Graphical abstract
We demonstrate that a significant improvement in the spectral resolution may be achieved in solid-state NMR experiments of proteins in inhomogeneously disordered oriented lipid bilayers. Using 1H homonuclear decoupling instead of standard 1H heteronuclear decoupling, the 15N linewidths may be reduced by up to seven times for such samples. For large oriented membrane proteins such resolution-enhancements may be crucial for assignment and structural interpretation.
Over the past decades solid-state NMR spectroscopy on macroscopically oriented lipid-bilayer or magnetically oriented bicelle samples has attracted considerable attention for determination of the structure of membrane-bound proteins.1,2 So far, most studies have addressed smaller membrane proteins and peptides for which it has been possible to obtain well-resolved peaks in 2D separated-local-field (SLF) spectra correlating the amide 1H-15N dipole-dipole couplings and 15N chemical shifts. In less favorable cases, in particular for larger proteins and high protein:lipid ratios, the resonances are substantially broader and display significant overlap.2–4 Despite large efforts in sample preparation,3,5 it is believed that a major cause of linebroadening is imperfect sample alignment, e.g., mosaic spread, which in many cases appears to be an intrinsic and unavoidable property of the system. The inevitable consequences are low signal-to-noise ratio and increased risk for spectral overlap.
In this Communication, we demonstrate a new method that may significantly improve the resolution in the 15N dimension of 1D 15N and 2D 1H −15N SLF experiments for oriented membrane protein systems. The method, relying on orientation-disorder induced differential linebroadening, bears resemblance to the TROSY type experiments6 where differential linebroadening, induced indirectly from anisotropic interactions by relaxation, offered new capabilities for studying large proteins by liquid-state NMR. The orientation-induced differential linebroadening occurs when an inhomogeneous broadening from one anisotropic interaction (e.g., chemical shift) is partly cancelled by inhomogeneous broadening from another anisotropic interaction (e.g., heteronuclear dipolar coupling) with opposite sign. In the present work, this effect is observed in 1D and 2D 15N experiments using 1H homonuclear dipolar decoupling instead of standard 1H heteronuclear decoupling.
To experimentally demonstrate the spectral effects of orientational disorder in oriented lipid-bilayer samples, we prepared a sample of 15N-Aib8 alamethicin in oriented DMPC bilayers. With the high peptide:lipid ratio (1:15 molar ratio) used here, the resonances in a 1D spectrum (detected with 1H heteronuclear SPINAL-647 decoupling) displays significant linebroadening as evidenced by the spectrum in Fig. 1a. Indeed, the spectrum shows accumulation of intensity at ~200 ppm which is characteristic for oriented peptides with a transmembrane α-helical conformation.8 The measured linewidth of 1.9 kHz, however, is significantly larger than the values observed for perfectly oriented peptides. Fig. 1d illustrates the appearance of the 15N spectrum recorded using 1H homonuclear frequency-switched Lee-Goldburg (FSLG) decoupling.9 The spectrum shows the expected dipolar doublet, the peak at ~150 ppm is relatively sharp (1.2 kHz linewidth) compared to the regular 1D spectrum, while the peak at ~270 ppm is much broader and reaches only about half the peak height with a pronounced upfield tail extending down to ~200 ppm.
Figure 1.
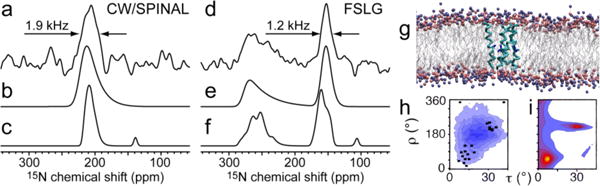
(a,d) Experimental and (b,c,e,f) simulated 16.45-T 1H-15N spectra for a sample of 15N-Aib8 alamethicin in oriented lipid bilayers. The spectra are obtained using (a–c) heteronuclear SPINAL-64 1H decoupling and (d–f) 1H homonuclear FSLG decoupling. (b,e) Simulations assuming a single molecular conformation (corresponding to an α-helix conformation of τ,ρ = 7.85°, 52°) and Δβ = 18° for the mosaic spread. (c,f) Simulations obtained using the average chemical-shift and dipole-dipole coupling frequencies for the 25 molecules in the MD simulation. (g) Snapshot of an alamethicin ion channel-like aggregate with four alamethicin molecules resulting from the MD simulation. (h) Distribution of helix conformations in the MD simulation. Blue contours represent conformation counts, while the black points represent average conformations back-calculated from averaging the nuclear spin interaction frequencies for each peptide conformation. (i) Restriction plot showing peptide conformations in agreement with the FSLG spectrum (d), assuming an ideal α-helical secondary structure.
The observation that the FSLG-decoupled spectrum displays a resonance with only about half the linewidth of the regular 1D spectrum renders homonuclear decoupling a very appealing alternative to standard 1H heteronuclear decoupling in the design of new experiments for samples with orientational disorder, provided we can justify the origin of the differential linebroadening effect. Because of the asymmetric lineshape of the downfield peak in the present spectrum, we can rule out transverse relaxation as a main reason for the spectral appearance. Relaxation would only broaden the resonances, but cannot account for the lineshape of the downfield peak. On the other hand, considering that the two peaks appear at the frequency of the orientation-dependent chemical shift plus or minus the scaled dipolar coupling, the two peaks will be influenced by the sum or difference, respectively, of an inhomogeneous distribution of anisotropic parts of the chemical shift and dipolar coupling, leading to different net anisotropies for the two peaks.
To get insight into the behavior of alamethicin in a bilayer at high peptide concentrations, we performed a coarse-grained (CG) molecular dynamics (MD) simulation including an ensemble of 25 alamethicin peptides in a bilayer consisting of 330 DMPC lipids (1:13.2 peptide:lipid ratio). Fig. 1g shows a snapshot from this simulation, which illustrates the channel-like assembly of four of the peptides. For this snapshot, we make two interesting observations. First, the four monomers do not have identical conformations and second, the bilayer is not perfectly aligned. Based on this, we will use two models for the orientational disorder assuming: (i) that all peptide conformations average to a single conformation in the bilayer on the timescale of the NMR experiment, with a static disorder (mosaic spread) of the bilayer and (ii) the presence of multiple different molecular conformations in the bilayer.
The conformation-dependent nuclear spin interactions will be averaged around the local bilayer normal due to fast rotational diffusion at the present temperature well above the DMPC phase transition.10 The local bilayer normals are assumed to have a Gaussian distribution of width Δβ with respect to the external magnetic field due to mosaic spread.4,11 The NMR spectra, simulated using SIMPSON and SIMMOL,12 and for the first model using the peptide conformation and mosaic spread (Δβ) as free variables, are shown in Fig. 1b,e. The corresponding restriction plot, representing peptide conformations being in agreement with the experimental spectrum, is shown in Fig. 1i. We observe a good agreement between experimental and simulated spectra, which supports the validity of this model.
For the second model, we use the molecular conformations from the MD simulation. To eliminate the effects of fast fluctuations, which would not be directly observed in the NMR experiments, we have calculated the time-averaged 15N chemical shift and effective 1H-15N dipole-dipole coupling for each of the 25 alamethicin molecules and used these parameters with additional 10° mosaic spread as input for the simulations in Fig. 1c,f. Again, we observe a good agreement with the experimental data, although the spectra are quite ragged due to the small number of alamethicin peptides in the MD simulation. The two models support each other by the presence of more than a single unique peptide conformation. This is further substantiated in Fig. 1h, which shows (with black dots) the back-calculated peptide conformations from the 25 average nuclear spin interaction parameters. These conformations compare well with the restrictions found by the first model (Fig. 1i).
Based on the good agreement between the experimental and simulated spectra, it is evident that direct effects from differential linebroadening can be rationalized and used experimentally by recording spectra with homonuclear instead of heteronuclear decoupling. It is relevant to exploit this for multidimensional experiments, which are indispensable for multiply labeled samples. The immediate gain of homonuclear decoupling is demonstrated in Fig. 2 by simulated 2D SLF spectra for a uniformly 15N labeled α-helical peptide with a mosaic spread of Δβ = 8° using standard heteronuclear (CW or SPINAL-64) or FSLG decoupling during acquisition. While both spectra show the expected wheel-like patterns for the resonances, the FSLG-decoupled spectrum splits into a doublet in the 15N chemical shift dimension due to the 1H-15N dipolar coupling, and, more importantly, displays significantly better resolution than the standard SLF spectrum. We note that only the horizontal 15N chemical shift dimension is affected by the homonuclear decoupling. Nonetheless, while the standard SLF experiments displays severe overlap of all resonances, the FSLG spectrum shows significantly better resolution. A more systematic investigation of the gain in resolution is presented in Fig. 2e, which shows the relative linewidth of the narrow peak in the 15N dimension of the FSLG spectrum vs. the linewidth in the CW-decoupled spectrum as a function of the mosaic spread and magnetic field strength. This demonstrates that it is possible to achieve up to seven-fold gain in resolution by using homonuclear decoupling.
Figure 2.
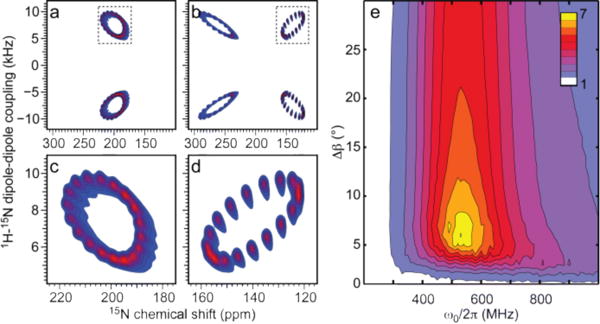
Simulated 16.45-T 15N SLF spectra for an ideal uniformly 15N labeled α-helix with a tilt of τ = 15° relative to the bilayer normal and with Δβ = 8°. (a,c) Regular SLF spectrum and (b,d) SLF spectrum using 1H FSLG decoupling during acquisition. The representations in (c,d) are expansions illustrated by dashed boxes in (a,b). (e) Average resolution enhancement for the 18 residues of an ideal α-helix (τ = 15°) obtained using FSLG decoupling as compared to standard heteronuclear decoupling as a function of the mosaic spread and magnetic field strength (1H MHz).
In conclusion, we have demonstrated that significant improvement in the resolution of spectra for inhomogeneously disordered oriented samples may be obtained by recording spectra with homonuclear proton decoupling instead of conventional heteronuclear proton decoupling. This resolution enhancement may be essential for assignment and structural exploitation of spectra for large oriented membrane proteins.
Supplementary Material
Acknowledgments
Support from the Danish National Research Foundation, the Danish Natural Science Research Council, Carlsbergfondet, Lundbeckfonden, National Institutes of Health (R01-GM067887), the Danish Center for Scientific Computing, TeraGrid (MCA06N060) at Indiana University, and the Danish Biotechnology Instrument Centre (DABIC) is acknowledged.
Footnotes
Supporting Information Available: Details on the sample preparation, the experimental setup of the solid-state NMR experiments, the numerical simulations, and the MD simulation. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Cornell BA, Separovic F, Baldassi AJ, Smith R. Biophys J. 1988;53:67. doi: 10.1016/S0006-3495(88)83066-2. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ketchem RR, Hu W, Cross TA. Science. 1993;261:1457. doi: 10.1126/science.7690158. [DOI] [PubMed] [Google Scholar]; (c) Opella SJ. Nature Struct Biol. 1997;4:845. [PubMed] [Google Scholar]; (d) Opella SJ, Marassi FM. Chem Rev. 2004;104:3587. doi: 10.1021/cr0304121. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Kovacs F, Quine J, Cross TA. Proc Natl Acad Sci. 1999;96:7910. doi: 10.1073/pnas.96.14.7910. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Lambotte S, Jasperse P, Bechinger B. Biochemistry. 1998;37:16. doi: 10.1021/bi9724671. [DOI] [PubMed] [Google Scholar]; (g) Durr UH, Yamamoto K, Im SC, Waskell L, Ramamoorthy A. J Am Chem Soc. 2007;129:6670. doi: 10.1021/ja069028m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(a) Kamihira M, Vosegaard T, Mason AJ, Straus SK, Nielsen NC, Watts A. J Struct Biol. 2005;149:7. doi: 10.1016/j.jsb.2004.10.002. [DOI] [PubMed] [Google Scholar]; (b) Vosegaard T, Kamihira-Ishijima M, Watts A, Nielsen NC. Biophys J. 2008;94:241. doi: 10.1529/biophysj.107.116004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grobner G, Taylor A, Williamson PT, Choi G, Glaubitz C, Watts JA, de Grip WJ, Watts A. Anal Biochem. 1997;254:132. doi: 10.1006/abio.1997.2415. [DOI] [PubMed] [Google Scholar]
- 4.(a) Bechinger B, Sizun C. Concepts Magn Reson. 2003;18A:130. [Google Scholar]; (b) Aisenbrey C, Bechinger B. Biochemistry. 2004;43:10502. doi: 10.1021/bi049409h. [DOI] [PubMed] [Google Scholar]
- 5.(a) Rainey JK, Sykes BD. Biophys J. 2005;89:2792. doi: 10.1529/biophysj.105.063800. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hallock KJ, Henzler Wildman K, Lee DK, Ramamoorthy A. Biophys J. 2002;82:2499. doi: 10.1016/S0006-3495(02)75592-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pervushin K, Riek R, Wider G, Wuthrich K. Proc Natl Acad Sci U S A. 1997;94:12366. doi: 10.1073/pnas.94.23.12366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fung BM, Khitrin AK, Ermolaev K. J Magn Reson B. 2000;142:97. doi: 10.1006/jmre.1999.1896. [DOI] [PubMed] [Google Scholar]
- 8.(a) Wang J, Denny J, Tian C, Kim S, Mo Y, Kovacs F, Song Z, Nishimura K, Gan Z, Fu R, Quine JR, Cross TA. J Magn Reson. 2000;144:162. doi: 10.1006/jmre.2000.2037. [DOI] [PubMed] [Google Scholar]; (b) Marassi FM, Opella SJ. J Magn Reson. 2000;144:150. doi: 10.1006/jmre.2000.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Vosegaard T, Nielsen NC. J Biomol NMR. 2002;22:225. doi: 10.1023/a:1014987227285. [DOI] [PubMed] [Google Scholar]
- 9.Bielecki A, Kolbert AC, de Groot HJM, Griffin RG, Levitt MH. Adv Magn Reson. 1990;14:111. [Google Scholar]
- 10.(a) Smith R, Thomas DE, Separovic F, Atkins AR, Cornell BA. Biophys J. 1989;56:307. doi: 10.1016/S0006-3495(89)82677-3. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Prongidi-Fix L, Bertani P, Bechinger B. J Am Chem Soc. 2007;129:8430. doi: 10.1021/ja072668k. [DOI] [PubMed] [Google Scholar]
- 11.Nevzorov AA, Moltke S, Heyn MP, Brown MF. J Am Chem Soc. 1999;121:7636. [Google Scholar]
- 12.(a) Bak M, Rasmussen JT, Nielsen NC. J Magn Reson. 2000;147:296. doi: 10.1006/jmre.2000.2179. [DOI] [PubMed] [Google Scholar]; (b) Bak M, Schultz R, Vosegaard T, Nielsen NC. J Magn Reson. 2002;154:28. doi: 10.1006/jmre.2001.2454. [DOI] [PubMed] [Google Scholar]; (c) Vosegaard T, Malmendal A, Nielsen NC. Chem Monthly. 2002;133:1555. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


