Abstract
Several strains of a new aflatoxigenic species of Aspergillus, A. korhogoensis, were isolated in the course of a screening study involving species from section Flavi found contaminating peanuts (Arachis hypogaea) and peanut paste in the Côte d’Ivoire. Based on examination of four isolates, this new species is described using a polyphasic approach. A concatenated alignment comprised of nine genes (ITS, benA, cmdA, mcm7, amdS, rpb1, preB, ppgA, and preA) was subjected to phylogenetic analysis, and resulted in all four strains being inferred as a distinct clade. Characterization of mating type for each strain revealed A. korhogoensis as a heterothallic species, since three isolates exhibited a singular MAT1-1 locus and one isolate exhibited a singular MAT1-2 locus. Morphological and physiological characterizations were also performed based on their growth on various types of media. Their respective extrolite profiles were characterized using LC/HRMS, and showed that this new species is capable of producing B- and G-aflatoxins, aspergillic acid, cyclopiazonic acid, aflavarins, and asparasones, as well as other metabolites. Altogether, our results confirm the monophyly of A. korhogoensis, and strengthen its position in the A. flavus clade, as the sister taxon of A. parvisclerotigenus.
Keywords: Aspergillus section Flavi, aflatoxins, cyclopiazonic acid, polyphasic approach, versicolorins
1. Introduction
The presence of mycotoxins in agricultural commodities poses serious economic and health risks [1,2,3]. Among the mycotoxins, aflatoxins are by far the most studied since their ingestion can cause deleterious health effects in humans and animals including hepatic cancer and, in some instances, death [4]. Aflatoxin B1 is the potent compound of this chemical family as it displays mutagenic, teratogenic and hepatocarcinogenic effects in humans and animals [5]. To date, it is considered as the most carcinogenic, teratogenic and genotoxic substance of natural origin [6,7].
Species that have so far been reported to produce aflatoxins are all classified in Aspergilllus subgenus Circumdati and section Flavi, with the exception of two other species originally sampled in the Côte d’Ivoire, A. ochraceoroseus and A. rambelli [8,9]. Species from Aspergillus section Flavi represent a well-known group of saprophytic filamentous fungi, several of which have the ability to produce beneficial secondary metabolites or enzymes used in food fermentation and biotechnology, such as kojic acid and α-amylase [10]. Conversely, some of these species have the potential to produce one or more harmful mycotoxins, such as aflatoxins, cyclopiazonic acid, versicolorins, and aflatrems [11,12]. Due to extensive research into their aflatoxin production, A. flavus, A. parasiticus, and A. nomius are considered major species in section Flavi. Among them, A. flavus is the most important because of its worldwide distribution, and it represents the largest source of aflatoxin B1 contamination of several staple crops, including maize, tree nuts, peanuts, cottonseed, grains, cassava, and spices [13,14,15,16].
Although some species relationships in section Flavi are still unclear, the section can be separated into one of seven main clades based on a polyphasic approach: A. flavus clade, A. parasiticus clade, A. tamarii clade, A. nomius clade, A. alliaceus clade, A. togoensis clade, or A. avenaceus clade, and A. mottae and A. bertholletius [17,18,19]. Each clade may contain cryptic species that are difficult to identify, based solely on morphological characters or extrolite profiles, but can be delineated using a polyphasic approach that also includes molecular analyses [12,17,18]. It has been suggested that two cryptic species, A. minisclerotigenes and A. parvisclerotigenus, belong to the A. flavus clade [9,20]. However, in addition to B-aflatoxins these species produce G-aflatoxins, which A. flavus is incapable of producing [17,21] due to a deletion of genomic sequence between the aflatoxin pathway genes, norB (aflF) and cypA (aflU), thereby altering the promoter and coding regions [22].
In this paper, we describe A. korhogoensis sp. nov. as a novel cryptic species within the A. flavus clade, based on a polyphasic analysis of four strains isolated from peanuts collected in the region of Korhogo, Côte d’Ivoire.
2. Results
2.1. Molecular Analyses
2.1.1. Multilocus Phylogenetic Analysis
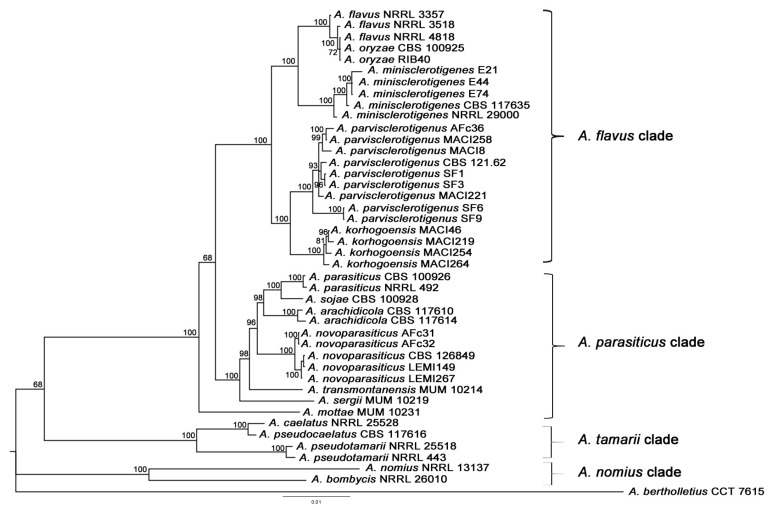
The phylogenetic tree inferred from nine concatenated genes (ITS, benA, cmdA, mcm7, amdS, rpb1, preB, ppgA, and preA), obtained from Bayesian and ML analyses, yielded largely similar topologies, particularly congruent for Aspergillus flavus clade. Here, we chose the Bayesian topology as hypothesis of phylogenetic relationships because the results were of greater robustness (Figure 1). Our results support previous phylogenetic inferences involving species from section Flavi. Aspergillus bertholletius was used as the outgroup taxon. The A. nomius clade, which included A. bombycis and A. nomius, was monophyletic and appeared as a basal group (Posterior Probability, PP = 1). The topology then split in two robust groups, one formed by the A. tamarii clade, which included A. caelatus, A. pseudocaelatus and A. pseudotamarii, and a second monophyletic group, which included the A. parasiticus and A. flavus clades, as well as A. mottae. This latter was placed as the ancestral taxon of the group including A. parasiticus and A. flavus clades. Aspergillus parasiticus clade was consistent with Soares et al. results [18] and included A. parasiticus, A. sojae, A. arachidicola, A. novoparasiticus, A. sergii and A. transmontanensis. A. sergii and A. transmontanensis are basal taxa, respectively. The A. flavus clade included A. flavus, A. oryzae, A. minisclerotigenes, A. parvisclerotigenus and A. korhogoensis, the herein described new species.
Figure 1.
Phylogenetic tree of Aspergillus section Flavi based on concatenated sequences from nine genomic loci (ITS, benA, cmdA, mcm7, amdS, rpb1, preB, ppgA, and preA). Bayesian tree was calculated from 41 strains, and includes the Type strain for most species. Strong bootstrap values are shown at branch nodes. Species isolate numbers are indicated at branch tips. A. bertholletius CCT 7615 was used as the outgroup taxon.
The A. flavus clade is comprised of two main groups: one that includes A. flavus, its domesticated species A. oryzae and A. minisclerotigenes (PP = 1); and the other group encompasses A. parvisclerotigenus and A. korhogoensis sp. nov. (PP = 1). The four isolates (MACI46, MACI219, MACI254 and MACI264) putatively identified as A. korhogoensis were tightly clustered, suggesting they were a distinct species from A. parvisclerotigenus. Strains from the latter species included isolates from different populations, which were clustered together, suggesting a monophyletic group with no major differences among populations.
Aspergillus novoparasiticus was represented by two well-supported groups, which segregated based on geography since one group corresponds to the strains isolated from Brazil [23], while the other group corresponds to strains isolated from Benin [14]. Besides their geographical distributions, both groups were isolated from different environments, such as hospital environments (Brazil samples) or foodstuffs (Benin samples).
2.1.2. Mating Type Analysis
Results from our mating type diagnostic PCR revealed that isolates MACI46, MACI254 and MACI264 contained a single Mat1-1 gene, and that isolate MACI219 contained a single Mat1-2 gene. These findings demonstrate that A. korhogoensis sp. nov. is likely a heterothallic (self-infertile) fungus. Whether these mating-type genes are functional is unknown. Future mating tests will be required to determine this.
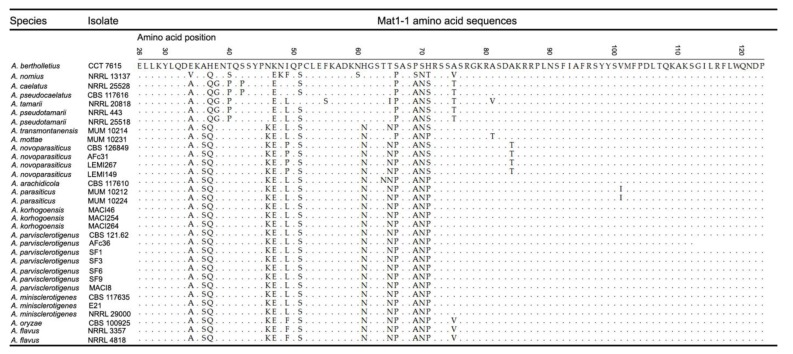
The MAT1-1 amino acid sequence of A. bertholletius was used as the reference sequence to compare with Mat1-1 genes from other examined taxa. Basal taxa (A. bertholletius, A. nomius, A. caelatus, A. pseudocaelatus, A. tamarii and A. pseudotamarii) presented alanine, asparagine, histidine and threonine at position 36, 46, 61 and 65, respectively, which changed in derived species into serine, lysine, asparagine and asparagine (Figure 2). The A. tamarii clade presented four apomorphies that are specific to the clade, and one apomorphy that was fixed in the derived species. The A. parasiticus and A. flavus clades exhibited a highly conserved MAT1-1 amino acid sequence, except for one amino acid substitution in A. parasiticus at position 101, and two substitutions in both A. flavus and A. oryzae at positions 49 and 75. Although haplotypes of A. minisclerotigenes, A. parvisclerotigenus and A. korhogoensis sp. nov. shared identity for their respective MAT1-1 amino acid sequences (Figure 2), there were single nucleotide polymorphism (SNP) differences that did not result in an amino acid replacement. Aspergillus minisclerotigenes exhibited two apomorphies, whereas A. parvislerotigenus and A. korhogoensis sp. nov. each exhibited only one (except MACI264, which exhibited the conserved ancestral state).
Figure 2.
Amino acid sequence alignment for the Mat1-1 locus in examined strains representing several Aspergillus species. The amino acid positions were determined based on the complete amino acid sequence of A. flavus NRRL 3357 strain (accession number = EED46656). Accession numbers recovered from GenBank: A. parasiticus MUM 10.212 = HM80303, A. parasiticus MUM 10.224 = HM803058, and A. tamarii NRRL 20818 = HM803044. Other accession numbers are given in Table S2.
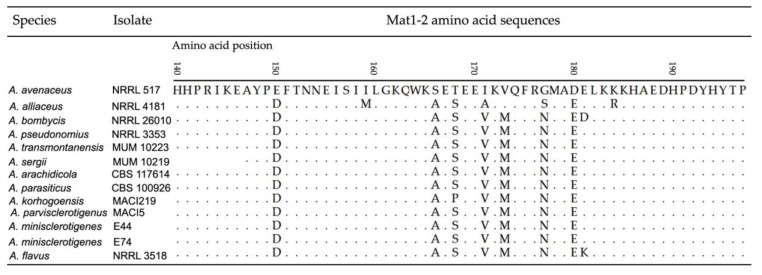
In the case of Mat1-2 gene, amino acid sequences were identical for A. nomius, A. pseudonomius, A. sergii, A. transmontanensis, A. arachidicola, A. parasiticus, A. parvisclerotigenus and A. minisclerotigenes. There was one substitution in the amino acid sequence for A. korhogoensis sp. nov. (S168P), and one for A. flavus (E181K) (Figure 3). Basal taxa, A. avenaceus and A. alliaceus, exhibited several differences in their amino acid sequences.
Figure 3.
Amino acid sequence alignment for the Mat1-2 locus in examined strains representing several Aspergillus species. The amino acid positions were determined based on the complete amino acid sequence of A. bombycis NRRL 26010 strain (accession number = OGM45987). Accession numbers recovered from GenBank: A. avenaceus NRRL 517 = HM802955, A. alliaceus NRRL 4181 = HM802964, and A. transmontanensis MUM 10223 = HM802958. Other accession numbers are given in Table S2 except for Aspergillus parvisclerotigenus MACI5 (MF966968). Aspergillus parvisclerotigenus MACI5 species identification was based on genomic sequences from ITS (KY689161), benA (KY628772) and cmdA (KY661269). The A. sergii sequence available from GenBank is shorter.
2.2. Secondary Metabolism Characterization
An analysis of secondary metabolites produced by the four A. korhogoensis strains was performed and the results are summarized in Table 1. Metabolites were identified according to the Metabolomics Standard Initiative level definitions [24]. Metabolites were identified at level 1 when they displayed the same retention time, and UV and MS/MS spectra as the authentic standard. They were identified at level 2 when the metabolites shared the same UV spectrum and/or the same MS/MS fragmentation pattern in accordance with the literature.
Table 1.
Principal secondary metabolites produced by Aspergillus korhogoensis.
| Metabolite | Elemental Composition | m/z | Ion | Retention Time (min) | MS/MS | Error (ppm) | ID Level * | References |
| AFLATOXIN BIOSYNTHESIS PATHWAY | ||||||||
| Aflatoxin B1 | C17H12O6 | 313.07 | [M + H]+ | 17.37 | 285 (100), 298, 284, 270, 257, 243, 229 | −0.398 | 1, 2 | [25] |
| Aflatoxin B2 | C17H14O6 | 315.07 | [M + H]+ | 14.95 | 297, 287 (100), 259, 269, 273 | −5.920 | 1, 2 | [25] |
| Aflatoxin G1 | C17H12O7 | 329.08 | [M + H]+ | 15.25 | 311 (100), 301, 300, 283, 243 | −0.119 | 1, 2 | [25] |
| Aflatoxin G2 | C17H14O7 | 331.08 | [M + H]+ | 12.84 | 313 (100), 303, 285, 275, 257, 245 | −0.511 | 1, 2 | [25] |
| O-methyl-sterigmatocystin | C19H14O6 | 339.08 | [M + H]+ | 24.21 | 324 (100), 311, 306, 295 | 2.817 | 1 | |
| Sterigmatocystin | C18H12O6 | 325.07 | [M + H]+ | 33.59 | 310 (100), 297, 282 | 0.570 | 1, 2 | [26] |
| Versicolorin A | C18H10O7 | 337.03 | [M − H]− | 35.95 | 309 (100), 319, 308, 293, 265, 253 | −2.094 | 1, 2 | [27] |
| Versicolorin B | C18H12O7 | 339.05 | [M − H]− | 34.40 | 311 (100) 310, 309, 295, 297, 283 | −0.578 | 1, 2 | [27] |
| Norsolorinic acid | C20H18O7 | 369.10 | [M − H]− | 42.07 | 351 (100), 341, 325, 308, 297, 270 | 1.528 | 1 | |
| CYCLOPIAZONIC ACID BIOSYNTHETIC PATHWAY | ||||||||
| α-cyclopiazonic acid | C20H20N2O3 | 337.15 | [M + H]+ | 36.77 | 182 (100), 196, 154, 140 | 0.561 | 1, 2 | [28] |
| β-cyclopiazonic acid | C20H22N2O3 | 339.17 | [M + H]+ | 37.58 | 198 (100), 324, 283, 183, 144, 130 | −1.289 | 2 | [28] |
| 2′-oxo-cyclopiazonic acid | C20H20N2O4 | 353.15 | [M + H]+ | 36.20 | 335 (100), 311, 293, 252, 224, 212 | −1.174 | 2 | [28] |
| 3′-hydroxy-speradine A | C21H22 N2O5 | 383.16 | [M + H]+ | 21.19 | 355 (100), 365, 182, 184, 226, 254, 323, 347, 337 | −1.144 | 2 | [28] |
| Speradine C | C20H22 N2O5 | 371.16 | [M + H]+ | 18.19 | 353 (100), 287, 269, 259, 226, 184 | 2.780 | 2 | [28] |
| Speradine D | C20H22 N2O6 | 387.16 | [M + H]+ | 20.80 | 369 (100), 269, 226, 184 | 2.679 | 2 | [28] |
| Speradine F | C21H22 N2O7 | 415.15 | [M + H]+ | 18.99 | 397 (100), 379, 369, 355, 353, 337, 311, 297, 281, 269, 253, 226, 184 | −0.644 | 2 | [28] |
| Cyclopiamide J | C22H24N2O7 | 429.17 | [M + H]+ | 23.96 | 287 (100), 411, 497, 379, 369, 337, 269, 259, 226, 184 | −0.693 | 2 | [28] |
| KOJIC ACID BIOSYNTHETIC PATHWAY | ||||||||
| Kojic acid | C6H6O4 | 143.03 | [M + H]+ | 1.87 | 143 (100) 125, 113, 97 | 1.432 | 1, 2 | [29] |
| AFLATREM BIOSYNTHETIC PATHWAY | ||||||||
| α-aflatrem | C32H39NO4 | 502.29 | [M + H]+ | 41.45 | 444 (100), 484, 426, 412, 376, 198 | 1.144 | ||
| Paspalinine | C27H31NO4 | 434.23 | [M + H]+ | 39.22 | 376 (100), 416, 419, 362, 358, 344, 130 | 0.726 | 2 | [30] |
| Paspaline | C28H39NO2 | 422.31 | [M + H]+ | 43.96 | 130 (100), 404, 407 | −0.583 | 2 | [30] |
| Hydroxyaflatrem | C32H39NO5 | 518.29 | [M + H]+ | 38.22 | 460 (100), 500, 482, 442, 446, 428 | −0.347 | ||
| Paxilline | C27H33NO4 | 436.25 | [M + H]+ | 38.64 | 418 (100), 421, 400, 378, 360, 346, 130 | −2.762 | 1, 2 | [30] |
| 13′-desoxypaxilline | C27H33NO3 | 420.25 | [M + H]+ | 40.31 | 402 (100), 405, 362, 130 | −0.320 | 2 | [30] |
| ASPARASONE BIOSYNTHESIS PATHWAY | ||||||||
| Asparasone A | C18H14O8 | 357.06 | [M − H]− | 22.13 | 339 (100) 299 | 1.315 | 2 | [31] |
| 1,3,4,6,8 pentahydroxy-2-(1′-hydroxy-3′-oxobuty)anthraquinone | C18H14O9 | 373.04 | [M − H]− | 9.36 | 355 (100) 315 | 0.629 | 2 | [31] |
| 1,3,6,8 tetrahydroxy-2-(1′-hydroxyethyl) anthraquinone | C16H1207 | 315.05 | [M − H]− | 27.98 | 297 (100) | 0.775 | 2 | [31] |
| 1,3,6,8 tetrahydroxy-2-(3′ oxobut 1′-en-1′-yl) anthraquinone | C18H1207 | 339.05 | [M − H]− | 29.77 | 297 (100) 321, 296, 295, 311, 306 | 1.428 | 2 | [31] |
| LEPORINS BIOSYNTHESIS PATHWAY | ||||||||
| Leporin B | C22H25NO3 | 352.19 | [M + H]+ | 40.78 | 216 (100), 230, 244, 258, 270, 282, 296, 306 | −1.505 | 2 | [25] |
| Leporin B precursor | C22H25NO2 | 336.20 | [M + H]+ | 37.97 | 200 (100), 214, 228, 242, 254, 266, 280 | 0.102 | 2 | [25] |
| AFLAVARIN BIOSYNTHESIS PATHWAY | ||||||||
| Aflavarin | C24H22O9 | 455.13 | [M + H]+ | 18.22 | 413 (100), 425, 437, 395, 379, 364, 348, 303 | −3.732 | 1, 2 | [32] |
| 7′-demethyl-siderin | C11H10O4 | 207.07 | [M + H]+ | 13.58 | 163 (100), 177, 175, 148, 147, 135, 133, 131, 115, 107 | 0.312 | 2 | [32] |
| Aflavarin precursor 6 | C22H18O8 | 411.11 | [M + H]+ | 20.69 | 369 (100), 381, 379, 352, 343, 337, 279, 207, 177, 147 | −0.569 | 2 | [32] |
| Aflavarin precursor 5 | C23H20O8 | 425.12 | [M + H]+ | 26.75 | 383 (100), 393, 369, 363, 357, 349 | 0.484 | 2 | [32] |
| Aflavarin precursor 4 | C24H22O8 | 439.14 | [M + H]+ | 30.52 | 397 (100), 383, 371, 367, 365, 351, 341, 321 | −0.624 | 2 | [32] |
| AFLAVININE BIOSYNTHESIS PATHWAY | ||||||||
| 20′-hydroxyaflavinine | C28H39O2N | 404.29 | [M − H2O + H]+ | 37.53 | 386 (100), 287, 269, 243, 144, 130 | 0.071 | 1 | |
| Unknown aflavanine | C28H39O2N | 404.29 | [M − H2O + H]+ | 38.14 | 386 (100), 287, 269, 224 | 0.170 | ||
* ID Level 1: Metabolites that displayed the same retention time, UV and MS/MS spectra than the authentic standard. Level 2: Metabolites that displayed the same UV spectrum and/or the same MS/MS fragmentation pattern in accordance with the literature.
All strains produced aflatoxins B1, B2, G1, and G2, as well as several aflatoxin biosynthetic intermediates including 3-O-methylsterigmatocystin, sterigmatocystin and versicolorins A and B. Cyclopiazonic acid (CPA) and different other members of the CPA family were detected in the whole fungal extract of each strain. This new species also produced kojic acid, aflatrem, and its precursors or related compounds (paspaline, paspalinine, paxilline, and 13-desoxypaxilline). Aflatrem and paspaline were also present in the sclerotium extracts, as well as another related compound that appears to be an uncharacterized hydroxylated form of aflatrem ([M + H]+/z = 518.28992; deviation = −0.347 ppm) previously evoked by Nicholson et al. [33]. Leporin B and its precursor were previously detected in culture extracts [25].
The HPLC-DAD analysis of sclerotium extracts revealed the presence of members of at least three families. First, five compounds showed a typical anthraquinone UV spectrum (nm): 223 (100%), 269sh, 293, 319sh, 455. On the bases of UV and MS/MS fragmentation patterns, this compound was identified as asparasone A [31,34]. Three other asparasone-derived anthraquinones were identified in sclerotium extracts (Table 1). Six compounds displayed an aflavarin UV spectrum (nm): 221 (100%), 238sh, 291, 310, 322sh [35]. The LC-HRMS allowed the identification of aflavarin and four aflavarin-associated compounds previously reported by Cary et al. [32]. The last chemical family identified in A. korhogoensis sclerotia were aflavinines [36,37]. Indeed, three compounds with a typical aflavinine UV spectrum (nm): 224 (100%), 283, and 291, were present in the sclerotium extracts of each tested strain. The comparison with the 20-hydroxyaflavinine standard confirmed the presence of this metabolite.
2.3. Taxonomy
Aspergillus korhogoensis A. Carvajal-Campos, A.L. Manizan, S. Tadrist, D.K. Akaki, R. Koffi-Nevry, G.G. Moore, S.O. Fapohunda, S. Bailly, D. Montet, I.P. Oswald, S. Lorber, C. Brabet and O. Puel sp. nov. (Figure 4, Figure 5, Figure 6 and Figure 7).
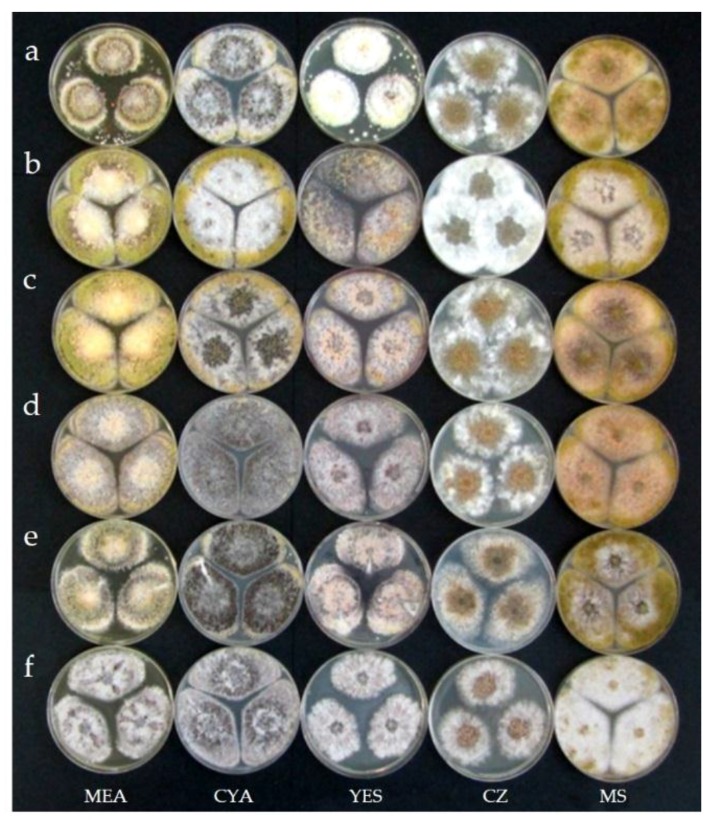
Figure 4.
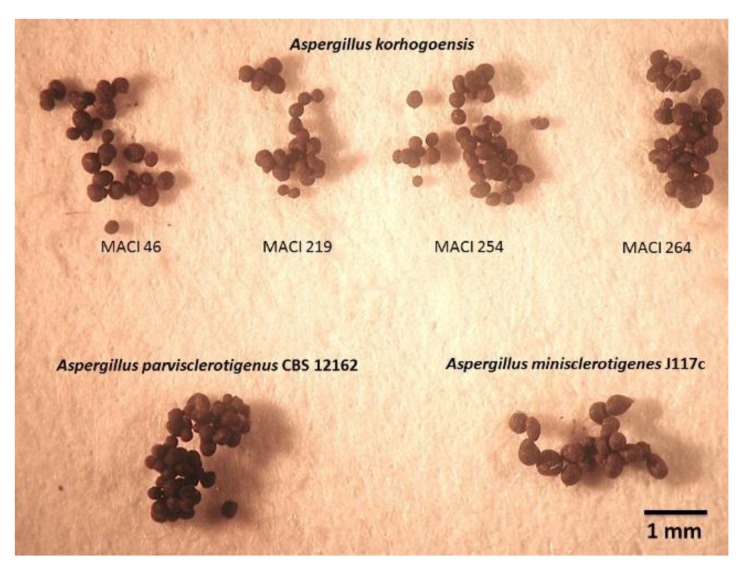
Comparison between cultures of Aspergillus korhogoensis sp. nov. and other species from the A. flavus clade: (a) A. korhogoensis MACI46; (b) A. korhogoensis MACI219; (c) A. korhogoensis MACI254; (d) A. korhogoensis MACI264; (e) A. parvisclerotigenus CBS 121.62; and (f) A. minisclerotigenes J117c. Cultures were grown on MEA, CYA, YES, CZ, and MS at 25 °C for Seven days.
Figure 5.
Comparison between sclerotia of A. korhogoensis sp. nov. and other species from A. flavus clade. Sclerotia recovered from cultures grown on MEA at 25 °C for seven days.
Figure 6.
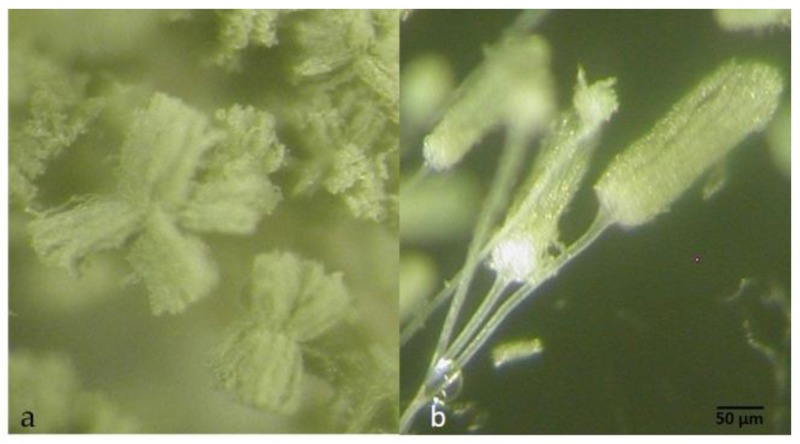
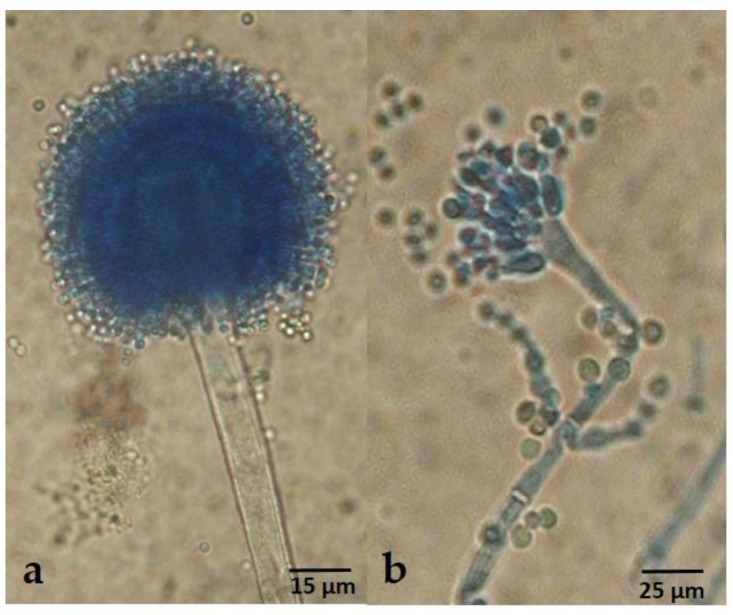
Conidial heads of A. korhogoensis MACI254 (100×): (a) radiate splitting conidial heads; and (b) columnar conidial heads (100×).
Figure 7.
Conidiophores of A. korhogoensis MACI254 (400×): (a) typical conidiophore, radiate and biseriate, mostly observed in basal mycelium; and (b) atypical conidiophore uniseriate, found in aerial mycelium.
Etymology: The specific epithet “korhogoensis” is a noun in the genitive case and refers to the Korhogo region located in the Côte d’Ivoire, from where the new species was isolated.
Diagnosis: Colonies on MEA deeply floccose with a dominant white aerial mycelium. Sporulation dull yellowish green. Abundant sclerotia (especially on MEA and CYA), mostly at the colony surface, small size (<400 µm), dark brown at mature state; conspicuous amber exudate produced by sclerotia. Reverse orange to brownish orange, more conspicuous on MS, and on MEA and CYA presence of concentric rings on orange shades. Conidial heads typically radiate, fertile upper 75% of their surface and splitting, less frequent narrow and long columnar to short columnar, rarely micro-heads. Conidiophores of radiate heads are hyaline, long, large and slightly roughened, whereas conidiophores of columnar heads and micro-heads are short, narrow and smooth. Conidial heads biseriate for radiate heads, and uniseriate for the others. Vesicles oblong to spatulate, 25–47 µm in diam; metulae 6.7–11.2 µm X 4–5.5 µm; phialides 7–10 µm X 3–5.7 µm; conidia yellowish green to green, oblong and smooth or slightly rough, 3–5 µm diam.
Colony diameters: After seven days at 25 °C, colonies reached 37–60 mm on MEA, 59–67 mm on MS, 36–57 mm on YES, and 57–80 mm on CYA. Colonies kept seven days at 37 °C on MEA reached 38–57 mm, whereas colonies kept at 42 °C reached 7.5–12 mm.
Physiological studies: All strains analyzed on AFPA showed a bright orange reverse, a sign of aspergillic acid production. The colonies did not sporulate and presented reduced aerial mycelia. On CREA, the strains showed a positive production of organic acids, except for MACI219.
Extrolite production: Aflatoxins B1, B2, G1, G2, 3-O-methylsterigmatocystin, versicolorins A and B, aspergillic acid, α- and β-CPA, 2 oxo-CPA, aflatrem, paspaline, paspalinine, aflavarins, asparasones, aflavinines, leporin B.
Aspergillus korhogoensis sp. nov. exhibited phenotypic characters that place it within the A. flavus clade, such as conidial heads typically radiate that split into several columns in green shades. Phenotypically, the new species resembled A. parvisclerotigenus. Both species shared several common traits, making difficult to distinguish between them; however, some subtle differences were observed. The new species grew faster on MEA and CYA at 25 °C than A. parvisclerotigenus, and the reverse coloration on MEA and MS was orange for A. korhogoensis and cream for A. parvisclerotigenus. The size of sclerotia was also comparatively smaller in A. korhogoensis. On MEA at 42 °C, A. parvisclerotigenus grew faster (15.5–20 mm) than A. korhogoensis, for which growth was reduced (7.5–12 mm) or inexistent in strain MACI219. On AFPA, A. parvisclerotigenus colonies were mildly to highly floccose, produced profuse sclerotia and conidia in yellowish shades, whereas A. korhogoensis sp. nov. colonies exhibited sparse aerial mycelium and sclerotia, and conidia were almost non-existent.
Holotype: Isolated from Gbandokaha. Deposited in the NRRL collection.
Isolates examined: MACI254 (NRRL 66710), Côte d’Ivoire, Gbandokaha (9°32′ N, 5°33′ W), from peanut pods, 15 November 2014, A.L. Manizan MACI254. MACI46 (NRRL 66708), Côte d’Ivoire, Korhogo (9°29′ N, 6°49′ W), from peanut seeds, 19 November 2014, A.L. Manizan MACI46. MACI219 (NRRL 66709), Côte d’Ivoire, Pokaha (9°24′ N, 5°30′ W), from peanut pods, 15 November 2014, A.L. Manizan MACI219. MACI264 (NRRL 66711), Côte d’Ivoire, Gbandokaha (9°32′ N, 5°33′ W), from peanut pods, 17 November 2014, A.L. Manizan MACI264.
Habitat: Found on peanuts.
Distribution: Korhogo region, North Côte d’Ivoire.
3. Discussion
The number of species in section Flavi, in direct correlation with the number of species capable of producing aflatoxins, has increased over the last decade [17,18,19,23]. Although unable to produce aflatoxins, a 26th species, A. hancockii sp. nov., was very recently identified and grouped with section Flavi species [38]. The use of a polyphasic approach to characterize a species, based on the unified species concept [39,40], has enabled mycologists to acknowledge cryptic diversity in Aspergillus section Flavi. By using this approach, morphological, physiological, and molecular characters are integrated to understand species relationships [12,17,18,19,20,23,41]. It is accepted that the use of a single approach will mask the exact relationships among species, not only in Aspergilli, but also in other fungi [12].
In the present study, we included an ensemble of six genomic regions already tested to be informative for section Flavi (ITS, benA, cmdA, mcm7, rpb1 and amdS). Three other genes (ppgA, preA, preB), reportedly involved in sexual development, were added and the set resulted in a concatenated sequence of 4624 bp. These three genes are required in the specific mating recognition. PreA and preB are MAT target genes that encode a-pheromone and α-pheromone receptors, respectively [42]. PpgA encodes the α-pheromone precursor that binds to PreB [43]. To our knowledge, this is the first study to make a phylogenetic inference that includes genes PreA, PreB and PpgA. Some authors have suggested that the accuracy of the phylogenetic approach could be increased by adding molecular data with different evolutive rates, diminishing possible artifacts caused by polymorphic haplotypes [44,45] and providing more robust information to elucidate potential complexity within a clade. The use of nine concatenated genes resulted in a robust phylogenetic tree topology, which includes the most important species of the section in terms of economic and public health impact, as well as species described in the last decade (not basal taxa). The results of the present study were congruent with several studies performed involving species from section Flavi [17,18,19,20,23]. The tree obtained from nine concatenated genes showed a clear partition of A. novoparasiticus strains in two, one subgroup containing South American isolates and another containing African isolates. More A. novoparasiticus strains isolated from both continents would be needed in order to confirm this observation. Additionally, the ensemble allowed to determine a cryptic species, A. korhogoensis sp. nov.
Most species in section Flavi are considered heterothallic, containing either the MAT1-1 or MAT1-2 idiomorph [15,18,46], and from the present study so is A. korhogoensis. Thus far, A. alliaceus is the only homothallic species in section Flavi [46]. Reportedly, strains of A. nomius may contain both idiomorphs, but only one is functional [11]. Diversity from sexual reproduction in these fungi is expected to arise from out-crossing of heterothallic species, between complementary strains that are able to produce sclerotia [47,48]. Laboratory crosses between sexually compatible strains showed that sexual reproduction was possible in A. parasiticus, A. flavus, and A. nomius [11,49,50]. Inter-specific hybridization was shown to be a possibility via laboratory crosses that resulted in viable ascospores, including recombinant offspring, being produced [51]. Moreover, sexual reproduction is more likely to occur within populations having a 1:1 ratio of both idiomorphs [11,42,48], although asexual reproduction is still a large component to the life cycle of micro-fungi such as the Aspergilli [52]. Presence of both idiomorphs in A. parvisclerotigenus, and A. korhogoensis suggests that cryptic sexuality might occur in natural populations, yet laboratory mating experiments involving these species is necessary to yield conclusions. As well, more population-scale field sampling of strains from these species are necessary to determine if they have a history of recombination as observed in A. flavus and A. parasiticus populations [53,54]. Indeed, the ratio 1:1 is not discernable due to the few strains isolated and curated in different collections.
The present study increases the number of species in the A. flavus clade, which is comprised of many heterothallic species that share common morphological characters, such as biseriate heads, greenish to brownish colony coloration, ability to produce sclerotia, among others [17]. Likewise, species in this clade are capable of producing aflatoxins, aspergillic acid, CPA, kojic acid, versicolorins, aflatrem, etc. [20,55]. Within the clade, the main difference between A. flavus and the remaining species (A. minisclerotigenes, A. parvisclerotigenus and A. korhogoensis sp. nov.), is that A. flavus has lost the ability to produce G-aflatoxins [22]. Another important difference is that A. flavus is comprised of two morphotypes: small sclerotium producers and those that are able to produce larger sclerotia than the other three species. Aspergillus flavus is a ubiquitous fungus, being readily sampled across the globe, but A. minisclerotigenes, A. parvisclerotigenus, and A. korhogoensis sp. nov. have smaller geographic distributions. For example, A. minisclerotigenes has been isolated from Africa, South and North America, Europe, and Australia, whereas A. parvisclerotigenus has been isolated from Guinea Gulf [16], and A. korhogoensis has only been found in the Côte d’Ivoire.
Herein, we proposed that Aspergillus korhogoensis sp. nov. is the sister taxon of A. parvisclerotigenus, based on secondary metabolite analyses, morphology and molecular evidence. Both species share a similar secondary metabolic profile according to Frisvad et al. [9]. However, there are some differences in their secondary metabolite production. Unlike A. parvisclerotigenus, the production of A-30461 (aspirochlorin) was not observed in any A. korhogoensis extracts. On the other hand, A. korhogoensis produced aflavinines, asparasones and leporin B. A pattern close to each other could be appreciated while comparing morphological characters, though subtle differences were observed between strains of both species. On AFPA, A. parvisclerotigenus had a trend to produce yellowish spores and highly floccose colonies, whereas A. korhogoensis sp. nov. tended to have flatter colonies and reduced sporulation. At the molecular level, the concatenation of nine different loci strongly suggested that they were two different species. The inclusion of strains from different populations of A. parvisclerotigenus (Benin, Nigeria and Côte d’Ivoire) reduced the possibility of any artifact linked with polymorphisms.
Sub-Saharan West Africa, and especially the Guinea Gulf, displays an interesting diversity of species from section Flavi, including several cryptic species, although A. flavus continues to be the most frequent species sampled [56]. Despite the prevalence of A. flavus, it is noteworthy that other S-strain species are present at lower rates, are usually G-aflatoxin producers, and their production of aflatoxins is usually higher than that of A. flavus sensu stricto [57,58]. Some strains, previously characterized as A. flavus SBG, are nowadays being classified as A. minisclerotigenes, A. parvisclerotigenus, and in this study as A. korhogoensis. In different countries of the Guinea Gulf, strains exhibiting the SBG chemotype have been associated with drier agroecological zones bordering the Sahara desert [14,59,60]. Cadwell and Cotty [59] suggested that production of G-aflatoxins in Northern Benin could be mainly due to SBG strains. Likewise, in this area, the environmental conditions could allow the presence of species that could be more sensitive to climate changes, resulting in a shift of the frequency of these strains [59]. Inter-specific sex is possible for these fungi, which has been shown via laboratory crosses [51]. All that is required for these strains to override heterokaryon incompatibility, or even species boundaries, is the need to circumvent an unfavorable environmental situation [61]. It may eventually be determined that many of the recently characterized novel species, with such similar morphological, genetic, metabolic and physiological characteristics, are hybrids resulting from cryptic inter-specific sex that comprise a species complex. However, much more research is required before this can be proven or refuted. Moore and co-workers [62,63,64] are sequencing the genomes of aflatoxigenic fungi in an effort to determine the relatedness of these fungi and to elucidate the evolution of aflatoxin production. The comparison of the genomes of species close to A. flavus such as A. minisclerotigenes, A. parvisclerotigenus and A. korhogoensis will also help to understand the genetic determinants of the A. flavus success.
4. Materials and Methods
4.1. Chemicals
Solvents (phenol, chloroform, ethanol, ethyl acetate, methanol, and acetonitrile) of analytical grade used in the extraction and high-performance liquid chromatography (HPLC) were obtained from ThermoFisher Scientific (Illkirch, France). Ultrapure water used for HPLC with Diode Array Detector (DAD), LC/MS analyses, and for molecular biology experiments was purified from a MilliQ purification system (Millipore, Billerica, MA, USA). Unless otherwise specified, chemicals were purchased from Sigma-Aldrich (Saint Quentin Fallavier, France).
4.2. Fungal Isolates and Culture Conditions
Seven atypical SBG A. flavus strains were isolated in 2014 from peanuts in the Northern Korhogo region of Côte d’Ivoire. Four other atypical SBG strains isolated from food, decaying leaves, and logs of wood collected in Southwest Nigeria [65] were added. To identify these eleven SBG strains to species level, we compared them against a dataset comprised of strains obtained from different international collections and stored under controlled conditions at Research Center in Food Toxicology TOXALIM, Toulouse. We included at least one strain belonging to most species within the section Flavi (Table 2). The isolates were cultured on Malt Extract Agar (MEA) (Biokar Diagnostics, Allone, France) at 25 °C for seven days, and stored as spore suspensions on 20% glycerol for further analyses. The A. korhogoensis Type strain, along with three other strains, were deposited at Agricultural Research Service Culture Collection (NRRL) (Peoria, IL, USA).
Table 2.
Aspergillus isolates used in this study.
| Strain | Sampling Data | Reference | |
| Substrate | Country | ||
| A. arachidicola | |||
| CBS 117610T = IBT 25020 | Arachis glabatra leaf | Argentina | [20] |
| CBS 117614 = IBT 27183 | Arachis glabatra leaf | Argentina | [20] |
| A. bertholletius | |||
| CCT 7615T | Soil near Bertholletia excelsa trees | Brazil | [19] |
| A. bombycis | |||
| NRRL 26010T = CBS 117187 | Frass, silkworm rearing house | Japan | [66] |
| A. caelatus | |||
| NRRL 25528T = ATCC 201128 = CBS 763.97 = JCM 10151 | Peanut field soil | Georgia, USA | Horn B.W., National Peanut Lab, Dawson, GA (in NRRL database) |
| A. flavus | |||
| NRRL 3518 | Wheat flour | Illinois, USA | Graves NRRL isolate (in NRRL database) |
| NRRL 4818 = CBS 16870 | Food, butter | USA | Fennell D.I., University of Wisconsin, Madison, Wisconsin (in NRRL database) |
| NRRL 3357 = CBS 128202 | Peanut cotyledons | USA | [67] |
| A. minisclerotigenes | |||
| CBS 117635T | Arachis hypogaea seed | Argentina | [20] |
| NRRL 29000 | Peanut soil | Australia | Geiser D., Pennsylvania State University (in [21]) |
| E21 | Cumin | Morocco | [15] |
| E44 | White pepper | Morocco | [15] |
| E74 | Paprika | Morocco | [15] |
| A. mottae | |||
| MUM 10.231T = CBS 130016 | Maize seed | Portugal | [18] |
| A. nomius | |||
| NRRL 13137T = CBS 260.88 | Wheat | Illinois, USA | Schindler A.F., FDA, Washington D.C. (in NRRL database) |
| A. novoparasiticus | |||
| CBS 126849T = LEMI 250 | Sputum, leukemic patient | São Paulo, Brazil | [23] |
| LEMI 149 | Hospital air | São Paulo, Brazil | [23] |
| LEMI 267 | Sputum, leukemic patient | São Paulo, Brazil | [23] |
| AFc31 = NRRL 62794 | Cassava | Benin | [14] |
| AFc32 = NRRL 62795 | Cassava | Benin | [14] |
| A. oryzae | |||
| CBS 100925T = IMI 16266 = NRRL 447 | Unknown source | Japan | [17] |
| RIB40 | Cereal (broad bean) | Kyoto, Japan | [68] |
| A. parasiticus | |||
| CBS 100926T | Pseudococcus calceolariae, sugar cane mealy bug | Hawaii, USA | [17] |
| NRRL 492 | Unknown source | China | [23] |
| A. parvisclerotigenus | |||
| CBS 121.62T | Arachis hypogea | Nigeria | [9] |
| AFc36 = NRRL 62796 | Cassava | Benin | [14] |
| MACI8 | Peanuts | Côte d’Ivoire | This study |
| MACI221 | Peanuts | Côte d’Ivoire | This study |
| MACI258 | Peanuts | Côte d’Ivoire | This study |
| SF1 | Rain forest soil | Nigeria | [65] |
| SF3 | Rain forest soil | Nigeria | [65] |
| SF6 | Rain forest soil | Nigeria | [65] |
| SF9 | Food item | Nigeria | [65] |
| A. pseudocaelatus | |||
| CBS 117616T | Arachis burkartii leaf | Argentina | [17] |
| A. pseudotamarii | |||
| NRRL 443 | Soil | Brazil | [69] |
| NRRL 25518 | Tea field soil | Miyazaki, Japan | [70] |
| A. sergii | |||
| MUM 10.219T = CBS 130017 | Almond shell | Portugal | [18] |
| A. sojae | |||
| CBS 100928T | Soy sauce | Japan | [17] |
| A. transmontanensis | |||
| MUM 10.214T = CBS 130015 | Almond shell | Portugal | [18] |
| A. korhogoensis sp. nov. | |||
| MACI254T | Peanuts | Côte d’Ivoire | This study |
| MACI46 | Peanuts | Côte d’Ivoire | This study |
| MACI219 | Peanuts | Côte d’Ivoire | This study |
| MACI264 | Peanuts | Côte d’Ivoire | This study |
CBS, Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands; NRRL: National Center for Agricultural Utilization Research, U.S. Department of Agriculture, Peoria, IL, USA; LEMI: Laboratório Especial de Micologia, São Paulo, Brazil; MUM: Micoteca da Universidade de Minho, Braga, Portugal; CCT: Coleção de Cultura Tropical, Campinas, Brazil; SF: Southern Regional Research Center, U.S. Department of Agriculture, New Orleans, USA.
4.3. DNA Extraction and Amplification
A loopful of spores from each of the examined Aspergillus isolates was inoculated on Yeast Extract Sucrose (YES) liquid medium, and kept in agitation in an orbital incubator at 170 rpm at 27 °C for five days. DNA extraction was performed according to Girardin et al. [71] by grinding a portion of mycelium in a 5 mL mortar on ice, followed by the addition of 5.5 mL lysis buffer 2 (5 mL Tris-HCL 1 M, 3.65 g NaCl, 12.5 mL EDTA 0.5 M pH 8, 2.5 g SDS, H2O qsp 250 mL). The content was transferred to a 15 mL tube, and 12.5 µL of proteinase K were added, before being incubated from 30 min to 1 h at 37 °C, and then incubated for 10 min at 65 °C. Afterwards, one volume of phenol/chloroform (7:3, v/v) was added and samples were then centrifuged at 3000× g for 1 h. The supernatant was recovered into a new tube, where 6 µL RNAse were added, and it was subsequently incubated for 2–3 h at 37 °C. Next, one volume of chloroform was added and centrifuged at 3000× g for 10 min. The supernatant was recovered into a new tube and one volume of isopropanol was added. At this point, samples were softly shaken for 2 h in a horizontal shaker and kept overnight at 4 °C. The following day, samples were centrifuged at 10,000× g for 30 min. The supernatant was eliminated and the pellet carefully washed with 300 µL of 70% ethanol, and centrifuged at 10,000× g for 15 min, followed by a gentle aspiration of the supernatant. Finally, the pellet was resuspended with 30 µL of pure water. DNA of samples was quantified using a NanoDrop ND-1000 (NanoDrop Technologies, Wilmington, DE, USA).
4.4. Amplification and Sequencing of Genomic Loci
Genes were amplified as follows: (1) pre-denaturation at 94 °C for 5 min; (2) denaturation at 94 °C for 45 s; (3) annealing at 55–57.3 °C for 1 min (55 °C = ITS, benA, cmdA, mcm7, preB and preA; 56 °C = ppgA; 57.3 °C = amdS and rpb1); (4) extension at 72 °C for 1 min (Steps 2 to 4 were carried out for 40 cycles); (5) final extension at 72 °C for 10 min; and (6) final temperature hold at 4 °C. Primers used in the study are shown in Table S1. Polymerase Chain Reaction (PCR) amplifications were performed in a C1000 TouchTM thermal cycler (BioRad, Marnes-la-Coquette, France). PCR amplicons were purified with GeneEluteTM PCR Clean-Up Kit (Sigma-Aldrich, Saint Quentin Fallavier, France). Sanger sequences were obtained by using the Applied Biosystems Big Dye Terminator v3.1 chemistry (ThermoFisher Scientific, Illkirch, France), they were then purified with the Applied Biosystems Big Dye XTerminator protocol (Thermofisher Scientific, Illkirch, France) and finally processed on the ABI 3130xl Genetic Analyzer (Thermofisher Scientific, Illkirch, France), available on the GeT-Purpan technological facility (Genome and Transcriptome, GenoToul, Toulouse, France). New sequences were deposited in GenBank and accession numbers are reported in Table S2. Sequence data of some isolates, obtained from previously accessioned data in the GenBank database, were included for constructing phylogenetic trees (Table S2).
4.5. Alignment, Model Selection, and Molecular Analyses
Data were assembled, aligned and trimmed in BioEdit/ClustalW (http://www.mbio.ncsu.edu/bioedit/bioedit.html). Gene regions with multiple gaps were aligned to minimize indels and optimize nucleotide identities among different strains. Sequences from multiple genomic regions were concatenated using Mesquite v3.2 [72], but the mating type loci were analyzed independently. For concatenated data, the best-fit nucleotide substitution models and partitioning scheme were chosen using PartitionFinder v2.0.0 [73] under BIC. To search for the best-fit scheme, a greedy algorithm with linked branch lengths of alternative partitions was used. Partitions obtained consisted of four subsets that corresponded to a specific model (noted in parentheses): Subset 1 included ppgA, cmdA, benA, rpb1 and mcm7 = 2129 bp (K80+G); Subset 2 included ITS = 778 bp (TRNEF+I); Subset 3 included preB and preA = 1223 bp (HKY+G); and Subset 4 included amdS = 491 bp (K80+G).
Bayesian inference statistical methods were used to obtain tree topologies for concatenated data, using the best-fit substitution models listed above. For Bayesian analyses, MrBayes v3.2 [74] was used, and four independent runs were carried out for 107 generations, each with four chains, Markov Chain Monte Carlo, and sampling every 103 generations. We confirmed for each analysis that the average standard deviation of split frequencies between chains approached values of ≤0.01, and the potential scale factor reduction factor (PSRF) to 1. For all the analyses, and from the total number of trees per run, 25% were arbitrarily discarded as “burn-in”. The remaining trees were used to calculate posterior probabilities (PP) for each bipartition in a 50% majority-rule consensus tree using Tracer v1.6 [75]. Phylogenetic trees were visualized and edited with FigTree v1.4.2 [76].
4.6. Morphological and Physiological Studies
Morphological and growth analyses were carried on MEA, High Salt MEA (MS) (MEA complemented with NaCl 60 g/L), Czapek agar (CZ) (Oxoid, Dardly, France), Czapek Yeast Autolysate agar (CYA), YES agar. Physiological analyses were carried out on creatine agar (CREA) [77], and Aspergillus flavus/parasiticus agar (AFPA) [78]. Strains MACI46 (NRRL 66708), MACI219 (NRRL 66709), MACI254 (NRRL 66710), MACI264 (NRRL 66711), A. parvisclerotigenus CBS 121.62 and A. minisclerotigenes J117e were used for the analyses on MS, CYA, CZ, YES, and CREA. In addition, four strains belonging to A. parvisclerotigenus were included for the analyses on MEA and AFPA: MACI8, MACI258, MACI221, and AFc36.
Cultures on MEA, MS, CZ, CYA, YES, CREA, and AFPA were seeded with three calibrated inoculates of 500 spores, and incubated in the dark at 25 °C for seven and ten days. Macroscopic characters were observed with a stereomicroscope SZX9—X12-120 (Olympus, Rungis, France). Microscopic characters were observed on MEA at 7 and 10 days using a microscope CX41—X400 and X1000 (Olympus, Rungis, France). In addition, growth analyses were calculated from MEA cultures, which were centrally inoculated with 103 spores, and incubated at 25 °C, 37 °C and 42 °C for seven days [17,79,80].
4.7. LC/MS Secondary Metabolic Characterization
4.7.1. Secondary Metabolic Characterization of Whole Fungal Culture
Pre-cultures of strains MACI46 (NRRL 66708), MACI219 (NRRL 66709), MACI254 (NRRL 66710) and MACI264 (NRRL 66711) incubated in the dark at 27 °C for seven days. For metabolite profile characterization, isolates were cultured in four different media: MEA, CYA, YES agar, and Potato Dextrose Agar medium (PDA) (Sigma-Aldrich, Saint Quentin Fallavier, France). For each medium, three biological replicates were inoculated centrally with 10 µL of calibrated spore suspensions (105 spores/mL) prepared from seven-day cultures on 7.5 cm Petri dishes. The samples were incubated in the dark at 27 °C for seven days.
To perform extrolite extractions, culture media were macerated and placed in 50 mL sterilized tubes, and thereafter 35 mL of ethyl acetate was added to each sample. Samples were agitated 48 h in an orbital incubator at 170 rpm at room temperature. Ethyl acetate was filtered through a Whatman 1PS phase separator (GE Healthcare Life Sciences, Vélizy-Villacoublay, France) and evaporated at 60 °C until dry. Samples were then dissolved in 400 µL of methanol. To eliminate possible impurities, each sample was filtered through a 0.45 µm disk filter (ThermoFisher Scientific, Illkirch, France) [81].
4.7.2. Secondary Metabolic Characterization of Sclerotia
The isolates were cultured on MEA whereby a loopful of spores was taken from seven-day cultures and streaked onto 9 cm Petri dishes. The MEA samples were incubated in the dark at 27 °C for eight days. To recover sclerotia from culture media, 10 mL of 0.01% Triton-X solution were added to each Petri dish. Sclerotia were gently scraped and transferred into 15 mL tubes. To remove mycelium and conidium debris, 10 mL of 0.01% Triton-X were added to each tube and homogenized in a vortex. Once the sclerotia precipitated, the supernatant was discarded. This step was carried out 4 to 5 times to eliminate possible residual debris [82].
Sclerotia were transferred into a 5 mL mortar. Then 5 mL of ethyl acetate were gently added while grinding the sclerotia. This step was carried out three times. Next, 5 mL of chloroform were gently added and the same procedure followed. This step was repeated three times. Samples were evaporated at 60 °C until dry, and resuspended in 0.5 mL solution methanol/acetonitrile/H2O qsp (30:30:40, v/v/v) [31,82]. To remove possible impurities, samples were filtered through 0.45 µm disk filters (ThermoFisher Scientific, Illkirch, France).
4.7.3. Secondary Metabolic Analysis
Extrolite analyses were carried out on a HPLC apparatus coupled to an LTQ Orbitrap XL high-resolution mass spectrometer (HRMS) (ThermoFisher Scientific, Illkirch, France). Extracted samples contained 10 µL of each replicate diluted with 170 µL methanol. For the analyses, 10 µL of each sample were injected into a reverse-phase 5 µm Luna C18 column (150 mm × 2.0 mm) (Phenomenex, Torrance, CA, USA) operated at a flow rate of 0.2 mL/min. A gradient program of 0.1% formic acid in water (phase A) and 100% acetonitrile (phase B) was executed as follows: 0 min 20% B, 30 min 50% B, from 35 to 45 min 90% B, from 50 to 60 min 20% B. HRMS acquisitions were obtained by electrospray ionization (ESI) in the positive and negative modes under the subsequent parameters: (1) positive mode: spray voltage + 4.5 kV, capillary temperature 350 °C, sheath gas (N2) flow rate 40 au (arbitrary units), auxiliary gas (N2) flow rate 6 au; and (2) negative mode: spray voltage—3.7 kV, capillary temperature 350 °C, sheath gas (N2) flow rate 30 au, auxiliary gas (N2) flow rate 10 au. Full MS spectra were accomplished at a resolution of 60,000 with a mass-to-charge ratio (m/z) range 50–800. The MS/MS spectra were generated by collision-induced dissociation (CID) according the following parameters: collision energy = 35 eV, resolution = 7500, isolation width = 1.5 Da, activation Q = 0.250, and activation time = 30 ms.
Acknowledgments
The authors would like to sincerely thank J.C. Frisvad for providing us the strain of Aspergillus bertholletius CCT 7615. Aflavarin standard was a generous gift from J. Diana Di Mavungu. The isolation of the four A. korhogoensis spp. nov. and three A. parvisclerotigenus strains from peanuts in Korhogo region, Côte d’Ivoire is resulting from the EU project 3CIvoire (EuropeAid/129596/L/ACT/CI DCI-NSAPVD/2010/64), and was also supported by the Service of co-operation and cultural action of the French Embassy in Côte d’Ivoire. The views expressed are not necessarily those of the EU. This work was funded by Aflafree (ANR-11-ALID-0003). Doctoral studies of Amaranta Carvajal-Campos were funded by the Secretaría de Educación Superior, Ciencia, Tecnología e Inovación (SENESCYT), Ecuador. We would like to thank H. Gryta for helpful advice on phylogeny.
Supplementary Materials
The following are available online at www.mdpi.com/2072-6651/9/11/353/s1, Table S1: Primers used to amplify multiple genomic regions within Aspergillus species, Table S2: Isolates examined and accession numbers deposited in GenBank.
Author Contributions
Isabelle P. Oswald, Olivier Puel, Sophie Lorber and Catherine Brabet conceived, supervised, and designed the experiments. Amaranta Carvajal-Campos performed the experiments, analyzed the data and contributed to experiment design. Souria Tadrist performed experiments. Ama Lethicia Manizan, David Koffi Akaki and Rose Koffi-Nevry performed the fieldwork in Côte d’Ivoire. Ama Lethicia Manizan isolated the new species strains from the samples of Côte d’Ivoire. Geromy G. Moore and Stephen O. Fapohunda isolated the strains of A. parvisclerotigenus from Nigeria. Sylviane Bailly performed morphological analyses. Olivier Puel performed the HPLC-DAD and LC-MS analyzes. Didier Montet coordinated the partnership between Côte d’Ivoire and France. Amaranta Carvajal-Campos, Geromy G. Moore, Sophie Lorber and Olivier Puel wrote the paper.
Conflicts of Interest
The authors declare that they have no competing interest. The funding sponsors had no role in the design of the project; collection, analyses or data interpretation; in writing the manuscript; or in the decision to publish the results.
References
- 1.Horn B.W. Ecology and Population Biology of Aflatoxigenic Fungi in Soil. Toxin Rev. 2003;22:351–379. doi: 10.1081/TXR-120024098. [DOI] [Google Scholar]
- 2.Mitchell N.J., Bowers E., Hurburgh C., Wu F. Potential economic losses to the US corn industry from aflatoxin contamination. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2016;33:540–550. doi: 10.1080/19440049.2016.1138545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu F., Groopman J.D., Pestka J.J. Public health impacts of foodborne mycotoxins. Annu. Rev. Food Sci. Technol. 2014;5:351–372. doi: 10.1146/annurev-food-030713-092431. [DOI] [PubMed] [Google Scholar]
- 4.Azziz-Baumgartner E., Lindblade K., Gieseker K., Rogers H.S., Kieszak S., Njapau H., Schleicher R., McCoy L.F., Misore A., DeCock K., et al. Aflatoxin Investigative Group. Case-control study of an acute aflatoxicosis outbreak, Kenya, 2004. Environ. Health Perspect. 2005;113:1779–1783. doi: 10.1289/ehp.8384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cano P., Puel O., Oswald I.P. Mycotoxins: Fungal Secondary Metabolites with Toxic Properties. In: Deshmukh S.K., Misra J.K., Tewari J.P., Papp T., editors. Fungi: Applications and Management Strategies. CRC Press; Boca Raton, FL, USA: 2016. pp. 318–371. [Google Scholar]
- 6.Bennett J.W., Klich M. Mycotoxins. Clin. Microbiol. Rev. 2003;16:497–516. doi: 10.1128/CMR.16.3.497-516.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Ruyck K., De Boevre M., Huybrechts I., De Saeger S. Dietary mycotoxins, co-exposure, and carcinogenesis in humans: Short review. Mutat. Res. Rev. Mutat. Res. 2015;766:32–41. doi: 10.1016/j.mrrev.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Bartoli A., Maggi O. Four new species of Aspergillus from Ivory Coast soil. Trans. Br. Mycol. Soc. 1978;71:383–394. doi: 10.1016/S0007-1536(78)80064-3. [DOI] [Google Scholar]
- 9.Frisvad J.C., Skouboe P., Samson R.A. Taxonomic comparison of three different groups of aflatoxin producers and a new efficient producer of aflatoxin B1, sterigmatocystin and 3-O-methylsterigmatocystin, Aspergillus rambellii sp. nov. Syst. Appl. Microbiol. 2005;28:442–453. doi: 10.1016/j.syapm.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 10.Abe K., Gomi K., Hasegawa F., Machida M. Impact of Aspergillus oryzae genomics on industrial production of metabolites. Mycopathologia. 2006;162:143–153. doi: 10.1007/s11046-006-0049-2. [DOI] [PubMed] [Google Scholar]
- 11.Horn B.W., Ramirez-Prado J.H., Carbone I. The sexual state of Aspergillus parasiticus. Mycologia. 2009;101:275–280. doi: 10.3852/08-205. [DOI] [PubMed] [Google Scholar]
- 12.Samson R.A., Varga J. What is a species in Aspergillus? Med. Mycol. 2009;47:S13–S20. doi: 10.1080/13693780802354011. [DOI] [PubMed] [Google Scholar]
- 13.Cotty P.J., Bhatnagar D. Variability among atoxigenic Aspergillus flavus strains in ability to prevent aflatoxin contamination and production of aflatoxin biosynthetic pathway enzymes. Appl. Environ. Microbiol. 1994;60:2248–2251. doi: 10.1128/aem.60.7.2248-2251.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adjovi Y.C.S., Bailly S., Gnonlonfin B.J.G., Tadrist S., Querin A., Sanni A., Oswald I.P., Puel O., Bailly J.D. Analysis of the contrast between natural occurence of toxigenic Aspergilli of the Flavi section and aflatoxin B1 in cassava. Food Microbiol. 2014;38:151–159. doi: 10.1016/j.fm.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 15.El Mahgubi A.E., Puel O., Bailly S., Tadrist S., Querin A., Ouadia A., Oswald I.P., Bailly J.D. Distribution and toxigenicity of Aspergillus section Flavi in spices marketed in Morocco. Food Control. 2013;32:143–148. doi: 10.1016/j.foodcont.2012.11.013. [DOI] [Google Scholar]
- 16.Perrone G., Gallo A., Logrieco A.F. Biodiversity of Aspergillus section Flavi in Europe in relation to the management of aflatoxin risk. Front. Microbiol. 2014;5:377. doi: 10.3389/fmicb.2014.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Varga J., Frisvad J.C., Samson R.A. Two new aflatoxin producing species, and an overview of Aspergillus section Flavi. Stud. Mycol. 2011;69:57–80. doi: 10.3114/sim.2011.69.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soares C., Rodrigues P., Peterson S.W., Lima N., Venâncio A. Three new species of Aspergillus section Flavi isolated from almonds and maize in Portugal. Mycologia. 2012;104:682–697. doi: 10.3852/11-088. [DOI] [PubMed] [Google Scholar]
- 19.Taniwaki M.H., Pitt J.I., Iamanaka B.T., Sartori D., Copetti M.V., Balajee A., Fungaro M.H.P., Frisvad J.C. Aspergillus bertholletius sp. nov. from Brazil Nuts. PLoS ONE. 2012;7:e42480. doi: 10.1371/journal.pone.0042480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pildain M.B., Frisvad J.C., Vaamonde G., Cabral D., Varga J., Samson R.A. Two novel aflatoxin-producing Aspergillus species from Argentinean peanuts. Int. J. Syst. Evol. Microbiol. 2008;58:725–735. doi: 10.1099/ijs.0.65123-0. [DOI] [PubMed] [Google Scholar]
- 21.Rodrigues P., Santos C., Venâncio A., Lima N. Species identification of Aspergillus section Flavi isolates from Portuguese almonds using phenotypic, including MALDI-TOF ICMS, and molecular approaches. J. Appl. Microbiol. 2011;111:877–892. doi: 10.1111/j.1365-2672.2011.05116.x. [DOI] [PubMed] [Google Scholar]
- 22.Ehrlich K.C., Chang P.-K., Yu J., Cotty P.J. Aflatoxin biosynthesis cluster gene cypA is required for G aflatoxin formation. Appl. Environ. Microbiol. 2004;70:6518–6524. doi: 10.1128/AEM.70.11.6518-6524.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonçalves S.S., Stchigel A.M., Cano J.F., Godoy-Martinez P.C., Colombo A.L., Guarro J. Aspergillus novoparasiticus: A new clinical species of the section Flavi. Med. Mycol. 2012;50:152–160. doi: 10.3109/13693786.2011.593564. [DOI] [PubMed] [Google Scholar]
- 24.Sumner L.W., Amberg A., Barrett D., Beale M.H., Beger R., Daykin C.A., Fan T.W.-M., Fiehn O., Goodacre R., Griffin J.L., et al. Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI) Metabolomics. 2007;3:211–221. doi: 10.1007/s11306-007-0082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arroyo-Manzanares N., Di Mavungu J.D., Uka V., Malysheva S.V., Cary J.W., Ehrlich K.C., Vanhaecke L., Bhatnagar D., De Saeger S. Use of UHPLC high-resolution Orbitrap mass spectrometry to investigate the genes involved in the production of secondary metabolites in Aspergillus flavus. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2015;32:1656–1673. doi: 10.1080/19440049.2015.1071499. [DOI] [PubMed] [Google Scholar]
- 26.Paguigan N.D., El-Elimat T., Kao D., Raja H.A., Pearce C.J., Oberlies N.H. Enhanced dereplication of fungal cultures via use of mass defect filtering. J. Antibiot. 2017;70:553–561. doi: 10.1038/ja.2016.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jakšić D., Puel O., Canlet C., Kopjar N., Kosalec I., Klarić M.Š. Cytotoxicity and genotoxicity of versicolorins and 5-methoxysterigmatocystin in A549 cells. Arch. Toxicol. 2012;86:1583–1591. doi: 10.1007/s00204-012-0871-x. [DOI] [PubMed] [Google Scholar]
- 28.Uka V., Moore G.G., Arroyo-Manzanares N., Nebija D., De Saeger S., Di Mavungu J.D. Unravelling the diversity of the cyclopiazonic acid family of mycotoxins in Aspergillus flavus by UHPLC Triple-TOF HRMS. Toxins. 2017;9:35. doi: 10.3390/toxins9010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang J., Liu Y., Ding T., Zhang X., Chen H., Shen C., Wu B., Niu W. Determination of kojic acid in foods using high performance liquid chromatography-tandem mass spectrometry. Chin. J. Chromatogr. 2012;30:578–583. doi: 10.3724/SP.J.1123.2012.02002. [DOI] [PubMed] [Google Scholar]
- 30.Uhlig S., Egge-Jacobsen W., Vrålstad T., Miles C.O. Indole–diterpenoid profiles of Claviceps paspali and Claviceps purpurea from high-resolution Fourier transform Orbitrap mass spectrometry. Rapid Commun. Mass Spectrom. 2014;28:1621–1634. doi: 10.1002/rcm.6938. [DOI] [PubMed] [Google Scholar]
- 31.Malysheva S.V., Arroyo-Manzanares N., Cary J.W., Ehrlich K.C., Vanden Bussche J., Vanhaecke L., Bhatnagar D., Di Mavungu J.D., De Saeger S. Identification of novel metabolites from Aspergillus flavus by high resolution and multiple stage mass spectrometry. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2014;31:111–120. doi: 10.1080/19440049.2013.859743. [DOI] [PubMed] [Google Scholar]
- 32.Cary J.W., Han Z., Yin Y., Lohmar J.M., Shantappa S., Harris-Coward P.Y., Mack B., Ehrlich K.C., Wei Q., Arroyo-Manzanares N., et al. Transcriptome analysis of Aspergillus flavus reveals veA-dependent regulation of secondary metabolite gene clusters, including the novel aflavarin cluster. Eukaryot. Cell. 2015;14:983–997. doi: 10.1128/EC.00092-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nicholson M.J., Koulman A., Monahan B.J., Pritchard B.L., Payne G.A., Scott B. Identification of two aflatrem biosynthesis gene loci in Aspergillus flavus and metabolic engineering of Penicillium paxilli to elucidate their function. Appl. Environ. Microbiol. 2009;75:7469–7481. doi: 10.1128/AEM.02146-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sobolev V.S., Cole R.J., Dorner J.W., Horn B.W., Harrigan G.G., Gloer J.B. Isolation and Structure Elucidation of a New Metabolite Produced by Aspergillus parasiticus. J. Nat. Prod. 1997;60:847–850. doi: 10.1021/np970131m. [DOI] [Google Scholar]
- 35.TePaske M.R., Gloer J.B., Wicklow D.T., Dowd P.F. Aflavarin and β-Aflatrem: New Anti-Insectan Metabolites from the Sclerotia of Aspergillus flavus. J. Nat. Prod. 1992;55:1080–1086. doi: 10.1021/np50086a008. [DOI] [Google Scholar]
- 36.Gloer J.B., TePaske M.R., Sima J.S., Wicklow D.T., Dowd P.F. Antiinsectan aflavinine derivative from sclerotia of Aspergillus flavus. J. Org. Chem. 1988;53:5457–5460. doi: 10.1021/jo00258a011. [DOI] [Google Scholar]
- 37.TePaske M.R., Gloer J.B., Wicklow D.T., Dowd P.F. Three new aflavinines from the sclerotia of Aspergillus tubingensis. Tetrahedron. 1989;45:4961–4968. doi: 10.1016/S0040-4020(01)81077-2. [DOI] [Google Scholar]
- 38.Pitt J.I., Lange L., Lacey A.E., Vuong D., Midgley D.J., Greenfield P., Bradbury M.I., Lacey E., Busk P.K., Pilgaard B., et al. Aspergillus hancockii sp. nov., a biosynthetically talented fungus endemic to southeastern Australian soils. PLoS ONE. 2017;12:e0170254. doi: 10.1371/journal.pone.0170254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Queiroz K. Species concepts and species delimitation. Syst. Biol. 2007;56:879–886. doi: 10.1080/10635150701701083. [DOI] [PubMed] [Google Scholar]
- 40.Giraud T., Refrégier G., Le Gac M., de Vienne D.M., Hood M.E. Speciation in fungi. Fungal Genet. Biol. 2008;45:791–802. doi: 10.1016/j.fgb.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 41.Bosso L., Lacatena F., Varlese R., Nocerino S., Cristinzio G., Russo D. Plant pathogen but not antagonists change in soil fungal communities across a land abandonment gradient in a Mediterranean landscape. Acta Oecol. (Montrouge) 2017;78:1–6. doi: 10.1016/j.actao.2016.11.002. [DOI] [Google Scholar]
- 42.Dyer P.S., Kück U. Sex and the Imperfect Fungi. Microbiol. Spectr. 2017;5 doi: 10.1128/microbiolspec.FUNK-0043-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dyer P.S., Paoletti M., Archer D.B. Genomics reveals sexual secrets of Aspergillus. Microbiology. 2003;149:2301–2303. doi: 10.1099/mic.0.C0119-0. [DOI] [PubMed] [Google Scholar]
- 44.Taylor J.W., Jacobson D.J., Kroken S., Kasuga T., Geiser D.M., Hibbett D.S., Fisher M.C. Phylogenetic Species Recognition and Species Concepts in Fungi. Fungal Genet. Biol. 2000;31:21–32. doi: 10.1006/fgbi.2000.1228. [DOI] [PubMed] [Google Scholar]
- 45.Dupuis J.R., Roe A.D., Sperling F.A.H. Multi-locus species delimitation in closely related animals and fungi: one marker is not enough. Mol. Ecol. 2012;21:4422–4436. doi: 10.1111/j.1365-294X.2012.05642.x. [DOI] [PubMed] [Google Scholar]
- 46.Ramirez-Prado J.H., Moore G.G., Horn B.W., Carbone I. Characterization and population analysis of the mating-type genes in Aspergillus flavus and Aspergillus parasiticus. Fungal Genet. Biol. 2008;45:1292–1299. doi: 10.1016/j.fgb.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 47.Dyer P.S., O’Gorman C.M. Sexual development and cryptic sexuality in fungi: Insights from Aspergillus species. FEMS Microbiol. Rev. 2012;36:165–192. doi: 10.1111/j.1574-6976.2011.00308.x. [DOI] [PubMed] [Google Scholar]
- 48.Moore G.G., Elliott J.L., Singh R., Horn B.W., Dorner J.W., Stone E.A., Chulze S.N., Barros G.G., Naik M.K., Wright G.C., et al. Sexuality generates diversity in the aflatoxin gene cluster: Evidence on a global scale. PLoS Pathog. 2013;9:e1003574. doi: 10.1371/journal.ppat.1003574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Horn B.W., Moore G.G., Carbone I. Sexual reproduction in Aspergillus flavus. Mycologia. 2009;101:423–429. doi: 10.3852/09-011. [DOI] [PubMed] [Google Scholar]
- 50.Horn B.W., Moore G.G., Carbone I. Sexual reproduction in aflatoxin-producing Aspergillus nomius. Mycologia. 2011;103:174–183. doi: 10.3852/10-115. [DOI] [PubMed] [Google Scholar]
- 51.Olarte R.A., Worthington C.J., Horn B.W., Moore G.G., Singh R., Monacell J.T., Dorner J.W., Stone E.A., Xie D., Carbone I. Enhanced diversity and aflatoxigenicity in interspecific hybrids of Aspergillus flavus and Aspergillus parasiticus. Mol. Ecol. 2015;24:1889–1909. doi: 10.1111/mec.13153. [DOI] [PubMed] [Google Scholar]
- 52.Sweany R.R., Damann K.E.J., Kaller M.D. Comparison of soil and corn kernel Aspergillus flavus populations: Evidence for niche specialization. Phytopathology. 2011;101:952–959. doi: 10.1094/PHYTO-09-10-0243. [DOI] [PubMed] [Google Scholar]
- 53.Carbone I., Jakobek J.L., Ramirez-Prado J.H., Horn B.W. Recombination, balancing selection and adaptive evolution in the aflatoxin gene cluster of Aspergillus parasiticus. Mol. Ecol. 2007;16:4401–4417. doi: 10.1111/j.1365-294X.2007.03464.x. [DOI] [PubMed] [Google Scholar]
- 54.Moore G.G., Singh R., Horn B.W., Carbone I. Recombination and lineage-specific gene loss in the aflatoxin gene cluster of Aspergillus flavus. Mol. Ecol. 2009;18:4870–4887. doi: 10.1111/j.1365-294X.2009.04414.x. [DOI] [PubMed] [Google Scholar]
- 55.Varga J., Baranyi N., Chandrasekaran M., Vágvölgyi C., Kocsubé S. Mycotoxin producers in the Aspergillus genus: An update. Acta Biol. Szeged. 2015;59:151–167. [Google Scholar]
- 56.Probst C., Bandyopadhyay R., Cotty P.J. Diversity of aflatoxin-producing fungi and their impact on food safety in sub-Saharan Africa. Int. J. Food Microbiol. 2014;174:113–122. doi: 10.1016/j.ijfoodmicro.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 57.Cotty P.J., Cardwell K.F. Divergence of West African and North American Communities of Aspergillus Section Flavi. Appl. Environ. Microbiol. 1999;65:2264–2265. doi: 10.1128/aem.65.5.2264-2266.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cotty P.J., Jaime-Garcia R. Influences of climate on aflatoxin producing fungi and aflatoxin contamination. Int. J. Food Microbiol. 2007;119:109–115. doi: 10.1016/j.ijfoodmicro.2007.07.060. [DOI] [PubMed] [Google Scholar]
- 59.Cardwell K.F., Cotty P.J. Distribution of Aspergillus section Flavi among field soils from the four agroecological zones of the Republic of Bénin, West Africa. Plant Dis. 2002;86:434–439. doi: 10.1094/PDIS.2002.86.4.434. [DOI] [PubMed] [Google Scholar]
- 60.Donner M., Atehnkeng J., Sikora A.R., Bandyopadhyay R., Cotty P.J. Distribution of Aspergillus section Flavi in soils of maize fields in three agroecological zones of Nigeria. Soil Biol. Biochem. 2009;41:37–41. doi: 10.1016/j.soilbio.2008.09.013. [DOI] [Google Scholar]
- 61.Moore G.G. Sex and recombination in aflatoxigenic Aspergilli: Global implications. Front. Microbiol. 2014;5:32. doi: 10.3389/fmicb.2014.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moore G.G., Mack B.M., Beltz S.B. Genomic sequence of the aflatoxigenic filamentous fungus Aspergillus nomius. BMC Genom. 2015;16:551. doi: 10.1186/s12864-015-1719-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moore G.G., Mack B.M., Beltz S.B. Draft genome sequences of two closely related aflatoxigenic Aspergillus species obtained from the Ivory Coast. Genome Biol. Evol. 2015;8:729–732. doi: 10.1093/gbe/evv246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moore G.G., Mack B.M., Beltz S.B., Gilbert M.K. Draft genome sequence of an aflatoxigenic Aspergillus species, A. bombycis. Genome Biol. Evol. 2016;8:3297–3300. doi: 10.1093/gbe/evw238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fapohunda S.O., Moore G.G., Ganiyu O.T., Beltz S.B. Toxigenic Aspergillus flavus and other fungi of public health concern in food and organic matter in southwest Nigeria. Mycology. 2012;3:210–219. [Google Scholar]
- 66.Peterson S.W., Ito Y., Horn B.W., Goto T. Aspergillus bombycis, a New Aflatoxigenic Species and Genetic Variation in Its Sibling Species, A. nomius. Mycologia. 2001;93:689–703. doi: 10.2307/3761823. [DOI] [Google Scholar]
- 67.Nierman W.C., Yu J., Fedorova-Abrams N.D., Losada L., Cleveland T.E., Bhatnagar D., Bennett J.W., Dean R., Payne G.A. Genome sequence of Aspergillus flavus NRRL3357, a strain that causes aflatoxin contamination of food and feed. Genome Announc. 2015;3:e00168-15. doi: 10.1128/genomeA.00168-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Machida M., Asai K., Sano M., Tanaka T., Kumagai T., Terai G., Kusumoto K., Arima T., Akita O., Kashiwagi Y., et al. Genome sequencing and analysis of Aspergillus oryzae. Nature. 2005;438:1157–1161. doi: 10.1038/nature04300. [DOI] [PubMed] [Google Scholar]
- 69.Ito Y., Peterson S.W., Wicklow D.T., Goto T. Aspergillus pseudotamarii, a new aflatoxin producing species in Aspergillus section Flavi. Mycol. Res. 2001;105:233–239. doi: 10.1017/S0953756200003385. [DOI] [Google Scholar]
- 70.Goto T., Wicklow D.T., Ito Y. Aflatoxin and cyclopiazonic acid production by a sclerotium-producing Aspergillus tamarii strain. Appl. Environ. Microbiol. 1996;62:4036–4038. doi: 10.1128/aem.62.11.4036-4038.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Girardin H., Latgé J.P., Srikantha T., Morrow B., Soll D.R. Development of DNA probes for fingerprinting Aspergillus fumigatus. J. Clin. Microbiol. 1993;31:1547–1554. doi: 10.1128/jcm.31.6.1547-1554.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maddison W.P., Maddison D.R. Mesquite: A Modular System for Evolutionary Analysis. [(accessed on 7 March 2017)];2017 Version 3.2. Available online: http://mesquiteproject.org.
- 73.Lanfear R., Frandsen P.B., Wright A.M., Senfeld T., Calcott B. PartitionFinder 2: New methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol. Biol. Evol. 2017;34:772–773. doi: 10.1093/molbev/msw260. [DOI] [PubMed] [Google Scholar]
- 74.Ronquist F., Teslenko M., van der Mark P., Ayres D.L., Darling A., Höhna S., Larget B., Liu L., Suchard M.A., Huelsenbeck J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Syst. Biol. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rambaut A., Suchard M.A., Xie D., Drummond A.J. Tracer v1.6. [(accessed on 1 December 2014)];2014 Available online: http://tree.bio.ed.ac.uk/software/tracer.
- 76.Rambaut A. FigTree v1.4.2, A Graphical Viewer of Phylogenetic Trees. [(accessed on 1 December 2014)];2014 Available online: http://tree.bio.ed.ac.uk/software/figtree/
- 77.Frisvad J.C., Samson R.A. Polyphasic taxonomy of Penicillium subgenus Penicillium. A guide to identification of food and air-borne terverticilliate Penicillia and their mycotoxins. Stud. Mycol. 2004;49:1–174. [Google Scholar]
- 78.Pitt J.I., Hocking A.D., Glenn D.R. An improved medium for detection of Aspergillus flavus and A. parasiticus. J. Appl. Bacteriol. 1983;54:109–114. doi: 10.1111/j.1365-2672.1983.tb01307.x. [DOI] [PubMed] [Google Scholar]
- 79.Raper K.B., Farnell D.I. The Genus Aspergillus. The Williams & Wilkins Company; Baltimore, MD, USA: 1965. pp. 1–686. [Google Scholar]
- 80.Pitt J.I., Hocking A.D. Fungi and Food Spoilage. 3rd ed. Springer; New York, NY, USA: 2009. pp. 1–519. [Google Scholar]
- 81.Cano P.M., Jamin E.L., Tadrist S., Bourdaud’hui P., Péan M., Debrauwer L., Oswald I.P., Delaforge D., Puel O. New Untargeted Metabolic Profiling Combining Mass Spectrometry and Isotopic Labeling: Application on Aspergillus fumigatus Grown on Wheat. Anal. Chem. 2013;85:8412–8420. doi: 10.1021/ac401872f. [DOI] [PubMed] [Google Scholar]
- 82.Chang P.K., Scharfenstein L.L., Li R.B., Arroyo-Manzanares N., De Saeger S., Di Mavungu J.D. Aspergillus flavus aswA, a gene homolog of Aspergillus nidulans oefC, regulates sclerotial development and biosynthesis of sclerotium-associated secondary metabolites. Fungal Genet. Biol. 2017;104:29–37. doi: 10.1016/j.fgb.2017.04.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.