Abstract
Historically, high-energy extremity injuries resulting in significant soft-tissue trauma and bone loss were often deemed unsalvageable and treated with primary amputation. With improved soft-tissue coverage and nerve repair techniques, these injuries now present new challenges in limb-salvage surgery. High-energy extremity trauma is pre-disposed to delayed or unpredictable bony healing and high rates of infection, depending on the integrity of the soft-tissue envelope. Furthermore, orthopedic trauma surgeons are often faced with the challenge of stabilizing and repairing large bony defects while promoting an optimal environment to prevent infection and aid bony healing. During the last decade, nanomedicine has demonstrated substantial potential in addressing the two major issues intrinsic to orthopedic traumas (i.e., high infection risk and low bony reconstruction) through combatting bacterial infection and accelerating/increasing the effectiveness of the bone-healing process. This review presents an overview and discusses recent challenges and opportunities to address major orthopedic trauma through nanomedical approaches.
Keywords: Nanomedicine, Bone regeneration, Antibacterial properties, Stem cells
1. Introduction
Complications from significant orthopedic extremity trauma include infection, nonunion, and the presence of large bony defects. Large segments of bone loss is a critical clinical problem still awaiting a reliable and consistent solution, leading to poor quality of life and high healthcare costs [1]. Current treatments for addressing bone loss, especially in the context of trauma and/or infection, are primitive at best. The most common method involves placing non-degradable polymeric cement spacers imbedded with antibiotics into the bony defect in preparation for secondary and tertiary reconstructive procedures. However, there are problems with this approach: 1) only 25% of antibiotics are released locally, 2) a second operation for cement removal is required, 3) the cement acts as a foreign body that may propagate secondary infection (bacterial biofilms), and 4) bony reconstruction remains a challenge.
During the last decade, new strategies to address these challenges have included tissue-engineering approaches and nano-medicine, or the application of nanotechnology principles to medicine. For example, nanoparticle (NP) carriers have been engineered to selectively deliver a range of drugs to sites of disease [2,3]. This highly multidisciplinary approach has been driven in large part by advances/discoveries in nanomaterials design and engineering, which have yielded the following milestones: 1) development of biocompatible and biodegradable nanocarriers for the delivery of diverse therapeutics (e.g., small molecules, nucleic acids, proteins, and peptides) of various sizes and solubility, 2) selective accumulation of therapeutics at disease sites with pharmacokinetics (PK), biodistribution (BD) and degradation profiles superior to previous modalities, achieved through careful optimization and engineering of NP biophysicochemical properties, 3) the ability to target any disease at the organ, tissue, cellular, or sub-cellular level, and 4) clinical successes spanning a 40-year period [4].
Nanomedicine demonstrates great promise in overcoming the main issues facing orthopedic extremity trauma, including high infection risk and low bony reconstruction. The potential of nano-materials in this area stems not only from the substantial antibacterial properties but also from their capacity to offer mechanical, biochemical, and physicochemical properties necessary to accelerate the healing process in injured tissue [5–7]. In this review, we will discuss recent progress and current challenges in applying nanomedicine to the multidisciplinary field of orthopedic extremity trauma, including bone healing and infection control.
2. Bone fracture repair
2.1. Bone composition
Bone is not uniformly solid, but is composed of living cells embedded in a biomineral matrix. In fact, bone is formed by the hardening of this matrix around entrapped cells.
2.1.1. Bone biomineral matrix
The biomineral matrix of bone can be referred to as a “biogenic hierarchical composite materials” that consists of both organic (∼30%) and inorganic (∼70%) phases [8–10]. Nearly 90% of this organic material is collagen type I fibrils (which give bone its tensile strength), while the remaining 10% is mainly made up of proteoglycan molecules [11], osteopontin (OPN) [12], and other bone matrix proteins (BMPs) [10,13]. BMPs act as ‘glue’ and play a crucial role in mechanical strength [14]. The inorganic portion of bone is predominantly carbonated hydroxyapatite (HA) with a hexagonal crystal structure [15]. The unit cell of crystalline HA possesses the chemical formula Ca10(PO4)6(OH)2 with an ideal Ca: P stoichiometric ratio of 1.67: 1. However, bone actually exhibits a Ca: P ratio ranging from 1.3:1 to 1.9:1 [9]. This deviation is mainly attributable to the carbonated groups, substitution of other cations (e.g., Ca2+ with Mg2+), and protonation of in the crystal lattice [16,17]. Because the superficial Ca2+ and provide binding sites in the HA structure, electrostatic interactions are considered the main driver of surface binding. The HA crystals arrange themselves parallel to the long axes of collagen fibrils that are formed by self-assembly of collagen triple helices [18]. In fact, with 80–100 nm thickness and length of a few to tens of microns, collagen fibrils are the distinctive building blocks of bone and determine the mechanical properties of bone at the nanometric scale. Although the nature of the collagen-mineral interaction is still not well understood, it was found that the location of charged amino acid residues of collagen determines the sites uniquely suited as potential apatite nucleation centers following the binding of calcium and phosphate ions [19,20].
2.1.2. Bone cells
In addition to mineralized bone matrices, bone cells are also crucial to the function of bones. Bone exerts numerous functions in the body including locomotion, support of soft tissue structures, regulation of calcium and phosphate, as well as housing the bone marrow and the hematopoietic system. Though a full review of the hematopoietic system is beyond the scope of this article, this section focuses on the four major bone cells involved in bony remodeling and architecture: osteogenic, osteoblasts, osteocytes, and osteoclasts (Fig. 1). The coordinated actions of these four cell types regulate the complex process of bony remodeling; together these cells form a temporary anatomic structure known as the basic multicellular unit (BMU) [21–24].
Fig. 1.

Four types of cells are found within bone tissue. Osteogenic cells are undifferentiated and develop into osteoblasts, which form new bone. Osteocytes maintain the mineral concentration of bone matrix. Osteoclasts, which develop from monocytes and macrophages and differ in appearance from other bone cells, are responsible for bone resorption. Reproduced with permission from Ref. [41].
2.1.2.1. Osteoblasts
Osteoblasts are derived from pluripotent mesenchymal stem/stromal cells that differentiate into an osteoprogenitor lineage. Expression of osteoprogenitor-specific genes such as Runx2, Osterix, and distal-less homeobox 5 are critical to this osteoblastic differentiation. [25,26] Runx2 is considered a master osteogenic regulatory gene, and Runx2-knockout mice are devoid of osteoblasts. [26,27] Upon expression of osteoblast progenitor genes, osteoblast progenitor cells transition from pre-osteoblasts into mature osteoblasts. This transition is mediated by an increase in expression of alkaline phosphatase, osterix (OSX), osteocalcin (OCN), bone sialoprotein (BSP 1), and type 1 collagen [22]. Fully differentiated osteoblasts then deposit bony matrix in two steps. First osteoblasts secrete type 1 collagen, noncollagen proteins (OCN, OPN, osteonectin), and proteoglycans to form an organic matrix. Next, this matrix is mineralized through two complex processes known as the vesicular and fibrillar phases. Further detail regarding this mineralization process is beyond the scope of this review, but Anderson et al. and Glimcher et al. provide a good review [28,29]. Mature osteoblasts finally appear as a layer of cuboidal cells that can then undergo apoptosis or become osteocytes or bone-lining cells.
2.1.2.2. Osteocytes
Osteoblasts are the most plentiful cell type in bone, making up to 90–95% of the total number of bone cells. They are characterized by a star-shaped morphology with a lifespan of up to 25 years [30]. Osteocytes are derived from MSCs that undergo osteoblastic differentiation. At the end of the differentiation/bone formation process, a subset of osteoblasts becomes incorporated into the bony matrix to become osteocytes. This process is accompanied by distinctive cellular changes involving reduction in osteoblast size, decrease in endoplasmic reticulum, increase in nucleus-to-cytoplasm ratio, and emergence of cytoplasmic processes [25,31]. Via the lacunocanalicular system, osteocytes then act as mechanosensors that detect dynamic mechanical pressures and loads [22]. Through this mechanotransduction, osteocytes are able to regulate certain osteoblastic and osteoclastic activities based on mechanical stresses. Additionally, osteocytes have been shown to respond to numerous chemical signaling pathways and participate in regulation of calcium and phosphate homeostasis [32,33]. Malfunction of the osteocyte network increases bone fragility and can lead to osteoporosis.
2.1.2.3. Lining cells
Bone lining cells are quiescent osteoblasts that reside on bony surfaces. These cells are flattened and possess cytoplasmic processes that extend into bony canaliculi as well as gap junctions between these cells and osteocytes. While the function of lining cells remains to be elucidated, they seem to play an important role in coupling bone resorption to bone formation and in calcium hemostasis [34,35]. Furthermore, they provide an important function as a barrier that prevents direct interaction between osteoclasts and bone matrix. Further studies have also shown that these lining cells participate in osteoclastic differentiation, secreting both OPG and receptor activator of nuclear factor kappa-B ligand (RANKL) [28,36]. Further studies continue to elucidate the function of these lining cells, but it is known that they are a critical component of the BMU.
2.1.2.4. Osteoclasts
Osteoclasts are terminally differentiated mononuclear cells that arise from the hematopoietic lineage. Osteoclastic differentiation is mediated by binding of macrophage colony-stimulating factor (M-CSF) to osteoclastic precursors to stimulate proliferation. [37] Further regulation of osteoclastogenesis is regulated by RANKL and OPG. RANKL is expressed by osteoblasts, osteocytes, and stromal cells; binding to the RANK receptor on osteoclast precursor cells induces osteoclast formation. OPG is released from a variety of cells and binds directly to RANKL, preventing activation of the RANK/RANKL pathway. Upon activation, osteoclasts undergo polarization to form four distinct membrane domains: a sealing zone and ruffled border in contact with bony matrix, and basolateral and sensory domains that are not in contact with the bony matrix [22]. The sealing domain contains integrin complexes that bind to arginine-glycine-aspartate (RGD)-containing sequences in the bony matrix such as osteopontin, vitronectin, and bone sialoprotein. The ruffled border is a critical component of osteoclastic function and works by resorbing bony lacuna and dissolving hydroxyapatitie crystals. Proteolytic enzymes including Cathepsin K, tartrate-resistant acid phosphatase (TRAP), and matrix metalloproteases are secreted into Howship lacunae to induce bony degradation [38–40]. Abnormalities in osteoclastic activity characterize diseases such as osteoporosis (increased osteoclast activity) and osteopetrosis (inability of osteoclasts to acidify Howship lacunae).
2.2. Repair process
Fracture healing is a complex process that has been extensively studied [42–45]. Following initial trauma, bony regeneration follows either direct intramembranous healing or indirect fracture healing; direct bone healing occurs much less frequently. In order for direct healing to occur, the fracture ends must be anatomically aligned without any gaps and held together with stable fixation, preferably under conditions of compression [46]. In the presence of these conditions, cutting cones and osteoclasts cross the fracture line and create cavities that are later filled by osteoblastic bone production. This process results in bony union and restoration of the native Haversian system (i.e., the fundamental functional unit of compact bone) found in bone.
Following trauma, indirect fracture healing is most common and consists of both endochondral and intramembranous ossification. Unlike direct bone healing, it does not require anatomic fracture alignment or absolute stability. In fractures with bone loss or comminution, indirect healing is the predominant mechanism of achieving bony union. Immediately following trauma, there is localized secretion of pro-inflammatory molecules for the first 24 h, which often lasts up to 7 days after injury [47]. Pro-inflammatory interleukins (IL-1, IL-6, IL-11, and IL-18) and tumor necrosis factor alpha (TNF-a) recruit and stimulate both osteogenic differentiation of mesenchymal stem/stromal cells (MSCs) and angiogenesis near the fracture site [48,49]. Following this complex initial inflammatory response, MSCs are recruited to the fracture site, proliferate, and differentiate into osteogenic cells [50,51]. The initial phase of healing is the formation of a cartilaginous callous that subsequently undergoes mineralization. Recruited MSCs undergo a molecular cascade influenced by the transforming growth factor beta (TGF-B) superfamily, resulting in production of collagen I and collagen II matrix. BMP-2 has also been shown to be crucial in initiating this cartilaginous healing cascade, as mice with inactivating mutations in BMP-2 are unable to form callus [52]. The peak of soft callus formation typically occurs 7–9 days post trauma in animal models [50]. The relatively avascular cartilaginous matrix is then revascularized through a pathway dependent on the presence and activation of vascular endothelial growth factor (VEGF). VEGF has been consistently documented as an important promotor of fracture healing, while inhibition of VEGF receptors blocks the regenerative process [53,54].
Following angiogenesis and vascularization of the fracture callus, chondrocytes become hypertrophic and undergo apoptosis. At this point the extracellular matrix becomes mineralized to create a hard callus that is solid and mechanically rigid. This process occurs by 14 days post-injury in animal models and is mediated by macrophage-colony stimulating factor (M-CSF), receptor activator of nuclear factor kappa B ligand (RANKL), osteoprotegrin (OPG), and TNF-a [55,56]. The final step is remodeling of the hard callus into organized lamellar bone, which fully restores the biomechanical properties of normal bone. This process is typically initiated at 3–4 weeks post-injury in animal models, but may take years to be fully completed. IL-1, TNF-a, and BMPs (especially BMP-2) have been shown to be critical in this process [53,57], and both adequate blood supply and mechanical stability are required. This complex pathway continues to be the target of various molecular and therapeutic interventions aimed at improving bone regeneration after trauma.
3. Clinical problems/limitations
Open bone fractures are complex injuries typically resulting from high-energy trauma and are accompanied by varying degrees of injury to muscle, tendon, nerve, and other soft tissue. The combination of compromised vascularity and contamination with microorganisms predisposes these fractures to higher rates of bone infection. Furthermore, the high-energy mechanisms of injury can often result in complete loss of bony segments, frequently resulting in complications in fracture healing [58]. The Gustilo-Anderson classification was designed to describe and classify open fractures and help guide treatment decisions [59,60]. Type 1 injuries include puncture wounds ≤1 cm with minimal contamination. Type 2 injuries include lacerations >1 cm with soft-tissue injury. Type 3A injuries have more-extensive soft-tissue damage and/or periosteal stripping, with adequate bone coverage. Type 3B injuries involve heavy soft-tissue contamination, soft-tissue injury and periosteal stripping, and require free muscular flaps for definitive soft-tissue coverage. Type 3C refers to any open fracture with an associated vascular injury requiring repair. Though this classification system has been widely accepted, it has poor reliability, e.g., Brumback et al. reported that the average agreement amongst orthopedic surgeons on classification of tibial fractures was only 60% [61]. Furthermore, Kanakaris et al. described the high healthcare costs and poor quality of life associated with long bone nonunions [1]. Therefore, treatment of bone infections and large bone defects following open fractures remains a challenge in orthopedic traumatology.
3.1. Bone loss in open fractures
Historically, due to technical difficulties and other problems associated with limb reconstruction in the setting of significant bone loss, primary amputation was long the preferred treatment for severe fractures. However, modern surgical techniques of fracture stabilization as well as vascular and soft-tissue reconstruction have made limb salvage surgery more common [62]. A prospective study of admissions to a level-1 trauma center found that 11.4% of open fractures were associated with bone loss [59,60]. The majority of these injuries were classified as Grade IIIB or Grade IIIC based on the Gustilo-Anderson system. Bone loss occurs from loss of bony fragments at the time of injury or through debridement of devitalized tissue during the initial surgery. Typically, the extent of the bony defect is characterized by the length of the bone involved and degree of circumferential bone loss [62]. Segmental defects greater than 2 cm or 50% of the circumference are unlikely to heal spontaneously following skeletal stabilization, and commonly require additional treatment to induce bone healing [62]. Furthermore, the degree of soft-tissue injury, patient age and medical comorbidities, as well as local biology influence both the rate and quality of bony healing. While various surgical techniques have evolved to treat these injuries, the ultimate goals remain the same: stabilization of the fracture, restoration of limb alignment, achievement of bony union, and improvement of patient function. Intramedullary rodding, plate fixation, and external fixation have all shown reasonable outcomes when utilized for skeletal stabilization [63,64]. However, one persistent challenge is the application of osteogenic and osteoinductive materials to stimulate new bone formation, especially in the setting of bone loss. Various animal studies have shown that bone morphogenetic proteins (BMPs) can stimulate new bone formation, but their clinical utility in fracture surgery has not been consistently demonstrated. In a trial comparing bone graft to osteogenic protein-1 (BMP-7) in treatment of tibial nonunion, success rates were identical. Other osteoinductive materials such as calcium phosphate have been utilized to fill small bony defects in the distal radius, tibia, and humerus, but cannot be applied to large segments of bone loss [65–69]. Therefore, there remains a critical need for the development of structurally competent osteoinductive materials for treatment of large bony defects.
3.2. Bacterial infection following orthopedic trauma surgery
Infection remains one of the major challenges in the treatment of open fractures. The breakdown of the tissue barrier between the fracture site and the external environment leaves bone susceptible to bacterial contamination and infection. Wound contamination is dependent on the severity of soft-tissue injury, with rates of primary colonization as high as 70–80% [59]. Fractures classified as Gustilo-Anderson Type 3 have demonstrated infection rates of up to 50% after injury [60,70,71]. Lack of vascularity at the fracture site, especially in Gustilo-Anderson IIIC type fractures, further contributes to the risk of bone infection [72–74]. Such infectious complications disrupt soft-tissue and bony healing and often require multiple surgical debridements and long-term antibiotics; in the most severe cases, limb amputation becomes necessary. Therefore, it is no surprise that significant morbidity and cost could be avoided by the prevention and treatment of these infections. Furthermore, Ellington et al. demonstrated that Staph. aureus can invade intracellular locations within osteoblasts, leading to difficulties in bacterial eradication and increased susceptibility to chronic osteomyelitis following infection [75].
Treatment of contaminated open fractures requires surgical debridement, antibiotics, and skeletal stabilization. Successful debridement of all devitalized tissues and bony segments along with copious irrigation can decrease bacterial loads up to 80% [60]. Timely administration of appropriate antibiotics (based on type of injury and suspected contaminant) is critical in initial management. Patzakis et al. demonstrated a dramatic decrease in infection rates (from 13.9% to 2.3%) with the administration of cephalothin [76]. Skeletal stabilization in the form of intramedullary rodding, plate fixation, or external fixation has also been shown to decrease the rate of septic complications; however, insertion of a stabilizing implant may contribute to secondary soft-tissue trauma and may ultimately serve as a sanctuary for micro-colonization of existing bacteria [77].
In grossly contaminated wounds, local antibiotic carriers are frequently used to deliver high levels of antibiotic. Most often, antibiotic-impregnated cement beads or spacers made of polymethylmethacrylate (PMMA) are implanted to complement the antibacterial activity of parenterally administered antibiotics [78]. Osterman et al. found a decrease in infection rate from 12% to 3.7% when the systemic intravenous treatment of open fractures was supplemented with local placement of antibiotic beads [79]. However, this treatment strategy is still fraught with limitations. Only 25% of antibiotics are released locally from the PMMA spacer, which may be inadequate for the treatment of grossly contaminated wounds or chronic infections. Since the cement spacer is a foreign body, glycocalyx-producing bacteria create biofilms that allow them to adhere directly to the PMMA and serve as a nidus for recurrent infection and secondary contamination. As PMMA is not degradable and does not aid in bone repair, a second operation is needed to remove the spacer, which increases both morbidity and cost. Given the limitations of this strategy, biodegradable carriers capable of delivering high levels of antibiotics represent an attractive alternative to current treatment regimens.
4. Nanotherapeutics for healing bone after trauma
The development and differentiation of osteoblasts and osteoclasts are dominated by growth factors, cytokines generated in the bone-marrow microenvironment, and adhesion molecules that mediate cell-cell and cell-matrix interactions. Osteogenic growth factors typically include transforming growth factors β (TGF-β), various bone morphogenetic proteins (BMPs, such as BMP-2, BMP-6, BMP-7, and BMP-9), platelet-derived growth factor (PDGF), and insulin-like growth factor (IGF) [9,80,81]. BMPs, members of the TGF-β superfamily, are considered the most potent osteo-inductive growth factors [82]. Recombinant human bone morphogenetic protein-2 (BMP-2) has an especially remarkable ability to induce new bone formation in vivo [83]. The most severe limitation of these factors for bone-healing applications is that they (e.g., BMP-2) are rapidly degraded in the body by several bio-processes (e.g., enzymatic activities). One possibility to overcome this issue is to use high doses of bone-healing factors, though their side effects include ectopic bone formation and immunological reactions [84]. Those limitations have prompted the development of alternative strategies (e.g., nano-structured scaffolds and nanocarriers) to reduce protein diffusion from the integration site and increase effectiveness [85–87].
One of the strategies to improve the therapeutic efficacy of bone-healing factors is to encapsulate them in micro- to nano-scale carriers [88]. For example, it was shown that a fibrin scaffold containing the growth factor (BMP-2) loaded into heparin/poly (l-lysine) NPs preserved BMP-2 bioactivity; while the α-helical structure of BMP-2-loaded fibrin constructs without NPs had partially been transformed into β-sheets after 21 days. Consequently, the retention of BMP-2 bioactivity resulted in osteogenic differentiation of embedded human mesenchymal stem/stromal cells (hMSCs) into the fibrin hydrogel [85]. Reverse transcription polymerase chain reaction (RT-PCR) results demonstrated that the expression of collagen type I and bone sialoprotein from hMSCs from NPs-BMP increased about 500% and 80% (respectively) compared to the fibrin hydrogel containing native BMP-2. Additionally, when NPs-BMP were loaded with about six-fold higher concentration of osteocalcin (OC), carboxyglutamyl residues formed, stabilizing the α-helical structure of the protein and conferring a greater affinity for the binding of calcium and hydroxyapatite after 21 days, which implies a significant augmentation of osteoblast differentiation [85].
Both the size of the nanocarrier and the composition of the growth factor have crucial roles in burst effect and long-term release of the healing molecules. For example, Wang et al. compared the release performance of colloidal gelatin gels made of either microspheres (26 μm) or nanospheres (150–200 nm) containing BMP-2 and ALP. For nanosphere-based gels, 90% of BMP-2 was released after 4 weeks with nearly zero-order release kinetics, including an initial burst release of about 25% after 24 h. The release profile of the microspheres included 30% burst release after 24 h followed by a relatively slow release (60% after 4 weeks). The differences between the release profiles of BMP-2 from gelatin micro- and nanospheres were attributed to more than 10-fold faster enzymatic degradation profiles for gelatin nanospheres compared to gelatin microspheres, given the larger surface area of the nanospheres when the total mass for the preparation of the nanospheres and the microspheres was kept constant (Fig. 2) [86]. In fact, BMP-2 release from nanospherebased gels reflects a degradation profile, while release from microsphere-based colloidal gels was controlled by a combination of gelatin degradation and BMP-2 diffusion. In contrast to BMP-2, the release kinetics of ALP followed a desorption-controlled profile consisting of a high initial burst release (70%) irrespective of the particle size of gelatin spheres [86].
Fig. 2.

Correlation between BMP-2 release and degradation of colloidal gels: (a) nanoparticles (NP) and (b) microspheres. Reproduced with permission from Ref. [86].
Wei et al. investigated the release kinetics of rhBMP-7 from three types of PLGA NPs (i.e., PLGA50-6.4KDa, PLGA50-64KDa, and PLGA75-113KDa) (d = 300 nm and encapsulation efficiency (EE) = 78–81%) immobilized on a nanofibrous (d = 50–500 nm) PLLA scaffold using a post-seeding technique [87]. With the increase of the LA/GA ratio in PLGA copolymer NPs and/or PLGA molecular weight, the rhBMP-7 release profiles varied: from initial burst release to biphasic and to sustained slow release. Although cells and tissues penetrated throughout the scaffolds onto which rhBMP-7 had been adsorbed as well as those treated with rhBMP-7 NPs, histologic analysis revealed that the incorporation of rhBMP-7 into the NPs protected the protein's bioactivity, prolonged delivery, and subsequently induced ectopic cone formation; in contrast, simple adsorption of rhBMP-7 onto the scaffolds failed to stimulate bone formation [87]. Rodriguez-Evora et al. investigated the bone-inducing efficacy of a composite composed of a porous ring of tribasic calcium phosphate (β-TCP) filled with a pasty core of segmented polyurethane (SPU) and PLGA microspheres (212 μm) encapsulating rhBMP-2 [89]. In vivo experiments showed that 65% of rhBMP-2 was cleared from the scaffolds containing non-loaded PLGA microspheres and rhBMP-2 in solution during the first day, in comparison with 25% of the protein from the scaffolds containing rhBMP-2-loaded PLGA microspheres. In the case of the latter scaffolds, 60% of rhBMP-2 was released during the first 7 days followed by a constant rate of 1% per day for the next 5 weeks. The scaffolds containing rhBMP-2-loaded PLGA (1.6 μg of rhBMP-2 in microspheres) exhibited high bone-forming activity with repair rates of 13%, 31%, and 60% at 4, 8, and 12 weeks, respectively. In contrast, scaffolds containing non-loaded PLGA microspheres and rhBMP-2 in solution did not respond to treatment: the PLGA microspheres in the core of scaffolds were replaced with connective tissue (CT) and blood vessels after complete degradation of PLGA (Fig. 3). These results demonstrate that controlling the presence of rh-BMP-2 is necessary to induce bone regeneration at the defect site [89].
Fig. 3.
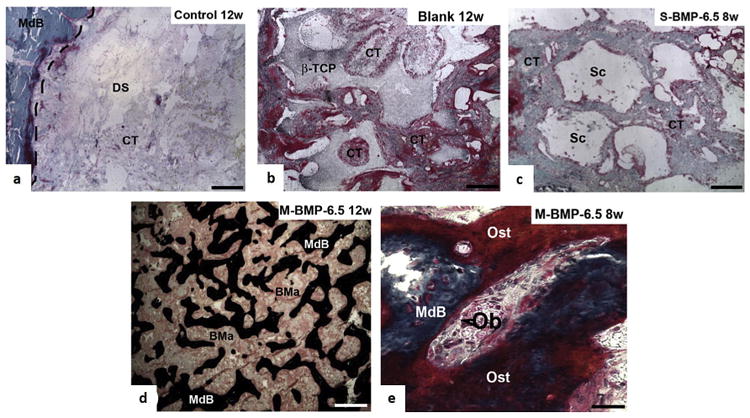
Histological analysis: Representative images of undecalcified histological sections from rat calvariae from (a) Control (i.e., empty defect) 12 weeks postimplantation (Masson-Goldner's Trichrome staining); the dashed line shows the host-to-bone transition zone. (b) Blank (i.e., non-loaded microspheres) 12 weeks postimplantation (Masson-Goldner's Trichrome staining), showing the presence of fibrous tissue inside the βTCP structure. (c) S-BMP-6.5 (10 μl of BMP-2 solution (0.16 μg/mL) + blank system) group 12 weeks postimplantation, where voids represent the space originally occupied by βTCP, which has most likely been removed during sectioning (Masson-Goldner's Trichrome staining). (d) M-BMP-6.5 (i.e., system with 6.5 of BMP-2 microspheres) 12 weeks postimplantation (von Kossa staining). (e) High-magnification images of M-BMP-6.5 8 weeks postimplantation, showing structural details and the repair process (Masson-Goldner's trichrome staining). CT: Connective tissue. DS: Defect site. MdB: Mineralized bone. βTCP: β-tricalcium phosphate. SPU: Segmented polyurethane. GC: Foreign body giant cells. Sc: Scaffold. V: Blood vessels. Ob: Osteoblast (arrowhead). Ost: Osteoid. Scale bars: a-d = 500 μm, f = 30 μm. Reproduced with permission from Ref. [89].
Besides the use of growth factors, synthetic peptides that mimic important residues of the therapeutic molecules have demonstrated great potential in the bone-healing process. For example, osteogenic BMP peptides, e.g., the synthetic peptide KIPKASSVP-TELSAISTLYL, corresponding to residues 73–92 of the knuckle epitope of BMP-2, have been utilized as an alternative to decrease the negative side effects of high doses of BMPs [90–93]. This strategy has gained great attention for application in drug-delivery systems shown to have a high burst release. Zhou et al. investigated the influence of simultaneous incorporation of BMP-2 peptide and DEX into mesoporous silica NPs (DEX/Pep/NPs) on the osteogenic differentiation of bone mesenchymal stem/stromal cells (BMSCs) [94]. While the BMP-2 peptides were covalently attached onto the silica NPs' surface, DEX loading was carried out by immersing the NPs into DEX solution. Experiments on rats demonstrated that the amount of new bone in the DEX/Pep/NPs group was greater than the peptide-functionalized and bare-NPs groups; however, this difference was inconsistent with the highest levels of ALP, RUNX2, and OC observed for BMSCs treated with DEX/Pep/NPs. Thus, the immobilization of BMP peptide, on the one hand, could promote osteogenic differentiation; on the other hand, it could significantly enhance cellular uptake efficiency. In addition, DEX was released with a sustained profile, maintaining the concentration required to induce the osteogenic differentiation of BMSCs (DEX concentration over 10–7 M at day 3) [94]. In another study, bone-forming peptides derived from BMP-7 were encapsulated into the mesoporous channels of silica NPs. An initial burst release of the peptide was found to be 37% of the total encapsulated peptide after 2 days, while another 46% of peptide was released more slowly over 6 days. In spite of the relatively fast release, these NPs induced the expression of ALP after 7 and 14 days [95]. It has been reported that HA NPs inhibit growth and promote apoptosis in osteoblasts [96]. Sun et al. used catechol chemistry to graft BMP-7-derived bone-forming peptides onto the surface of HA NPs coated with polydopamine (PDA) (Pep/PDA/HA NPs) [97]. MG-63 cells incubated with peptide-functionalized HA NPs showed significantly increased viability in a dose-dependent manner compared to the pristine HA NPs, indicating that peptide decoration strongly promoted cell proliferation. Furthermore, the apoptosis rate of MG-63 cells incubated with HA NPs was 17%, which decreased to 1% for Pep/PDA/HA NPs. Moreover, the presence of large numbers of cellular filopodia showed that Pep/PDA/HA NPs elicited better spreading and adhesion of osteoblast-like cells. In comparison with the bare nano-HA, the HA NPs decorated with bone-forming peptide enhanced both cell proliferation and differentiation in terms of ALP activity [97].
In addition to the growth factors, some drugs (e.g., dexamethasone; Dex) have the capacity to stimulate the osteogenic differentiation of MSCs at the early stage and direct the cells towards maturation in the late stage of differentiation and matrix mineralization [98]. To that end, the effect of Dex-loaded carboxymethylchitosan/poly (amidoamine) dendrimer NPs on the osteogenic differentiation of rat bone marrow stromal cells seeded onto the surface of either HA or starch-p Though many engineered materials olycaprolactone scaffolds was probed [99]. Evaluation of ALP and OC expression levels as early- and late-stage markers of osteogenic differentiation showed that the synthesized NPs promoted proliferation and osteogenic differentiation as well as a culture medium containing Dex [99].
Another therapeutic strategy is to use NPs to provoke cells to secrete healing factors for bone regeneration. Inspired by the effect of magnetic fields on bone wound healing [100], magnetic NPs have been used as dynamic mechanical stimuli (which are often lacking in therapeutic approaches applied for bone regeneration) by directly targeting cell-specific cell receptors, such as mechanically gated ion channel TREK1 and the arginine-glycine-aspartate (RGD)-binding site of integrin, and transducing forces from an external magnetic field [101–104]. Thereby, mechanotransduction can be remotely stimulated without causing mechanical stress to the scaffold or surrounding tissues. Russo et al. studied the osteointe-grative properties of two HA/Collagen (70-30 wt %) magnetic scaffolds magnetized with two methods: (i) direct nucleation of biomimetic phase and iron oxide NPs (200 nm) on self-assembling collagen fibers (MAG-A) and (ii) scaffold impregnation in ferro-fluid solution (MAG-B) [101]. No inflammatory reaction or bone reabsorption activity due to magnetic nanoparticles was observed in the newly formed bone tissue at the implant site. In addition, bone healing was significantly faster in MAG-A than in MAG-B. The porosity of the MAG-A scaffold, the homogeneous distribution of the iron oxide NPs, and the low concentration of these NPs in the scaffold could account for the difference in healing speed [101]. Meng et al. reported the effects of applying a magnetic field and nanoparticles in a synergistic way to enhance bone regeneration. While the authors noted a significant increase in the proliferation rate of osteoblasts seeded on both HA NPs/PLA/γ-Fe203 NPs and HA NPs/PLA nanofibrous films under a magnetic field, only the cells growing on the HA NPs/PLA/γ-Fe203 NPs films produced significantly greater ALP [104]. Henstock et al. showed that the combination of sustained release of BMP-2 from polymer microspheres and magnetic nanoparticle-mediated mechanotransduction of MSCs can be applied synergistically to enhance both volume and density of newly formed bone (Fig. 4) [102]. In fact, mechano-transduction increases the intensity and duration of Smad phosphorylation early in the BMP signaling pathway, amplifying intracellular cascades and potentially improving the clinical potential of therapeutic BMP treatments for various bone pathologies [105,106].
Fig. 4.

Combinations of magnetic NP-labeled hMSCs and the release of BMP2 from polymer microparticles in 2.0% collagen hydrogels in comparison with either NPs or BMP2 alone and controls, which were hMSCs alone (t = 72 h). Both bone volume and density significantly increased when the microparticles containing BMP2 and magnetic NPs were combined. a) The volume of the mineralizing high-density material was similar for BMP2 alone and the control, while cells treated with either NP in combination with BMP2 were significantly more mineralized. NP-only and NPs + BMP combinations resulted in the formation of more numerous and thicker mineralized areas within the hydrogels. b) These mineralized nodular regions were significantly less interconnected in the TREK-NP-containing groups compared with the control group and are highlighted in green in the microtomography reconstructions, shown as cross-sections through the center of hMSC-seeded hydrogels containing BMP2 microparticles alone (c), compared with hydrogels containing BMP2 particles plus TREK-nanoparticle-labeled hMSC (d). RGD: Arg-Gly-Asp tripeptide. Reproduced with permission form Ref. [102]. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
5. Nanostructure scaffolds for bone-healing applications
Scaffolds are implants that act as carriers to deliver cells, drugs, and genes into the body [107]; however, they can also provide a suitable environment for bone cells to make their own tissue. An ideal scaffold for bone regeneration must be able to induce rapid cell adhesion, proliferation, and differentiation while possessing adequate mechanical strength to sustain physiological loads [108,109]. Natural bone owes its resistance to fracture to a unique combination of structural features that range from micro- to nanoscale with a meticulous interface between various components [110]. Though many engineered materials (e.g., biodegradable polymers and ceramics) have been designed to mimic natural bone, these materials have several limitations including inadequate mechanical strength. In a very recent review, Bobbert and Zadpoor thoroughly reviewed a wide range of scaffolds with various mechanical and physicochemical properties for bone healing applications [111]. NP-modified composite scaffolds have recently attracted great interest as promising candidates for supporting bone regeneration. In fact, the incorporation of nanoscale organic and inorganic materials into biodegradable polymer scaffolds may result in changes in micro- and nanostructure to improve mechanical properties as well as surface modifications to promote cellular adhesion, differentiation, and integration into the surrounding environment, allowing for eventual replacement with functional tissue [108,109].
For bone applications, therapeutic scaffolds should have proper mechanical properties. Besides approaches presented in a recent review by Bobbert and Zadpoor [111], another strategy is to use plastic compression (PC) of hydrogels [112–115]. For example, cellular collagen hydrogels had previously been used as 3D structures for model creation, but their culture-dependent growth is slow, and they cannot provide sufficient mechanical strength [116]. Brown et al. engineered a means for rapid, controlled manipulation of fibril density for cell-independent tissue engineering through the PC of native collagen gels [116]. Their protocol involves the controlled expulsion of interstitial liquid inside collagen gels by applying a compressive mechanical load and/or capillary suction into porous layers. PC led to a drastic reduction in initial construct thickness, from a gel of about 3.6 mm to a collagen sheet of 23–48 μm thickness, increasing collagen density through decreasing fluid content. This approach formed the foundation for further applications of sufficiently dense collagen structures in tissue engineering. One such application was the investigation of the effects of the hydraulic permeability (k) of collagen gels (produced by unconfined PC under increasing load) on the cell-scaffold interaction, including MSC-induced gel contraction, metabolism, and differentiation [114]. The outcomes demonstrated visually distinct gel contraction patterns under osteogenic and non-osteogenic medium, whereby contraction was more extensive in the latter than the former. Gel contraction decreased as a direct function of increasing the collagen fibril density (CFD) of the gels, resulting in decreased k. Additionally, MSC differentiation increased strongly with decreasing k within samples that underwent PC, in comparison to a 0 Nm–2 static stress control sample exposed to osteogenic medium and monitored by ALP staining [114]. In another study, Chamieh et al. investigated the osteogenic effects of collagen scaffolds produced by PC and seeded with mesenchymal dental pulp stem cells on bone regeneration in a calvarial defect model. The dense collagen-based scaffolds supported the long-term metabolic activity of the stem cells, eliciting an increase in fibrous connective and mineralized tissue volume [115].
6. Antibacterial nanotechnologies to combat orthopedic infections
Bacterial infection continues to be a major challenge in orthopedic trauma surgery, compounded by resistance against many common antibacterial agents. Drug resistance propels high-dose administration of antibiotics, often generating adverse side effects including end-organ toxicity. This has necessitated the development of alternative strategies to treat bacterial diseases [117]. Among them, nanoscale materials have attracted great interest as novel antimicrobial agents to combat the bacterial resistance threat [5,118]. Several classes of antimicrobial NPs and nano-sized carriers for antibiotic delivery have proven effective in treating infectious diseases, including antibiotic-resistant ones, in vitro as well as in animal models [117]. Very recently, we demonstrated that even multidrug-resistant bacterial infection can be treated by synergistic antimicrobial therapy using NPs and antibiotics [119]. NPs with different physicochemical properties use different pathways to attack the bacterial infection. For example, some NPs can attach to the membrane of bacteria by electrostatic interaction and damage the integrity of the bacterial membrane [120]. As an another example, engineered NPs can induce massive oxidative stress to bacteria by free radical formation (i.e., reactive oxygen species; ROS) and diminish their infection risk (Fig. 5) [121,122]. We have thoroughly reviewed the antibacterial properties of a wide range of NPs [119]; readers are referred to that review for details on specific types of NPs and their corresponding mechanisms of action. Here, we will focus solely on NP platforms designed to impede the bacterial activity associated with orthopedic trauma surgery.
Fig. 5.
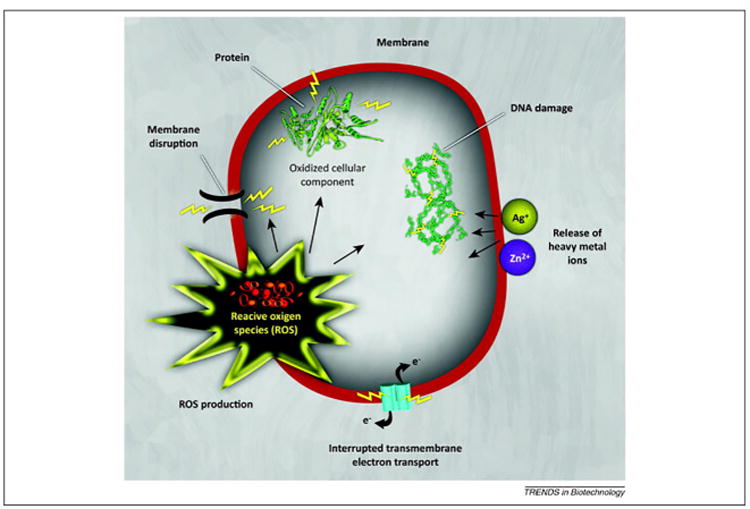
Toxicity mechanisms of NPs against bacteria. NPs and their ions (e.g., silver and zinc) can induce oxidative stress via the production of reactive oxygen species (ROS). The ROS produced can irreversibly damage bacteria (e.g., their membrane, DNA, and mitochondria) culminating in bacterial death. Reproduced with permission from Ref. [6].
Some of the most commonly employed NPs for reduction of infection risk in orthopedic trauma are silver (Ag) NPs. Their broad antibacterial activity is well known, and they have long been used to combat different bacteria including Staphylococcus aureus [123], Bacillus subtilis [124], Klebsiella pneumoniae [125], Pseudomonas aeruginosa [126], and Escherichia coli [126]. The mechanisms of action of the Ag NPs in killing bacteria and/or slowing down their growth include damaging the bacterial membrane and induction of oxidative stress via the formation of free radicals [123–126]. The use of Ag NPs in bone-healing cements can substantially reduce the risk of infection compared to conventional antibacterial drugs. For example, Alt et al. reported that poly(methyl methacrylate) (PMMA) bone cement, consisting of 1.0% Ag NPs (5–50 nm), entirely inhibited the proliferation of Staphylococcus epidermidis, methicillin-resistant S. epidermidis (MRSE), and methicillin-resistant S. aureus (MRSA), while PMMA bone cement loaded with 2% of gentamicin sulphate inhibited only the proliferation of S. epidermidis [127]. Similar to other applications of nanomedicine, the safety of these NPs together with their minimal side effects have drawn considerable notice. However, one of the main adverse effects of bone cement Ag NPs is their contribution to central and peripheral nervous system dysfunction [128,129]. As one might expect, the antibacterial properties of the NPs are strongly dependent to their concentration in the scaffold/cement. Rameshbabu et al. investigated the antibacterial effect of silver-substituted (0.5–1.5%) hydroxyapatite particles with needle-like morphology (width: 15–20 nm and length: 60–70 nm) against E. coli and S. aureus. These particles completely inhibited S. aureus even at high cell concentrations (108 cells/mL), while E. coli colonies were observed at the same cell concentrations for samples containing less than 1.5% silver. Importantly, osteoblast adherence and spreading were found to be remarkably greater with particles with 0.5% silver substitution compared to 1.0 or 1.5%, suggesting that hydroxyapatite with lower amounts of silver are most suitable for implant applications [130].
Repair of orthopedic trauma factures in clinics may call for metallic/polymeric/ceramic scaffolds [e.g., titanium (Ti) and chitosan) with/without suitable coating [e.g., hydroxyapatite coating (HAC)]. However, various bacterial infections can affect long-term in vivo performance of these implants [131–133]. One possibility to overcome this issue is to incorporate antibacterial NPs either in the composition of implantable materials or their coatings. To this end, Tian et al. used a Ag mirror-reaction method (i.e., deposition of Ag on the surface of scaffold in the form of a “mirror”) to decorate the surface of hydroxyapatite coatings on Ti-6Al-4V with Ag NPs (10–30 nm) [134]. The formation of Ag NPs required two steps: i) adsorption of Ag+ ions onto the HAC surface via electrostatic interactions between negatively charged and positively charged Ag+ ions, and ii) reduction of Ag ions to Ag NPs and HAC attachment. The authors developed HAC with block morphology to enhance Ag load efficiency and antibacterial activity. Antibacterial tests showed that there were significantly fewer living E. coli and S. aureus organisms (t = 24 h) when Ag NPs were doped on the surface of hydroxyapatite. In another study, a biocompatible scaffold composed of chitosan (CS) and hydroxyapatite NPs (< 200 nm) was fabricated, and antibacterial effects were achieved via the addition of Ag NPs (80–120 nm) [135]. Ag NPs were formed by immersion of the scaffold into silver nitrate solution following the reduction of the Ag ions to metallic silver by the nitrogen and oxygen atoms of the functional groups in CS without the use of any external chemical reducing agent. The Ag NPs' incorporation into the scaffold decreased the swelling percentage, indicating an improvement in mechanical strength supporting bone tissue ingrowth. Antibacterial tests using S. aureus and E. coli showed a greater zone of inhibition (∼ 3-fold) for the scaffold containing Ag NPs [135]. Stevanovic et al. used selenium (Se) NPs, which has been reported to have antibacterial activity [136], to coat a bioactive glass-based scaffold (SiO2 = 45 wt% and molar ratio of Ca to P = 5) prepared by the foam replica method [137]. Broth microdilution assays against S. epidermidis and S. aureus showed decreases in minimum inhibitory concentration (MIC) of about 50% and 75% (respectively) when Se NPs were added to the scaffold, whereas Se NPs were not effective against B. subtilis or K. pneumoniae.
Chitosan (CS), a linear polysaccharide produced from crustacean shells, has a great potential for incorporation into bone-healing scaffolds mainly due to its intrinsic antibacterial activity and its low toxicity towards mammalian cells [138]. In addition, CS has a cationic nature due to the presence of amino groups in its linear structure and can interact with different crosslinkers to form NPs. While chemical crosslinkers (e.g., glutaraldehyde) can be toxic to biological systems, products incorporating CS are safe, since it can be ionically crosslinked with multivalent anions such as tripoly-phosphate. This process, known as ionic gelation, has been successfully applied to fabricate CS NPs with sizes <200 nm [139,140]. However, one concern is that CS is insoluble in neutral and basic environments, which may pose technical limitations (e.g., difficulties in infusing CS) for bone cement production. To address this concern, CS derivatives containing ammonium salt have been synthesized, and their antibacterial activities were shown to be enhanced by increasing the chain length of the alkyl substituent [141]. Retention of mechanical properties is another matter of concern in CS modification of bone cement, since CS solubilizes and degrades in an acidic environment; a localized pH drop is usually a consequence of infections and inflammation, exactly when maximal effectiveness is most required [142,143]. Shi et al. assessed the influence of the incorporation of CS NPs (d = 70 nm, average zeta potential = 48 mV) and quaternary ammonium CS derivative (QCS) NPs (d = 100 nm, average zeta potential = 67 mV) on antibacterial activity of commercial bone cements against S. aureus and S. epidermidis [144]. A comparison of viable bacteria after incubation with different substrates demonstrated decreases of about 100- and 1000-fold (respectively) when CS NPs and QCS NPs were incorporated. The higher antibacterial activity of QCS NPs was attributed to the presence of the quaternary ammonium groups that increase i) the affinity for the negatively charged bacterial cell membrane and ii) the efficiency of penetration through the bacterial cell membrane. In addition, the incorporation of CS NPs and QCS NPs improved the antibacterial efficiency of gentamicin-loaded bone cements. Importantly, antibacterial effectiveness of gentamicin-loaded bone cements showed a much smaller decrease after 3 weeks when CS NPs and QCS NPs were incorporated [144].
Biodegradable nanoplatforms have the advantages of higher efficacy and lower side effects compared to the local delivery of antibacterial drugs to the site of infection. To that end, synthetic biodegradable polymers are a popular choice for materials, since the degradation profile of the polymer can be tailored to obtain a specific antibiotic release profile. Several biodegradable polymers approved by the FDA have been designed and applied for clinical trials, including poly(d,l-lactic acid), poly(lactic-co-glycolic acid) (PLGA), and copolymers of l-lactide and d, l-lactide [118,145]. These polymers, after being engineered/processed, demonstrated the capacity to release antibiotics for several hours to 40 weeks in vitro and are efficient for several weeks in vivo [146,147]. However, the hydrophobic surface of the synthetic polymer largely impedes biological interactions, limiting their broader application. Feng et al. reported the application of PLGA NPs containing doxycycline (DOXY, a hydrophilic antibiotic) uniformly distributed throughout a poly (l-lactic acid) (PLLA) scaffold to achieve sustained release of DOXY with reduced initial burst release compared to PLLA scaffold laden with DOXY. In vitro release kinetics of DOXY from PLGA NPs revealed that a simultaneous increase in lactic acid (LA): glycolic acid (GA) ratio from 50: 50 to 85: 15 and PLGA molecular weight from 6.5 K to 142 K led to an extended-release profile with lower burst release. While the antibacterial activity of the DOXY absorbed onto the PLLA scaffold against S. aureus and E. coli rapidly diminished from 100% to around 10% after 42 days, the scaffold containing DOXY-loaded PLGA NPs showed 70% and 35% inhibition for S. aureus and E. coli (respectively) after the same time [148]. Ti Peng et al. demonstrated that gels formed via aggregation of amphiphilic poly(ethylene glycol) monomethyl ether (mPEG)-PLGA diblock copolymer micelles (100 nm) were effective in treating rabbits infected with S. aureus (Fig. 6). In fact, covalent coupling of the hydrophilic mPEG to PLGA rendered the copolymer aqueous under its phase-transition temperature (19–21 °C). Therefore, teicoplanin, a glycopeptide antibiotic, could be completely encapsulated (100%). The release of teicoplanin in vitro did not show significant burst release but was steady, with a diffusion-controlled profile. Furthermore, the total released amount of teicoplanin was 60% at day 31 in vitro [149].
Fig. 6.

Treatment of S. aureus-induced osteomyelitis in rabbits with (A) the teicoplanin-impregnated PMMA cement beads: (a) osteomyelitis induction, (b and c) after 4- and 8-week treatment, (d) The PMMA cement beads act as physical barriers to new bone growth into the defect. (B) teicoplanin-loaded mPEGePLGA hydrogel micelles: (a) osteomyelitis induction, (b and c) after 4- and 8-week treatment, (d) In vivo study confirmed that the mPEGePLGA hydrogel micelles efficiently treated osteomyelitis in rabbits without the drawbacks of PMMA cement beads. Reproduced with permission from Ref. [149].
In another study, Gonzalez-Sanchez et al. used methacrylate-based gel matrix containing calcium phosphate microparticles to incorporate Ag NPs (60–80 nm). Three different preparation methods were used: i) mixing the Ag NPs with the monomers before gel synthesis, ii) diffusion of the Ag NPs into the composite and iii) simple adsorption. Unlike the two first preparation methods, the adsorption of Ag NPs on the gel composite showed antibacterial activity only against S. epidermidis and MRSA. In the case of the first preparation method, the formation of a calcium phosphate and/or silver phosphate film might have prevented silver ion flow across the surface of the composite; while with the second preparation method, the precipitation of the silver ions with the phosphate ions to the bottom of the reaction vessel was identified as the main cause for the reduced antibacterial activity of the Ag NPs [150]. A similar trend was observed with superparamagnetic iron oxide NPs embedded in collagen scaffolds [151]. More specifically, the magnetic NPs demonstrated stronger antibacterial effects when they were physically adsorbed to the collagen scaffolds, compared to when they were chemically incorporated into the collagen structure. Table 1 offers examples of important reports on the antibacterial properties of nanostructured materials against bone infections.
Table 1.
Different nanostructured materials and their toxic effect on bone infections.
| Bacteria | Bacterial property | Scaffold | NPs | Applied dosage | Remarks | Ref. |
|---|---|---|---|---|---|---|
| S. epidermidis | Gram positive bacteria, Biofilm formation, normal of flora of skin, Gentamicin-resistant | Poly (methyl methacrylate) (PMMA) | Ag 5–50 nm | 0.1–1.0 wt% of the bone cement | The proliferation of S. epidermidis and methicillin-resistant S. epidermidis completely inhibited at the highest NP concentration | [127] |
| 45S5Bioglass scaffold | Selenium (Se) | ND | The scaffolds were coated by soaking/immersing into solution containing Se NPs. 50% decrease in minimum inhibitory concentration of bacteria | [137] | ||
| Methyl methacrylate methacrylate copolymer cement | CS NPs 70 nm and quaternary ammonium CS derivative (QCS) NPs -100 | CS to bone cement powder ratio of 15% (w/w) | 100- and 1000-fold decrease in bacteria viability when CS NPs and QCS NPs were incorporated, respectively. | [144] | ||
| Methacrylate-based gel matrix containing calcium phosphate microparticles | Ag 60–80 nm | 0.5 mM | Ag NPs were integrated into the composite matrix via three different methods: i) entrapment in the polymeric hydrogel before mineralization; ii) diffusion during the process of calcium phosphate crystallization and iii) adsorption post-mineralization. The adsorption of Ag NPs was the most effective method to decrease antibacterial activity longer than 48 h | [150] | ||
| S. aureus | Gram positive bacteria, Biofilm formation, normal of flora of skin | Poly (methyl methacrylate) (PMMA) | Ag 5–50 nm | 0.1–1.0 wt% of the bone cement | The proliferation of S. aureus and methicillin-resistant S. aureus (MRSA) completely inhibited at the highest concentration of the NPs | [127] |
| – | silver-substituted (0.5–1.5%) hydroxyapatite NPs - needle-like morphology (width: 15–20 nm and length: 60–70 nm) | 1 mg/mL | Complete abrogation of S. aureus proliferation at high cell concentration (108 cells/mL) | [130] | ||
| Hydroxyapatite coated titanium (Ti) implant | Ag 10-30 | ND | Ag mirror reaction method was used to decorate the surface of the implant. A significant decrease in the number of living S. aureus organisms. | [134] | ||
| Chitosan (CS) containing hydroxyapatite NPs | Ag 80–120 nm | ND | Ag NPs were formed by immersion of the scaffold into silver nitrate solution. 3× increase in the zone of inhibition | [135] | ||
| 45S5Bioglass scaffold | Selenium (Se) | ND | The scaffolds were coated by soaking/immersion in solution containing Se NPs. 75% decrease in minimum inhibitory concentration of bacteria | [137] | ||
| Methylmethacrylate methacrylate copolymer cement | CS NPs 70 nm and quaternary ammonium CS derivative (QCS) NPs -100 | CS to bone cement powder ratio of 15% (w/w) | 100- and 1000-fold decrease in bacteria viability when CS NPs and QCS NPs (respectively) were incorporated. | [144] | ||
| Nanofibrous PLLA scaffold | Poly (lactic-co-glycolic acid) (PLGA) laden with doxycycline | ND | The scaffold containing DOXY-loaded PLGA NPs showed 7 times more inhibition for E. coli after 42 days compared to the PLLA scaffold | [148] | ||
| Methacrylate-based gel matrix containing calcium phosphate microparticles | Ag 60–80 nm | 0.5 mM | Ag NPs were integrated into the composite matrix via three different methods: i) entrapment in the polymeric hydrogel before mineralization; ii) diffusion during the process of calcium phosphate crystallization and iii) adsorption post-mineralization. The adsorption of Ag NPs was the most effective method to decrease antibacterial activity against MRSA longer than 48 h | [150] | ||
| – | PLGA microparticles (20–25 μm) containing either nafcillin or levofloxacin coated with coated with calcium phosphate (CP) NPs (100 nm) | The final concentration of the antibiotics ranged from 64 μg/mL to 0.03 μg/mL | The CP coating reduced the burst release of the antibiotics, while providing sustained release for up to 4 weeks. Complete inhibition of S. aureus biofilm formation as well as deterioration of the established biofilm were achieved | [80] | ||
| CS | Clindamycin-loaded hydroxyapatite (HA) NPs 2–10 nm | 1 mL to 37 mg/mL | Although the addition of CS to the NPs mitigated the burst release of the antibiotic, it significantly reduced the antibacterial efficacy against S. aureus | [163] | ||
| PLGA-coated calcium Phosphate containing clindamycin 20–60 nm | Clindamycin: 1 and 5 wt% | The antibiotic containing NPs showed sustained drug release contingent upon the degradation rate of the carrier, while they were highly effective in suppressing the growth of S. aureus | [164] | |||
| PLGA nanofibers (NFs) | amoxicillin (AMX)-loaded HA NPs | 5 wt% HA NPs and 1% AMX both relative to PLGA | The loaded AMX within the HA NPs/PLGA hybrid nanofibers exhibited a sustained release profile and a non-compromised activity to suppress the growth of S. aureus | [165] | ||
| Porous beta-tricalcium phosphate (β-TCP) | Gentamicin sulfates (GS)-loaded liposomes 100 nm to 5 μm | GS content ranges from 2.5 to 800 μg/mL | The GS release from the liposome combined β-TCP was recognized as an initial high dose of liposomal GS released from the matrix and a further sustained release of free GS from the liposome. The liposome-combined β-TCP showed a potential anti-biofilm activity against S. aureus even at the lowest GS concentration (2.5 μg/mL), and the regrowth time was extended from 5 h to 9.5 h | [166] | ||
| E. Coli | Gram positive bacteria, normal flora of the gut | – | Silver-substituted (0.5–1.5%) hydroxyapatite NPs - needle-like morphology (width: 15–20 nm and length: 60–70 nm) | 1 mg/mL | NPs containing less than 1.5% silver could not inhibit E. coli growth | [130] |
| Hydroxyapatite coated titanium (Ti) implant | Ag 10-30 | ND | Ag mirror reaction method was used to decorate the surface of the implant. A significant decrease in the number of living S. aureus organisms. | [134] | ||
| Chitosan (CS) containing hydroxyapatite NPs | Ag 80–120 nm | ND | Ag NPs were formed by immersion of the scaffold into silver nitrate solution. 3× the zone of inhibition | [135] | ||
| Nanofibrous PLLA scaffold | Poly (lactic-co-glycolic acid) (PLGA) laden with doxycycline | ND | The scaffold containing DOXY-loaded PLGA NPs showed 3.5 times more inhibition for E. coli after 42 days compared to the PLLA scaffold. In fact, the biodegradable polymer provides controlled release of DOXY | [148] |
Abbreviation: ND, No data.
Controlled release of payloads (e.g., drugs and antibiotics) is the definitive function of micro- to nano-scale carriers. The term “controlled release” includes a range of different release profiles and mechanisms such as triggered release and sustained (or extended) release [152–157]. An ideal bone-drug delivery system should provide sustained and controlled release of drugs to both promote bone regeneration and reduce infection risk. Composites consisting of calcium phosphate (CP) and a biodegradable polymer (e.g. PLGA) have shown great potential in the reconstruction of damaged bone tissue. In fact, the biodegradable polymer provides controlled release of drugs, which is often a challenge for CP due to relatively slow degradation; while the CP coating governs the interfacial process, including adsorption of biomolecules and apatite crystal nucleation and growth. In general, this type of composite causes low cytotoxicity [158], provides adhesion of an osteoprogenitor with the final goal of heightening differentiation and osteogenesis [159], and reduces inflammatory processes in human tissue in combination with tetracycline antibiotic [160]. Xu and Czernuszka synthesized hydroxyapatite-coated PLGA microspheres encapsulating amoxi-cillin (AMX) with EE = 40%. The presence of HA on the surface was expected to improve the bone-bioactivity of these microspheres and did not significantly alter the release profile of amoxicillin [161]. In another study by Bastari et al., PLGA microparticles (20–25 μm) were coated with CP NPs (100 nm) via surface-adsorption, while either nafcillin (hydrophilic antibiotic) or levofloxacin (hydrophobic antibiotic) was encapsulated. Uncoated nafcillin-loaded PLGA microparticles exhibited a burst release (75%, t = 24 h), whereas their CP-coated counterparts showed more-sustained release of nafcillin (up to 4 weeks) with only 15% initial burst release. This difference was attributed to the CP coating, which acted as a barrier against rapid drug release. Unlike nafcillin-loaded particles, there was a marginal difference in the release profiles of levofloxacin from CP-coated PLGA and PLGA particles, i.e., a small initial burst release followed by 5–6 weeks of sustained release. Unlike nafcillin, the amphiphilic nature of levofloxacin may prevent the drug from escaping to the external aqueous medium during NP preparation. Furthermore, levofloxacin-loaded PLGA microparticles (either with or without CP coating) effectively eliminated 100% of S. aureus-established biofilm within 7 days [80]. These findings highlight the importance of design and composition of micro- to nano-scale particles to create efficient delivery systems against bone infections. Conversely, Ignjatovic et al. reported the fabrication of a two-step NP system consisting of CP NPs coated with PLGA polymer loaded with tigecycline (d = 65–95 nm). In the first step, controlled release of tigecycline occurred due to PLGA resorption; in the second step, CP NPs that remained after the resorption of the polymer acted as filler to counteract potential damage to bone tissue. More than 90% of the total amount of tigecycline was released over 20 days, while the release rate after the first day varied from 8% to 20% depending largely on the initial concentration of tigecycline (0.6–5 wt%). According to an in vivo study, the sample with the lowest antibiotic concentration (i.e., 0.6 wt%) satisfied MICs for the most multi-resistant coagulase-negative staphylococci, while the concentration of tigecycline in surrounding tissues, leg muscles, and kidneys was found to be zero [162]. Uskokovic and Desai showed that the addition of CS to clindamycin-loaded HA NPs (2–10 nm) decreased the burst release from 60% to 15% after 24 h and promoted more-sustained drug release for three weeks. However, the surface layer of CS significantly decreased the antibacterial activity of clindamycin-loaded HA NPs against S. aureus (32-fold greater MIC). The amount of antibiotic released after 3 weeks was the same for coated and uncoated HA NPs, implying that the lower initial release of the antibiotic in the presence of CS coating was the main reason for the lower antibacterial efficiency [163]. However, an earlier study demonstrated the antibacterial effectiveness of clindamycin-loaded HA NPs coated with PLGA [164]. One explanation for this discrepancy could be that alkaline residues of CS might be less effective than the acidic residues of PLGA against S. aureus. Unlike CS, PLGA is negatively charged under physiological conditions. Another possibility is that the synergy between the antibiotic and its carrier resulted in the retention of the antibiotic in the surface layers of HA NPs. Electrospun antibiotic-loaded organic/inorganic nano-fibers (NFs) were designed to alleviate the burst release of the antibiotic loaded onto HA NPs. Zheng et al. fabricated composite NFs (d = 777 ± 204 nm) composed of PLGA and AMX-loaded rod-like HA NPs (d = 37 nm and L = 118 nm) (5 wt% HA NPs and 1% AMX both relative to PLGA). While AMX-loaded HA NPs and AMX-loaded PLGA NFs exhibited an initial burst release (50%) within 24 h, AMX/HA NPs/PLGA NFs showed a relatively sustained release profile, while the amount of AMX released was 16% after 24 h and 35% after 18 days [165]. In contrast, in the case of AMX-loaded HA NPs (AMX/HA) and PLGA NFs (AMX/PLGA), more than 90% of AMX was released within 6 days with a considerable burst release (50%). The relatively slow release of AMX from AMX/HA NPs or PLGA NFs and the resulting low concentration of AMX within 24 h meant that these fibers were inefficient in inhibition of bacterial (S. aureus) growth when the concentration of AMX was lower than 60 μg/mL in bacterial liquid medium. In contrast, free AMX and AMX-loaded PLGA NFs efficiently inhibited bacterial growth when the concentration was 40 μg/mL. However, after 9 days' release, AMX/PLGA NFs had much less antibacterial activity than AMX/HA NPs/PLGA NFs (5% vs. 43%), likely due to a significant amount of AMX released on day 9 [165].
In addition to NPs, other types of nanocarriers can be used to prevent bone infections. Zhu et al. reported the use of liposomes, defined as spherical vesicles of phospholipid molecules enclosing a water droplet, as drug carriers. Gentamicin sulfates (GS)-loaded liposomes of different particle sizes (100 nm–5 μm) were combined with beta-tricalcium phosphate (β-TCP). The release of GS had two stages i) fast release of GS-loaded liposomes from β-TCP (90% at t = 24 h) and ii) slow release of GS from the liposomes (5% at t = 24 h). In other words, the scaffold released the liposomes in sac form at the infection site, followed by release of GS itself. This release mechanism achieved significant inhibition of bacterial regrowth of S. aureus biofilms. However, the main drawback of this liposomal GS scaffold is low EE for the antibiotic (<10%) [166].
7. Conclusions and future perspectives
Treatment of bony defects and eradication of bony infection are central issues in orthopedic trauma surgery. Here we reviewed the substantial impact of nanomedicine in both accelerating the healing process and diminishing the risk of infection in such surgery. These novel approaches may soon reduce the need for follow-up surgeries and even prevent amputations. There are promising trends of combined improvements in bone healing and the antibacterial activity of nanoplatforms. However, individual studies are difficult to generalize, mainly because poorly defined and characterized nanoplatforms are used, making correlation with basic physicochemical properties impossible. Furthermore, the roles and temporal parameters of expression of the molecular mediators in fracture healing need to be further elucidated. Without agreement on standard nanoplatforms and biological outcomes (e.g., the bone healing process and antibacterial activity) as reference systems for future studies, there is still a long way to go before we develop highly efficient nanoplatforms that overcome the present challenges in the field of orthopedic surgery.
Acknowledgments
This work was supported by the US National Institutes of Health grants HL127464-01A1(O.C.F.), EB015419 (O.C.F.), Department of Defense grant PC140318 (O.C.F.), and The BWH Stepping Strong Center Award for Trauma Innovation.
Footnotes
Competing financial interests: O.C.F. declare financial interests in Selecta Biosciences, Tarveda Therapeuticsand Placon Therapeutics.
References
- 1.Kanakaris NK, Giannoudis PV. The health economics of the treatment of long-bone non-unions. Injury. 2007;38:S77–S84. doi: 10.1016/s0020-1383(07)80012-x. [DOI] [PubMed] [Google Scholar]
- 2.Farokhzad OC, Langer R. Nanomedicine: developing smarter therapeutic and diagnostic modalities. Adv Drug Deliv Rev. 2006;58:1456–1459. doi: 10.1016/j.addr.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nano-carriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 4.Shi J, Xiao Z, Kamaly N, Farokhzad OC. Self-assembled targeted nano-particles: evolution of technologies and bench to bedside translation. Acc Chem Res. 2011;44:1123–1134. doi: 10.1021/ar200054n. [DOI] [PubMed] [Google Scholar]
- 5.Mahmoudi M, Serpooshan V. Silver-coated engineered magnetic nano-particles are promising for the success in the fight against antibacterial resistance threat. ACS Nano. 2012;6:2656–2664. doi: 10.1021/nn300042m. [DOI] [PubMed] [Google Scholar]
- 6.Hajipour MJ, Fromm KM, Ashkarran AA, de Aberasturi DJ, de Larra-mendi IR, Rojo T, Serpooshan V, Parak WJ, Mahmoudi M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012;30:499–511. doi: 10.1016/j.tibtech.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Vallet-Regí M, Ruiz-Hernández E. Bioceramics: from bone regeneration to cancer nanomedicine. Adv Mater. 2011;23:5177–5218. doi: 10.1002/adma.201101586. [DOI] [PubMed] [Google Scholar]
- 8.Aizenberg J, Weaver JC, Thanawala MS, Sundar VC, Morse DE, Fratzl P. Skeleton of Euplectella sp.: structural hierarchy from the nanoscale to the macroscale. Science. 2005;309:275–278. doi: 10.1126/science.1112255. [DOI] [PubMed] [Google Scholar]
- 9.Boskey AL. Bone Tissue Engineering. CRC Press; 2005. [Google Scholar]
- 10.Young MF. Bone matrix proteins: their function, regulation, and relationship to osteoporosis. Osteoporos Int. 2003;14:35–42. doi: 10.1007/s00198-002-1342-7. [DOI] [PubMed] [Google Scholar]
- 11.Raspanti M, Congiu T, Alessandrini A, Gobbi P, Ruggeri A. Different patterns of collagen-proteoglycan interaction: a scanning electron micro-scopyand atomic force microscopy study. Eur J Histochem. 2000;44:335. [PubMed] [Google Scholar]
- 12.McKee M, Nanci A. Osteopontin at mineralized tissue interfaces in bone, teeth, and osseointegrated implants: ultrastructural distribution and implications for mineralized tissue formation, turnover, and repair. Microsc Res Tech. 1996;33:141–164. doi: 10.1002/(SICI)1097-0029(19960201)33:2<141::AID-JEMT5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 13.Robey PG, Fedarko NS, Hefferan TE, Bianco P, Vetter UK, Grzesik W, Friedenstein A, Van Der Pluijm G, Mintz KP, Young MF. Structure and molecular regulation of bone matrix proteins. J Bone Miner Res. 1993;8:S483–S487. doi: 10.1002/jbmr.5650081310. [DOI] [PubMed] [Google Scholar]
- 14.Fantner GE, Hassenkam T, Kindt JH, Weaver JC, Birkedal H, Pechenik L, Cutroni JA, Cidade GAG, Stucky GD, Morse DE, Hansma PK. Sacrificial bonds and hidden length dissipate energy as mineralized fibrils separate during bone fracture. Nat Mater. 2005;4:612–616. doi: 10.1038/nmat1428. [DOI] [PubMed] [Google Scholar]
- 15.Zhang S, Gangal G, Uludağ H. Magic bullets' for bone diseases: progress in rational design of bone-seeking medicinal agents. Chem Soc Rev. 2007;36:507–531. doi: 10.1039/b512310k. [DOI] [PubMed] [Google Scholar]
- 16.Brown PW, Martin RI. An analysis of hydroxyapatite surface layer formation. J Phys Chem B. 1999;103:1671–1675. [Google Scholar]
- 17.Dorozhkin SV, Epple M. Biological and medical significance of calcium phosphates. Angew Chem, Int Ed. 2002;41:3130–3146. doi: 10.1002/1521-3773(20020902)41:17<3130::AID-ANIE3130>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 18.Weiner S, Wagner HD. The material bone: structure-mechanical function relations. Annu Rev Mater Res. 1998;28:271–298. [Google Scholar]
- 19.Landis WJ, Silver FH. Mineral deposition in the extracellular matrices of vertebrate tissues: identification of possible apatite nucleation sites on type I collagen. Cells Tissues Organs. 2008;189:20–24. doi: 10.1159/000151454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hartgerink JD, Beniash E, Stupp SI. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science. 2001;294:1684–1688. doi: 10.1126/science.1063187. [DOI] [PubMed] [Google Scholar]
- 21.Frost HM. Tetracycline-based histological analysis of bone remodeling. Calcif Tissue Res. 1969;3:211–237. doi: 10.1007/BF02058664. [DOI] [PubMed] [Google Scholar]
- 22.Florencio-Silva R, Sasso GR, Sasso-Cerri E, Simoes MJ, Cerri PS. Biology of bone tissue: structure, function, and factors that influence bone cells. Biomed Res Int. 2015;2015:421746. doi: 10.1155/2015/421746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hauge EM, Qvesel D, Eriksen EF, Mosekilde L, Melsen F. Cancellous bone remodeling occurs in specialized compartments lined by cells expressing osteoblastic markers. J Bone Miner Res. 2001;16:1575–1582. doi: 10.1359/jbmr.2001.16.9.1575. [DOI] [PubMed] [Google Scholar]
- 24.Andersen TL, Sondergaard TE, Skorzynska KE, Dagnaes-Hansen F, Plesner TL, Hauge EM, Plesner T, Delaisse JM. A physical mechanism for coupling bone resorption and formation in adult human bone. Am J Pathol. 2009;174:239–247. doi: 10.2353/ajpath.2009.080627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Capulli M, Paone R, Rucci N. Osteoblast and osteocyte: games without frontiers. Arch Biochem Biophys. 2014;561:3–12. doi: 10.1016/j.abb.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 27.Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 28.Anderson HC. Matrix vesicles and calcification. Curr Rheumatol Rep. 2003;5:222–226. doi: 10.1007/s11926-003-0071-z. [DOI] [PubMed] [Google Scholar]
- 29.Glimcher MJ. The nature of the mineral component of bone and the mechanism of calcification. Instr Course Lect. 1987;36:49–69. [PubMed] [Google Scholar]
- 30.Nijweide Peter J, van der Plas Arie, Alblas Marcel J, Klein-Nulend Jenneke. The osteocyte. In: Bilezikian, Rodan JPRL, editor. Principles of Bone Biology. Academic Press; 1996. [Google Scholar]
- 31.Schaffler MB, Cheung WY, Majeska R, Kennedy O. Osteocytes: master orchestrators of bone. Calcif Tissue Int. 2014;94:5–24. doi: 10.1007/s00223-013-9790-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aarden EM, Nijweide PJ, Burger EH. Function of osteocytes in bone. J Cell Biochem. 1994;55:287–299. doi: 10.1002/jcb.240550304. [DOI] [PubMed] [Google Scholar]
- 33.Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J Clin Invest. 1998;102:274. doi: 10.1172/JCI2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis 1. Endocr Rev. 2000;21:115–137. doi: 10.1210/edrv.21.2.0395. [DOI] [PubMed] [Google Scholar]
- 35.Everts V, Delaisse JM, Korper W, Jansen DC, Tigchelaar-Gutter W, Saftig P, Beertsen W. The bone lining cell: its role in cleaning Howship's lacunae and initiating bone formation. J Bone Miner Res. 2002;17:77–90. doi: 10.1359/jbmr.2002.17.1.77. [DOI] [PubMed] [Google Scholar]
- 36.Mosley JR. Osteoporosis and bone functional adaptation: mechanobiological regulation of bone architecture in growing and adult bone, a review. J Rehabil Res Dev. 2000;37:189–199. [PubMed] [Google Scholar]
- 37.Yavropoulou MP, Yovos JG. Osteoclastogenesis–current knowledge and future perspectives. J Musculoskelet Neuronal Interact. 2008;8:204–216. [PubMed] [Google Scholar]
- 38.Nesbitt SA, Horton MA. Trafficking of matrix collagens through bone-resorbing osteoclasts. Science. 1997;276:266–269. doi: 10.1126/science.276.5310.266. [DOI] [PubMed] [Google Scholar]
- 39.Salo J, Lehenkari P, Mulari M, Metsikkö K, Väänänen HK. Removal of osteoclast bone resorption products by transcytosis. Science. 1997;276:270–273. doi: 10.1126/science.276.5310.270. [DOI] [PubMed] [Google Scholar]
- 40.Udagawa N, Takahashi N, Akatsu T, Tanaka H, Sasaki T, Nishihara T, Koga T, Martin TJ, Suda T. Origin of osteoclasts: mature monocytes and macrophages are capable of differentiating into osteoclasts under a suitable microenvironment prepared by bone marrow-derived stromal cells. Proc Natl Acad Sci U S A. 1990;87:7260–7264. doi: 10.1073/pnas.87.18.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Betts JG. Bone Tissue and The Skeletal System. Anatomy & Physiology, Open Stax College. 2013:207–242. [Google Scholar]
- 42.Bigham-Sadegh A, Oryan A. Basic concepts regarding fracture healing and the current options and future directions in managing bone fractures. Int Wound J. 2015;12:238–247. doi: 10.1111/iwj.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gruber R, Koch H, Doll BA, Tegtmeier F, Einhorn TA, Hollinger JO. Fracture healing in the elderly patient. Exp Gerontol. 2006;41:1080–1093. doi: 10.1016/j.exger.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 44.Niyibizi C, Kim M. Novel approaches to fracture healing. Expert Opin Investig Drugs. 2000;9:1573–1580. doi: 10.1517/13543784.9.7.1573. [DOI] [PubMed] [Google Scholar]
- 45.Doblaré M, García JM, Gomez MJ. Modelling bone tissue fracture and healing: a review. Eng Fract Mech. 2004;71:1809–1840. [Google Scholar]
- 46.Marsell R, Einhorn TA. The biology of fracture healing. Injury. 2011;42:551–555. doi: 10.1016/j.injury.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cho TJ, Gerstenfeld LC, Einhorn TA. Differential temporal expression of members of the transforming growth factor beta superfamily during murine fracture healing. J Bone Miner Res. 2002;17:513–520. doi: 10.1359/jbmr.2002.17.3.513. [DOI] [PubMed] [Google Scholar]
- 48.Cho HH, Kyoung KM, Seo MJ, Kim YJ, Bae YC, Jung JS. Overexpression of CXCR4 increases migration and proliferation of human adipose tissue stromal cells. Stem Cells Dev. 2006;15:853–864. doi: 10.1089/scd.2006.15.853. [DOI] [PubMed] [Google Scholar]
- 49.Yang X, Ricciardi BF, Hernandez-Soria A, Shi Y, Pleshko Camacho N, Bostrom MP. Callus mineralization and maturation are delayed during fracture healing in interleukin-6 knockout mice. Bone. 2007;41:928–936. doi: 10.1016/j.bone.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Einhorn TA. The cell and molecular biology of fracture healing. Clin Orthop Relat Res. 1998:S7–S21. doi: 10.1097/00003086-199810001-00003. [DOI] [PubMed] [Google Scholar]
- 51.Granero-Molto F, Weis JA, Miga MI, Landis B, Myers TJ, O'Rear L, Longobardi L, Jansen ED, Mortlock DP, Spagnoli A. Regenerative effects of transplanted mesenchymal stem cells in fracture healing. Stem Cells. 2009;27:1887–1898. doi: 10.1002/stem.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsuji K, Bandyopadhyay A, Harfe BD, Cox K, Kakar S, Gerstenfeld L, Einhorn T, Tabin CJ, Rosen V. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat Genet. 2006;38:1424–1429. doi: 10.1038/ng1916. [DOI] [PubMed] [Google Scholar]
- 53.Ai-Aql ZS, Alagl AS, Graves DT, Gerstenfeld LC, Einhorn TA. Molecular mechanisms controlling bone formation during fracture healing and distraction osteogenesis. J Dent Res. 2008;87:107–118. doi: 10.1177/154405910808700215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Keramaris NC, Calori GM, Nikolaou VS, Schemitsch EH, Giannoudis PV. Fracture vascularity and bone healing: a systematic review of the role of VEGF. Injury. 2008;39:S45–S57. doi: 10.1016/S0020-1383(08)70015-9. [DOI] [PubMed] [Google Scholar]
- 55.Barnes GL, Kostenuik PJ, Gerstenfeld LC, Einhorn TA. Growth factor regulation of fracture repair. J Bone Miner Res. 1999;14:1805–1815. doi: 10.1359/jbmr.1999.14.11.1805. [DOI] [PubMed] [Google Scholar]
- 56.Gerstenfeld LC, Cullinane DM, Barnes GL, Graves DT, Einhorn TA. Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. J Cell Biochem. 2003;88:873–884. doi: 10.1002/jcb.10435. [DOI] [PubMed] [Google Scholar]
- 57.Mountziaris PM, Mikos AG. Modulation of the inflammatory response for enhanced bone tissue regeneration. Tissue Eng Part B Rev. 2008;14:179–186. doi: 10.1089/ten.teb.2008.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zalavras CG, Patzakis MJ. Open fractures: evaluation and management. J Am Acad Orthop Surg. 2003;11:212–219. doi: 10.5435/00124635-200305000-00008. [DOI] [PubMed] [Google Scholar]
- 59.Gustilo RB, Mendoza RM, Williams DN. Problems in the management of type III (severe) open fractures: a new classification of type III open fractures. J Trauma. 1984;24:742–746. doi: 10.1097/00005373-198408000-00009. [DOI] [PubMed] [Google Scholar]
- 60.Gustilo RB, Anderson JT. Prevention of infection in the treatment of one thousand and twenty-five open fractures of long bones: retrospective and prospective analyses. J Bone Jt Surg Am. 1976;58:453–458. [PubMed] [Google Scholar]
- 61.Brumback RJ, Jones AL. Interobserver agreement in the classification of open fractures of the tibia. The results of a survey of two hundred and forty-five orthopaedic surgeons. J Bone Jt Surg Am. 1994;76:1162–1166. doi: 10.2106/00004623-199408000-00006. [DOI] [PubMed] [Google Scholar]
- 62.Keating JF, Simpson AH, Robinson CM. The management of fractures with bone loss. J Bone Jt Surg Br. 2005;87:142–150. doi: 10.1302/0301-620x.87b2.15874. [DOI] [PubMed] [Google Scholar]
- 63.Perren SM. Evolution of the internal fixation of long bone fractures. The scientific basis of biological internal fixation: choosing a new balance between stability and biology. J Bone Jt Surg Br. 2002;84:1093–1110. doi: 10.1302/0301-620x.84b8.13752. [DOI] [PubMed] [Google Scholar]
- 64.Simpson AH, Andrews C, Giele H. Skin closure after acute shortening. J Bone Jt Surg Br. 2001;83:668–671. doi: 10.1302/0301-620x.83b5.11585. [DOI] [PubMed] [Google Scholar]
- 65.Kopylov P, Jonsson K, Thorngren KG, Aspenberg P. Injectable calcium phosphate in the treatment of distal radial fractures. J Hand Surg Br. 1996;21:768–771. doi: 10.1016/s0266-7681(96)80184-7. [DOI] [PubMed] [Google Scholar]
- 66.Sanchez-Sotelo J, Munuera L, Madero R. Treatment of fractures of the distal radius with a remodellable bone cement: a prospective, randomised study using Norian SRS. J Bone Jt Surg Br. 2000;82:856–863. doi: 10.1302/0301-620x.82b6.10317. [DOI] [PubMed] [Google Scholar]
- 67.Schildhauer TA, Bauer TW, Josten C, Muhr G. Open reduction and augmentation of internal fixation with an injectable skeletal cement for the treatment of complex calcaneal fractures. J Orthop Trauma. 2000;14:309–317. doi: 10.1097/00005131-200006000-00001. [DOI] [PubMed] [Google Scholar]
- 68.Keating JF, Hajducka CL, Harper J. Minimal internal fixation and calcium-phosphate cement in the treatment of fractures of the tibial plateau. A pilot study. J Bone Jt Surg Br. 2003;85:68–73. doi: 10.1302/0301-620x.85b1.12575. [DOI] [PubMed] [Google Scholar]
- 69.Robinson CM, Page RS. Severely impacted valgus proximal humeral fractures. Results of operative treatment. J Bone Jt Surg Am. 2003;85-A:1647–1655. doi: 10.2106/00004623-200309000-00001. [DOI] [PubMed] [Google Scholar]
- 70.Fischer MD, Gustilo RB, Varecka TF. The timing of flap coverage, bone-grafting, and intramedullary nailing in patients who have a fracture of the tibial shaft with extensive soft-tissue injury. J Bone Jt Surg Am. 1991;73:1316–1322. [PubMed] [Google Scholar]
- 71.Weitz-Marshall AD, Bosse MJ. Timing of closure of open fractures. J Am Acad Orthop Surg. 2002;10:379–384. doi: 10.5435/00124635-200211000-00001. [DOI] [PubMed] [Google Scholar]
- 72.DeLong WG, Jr, Born CT, Wei SY, Petrik ME, Ponzio R, Schwab CW. Aggressive treatment of 119 open fracture wounds. J Trauma. 1999;46:1049–1054. doi: 10.1097/00005373-199906000-00012. [DOI] [PubMed] [Google Scholar]
- 73.Suedkamp NP, Barbey N, Veuskens A, Tempka A, Haas NP, Hoffmann R, Tscherne H. The incidence of osteitis in open fractures: an analysis of 948 open fractures (a review of the Hannover experience) J Orthop Trauma. 1993;7:473–482. doi: 10.1097/00005131-199310000-00011. [DOI] [PubMed] [Google Scholar]
- 74.Templeman DC, Gulli B, Tsukayama DT, Gustilo RB. Update on the management of open fractures of the tibial shaft. Clin Orthop Relat Res. 1998:18–25. [PubMed] [Google Scholar]
- 75.Ellington JK, Harris M, Webb L, Smith B, Smith T, Tan K, Hudson M. Intracellular Staphylococcus aureus. A mechanism for the indolence of osteomyelitis. J Bone Jt Surg Br. 2003;85:918–921. [PubMed] [Google Scholar]
- 76.Patzakis MJ, Harvey JP, Jr, Ivler D. The role of antibiotics in the management of open fractures. J Bone Jt Surg Am. 1974;56:532–541. [PubMed] [Google Scholar]
- 77.Worlock P, Slack R, Harvey L, Mawhinney R. The prevention of infection in open fractures: an experimental study of the effect of fracture stability. Injury. 1994;25:31–38. doi: 10.1016/0020-1383(94)90181-3. [DOI] [PubMed] [Google Scholar]
- 78.Moehring HD, Gravel C, Chapman MW, Olson SA. Comparison of antibiotic beads and intravenous antibiotics in open fractures. Clin, Orthop Relat Res. 2000:254–261. doi: 10.1097/00003086-200003000-00028. [DOI] [PubMed] [Google Scholar]
- 79.Ostermann PA, Seligson D, Henry SL. Local antibiotic therapy for severe open fractures. A review of 1085 consecutive cases. J Bone Jt Surg Br. 1995;77:93–97. [PubMed] [Google Scholar]
- 80.Bastari K, Arshath M, Ng ZHM, Chia JH, Yow ZXD, Sana B, Tan MFC, Lim S, Loo SCJ. A controlled release of antibiotics from calcium phosphate-coated poly (lactic-co-glycolic acid) particles and their in vitro efficacy against Staphylococcus aureus biofilm. J Mater Sci Mater Med. 2014;25:747–757. doi: 10.1007/s10856-013-5125-9. [DOI] [PubMed] [Google Scholar]
- 81.Boskey AL. Biomineralization: conflicts, challenges, and opportunities. J Cell Biochem. 1998;72:83–91. doi: 10.1002/(SICI)1097-4644(1998)72:30/31+<83::AID-JCB12>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 82.David L, Feige JJ, Bailly S. Emerging role of bone morphogenetic proteins in angiogenesis. Cytokine Growth Factor Rev. 2009;20:203–212. doi: 10.1016/j.cytogfr.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 83.Yasko AW, Lane J, Fellinger E, Rosen V, Wozney J, Wang E. The healing of segmental bone defects, induced by recombinant human bone morphogenetic protein (rhBMP-2). A radiographic, histological, and biomechanical study in rats. J Bone Jt Surg Am. 1992;74:659–670. [PubMed] [Google Scholar]
- 84.Shimer AL, Öner FC, Vaccaro AR. Spinal reconstruction and bone morphogenetic proteins: open questions. Injury. 2009;40:S32–S38. doi: 10.1016/S0020-1383(09)70009-9. [DOI] [PubMed] [Google Scholar]
- 85.Park KH, Kim H, Moon S, Na K. Bone morphogenic protein-2 (BMP-2) loaded nanoparticles mixed with human mesenchymal stem cell in fibrin hydrogel for bone tissue engineering. J Biosci Bioeng. 2009;108:530–537. doi: 10.1016/j.jbiosc.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 86.Wang H, Boerman OC, Sariibrahimoglu K, Li Y, Jansen JA, Leeuwenburgh SC. Comparison of micro-vs. nanostructured colloidal gelatin gels for sustained delivery of osteogenic proteins: bone morphogenetic protein-2 and alkaline phosphatase. Biomaterials. 2012;33:8695–8703. doi: 10.1016/j.biomaterials.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 87.Wei G, Jin Q, Giannobile WV, Ma PX. The enhancement of osteogenesis by nano-fibrous scaffolds incorporating rhBMP-7 nanospheres. Biomaterials. 2007;28:2087–2096. doi: 10.1016/j.biomaterials.2006.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Facca S, Cortez C, Mendoza-Palomares C, Messadeq N, Dierich A, Johnston A, Mainard D, Voegel JC, Caruso F, Benkirane-Jessel N. Active multilayered capsules for in vivo bone formation. Proc Natl Acad Sci U S A. 2010;107:3406–3411. doi: 10.1073/pnas.0908531107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rodríguez-Évora M, Delgado A, Reyes R, Hernández-Daranas A, Soriano I, San Román J, Évora C. Osteogenic effect of local, long versus short term BMP-2 delivery from a novel SPU–PLGA–βTCP concentric system in a critical size defect in rats. Eur J Pharm Sci. 2013;49:873–884. doi: 10.1016/j.ejps.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 90.Saito A, Suzuki Y, Ogata SI, Ohtsuki C, Tanihara M. Activation of osteoprogenitor cells by a novel synthetic peptide derived from the bone morphogenetic protein-2 knuckle epitope. Biochim Biophys Acta. 2003;1651:60–67. doi: 10.1016/s1570-9639(03)00235-8. [DOI] [PubMed] [Google Scholar]
- 91.Kim HK, Kim JH, Park DS, Park KS, Kang SS, Lee JS, Jeong MH, Yoon TR. Osteogenesis induced by a bone forming peptide from the pro-domain region of BMP-7. Biomaterials. 2012;33:7057–7063. doi: 10.1016/j.biomaterials.2012.06.036. [DOI] [PubMed] [Google Scholar]
- 92.Saito A, Suzuki Y, Kitamura M, Ogata SI, Yoshihara Y, Masuda S, Ohtsuki C, Tanihara M. Repair of 20-mm long rabbit radial bone defects using BMP-derived peptide combined with an α-tricalcium phosphate scaffold. J Biomed Mater Res Part A. 2006;77:700–706. doi: 10.1002/jbm.a.30662. [DOI] [PubMed] [Google Scholar]
- 93.Li Y, Chen X, Gu N. Computational investigation of interaction between nanoparticles and membranes: hydrophobic/hydrophilic effect. J Phys Chem B. 2008;112:16647–16653. doi: 10.1021/jp8051906. [DOI] [PubMed] [Google Scholar]
- 94.Zhou X, Feng W, Qiu K, Chen L, Wang W, Nie W, Mo X, He C. BMP-2 derived peptide and dexamethasone incorporated mesoporous silica nanoparticles for enhanced osteogenic differentiation of bone mesenchymal stem cells. ACS Appl Mater Interfaces. 2015;7:15777–15789. doi: 10.1021/acsami.5b02636. [DOI] [PubMed] [Google Scholar]
- 95.Luo Z, Deng Y, Zhang R, Wang M, Bai Y, Zhao Q, Lyu Y, Wei J, Wei S. Peptide-laden mesoporous silica nanoparticles with promoted bioactivity and osteo-differentiation ability for bone tissue engineering. Colloids Surf B Biointerfaces. 2015;131:73–82. doi: 10.1016/j.colsurfb.2015.04.043. [DOI] [PubMed] [Google Scholar]
- 96.Motskin M, Wright DM, Muller K, Kyle N, Gard TG, Porter AE, Skepper JN. Hydroxyapatite nano and microparticles: correlation of particle properties with cytotoxicity and biostability. Biomaterials. 2009;30:3307–3317. doi: 10.1016/j.biomaterials.2009.02.044. [DOI] [PubMed] [Google Scholar]
- 97.Sun Y, Deng Y, Ye Z, Liang S, Tang Z, Wei S. Peptide decorated nano-hydroxyapatite with enhanced bioactivity and osteogenic differentiation via polydopamine coating. Colloids Surf B Biointerfaces. 2013;111:107–116. doi: 10.1016/j.colsurfb.2013.05.037. [DOI] [PubMed] [Google Scholar]
- 98.Eijken M, Koedam M, van Driel M, Buurman CJ, Pols HAP, van Leeuwen JPTM. The essential role of glucocorticoids for proper human osteoblast differentiation and matrix mineralization. Mol Cell Endocrinol. 2006;248:87–93. doi: 10.1016/j.mce.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 99.Oliveira JM, Sousa RA, Kotobuki N, Tadokoro M, Hirose M, Mano JF, Reis RL, Ohgushi H. The osteogenic differentiation of rat bone marrow stromal cells cultured with dexamethasone-loaded carboxymethylchitosan/poly (amidoamine) dendrimer nanoparticles. Biomaterials. 2009;30:804–813. doi: 10.1016/j.biomaterials.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 100.Strauch B, Patel MK, Navarro JA, Berdichevsky M, Yu HL, Pilla AA. Pulsed magnetic fields accelerate cutaneous wound healing in rats. Plast Reconstr Surg. 2007;120:425–430. doi: 10.1097/01.prs.0000267700.15452.d0. [DOI] [PubMed] [Google Scholar]
- 101.Panseri S, Russo A, Giavaresi G, Sartori M, Veronesi F, Fini M, Salter D, Ortolani A, Strazzari A, Visani A. Innovative magnetic scaffolds for orthopedic tissue engineering. J Biomed Mater Res Part A. 2012;100:2278–2286. doi: 10.1002/jbm.a.34167. [DOI] [PubMed] [Google Scholar]
- 102.Henstock JR, Rotherham M, Rashidi H, Shakesheff KM, El Haj AJ. Remotely activated mechanotransduction via magnetic nanoparticles promotes mineralization synergistically with bone morphogenetic protein 2: applications for injectable cell therapy. Stem Cells Trans l Med. 2014;3:1363–1374. doi: 10.5966/sctm.2014-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bock N, Riminucci A, Dionigi C, Russo A, Tampieri A, Landi E, Goranov VA, Marcacci M, Dediu V. A novel route in bone tissue engineering: magnetic biomimetic scaffolds. Acta Biomater. 2010;6:786–796. doi: 10.1016/j.actbio.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 104.Meng J, Zhang Y, Qi X, Kong H, Wang C, Xu Z, Xie S, Gu N, Xu H. Paramagnetic nanofibrous composite films enhance the osteogenic responses of pre-osteoblast cells. Nanoscale. 2010;2:2565–2569. doi: 10.1039/c0nr00178c. [DOI] [PubMed] [Google Scholar]
- 105.Rhee ST, Buchman SR. Colocalization of c-Src (pp60src) and bone morphogenetic protein 2/4 expression during mandibular distraction osteogenesis: in vivo evidence of their role within an integrin-mediated mechanotransduction pathway. Ann Plast Surg. 2005;55:207–215. doi: 10.1097/01.sap.0000164576.10754.aa. [DOI] [PubMed] [Google Scholar]
- 106.Kopf J, Petersen A, Duda GN, Knaus P. BMP2 and mechanical loading cooperatively regulate immediate early signalling events in the BMP pathway. BMC Biol. 2012;10:37. doi: 10.1186/1741-7007-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Garg T, Singh O, Arora S, Murthy R. Scaffold: a novel carrier for cell and drug delivery. Crit Rev Ther Drug Carr Syst. 2012;29 doi: 10.1615/critrevtherdrugcarriersyst.v29.i1.10. [DOI] [PubMed] [Google Scholar]
- 108.Gusić N, Ivković A, Va Faye J, Vukasović A, Ivković J, Hudetz D, Janković S. Nanobiotechnology and bone regeneration: a mini-review. Int Orthop. 2014;38:1877–1884. doi: 10.1007/s00264-014-2412-0. [DOI] [PubMed] [Google Scholar]
- 109.Walmsley GG, McArdle A, Tevlin R, Momeni A, Atashroo D, Hu MS, Feroze AH, Wong VW, Lorenz PH, Longaker MT. Nanotechnology in bone tissue engineering. Nanomedicine. 2015;11:1253–1263. doi: 10.1016/j.nano.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zimmermann EA, Barth HD, Ritchie RO. The multiscale origins of fracture resistance in human bone and its biological degradation. Jom. 2012;64:486–493. [Google Scholar]
- 111.Bobbert FSL, Zadpoor AA. Effects of bone substitute architecture and surface properties on cell response, angiogenesis, and structure of new bone. J Phys Chem B. 2017;5:6175–6192. doi: 10.1039/c7tb00741h. [DOI] [PubMed] [Google Scholar]
- 112.Brown RA, Wiseman M, Chuo CB, Cheema U, Nazhat SN. Ultrarapid engineering of biomimetic materials and tissues: fabrication of nano-and microstructures by plastic compression. Adv Funct Mater. 2005;15:1762–1770. [Google Scholar]
- 113.Wei K, Serpooshan V, Hurtado C, Diez-Cuñado M, Zhao M, Maruyama S, Zhu W, Fajardo G, Noseda M, Nakamura K. Epicardial FSTL1 reconstitution regenerates the adult mammalian heart. Nature. 2015;525:479–485. doi: 10.1038/nature15372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Serpooshan V, Julien M, Nguyen O, Wang H, Li A, Muja N, Henderson JE, Nazhat SN. Reduced hydraulic permeability of three-dimensional collagen scaffolds attenuates gel contraction and promotes the growth and differentiation of mesenchymal stem cells. Acta Biomater. 2010;6:3978–3987. doi: 10.1016/j.actbio.2010.04.028. [DOI] [PubMed] [Google Scholar]
- 115.Chamieh F, Collignon AM, Coyac BR, Lesieur J, Ribes S, Sadoine J, Llorens A, Nicoletti A, Letourneur D, Colombier ML. Accelerated craniofacial bone regeneration through dense collagen gel scaffolds seeded with dental pulp stem cells. Sci Rep. 2016;6 doi: 10.1038/srep38814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Feng Z, Yamato M, Akutsu T, Nakamura T, Okano T, Umezu M. Investigation on the mechanical properties of contracted collagen gels as a scaffold for tissue engineering. Artif Organs. 2003;27:84–91. doi: 10.1046/j.1525-1594.2003.07187.x. [DOI] [PubMed] [Google Scholar]
- 117.Huh AJ, Kwon YJ. “Nanoantibiotics”: a new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J Control Release. 2011;156:128–145. doi: 10.1016/j.jconrel.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 118.Radovic-Moreno AF, Lu TK, Puscasu VA, Yoon CJ, Langer R, Farokhzad OC. Surface charge-switching polymeric nanoparticles for bacterial cell wall-targeted delivery of antibiotics. ACS Nano. 2012;6:4279–4287. doi: 10.1021/nn3008383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gupta A, Saleh NM, Das R, Landis RF, Bigdeli A, Motamedchaboki K, Campos AR, Pomeroy K, Mahmoudi M, Rotello VM. Synergistic antimicrobial therapy using nanoparticles and antibiotics for the treatment of multidrug-resistant bacterial infection. Nano Futur. 2017;1:015004. [Google Scholar]
- 120.Thill A, Zeyons O, Spalla O, Chauvat F, Rose J, Auffan M, Flank AM. Cytotoxicity of CeO2 nanoparticles for Escherichia coli. Physico-chemical insight of the cytotoxicity mechanism. Environ Sci Technol. 2006;40:6151–6156. doi: 10.1021/es060999b. [DOI] [PubMed] [Google Scholar]
- 121.Soenen SJ, Rivera-Gil P, Montenegro JM, Parak WJ, De Smedt SC, Braeckmans K. Cellular toxicity of inorganic nanoparticles: common aspects and guidelines for improved nanotoxicity evaluation. Nano Today. 2011;6:446–465. [Google Scholar]
- 122.Sharifi S, Behzadi S, Laurent S, Forrest ML, Stroeve P, Mahmoudi M. Toxicity of nanomaterials. Chem Soc Rev. 2012;41:2323–2343. doi: 10.1039/c1cs15188f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Prema P, Raju R. Fabrication and characterization of silver nanoparticle and its potential antibacterial activity. Biotechnol Bioprocess Eng. 2009;14:842–847. [Google Scholar]
- 124.Ruparelia JP, Chatterjee AK, Duttagupta SP, Mukherji S. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater. 2008;4:707–716. doi: 10.1016/j.actbio.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 125.Khan SS, Mukherjee A, Chandrasekaran N. Studies on interaction of colloidal silver nanoparticles (SNPs) with five different bacterial species. Colloids Surf, B Biointerfaces. 2011;87:129–138. doi: 10.1016/j.colsurfb.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 126.Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramírez JT, Yacaman MJ. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16:2346. doi: 10.1088/0957-4484/16/10/059. [DOI] [PubMed] [Google Scholar]
- 127.Alt V, Bechert T, Steinrücke P, Wagener M, Seidel P, Dingeldein E, Domann E, Schnettler R. An in vitro assessment of the antibacterial properties and cytotoxicity of nanoparticulate silver bone cement. Biomaterials. 2004;25:4383–4391. doi: 10.1016/j.biomaterials.2003.10.078. [DOI] [PubMed] [Google Scholar]
- 128.Dueland R, Spadaro J, Rahn B. Silver antibacterial bone cement: comparison with gentamicin in experimental osteomyelitis. Clin Orthop Relat Res. 1982;169:264–268. [PubMed] [Google Scholar]
- 129.Vik H, Andersen K, Julshamn K, Todnem K. Neuropathy caused by silver absorption from arthroplasty cement. Lancet. 1985;325:872. doi: 10.1016/s0140-6736(85)92230-5. [DOI] [PubMed] [Google Scholar]
- 130.Rameshbabu N, Sampath Kumar T, Prabhakar T, Sastry V, Murty K, Prasad Rao K. Antibacterial nanosized silver substituted hydroxyapatite: synthesis and characterization. J Biomed Mater Res Part A. 2007;80:581–591. doi: 10.1002/jbm.a.30958. [DOI] [PubMed] [Google Scholar]
- 131.Rahim MI, Rohde M, Rais B, Seitz JM, Mueller PP. Susceptibility of metallic magnesium implants to bacterial biofilm infections. J Biomed Mater Res Part A. 2016;104:1489–1499. doi: 10.1002/jbm.a.35680. [DOI] [PubMed] [Google Scholar]
- 132.Monzón M, García-Álvarez F, Laclériga A, Amorena B. Evaluation of four experimental osteomyelitis infection models by using precolonized implants and bacterial suspensions. Acta Orthop Scand. 2002;73:11–19. doi: 10.1080/000164702317281341. [DOI] [PubMed] [Google Scholar]
- 133.Aviv M, Berdicevsky I, Zilberman M. Gentamicin-loaded bioresorbable films for prevention of bacterial infections associated with orthopedic implants. J Biomed Mater Res Part A. 2007;83:10–19. doi: 10.1002/jbm.a.31184. [DOI] [PubMed] [Google Scholar]
- 134.Tian B, Chen W, Yu D, Lei Y, Ke Q, Guo Y, Zhu Z. Fabrication of silver nanoparticle-doped hydroxyapatite coatings with oriented block arrays for enhancing bactericidal effect and osteoinductivity. J Mech Behav Biomed Mater. 2016;61:345–359. doi: 10.1016/j.jmbbm.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 135.Saravanan S, Nethala S, Pattnaik S, Tripathi A, Moorthi A, Selvamurugan N. Preparation, characterization and antimicrobial activity of a bio-composite scaffold containing chitosan/nano-hydroxyapatite/nano-silver for bone tissue engineering. Int J Biol Macromol. 2011;49:188–193. doi: 10.1016/j.ijbiomac.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 136.Tran PA, Webster TJ. Selenium nanoparticles inhibit Staphylococcus aureus growth. Int J Nanomedicine. 2011;6:1553–1558. doi: 10.2147/IJN.S21729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Stevanović M, Filipović N, Djurdjević J, Lukić M, Milenković M, Boccaccini A. 45S5Bioglass®-based scaffolds coated with selenium nanoparticles or with poly (lactide-co-glycolide)/selenium particles: processing, evaluation and antibacterial activity. Colloids Surf B Biointerfaces. 2015;132:208–215. doi: 10.1016/j.colsurfb.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 138.Kumar MNVR, Muzzarelli RAA, Muzzarelli C, Sashiwa H, Domb AJ. Chitosan chemistry and pharmaceutical perspectives. Chem Rev. 2004;104:6017–6084. doi: 10.1021/cr030441b. [DOI] [PubMed] [Google Scholar]
- 139.Calvo P, Remunan-Lopez C, Vila-Jato J, Alonso M. Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J Appl Polym Sci. 1997;63:125–132. [Google Scholar]
- 140.Hosseinzadeh H, Atyabi F, Dinarvand R, Ostad SN. Chitosan–Pluronic nanoparticles as oral delivery of anticancer gemcitabine: preparation and in vitro study. Int J Nanomedicine. 2012;7:1851. doi: 10.2147/IJN.S26365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kim CH, Choi JW, Chun HJ, Choi KS. Synthesis of chitosan derivatives with quaternary ammonium salt and their antibacterial activity. Polymer Bull. 1997;38:387–393. [Google Scholar]
- 142.Fuchs S, Pane-Farre J, Kohler C, Hecker M, Engelmann S. Anaerobic gene expression in Staphylococcus aureus. J Bacteriol. 2007;189:4275–4289. doi: 10.1128/JB.00081-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dubos R. The micro-environment of inflammation or Metchnikoff revisited. Lancet. 1955;266:xxiv–5. doi: 10.1016/s0140-6736(55)93374-2. [DOI] [PubMed] [Google Scholar]
- 144.Shi Z, Neoh K, Kang E, Wang W. Antibacterial and mechanical properties of bone cement impregnated with chitosan nanoparticles. Biomaterials. 2006;27:2440–2449. doi: 10.1016/j.biomaterials.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 145.Kamaly N, Xiao Z, Valencia PM, Radovic-Moreno AF, Farokhzad OC. Targeted polymeric therapeutic nanoparticles: design, development and clinical translation. Chem Soc Rev. 2012;41:2971–3010. doi: 10.1039/c2cs15344k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Abazinge M, Jackson T, Yang Q, Owusu-Ababio G. Comparison of in vitro and in vivo release characteristics of sustained release ofloxacin microspheres. Drug Deliv. 2000;7:77–81. doi: 10.1080/107175400266632. [DOI] [PubMed] [Google Scholar]
- 147.Yeni˙ ce İ, Caliş S, Ati˙ lla B, Kaş H, Özalp M, Eki˙ zoglu M, Bi˙ lgi˙ li˙ H, Hincal A. In vitro/in vivo evaluation of the efficiency of teicoplanin-loaded biodegradable microparticles formulated for implantation to infected bone defects. J Microencapsul. 2003;20:705–717. doi: 10.1080/0265204031000154179. [DOI] [PubMed] [Google Scholar]
- 148.Feng K, Sun H, Bradley MA, Dupler EJ, Giannobile WV, Ma PX. Novel antibacterial nanofibrous PLLA scaffolds. J Control Release. 2010;146:363–369. doi: 10.1016/j.jconrel.2010.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Peng KT, Chen CF, Chu IM, Li YM, Hsu WH, Hsu RWW, Chang PJ. Treatment of osteomyelitis with teicoplanin-encapsulated biodegradable thermosensitive hydrogel nanoparticles. Biomaterials. 2010;31:5227–5236. doi: 10.1016/j.biomaterials.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 150.González-Sánchez MI, Perni S, Tommasi G, Morris NG, Hawkins K, López-Cabarcos E, Prokopovich P. Silver nanoparticle based antibacterial methacrylate hydrogels potential for bone graft applications. Mater Sci Eng C. 2015;50:332–340. doi: 10.1016/j.msec.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mahmoudi M, Zhao M, Matsuura Y, Laurent S, Yang PC, Bernstein D, Ruiz-Lozano P, Serpooshan V. Infection-resistant MRI-visible scaffolds for tissue engineering applications. BioImpacts BI. 2016;6:111. doi: 10.15171/bi.2016.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Behzadi S, Gallei M, Elbert J, Appold M, Glasser G, Landfester K, Crespy D. A triblock terpolymer vs. blends of diblock copolymers for nanocapsules addressed by three independent stimuli. Chem. 2016;7:3434–3443. [Google Scholar]
- 153.Behzadi S, Rosenauer C, Kappl M, Mohr K, Landfester K, Crespy D. Osmotic pressure-dependent release profiles of payloads from nanocontainers by co-encapsulation of simple salts. Nanoscale. 2016;8:12998–13005. doi: 10.1039/c6nr01882c. [DOI] [PubMed] [Google Scholar]
- 154.Behzadi S, Steinmann M, Estupiñán D, Landfester K, Crespy D. The proactive payload strategy significantly increases selective release from mesoporous nanocapsules. J Control Release. 2016;242:119–125. doi: 10.1016/j.jconrel.2016.08.040. [DOI] [PubMed] [Google Scholar]
- 155.Mendoza-Palomares C, Ferrand A, Facca S, Fioretti F, Ladam G, Kuchler-Bopp S, Regnier T, Mainard D, Benkirane-Jessel N. Smart hybrid materials equipped by nanoreservoirs of therapeutics. ACS Nano. 2012;6:483–490. doi: 10.1021/nn203817t. [DOI] [PubMed] [Google Scholar]
- 156.Ferrand A, Eap S, Richert L, Lemoine S, Kalaskar D, Demoustier-Champagne S, Atmani H, Mély Y, Fioretti F, Schlatter G. Osteogenetic properties of electrospun nanofibrous PCL scaffolds equipped with chitosan-based nano-reservoirs of growth factors. Macromol Biosci. 2014;14:45–55. doi: 10.1002/mabi.201300283. [DOI] [PubMed] [Google Scholar]
- 157.Behzadi S, Stadler J, Hosseinpour S, Crespy D, Landfester K. Suppressing non-controlled leakage of hydrophilic payloads from redox-responsive nanocapsules. Colloids Surf A Physicochem Eng Asp. 2017 http://dx.doi.org/10.1016/j.colsurfa.2017.07.077.
- 158.Ignjatović N, Ninkov P, Kojić V, Bokurov M, Srdić V, Krnojelac D, Selaković S, Uskoković D. Cytotoxicity and fibroblast properties during in vitro test of biphasic calcium phosphate/poly-dl-lactide-co-glycolide biocomposites and different phosphate materials. Microsc Res Tech. 2006;69:976–982. doi: 10.1002/jemt.20374. [DOI] [PubMed] [Google Scholar]
- 159.Ignjatovic N, Ninkov P, Ajdukovic Z, Vasiljevic-Radovic D, Uskokovic D. Biphasic calcium phosphate coated with poly-d,l-lactide-co-glycolide biomaterial as a bone substitute. J Eur Ceram Soc. 2007;27:1589–1594. [Google Scholar]
- 160.Pataro AL, Oliveira MF, Teixeira KIR, Turchetti-Maia RMM, Lopes MTP, Wykrota FHL, Sinisterra RD, Cortés ME. Polymer: bioceramic composites optimization by tetracycline addition. Int J Pharm. 2007;336:75–81. doi: 10.1016/j.ijpharm.2006.11.038. [DOI] [PubMed] [Google Scholar]
- 161.Xu Q, Czernuszka JT. Controlled release of amoxicillin from hydroxyapatitecoated poly (lactic-co-glycolic acid) microspheres. J Control Release. 2008;127:146–153. doi: 10.1016/j.jconrel.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 162.Ignjatović NL, Ninkov P, Sabetrasekh R, Uskoković DP. A novel nano drug delivery system based on tigecycline-loaded calciumphosphate coated with poly-DL-lactide-co-glycolide. J Mater Sci Mater Med. 2010;21:231–239. doi: 10.1007/s10856-009-3854-6. [DOI] [PubMed] [Google Scholar]
- 163.Uskoković V, Desai TA. In vitro analysis of nanoparticulate hydroxyapatite/chitosan composites as potential drug delivery platforms for the sustained release of antibiotics in the treatment of osteomyelitis. J Pharm Sci. 2014;103:567–579. doi: 10.1002/jps.23824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Uskoković V, Hoover C, Vukomanović M, Uskoković DP, Desai TA. Osteogenic and antimicrobial nanoparticulate calcium phosphate and poly-(D, L-lactide-co-glycolide) powders for the treatment of osteomyelitis. Mater Sci Eng C. 2013;33:3362–3373. doi: 10.1016/j.msec.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zheng F, Wang S, Wen S, Shen M, Zhu M, Shi X. Characterization and antibacterial activity of amoxicillin-loaded electrospun nano-hydroxyapatite/poly (lactic-co-glycolic acid) composite nanofibers. Biomaterials. 2013;34:1402–1412. doi: 10.1016/j.biomaterials.2012.10.071. [DOI] [PubMed] [Google Scholar]
- 166.Zhu CT, Xu YQ, Shi J, Li J, Ding J. Liposome combined porous β-TCP scaffold: preparation, characterization, and anti-biofilm activity. Drug Deliv. 2010;17:391–398. doi: 10.3109/10717541003762870. [DOI] [PubMed] [Google Scholar]


