Abstract
Fermented food samples (n = 191) including maize gruel (ogi), sorghum gruel (ogi-baba), melon seed (ogiri), locust bean (iru) and African oil bean seed (ugba) from Southwest Nigeria were quantified for 23 mycotoxins, including aflatoxin B1 (AFB1), fumonisin B1 (FB1), and sterigmatocystin (STE) using liquid chromatography-tandem mass spectrometry. The practices, perceived understanding and health risks related to fungal and mycotoxin contamination amongst fermented food sellers was also established. Data obtained revealed that 82% of the samples had mycotoxins occurring singly or in combination. FB1 was present in 83% of ogi-baba samples, whereas 20% of ugba samples contained AFB1 (range: 3 to 36 µg/kg) and STE was present in 29% of the ogi samples. In terms of multi-mycotoxin contamination, FB1 + FB2 + FB3 + STE + AFB1 + alternariol + HT-2 co-occurred within one sample. The awareness study revealed that 98% of respondents were unaware of mycotoxin contamination, and their education level slightly correlated with their level of awareness (p < 0.01, r = 0.308). The extent to which the analyzed mycotoxins contaminated these food commodities, coupled with the poor perception of the population under study on fungi and mycotoxins, justifies the need to enact fungal and mycotoxin mitigation strategies along the food chain.
Keywords: fermented foods, mycotoxins, awareness, food safety, LC-MS/MS
1. Introduction
Processing of food relies on a series of preservative technologies developed to enhance quality, safety, and acceptability, one of which is fermentation. Fermentation is the oxidation of carbohydrates to produce a wide range of products principally alcohol, organic acids and carbon dioxide through microbial activities [1]. Fermentation being a low-cost technology improves the digestibility and functionality of foods and facilitates food detoxification [2]. So far, most microorganisms involved in the fermentation of foods (cereals, legumes, oil seeds, etc.) belong mainly to the Lactobacillus, Leuconostoc, Lactococcus, Pediococcus, Bacillus and Saccharomyces genera. Iru is a condiment that is produced via the fermentation of African locust bean (Parkia biglobosa) by B. substilis, B. licheniformis and B. pumilis [3], whereas ogiri is from melon (Citrullus colocynthis) seed with Bacillus, Escherichia and Pediococcus spp. as the fermenting organisms [3]. The solid-state alkaline fermented proteinous product of the African oil bean seed (Pentaclethra macrophylla) is known as ugba [4], while ogi is a product of lactic acid fermentation of maize or sorghum and principally consumed as weaning food. Ogiri and ugba like iru, are principal condiments used to flavor stews and soups [5]. Ugba is also consumed as snack and used in the preparation of porridge.
These products amongst variants such as injera, banku, amasi, fufu, garri, kenkey, uji, and mawe are indigenous to Africa. In Africa, they are typically manufactured in homes under spontaneous conditions with little or no process control [2]. Their production is also dominated by informal processing sectors (cottage and rural small-scale processors) that make use of different traditional processing methods thereby, bringing about variation in substrates used, processing conditions (time, temperature, moisture etc.), packaging materials, handling and storage practices [6]. These factors determine the quality and safety of the final products. However, irrespective of the processing method employed, it is expedient for food marked for sale to be of good quality and free from pathogenic and spoilage microorganisms such as fungi and associated toxins.
For fungi, a number of strains belonging mainly to the Aspergillus, Fusarium and Penicillium genera that attack various food commodities are toxigenic, producing various types of mycotoxins. About 25% of the global food output is contaminated by mycotoxins, causing significant economic losses [7]. Moreover, they are a serious health hazard, as they are known to be carcinogenic, nephrotoxic and immunotoxic. Mycotoxins of significance in sub-Saharan Africa (SSA) in terms of health and economy are fumonisins (FBs), aflatoxins (AFs), ochratoxin A (OTA), zearalenone (ZEN), and trichothecenes (TCs) [8]. They have been found present in different food categories mainly in cereals such as maize and oil seeds such as melon that are substrates used in the production of fermented foods. Therefore, the presence of mycotoxins in fermented foods (i.e., iru, ogi, ogi baba, ugba and ogiri) cannot be undermined. Even though fermentation could play a role in the degradation or detoxification of mycotoxins in foods, there are increasing reports of mycotoxins in fermented foods [9,10,11]. In Nigeria, there are no in-depth studies that report multiple mycotoxin contamination in the selected fermented foods, hence the need for this study.
On the other hand, a model proposed for the management of mycotoxins in SSA, identified awareness creation and enlightenment of people on mycotoxins as a principal strategy that can contribute to limit mycotoxin contamination in foods [12]. However, in recent years, increasing reports on the prevalence of multiple mycotoxins in foods consumed in SSA [8,9,11,13,14,15,16,17] suggests that only minimal efforts are deployed towards mycotoxin management particularly amongst food processors and sellers. Though, their main goal is to generate income, chances are high that adequate understanding of health implications of mycotoxin contamination through experiential learning will prompt behavioral changes and the enactment of necessary mitigation actions. Strategies such as sorting of moldy grains, utilization of adequate packaging materials, proper drying, implementation of appropriate storage methods and facilities can reduce mycotoxin contamination to a large extent. It is therefore crucial to investigate the practices, understanding and perceived health risks of fungal and mycotoxin contamination amongst stakeholders along the food chain including fermented food sellers to ascertain the level of awareness. It was also imperative to establish the magnitude to which multiple mycotoxins contaminate the fermented foods offered for sale. In a clear context, the objective of this study was to determine the level of awareness of fungal and mycotoxin contamination amongst selected fermented food sellers in Southwest Nigeria and to assess the level of mycotoxin contamination in the products (iru, ogiri, ogi, ogi-baba and ugba) they offer for sale.
2. Results and Discussion
2.1. Perception Studies
An appraisal was carried out on fermented food sellers perceived attitudes, practices and knowledge of fungal colonization of foodstuffs, being an antecedent of mycotoxins in Southwest Nigeria. The result demonstrated a wide knowledge gap amongst those under study (n = 86), as 98% could not link fungi to mycotoxin contamination and perceived associated health risks. However, these findings corroborate those of other studies [14,18,19]. According to Siegrist and Cvetkovich [20], a significant number of people in both developed and developing nations are not well informed on contaminants in foods. Majority (93%) of participants were females (n = 80) as shown in Table 1, which accentuates the role of women under the spotlight of food production and processing in Africa. Amongst the respondents, 57% store their finished products in polyethylene bags and 20% in leaves for an average of seven days. Fermented foods stored in leaves are more predisposed to fungal and mycotoxin contamination because of the indigenous microflora of the leaves and the deployment of little or no effort to clean or sterilize the leaves before use. In the study of Adegunloye et al. [21], Thaumatococcus daniellii and Musa paradisiaca leaves which are popularly used in packaging or storing fermented foods had high fungal load and toxigenic fungi such as A. niger, A.flavus and P. expansum were prevalent. Although, fermented foods are perceived to be safe, their mode of storage could predispose them to fungal and mycotoxin contamination if the storage methods are not complemented by other means of preservation.
Table 1.
Descriptive statistics and knowledge of fungal and mycotoxin contamination amongst fermented food sellers.
| Parameters | Incidence (%) | Parameters | Incidence (%) | Parameters | Incidence (%) |
|---|---|---|---|---|---|
| Sociodemographic Variables | |||||
| Gender | Education level | Age | |||
| Male | 6 (7) | None | 9 (11) | <30 years | 6 (7) |
| Female | 80 (93) | Primary | 52 (61) | 31–50 years | 74 (86) |
| Secondary | 23 (27) | >50 years | 6 (7) | ||
| Tertiary | 2 (2) | ||||
| Fermented Food Characteristics | |||||
| Mode of consumption | Food type | Food source | |||
| Direct consumption | 28 (33) | Ogi | 28 (33) | Home processed | 4 (5) |
| Food Ingredient | 46 (54) | Iru | 21 (24) | Market | 32 (37) |
| Both | 12 (14) | Ogiri | 19 (22) | Processors | 50 (58) |
| Ugba | 18 (21) | ||||
| Storage Variables | |||||
| Storage method of raw materials | Storage duration of raw materials | Average shelf life of raw material | |||
| Bags | 13 (15) | 1–3 months | 13 (15) | 1–4 weeks | 1 (1) |
| Containers | 5 (6) | >3 months | 5 (6) | >4 weeks | 17 (20) |
| Not applicable | 68 (79) | Not applicable | 68 (79) | Not applicable | 68 (79) |
| Storage method of finished product | Storage duration of finished product | Average shelf life of finished product | |||
| Polyethylene bags | 49 (57) | 1–7 days | 86 (100) | 1–3 days | 14 (16) |
| Containers | 14 (16) | >7 days | - | 3–7 days | 42 (49) |
| Paper | 3 (4) | >7 days | 30 (35) | ||
| Leaves | 17 (20) | ||||
| Wooden Boxes | 3 (4) | ||||
| Knowledge of Fungi and Mycotoxins | |||||
| Knowledge of fungi | Identification of fungal contamination in food | Frequency of contamination | |||
| Yes | 63 (73) | Rarely | 36 (42) | ||
| No | 16 (19) | Yes | 59 (68) | Frequently | 19 (22) |
| Not sure | 7 (8) | No | 27(32) | Not applicable | 31 (36) |
| Perception of reasons of fungi occurrence | Knowledge of health risks associated with fungal contamination | Knowledge of production of toxins by fungi | |||
| Storage | 21 (24) | Yes | 7 (8) | Yes | 3 (4) |
| Bad raw materials | 19 (22) | No | 79 (92) | No | 83 (96) |
| Insect infestation | 18 (21) | ||||
| All of the Above | 21 (24) | ||||
| Not sure | 7 (8) | ||||
| Knowledge of mycotoxin contamination | Willingness to attend training on mycotoxin mitigation | ||||
| Yes | 2 (2) | Yes | 83 (97) | ||
| No | 84 (98) | No | 3 (3) |
Number of respondents: 86.
The sellers (95%) obtained foods from different sources (markets or processors) as retailers while a few process their products themselves, the products were also stored over varying length of time e.g., up to seven days for finished products and up to three months for raw materials. Storage and marketing practices employed amongst the sellers also have the tendency to facilitate variations in mycotoxin contamination [22]. Knowledge of environmental factors such as humidity, temperature, insect infestation, pre- and post-harvest practices that affect fungal growth and mycotoxin production in foods are particularly important when developing and implementing strategies for the control of fungi and mycotoxins along the food chain [23]. It was evident in the study that the majority of study participants (92%) could attribute these factors to the persistence of fungi in foods.
Amongst the respondents, 22% expressed that they frequently experience fungal contamination. Fungi can thrive on varieties of foods but some foods are better substrate for their growth than the others. For example, ugba will favor the growth of fungi based on its alkaline pH than ogi which has an acidic pH [24]. Therefore the contrast amongst the respondents in terms of the frequency of contamination is expected. Ugba and ogiri were observed to be more susceptible to fungal contamination amongst the foods but were still offered for sale. Ogiri upon fungal invasion had characteristic black color while ugba overgrown with mold were posited to be more suitable as an ingredient for porridge than its principal use as condiment. Particularly worthy of note was the willingness expressed by 97% of the respondents to attend training on mycotoxin mitigation. Public awareness trainings drives attitudinal transformation if target groups have confidence in the lessons received and apprehend the problem well enough to be persuaded to revise old practices and habits [25].
Few respondents had no formal education (11%), whereas most of those under study had primary education (61%). Table 2 presents important information on the association between the level of education and knowledge on fungi and mycotoxins amongst respondents. Their knowledge of fungi correlated positively (p < 0.01, r = 0.355) with their ability to identify foodstuffs contaminated with fungi which could be due to their experience of fungal contamination as shown in Table 2. Moreover, findings also revealed that the level of education had a significant but slightly positive influence (p < 0.01, r = 0.296) on their apprehension of fungi and mycotoxin contamination (p < 0.01, r = 0.308). Dosman et al. [26] highlighted that individuals with higher education levels are likely to more knowledgeable and aware of some food contaminants than individuals with less education because they have more access and tend to seek for more information on food safety and related issues [27]. Also in Nigeria, studies have posited that educational attainment is crucial for public awareness of food safety [28]. For individuals with little or no formal education as observed in this study, more strength lies with this as an easily transmittable skill since mycotoxin related issues are not precisely covered in the curricular of any primary or secondary school in Nigeria as the case may be for other countries in Africa and beyond. Even though our findings reveal that education level correlates positively with awareness, knowledge and recognized benefits, it is expedient to make the problem known to all categories of individuals.
Table 2.
Kendall’s tau-b correlation between education and awareness level of fungi and mycotoxins amongst respondents.
| Correlations | Level of Education | Do You Know What Fungi Is | Can You Identify Food with Fungi | Does Fungi Contamination of Foodstuffs Cause Health Problems | Do You Know Fungi Produce Toxins | Have You Heard of Mycotoxin Contamination |
|---|---|---|---|---|---|---|
| Level of Education | 1.000 | 0.296 ** | −0.172 | 0.014 | 0.048 | 0.308 ** |
| Do you know what fungi is | 0.296 ** | 1.000 | 0.355 ** | 0.249 * | 0.075 | −0.139 |
| Can you identify food with fungi | −0.172 | 0.355 ** | 1.000 | 0.069 | 0.190 | 0.100 |
| Does fungi contamination of foodstuffs cause health problems | 0.014 | 0.249 * | 0.069 | 1.000 | 0.122 | 0.221 * |
| Do you know fungi produce toxins | 0.048 | 0.075 | 0.190 | 0.122 | 1.000 | 0.109 |
| Have you heard of mycotoxin contamination | 0.308 ** | −0.139 | 0.100 | 0.221 * | 0.109 | 1.000 |
** Correlation is significant at p < 0.01 (2-tailed); * correlation is significant at p < 0.05 level (2-tailed); n: number of respondents (86); values along each column are correlation coefficients.
Mycotoxins are at the forefront amongst chronic food toxicants [19], usually occurring below levels that elicit acute health effects, but such levels could provoke long-term health implications amongst humans and animals [29]. It may therefore be difficult to associate several health complications to mycotoxin exposure, which strongly supports the poor perception on the subject demonstrated by respondents in this study. In addition to this, because mycotoxin can be present in foods after the dissipation of fungi, it was therefore unexpected for respondents to physically discern a food that is contaminated with mycotoxins in addition to fact they seem not to alter the taste or flavor of foods. These variations need to be considered and communicated particularly during the implementation of trainings aimed at fungal and mycotoxin mitigation.
The findings of our research have profound implications for strategies directed towards mycotoxin management in fermented foods and other food categories in Nigeria and SSA, based on studies [12,25,28] that have established that awareness and education are critical elements in reducing the menace of mycotoxins in developing countries. The study was particularly targeted towards fermented food sellers but has extensive inference for the curtailment of food hazards/contaminants in evolving economies. It was therefore imperative to further establish the extent of mycotoxin contamination in fermented foods (i.e., ogi, ugba, iru, ogiri, and ogi-baba) based on the feasibility of human exposure revealed through the poor perception of mycotoxin contamination amongst their sellers.
2.2. Method Performance Characteristics
Table 3 shows the results of the method validation parameters including the LOD, LOQ and AR of the different fermented food matrices. The calibration curves for the analytes were linear and the AR of all the analyzed mycotoxins varied between 89% and 109%, which aligns within the range set by the EC [30]. The LODs for 3-ADON 15-ADON, AFB1, AFB2, AFG1, AFG2, DAS, and ROQ C were <6 μg/kg while the LOQs of STE, and ZEN were ≤20 μg/kg in all the tested fermented food.
Table 3.
Method performance parameters of the fermented food matrices.
| Mycotoxins | Calibration Range µg/kg | Fermented Melon (ogiri) | Fermented Locust Bean (iru) | Fermented African Oil Bean (ugba) | Fermented Maize Gruel (ogi) | Fermented Sorghum Gruel (ogi baba) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LOD | LOQ | AR | LOD | LOQ | AR | LOD | LOQ | AR | LOD | LOQ | AR | LOD | LOQ | AR | ||
| Deoxynivalenol | 200–800 | 11 | 22 | 100 | 4.9 | 9.8 | 99 | 15 | 30 | 101 | 7 | 14 | 97 | 12 | 24 | 101 |
| Nivalenol | 100–400 | 48 | 96 | 100 | 11 | 22 | 100 | 21 | 42 | 103 | 35 | 70 | 101 | 87 | 175 | 99 |
| Neosolaniol | 50–200 | 20 | 40 | 95 | 16 | 32 | 99 | 24 | 48 | 96 | 2.2 | 4.4 | 103 | 3.0 | 6.0 | 100 |
| Fusarenon-X | 100–400 | 39 | 78 | 97 | 8.1 | 16 | 101 | 25 | 50 | 96 | 21 | 42 | 100 | 45 | 90 | 100 |
| 3-Acetyldeoxynivalenol | 25–100 | 2.3 | 4.6 | 96 | 2.0 | 4.0 | 102 | 1.2 | 2.4 | 101 | 5.0 | 10 | 105 | 12 | 24 | 97 |
| 15-Acetyldeoxynivalenol | 12.5–50 | 1.7 | 3.5 | 94 | 3.9 | 7.9 | 101 | 1.8 | 3.7 | 96 | 10 | 20 | 95 | 7.0 | 14 | 99 |
| Aflatoxin B1 | 10–40 | 2.0 | 4.0 | 96 | 1.2 | 3.3 | 96 | 1.5 | 3.0 | 100 | 3.8 | 7.5 | 100 | 5.0 | 10 | 100 |
| Aflatoxin B2 | 10–40 | 2.3 | 4.6 | 96 | 1.8 | 3.3 | 94 | 1.4 | 2.8 | 96 | 1.8 | 3.5 | 99 | 2.5 | 5.0 | 102 |
| Aflatoxin G1 | 10–40 | 3.9 | 7.8 | 99 | 1.7 | 3.3 | 95 | 1.9 | 3.9 | 98 | 1.8 | 3.5 | 98 | 2.5 | 5.0 | 101 |
| Aflatoxin G2 | 10–40 | 3.7 | 7.4 | 96 | 1.2 | 2.3 | 91 | 2.2 | 4.4 | 94 | 3.8 | 7.5 | 100 | 5.0 | 10 | 99 |
| Diacetoxyscirpenol | 2.5–10 | 0.9 | 1.8 | 97 | 0.7 | 1.4 | 97 | 1.0 | 2.0 | 89 | 0.3 | 0.6 | 99 | 0.5 | 1.0 | 94 |
| Alternariol | 50–200 | 6.5 | 13 | 98 | 9.7 | 20 | 100 | 5.9 | 11 | 98 | 40 | 80 | 92 | 40 | 80 | 99 |
| Alternariol Methyl Ether | 100–400 | 54 | 107 | 96 | 5.0 | 10 | 96 | 4.6 | 9.2 | 98 | 5.0 | 10 | 109 | 6.3 | 12 | 96 |
| HT-2 Toxin | 50–200 | 6.5 | 13 | 98 | 7.4 | 14 | 98 | 15 | 30 | 94 | 6.5 | 13 | 85 | 6.5 | 13 | 95 |
| T-2 Toxin | 50–200 | 12 | 24 | 98 | 14 | 28 | 94 | 13 | 26 | 100 | 3.6 | 7.2 | 87 | 8.0 | 16 | 94 |
| Fumonisin B1 | 200–800 | 24 | 48 | 97 | 22 | 44 | 100 | 38 | 76 | 97 | 8.2 | 16 | 87 | 10 | 20 | 98 |
| Fumonisin B2 | 200–800 | 11 | 22 | 99 | 9.4 | 18 | 99 | 43 | 87 | 95 | 12 | 23 | 89 | 11 | 22 | 100 |
| Fumonisin B3 | 25–100 | 13 | 26 | 97 | 21 | 42 | 97 | 33 | 66 | 94 | 14 | 28 | 89 | 14 | 28 | 96 |
| Ochratoxin A | 25–100 | 11 | 22 | 89 | 1.2 | 2.4 | 93 | 3.6 | 7.2 | 90 | 1.5 | 3.0 | 99 | 2.5 | 5.0 | 95 |
| Sterigmatocystin | 25–100 | 5.5 | 11 | 100 | 1.7 | 3.3 | 97 | 1.9 | 3.8 | 95 | 1.3 | 2.5 | 100 | 2.5 | 5.0 | 101 |
| Roquefortine C | 5–20 | 4.9 | 9.7 | 101 | 1.2 | 2.3 | 99 | 1.0 | 2.0 | 99 | 4.0 | 8.0 | 97 | 6.0 | 12 | 98 |
| Zearalenone | 50–200 | 9.8 | 20 | 96 | 2.9 | 5.9 | 92 | 4.4 | 8.8 | 104 | 3.3 | 6.5 | 102 | 3.8 | 7.6 | 93 |
| Enniatin B | 40–160 | 26 | 52 | 93 | 6.4 | 13 | 94 | 5.6 | 11 | 99 | 6.3 | 12 | 82 | 7.9 | 16 | 91 |
LOD: limit of detection (µg/kg); LOQ: limit of quantification (µg/kg); AR: Apparent recovery (%).
2.3. Mycotoxin Contamination
In this study, the multi-mycotoxin profile of Nigerian fermented food products including ogiri, ugba and iru, intended for use as condiments, as well as ogi and ogi-baba, popularly consumed as breakfast cereals, was delineated (Table 4). Generally, 56% of the 34 fermented food samples positive for AFB1 had levels above the maximum regulatory limit of 2 µg/kg in foods according to the EC [31]. Data obtained via LC-MS/MS showed that ogiri samples had a higher incidence of AFB1 (48%) (range: 3–4 µg/kg) compared to others. Co-occurrence of AFB2 and AFG2 was established in ogiri. We also noted the prevalence of these important analogues AFs (including others, i.e., AFB1 and AFG1), singly or in combination in all the samples, which might be due to the presence of AF-producing fungi in similar samples of ogiri, ugba, ogi, ogi-baba and iru [24]. It has been established that chronic exposure to AF from fermented foods affects close to 4.5 billion persons in the developing countries [32]. The presence of Aspergillus spp. and AF in some raw materials used in the manufacture of the tested fermented foods has been previously established. Ezekiel et al. [33] recovered AFB1 and total AF from melon seeds used in the manufacture of ogiri at a mean level of 37.5 µg/kg and 142 µg/kg, respectively, meanwhile Makun et al. [15] reported the presence of A. flavus and AFB1 in 54% of sorghum for ogi baba production. The biosynthetic pathway of AFB1 has also been studied [9] with averantin, averufin, norsolorinic acid, versicolorin A and STE established as precursors/intermediate compounds. STE and AFB1 are synthesized by the same Aspergillus spp. and the presence of AF in the fermented foods can as well be attributed to the presence of such a precursor as STE. STE was detected in ugba (range: 22–27 µg/kg) and ogi (range: 4–7 µg/kg), was <LOQ in ogiri and ogi and absent in iru.
Table 4.
Contamination of mycotoxins (µg/kg) in fermented foods from Southwest Nigeria.
| Mycotoxins | Fermented Melon (n = 31) (ogiri) | Fermented Locust Bean (n = 60) (iru) | Fermented African Oil Bean (n = 30) (ugba) | Fermented Maize Gruel (n = 35) (ogi) | Fermented Sorghum Gruel (n = 35) (ogi baba) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % +ve | Range | Mean | % +ve | Range | Mean | % +ve | Range | Mean | % +ve | Range | Mean | % +ve | Range | Mean | |
| Deoxynivalenol | 3 (10) | <LOQ-54 | 31 | 4 (7) | <LOQ-118 | 62 | 4 (13) | 36–38 | 37 | 4 (11) | <LOQ-55 | 32 | 3 (9) | 32–112 | 60 |
| Nivalenol | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 (9) | <LOQ | <LOQ |
| Neosolaniol | 1 (3) | 0 | <LOQ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fusarenon-X | 0 | 0 | 0 | 3 (5) | 40–76 | 62 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3-Acetyldeoxynivalenol | 0 | 0 | 0 | 1 (2) | 0 | 19 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 15-Acetyldeoxynivalenol | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Aflatoxin B1 | 15 (48) | 3–4 | <LOQ | 1 (2) | 0 | 6 | 6 (20) | 3–36 | 20 | 8 (23) | <LOQ-17 | <LOQ | 4 (11) | 10–24 | 11 |
| Aflatoxin B2 | 5 (16) | <LOQ | <LOQ | 1 (2) | 0 | <LOQ | 1 (3) | 0 | <LOQ | 7 (20) | <LOQ-7 | 6 | 0 | 0 | 0 |
| Aflatoxin G1 | 0 | 0 | 0 | 2 (3) | 8–8 | 8 | 0 | 0 | 0 | 2 (6) | 0 | 0 | 1 (3) | 0 | 16 |
| Aflatoxin G2 | 2 (7) | <LOQ | <LOQ | 3 (5) | 3–6 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Σ Aflatoxins | 17 (55) | 3-12 | 4 | 7 (12) | 3–8 | 5 | 7 (23) | 3–36 | 18 | 9 (26) | <LOQ-17 | 8 | 4 (11) | <LOQ-40 | 16 |
| Diacetoxyscirpenol | 1 (3) | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 (11) | 1–2 | 1 |
| Alternariol | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 (9) | <LOQ | <LOQ | 0 | 0 | 0 |
| Alternariol Methyl Ether | 6 (19) | 64–153 | 115 | 6 (10) | 19–77 | 38 | 4 (13) | 25–193 | 109 | 0 | 0 | 0 | 8 (23) | 30–35 | 33 |
| HT-2 Toxin | 4 (13) | 18–35 | 27 | 9 (15) | 17–51 | 33 | 1 (3) | 0 | 17 | 3 (9) | 20-21 | 21 | 0 | 0 | 0 |
| T-2 Toxin | 0 | 0 | 0 | 7 (12) | 28–31 | 29 | 1 (3) | 0 | 15 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fumonisin B1 | 0 | 0 | 0 | 4 (7) | 61–167 | 113 | 0 | 0 | 0 | 25 (71) | 68–2492 | 384 | 14 (40) | <LOQ-68 | 39 |
| Fumonisin B2 | 0 | 0 | 0 | 4 (7) | 32–42 | 38 | 0 | 0 | 0 | 23 (66) | 94–659 | 250 | 9 (25) | <LOQ-65 | 34 |
| Fumonisin B3 | 0 | 0 | 0 | 4 (7) | 76–89 | 84 | 0 | 0 | 0 | 18 (51) | 42–404 | 112 | 26 (74) | <LOQ-42 | 28 |
| Σ Fumonisin B1, B2 | 0 | 0 | 0 | 8 (13) | 32–167 | 76 | 0 | 0 | 0 | 25 (71) | 68–3151 | 645 | 18 (52) | <LOQ-129 | 48 |
| Σ Fumonisin B1, B2, B3 | 0 | 0 | 0 | 12 (20) | 32–167 | 78 | 0 | 0 | 0 | 27 (77) | 42–3555 | 672 | 29 (83) | <LOQ-168 | 55 |
| Ochratoxin A | 6 (19) | <LOQ-27 | <LOQ | 7 (12) | <LOQ-21 | 9 | 1 (3) | 0 | 9 | 0 | 0 | 0 | 2 (6) | 5–6 | 6 |
| Sterigmatocystin | 6 (19) | <LOQ | <LOQ | 0 | 0 | 0 | 2 (7) | 22–27 | 25 | 10 (29) | 4–7 | 4 | 8 (23) | <LOQ | <LOQ |
| Roquefortine C | 0 | 0 | 0 | 2 (3) | 10–14 | 12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Zearalenone | 8 (25) | 21–45 | 33 | 5 (8) | 11–33 | 18 | 4 (13) | 39–117 | 72 | 3 (9) | <LOQ | <LOQ | 0 | 0 | 0 |
| Enniatin B | 0 | 0 | 0 | 0 | 0 | 0 | 4 (13) | <LOQ | <LOQ | 5 (14) | 12–14 | 13 | 0 | 0 | 0 |
Only concentrations higher than LOQ are recorded and samples containing concentrations higher than LOD were considered positive/contaminated; % +ve: percentage of positive samples.
The vulnerability of food products such as maize to OTA contamination worldwide is documented [16]. The data presented in this study show OTA being present in ogi-baba, iru, and ugba, at mean levels of 6, 6, and 9 µg/kg, respectively. OTA was not found in ogi, which is contrary to the report of Oyelami et al. [17] on the presence of OTA in maize-based foods. All the ugba and ogiri samples were positive for OTA, containing levels that were above the 5 µg/kg recommended for foodstuffs by EC [31]. OTA is a potent secondary metabolite synthesized in foods by more than ten fungal species with A. ochraceus and P. verrucosum as principal producers in the tropics and in the temperate regions, respectively [34]. The same toxin is associated with kidney and liver impairment, Balkan endemic nephropathy, oxidative DNA damage and has been classified by the International Agency for Research on Cancer (IARC) [35] as a Group 2B carcinogen. ROQ C is often regarded as one of the most important fungal contaminants in fermented foods and beverages [36] and in this study, it was only detected in iru and at a low incidence rate (range: 10–14 µg/kg). Being a potent neurotoxin at high concentrations above 1500 µg/kg [31], it can therefore be deduced that the same samples are considered safe for consumers in terms of ROQ C concentrations. Like AF, STE, and OTA, the occurrence of ROQ C in these analyzed foods could be due to the participation of various fungi during fermentation, which is principally by chanced inoculation. Odunfa and Adeyele [37] identified Aspergillus and Penicillium fungi during the fermentation of ogi-baba.
The TCs are a large family of over 150 chemically related toxins produced principally by the Fusarium genera [38]. Based on their core structures, they are classified into four types: A, B, C and D. Type A includes mainly T-2 toxin and HT-2 toxin together with NEO, and DAS in this list. DON, NIV, 3-ADON, 15-ADON and FUS-X are the Type B TCs mycotoxins. In relation to toxicity according to Schollenberger et al. [39], type A TCs are more toxic when compared to type B TCs. In terms of geographical locations Type A TCs are not commonly reported in Africa, but in our study, the type A TH-HT-2 was the most frequently occurring, and it levels in ogi (range: 20–21 µg/kg) exceeded the recommended level of HT-2 + T-2 (15 µg/kg) in infant foods by EC [40]. Concerning iru, 9 samples were positive for HT-2 within the range of 17 to 51 μg/kg (mean: 33 μg/kg) (Table 4) and T-2 was also detected in iru. Generally, T-2 and HT-2 toxins are of great concern based on their capacity to induce oxidative stress, inhibit DNA, RNA, and protein synthesis as well as mitochondrial performance [40]. Furthermore, the contamination of both TCs mycotoxins can co-occur together with DAS, because of similarity in biosynthesis at the side branch of the pathway of T-2 [41], which was only observed in ogiri. The level of DON in all the positive samples (n = 18) reach a maximum of 118 µg/kg, which was far less than the maximum limit for DON in processed cereals (750 µg/kg). The frequency and contamination level found for the acetylated derivative of DON (3-ADON) was low, whereas no analyzed samples contained 15-ADON. Chilaka et al. [11] found much higher contamination levels of DON and 3-ADON than any reported in our study.
The FBs were the dominant mycotoxins in ogi and ogi-baba, with most of the ogi samples having FB1 and FB2 higher than maximum set limit for FB1 + FB2 (200 µg/kg) in maize-based infant foods by EC [31]. This suggests the high exposure of infants to FB1 and FB2. The high incidence of this toxin group observed in ogi, a maize-based product substantiates the vulnerability of the maize crops to FB producing fungi such as F. proliferatum and F. verticillioides. Additionally, the low levels of FBs observed in ogi baba as a fermented processed sorghum product also correlates well with the findings that sorghum is less susceptible to fungal infestation when compared to maize [11]. Whilst, data on the occurrence of the studied mycotoxins in ogi baba is scarce, studies from Nigeria have reported the presence of FBs in sorghum grains [11,15]. Amongst the alkaline fermented food studied, only iru (7%) was positive for FB1, FB2 and FB3 with mean values of 113, 38 and 84 µg/kg, respectively. FB1 has been linked to liver and esophageal cancer, and on that note was classified as a group 2B carcinogen [35]. Recently in Tanzania, Shirima et al. [42] studied child growth during early childhood in relation to AF and FBs exposure and established that exposure to FBs alone or together with AF is a factor of growth impairment in children. As established in our study, ogi as a weaning food in Nigeria can be considered unsafe for consumption by infants.
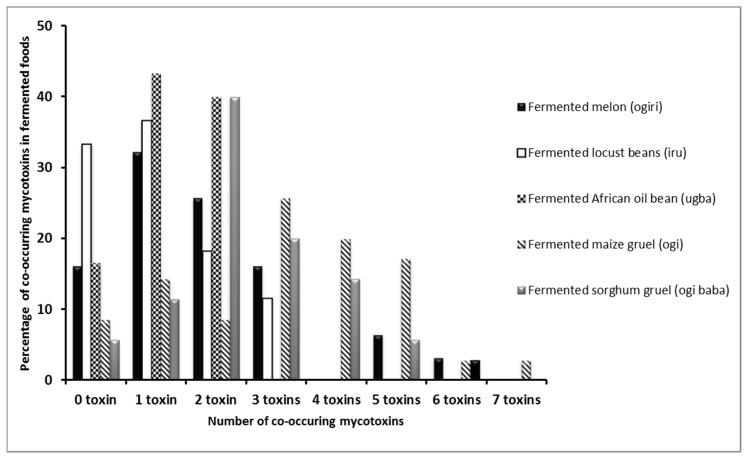
In 90% of the samples positive for ZEN (n = 20), levels recovered were less than 50 µg/kg, which is insignificant when compared with the maximum limit of 50–1000 µg/kg in foods in 16 countries where ZEN is being regulated [43]. According to Kpodo et al. [9], ZEN is largely produced by some Fusarium spp. in cool dry climates between 10 °C and 15 °C, whereas temperatures from 27 °C to 40 °C commonly persist yearly in Nigeria. Howbeit, the low levels of ZEN detected in the samples may be due to the persistence of such climatic conditions that are unfavorable for the production of the toxin in Nigeria. Amongst all these fermented food types, 18% (n = 35) was devoid of the tested mycotoxins, ugba was the least contaminated in terms of the number of mycotoxins (10) found compared to others including ogiri (11), ogi-baba (12), ogi (13) and iru (16). Ugba is encapsulated in extremely hard seed coats, which make it less prone to fungi and mycotoxin contamination than others with less formidable coats. This might account for the low levels in the number of mycotoxins found in ugba in comparison with other samples. Co-occurrence of several mycotoxins within the fermented foods analyzed was also observed (Figure 1).
Figure 1.
Percentage co-occurrence of mycotoxins in fermented foods from Southwest Nigeria.
Out of the 191 samples analyzed, 82% (n = 156) had mycotoxins occurring singly or in combination. For the 23 different mycotoxins analyzed in each food matrix, 3 different toxins co-occurred in 16% ogiri, 12% iru, 26% ogi and 26% ogi samples tested. This phenomenon has been demonstrated for several mycotoxins in foods consumed in Africa [11,14,15]. DON usually co-occurs with its acetylated forms but this was not the case in this study. The co-occurrence of DON and ZEN has also been established, but with ZEN mostly occurring at a lower concentration than DON [38]. This relationship was observed in some ogiri samples that were positive for both mycotoxins. Also, the co-occurrence of up to seven metabolites (FB1 + FB2 + FB3 + STE + AFB1 + AOH + HT-2) in ogi could be due to its susceptibility to fungal contamination when compared to other samples. The multiple mycotoxins observed within the same sample could exacerbate the health risks amongst humans since they can elicit some synergistic and additive effects especially at levels above those accepted by various regulatory bodies.
The varying contamination levels, incidence and co-occurrence of mycotoxins in the fermented foods analyzed are a result of many cogent factors. It however, remains unclear whether the mycotoxins recovered from the samples analyzed are due to carry-over from the raw materials used in processing them, or due to fungal contamination of processed foods tested in this study. In any case, ignorance as well as climatic conditions that prevail in the study sites, seems to also play a role. Unacceptable trade activities of mixing moldy food products with high-quality products to maximize profit also persists due to non-enforcement of regulatory limits on locally grown crops or locally produced products sold in the markets, and ignorance on the existence of mycotoxins. The importance of the right processing environment and conditions cannot be underrated as most of these foods were manufactured in homes under unhygienic conditions. Besides an unhygienic processing environment, improper storage practices, which provide optimal conditions for mould development and subsequent mycotoxin accumulation, may exacerbate the situation. Bearing in mind that, these fermented products are not the only dietary sources of mycotoxin exposure amongst humans, the overall daily exposure to these mycotoxins can be high.
3. Conclusions
This study gave an insight into the safety of fermented foods produced in Nigeria and equally established the awareness of the sellers towards fungal and mycotoxin contamination and associated health risks. We observed that there exists a wide knowledge gap amongst participants on this aspect of food safety. In terms of the food analyzed, a significant fraction of the samples (156/191) had mycotoxins occurring singly or in combination though relatively at low incidence and contamination levels. Ogi was the most contaminated sample based on the total number of samples positive (94%, n = 35) for the analyzed toxins which makes the risk of mycotoxin exposure higher amongst its consumers. Some of the samples exceeded the maximum limit for FB, AF, OTA and ZEN in foods as regulated by the EC. In broad terms, the incidence of type A TCs was slightly higher than type B TCs. All the samples were negative for 15-ADON. AOH, AME, STE, ENN B and ROQ C were also present at low levels in few samples. Ogi-baba and ogi had the highest number of co-occurring fungal metabolites. To the best of our knowledge, this is the first study that assessed the presence of mycotoxins in ugba and ogi-baba. As well as the first to report a wide range of previously unreported mycotoxins in iru, ogiri and ogi consumed in Nigeria.
Considering the high level of consumption of these fermented foods in Nigeria, strategies towards mycotoxin mitigation should be a priority. Awareness needs to capture good agricultural practices aimed at reducing fungal infestation of the raw materials during growth and storage, while executing training on ways of selecting high-quality raw materials. Hands-on learning activities needs to be integrated with awareness campaigns to create more opportunities for the target groups to adopt the recommendations provided. Awareness can also be created from a gender focal point where women being at the forefront of food production are involved in the formulation of education programs on mycotoxin management. Proper understandings of the economic and health effects of mycotoxins are important drivers as individuals are most likely to take steps towards mycotoxin reduction if effects are known. Proposed strategies should therefore emphasize benefits. To pave the way forward, there is need for enforcement of risk-based food laws, encouragement of dietary diversity, sustained use of intervention technologies and more surveillance programs that could be implemented to provide toxicological and exposure data.
4. Materials and Methods
4.1. Sampling
The cluster sampling method was used to obtain fermented foods namely; maize gruel (ogi), sorghum gruel (ogi-baba), locust bean (iru), African oil bean seed (ugba) and melon seed (ogiri) from various fermented food sellers in Southwest Nigeria between February 2015 and July 2016. Composite samples of each fermented food: ogi (n = 35), ogi-baba (n = 35), iru (n = 60), ugba (n = 30) and ogiri (n = 31) were taken to obtain a total of 191 composite samples. Each composite sample of about 270 g was an aggregate of sub samples obtained from three different fermented food sellers. All samples were collected in sterile containers and immediately transported to the laboratory. Afterwards, each composite sample was properly mixed and trisected twice to obtain a representative sample of 30 g, after which they were stored at −18 °C for mycotoxin analysis at the Laboratory of Food Analysis, Ghent University, Belgium.
4.2. Awareness Studies
A descriptive cross-sectional study was carried out amongst some of the fermented food sellers within the sampling area in February 2015 using a questionnaire that consisted of closed- and open-ended questions. The questionnaire (Table S1) was designed to capture the demographics, practices, understanding and perceived health risk of food contamination by fungi and mycotoxins amongst fermented food sellers. The sellers were informed about the purpose of the study and their verbal consent was obtained with a standardized consent form. In total, 86 respondents willingly participated in the study.
4.3. Mycotoxin Analysis
4.3.1. Materials and Chemicals
Methanol, glacial acetic acid, and acetonitrile (LC-MS/MS grade) were purchased from Biosolve B.V. (Valkenswaard, The Netherlands). Ammonium acetate and acetic acid (analytical grade) were supplied by Merck (Darmstadt, Germany). HPLC grade methanol and n-hexane in addition to Whatman® (Maidstone, UK) glass microfiber filters were obtained from VWR International (Zaventem, Belgium). Ultrafree-MC centrifugal filter devices (0.22 μm) were obtained from Millipore (Brussels, Belgium). MultiSep®226 AflaZon+ immunoaffinity columns and C18 solid phase extraction (SPE) columns were obtained from Romer Labs (Gernsheim, Germany) and Grace Discovery Sciences (Lokeren, Belgium), respectively. Water was purified in a Milli-Q Gradient apparatus (Millipore, Brussels, Belgium). All other reagents and chemicals were of analytical grade.
4.3.2. Mycotoxin Standards
Mycotoxin standards comprising of aflatoxin B1 (AFB1), aflatoxin B2 (AFB2), aflatoxin G1 (AFG1), aflatoxin G2 (AFG2), fumonisin B1 (FB1), fumonisin B2 (FB2), deepoxy-deoxynivalenol (DOM), 15-acetyl-deoxynivanelol (15-ADON), neosolaniol (NEO), OTA, alternariol (AOH), alternariol monomethyl ether (AME), zearalenone (ZEN), nivalenol (NIV), deoxynivanelol (DON), 3-acetyl-deoxynivanelol (3-ADON), sterigmatocystin (STE), roquefortine C (ROQ C), enniatin B (ENN B), fusarenon-X (FUS-X), HT-2 toxin (HT-2) and zearalanone (ZAN) were obtained from Sigma-Aldrich (Bornem, Belgium). Fumonisin B3 (FB3) was procured at Promec Unit (Tynberg, South Africa), while T-2 toxin (T-2) and diacetoxyscirpenol (DAS) were purchased from Biopure Referenzsubstanze (Tulln, Austria).
4.3.3. Sample Preparation
All samples were dried in a hot air oven (UM200, Memmert, Schwabach, Germany) and milled to a particle size between 0.5 and 1 mm. Milled samples were accurately measured (5 ± 0.005 g), and reinforced with internal standards (ZAN-10 µg/mL and DOM-50 µg/mL), and allowed to equilibrate for 15 min in the dark. Extraction of mycotoxins in the sample was done using 20 mL of acetonitrile/acetic acid/water (79/1/20, v/v/v). The mixture was vortexed for 10 s, placed on an overhead shaker (Agitelec, Paris, France) for 1 h and centrifuged for 15 min at 3500 rpm. All the supernatant was transferred into a pre-conditioned SPE C18 column and defatted (2×) using 10 mL n-hexane. Two cleanup procedures were applied to recover the 23 mycotoxins. First, 27.5 mL of acetonitrile/acetic acid (99/1, v/v) was added to 12.5 mL of the defatted extract, and passed through a MultiSep 226 AflaZon+ immunoaffinity column. Second, using a glass micro filter (General Electric, Coventry, UK), 2 mL of defatted extract was filtered and combined with the MultiSep 226 eluate and evaporated to dryness. The residue was reconstituted in 150 µL of injection solvent consisting of methanol/water/acetic acid (57.2/41.8/1, v/v/v) and 5 mM ammonium acetate (0.385 g/L). The reconstituted extract was placed in an Ultrafree® PVDF centrifuge filter (Merck, Darmstadt, Germany), and centrifuged at 10,000 rpm for 10 min. The eluent was transferred into an LC-MS/MS injection vial prior to analysis.
4.3.4. Liquid Chromatography-Tandem Mass Spectrometry
A Waters Acquity UPLC apparatus paired to a Quattro Premier XE Tandem Mass Spectrometer (Waters, Milford, MA, USA) was utilized for the identification and quantification of the analytes. Data acquisition and processing utilities included the use of the MassLynx™ (V. 4.1) and QuanLynx® (V. 4.1) software (Micromass, Manchester, UK). The column used to separate the analytes of interest was a Symmetry C18 column (150 mm × 2.1 mm i.d. 5 μm particle size) with a guard column (10 mm × 2.1 mm i.d.) of the same material (Waters, Zellik, Belgium). The chromatographic conditions set were similar to those of Ediage et al. [44]. Mobile phase A contained acetic acid/methanol/water (1/5/94, v/v/v) and 5 mM ammonium acetate (0.385 g/L), and mobile phase B contained acetic acid/water/methanol (1/2/97, v/v/v) and 5 mM ammonium acetate (0.385 g/L). With a sample injection volume set at 10 μL, the total analytical run time was 28 min with a pressure that varied between 0 and 5000 psi. The mass spectrometer was operated using selected reaction monitoring (SRM) channels in positive electrospray ionization (ESI+) mode. Further details on the mycotoxin transitions are reported by De Boevre et al. [45] and Monbaliu et al. [46]. For the identification of the targeted mycotoxins, the criteria of the Commission Regulation 657/2002/EC [47] were followed.
4.3.5. Method Validation
The Commission Regulation 401/2006/EC [30] was used for the validation studies. Variables including limit of quantification (LOQ), limit of detection (LOD) and apparent recovery (AR) were accessed by spiking mycotoxin-free samples (blank) with the different mycotoxins in triplicates. ZAN and DOM were used as internal standards and matrix matched calibration curves (MMC) were constructed from the ratio of the peak area of each analyte to the internal standard. The linearity of each analyte was estimated graphically using a scatter plot, and the linear regression model evaluated using a lack-of-fit test, while apparent recoveries were established by dividing the calculated concentration by the theoretical concentration.
4.4. Data Analysis
A descriptive statistics (mean, range, frequencies, and percentages) of the data generated in this study was performed using Microsoft Office Excel 2010 (Redmond, WA, USA). In addition, the degree of awareness of fungal and mycotoxin contamination amongst the fermented food sellers was correlated with their level of education using Kendall’s tau-b test on SPSS version 23.0 (IBM Corporation, NY, USA).
Acknowledgments
The authors wish to sincerely thank the following: Organisation for Women in Science in the Developing World (OWSD), Italy; Centre of Excellence (CoE) in Food Security co-hosted by the University of Pretoria and the University of the Western Cape, South Africa; African Women in Agricultural Research and Development (AWARD), Kenya; and the MYTOX-SOUTH, hosted in the Laboratory of Food Analysis, Ghent University, Belgium for their outstanding contributions to this study.
Supplementary Materials
The following are available online at www.mdpi.com/2072-6651/9/11/363/s1, Table S1: Questionnaire on Demographics, Practices, Understanding and Perceived Health Risk of Fungal and Mycotoxins Contamination amongst Fermented Food Sellers in Nigeria.
Author Contributions
I.A. and A.O. conceived and designed the experiment; I.A. performed the experiment, analyzed the data and wrote the manuscript; C.C. assisted with the experiment and edited the manuscript; P.N., A.O., S.O., M.D.B. and S.D.S. supervised the researched, edited and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Ross P., Morgan S., Hill C. Preservation and fermentation: Past, present and future. Int. J. Food Microbiol. 2002;79:3–16. doi: 10.1016/S0168-1605(02)00174-5. [DOI] [PubMed] [Google Scholar]
- 2.Oyewole O.B. Lactic fermented foods in Africa and their benefits. Food Control. 1997;8:289–297. doi: 10.1016/S0956-7135(97)00075-3. [DOI] [Google Scholar]
- 3.Aworh O.C. The role of traditional food processing technologies in national development: The West African experience. In: Robertson G.L., Lupien J.R., editors. Using Food Science and Technology to Improve Nutrition and Promote National Development. International Union of Food Science & Technology; Toronto, ON, Canada: 2008. pp. 1–18. [Google Scholar]
- 4.Olotu I.O., Enujiugha V., Obadina A.O. The effect of γ-irradiation and cooking on the amino acid profile of African oil bean seed (Pentaclethra macrophylla Benth) J. Food Process. Preserv. 2014;38:2020–2026. doi: 10.1111/jfpp.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Onyenekwe P.C., Odeh C., Nweze C.C. Volatile constituents of ogiri, soybean daddawa and locust bean daddawa three fermented Nigerian food flavour enhancers. Electron. J. Environ. Agric. Food Chem. 2012;11:15–22. [Google Scholar]
- 6.Babajide J.M., Oyewole O.B., Obadina O.A. An assessment of the microbiological safety of dry yam (gbodo) processed in South West Nigeria. Afr. J. Biotechnol. 2006;5:157–161. [Google Scholar]
- 7.Ostry V., Malir F., Grosse Y. Mycotoxins as human carcinogens—The IARC monographs classification. Mycotoxin Res. 2017;33:65–73. doi: 10.1007/s12550-016-0265-7. [DOI] [PubMed] [Google Scholar]
- 8.Darwish W.S., Ikenaka Y., Nakayama S.M.M., Ishizuka M. An overview on mycotoxin contamination of foods in Africa. J. Vet. Med. Sci. 2014;76:789–797. doi: 10.1292/jvms.13-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kpodo K., Sørensen A.K., Jakobsen M. The occurrence of mycotoxins in fermented maize products. Food Chem. 1996;56:147–153. doi: 10.1016/0308-8146(95)00155-7. [DOI] [Google Scholar]
- 10.Colak H., Hampikyan H., Bingol E.B., Cetin O., Akhan M., Turgay S.I. Determination of mould and aflatoxin contamination in tarhana, a Turkish fermented food. Sci. World J. 2012;2012:1–6. doi: 10.1100/2012/218679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chilaka C.A., De Boevre M., Atanda O.O., De Saeger S. Occurrence of Fusarium mycotoxins in cereal crops and processed products (ogi) from Nigeria. Toxins (Basel) 2016;8 doi: 10.3390/toxins8110342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strosnider H., Azziz-Baumgartner E., Banziger M., Bhat R.V., Breiman R., Brune M.N., DeCock K., Dilley A., Groopman J., Hell K., et al. Workgroup report: Public health strategies for reducing aflatoxin exposure in developing countries. Environ. Health Perspect. 2006;114:1898–1903. doi: 10.1289/ehp.9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdallah M.F., Krska R., Sulyok M. Mycotoxin contamination in sugarcane grass and juice: First report on detection of multiple mycotoxins and exposure assessment for aflatoxins B1 and G1 in Humans. Toxins (Basel) 2016;8:343. doi: 10.3390/toxins8110343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ezekiel C.N., Sulyok M., Babalola D.A., Warth B., Ezekiel V.C., Krska R. Incidence and consumer awareness of toxigenic Aspergillus section Flavi and aflatoxin B1 in peanut cake from Nigeria. Food Control. 2013;30:596–601. doi: 10.1016/j.foodcont.2012.07.048. [DOI] [Google Scholar]
- 15.Makun H.A., Adeniran A.L., Mailafiya S.C., Ayanda I.S., Mudashiru A.T., Ojukwu U.J., Jagaba A.S., Usman Z., Salihu D.A. Natural occurrence of ochratoxin A in some marketed Nigerian foods. Food Control. 2013;31:566–571. doi: 10.1016/j.foodcont.2012.09.043. [DOI] [Google Scholar]
- 16.Njobeh P.B., Dutton M.F., Koch S.H., Chuturgoon A.A., Stoev S.D., Mosonik J.S. Simultaneous occurrence of mycotoxins in human food commodities from Cameroon. Mycotoxin Res. 2010;26:47–57. doi: 10.1007/s12550-009-0039-6. [DOI] [PubMed] [Google Scholar]
- 17.Oyelami O.A., Maxwell S.M., Adeoba E. Aflatoxins and ochratoxin A in the weaning food of Nigerian children. Ann. Trop. Paediatr. 1996;16:137–140. doi: 10.1080/02724936.1996.11747816. [DOI] [PubMed] [Google Scholar]
- 18.Kumar G.D.S., Popat M.N. Farmers’ perceptions, knowledge and management of aflatoxins in groundnuts (Arachis hypogaea L.) in India. Crop Prot. 2010;29:1534–1541. doi: 10.1016/j.cropro.2010.08.019. [DOI] [Google Scholar]
- 19.Matumba L., Monjerezi M., Kankwamba H., Njoroge S.M.C., Ndilowe P., Kabuli H., Kambewa D., Njapau H. Knowledge, attitude, and practices concerning presence of molds in foods among members of the general public in Malawi. Mycotoxin Res. 2016;32:27–36. doi: 10.1007/s12550-015-0237-3. [DOI] [PubMed] [Google Scholar]
- 20.Siegrist M., Cvetkovich G. Better negative than positive? Evidence of a bias for negative information about possible health dangers. Risk Anal. 2001;21:199–206. doi: 10.1111/0272-4332.211102. [DOI] [PubMed] [Google Scholar]
- 21.Adegunloye D.V., Agarry O.O., Adebolu T.T., Adetuyi F. Effect of leaf-packaging on the microbiological assessment of some food items. J. Biotechnol. 2006;5:445–447. [Google Scholar]
- 22.Hell K., Cardwell K.F., Poehling H.M. Relationship between management practices, fungal infection and aflatoxin for stored maize in Benin. J. Phytopathol. 2003;151:690–698. doi: 10.1046/j.1439-0434.2003.00792.x. [DOI] [Google Scholar]
- 23.Milani J. Ecological conditions affecting mycotoxin production in cereals: A review. Vet. Med. (Praha) 2013;58:405–411. [Google Scholar]
- 24.Adekoya I., Obadina A., Phoku J., Nwinyi O., Njobeh P. Contamination of fermented foods in Nigeria with fungi. LWT Food Sci. Technol. 2017;86:76–84. doi: 10.1016/j.lwt.2017.07.044. [DOI] [Google Scholar]
- 25.James B., Adda C., Cardwell K., Annang D., Hell K., Korie S., Edorh M., Gbeassor F., Nagatey K., Houenou G. Public information campaign on aflatoxin contamination of maize grains in market stores in Benin, Ghana and Togo. Food Addit. Contam. 2007;24:1283–1291. doi: 10.1080/02652030701416558. [DOI] [PubMed] [Google Scholar]
- 26.Dosman D.M., Adamowicz W., Hrudey S.E. Socioeconomic determinants of health and food safety related risk perceptions. Risk Anal. 2001;21:307–318. doi: 10.1111/0272-4332.212113. [DOI] [PubMed] [Google Scholar]
- 27.Jolly C.M., Bayard B., Awuah R.T., Fialor S.C., Williams J.T. Examining the structure of awareness and perceptions of groundnut aflatoxin among Ghanaian health and agricultural professionals and its influence on their actions. J. Socio Econ. 2009;38:280–287. doi: 10.1016/j.socec.2008.05.013. [DOI] [Google Scholar]
- 28.Oni O.A., Inedia O.F. Consumer willingness to pay for safety labels in Nigeria: A case study. J. Cent. Eur. Agric. 2005;6:381–388. [Google Scholar]
- 29.Smith J.E., Solomons G., Lewis C., Anderson J.G. Role of mycotoxins in human and animal nutrition and health. Nat. Toxins. 1995;3:187–192. doi: 10.1002/nt.2620030404. [DOI] [PubMed] [Google Scholar]
- 30.European Commission (EC) Commision Regulation (EC) No 401/2006 of 23 February 2006 laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs. Off. J. Eur. Union. 2006;L70:12–34. [Google Scholar]
- 31.European Commission (EC) Commision Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union. 2006;L364:5–24. [Google Scholar]
- 32.Williams J.H., Phillips T.D., Jolly P.E., Stiles J.K., Jolly C.M., Aggarwal D. Human aflatoxicosis in developing countries: A review of toxicology, exposure, potential health consequences, and interventions. Am. J. Clin. Nutr. 2004;80:1106–1122. doi: 10.1093/ajcn/80.5.1106. [DOI] [PubMed] [Google Scholar]
- 33.Ezekiel C.N., Sulyok M., Somorin Y., Odutayo F.I., Nwabekee S.U., Balogun A.T., Krska R. Mould and mycotoxin exposure assessment of melon and bush mango seeds, two common soup thickeners consumed in Nigeria. Int. J. Food Microbiol. 2016;237:83–91. doi: 10.1016/j.ijfoodmicro.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 34.Kumar V., Basu M.S., Rajendran T.P. Mycotoxin research and mycoflora in some commercially important agricultural commodities. Crop Prot. 2008;27:891–905. doi: 10.1016/j.cropro.2007.12.011. [DOI] [Google Scholar]
- 35.International Agency for Research on Cancer (IARC) Some naturally occurring substances: Food items and constituents, heterocyclic aromatic amines and mycotoxins. IARC Monogr. Eval. Carcinog. Risks Hum. 2002;96:1–390. doi: 10.1002/food.19940380335. [DOI] [Google Scholar]
- 36.Finoli C., Vecchio A., Galli A., Dragoni I. Roquefortine C occurrence in blue cheese. J. Food Prot. 2001;64:246–251. doi: 10.4315/0362-028X-64.2.246. [DOI] [PubMed] [Google Scholar]
- 37.Odunfa S.A., Adeyele S. Microbiological changes during the traditional production of ogi-baba, a West African fermented sorghum gruel. J. Cereal Sci. 1985;3:173–180. doi: 10.1016/S0733-5210(85)80027-8. [DOI] [Google Scholar]
- 38.Mbundi L., Gallar-Ayala H., Khan M.R., Barber J.L., Losada S., Busquets R. Advances in the analysis of challenging food contaminants: Nanoparticles, bisphenols, mycotoxins, and brominated flame retardants. Adv. Mol. Toxicol. 2014;8:35–105. doi: 10.1016/B978-0-444-63406-1.00002-7. [DOI] [Google Scholar]
- 39.Schollenberger M., Müller H.M., Ernst K., Sondermann S., Liebscher M., Schlecker C., Wischer G., Drochner W., Hartung K., Piepho H.P. Occurrence and distribution of 13 trichothecene toxins in naturally contaminated maize plants in Germany. Toxins (Basel) 2012;4:778–787. doi: 10.3390/toxins4100778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.European Food Safety Authority (EFSA) Scientific Opinion on the risks for animal and public health related to the presence of T-2 and HT-2 toxin in food and feed. EFSA J. 2011;9:1–187. doi: 10.2903/j.efsa.2011.2481. [DOI] [Google Scholar]
- 41.Kimura M., Tokai T., Takahashi-Ando N., Ohsato S., Fujimura M. Molecular and genetic studies of fusarium trichothecene biosynthesis: Pathways, genes, and evolution. Biosci. Biotechnol. Biochem. 2007;71:2105–2123. doi: 10.1271/bbb.70183. [DOI] [PubMed] [Google Scholar]
- 42.Shirima C.P., Kimanya M.E., Routledge M.N., Srey C., Kinabo J.L., Humpf H.U., Wild C.P., Tu Y.K., Gong Y.Y. A prospective study of growth and biomarkers of exposure to aflatoxin and fumonisin during early childhood in Tanzania. Environ. Health Perspect. 2015;123:173–178. doi: 10.1289/ehp.1408097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Food and Agriculture Organization of the United Nations . Worldwide Regulations for Mycotoxins in Food and Feed. Food and Agriculture Organization of the United Nations; Rome, Italy: 2004. Mycotoxin regulations in 2003 and current developments; pp. 9–28. [Google Scholar]
- 44.Ediage E.N., Di Mavungu J.D., Monbaliu S., Van Peteghem C., De Saeger S. A validated multianalyte LC-MS/MS method for quantification of 25 mycotoxins in cassava flour, peanut cake and maize samples. J. Agric. Food Chem. 2011;59:5173–5180. doi: 10.1021/jf2009364. [DOI] [PubMed] [Google Scholar]
- 45.De Boevre M., Di Mavungu J.D., Landschoot S., Audenaert K., Eeckhout M., Maene P., Haesaert G., De Saeger S. Natural occurrence of mycotoxins and their masked forms in food and feed products. World Mycotoxin J. 2012;5:207–219. doi: 10.3920/WMJ2012.1410. [DOI] [Google Scholar]
- 46.Monbaliu S., Van Poucke C., Van Peteghem C., Van Poucke K., Heungens K., De Saeger S. Development of a multi-mycotoxin liquid chromatography/tandem mass spectrometry method for sweet pepper analysis. Rapid Commun. Mass Spectrom. 2009;23:3–11. doi: 10.1002/rcm.3833. [DOI] [PubMed] [Google Scholar]
- 47.European Commission (EC) Commission Decision 2002/657/EC implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Off. J. Eur. Union. 2002;L221:8–36. doi: 10.1017/CBO9781107415324.004. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.