Abstract
Purpose
Intraperitoneal (IP) therapy improves survival compared to intravenous (IV) treatment for women with newly diagnosed, optimally cytoreduced, ovarian cancer. However, the role of IP therapy in recurrent disease is unknown. Preclinical data demonstrated IP administration of the proteasome inhibitor, bortezomib prior to IP carboplatin increased tumor platinum accumulation resulting in synergistic cytotoxicity. We conducted this phase I trial of IP bortezomib and carboplatin in women with recurrent disease.
Methods
Women with recurrent ovarian cancer were treated with escalating doses of IP bortezomib - in combination with IP carboplatin (AUC 4 or 5) every 21 days for 6 cycles. Pharmacokinetics of both agents were evaluated in cycle 1.
Results
Thirty-three women participated; 32 were evaluable for safety. Two patients experienced dose-limiting toxicity (DLT) at the first dose level (carboplatin AUC 5, bortezomib 0.5 mg/m2), prompting carboplatin reduction to AUC 4 for subsequent dose levels. With carboplatin dose fixed at AUC 4, bortezomib was escalated from 0.5 to 2.5 mg/m2 without DLT. Grade 3/4 related toxicities included abdominal pain, nausea, vomiting, and diarrhea which were infrequent. The overall response rate in patients with measurable disease (n = 21) was 19% (1 complete, 3 partial). Cmax and AUC in peritoneal fluid and plasma increased linearly with dose, with a favorable exposure ratio of the peritoneal cavity relative to peripheral blood plasma.
Conclusion
IP administration of this novel combination was feasible and showed promising activity in this phase I trial of heavily pre-treated women with ovarian cancer. Further evaluation of this IP combination should be conducted.
Keywords: Intraperitoneal, Ovarian cancer, Proteasome inhibition, Platinum
1. Introduction
Epithelial ovarian cancer affects over 20,000 women annually, and over 14,000 are projected to die of the disease each year in the United States alone [1]. For women with newly diagnosed, optimally cytoreduced, advanced ovarian cancer, adjuvant treatment that incorporates the intraperitoneal (IP) administration of chemotherapy affords a survival advantage [2–6]. The magnitude of the pharmacologic advantage of administering a drug IP is a function of the rate of clearance of the drug from the peritoneal cavity relative to its clearance from the systemic circulation, referred to as the AUC ratio [7]. In the case of carboplatin, the advantage, expressed as the AUC ratio, is in the range of eighteen- to nineteen-fold [8]. Despite the survival advantages, treatments that includes IP therapy have not been widely adopted [9]. This is likely due to concerns for excess toxicities with IP treatment, related to both drug and catheter-specific issues [10]. In addition, the role of IP therapy for women with relapsed disease is relatively unexplored.
Bortezomib, a dipeptidyl boronic acid, potently inhibits the 20S subunit of the proteasome by which ubiquitinated proteins are degraded. It is currently approved for the treatment of multiple myeloma and mantle cell lymphoma [11–12]. However, there have been limited investigations of this agent administered IV in women with ovarian cancer [13,14]. Pharmacokinetic studies of bortezomib indicate that over 90% of the drug is cleared from the plasma within 15 min of systemic administration following a single dose [15]. However, after multiple doses, the elimination half-life is prolonged, and plasma clearance is decreased [16]. Multiple pharmacodynamic studies of IV bortezomib using an assay for 20S proteasome inhibition [17] demonstrate that proteasome inhibition is dose-dependent, is highest after one hour (~65–70%), and recovers toward baseline levels after approximately 72 h. No significant changes are seen in the levels of 20S proteasome inhibition with repeated dosing (on Days 1, 4, 8 and 11), which suggests that 72 h is sufficient for recovery of proteasome function in normal tissues [18].
Jandial et al. performed preclinical studies of bortezomib using a human ovarian intraperitoneal xenograft model and administering the agent by the IP route rather than IV [19]. They reported that IP bortezomib administration resulted in a 252-fold greater exposure (AUC) for the peritoneal cavity than the plasma. In addition, bortezomib acted as a pharmacologic modulator of platinum uptake in human ovarian cancer cells and tumors in vivo. Furthermore, pretreatment with bortezomib prior to a single dose of cisplatin in mice resulted in significantly higher cellular platinum accumulation within tumor nodules growing on the peritoneal surface of mice by 33% (p = 0.0006). Median effect analysis demonstrated the accumulation was associated with synergistic cytotoxicity. In addition, IP carboplatin results in similar tumor penetration to IP cisplatin in this model, when each agent is given at equitoxic doses [20].
Taken together, these preclinical findings, which demonstrate both the ability of bortezomib to enhance platinum tumor uptake and a potential pharmacologic advantage of intraperitoneally administered bortezomib, supported the hypothesis that dual IP treatment may be an effective treatment option for women with recurrent ovarian cancer, thus providing the rationale for this Phase I trial.
2. Methods
2.1. Study drugs
Bortezomib and carboplatin were administered IP on Day 1 of a 21-day cycle for a planned total of 6 cycles, though patients having a clinical benefit were allowed to continue for more cycles. Patients were treated with bortezomib starting at a dose of 0.5 mg/m2, escalating by a fixed amount each cohort to a maximum dose of 2.5 mg/m2. Initially, carboplatin was fixed at AUC 5. However, at the first dose level (referred to as dose level 1a in Table 1), toxicity was noted and carboplatin was reduced to AUC 4 for all further dose levels (dose levels 1–6).
Table 1.
Dose escalation plan.
| Dose level | Number treated | IP bortezomib (mg/m2) | IP carboplatin (AUC) |
|---|---|---|---|
| 1a | 6 | 0.5 | 5 |
| 1 | 6 | 0.5 | 4 |
| 2 | 5 | 0.9 | 4 |
| 3 | 3 | 1.3 | 4 |
| 4 | 3 | 1.7 | 4 |
| 5 | 3 | 2.1 | 4 |
| 6 | 6 | 2.5 | 4 |
Placement of an IP port was required and performed following the guidelines from the Gynecologic Oncology Group (GOG) Surgical Procedures Manual. Bortezomib was diluted in a volume of 500 mL of 0.9% NaCl and instilled into the peritoneal cavity. Carboplatin was mixed in up to 2 L of normal saline and administered IP one hour after bortezomib administration. Standard antiemetic regimens were used, following guidelines put forth by the National Comprehensive Cancer Network (REF: NCCN Guidelines for Supportive care: Antiemesis V.1.2017. Available at: https://www.nccn.org/professionals/physician_gls/recently_updated.asp).
2.2. Eligibility criteria
Eligible patients must have had recurrent or persistent epithelial ovarian, fallopian tube, or primary peritoneal cancer. Either measurable disease (as defined by RECIST 1.121) or detectable disease (e.g., ascites, pleural effusion, non-measurable disease, or a baseline values of CA-125 at least twice the upper limit of normal) was required. Tumor debulking prior to protocol entry was not required. All patients must have had a GOG Performance Status of 0 to 2.
Patients must have had at least two and were allowed up to four prior regimens (inclusive of the primary treatment). Prior treatment with biologic (non-cytotoxic) therapy either alone or as part of the cytotoxic regimens for management of recurrent or persistent disease was allowed, but prior treatment with bortezomib was not allowed. All prior anti-cancer treatment must have been discontinued three weeks prior to registration. Laboratory criteria for eligibility included an absolute neutrophil count (ANC) ≥ 1500/mcl, platelets ≥ 100,000/mcl, creatinine ≤ 1.5 times the institutional upper limit of normal (ULN), bilirubin <1.2 times the ULN, alanine transaminase and aspartate transaminase ≤ 3.0 times the ULN, and alkaline phosphatase ≤ 2.5 times the ULN. Patients must have had a baseline neuropathy (sensory and motor) ≤ Grade 1 (NCI CTCAE Version 4.
This study was reviewed and approved by the Cancer Therapy Evaluation Program (CTEP) of the National Cancer Institute. All patients gave written informed consent before study entry in compliance with institutional, state, and federal regulations.
2.3. Evaluation of toxicity and of dose-limiting toxicity
Patients underwent weekly laboratory evaluations and toxicity assessments. Dose limiting toxicities (DLTs) were defined by study-related adverse events which occurred in association with the first cycle of treatment. Criteria for hematologic DLTs included dose delay of >3 weeks due to persistent low blood counts; febrile neutropenia; grade 4 neutropenia lasting >7 days; grade 4 thrombocytopenia, or grade 3 thrombocytopenia with clinically significant bleeding. Criteria for non-hematologic DLTs included drug-related death and any grade 3 or 4 adverse event with the exception of the following grade 3 toxicities: abdominal pain deemed related to the port as determined by the treating physician; anorexia; fatigue; nausea and/or vomiting, or diarrhea (lasting ≤ 48 h despite maximal medical management); dehydration (as a result of nausea and vomiting); constipation; metabolic abnormalities (hypokalemia, hypomagnesemia, hypocalcemia, hypophosphatemia that recovered to grade 2 or less within 48 h with or without medical management).
2.4. Dose modifications
Initial treatment modifications consisted of cycle delay or dose reduction with treatment decisions for hematologic toxicity based on the ANC. For the start of a cycle, treatment was delayed for a maximum of three weeks until the ANC was ≥ 1500 cells/mm3 and the platelet count was ≥ 100,000/mm3. Prophylactic use of hematopoietic cytokines and protective reagents was not allowed. Bortezomib dose was reduced by one dose level for patients who experienced dose-limiting neutropenia in the first cycle. Both bortezomib and carboplatin were reduced by one dose level for dose-limiting thrombocytopenia with or without dose-limiting neutropenia. There were no dose modifications on the basis of uncomplicated ANC nadirs lasting <7 days. For dose-limiting non-hematologic toxicities, bortezomib was reduced by one dose level for up to two occurrences. For all patients treated on dose levels 1 through 6, no dose reduction of carboplatin was allowed.
2.5. Pharmacokinetics
All patients underwent plasma collection for quantitative analysis of bortezomib and ultrafilterable platinum. Samples were taken from both the blood (EDTA anticoagulated) and peritoneal fluid at the following time points: pre-infusion of bortezomib, immediately following infusion, 15 min after infusion, then 30 min (corresponding to time prior to carboplatin infusion), 60 min, 90 min, 2 h, 4 h, and 6 h post-bortezomib infusion. An additional collection was obtained immediately following the infusion of carboplatin. Concentrations of total platinum and ultrafilterable platinum (platinum not bound to macromolecules) were quantitated by atomic absorption spectrophotometry (AAS) [22]. Bortezomib concentrations were quantitated by LC-MS/MS as previously described [22] and implemented in the Gynecologic Oncology Group (GOG) Pharmacokinetic Core Facility in Pittsburgh. Based on quality control samples at 0.3, 2, 10, 20, and a dilutional QC at 50 ng/mL, the precision (CV) ranged from 1.3% to 4.0% and the accuracy ranged from 3.7% to 8.3%. Pharmacokinetic parameters were derived by non-compartmental modeling using PK Solutions 2.0 (Summit Research Services, Montrose, CO; www.summitPK.com).
2.6. Response assessment
For patients with measurable disease, RECIST 1.1 criteria were used to define response [21].
2.7. Statistical considerations
This trial used a standard 3 + 3 design during dose escalation trial. If one patient out of three experienced a DLT, an additional three patients were enrolled at that dose level. The MTD was determined by the maximum dose level achieved at which ≤ 1 patient (among six) experienced a DLT. No intra-patient dose escalation was allowed. No patients were enrolled at the next higher level of dose until all patients at the previous dose level had been followed through the end of the first cycle. In addition, patients who did not complete one cycle of therapy and who did not have a DLT were replaced. Patients who discontinued treatment due to port-related complications were also replaced. Progression-free survival was summarized using Kaplan-Meier method. Descriptive analysis was used to characterize the pharmacokinetic parameters of bortezomib and carboplatin.
3. Results
3.1. Demographics
Thirty-three patients participated in this study, 32 of whom were treated and thus evaluable for safety. One subject was unevaluable due to technical issues related to IP port placement after enrollment and was removed from the study. The median age of participants was 60 years (range, 24 to 79). Measurable disease was present in 21 patients (66%). Demographic data is summarized in Table 2.
Table 2.
Demographics.
| Characteristic | N = 32 n (%) |
|---|---|
| Age (y) | |
| <60 | 16 (50) |
| >60 | 16 (50) |
| Ethnicity | |
| Hispanic | 2 (6) |
| Non-Hispanic | 25 (78) |
| Unknown | 5 (16) |
| Race | |
| White | 30 (94) |
| Black | 1 (3) |
| American Indian | 1 (3) |
| Performance status | |
| 0 | 21 (66) |
| 1 | 10 (31) |
| 2 | 1 (3) |
| Prior radiotherapy | 1 (3) |
| Number of prior chemotherapy regimens | |
| 1 | 1 (3) |
| 2 | 12 (38) |
| 3 | 17 (53) |
| 4 | 2 (6) |
| Number of prior platinum-containing regimens | |
| 1 | 16 (50) |
| 2 | 12 (38) |
| 3 | 4 (13) |
| Histology | |
|
| |
| Serous adenocarcinoma | 20 (63) |
| Adenocarcinoma, unspecified | 5 (16) |
| Endometrioid adenocarcinoma | 3 (9) |
| Mixed epithelial carcinoma | 2 (6) |
| Clear cell carcinoma | 1 (3) |
| Mucinous adenocarcinoma | 1 (3) |
3.2. Adverse events
At dose level (DL) 1a, two patients experienced DLT. One patient had grade 3 abdominal pain, and the other patient experienced grade 4 thrombocytopenia and a grade 3 lung infection. Due to these toxicities, the dose of carboplatin was reduced to a fixed dose of AUC 4 for subsequent patients, and dose escalation of bortezomib proceeded. The dose of bortezomib was then escalated from DL1 to DL6 (Table 1) with no DLTs noted. While this trial did not establish the maximum tolerated dose (MTD), carboplatin AUC 4 plus bortezomib 2.5 mg/m2 was tolerable and may be investigated further.
Adverse events (AEs) are presented overall and by dose level (Supplemental file). Grade 3/4 toxicities associated with treatment were infrequent but included the following (incidence ≥ 10%): neutropenia (16%), nausea (16%), thrombocytopenia (13%), anemia (13%), abdominal pain (13%), diarrhea (13%) and vomiting (13%).
Four patients (13%) experienced carboplatin hypersensitivity reactions (grade 3 after cycle 2 in two patients, grade 2 after cycle 2 in one patient, and grade 1 after cycle 4 in one patient). Three of these patients had received 12 prior cycles of platinum chemotherapy, and one had received eight. Reactions were similar to those typically encountered with IV carboplatin and all patients responded well to conservative management. None of these patients were re-challenged with IP carboplatin.
3.3. Evaluation of activity
Among 21 patients with measurable disease, 4 responses (19%, 95% CI: 5%, 42%) were seen including one complete responder treated at DL5. Partial responses were seen at DL1, DL4, and DL6. Of the four responses, three patients had a serous adenocarcinoma, and one had a mixed epithelial carcinoma. An additional 14 patients (67%) experienced stable disease. In all treated patients, median progression-free survival (PFS) was 4.9 months (95% CI: 2.9, 6.7 months).
3.4. Pharmacokinetic data
Bortezomib pharmacokinetic data was available from plasma in 29 patients, from peritoneal fluid in 28 patients and from both in 26 patients (Table 3 and Figs. 1A, 2). Bortezomib peritoneal fluid concentrations peaked at the end of instillation, while bortezomib plasma concentrations peaked approximately half an hour later at approximately 2 orders of magnitude lower levels. Bortezomib exposure in both the peritoneal fluid and peripheral blood plasma increased with dose while the ratio of peritoneal fluid to plasma exposure appeared to decrease somewhat yet remained relatively high across the dose range studied.
Table 3.
Bortezomib geometric mean (SD) peripheral blood plasma (PL) and peritoneal fluid (PF) pharmacokinetic parameters.
| Dose (mg/m2) | Peritoneal fluid | Plasma | PF/PL-ratio | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||||
| Cmax (μg/mL) | Tmax (min) | AUC0 – t (μg/mL·min) | AUC0 – infa (μg/mL·min) | t½ (min) | Cl/F (mL/h/m2) | Vss/F (mL/m2) | Cmax (ng/mL) | Tmax (min) | AUC0 – t (μg/mL·min) | t½ (min) | Cmax | AUC0 – t | |
| 0.5 (N = 9) | 0.86 (0.33) | 44 (19) | 55 (32) | 57 (34) | 46 (62) | 524 (452) | 695 (453) | 1.59 (1.14) | 94 (48) | 0.27 (0.18) | 210 (85) | 586 (525) | 224 (204) |
| 0.9 (N = 4) | 1.62 (0.62) | 43 (31) | 125 (30) | 140 (58) | 153 (182) | 385 (127) | 744 (277) | 2.33 (4.44) | 81 (67) | 0.45 (0.50) | 296 (169) | 633 (1280) | 271 (853) |
| 1.3 (N = 3) | 1.07 (1.31) | 60 (24) | 88 (104) | 88 (104) | 10 (2) | 884 (2337) | 988 (3532) | 7.13 (3.76) | 73 (14) | 1.04 (0.44) | 192 (225) | 150 (172) | 85 (141) |
| 1.7 (N = 3) | 1.21 (1.89) | 50 (25) | 126 (116) | 127 (115) | 47 (18) | 804 (1091) | 1354 (4814) | 12.4 (0.7) | 73 (10) | 1.83 (0.34) | 122 (12) | 98 (163) | 69 (83) |
| 2.1 (N = 3) | 4.03 (0.99) | 43 (13) | 246 (138) | 258 (136) | 95 (20) | 488 (269) | 769 (954) | 17.7 (2.5) | 70 (25) | 2.89 (0.53) | 135 (47) | 228 (87) | 85 (62) |
| 2.5 (N = 4) | 3.27 (1.98) | 70 (27) | 222 (154) | 298 (195) | 52 (151) | 503 (555) | 1041 (1036) | 16.9 (11.8) | 105 (35) | 2.46 (2.43) | 113 (40) | 164 (147) | 87 (110) |
| All (N = 26) | – | 50 (23) | – | – | 51 (109) | 547 (876) | 850 (1922) | – | 86 (43) | – | 179 (128) | 304 (665) | 139 (366) |
SD, standard deviation; Cmax, peak drug concentration; Tmax, time to peak concentration; t1/2, elimination half-life; Cl/F, apparent total clearance of the drug from plasma; Vss/F, apparent volume of distribution at steady state.
mean extrapolated portion represented 6.3% of AUC0 – inf (range 0.004–75.6%).
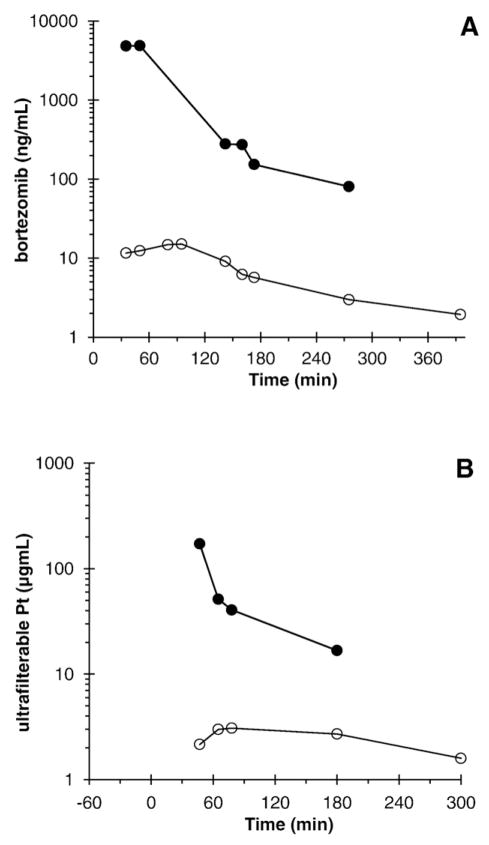
Fig. 1.
Peritoneal fluid (●) and plasma (○) bortezomib (A) and ultrafilterable platinum (B) concentrations of a patient treated with 2.1 mg/m2 bortezomib and carboplatin AUC 4.
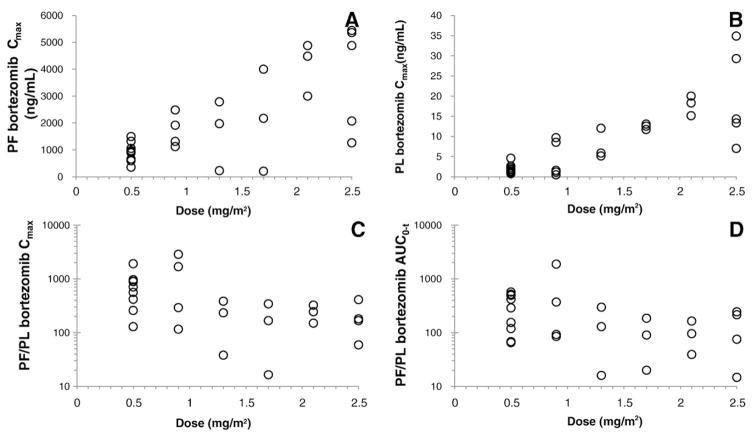
Fig. 2.
Bortezomib Cmax (A) and AUC0 – t (B) as a function of bortezomib dose, and the peritoneal fluid (PF) to peripheral blood plasma exposure ratios based on Cmax (C) and AUC0 – t (D) plotted on a semi-log y-axis as a function of bortezomib dose.
Useable platinum pharmacokinetic data was available from 10 patients, all treated at AUC 4 (Table 4 and Fig. 1B). Ultrafilterable platinum concentrations peaked at the end of instillation, while plasma concentrations peaked approximately 40 min later at approximately 1 order of magnitude lower levels. The ultrafilterable platinum concentrations represented most of the total platinum concentrations both in the peritoneal compartment and the plasma compartment.
Table 4.
Ultrafilterable platinum geometric mean (SD) peripheral blood plasma (PL) and peritoneal fluid (PF) pharmacokinetic parameters.
| Dose (AUC) | Peritoneal fluid | Plasma | PF/PL-ratio | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| Cmax (μg/mL) | Tmax (min) | AUC0 – t (mg/mL·min) | Cmax (ng/mL) | Tmax (min) | AUC0 – t (mg/mL·min) | Cmax | AUC0 – t | |
| 4 (N = 10) | 157 (93) | 38 (19) | 21.6 (17.0) | 3.7 (1.4) | 77 (50) | 1.45 (0.55) | 42.4 (35.5) | 14.9 (17.6) |
SD, standard deviation.
All weight units are expressed as elemental platinum, except AUC, which is expressed as carboplatin.
PK analysis shows that the bortezomib maximal concentration (Cmax) increased linearly with dose in both the plasma (PL) (Table 3 and Fig. 2) and peritoneal fluid (PF) with an excess of 100-fold greater concentration in the peritoneal cavity than in plasma (Figs. 1 and 2). In addition, when the therapeutic ratio (PF/PL) between bortezomib and ultrafilterable platinum in the plasma and peritoneal fluid are compared (Fig. 2), we identified a large difference and high therapeutic ratio, indicating that bortezomib stays predominantly in the peritoneal fluid.
4. Discussion
This is the first prospective trial of a multidrug IP regimen conducted for women with recurrent ovarian cancer. IP bortezomib, in combination with IP carboplatin fixed at an AUC 4, was successfully escalated up to 2.5 mg/m2 without dose-limiting toxicities. Our pharmacokinetic analyses show that bortezomib exposure increased with dose in both the plasma and peritoneal fluid with an exposure ratio in excess of 100-fold in favor of the peritoneal cavity. Previously reported pharmacokinetic parameters of intravenous bortezomib at 1 mg/m2 resulted in plasma Cmax of 85–144 ng/mL and plasma AUC values of 6.5–10.3 μg/mL·min [23]. Similarly, the ultrafilterable platinum exposures documented in this trial suggest a favorable exposure ratio. The high proportion of total plasma platinum accounted for by ultrafilterable platinum suggests that the platinum detected in plasma, though ultrafilterable, no longer possesses the ability to platinate macromolecules, and is consequently inactive. Therefore, the already advantageous peritoneal/plasma AUC ratio of approximately 15–42 is likely an underestimate of the true exposure ratio of active platinum species.
An objective response rate of 19% was observed in this trial and 67% of patients were able to achieve stable disease in this heavily pretreated population. In the current trial, disease burden and location varied widely, but the overall response rate of 19% was attained in the face of substantial tumor burden in many patients, which runs counter to the presumed role of IP therapy for women with ovarian cancer, where it is normally an option only for women with optimally cytoreduced, advanced ovarian cancer [3,24,25].
In addition, this IP regimen was well tolerated by patients with a manageable toxicity profile, evidenced by six patients with stable disease electing to remain on study past the required six cycles of treatment. The AEs produced by this combination reflect the known myelosuppressive activity of these agents. However, the IP combination of these drugs did not have the toxicities seen with the IV combination of bortezomib and carboplatin seen in other trials in this population. In particular, grade 3 sensory peripheral neuropathy, a DLT with the IV combination, was not observed [13]. It is particularly noteworthy that even at the highest dose of bortezomib (2.5 mg/m2) in combination with carboplatin no overt peritonitis occurred and the frequency and severity of abdominal pain was similar to that observed in first line IP chemotherapy trials of cisplatin or carboplatin in combination with paclitaxel.
With respect to platinum hypersensitivity reactions, four patients (13%) experienced carboplatin hypersensitivity reactions. These patients had been heavily treated with platinum prior to enrollment in this study, with a median number of 11 prior platinum chemotherapy cycles. We are not able to find comparative data from other studies of bortezomib and carboplatin because it was not specifically reported in the two prior Phase 1 IV trials of this combination in a similar population [13,14]. However, the approximate rate of hypersensitivity reactions with retreatment using IV carboplatin is 12 to 16% [26,27]. Our data suggest that both the frequency and severity of carboplatin hypersensitivity reactions after IP therapy are similar to historically reported rates, and manifest similarly in terms of time to onset after carboplatin infusion as well as overall symptomatology.
It is important to note that this trial did not establish whether there is a true synergistic interaction between carboplatin and bortezomib. Additional studies of their interaction should be considered in the very high concentration ranges attainable in the peritoneal cavity. This trial points to the possibility of further escalating the IP dose of bortezomib which can be expected to enhance tumor penetration particularly in the case of small volume disease.
While IP treatment is associated with a survival advantage when used in the upfront setting, it is limited to patients with optimally cytoreduced disease [6,24]. This trial is unique in that it offers important insights into the use of IP in patients with advanced recurrent disease, including both measurable disease as well as nonmeasurable miliary disease and carcinomatosis. In addition, a Japanese study presented at the 2016 American Society of Clinical Oncology Annual Meeting suggests that patients with suboptimal residual ovarian cancer may derive benefit from an IP-containing regimen, contrary to our previously established standard recommendations against IP treatment in these patients [28].
Our data suggest that the use of bortezomib may indeed enhance the activity of carboplatin, as demonstrated by the response rates seen in our trial, proposing a potential role among women with newly diagnosed suboptimally cytoreduced ovarian cancer. The activity of the carboplatin and bortezomib combination in this setting also raises the question of whether second line debulking prior to IP therapy for recurrent disease might be considered. Whether it improves outcomes compared to standard therapies remains to be seen, and such a question can only be answered in a carefully conducted randomized trial. However, these data are sufficiently encouraging to warrant further investigation.
Supplementary Material
HIGHLIGHTS.
IP bortezomib + carboplatin can be safely combined in recurrent ovarian cancer patients.
An overall response rate of 19% was seen in this heavily pretreated population.
Intraperitoneal bortezomib exhibits a highly favorable plasma: peritoneal AUC ratio.
Acknowledgments
This study was supported by National Cancer Institute grants NRG Oncology SDMC grant U10 CA180822 and the NRG Oncology Operations grant U10CA 180868.
The following Gynecologic Oncology Group member institutions participated in the primary treatment studies: Washington University School of Medicine, University of Oklahoma Health Sciences Center, Women and Infants Hospital, University of Virginia, The Hospital of Central Connecticut, Virginia Commonwealth University, University of Iowa Hospitals and Clinics, Hillcrest Hospital Cancer Center, Hartford Hospital.
Footnotes
This project used the UPCI Cancer Pharmacokinetics and Pharmacodynamics Facility (CPPF) and was supported in part by award P30-CA47904. In addition, the following National Cancer Institute grants also supported this study: NRG Oncology Operations grant number U10 CA180868 as well as NRG SDMC grant U10 CA180822, Gynecologic Oncology Group (GOG) Administrative Office and the GOG Tissue Bank (CA 27469) and the GOG Statistical and Data Center (CA 37517).
This study was presented in abstract form at the ASCO meeting held in Chicago, IL on 5/29 to 6/2015. J Clin Oncol 33(15s): ASCO#5515, 2015. NCT01074411.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ygyno.2017.03.013.
Conflicts of interest
Dr. Bill Brady reports that he received grants from the NCI (1 U10 CA 180822-01, 5 U10 CA037517-29, P30 CA16056) during the conduct of the submitted work. Additionally, Dr. Brady reports that he has received a grant from Nektar Therapeutics and Advaxis outside the submitted work. Dr. Russell Schilder reports that has received personal fees from Celsion as Chair, IDMC. Jan Beumer reports that the pharmacologic work was supported in part by Millenium Pharmaceuticals. Dr. Matthew Powell reports serving as paid consultant/speaker for Roche/Genentech and AstraZeneca. Dr. Kathleen Moore repots serving on the Advisory Board of Genentech Roche, Astra Zeneca, Amgen, Immunogen, TESARO, Clovis, Advaxis.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Ma C, Mandrekar SJ, Alberts SR, Croghan GA, Jatoi A, Reid JM, et al. A phase I and pharmacologic study of sequences of the proteasome inhibitor, bortezomib (PS-341, Velcade), in combination with paclitaxel and carboplatin in patients with advanced malignancies. Cancer Chemother Pharmacol. 2007;59:207–215. doi: 10.1007/s00280-006-0259-9. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong DK, Bundy B, Wenzel L, Wenzel L, Huang HQ, Baergen R, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 4.Elit L, Oliver TK, Covens A, Kwon J, Fung MF, Hirte HW, et al. Intraperitoneal chemotherapy in the first-line treatment of women with stage III epithelial ovarian cancer: a systematic review with metaanalyses. Cancer. 2007;109:692–702. doi: 10.1002/cncr.22466. [DOI] [PubMed] [Google Scholar]
- 5.Markman M, Bundy BN, Alberts DS, Fowler JM, Clark-Pearson DL, Carson LF, et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol. 2001;19:1001–1007. doi: 10.1200/JCO.2001.19.4.1001. [DOI] [PubMed] [Google Scholar]
- 6.Tewari D, Java JJ, Salani R, Armstrong DK, Markman M, Herzog T, et al. Long-term survival advantage and prognostic factors associated with intraperitoneal chemotherapy treatment in advanced ovarian cancer: a gynecologic oncology group study. J Clin Oncol. 2015;33:1460–1466. doi: 10.1200/JCO.2014.55.9898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howell SB, Section XV. Principles of chemotherapy, 4. Regional chemotherapy. In: Holland JF, Frei EI, Bast RC Jr, Kufe D, Morton R, Weischselbaum RR, editors. Cancer Medicine. 3. Lea & Febiger; Philadelphia: 1993. pp. 640–652. [Google Scholar]
- 8.Howell SB, Pfeifle CL, Wung WE, Olshen RA, Lucas WE, Yon JL, et al. Intraperitoneal cisplatin with systemic thiosulfate protection. Ann Intern Med. 1982;97:845–851. doi: 10.7326/0003-4819-97-6-845. [DOI] [PubMed] [Google Scholar]
- 9.Wright AA, Cronin A, Milne DE, Bookman MA, Burger Ra, Cohn DE, et al. Use and effectiveness of intraperitoneal chemotherapy for treatment of ovarian cancer. J Clin Oncol. 2015;33:2841–2847. doi: 10.1200/JCO.2015.61.4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walker JL, Armstrong DK, Huang HQ, Fowler J, Webster K, Burger RA, et al. Intraperitoneal catheter outcomes in a phase III trial of intravenous versus intraperitoneal chemotherapy in optimal stage III ovarian and primary peritoneal cancer: a Gynecologic Oncology Group Study. Gynecol Oncol. 2006;100:27–32. doi: 10.1016/j.ygyno.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 11.Jagannath S, Barlogie B, Berenson J, Siegel D, Irwin D, Richardson PG, et al. A phase 2 study of two doses of bortezomib in relapsed or refractory myeloma. Br J Haematol. 2004;127:165–172. doi: 10.1111/j.1365-2141.2004.05188.x. [DOI] [PubMed] [Google Scholar]
- 12.Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348:2609–2617. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- 13.Aghajanian C, Dizon DS, Sabbatini P, Raizer JJ, Dupont J, Spriggs DR. Phase I trial of bortezomib and carboplatin in recurrent ovarian or primary peritoneal cancer. J Clin Oncol. 2005;23:5943–5949. doi: 10.1200/JCO.2005.16.006. [DOI] [PubMed] [Google Scholar]
- 14.Ramirez PT, Landen CN, Jr, Coleman RL, Milam MR, Levenback C, Johnston TA, et al. Phase I trial of the proteasome inhibitor bortezomib in combination with carboplatin in patients with platinum- and taxane-resistant ovarian cancer. Gynecol Oncol. 2008;108:68–71. doi: 10.1016/j.ygyno.2007.08.071. [DOI] [PubMed] [Google Scholar]
- 15.Adams J, Kauffman M. Development of the proteasome inhibitor Velcade (Bortezomib) Cancer Investig. 2014;22:304–311. doi: 10.1081/cnv-120030218. [DOI] [PubMed] [Google Scholar]
- 16.Ogawa Y, Suzuki K, Sakai A, Iida S, Ogura Tobinai K, et al. Phase I/II study of bortezomib-melphalan-prednisolone for previously untreated Japanese patients with multiple myeloma. Cancer Sci. 2013;104:912–919. doi: 10.1111/cas.12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lightcap ES, McCormack TA, Pien CS, Chau V, Adams J, Elliott PJ. Proteasome inhibition measurements: clinical application. Clin Chem. 2000;46:673–684. [PubMed] [Google Scholar]
- 18.Aghajanian C, Soignet S, Dizon DS, Pien CS, Adams J, Elliott PJ, et al. A phase I trial of the novel proteasome inhibitor PS341 in advanced solid tumor malignancies. Clin Cancer Res. 2002;8:2505–2511. [PubMed] [Google Scholar]
- 19.Jandial DD, Farshchi-Heydari S, Larson CA, Elliott GI, Wrasidlo WJ, Howell SB. Enhanced delivery of cisplatin to intraperitoneal ovarian carcinomas mediated by the effects of bortezomib on the human copper transporter 1. Clin Cancer Res. 2009;15:553–560. doi: 10.1158/1078-0432.CCR-08-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jandial DD, Messer K, Farshchi-Heydari S, Pu M, Howell SB. Tumor platinum concentration following intraperitoneal administration of cisplatin versus carboplatin in an ovarian cancer model. Gynecol Oncol. 2009;115:362–366. doi: 10.1016/j.ygyno.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 22.Colville H, Dzadony R, Kemp R, Stewart S, Zeh HJ, 3rd, Bartlett DL, et al. In vitro circuit stability of 5-fluorouracil and oxaliplatin in support of hyperthermic isolated hepatic perfusion. J Extra Corpor Technol. 2010;42:75–79. [PMC free article] [PubMed] [Google Scholar]
- 23.Venkatakrishnan K, Rader M, Ramanathan RK, Ramalingam S, Chen E, Riordan W, et al. Effect of the CYP3A inhibitor ketoconazole on the pharmacokinetics and pharmacodynamics of bortezomib in patients with advanced solid tumors: a prospective, multicenter, open-label, randomized, two-way crossover drug-drug interaction study. Clin Ther. 2009;31(Pt 2):2444–2458. doi: 10.1016/j.clinthera.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 24.Howell SB, Zimm S, Markman M, Abramson IS, Cleary S, Lucas WE, et al. Long-term survival of advanced refractory ovarian carcinoma patients with small-volume disease treated with intraperitoneal chemotherapy. J Clin Oncol. 1987;5:1607–1612. doi: 10.1200/JCO.1987.5.10.1607. [DOI] [PubMed] [Google Scholar]
- 25.Jaaback K, Johnson N. Intraperitoneal chemotherapy for the initial management of primary epithelial ovarian cancer. Cochrane Database Syst Rev. 2006 Jan 25;:CD005340. doi: 10.1002/14651858.CD005340.pub2. [DOI] [PubMed] [Google Scholar]
- 26.Markman M, Kennedy A, Webster K, Elson P, Peterson G, Kulp B, et al. Clinical features of hypersensitivity reactions to carboplatin. J Clin Oncol. 1999;17:1141. doi: 10.1200/JCO.1999.17.4.1141. [DOI] [PubMed] [Google Scholar]
- 27.Polyzos A, Tsavaris N, Kosmas C, Arnaouti T, Kalahanis N, Tsigris C, et al. Hypersensitivity reactions to carboplatin administration are common but not always severe: a 10-year experience. Oncology. 2001;61:129–133. doi: 10.1159/000055363. [DOI] [PubMed] [Google Scholar]
- 28.Hasegawa K, Shmiada M, Takeuchi S, Fujiwara H, Imai Y, Iwasa N, et al. Multicenter phase II study of intraperitoneal carboplatin plus intravenous dose-dense paclitaxel in patients with suboptimally debulked ovarian or primary peritoneal carcinoma. J Clin Oncol. 2016;33(Suppl) abstr 5004. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.