigate potential interactions between p75 and the components of the GDNF receptor complex, namely GFRα1 and Ret, we stained primary cultures of dissociated DRG neurons with antibodies against p75 and GFRα1. As shown in Figure 2A, punctate staining of p75 is often co-localized with GFRα1, although non-overlapping staining exists. Based on these findings, we next explored whether a functional interaction exists between p75 and GFRα1 and Ret. p75 was co-expressed with GFRα1 or Ret in HEK-293 cells and a co-immunoprecipitation assay was performed. As shown in Figures 2B and 2C, p75 co-immunoprecipitates with GFRα1 and Ret. GDNF treatment enhanced the association of p75 with GFRα1 (Figure 2B). On the other hand, GDNF had no effect when myc-tagged p75 were co-expressed with Ret alone in HEK293 cells. Quantification of the western blot signals from 3 independent experiments showed that no statistically significant changes in the amount of normalized Ret in the immuoprecipitate pulled down by an anti-myc antibody (fold change +GDNF/−GDNF=1.17, p=0.19) or an anti-p75 antibody (fold change +GDNF/−GDNF=1.08, p=0.58). The amount of normalized p75 in the immuoprecipitate pulled down by an anti-Ret antibody is also unchanged with GDNF treatment (fold change +GDNF/−GDNF = 0.86, p=0.43). This is likely due to the lack of GFRα1 expression. To determine whether p75 forms a complex with GFRα1 under physiological conditions in vivo, co-immunoprecipitation assays were performed using lysates from superior cervical ganglia (SCG). We found that p75 and GFRα1 were able to be co-immunoprecipitated regardless of which protein was isolated by IP (Figure 2D). Furthermore, to determine the time course of the interaction between p75 and Ret, primary DRG neuron cultures were generated from P0 rats and maintained in culture for 7–10 days. Importantly, DRG neurons cultured under similar conditions were previously shown to develop in a manner comparable to their in vivo development regarding the expression of ion channels and neurotrophic factor receptors (Molliver et al., 1997). 7–10 DIV DRG neurons were stimulated with GDNF for the indicated times, followed by immunoprecipitation of Ret and immunoblotting for p75 and Ret. Interestingly, we observed that GDNF led to a statistically significant increase in p75-Ret association at 15 minutes, 1 hour, and 6 hours (Figure 2E–F) after stimulation. These results suggest that p75 forms a complex with GFL receptor components that mediate GFL signaling.
Figure 2. p75 forms a receptor complex with GFRα1 and Ret.
(A) Primary DRG neurons were cultured from P14 mice and immunostained with anti-GFRα1 and anti-p75 antibodies without fixation. Images in the second row are magnified regions from the rectangles from images in the first row. Scale bar, 100µm (only for the top panels). (B) Immunoprecipitation assay of HEK293 cells co-transfected with GFRα1 and p75. When GFRα1 and p75 are co-expressed in HEK293 cells, they can be co-immunoprecipitated with an anti-GFRα1 antibody or an anti-p75 antibody. Quantification of the GFRα1 and p75 signals in co-IP experiments indicates GFRα1 and p75 association increased with GDNF treatments. (C) Immunoprecipitation assay of HEK-293 cells co-transfected with Ret and a myc-tagged p75. Ret and p75 can be co-immunoprecipitated with an anti-myc antibody, a rabbit anti-p75 antibody or a goat anti-Ret antibody. Treating the cells with GDNF did not increase the association between p75 and Ret. Normal rabbit (rab) or goat IgG was used as a negative control. (D) Immunoprecipitation assay of P1-2 SCG. GFRα1 can be co-immunoprecipitated with p75 using a rabbit anti-p75 antibody or a goat anti-GFRα1 antibody. Normal rabbit (rab) or goat IgG was used as control. (E) Primary DRG neurons were cultured from E18-P0 rats in the presence of 50 ng/ml NGF. After 7–10 DIV, the neurons were stimulated with medium alone or with GDNF for the indicated times. Ret receptor complexes were immunoprecipitated and immunoblotted for p75 (top panel; left), followed by Ret51 (middle; left). Lysates were immunoblotted for p75, Ret, and for actin (as a loading control). (F) Quantification of p75 co-immunoprecipitating with Ret at each timepoint.
p75 augments GDNF-mediated Ret activation by increasing cell surface localization of Ret
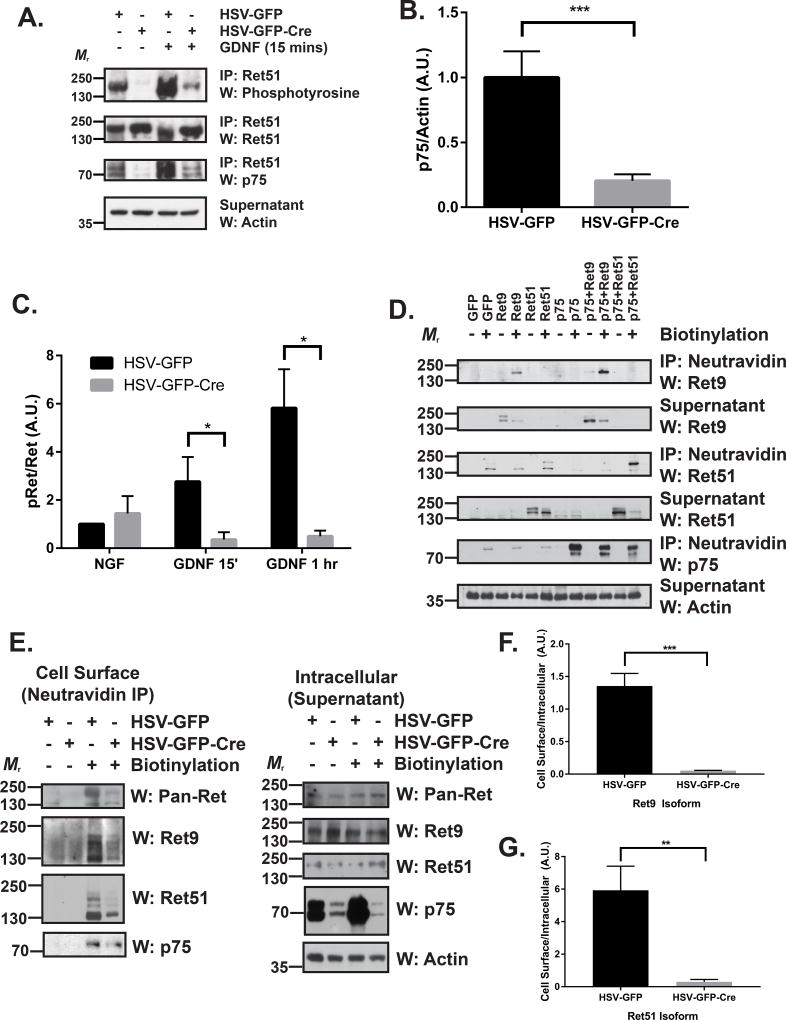
To determine whether p75 modulates GDNF-induced Ret autophosphorylation, primary DRG neuron cultures were generated from p75 conditional knockout mice (p75F/F), followed by infection with HSV-expressing Cre to knockdown their expression of p75, or with HSV-expressing GFP alone as a control. The cultures were then treated with GDNF, followed by immunoprecipitation to assess the level of phosphorylated Ret using an anti-phosphotyrosine antibody. As shown in Figure 3A, GDNF induces a rapid phosphorylation of Ret, which is sustained above baseline for at least 60 minutes. Transduction of primary DRG neurons with HSV-GFP-Cre resulted in successful knockdown of approximately 80% of p75 compared to HSV-GFP-transduced neurons (Figure 3A–B). Following knockdown of p75, GDNF-induced Ret phosphorylation is dramatically reduced at both 15 minutes and 60 minutes following GDNF treatment (Figure 3A, 3C). These results suggest that p75 augments GDNF-mediated Ret activation.
Figure 3. p75 augments GDNF-Ret signaling by increasing the cell surface localization of Ret.
(A) DRG neurons were cultured from P0 p75F/F mice in the presence of 50 ng/ml NGF. After two days, neurons were exposed to HSV-GFP or HSV-GFP-Cre viruses overnight (indicated above the blots). Neurons were maintained in culture until 7 DIV, followed by stimulation with medium alone or with 50 ng/ml GDNF for 15 minutes or 1 hour. Ret was immunoprecipitated and its level of activation was determined by phosphotyrosine immunoblotting. p75 immunoblotting confirmed that p75 protein levels were substantially reduced upon Cre expression. Actin immunoblotting of the supernatants served as a loading control. (B) Quantifications of p75 levels in 10 separate experiments demonstrate that approximately 80% of p75 is removed following treatment with HSV-GFP-Cre compared to HSV-GFP alone (*** p < 0.001). (C) Quantification of the mean proportion of phospho-Ret (pRet) over total Ret ± the standard error (5 experiments were quantified; * p < 0.05). (D) NIH/3T3 cells were transfected with GFP, Ret9, Ret51, p75, p75 and Ret9, or p75 and Ret51. After 24–36 hours, the cells were surface biotinylated using NHS-LC-Biotin in PBS (or treated with PBS alone as a control). The cells were then subjected to neutravidin precipitation. Precipitated cell surface proteins and supernatants (containing intracellular proteins) were then immunoblotted for Ret9, Ret51, and p75. Actin immunoblotting served as a loading control. (E) DRG neurons were cultured from P0 p75F/F mice and treated as described in A. Neurons were subsequently surface biotinylated as in D followed by neutravidin immunoprecipitation. Precipitated cell surface proteins (left) and supernatants (right; containing intracellular proteins) were analyzed by immunoblotting for pan-Ret (upper), Ret9 (2nd panel), and Ret51 (3rd panel). Actin immunoblotting served as a loading control. p75 immunoblotting confirmed efficient knockdown of greater than 70% of p75. (F) Quantifications of the cell surface levels of Ret9 (*** p < 0.001) and (G) Ret51 (** p < 0.01) indicate a highly significant difference in HSV-GFP-Cre treated neurons compared to HSV-GFP controls. IP, immunoprecipitation; W, western blot.
Given the striking loss of GDNF-mediated Ret activation, we hypothesized that loss of p75 may result in changes of cell surface localization of Ret. To explore this possibility, we first transfected NIH/3T3 cells with Ret9 or Ret51, with or without p75 co-transfection. Cell surface proteins were biotinylated using NHS-LC-Biotin and precipitated using neutravidin. Interestingly, we observed that co-transfection of p75 with Ret9 and Ret51 significantly enhanced cell surface levels of both isoforms (Figure 3D). To test whether p75 plays a similar role in DRG neurons, we performed cell surface biotinylation experiments in HSV-treated p75F/F DRG neurons. We observed a striking reduction in the amount of total cell surface Ret using an antibody that detects both isoforms, as well as a reduction of both individual Ret9 and Ret51 isoforms in HSV-Cre treated neurons compared to HSV-GFP controls (Figure 3E, quantifications in 3F, 3G).
p75 is required for GFL-mediated survival of Ret+ nonpeptidergic nociceptors in vitro
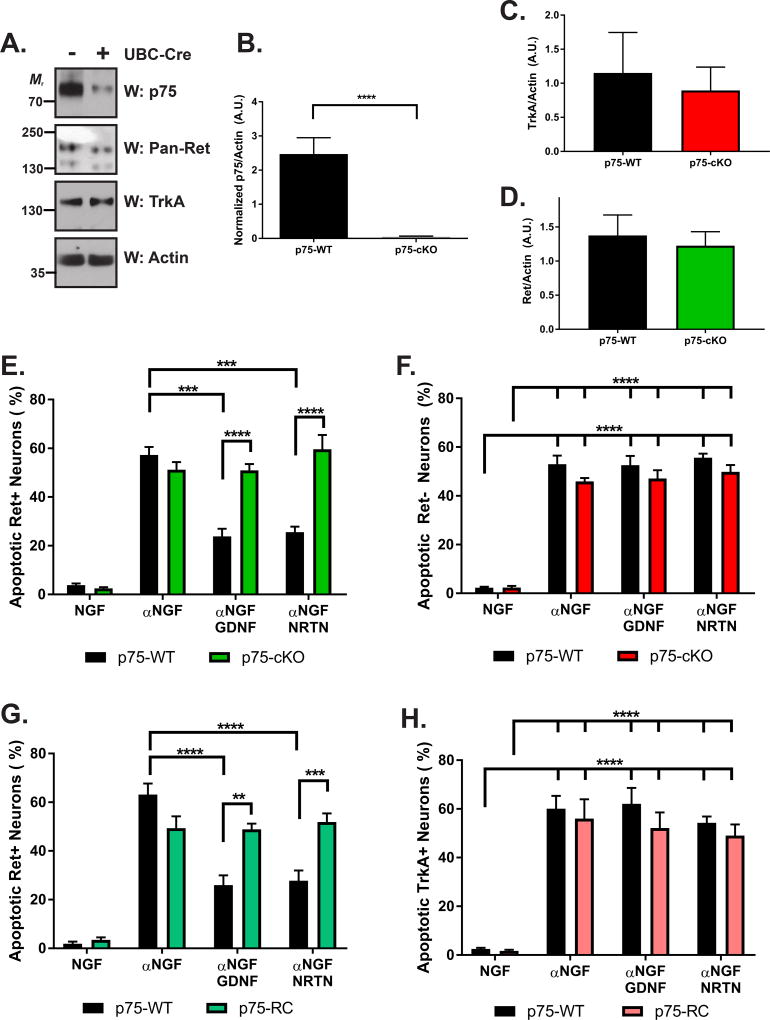
Given that p75 is required for GDNF-mediated Ret activation, we sought to determine whether loss of p75 impacted GFL-mediated DRG neuron survival. Further, because TrkA+ peptidergic nociceptors selectively depend on NGF, while Ret+ nonpeptidergic nociceptors depend on GDNF and NRTN (Molliver et al., 1997; Snider and Silos-Santiago, 1996) for their survival, we hypothesized that p75 deletion would selectively impair GFL-mediated survival within the nonpeptidergic nociceptor population. To this end, we crossed p75F/F mice with UBC-Cre/ERT2 mice, in which a tamoxifen (TMX)-inducible Cre is driven by the ubiquitin C promoter expressed in all cells (generating p75-WT and p75-cKO neurons) (Ruzankina et al., 2007), or Ret-Cre/ERT2 mice, in which TMX induces recombination selectively within Ret+ neurons (generating p75-WT and p75-RC neurons) (Luo et al., 2009). DRG neurons were cultured in the presence of NGF and 4-hydroxy-TMX to induce recombination. Importantly, we observed a substantial knockdown of p75 in p75-cKO neurons compared to p75-WT (p < 0.0001; greater than 98% reduction; Figures 4A–B). No changes were observed in the total amount of TrkA (Figure 4A, 4C) or Ret (Figure 4A, 4D) protein levels, further validating this system. Due to the difficulty of detecting GFRα1 and GFRα2 protein levels by western blotting in cultured DRG neurons, we performed immunostaining to determine whether loss of p75 alters the proportion of GFRα1 or GFRα2 neurons. While we observed no difference in the proportion of GFRα1+ neurons (p = 0.2723), a small but statistically significant reduction in the proportion of GFRα2+ neurons was observed (p = 0.0103; p75-WT: 80.12 ± 1.32 and p75-cKO: 66.76 ± 4.34%; Figure S2C–D).
Figure 4. p75 mediated Ret-mediated survival of nonpeptidergic nociceptors in vitro.
(A) Primary DRG neuron cultures were generated from P0 p75F/F; UBC-Cre/ERT2 (p75-cKO) or p75F/F control mice (p75-WT) and maintained in the presence of 50 ng/ml NGF and 4-OH-TMX. 7 DIV neurons were lysed and immunoblotted for p75 (top panel) to confirm efficacy of deletion, pan-Ret, TrkA, and actin (as a loading control). (B) Quantification of normalized p75 levels indicated a highly significant reduction of p75 levels in p75-cKO neurons (n=10) compared to p75-WT neurons (n=8; **** p < 0.0001). (C) No difference was observed in total levels of TrkA (n=5 p75-WT and n=7 p75-cKO; p = 0.6982) or (D) Ret protein levels (p=0.6737). (E, F) 7 DIV neurons were treated as described in A and subsequently treated with NGF, an anti-NGF blocking antibody (αNGF), αNGF with GDNF, or αNGF with NRTN (as indicated on the horizontal axis). 48 hours post-treatment, neurons were fixed and stained with Ret, TrkA, and DAPI (to determine nuclear pyknosis, indicating apoptosis) The number of apoptotic Ret+/TrkA(E) or Ret/TrkA+ neurons (F) was quantified for each treatment group (n=5 for p75-WT and n=3 for p75-cKO for each condition), with all statistical differences noted. GDNF and NRTN treatment was sufficient to recue Ret+, but not TrkA+ neurons from apoptosis in p75-WT, but not p75-cKO neurons. (G, H) DRG neuron cultures were generated from P0 p75F/F (p75-WT) and p75F/F; Ret-Cre/ERT2 (p75-RC) mice and treated, immunostained, and quantified as in E and F (treatments indicated on the horizontal axis). The number of apoptotic Ret+/TrkA (G) or Ret/TrkA+ neurons (H) was quantified for each treatment group (n=6 for p75-WT and n=5 for p75-RC for each condition). Similar results were noted as in E and F, with GFL treatment able to selectively rescue Ret+ neurons from apoptosis in p75-WT, but not p75-RC neurons.
To fully characterize how p75 deletion alters Ret and TrkA expression, we next treated 7–10 DIV, TMX-maintained p75-WT and p75-cKO DRG neurons with NGF, a NGF blocking antibody (αNGF), αNGF and GDNF, or αNGF and NRTN. 48 hours later, p75-WT and p75-cKO DRG neurons were fixed and immunostained for DAPI, TuJ1 (a pan-neuronal marker), Ret, and TrkA. The number of TrkA+/Ret− and Ret+/TrkA− neurons were quantified in each condition. We observed no genotype-dependent differences in the number of TrkA+ neurons regardless of treatment condition (Figure S2A), although we did observe a significant reduction of TrkA+ neurons following NGF deprivation, as expected (Snider and Silos-Santiago, 1996). In addition, we observed an increase in the percentage of Ret+ neurons following NGF-deprivation (p < 0.0001 for all genotypes) (Figure S2B), as expected due to the death of the TrkA+ population of neurons. Interestingly, the proportion of Ret+ neurons was increased in p75-cKO compared to p75-WT neurons treated with αNGF, αNGF and GDNF, and αNGF and NRTN, but not in NGF-maintained neurons. Collectively, these data indicate that removal of p75 does not substantially alter levels of TrkA, but enhances upregulation of Ret following NGF deprivation.
To determine whether loss of p75 altered GFL-mediated survival of TrkA+ peptidergic or Ret+ nonpeptidergic neurons, p75-WT and p75-cKO neurons were treated as described above and the number of apoptotic Ret+ (Figure 4E) and TrkA+ (Figure 4F) neurons was quantified by counting pyknotic nuclei. As expected, NGF deprivation led to an increase in the number of apoptotic Ret+ and TrkA+ neurons (Figures 4E–F) regardless of genotype. GDNF and NRTN treatments were able to partially rescue Ret+ neurons from apoptosis (Figure 4E; p < 0.001 for each), and this rescue affect was substantially diminished in p75-cKO (p < 0.0001 for each). As expected, GDNF and NRTN were not able to rescue TrkA+ neurons and loss of p75 had no effect (Figure 4F). To further substantiate these findings, these experiments were repeated using DRG neurons cultured from p75-RC, or p75-WT littermate mice. Interestingly, we observed that GDNF and NRTN were each able to rescue Ret+ neurons from apoptosis in p75-WT (p < 0.0001), but not p75-RC neurons, with a corresponding increase in apoptosis observed in p75-RC neurons treated with αNGF and GDNF (p < 0.01), and αNGF and NRTN (p < 0.001) compared to p75-WT neurons (Figure 4G). Additionally, as expected, GFL treatment did not rescue TrkA+ neurons from apoptosis, and no genotype-dependent effects were observed in TrkA+ neurons (Figure 4H). Collectively, these results indicate that p75 functions specifically to augment the GFL-mediated survival of Ret+ DRG neurons.
Removal of p75 expression in DRG neurons induces a loss of 20% of adult DRG neurons
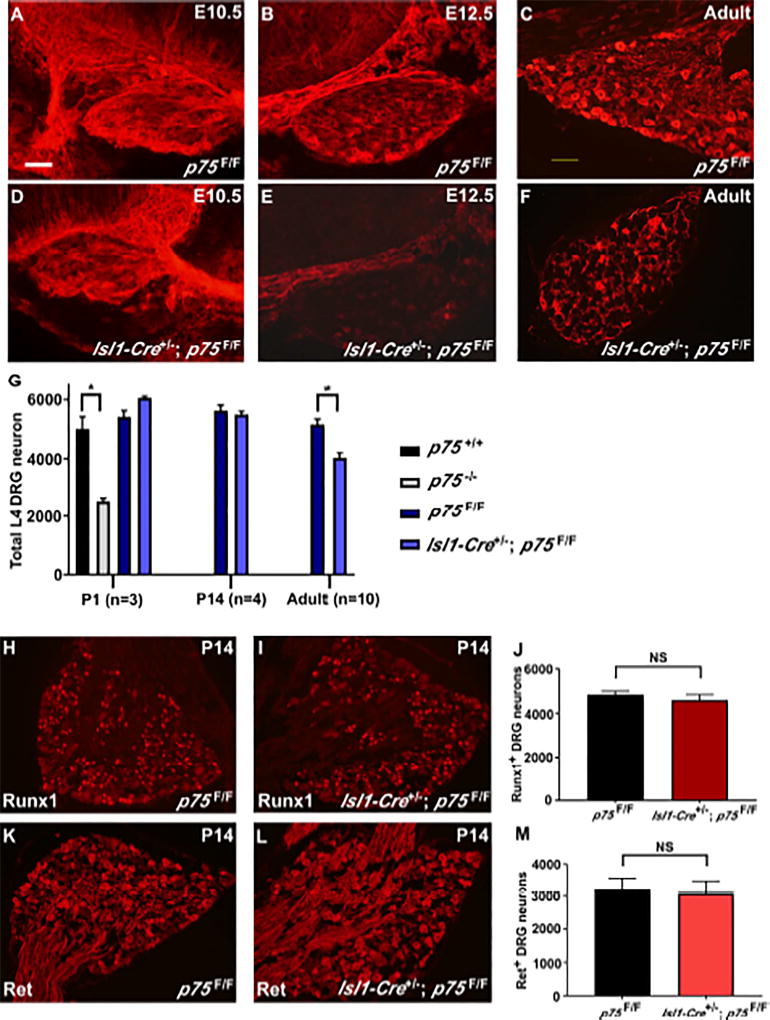
Based on our findings that p75 is necessary for GFL-mediated survival of DRG neurons in vitro, we asked whether it plays similar roles in vivo. Previous studies analyzing p75 germline knockout mice have demonstrated that 50% of DRG neurons are lost, and these losses occur across sensory modalities. It is unclear, however, whether this drastic phenotype reflects a cell-autonomous requirement for p75 within sensory neurons themselves. Thus, we generated p75F/F mice (described in Supplemental Experimental Procedures and Figure S3) and crossed them with an Islet1-Cre driver that expresses Cre in spinal motor and sensory neurons at later stages (Srinivas et al., 2001). Grossly, Isl1-Cre+/−; p75F/F mice are indistinguishable from their littermates. To determine at what stage p75 is deleted in these animals, p75 immunostaining was performed on E10.5, E12.5, and adult Isl1-Cre+/−; p75F/F mice (compared to p75F/F controls). We observed no reduction of p75 immunolabeling at E10.5 (Figure 5A, 5D), but a drastic loss of p75 by E12.5 (Figure 5B, 5E). At both E12.5 and adulthood (Figure 5C, 5F), the vast majority of neurons have lost p75 immunolabeling, with primarily non-neuronal cells expressing the residual p75. Therefore, Isl1-Cre+/−; p75F/F mice lose neuronal p75 expression between E10.5 and E12.5. To determine whether neuronal survival is altered in the Isl1-Cre+/−; p75F/F mice, we performed Nissl staining and counted total cell numbers in L4 DRGs from Isl1-Cre+/−; p75F/F (and p75F/F control) mice at P1, P14 or adulthood. As a control, we also analyzed L4 DRG counts in p75 germline knockout mice. As expected, we observed a 50% reduction in P1 p75−/− DRGs compared to p75+/+ controls (Figure 5G). Strikingly, given the drastic results observed in P1 p75−/− mice, we observed no difference in total L4 DRG neuron numbers at P1 or P14 in Isl1-Cre+/−; p75F/F mice (Figure 5G). We did, however, observe that approximately 20% of DRG neurons were lost in adult Isl1-Cre+/−; p75F/F mice compared to p75F/F controls (Figure 5G). Collectively, these results indicate that neuron-specific deletion of p75 by E12.5 is insufficient to impact neuronal survival during embryogenesis, and suggest neuronal p75 functions postnatally in sensory neuron diversification.
Figure 5. Isl1-Cre+/−; p75F/F mice lose 20% of DRG neurons.
p75 immunofluorescence staining of DRG sections from E10.5, E12.5 and adult p75F/F and Isl1-Cre+/−; p75F/F mice is shown in A-F. As shown in panel D, p75 immunoreactivity in DRG sensory neurons is not reduced at E10.5 in Isl1-Cre+/−; p75F/F mice. However, the number of p75-immunoreactive neurons is markedly reduced in E12.5 (panel E) and adult animals (panel F). The immunoreactivity observed in adult DRG is likely coming predominantly from satellite cells, which normally express p75. Scale bar, 100µm. (G) Total neuron numbers in L4 DRGs from p75F/F mice were compared to Isl1-Cre+/−; p75F/F mice at different postnatal ages. Adult Isl1-Cre+/−; p75F/F mice lose approximately 20% of DRG neurons. In contrast, p75−/− mice lose 50% of DRG neurons at P1 (* p < 0.05). At p14, Runx1 expression in p75F/F mice (H) is similar to Isl1-Cre+/−; p75F/F mice (I). The Ret expression level is also not changed between p75F/F (K) and Isl1-Cre+/−; p75F/F (L) mice. There is no change in the number of Runx1+ neurons (J) or Ret+ neurons (M) at p14 in Isl1-cre+/−; p75F/F mice compared to p75F/F mice (n=3).
Removal of p75 does not alter the generation of Ret+ or Runx1+ neurons
Loss of NGF-TrkA signaling in a Bax−/− background (thereby preventing apoptosis) results in significantly reduced levels of Ret, as well as reduced levels of the transcription factor Runx1 (Luo et al., 2007). Given that p75 has also been shown to enhance NGF signaling through TrkA, we tested whether the generation of Ret+ or Runx1+ neurons is affected in Isl1-Cre+/−; p75F/F mice. Upon immunostaining P14 DRG neurons for Ret or Runx1, we observed no differences in the intensity of immunostaining (Figure 5H–I, 5K–L) or total number of Runx1+ and Ret+ neurons in Isl1-Cre+/−; p75F/F compared to p75F/F control mice (Figure 5J, 5M), suggesting that the removal of p75 does not impact the NGF-TrkA-Runx1 signaling axis, thus unlikely to alter subsequent Ret expression. We further characterized the expression level of TrkA, TrkB, GFRα1 and GFRα2 at P0, P14 and adult DRGs. There is no obvious difference in any of these markers between Isl1-Cre+/−; p75F/F mice and controls at P0 and P14 (Figure S4, Figure S5). These results suggest that loss of p75 in the Isl1-Cre+/−; p75F/F mice does not impact the expression level of TrkA, TrkB, or the relevant GFL co-receptors while Ret+ nonpeptidergic nociceptors are being generated.
p75 is required for survival of a subset of nonpeptidergic nociceptors
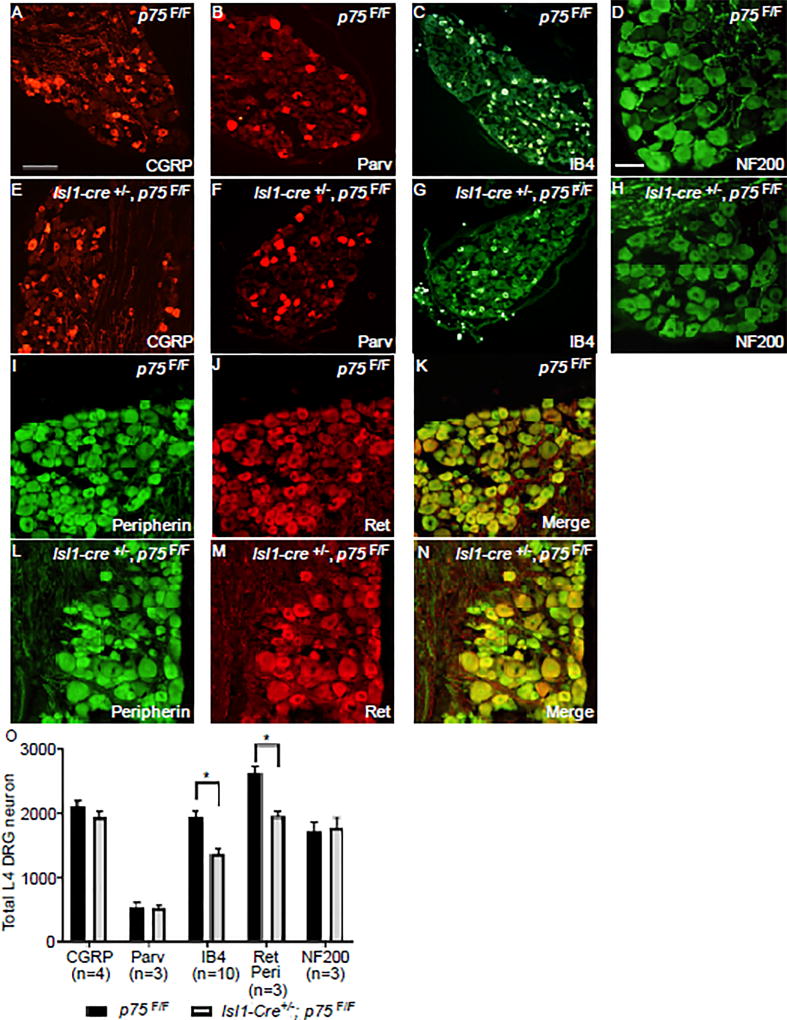
Given that we observed losses of approximately 20% of DRG neurons, and our in vitro findings linking p75 to GFL-Ret signaling, we hypothesized that nonpeptidergic nociceptors were selectively lost in Isl1-Cre+/−; p75F/F mice. To answer this question, adult p75F/F and Isl1-Cre+/−; p75F/F L4 DRG neurons were immunostained for NF200 (mechanoreceptors and proprioceptors), CGRP (peptidergic nociceptors), paravalbumin (proprioceptors), and IB4 (nonpeptidergic nociceptors). Interestingly, we observed no difference in the number of CGRP+ (Figure 6A, 6E), parvalbumin+ (Figure 6B, 6F), or NF200+ (Figure 6D, 6H) neurons between Isl1-Cre+/−; p75F/F and p75F/F mice (Figure 6O). In contrast, we observed a 35% decrease in IB4+ neurons (Figure 6C, 6G, 6O), indicating that p75 is selectively required for the survival of a subset of nonpeptidergic nociceptors. Given that the IB4+ immunolabeling may not sufficiently label all nonpeptidergic nociceptors, we sought to corroborate these results by performing co-immunostaining for peripherin and Ret to count the number of small diameter Ret+ nociceptors (Figure 6I–N). As shown in Figure 6O, we observed a 25% decrease in the total number of peripherin+/Ret+ neurons. We also observed ~30% decrease in the GFRα2+ population (Figure S5G) and ~50% decrease in small diameter GFRα1+ population (Figure S4N). Collectively, these results indicate that p75 is selectively required to promote the survival of nonpeptidergic Ret+ neurons, although we cannot rule out the possibility that p75 has additional functions in other unexplored subpopulations.
Figure 6. IB4+ nonpeptidergic nociceptors are selectively lost in Isl1-Cre+/−; p75F/F mice.
Immunofluorescence staining of adult L4 DRG with anti-CGRP (panel A and E), anti-Parvalbumin (panel B and F), anti-NF200 (panel D and H) antibody, IB4 (panel C and G) or a combination of anti-peripherin (I, L) and anti-Ret (J, M) antibodies from p75F/F (panel A–D, I–K) and Isl1-Cre+/−; p75F/F (panel E-H, L-N) mice. Scale bar for A-C, E-G, 200µm (located in panel A), Scale bar for D, H-N 100µm (located in panel D). (O) The quantification of CGRP+, Parvalbumin+, IB4+, Peripherin+/Ret+ and NF200+ neurons in adult L4 DRGs from p75F/F and Isl1-Cre+/−; p75F/F mice. Only the IB4+ and Peripherin+/Ret+ neurons were significantly reduced in the Isl1-Cre+/−; p75F/F mice. Values are expressed as mean ± SEM, * p<0.05. N indicates the number of animals being counted (each animal provides two DRGs).
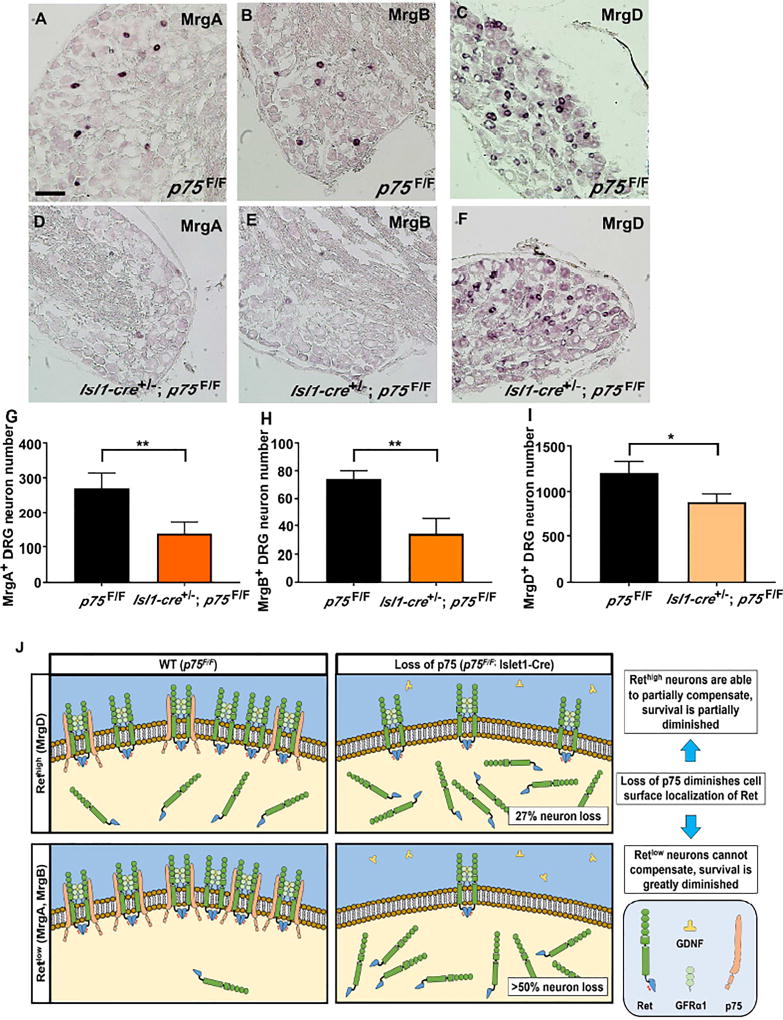
To characterize the neuron loss within the IB4+ population, we performed in situ hybridization using probes against MrgA, MrgB, and MrgD on adult (6 months old) L4 DRG sections (Dong et al., 2001). Importantly, MrgA+ and MrgB+ neurons are known to express Ret weakly (Retlow), while MrgD+ neurons express Ret more strongly (Rethigh) (Zylka et al., 2003). Interestingly, compared to p75F/F mice, we observed that Isl1-Cre+/−; p75F/F DRGs had a greater than 50% reduction in MrgA+ (Figure 7A–B, 7G) and MrgB+ neurons (Figure 7C–D, 7H), while only 27% of MrgD+ neurons were lost (Figure 7E–F, 7I). We cannot rule out the possibility that the loss of p75 in DRG neurons causes down regulation of Mrg family gene expression, as previous studies have shown a dependence on Ret signaling (Luo et al., 2007). Nonetheless, these data are consistent with the model that the p75-dependence of Ret+ neurons is correlated with the level of p75 expression in the Retlow and Rethigh neurons.
Figure 7. Nonpeptidergic neuron deficits in Isl1-Cre+/−; p75F/F mice correlates with level of p75-Ret co-expression.
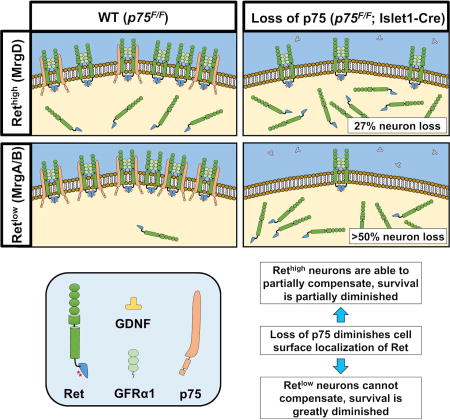
In situ hybridization of adult (6 months old) L4 DRG with MrgA (panel A and D), MrgB (panel B and E) and MrgD (panel C and F) in situ probes from p75F/F mice (panel A–C) and Isl1-Cre+/−; p75F/F (panel D–F). Scale bar, 100µm. Quantification of MrgA+ (panel G), MrgB+ (panel H) and MrgD+ (panel I) neurons showed that in Isl1-Cre+/−; p75F/F mice, over 50% of MrgA+ and MrgB+ neurons were lost, while approximately 20% of MrgD+ neurons were lost. Importantly, MrgA+ and MrgB+ neurons were shown to express low level of Ret while MrgD+ neurons express high level of Ret. The value is expressed as mean ± SEM (n=3 for each data point, * p<0.05, ** p<0.01). (J) The expression of p75 increases the level of Ret on the cell surface in p75F/F mice and potentiates GFL-induced Ret phosphorylation and its downstream survival effects. When p75 expression is lost in Isl1-Cre+/−; p75F/F mice, the level of Ret on the cell surface is reduced. For neurons that express high levels of Ret (MrgD+), the high level of Ret expression may compensate for the loss of p75 and results in more modest deficits. In neurons that express low levels of Ret (MrgA+ and MrgB+), however, the loss of Ret on the cell surface cannot be compensated for, leading to substantial cell loss.
Discussion
In this study, we demonstrate that p75 forms a GDNF-activated receptor complex with GFRα1 and Ret. Furthermore, knockdown of p75 levels within DRG neurons resulted in a drastic impairment in GDNF-mediated Ret activation, and a subsequent loss of cell surface localization of Ret, suggesting that p75 potentiates GDNF-Ret signal transduction through enhancing receptor availability to ligand, or by enhancing recycling. Correspondingly, p75 deletion results in a substantial reduction in GDNF and NRTN-mediated survival of Ret+ nonpeptidergic neurons, but not TrkA+ peptidergic neurons. Strikingly, especially when considering the 50% loss of DRG neurons during embryonic development in p75 germline null mutants, neuron-specific deletion of p75 by E12.5 resulted in no embryonic deficits. In these animals, 20% of neurons were lost postnatally, with losses occurring selectively within the IB4+/peripherin+/Ret+ class of nonpeptidergic nociceptors during the transition from P14 to adulthood. MrgA+ and MrgB+ neurons expressing the lowest levels of Ret had the highest degree of p75 expression, and were more significantly affected than Retlow MrgD+ neurons. In light of these findings, we propose the following model to explain the role of p75 modulation of Ret signaling in the survival of adult DRG neurons (Figure 7J). The expression of p75 increases the level of Ret on the cell surface in p75F/F mice, increasing sensitivity of these neurons to GDNF, thereby potentiating GFL-induced Ret activation and downstream survival. Upon loss of p75 expression in Isl1-Cre+/−; p75F/F mice, the level of Ret on the cell surface is reduced. Rethigh MrgD+ neurons may be better able to compensate for the loss of p75, resulting in lower neuron losses. However, in Retlow MrgA+ or MrgB+ neurons, the loss of cell surface Ret cannot be adequately buffered, leading to more substantial losses within the MrgA+ and MrgB+ neuron population. This model highlights the ability of p75 to fine-tune Ret signaling and maintain the balance of different types of DRG neurons in postnatal development. Our findings further expand the repertoire of receptor complexes that p75 modulates and the developmental processes that p75 impacts.
The developmental diversification of DRG neurons is an intricate process regulated by transcription factors and growth factors. Different types of sensory neurons rely on specific neurotrophic factors for their survival as they differentiate and mature. The p75 receptor can modulate both neurotrophin and GFL signaling pathways, playing a significant role in the development and maintenance of DRG neuron diversity. In the p75 knockout mice, 50% of lumbar DRG neurons are lost by the first week of postnatal life (Figure 5G) (Murray et al., 1999). This loss is across all DRG neuronal types and occurs early during embryonic DRG development. In Isl1-Cre+/−; p75F/F mice, p75 expression is removed at E12.5 specifically within nearly all sensory neurons, yet no neuronal deficits are observed before P14, and only 20% of the total neurons are lost in adult Isl1-Cre+/−; p75F/F mice. The striking disparity between neuronal losses in p75 knockout mice compared to Isl1-Cre+/−; p75F/F mice suggests that the early deficits in p75 knockout mice may be due to its function in non-neuronal cells. For example, previous studies have indicated that p75 is expressed in neural crest stem cells, which give rise to DRG neurons, satellite cells in the DRG, and Schwann cells. Given that germline p75 knockout animals lack p75 expression in all cell types from the onset of development, the previously observed loss of 50% of neurons across DRG subtypes may be due to defects in neural crest progenitors. Another possibility is that removing p75 from satellite cells and Schwann cells may create a less supportive environment for the survival of DRG neurons. While we cannot rule out the possibility that the small amount of p75 remaining following Islet1-Cre mediated deletion is responsible for the differences observed between germline p75 knockout mice and Isl1-Cre+/−; p75F/F mice, previous studies have also suggested a non-neuronal role for p75 early in sensory neuron development. For example, migration of p75-deficient Schwann cells is impaired and may thereby contribute to a reduced ability of developing sensory neurons to reach their proper target cells (Bentley and Lee, 2000). Nevertheless, the results reported here indicate that p75 has a postnatal function specifically serving to augment Ret signaling in nonpeptidergic nociceptors.
Another unexpected finding of this study is that p75 expression is required for the survival of nonpeptidergic nociceptors, but not other neuronal populations. In our analysis of Isl1-Cre+/−; p75F/F mice, we observed no deficits in the number of CGRP+ peptidergic nociceptive neurons, nor is their in vitro survival impaired, despite the fact that p75 is expressed in the majority of CGRP+ neurons and p75 expression is removed in most CGRP+ neurons in Isl1-Cre+/−; p75F/F mice at a developmental period during which these neurons are highly dependent on NGF/TrkA signaling (Figure S6). In addition, we observed no deficits in numbers of TrkA+, Runx1+, Ret+, GFRα1+, or GFRα2+ neurons at P14. These results are surprising because p75 has been shown in many studies to enhance NGF signaling via TrkA through increased NGF binding affinity (Esposito et al., 2001; Hempstead et al., 1991), as well as through reduced ubiquitination and subsequent degradation of TrkA (Makkerh et al., 2005). The pro-survival function of p75 in sensory neurons has been proposed by many (Davies et al., 1993; Lee et al., 1994) to be through modulation of NGF/TrkA signaling. Based on these results, we conclude that the intrinsic level of NGF signaling is sufficient to maintain their survival and, therefore, p75 is dispensable for NGF/TrkA-dependent survival of peptidergic neurons, although the trophic status of these neurons may still be impacted.
In conclusion, we have found that p75 physically associates with receptors in the GDNF family and plays a novel role in mediating the survival-promoting effects of the GFLs by enhancing cell surface localization of Ret, thereby augmenting GFL/GFRα/Ret signaling. Surprisingly, in vivo, deletion of p75 specifically within neurons resulted in no deficits until after P14, suggesting a potential early, non-neuronal role for p75 followed by a postnatal, neuron-specific function in nonpeptidergic nociceptors. Within the nonpeptidergic nociceptor population, neurons of the MrgA+ and MrgB+ subclasses demonstrate a greater dependence on p75 to potentiate Ret signaling compared to MrgD+ neurons. Thus, our data clarify the function of this enigmatic receptor in sensory neurons and identify a unique mechanism by which neurotrophic factor support can be fine-tuned to allow the selective survival of discrete subpopulations, ultimately expanding sensory neuron diversity.
Experimental Procedures
Statistics
Statistical tests were carried out by using GraphPad Prism (GraphPad Software); statistical tests used are indicated throughout the results section. A student’s t-test was used to assessing statistical significance between two conditions, while one-way ANOVA was utilized when multiple variables were compared. All data are reported as the mean ± s.e.m. *p < 0.05, **p < 0.01, ***p < 0.001, ****p <0.0001.
Supplementary Material
Acknowledgments
This research was supported by grants from the NIH (HD034534, NS060833, NS072031, NS089585, AG010435, CA014195, AG042985, AG047669, OD023076, and DE023479), the Clayton Foundation, the Schlink Foundation, the Gemcon Family Foundation and the Joe W. and Dorothy Dorsett Brown Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Additional methods are included in the Supplementary Materials.
Author contribution
Z.C., C.R.D., B.A.P., and K.F.L. designed experiments, interpreted the data, and wrote the manuscript. Z.C., C.R.D., A.S.H. and B.D. performed the experiments. Y. H. and W.L. generated p75 floxed mice. B.A.P. and K.F.L. were responsible for the overall direction and communication of the experiments.
References
- Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat. Rev. Neurosci. 2002;3:383–394. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- Bentley CA, Lee KF. p75 is important for axon growth and schwann cell migration during development. J. Neurosci. 2000;20:7706–7715. doi: 10.1523/JNEUROSCI.20-20-07706.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourane S, Garces A, Venteo S, Pattyn A, Hubert T, Fichard A, Puech S, Boukhaddaoui H, Baudet C, Takahashi S, et al. Low-threshold mechanoreceptor subtypes selectively express MafA and are specified by Ret signaling. Neuron. 2009;64:857–870. doi: 10.1016/j.neuron.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Ceni C, Unsain N, Zeinieh MP, Barker PA. Neurotrophins in the regulation of cellular survival and death. Handb. Exp. Pharmacol. 2014;220:193–221. doi: 10.1007/978-3-642-45106-5_8. [DOI] [PubMed] [Google Scholar]
- Chen CL, Broom DC, Liu Y, de Nooij JC, Li Z, Cen C, Samad OA, Jessell TM, Woolf CJ, Ma Q. Runx1 determines nociceptive sensory neuron phenotype and is required for thermal and neuropathic pain. Neuron. 2006;49:365–377. doi: 10.1016/j.neuron.2005.10.036. [DOI] [PubMed] [Google Scholar]
- Crowley C, Spencer SD, Nishimura MC, Chen KS, Pitts-Meek S, Armanini MP, Ling LH, McMahon SB, Shelton DL, Levinson AD, et al. Mice lacking nerve growth factor display perinatal loss of sensory and sympathetic neurons yet develop basal forebrain cholinergic neurons. Cell. 1994;76:1001–1011. doi: 10.1016/0092-8674(94)90378-6. [DOI] [PubMed] [Google Scholar]
- Davies AM, Lee KF, Jaenisch R. p75-deficient trigeminal sensory neurons have an altered response to NGF but not to other neurotrophins. Neuron. 1993;11:565–574. doi: 10.1016/0896-6273(93)90069-4. [DOI] [PubMed] [Google Scholar]
- Dong X, Han S, Zylka MJ, Simon MI, Anderson DJ. A diverse family of GPCRs expressed in specific subsets of nociceptive sensory neurons. Cell. 2001;106:619–632. doi: 10.1016/s0092-8674(01)00483-4. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Lee KF, Kucera J, Jaenisch R. Lack of neurotrophin-3 leads to deficiencies in the peripheral nervous system and loss of limb proprioceptive afferents. Cell. 1994;77:503–512. doi: 10.1016/0092-8674(94)90213-5. [DOI] [PubMed] [Google Scholar]
- Ernsberger U. The role of GDNF family ligand signalling in the differentiation of sympathetic and dorsal root ganglion neurons. Cell Tissue Res. 2008;333:353–371. doi: 10.1007/s00441-008-0634-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito D, Patel P, Stephens RM, Perez P, Chao MV, Kaplan DR, Hempstead BL. The cytoplasmic and transmembrane domains of the p75 and Trk A receptors regulate high affinity binding to nerve growth factor. J. Biol. Chem. 2001;276:32687–32695. doi: 10.1074/jbc.M011674200. [DOI] [PubMed] [Google Scholar]
- Franck MC, Stenqvist A, Li L, Hao J, Usoskin D, Xu X, Wiesenfeld-Hallin Z, Ernfors P. Essential role of Ret for defining non-peptidergic nociceptor phenotypes and functions in the adult mouse. Eur. J. Neurosci. 2011;33:1385–1400. doi: 10.1111/j.1460-9568.2011.07634.x. [DOI] [PubMed] [Google Scholar]
- Golden JP, Hoshi M, Nassar MA, Enomoto H, Wood JN, Milbrandt J, Gereau RWt, Johnson EM, Jr, Jain S. RET signaling is required for survival and normal function of nonpeptidergic nociceptors. J. Neurosci. 2010;30:3983–3994. doi: 10.1523/JNEUROSCI.5930-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L, Ma C, Liu Q, Weng HJ, Cui Y, Tang Z, Kim Y, Nie H, Qu L, Patel KN, et al. A subpopulation of nociceptors specifically linked to itch. Nat. Neurosci. 2013;16:174–182. doi: 10.1038/nn.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempstead BL, Martin-Zanca D, Kaplan DR, Parada LF, Chao MV. High-affinity NGF binding requires coexpression of the trk proto-oncogene and the low-affinity NGF receptor. Nature. 1991;350:678–683. doi: 10.1038/350678a0. [DOI] [PubMed] [Google Scholar]
- Inoue K, Ito K, Osato M, Lee B, Bae SC, Ito Y. The transcription factor Runx3 represses the neurotrophin receptor TrkB during lineage commitment of dorsal root ganglion neurons. J. Biol. Chem. 2007;282:24175–24184. doi: 10.1074/jbc.M703746200. [DOI] [PubMed] [Google Scholar]
- Jones KR, Farinas I, Backus C, Reichardt LF. Targeted disruption of the BDNF gene perturbs brain and sensory neuron development but not motor neuron development. Cell. 1994;76:989–999. doi: 10.1016/0092-8674(94)90377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R, Smeyne RJ, Wurst W, Long LK, Auerbach BA, Joyner AL, Barbacid M. Targeted disruption of the trkB neurotrophin receptor gene results in nervous system lesions and neonatal death. Cell. 1993;75:113–122. [PubMed] [Google Scholar]
- Kramer I, Sigrist M, de Nooij JC, Taniuchi I, Jessell TM, Arber S. A role for Runx transcription factor signaling in dorsal root ganglion sensory neuron diversification. Neuron. 2006;49:379–393. doi: 10.1016/j.neuron.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Lee KF, Davies AM, Jaenisch R. p75-deficient embryonic dorsal root sensory and neonatal sympathetic neurons display a decreased sensitivity to NGF. Development. 1994;120:1027–1033. doi: 10.1242/dev.120.4.1027. [DOI] [PubMed] [Google Scholar]
- Lee KF, Li E, Huber LJ, Landis SC, Sharpe AH, Chao MV, Jaenisch R. Targeted mutation of the gene encoding the low affinity NGF receptor p75 leads to deficits in the peripheral sensory nervous system. Cell. 1992;69:737–749. doi: 10.1016/0092-8674(92)90286-l. [DOI] [PubMed] [Google Scholar]
- Lindfors PH, Voikar V, Rossi J, Airaksinen MS. Deficient nonpeptidergic epidermis innervation and reduced inflammatory pain in glial cell line-derived neurotrophic factor family receptor alpha2 knock-out mice. J. Neurosci. 2006;26:1953–1960. doi: 10.1523/JNEUROSCI.4065-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Sikand P, Ma C, Tang Z, Han L, Li Z, Sun S, LaMotte RH, Dong X. Mechanisms of itch evoked by beta-alanine. J. Neurosci. 2012;32:14532–14537. doi: 10.1523/JNEUROSCI.3509-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Vrontou S, Rice FL, Zylka MJ, Dong X, Anderson DJ. Molecular genetic visualization of a rare subset of unmyelinated sensory neurons that may detect gentle touch. Nat. Neurosci. 2007;10:946–948. doi: 10.1038/nn1937. [DOI] [PubMed] [Google Scholar]
- Liu Y, Ma Q. Generation of somatic sensory neuron diversity and implications on sensory coding. Curr. Opin. Neurobiol. 2011;21:52–60. doi: 10.1016/j.conb.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Yang FC, Okuda T, Dong X, Zylka MJ, Chen CL, Anderson DJ, Kuner R, Ma Q. Mechanisms of compartmentalized expression of Mrg class G-protein-coupled sensory receptors. J. Neurosci. 2008;28:125–132. doi: 10.1523/JNEUROSCI.4472-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Enomoto H, Rice FL, Milbrandt J, Ginty DD. Molecular identification of rapidly adapting mechanoreceptors and their developmental dependence on ret signaling. Neuron. 2009;64:841–856. doi: 10.1016/j.neuron.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Wickramasinghe SR, Savitt JM, Griffin JW, Dawson TM, Ginty DD. A hierarchical NGF signaling cascade controls Ret-dependent and Ret-independent events during development of nonpeptidergic DRG neurons. Neuron. 2007;54:739–754. doi: 10.1016/j.neuron.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Makkerh JP, Ceni C, Auld DS, Vaillancourt F, Dorval G, Barker PA. p75 neurotrophin receptor reduces ligand-induced Trk receptor ubiquitination and delays Trk receptor internalization and degradation. EMBO Rep. 2005;6:936–941. doi: 10.1038/sj.embor.7400503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmigere F, Ernfors P. Specification and connectivity of neuronal subtypes in the sensory lineage. Nat. Rev. Neurosci. 2007;8:114–127. doi: 10.1038/nrn2057. [DOI] [PubMed] [Google Scholar]
- Molliver DC, Wright DE, Leitner ML, Parsadanian AS, Doster K, Wen D, Yan Q, Snider WD. IB4-binding DRG neurons switch from NGF to GDNF dependence in early postnatal life. Neuron. 1997;19:849–861. doi: 10.1016/s0896-6273(00)80966-6. [DOI] [PubMed] [Google Scholar]
- Murray SS, Bartlett PF, Cheema SS. Differential loss of spinal sensory but not motor neurons in the p75NTR knockout mouse. Neurosci. Lett. 1999;267:45–48. doi: 10.1016/s0304-3940(99)00330-4. [DOI] [PubMed] [Google Scholar]
- Ruzankina Y, Pinzon-Guzman C, Asare A, Ong T, Pontano L, Cotsarelis G, Zediak VP, Velez M, Bhandoola A, Brown EJ. Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell. 2007;1:113–126. doi: 10.1016/j.stem.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott A, Hasegawa H, Sakurai K, Yaron A, Cobb J, Wang F. Transcription factor short stature homeobox 2 is required for proper development of tropomyosin-related kinase B-expressing mechanosensory neurons. J. Neurosci. 2011;31:6741–6749. doi: 10.1523/JNEUROSCI.5883-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silos-Santiago I, Molliver DC, Ozaki S, Smeyne RJ, Fagan AM, Barbacid M, Snider WD. Non-TrkA-expressing small DRG neurons are lost in TrkA deficient mice. J. Neurosci. 1995;15:5929–5942. doi: 10.1523/JNEUROSCI.15-09-05929.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeyne RJ, Klein R, Schnapp A, Long LK, Bryant S, Lewin A, Lira SA, Barbacid M. Severe sensory and sympathetic neuropathies in mice carrying a disrupted Trk/NGF receptor gene. Nature. 1994;368:246–249. doi: 10.1038/368246a0. [DOI] [PubMed] [Google Scholar]
- Snider WD, Silos-Santiago I. Dorsal root ganglion neurons require functional neurotrophin receptors for survival during development. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 1996;351:395–403. doi: 10.1098/rstb.1996.0034. [DOI] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessarollo L, Vogel KS, Palko ME, Reid SW, Parada LF. Targeted mutation in the neurotrophin-3 gene results in loss of muscle sensory neurons. Proc Natl Acad Sci U S A. 1994;91:11844–11848. doi: 10.1073/pnas.91.25.11844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylka MJ, Dong X, Southwell AL, Anderson DJ. Atypical expansion in mice of the sensory neuron-specific Mrg G protein-coupled receptor family. Proc. Natl. Acad. Sci. U S A. 2003;100:10043–10048. doi: 10.1073/pnas.1732949100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylka MJ, Rice FL, Anderson DJ. Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to Mrgprd. Neuron. 2005;45:17–25. doi: 10.1016/j.neuron.2004.12.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.