Abstract
BACKGROUND
The previously published results of the Systolic Blood Pressure Intervention Trial showed that among participants with hypertension and an increased cardiovascular risk, but without diabetes, the rates of cardiovascular events were lower among those who were assigned to a target systolic blood pressure of less than 120 mm Hg (intensive treatment) than among those who were assigned to a target of less than 140 mm Hg (standard treatment). Whether such intensive treatment affected patient-reported outcomes was uncertain; those results from the trial are reported here.
METHODS
We randomly assigned 9361 participants with hypertension to a systolic blood-pressure target of less than 120 mm Hg or a target of less than 140 mm Hg. Patient-reported outcome measures included the scores on the Physical Component Summary (PCS) and Mental Component Summary (MCS) of the Veterans RAND 12-Item Health Survey, the Patient Health Questionnaire 9-item depression scale (PHQ-9), patient-reported satisfaction with their blood-pressure care and blood-pressure medications, and adherence to blood-pressure medications. We compared the scores in the intensive-treatment group with those in the standard-treatment group among all participants and among participants stratified according to physical and cognitive function.
RESULTS
Participants who received intensive treatment received an average of one additional anti-hypertensive medication, and the systolic blood pressure was 14.8 mm Hg (95% confidence interval, 14.3 to 15.4) lower in the group that received intensive treatment than in the group that received standard treatment. Mean PCS, MCS, and PHQ-9 scores were relatively stable over a median of 3 years of follow-up, with no significant differences between the two treatment groups. No significant differences between the treatment groups were noted when participants were stratified according to baseline measures of physical or cognitive function. Satisfaction with blood-pressure care was high in both treatment groups, and we found no significant difference in adherence to blood-pressure medications.
CONCLUSIONS
Patient-reported outcomes among participants who received intensive treatment, which targeted a systolic blood pressure of less than 120 mm Hg, were similar to those among participants who received standard treatment, including among participants with decreased physical or cognitive function. (Funded by the National Institutes of Health; SPRINT ClinicalTrials.gov number, NCT01206062.)
The systolic blood pressure intervention Trial (SPRINT) showed that among older adults with hypertension and a high risk of cardiovascular disease, but without prevalent diabetes or a history of stroke, blood-pressure treatment that targeted a systolic blood pressure of less than 120 mm Hg (intensive treatment) led to lower rates of cardiovascular events and death than treatment that targeted a systolic blood pressure of less than 140 mm Hg (standard treatment).1 The adoption of the lower blood-pressure target into clinical practice may be limited by concerns regarding its effect on patient-reported outcomes, such as health status, quality of life, and satisfaction with care.2–4 Although lower rates of cardiovascular events associated with intensive treatment could result in improved health status, serious adverse events associated with low end-organ perfusion, including symptomatic hypotension, syncope, and acute kidney injury, were more common among trial participants who were randomly assigned to intensive treatment.1 Reductions in cerebral blood flow, especially among older patients who have hypertension as well as physical and cognitive impairment, may lead to light-headedness, confusion, and depression.3,5,6 Participants with a target systolic blood pressure of less than 120 mm Hg received an average of one additional antihypertensive medication.1 Antihypertensive medications may have negative effects on health-related quality of life.7 Therefore, it is important to evaluate the effect of intensive treatment for hypertension, not only on the rates of cardiovascular events and death, but also on outcomes that are important to a patient’s perception of well-being and satisfaction.
Using patient-reported outcomes from the trial,1 we sought to address three questions. First, did patients randomly assigned to intensive blood-pressure control perceive their health status, as measured by patient-reported outcomes of physical and mental health, differently from patients randomly assigned to standard treatment? Second, did older patients who had hypertension as well as lower physical and cognitive function have different patient-reported outcomes depending on whether they were receiving intensive or standard antihypertensive treatment? Third, because adverse effects from medications may lead to poor adherence to treatment, did intensive treatment affect patient-reported adherence to treatment?
METHODS
TRIAL DESIGN AND OVERSIGHT
The trial design and primary results have been published previously.1,8 In brief, we conducted a multicenter, randomized, controlled trial that compared two strategies for managing systolic blood pressure in older adults with hypertension: an intensive strategy with a systolic blood-pressure target of less than 120 mm Hg versus a standard-care strategy targeting a systolic blood-pressure of less than 140 mm Hg. The primary outcome was the first occurrence of any component of the composite of myocardial infarction, acute coronary syndrome, stroke, heart failure, or death from cardiovascular disease. The population included participants 50 years of age or older who had a systolic blood pressure between 130 and 180 mm Hg at the screening visit, with the eligible blood-pressure range varying as a function of the number of antihypertensive medications the participants were receiving. Participants were considered to have an increased cardiovascular risk if they had clinical or subclinical cardiovascular disease, chronic kidney disease, or an elevated Framingham risk score, or if they were 75 years of age or older. Patients with diabetes mellitus or a history of stroke were excluded. Enrollment began in November 2010 and ended in March 2013. A total of 9361 participants at 102 clinical sites underwent randomization.
All the components of the trial protocol (which is available with the full text of this article at NEJM.org) were designed and implemented by the steering committee in collaboration with the investigators at the clinics, who collected the data. The steering committee vouches for the accuracy and completeness of the data and for the fidelity of the trial to the protocol. The writing committee analyzed the data, wrote and revised the manuscript, and, together with the steering committee, made the decision to submit the manuscript for publication. The policy of the National Institutes of Health (NIH, the sponsor of the trial) requires the sharing of data; although some data from SPRINT were previously made publicly available, it is anticipated that the complete results, including those provided in this article, will be available through the data repository of the National Heart, Lung, and Blood Institute in late 2018. The trial was approved by the institutional review board at each participating trial site. Two of the blood-pressure–lowering medications used in the trial (azilsartan and azilsartan combined with chlorthalidone) were donated by both Takeda Pharmaceuticals International and Arbor Pharmaceuticals; neither company had any other role in the study.
TRIAL MEASUREMENTS
Patient-reported outcomes were assessed by well-validated measures. The Veterans RAND 12-Item Health Survey (VR-12) was used to describe physical and mental health-related quality of life.9 Scores on the Physical Component Summary (PCS) and Mental Component Summary (MCS) of the VR-12 are standardized with a mean of 50 and a standard deviation of 10; scores range from 0 to 100, with higher scores denoting better physical health and mental health, respectively. Depressive symptoms were measured with the use of the Patient Health Questionnaire 9-item depression scale (PHQ-9).10 Scores on the PHQ-9 range from 0 to 27, with higher scores indicating greater severity of depressive symptoms and scores of 10 or higher suggesting moderate-to-severe depressive symptoms. Data regarding these outcomes were collected at baseline and annually thereafter.
Patient satisfaction with blood-pressure care and with blood-pressure medications were each assessed by means of responses to single questions scored on a five-point Likert scale that ranged from very satisfied to very dissatisfied. Adherence to medication was measured with the use of the eight-item Morisky Medication Adherence Scale; scores range from 0 to 8, with higher scores indicating better adherence. It has been suggested that a 2-point change over time on the Morisky Medication Adherence Scale represents a meaningful change in adherence to antihypertensive medication.11,12 Data on satisfaction with treatment and on adherence to medication were collected at baseline, 12 months, and 48 months. Participants who were not receiving antihypertensive medications at the time of assessment did not respond to the questions regarding satisfaction with medications and medication adherence.
We continued to collect data on patient-reported outcomes in our trial after the onset of nonfatal trial end points. The current analyses include data that were collected through August 20, 2015. On that date, the director of the National Heart, Lung, and Blood Institute accepted a recommendation from the data and safety monitoring board to inform the investigators and participants of the cardiovascular-outcome results after analyses of the primary outcome exceeded the monitoring boundary at two consecutive time points, thus initiating the process to end the blood-pressure intervention early.1 Because of the early termination of the intervention, only limited data on patient satisfaction and adherence to medication were available at 48 months; those data are not included in the current analyses.
Outcomes in the standard-treatment group and the intensive-treatment group were compared in unstratified analyses as well as after stratification according to physical and cognitive function at baseline. Stratification according to the number of physical coexisting conditions at baseline and according to health-related quality of life at baseline (lower vs. higher) was prespecified in the trial protocol. Lower physical function was operationalized as a score of less than 40 on the PCS. Coexisting conditions were reported by the participants with the use of the Selim comorbidity index, which assesses 30 medical conditions and 6 mental health conditions.13 The physical score on the Selim comorbidity index was calculated as the sum of the number of these 30 possible medical conditions reported, and the mental score was the sum of the number of these 6 possible mental health conditions reported. In exploratory analyses, we also considered additional stratifications according to age (<75 years vs. ≥75 years), Montreal Cognitive Assessment (MoCA) score (<25th percentile vs. ≥25th percentile, on the basis of normative data specific to age, educational level, and race from the Irish Longitudinal Study on Ageing; scores range from 0 to 30, with higher scores indicating better cognitive performance),14,15 and frailty status, which was based on a 36-item frailty index that aggregates information on coexisting conditions, laboratory test results, cognitive status, physical functioning, and health status. Frailty index scores range from 0 to 1, with higher scores indicating greater frailty.16
STATISTICAL ANALYSIS
Baseline characteristics were analyzed as means and standard deviations or medians and inter-quartile ranges for continuous variables and as frequency distributions for categorical variables. Differences at baseline were examined with the use of Student’s t-test or the Wilcoxon rank-sum test as appropriate for continuous variables and with the use of the chi-square test or Fisher’s exact test for categorical variables. Linear mixed models were used to compare longitudinal trajectories for each of the patient-reported outcomes between the two treatment groups, assuming linear change over time (see Supplementary Methods in the Supplementary Appendix, available at NEJM.org). The models included participant-specific and clinic-specific random effects to address within-participant correlations as a result of repeated assessments and correlations among participants at the same trial site. Fixed effects in the model included treatment group, follow-up time, and the interaction between treatment group and follow-up time. For each outcome, we also tested for interactions between treatment group and subgroups that were defined according to the number of medical coexisting conditions, the number of mental health coexisting conditions, PCS score (lower vs. higher), age, MoCA score, and frailty status. We also conducted sensitivity analyses using multiple imputation to examine the effect of missing data (see Supplementary Methods in the Supplementary Appendix). The analyses were performed with the use of SAS software, version 9.4 (SAS Institute), or the R statistical computing environment.17 All the hypothesis tests were two-sided, and P values of less than 0.05 were considered to indicate statistical significance. No adjustments for multiple comparisons were made.
RESULTS
TRIAL PARTICIPANTS
We randomly assigned 9361 participants to receive either intensive treatment (4678 participants) or standard treatment (4683 participants). Completion rates for the VR-12 PCS assessment were 99.6% at baseline, 92.0% at 12 months, and remained above 87% for the remaining follow-up visits (Table S1 in the Supplementary Appendix). The mean (±SD) PCS score at baseline was 44.7±10.3, and the mean MCS score was 53.1±9.6. The mean PHQ-9 score was 3.1±4.2. Overall, 85.0% of the participants were satisfied or very satisfied with their blood-pressure care at baseline. Among the participants who were receiving blood-pressure medications at baseline, 76.2% were satisfied or very satisfied with their medications, and 38.2% reported high adherence (Morisky Medication Adherence Scale score of 8). There were no significant differences between the two treatment groups at baseline with respect to a broad range of measures of coexisting conditions, physical function, and mental function (Table 1, and Table S2 in the Supplementary Appendix). The mean systolic blood pressure at baseline was 139.7±15.6 mm Hg, and the mean was similar in the two groups. At 1 year after randomization, the mean systolic blood pressure was 136.2 mm Hg in the standard-treatment group and 121.4 mm Hg in the intensive-treatment group, a difference of 14.8 mm Hg (95% confidence interval [CI], 14.3 to 15.4).
Table 1.
Baseline Characteristics of Trial Participants.*
| Variable | Intensive Treatment (N = 4678) | Standard Treatment (N = 4683) |
|---|---|---|
| VR-12 PCS† | ||
| Score | 44.6±10.4 | 44.8±10.2 |
| Score <40 — no./total no. (%) | 1420/4656 (30.5) | 1412/4662 (30.3) |
| VR-12 MCS† | ||
| Score | 53.2±9.6 | 53.1±9.5 |
| Score <40 — no./total no. (%) | 507/4653 (10.9) | 481/4659 (10.3) |
| PHQ-9 score‡ | ||
| Score | 3.1±4.1 | 3.1±4.2 |
| Score ≥10 — no./total no. (%) | 371/4654 (8.0) | 358/4659 (7.7) |
| Level of satisfaction with blood-pressure care — no. (%) | ||
| Very satisfied | 2403 (51.4) | 2371 (50.6) |
| Satisfied | 1570 (33.6) | 1611 (34.4) |
| Neutral | 504 (10.8) | 513 (11.0) |
| Dissatisfied | 135 (2.9) | 121 (2.6) |
| Very dissatisfied | 29 (0.6) | 26 (0.6) |
| Missing data | 37 (0.8) | 41 (0.9) |
| Level of satisfaction with medications received for blood pressure — no./total no. (%)§ | ||
| Very satisfied | 1399/4246 (32.9) | 1383/4233 (32.7) |
| Satisfied | 1825/4246 (43.0) | 1857/4233 (43.9) |
| Neutral | 724/4246 (17.1) | 727/4233 (17.2) |
| Dissatisfied | 187/4246 (4.4) | 162/4233 (3.8) |
| Very dissatisfied | 26/4246 (0.6) | 29/4233 (0.7) |
| Missing data | 85/4246 (2.0) | 75/4233 (1.8) |
| Morisky Medication Adherence Scale category — no./total no. (%)§¶ | ||
| High, score of 8 | 1641/4246 (38.6) | 1600/4233 (37.8) |
| Medium, score of 6 to <8 | 1646/4246 (38.8) | 1686/4233 (39.8) |
| Low, score of <6 | 885/4246 (20.8) | 879/4233 (20.8) |
| Missing data | 74/4246 (1.7) | 68/4233 (1.6) |
| Selim medical comorbidity index — no. (%) || | ||
| ≤2 | 1245 (26.6) | 1287 (27.5) |
| 3 or 4 | 1499 (32.0) | 1485 (31.7) |
| 5 or 6 | 1094 (23.4) | 1039 (22.2) |
| ≥7 | 823 (17.6) | 857 (18.3) |
| Missing data | 17 (0.4) | 15 (0.3) |
| Selim mental comorbidity index — no. (%) || | ||
| 0 | 3471 (74.2) | 3499 (74.7) |
| 1 | 739 (15.8) | 730 (15.6) |
| ≥2 | 456 (9.7) | 440 (9.4) |
| Missing data | 12 (0.3) | 14 (0.3) |
| Frailty status — no. (%)** | ||
| Fit, frailty index ≤0.10 | 856 (18.3) | 891 (19.0) |
| Less fit, frailty index >0.10 and ≤0.21 | 2488 (53.2) | 2511 (53.6) |
| Frail, frailty index >0.21 | 1304 (27.9) | 1256 (26.8) |
| Missing data | 30 (0.6) | 25 (0.5) |
| MoCA score†† | ||
| Median (IQR) | 23 (20–26) | 23 (20–26) |
| Score less than the normative 25th percentile — no./total no. (%) | 1875/4646 (40.4) | 1925/4650 (41.4) |
Plus-minus values are means ±SD. There were no significant differences (P<0.05) between the two treatment groups in the above characteristics evaluated at baseline. Percentages may not sum to 100 because of rounding. IQR denotes interquartile range.
Scores on the Physical Component Summary (PCS) and Mental Component Summary (MCS) of the Veterans RAND 12-Item Health Survey (VR-12) are standardized with a mean of 50 and a standard deviation of 10; scores range from 0 to 100, with higher scores denoting better physical health and mental health, respectively. Lower physical function and mental health were operationalized as a score of less than 40 on the PCS and MCS, respectively.
Scores on the Patient Health Questionnaire 9-item depression scale (PHQ-9) range from 0 to 27, with higher scores indicating greater severity of depressive symptoms and with scores of 10 or higher suggesting moderate-to-severe depressive symptoms.
The analysis of this variable included only participants who were receiving at least one antihypertensive agent at baseline.
Scores on the Morisky Medication Adherence Scale range from 0 to 8, with higher scores indicating better adherence.
Scores on the Selim medical comorbidity index range from 0 to 30, with higher scores indicating more coexisting medical conditions. Scores on the Selim mental comorbidity index range from 0 to 6, with higher scores indicating more coexisting mental health conditions.
Frailty index scores range from 0 to 1, with higher scores indicating greater frailty.
Montreal Cognitive Assessment (MoCA) scores range from 0 to 30, with higher scores indicating better cognitive performance. The normative 25th percentile was based on normative data specific to age, educational level, and race from the Irish Longitudinal Study on Ageing.
PATIENT-REPORTED OUTCOMES
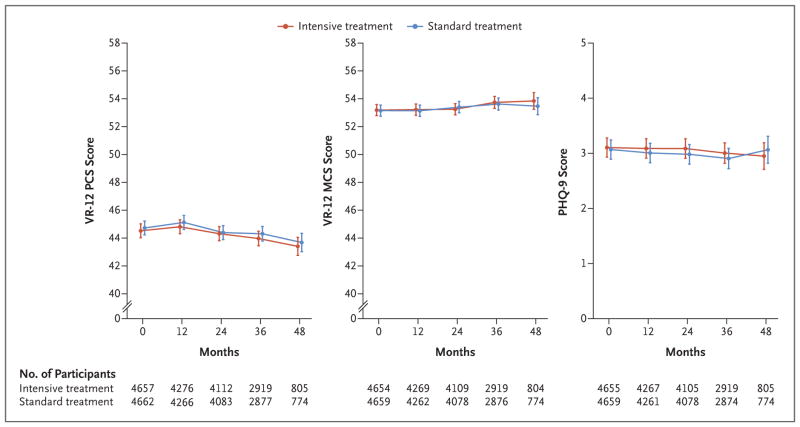
Despite the difference between the two groups in achieved blood pressure, the mean PCS, MCS, and PHQ-9 scores were relatively stable over the course of follow-up, with no significant differences between the treatment groups (Fig. 1). Assuming linear change over time, the mean PCS scores decreased slightly over the course of follow-up in both the intensive-treatment group (−0.23 points per year; 95% CI, −0.31 to −0.16) and the standard-treatment group (−0.23 points per year; 95% CI, −0.31 to −0.15) (P = 0.90) (Tables S3 through S5 in the Supplementary Appendix). The mean MCS scores increased slightly over time in both the intensive-treatment group (0.15 points per year; 95% CI, 0.07 to 0.24) and the standard-treatment group (0.14 points per year; 95% CI, 0.05 to 0.22) (P = 0.79). The mean PHQ-9 scores decreased slightly over the course of follow-up, with a mean change of −0.03 points per year (95% CI, −0.06 to 0) in the intensive-treatment group and −0.03 points per year (95% CI, −0.07 to 0) in the standard-treatment group (P = 0.86). The inclusion of additional follow-up data on patient-reported outcomes through December 1, 2015, did not alter the conclusions of the trial. Similarly, the censoring of data from participants at the time of an outcome event did not alter the trial conclusions (Tables S6 through S11 in the Supplementary Appendix).
Figure 1. Patient-Reported Outcomes in the Two Treatment Groups over Time.
The data points represent the estimated mean based on a linear mixed model; I bars denote 95% confidence intervals. The values below each graph indicate the number of participants assessed at each trial visit. Scores on the Physical Component Summary (PCS) and Mental Component Summary (MCS) of the Veterans RAND 12-Item Health Survey (VR-12) are standardized with a mean of 50 and a standard deviation of 10; scores range from 0 to 100, with higher scores denoting better physical health and mental health, respectively. Scores on the Patient Health Questionnaire 9-item depression scale (PHQ-9) range from 0 to 27, with higher scores indicating greater severity of depressive symptoms and scores of 10 or higher suggesting moderate-to-severe depressive symptoms.
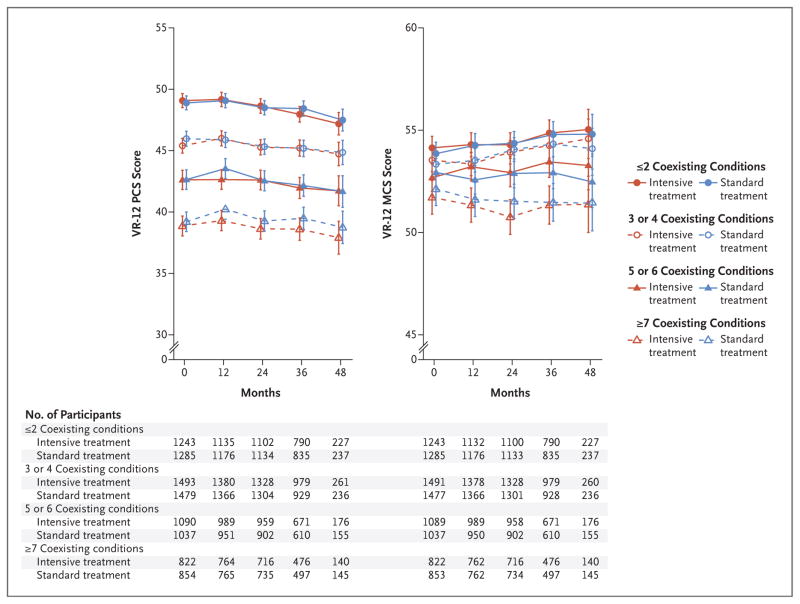
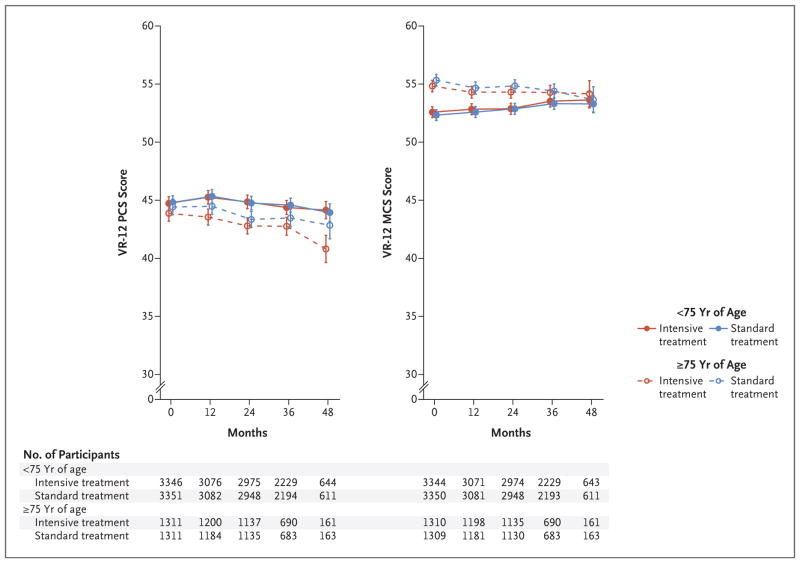
At baseline, lower mean PCS and MCS scores were associated with higher numbers of medical coexisting conditions (Fig. 2). No significant differences between the intensive-treatment group and the standard-treatment group over time were observed when participants were stratified according to the number of medical coexisting conditions. Overall, 28.2% of trial participants were 75 years of age or older. These participants had worse PCS scores but better MCS scores at baseline than did younger participants (Fig. 3). No significant differences between the intensive-treatment group and the standard-treatment group were noted over time within each of the two age strata. Similar results were noted when participants were stratified according to additional baseline measures of physical and cognitive function, including the number of mental health coexisting conditions, baseline PCS score (≥40 vs. <40), MoCA score (≥25th percentile vs. <25th percentile), and the frailty index score, with no significant interactions among these subgroups (Figs. S1 through S4 and Tables S3 through S5 in the Supplementary Appendix). Sensitivity analyses based on multiple imputation, under the assumption that missing data occurred at random, did not change the results appreciably (Table S12 and Fig. S5 in the Supplementary Appendix).
Figure 2. Scores on the VR-12 over Time, According to Treatment Group and Number of Medical Coexisting Conditions.
The burden of medical coexisting conditions was categorized as 2 or fewer, 3 or 4, 5 or 6, or 7 or more. The data points represent the estimated mean based on a linear mixed model, with I bars denoting 95% confidence intervals. The values below each graph indicate the number of participants assessed at each trial visit, according to the number of coexisting conditions participants had at baseline. Coexisting conditions were reported by the participants with the use of the Selim comorbidity index, which assesses 30 medical conditions and 6 mental health conditions. The physical score on the Selim comorbidity index was calculated as the sum of the number of these 30 possible medical conditions reported, and the mental score was the sum of the number of these 6 possible mental health conditions reported.
Figure 3. Scores on the VR-12 over Time, According to Treatment Group and Age Category.
The data points represent the estimated mean based on a linear mixed model, with I bars denoting 95% confidence intervals. The values below each graph indicate the number of participants assessed at each trial visit, according to age category.
At 12 months, small but significant differences were observed between the intensive-treatment group and the standard-treatment group with respect to the participants’ level of satisfaction with their blood-pressure care (P= 0.03) (Table 2). Despite these differences, a majority of participants in each group reported that they were satisfied or very satisfied with their blood-pressure care (88.6% in the intensive-treatment group and 88.2% in the standard-treatment group), and the percentage of participants who described an improvement in satisfaction from baseline was similar in the two groups (35.0% and 33.7%, respectively; P = 0.18). Small but significant differences between the two groups were noted with respect to satisfaction with the medications received for blood pressure; 62.1% of participants in the intensive-treatment group and 57.7% of participants in the standard-treatment group reported being very satisfied with the medications they received. Overall, 44.4% of participants reported high adherence to blood-pressure medications at 12 months, and no significant differences were noted between the two treatment groups with respect to medication adherence.
Table 2.
Patient-Reported Treatment Satisfaction and Medication Adherence.
| Treatment Satisfaction and Treatment Adherence | Intensive Treatment | Standard Treatment | P Value* |
|---|---|---|---|
| Response at 12-month visit | |||
| Level of satisfaction with blood-pressure care — no./total no. (%)† | 0.03 | ||
| Very satisfied | 3451/4641 (74.4) | 3293/4640 (71.0) | |
| Satisfied | 662/4641 (14.3) | 797/4640 (17.2) | |
| Neutral | 84/4641 (1.8) | 89/4640 (1.9) | |
| Dissatisfied | 11/4641 (0.2) | 14/4640 (0.3) | |
| Very dissatisfied | 25/4641 (0.5) | 30/4640 (0.6) | |
| Missing data | 408/4641 (8.8) | 417/4640 (9.0) | |
| Level of satisfaction with medications received for blood pressure — no./total no. (%)‡ | <0.001 | ||
| Very satisfied | 2856/4602 (62.1) | 2464/4274 (57.7) | |
| Satisfied | 1094/4602 (23.8) | 1123/4274 (26.3) | |
| Neutral | 179/4602 (3.9) | 204/4274 (4.8) | |
| Dissatisfied | 34/4602 (0.7) | 26/4274 (0.6) | |
| Very dissatisfied | 17/4602 (0.4) | 29/4274 (0.7) | |
| Missing data | 422/4602 (9.2) | 428/4274 (10.0) | |
| Morisky Medication Adherence Scale category — no./total no. (%)‡ | 0.21 | ||
| High, score of 8 | 2046/4602 (44.5) | 1893/4274 (44.3) | |
| Medium, score of 6 to <8 | 1562/4602 (33.9) | 1472/4274 (34.4) | |
| Low, score of <6 | 578/4602 (12.6) | 486/4274 (11.4) | |
| Missing data | 416/4602 (9.0) | 423/4274 (9.9) | |
| Change from baseline to 12-month visit | |||
| Satisfaction with blood-pressure care — no./total no. (%)† | 0.001 | ||
| Decline in satisfaction | 283/4641 (6.1) | 380/4640 (8.2) | |
| No change in satisfaction | 2300/4641 (49.6) | 2250/4640 (48.5) | |
| Improvement in satisfaction | 1625/4641 (35.0) | 1564/4640 (33.7) | |
| Missing data | 433/4641 (9.3) | 446/4640 (9.6) | |
| Satisfaction with medications received for blood pressure — no./total no. (%)§ | 0.02 | ||
| Decline in satisfaction | 324/4185 (7.7) | 366/3959 (9.2) | |
| No change in satisfaction | 1605/4185 (38.4) | 1549/3959 (39.1) | |
| Improvement in satisfaction | 1815/4185 (43.4) | 1610/3959 (40.7) | |
| Missing data | 441/4185 (10.5) | 434/3959 (11.0) | |
| Morisky Medication Adherence Scale — no./total no. (%)§ | 0.56 | ||
| Decline in adherence | 633/4185 (15.1) | 561/3959 (14.2) | |
| No change in adherence | 1969/4185 (47.0) | 1864/3959 (47.1) | |
| Improvement in adherence | 1161/4185 (27.7) | 1111/3959 (28.1) | |
| Missing data | 422/4185 (10.1) | 423/3959 (10.7) |
P values were calculated with the use of chi-square tests. Percentages may not sum to 100 because of rounding.
Included in the analysis were all participants who were alive as of the 12-month visit.
Included in the analysis were all participants who were alive as of the 12-month visit and either reported that they were receiving at least one antihypertensive agent at the 12-month visit or did not complete the assessment.
Included in the analysis were all participants who were alive as of the 12-month visit and either reported that they were receiving at least one antihypertensive agent at randomization and at the 12-month visit or did not complete the assessment.
DISCUSSION
Randomization to intensive blood-pressure control had little effect on changes in patient-reported outcomes and adherence to blood-pressure medication. Thus, the benefits seen with the intensive blood-pressure intervention with respect to cardiovascular events and death1 were not accompanied by worse physical function, mental function, or depressive symptoms, as perceived by the participants. In fact, satisfaction with both blood-pressure care and blood-pressure medication at 12 months was significantly higher among participants who received intensive treatment, although the differences between the two treatment groups were small.
We evaluated well-validated patient-reported outcomes that captured physical and mental health-related quality of life, depressive symptoms, and adherence to blood-pressure medication. We found that the two treatment groups did not differ significantly over a median period of 3 years of follow-up with respect to VR-12 and PHQ-9 scores or at 1 year with respect to scores on the Morisky Medication Adherence Scale. Moreover, we observed only small changes in the mean scores over time in both groups. On average, the mean PCS, MCS, and PHQ-9 scores changed less than 1 unit by the time of the 1-year follow-up visit; these differences were not considered to be clinically relevant.18–20
We hypothesized that intensive treatment might have adverse effects on patient-reported outcomes, despite the fact that it resulted in lower rates of cardiovascular events and death than those with standard treatment. Although decreased perfusion resulting from intensive treatment could affect a variety of organs, particular concern has been expressed that mean arterial blood pressure among patients receiving intensive antihypertensive treatment, especially among older patients with a widened pulse pressure, may be too low to maintain adequate cerebral perfusion.3 However, some studies have suggest-ed that long-term antihypertensive treatment results in increased cerebral blood flow, preserved autoregulation, and a reduction in orthostatic hypotension among older persons with hypertension.21–23
Intensive treatment typically results in the use of additional medications. Such medications could be associated with both physical and mental side effects. Antihypertensive medications that have been in use for decades, such as methyldopa, reserpine, and propranolol, have been associated with a diminished perception of quality of life.7 Studies of newer antihypertensive medications, however, have often shown little effect on health-related quality of life. In the Treatment of Mild Hypertension Study, the combination of lifestyle modifications plus medications was associated with greater improvements in health-related quality of life than lifestyle modifications plus placebo.24 The Action to Control Cardiovascular Risk in Diabetes (ACCORD) study, which, similar to our trial, compared intensive treatment with standard treatment, showed no significant differences between the groups with respect to the PHQ-9 scores and the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36) MCS scores.25 Although the PCS score in the ACCORD study was significantly lower with intensive treatment than with standard treatment, the 0.6-point difference was not considered to be clinically meaningful.
Controversy exists surrounding the benefits and risks of antihypertensive treatment in older patients and in patients with physical and cognitive impairment.26,27 Observational studies have suggested that impaired physical and cognitive function moderates the association between high blood pressure and adverse outcomes.28,29 However, subsequent analyses from our trial have shown that among participants 75 years of age or older, the benefits of intensive treatment over standard treatment in lowering the rates of cardiovascular events and death were similar across frailty and gait-speed subgroups.30 We now show that, on the basis of patient-reported outcomes, intensive treatment was associated with few side effects across the spectrum of physical and cognitive function included in our trial.
Our trial compared two different systolic blood-pressure targets rather than achieved blood pressure. Although a mean difference of 14.8 mm Hg was achieved, some participants had unacceptable side effects with a systolic blood-pressure target of less than 120 mm Hg. As part of our protocol, medications could have been tapered in participants in whom unacceptable symptoms developed. This finding emphasizes the need for clinicians to actively manage treatment for individual patients to maximize patient-reported health status while reducing a patient’s risk of cardiovascular events and death.
Our trial has several limitations. First, the greatest negative effect of intensive treatment on patient-reported outcomes could have occurred during the first several months of treatment intensification, in which case we would have missed such a transient effect. Second, because of the early termination of our trial, long-term follow-up was lacking for many participants, particularly with respect to satisfaction with blood-pressure care and adherence to blood-pressure medications. However, additional data on patient-reported outcomes collected over the course of an additional 4 months of follow-up did not alter the conclusions of the trial. Third, although our trial was successful in recruiting patients with hypertension who were at increased risk for cardiovascular events, questions could be raised regarding external validity. Yet it has been estimated that 16.8 million U.S. adults meet the eligibility requirements for our trial.31 Finally, the participants in our trial were aware of the treatment group to which they had been randomly assigned; knowledge regarding their treatment might have affected their perceptions of health.
The management of hypertension has been a major public health success; in large, integrated health care systems, blood-pressure control to a target of less than 140/90 mm Hg has been reported to be achieved in up to 80% of patients with hypertension.32,33 Translating the current findings into clinical practice presents a challenge and would result in changes in how clinicians manage hypertension in older patients.34 Before patients and clinicians adopt such changes, they will need to be reassured that intensive treatment not only reduces the risk of cardiovascular events and death, but will result in few side effects as shown in patient-reported assessments. The current results provide complementary evidence that supports the main findings of our trial.
Supplementary Material
Acknowledgments
Supported by contracts (HHSN268200900040C, HHSN268200 900046C, HHSN268200900047C, HHSN268200900048C, and HHSN268200900049C) and an interagency agreement (A-HL-13-002-001) from the NIH, including the National Heart, Lung, and Blood Institute, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute on Aging, and the National Institute of Neurological Disorders and Stroke. Several trial sites were supported by Clinical and Translational Science Awards funded by the National Center for Advancing Translational Sciences of the NIH (Case Western Reserve University: UL1TR000439; Ohio State University: UL1RR025755; University of Pennsylvania: UL1RR024134 and UL1TR000003; Boston University: UL1RR025771; Stanford University: UL1TR000093; Tufts University: UL1RR025752, UL1TR000073, and UL1TR001064; University of Illinois: UL1TR000050; University of Pittsburgh: UL1 TR000005; University of Texas Southwestern: 9U54TR000017-06; University of Utah: UL1TR000105-05; Vanderbilt University: UL1 TR000445; George Washington University: UL1TR000075; University of California, Davis: UL1TR000002; University of Florida: UL1TR000064; University of Michigan: UL1TR000433; and Tulane University: P30GM103337 COBRE Award NIGMS). The trial was also supported in part with respect to resources and the use of facilities by the Department of Veterans Affairs.
APPENDIX
From the Center for Healthcare Organization and Implementation Research, Bedford Veterans Affairs (VA) Hospital, Bedford (D.R.B., L.E.K.), and Boston University Schools of Medicine and Public Health (D.R.B., L.E.K.) and Tufts Medical Center (D.E.W.), Boston — all in Massachusetts; Wake Forest School of Medicine, Winston-Salem (C.G.F., J.N., N.M.P.), and East Carolina University College of Nursing (L.P.B.) and Brody School of Medicine (J.P.), East Carolina University, Greenville — both in North Carolina; the University of Pittsburgh, Pittsburgh (M.B.C.); Mayo Clinic Florida, Jacksonville (P.F.); the Ohio State Wexner Medical Center, Columbus (T.R.G.); the National Institute of Diabetes and Digestive and Kidney Diseases (P.L.K.) and the National Heart, Lung, and Blood Institute (J.S.), Bethesda, MD; the G.V. (Sonny) Montgomery VA Medical Center, Jackson, MS (K.K.); UCLA Fielding School of Public Health, Los Angeles (D.E.M.); Minneapolis VA Medical Center, Minneapolis (C.O.); University of Alabama at Birmingham, Birmingham (S.O., T.R.); University of Utah School of Medicine (D.L.S., M.A.S.) and VA Geriatric Research, Education and Clinical Center (M.A.S.), Salt Lake City; and the Clement J. Zablocki VA Medical Center, Milwaukee (J.W.).
Footnotes
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH), the Department of Veterans Affairs, or the U.S. Government. Use of the Morisky Medication Adherence Scale is protected by U.S. copyright laws. Permission for its use is required; address inquiries to Dr. Morisky at dmorisky@gmail.com. Use of the VR-12 is protected by U.S. copyright laws. Permission for its use is required (see www.bu.edu/sph/research/research-landing-page/vr-36-vr-12-and-vr-6d/about-the-vr-36-vr-12-and-vr-6d/).
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
The authors’ affiliations are listed in the Appendix.
References
- 1.The SPRINT Research Group. A randomized trial of intensive versus standard blood pressure control. N Engl J Med. 2015;373:2103–16. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ortiz E, James PA. Let’s not SPRINT to judgment about new blood pressure goals. Ann Intern Med. 2016;165:889–90. doi: 10.7326/L16-0442. [DOI] [PubMed] [Google Scholar]
- 3.Saper CB. How low can you go? Ann Neurol. 2015;78:665–6. doi: 10.1002/ana.24530. [DOI] [PubMed] [Google Scholar]
- 4.Schiffrin EL, Calhoun DA, Flack JM. SPRINT proves that lower is better for nondiabetic high-risk patients, but at a price. Am J Hypertens. 2016;29:2–4. doi: 10.1093/ajh/hpv190. [DOI] [PubMed] [Google Scholar]
- 5.Moonen JEF, Foster-Dingley JC, de Ruijter W, et al. Effect of discontinuation of antihypertensive treatment in elderly people on cognitive functioning — the DANTE Study Leiden. JAMA Intern Med. 2015;175:1622–30. doi: 10.1001/jamainternmed.2015.4103. [DOI] [PubMed] [Google Scholar]
- 6.Hildrum B, Mykletun A, Stordal E, Bjelland I, Dahl AA, Holmen J. Association of low blood pressure with anxiety and depression: the Nord-Trøndelag Health Study. J Epidemiol Community Health. 2007;61:53–8. doi: 10.1136/jech.2005.044966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Croog SH, Levine S, Testa MA, et al. The effects of antihypertensive therapy on the quality of life. N Engl J Med. 1986;314:1657–64. doi: 10.1056/NEJM198606263142602. [DOI] [PubMed] [Google Scholar]
- 8.Ambrosius WT, Sink KM, Foy CG, et al. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT) Clin Trials. 2014;11:532–46. doi: 10.1177/1740774514537404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Selim AJ, Rogers W, Fleishman JA, et al. Updated U.S. population standard for the Veterans RAND 12-item Health Survey (VR-12) Qual Life Res. 2009;18:43–52. doi: 10.1007/s11136-008-9418-2. [DOI] [PubMed] [Google Scholar]
- 10.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens (Greenwich) 2008;10:348–54. doi: 10.1111/j.1751-7176.2008.07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Munter P, Joyce C, Holt E, et al. Defining the minimal detectable change in scores on the eight-item Morisky Medication Adherence Scale. Ann Pharmacother. 2011;45(5):569–75. doi: 10.1345/aph.1P677. [DOI] [PubMed] [Google Scholar]
- 13.Selim AJ, Fincke G, Ren XS, et al. Comorbidity assessments based on patient report: results from the Veterans Health Study. J Ambul Care Manage. 2004;27:281–95. doi: 10.1097/00004479-200407000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–9. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 15.Kenny RA, Coen RF, Frewen J, Donoghue OA, Cronin H, Savva GM. Normative values of cognitive and physical function in older adults: findings from the Irish Longitudinal Study on Ageing. J Am Geriatr Soc. 2013;61(Suppl 2):S279–S290. doi: 10.1111/jgs.12195. [DOI] [PubMed] [Google Scholar]
- 16.Pajewski NM, Williamson JD, Apple-gate WB, et al. Characterizing frailty status in the Systolic Blood Pressure Intervention Trial. J Gerontol A Biol Sci Med Sci. 2016;71:649–55. doi: 10.1093/gerona/glv228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.R Development Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2015. ( https://www.R-project.org/) [Google Scholar]
- 18.Selim AJ, Berlowitz D, Kazis LE, et al. Comparison of health outcomes for male seniors in the Veterans Health Administration and Medicare Advantage plans. Health Serv Res. 2010;45:376–96. doi: 10.1111/j.1475-6773.2009.01068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ware JE, Jr, Bayliss MS, Rogers WH, Kosinski M, Tarlov AR. Differences in 4-year health outcomes for elderly and poor, chronically ill patients treated in HMO and fee-for-service systems: results from the Medical Outcomes Study. JAMA. 1996;276:1039–47. [PubMed] [Google Scholar]
- 20.Löwe B, Unützer J, Callahan CM, Perkins AJ, Kroenke K. Monitoring depression treatment outcomes with the Patient Health Questionnaire-9. Med Care. 2004;42:1194–201. doi: 10.1097/00005650-200412000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Tryambake D, He J, Firbank MJ, O’Brien JT, Blamire AM, Ford GA. Intensive blood pressure lowering increases cerebral blood flow in older subjects with hypertension. Hypertension. 2013;61:1309–15. doi: 10.1161/HYPERTENSIONAHA.112.200972. [DOI] [PubMed] [Google Scholar]
- 22.Gangavati A, Hajjar I, Quach L, et al. Hypertension, orthostatic hypotension, and the risk of falls in a community-dwelling elderly population: the Maintenance of Balance, Independent Living, Intellect, and Zest in the Elderly of Boston study. J Am Geriatr Soc. 2011;59:383–9. doi: 10.1111/j.1532-5415.2011.03317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lipsitz LA, Habtemariam D, Gagnon M, et al. Reexamining the effect of anti-hypertensive medications on falls in old age. Hypertension. 2015;66:183–9. doi: 10.1161/HYPERTENSIONAHA.115.05513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grimm RH, Jr, Grandits GA, Cutler JA, et al. Relationships of quality-of-life measures to long-term lifestyle and drug treatment in the Treatment of Mild Hypertension Study. Arch Intern Med. 1997;157:638–48. [PubMed] [Google Scholar]
- 25.O’Connor PJ, Narayan KM, Anderson R, et al. Effect of intensive versus standard blood pressure control on depression and health-related quality of life in type 2 diabetes: the ACCORD trial. Diabetes Care. 2012;35:1479–81. doi: 10.2337/dc11-1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wright JT, Jr, Fine LJ, Lackland DT, Ogedegbe G, Dennison Himmelfarb CR. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med. 2014;160:499–503. doi: 10.7326/M13-2981. [DOI] [PubMed] [Google Scholar]
- 27.Qaseem A, Wilt TJ, Rich R, et al. Pharmacologic treatment of hypertension in adults aged 60 years or older to higher versus lower blood pressure targets: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2017;166:430–7. doi: 10.7326/M16-1785. [DOI] [PubMed] [Google Scholar]
- 28.Sabayan B, van Vliet P, de Ruijter W, Gussekloo J, de Craen AJ, Westendorp RG. High blood pressure, physical and cognitive function, and risk of stroke in the oldest old: the Leiden 85-plus Study. Stroke. 2013;44:15–20. doi: 10.1161/STROKEAHA.112.663062. [DOI] [PubMed] [Google Scholar]
- 29.Odden MC, Peralta CA, Haan MN, Covinsky KE. Rethinking the association of high blood pressure with mortality in elderly adults: the impact of frailty. Arch Intern Med. 2012;172:1162–8. doi: 10.1001/archinternmed.2012.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williamson JD, Supiano MA, Apple-gate WB, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 years: a randomized clinical trial. JAMA. 2016;315:2673–82. doi: 10.1001/jama.2016.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bress AP, Tanner RM, Hess R, Colantonio LD, Shimbo D, Muntner P. Generalizability of SPRINT results to the U.S. adult population. J Am Coll Cardiol. 2016;67:463–72. doi: 10.1016/j.jacc.2015.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moser M. Hypertension treatment — a success study. J Clin Hypertens (Greenwich) 2006;8:312–4. doi: 10.1111/j.1524-6175.2005.05153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jaffe MG, Lee GA, Young JD, Sidney S, Go AS. Improved blood pressure control associated with a large-scale hypertension program. JAMA. 2013;310:699–705. doi: 10.1001/jama.2013.108769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chobanian AV. Time to reassess blood-pressure goals. N Engl J Med. 2015;373:2093–5. doi: 10.1056/NEJMp1513290. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.