Low-dose CT screening is expected to increase lung cancer diagnoses, shift stage at diagnosis toward earlier stages, and increase Medicare expenditures over a 5-year time horizon.
Abstract
Purpose:
The Centers for Medicare and Medicaid Services (CMS) recently issued a national coverage determination that provides reimbursement for low-dose computed tomography (CT) lung cancer screening for enrollees age 55 to 77 years with ≥ 30–pack-year smoking history who currently smoke or quit in the last 15 years. The clinical, resource use, and fiscal impacts of this change in screening coverage policy remain uncertain.
Methods:
We developed a simulation model to forecast the 5-year health outcome impacts of the CMS low-dose CT screening policy in Medicare compared with no screening. The model used data from the National Lung Screening Trial, CMS enrollment statistics and reimbursement schedules, and peer-reviewed literature. Outcomes included counts of screening examinations, patient cases of lung cancer detected, stage distribution, and total and per-enrollee per-month fiscal impact.
Results:
Over 5 years, we project that low-dose CT screening will result in 10.7 million more low-dose CT scans, 52,000 more lung cancers detected, and increased overall expenditure of $6.8 billion ($2.22 per Medicare enrollee per month). The most fiscally impactful factors were the average cost–per-screening episode, proportion of enrollees eligible for screening, and cost of treating stage I lung cancer.
Conclusion:
Low-dose CT screening is expected to increase lung cancer diagnoses, shift stage at diagnosis toward earlier stages, and substantially increase Medicare expenditures over a 5-year time horizon. These projections can inform planning efforts by Medicare administrators, contracted health care providers, and other stakeholders.
Introduction
Approximately 220,000 Americans are diagnosed with lung cancer annually, and only 16.8% survive 5 years after diagnosis.1 This poor survival prognosis is largely attributable to the fact that lung cancer is typically diagnosed at an advanced stage. A majority of lung cancer cases are diagnosed in patients age > 65 years, so the US Medicare program is particularly affected by this unfavorable stage distribution.1
Screening people who are at high risk of developing lung cancer (eg, those with history of heavy smoking) provides an opportunity to detect patient cases at an earlier stage and more effectively intervene with potentially curative treatment. After several earlier trials failed to show a benefit from screening, the National Lung Screening Trial (NLST) showed that screening with low-dose computed tomography (CT) imaging resulted in 50.0% of patient cases being diagnosed at stage I versus 31.1% with radiography.2,3 Those differences translated into a significant 20.0% (95% CI, 6.8% to 26.7%) reduction in lung cancer–specific mortality over 6.5 years of follow-up, as well as a 6.7% (95% CI, 1.2% to 13.6%) reduction in overall mortality.2 However, these low-dose CT screening benefits were balanced by a high frequency of false positives (96% of positive findings were false-positive results; 23% of all screening tests were false-positive results).2,4
On February 5, 2015, the Centers for Medicare and Medicaid Services (CMS) issued a national coverage determination that provides coverage of low-dose CT lung cancer screening for Medicare enrollees age 55 to 77 years with ≥ 30–pack-year smoking history who are current smokers or quit in the last 15 years (in accordance with NLST inclusion criteria) and who enroll in a CMS-approved registry. The objective of our study was to forecast the 5-year clinical, resource use, and fiscal implications of this screening policy in Medicare enrollees age 55 to 77 years. Our findings can inform planning by a variety of stakeholders and highlight opportunities for implementing and administering lung cancer screening programs.
Methods
Overview
We developed a simulation model in Microsoft Excel (Redmond, WA) to synthesize evidence from the NLST and other peer-reviewed data sources to project 5-year (2015 to 2019) aggregate health outcomes and costs related to low-dose CT lung cancer screening for the Medicare program. Our approach applied NLST stage-specific lung cancer incidence estimates to the Medicare population age 55 to 77 years under two scenarios: low-dose CT lung cancer screening coverage and no screening coverage. Costs included low-dose CT screening tests and associated primary care and other clinic visits, diagnostic work-up (imaging, bronchoscopy, biopsy), lung cancer treatment, and end-of-life care. Expenditure outcomes (total and per enrollee per month) were calculated from the Medicare payer perspective and discounted at 3% per year.
Population and Setting
The Medicare population evaluated in the model was based on Congressional Budget Office estimates for Medicare Part B enrollment from 2015 to 2019 (51 to 57 million enrollees).5 We assumed that the proportion of enrollees age 55 to 77 years eligible for low-dose CT screening was equal to the proportion of participants in the Prostate, Lung, Colorectal, and Ovarian screening trial who had ≥ 30–pack-year smoking history and were current smokers or had quit within the past 15 years (20.3%).6 Accordingly, 12.5% of the Medicare population is expected to meet screening criteria after adjusting for the proportion who do not qualify for screening because of age (< 55 or ≥ 77 years).
Low-Dose CT Screening Patient Flow
Lung cancer risk classification.
The simulation model tracked patient flow in each scenario over 5 years. First, the cohort was risk stratified (as high risk or low or moderate risk for lung cancer) using NLST criteria.3 Only high-risk enrollees are considered for screening, and we did not explicitly model health outcomes for low/moderate-risk individuals.
Uptake of low-dose CT screening.
We increased the proportion offered low-dose CT screening over the first 5 years of the program to reflect providers gradually building screening infrastructure and capacity. In the base case, we assumed that 30% of high-risk enrollees were offered screening in 2015, and an additional 15% of high-risk enrollees were offered low-dose CT screening in each subsequent year of the model time horizon (ie, 45% offered screening in year 2 to 90% offered screening in year 5). Among high-risk enrollees offered screening, we assumed that only a proportion proceeded to screening (50% in year 1 to 70% in year 5), similar to historic patient behavior with analogous screening technologies.7 These inputs collectively resulted in 15%, 25%, 36%, 49%, and 63% of high-risk enrollees receiving screening from 2015 to 2019, respectively.
Low-dose CT screening test performance.
Using positive (4.9%) and negative predictive value (99.9%) estimates from a subgroup analysis of NLST participants age ≥ 65 years, we calculated the proportion of those screened who were correctly classified as having (and not having) lung cancer.4 Among true-positive screens, we applied stage distribution estimates from patients age ≥ 65 years enrolled in the low-dose CT arm of NSLT.2,4,8 Additionally, we assumed that a proportion of false-negative screens and nonadherent high-risk patients went on to clinical detection of disease (with SEER stage distribution).2,9 Stage distributions for these groups are listed in Appendix Table A1 (online only). The remaining proportion of high-risk enrollees moved on to the subsequent screening interval in the model.
Overdiagnosis.
The model used NLST results to model overdiagnosis, the screen-detected lung cancers that would not have been diagnosed in the absence of screening over the 5-year time horizon of the model.10
No-Screening Patient Flow
In the no-screening scenario, the Medicare population flowed through the same calculations as the low-dose CT screening scenario, but lung cancer was only clinically detected.
Direct Medical Expenditure Inputs
Initial low-dose CT screening.
In accordance with the national coverage determination, high-risk enrollees in the Medicare population were offered low-dose CT screening once annually. The expenditures associated with this annual screening episode represent services involved in the patient-centered care process recommended by the American Lung Association, and costs were obtained from 2014 CMS reimbursement schedules (Appendix Table A1, online only).11 First, we assumed a prescreening low-complexity office visit (Current Procedural Terminology [CPT] code 99213) to assess smoking history, discuss benefit–risk tradeoffs, and determine if enrollees intended to undergo curative surgical treatment in the event of early-stage lung cancer diagnosis. Second, we assumed smoking cessation counseling (CPT code 99407) and use of a smoking-cessation intervention in 51% of those screened, the proportion of current smokers in NLST.2,12 Third, we assumed that enrollees received a low-dose CT screening scan at a nonfacility provider (CPT code 71250). Finally, we assumed a low-complexity office visit (CPT code 99213) for all positive screens to discuss the results and subsequent clinical actions. In the base case, this protocol cost an average of $422 per screening episode.
Diagnostic work-up for positive screening examinations.
Among enrollees who screened positive for lung cancer, we assumed that all had a moderate-complexity office visit (CPT code 99214), 70% received a follow-up low-dose CT scan (CPT code 71250), and 10% received a follow-up positron emission tomography–CT scan, based on the findings of NLST.8 True positives were assumed to be confirmed with bronchoscopy and biopsy, and expenditure for those procedures was derived from a prior study of low-dose CT screening based on NLST outcomes and CMS reimbursement schedules.13
Evaluation of incidental findings.
It is well established that low-dose CT screening will also detect other clinically relevant conditions, such as coronary artery calcifications, liver and kidney cysts, and emphysema.14 To reflect this reality, we included an average cost for incidental findings based on rates (9.2%) for participants age ≥ 65 years in the NLST (Appendix Table A1, online only). This cost only reflects additional diagnostic tests for characterization or medical and/or surgical intervention, not the cost of treatment itself.
Cancer care.
We derived the stage-specific costs of cancer care from a prior study that evaluated lung cancer–attributable expenditures using the SEER-Medicare linked database.15 The costs were partitioned into initial (first year), continuing, and end-of-life (last year of life) treatment phases (Appendix Table A1, online only).
Out-of-pocket costs.
In accordance with CMS screening policy, we assumed there were no copayments or coinsurance to offset Medicare program expenditures.
Mortality Inputs
Lung cancer mortality rates were derived from SEER stage–specific survival in individuals age ≥ 65 years from 2004 to 2010.1 Other cause-mortality rates were derived from smoker life-tables created as part of the National Cancer Institute CISNET (Cancer Intervention and Surveillance Modeling Network) project.16
Alternative Screening Uptake Scenarios
The rate of low-dose CT uptake is uncertain, but it may be highly influential on all model outcomes. Accordingly, we evaluated two additional scenarios in which the annual uptake rate was increased or decreased by 50% relative to the base case. These scenarios provide a plausible range of outcomes associated with variable screening uptake in the United States over the coming 5 years.
Sensitivity Analyses
We evaluated outcome uncertainty using one-way sensitivity analyses in which we propagated low- and high-value estimates through the model and obtained the resulting range of incremental early-stage lung cancer diagnoses and per-enrollee per-month cost impact for each model input. We also conducted a probabilistic sensitivity analysis using Monte Carlo simulation by specifying the distribution of model inputs, simultaneously sampling parameter sets from the distributions, and propagating the values through the model framework to calculate the joint distribution of model outcomes.17,18 We used these results to calculate 95% CIs around the base-case model outcomes.
Results
Base Case
Over a 5-year time horizon, the model projected 10.7 million (95% CI, 8.0 to 13.4) low-dose CT screening examinations among Medicare enrollees, including 2.9 million (95% CI, 2.2 to 3.7) false-positive screens and 4,300 (95% CI, 3,200 to 5,400) false-negative screens (Table 1). Additionally, approximately 980,000 incidental findings are expected to result in additional diagnostic work-up.
Table 1.
Base-Case Clinical and Resource Use 5-Year Results
| Scenario | Diagnoses |
Screenings |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stage I or II |
Stage III |
Stage IV |
Total Lung Cancer Diagnoses | False-Positive Screens |
False-Negative Screens |
Total Screening Episodes | ||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | |||
| Low-dose CT screening scenario | 124,000 | 52.1 | 56,000 | 23.5 | 58,000 | 24.4 | 238,000 | 2,926,000 | 27.3 | 4,300 | 0.04 | 10,722,000 |
| No-screening scenario | 42,000 | 22.6 | 63,000 | 33.9 | 81,000 | 43.5 | 186,000 | 0 | 0.0 | 0 | 0.0 | 0 |
| Difference | 82,000 | 29.5 | −7,000 | −10.3 | −23,000 | −19.2% | 52,000 | 2,926,000 | 27.3 | 4,300 | 0.04 | 10,722,000 |
Abbreviation: CT, computed tomography.
The expected lung cancer impacts of screening include 52,000 (95% CI, 38,000 to 65,000) more patient cases diagnosed and a stage shift, with 29.5% more patient cases diagnosed at an early stage (I or II), 10.3% fewer patient cases diagnosed at stage III, and 19.2% fewer patient cases diagnosed at stage IV (Table 1).
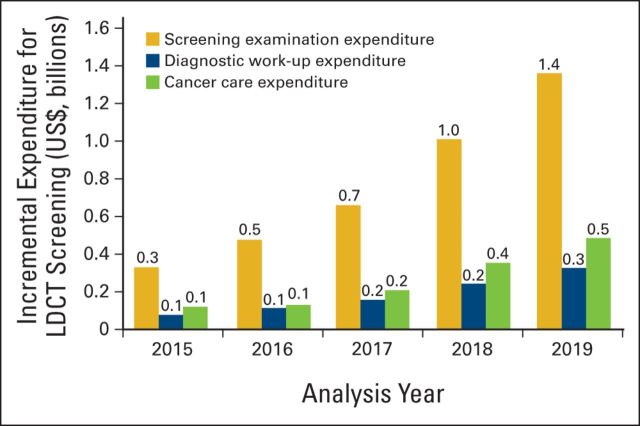
Medicare expenditures are estimated to increase by $4.3 billion (95% CI, $2.6 to $6.0 billion) for screening examinations, $1.0 billion (95% CI, $0.6 to $1.4 billion) for diagnostic work-up, and $1.5 billion (95% CI, $0.6 to $2.3 billion) for cancer care. In total, these expenditures amount to an increase of $6.8 billion (95% CI, $4.5 to $9.1 billion) and a per-enrollee per-month fiscal impact of $2.22 (95% CI, $1.47 to $2.98; Table 2). Across all years of the analysis, the majority of expenditure was attributable to screening examinations (Figure 1).
Table 2.
Base-Case Fiscal Impact 5-Year Results
| Scenario | Screening Episodes |
Diagnostic |
Cancer Care |
Total Expenditure (billion US$) | Per-Enrollee per-Month Expenditure (US$) | |||
|---|---|---|---|---|---|---|---|---|
| Cost (billion US$) | % | Cost (billion US$) | % | Cost (billion US$) | % | |||
| Low-dose CT screening scenario | 4.3 | 18.0 | 1.3 | 5.4 | 18.3 | 76.6 | 24.0 | $2.22 |
| No-screening scenario | 0.0 | 0.0 | 0.3 | 1.7 | 16.9 | 98.3 | 17.2 | — |
| Difference | 4.3 | 18.0 | 1.0 | 3.7 | 1.5 | −21.7 | 6.8 | — |
NOTE. 2014 US dollars.
Abbreviation: CT, computed tomography.
Figure 1.
Annual low-dose computed tomography (LDCT) screening, diagnostic work-up, and cancer care incremental expenditure. Incremental expenditure outcomes were calculated as difference between screening and no-screening strategies using base-case model inputs. Results are rounded, therefore the sum across all analysis years differs slightly from results in Table 2.
Scenario Analysis
Scenarios in which low-dose CT screening uptake was 50% lower and 50% higher resulted in 26,000 and 76,000 more patient cases of lung cancer diagnosed, 14.7% and 33.7% more patient cases diagnosed at an early stage, $2.2 and $6.3 billion more in screening examination expenditures, $0.5 and $1.5 billion more in diagnostic expenditures, $0.7 and $2.1 billion more in cancer care expenditures, $3.4 and $10.0 billion more in total expenditure, and per-enrollee per-month fiscal impacts of $1.12 and $3.27, respectively.
One-Way Sensitivity Analysis
The most influential parameters in the one-way sensitivity analyses for the increase in the proportion diagnosed at an early stage were the proportion of high-risk enrollees receiving low-dose CT screening (range, 28% to 31%) and the lung cancer incidence rate in high-risk enrollees not receiving low-dose CT screening (range, 29% to 31%). In one-way sensitivity analyses for total expenditure impact, the most influential parameters were the average cost per screening episode (range, $5.6 to $8.1 billion), proportion of Medicare enrollees eligible for screening by NLST criteria (range, $5.9 to $7.8 billion), and cost of treatment in the first year after diagnosis with stage I lung cancer (range, $6.3 to $7.4 billion).
Discussion
We estimated the 5-year clinical, resource, and fiscal impacts of implementing low-dose CT lung cancer screening in the Medicare program using recent evidence from a subgroup analysis of participants age ≥ 65 years in the NLST.4 Our findings suggest that low-dose CT screening will have important clinical impacts by shifting diagnoses toward earlier stages, in which curative treatment may be possible. However, this improvement in clinical outcomes will be accompanied by major expenditure increases for the Medicare program, because screening expenditure will greatly outpace any potential cancer care expenditure savings from a stage shift. Specifically, we project that establishing coverage for screening will increase total Medicare expenditure by approximately $6.8 billion—or $2.22 per enrollee per month over 5 years. This finding is lower than a prior estimate of the fiscal impact of breast cancer screening coverage ($2.50 per enrollee per month) and higher than estimates of the fiscal impact of colorectal and cervical cancer screening coverage ($1.10 and $0.95 per enrollee per month, respectively).19
Our findings have important implications for stakeholders involved in lung cancer screening, diagnosis, and care in the Medicare program. First, we demonstrate the potential for low-dose CT screening to precipitate a major shift in lung cancer diagnosis to earlier, more curable stages, even after accounting for suboptimal real-world screening uptake. Providers will diagnose increasing numbers of patients with stage I or II disease. This change has specific implications for surgical specialists and radiation oncologists, who may find rapidly increasing demand for lung cancer–related procedures over the coming 5 years. Second, implementing a screening program will dramatically increase demand for low-dose CT scans, health professionals, and associated information technology infrastructure. We estimate that, on average, implementing low-dose CT screening will require an additional 2.1 million low-dose CT scans per year from the Medicare population in the initial 5 years after implementation—or approximately 5,900 additional scans per day. It is unclear if the current CT scanner and health professional supply can meet this demand. Third, Medicare expenditure increases will require CMS to find cost offsets to balance its budget or to increase premiums to fund the implementation of lung cancer screening. Because the majority of increased expenditure is for screening episodes, there will still be a major fiscal impact even if false-positive rates are substantially improved relative to those from NLST. Nonetheless, the proportion of false-positive screening tests is a material driver of the fiscal impact of implementing low-dose CT screening. Development of screening protocols that increase the positive predictive value of low-dose CT screening, including considering larger nodule sizes for the threshold to define positive screens, could have a substantial impact on the cost of the Medicare screening program. Additionally, further refinement of CT imaging technology, intensive training on interpretation of low-dose CT imaging results for radiologists, and development of inexpensive supplemental methods to adjudicate positive screening results without invasive diagnostic procedures or additional CT imaging could have similar fiscal impacts.
A recent actuarial analysis from Pyenson et al20 also evaluated the fiscal impact of implementing low-dose CT lung cancer screening in Medicare. The authors' analysis assumed 50% screening uptake among enrollees classified as high risk and estimated that low-dose CT screening coverage would increase Medicare expenditure by $1.02 per enrollee per month over a 1-year time horizon.20 This figure is approximately half of our estimated cost per enrollee per month ($2.22), because Pyenson et al assumed a lower average cost per screening episode ($241), which only reflected a screening scan and smoking-cessation counseling, used screening effectiveness results that were not specific to the Medicare population age ≥ 65 years, and assumed no overdiagnosis with low-dose CT screening. We believe our analysis better represents the potential impacts of low-dose CT screening in Medicare, because our average cost per screening episode reflects the shared decision-making protocol specified by the CMS national coverage determination, our effectiveness estimates are derived from a subgroup analysis of NLST focused on participants age ≥ 65 years, and we considered the impact of overdiagnosis, a well-documented phenomenon in low-dose CT lung cancer screening and similar screening procedures.10 Additionally, our analysis has the advantage of considering a 5-year time horizon capturing the longer-term impacts of overdiagnosis, lung cancer treatment cost, and lung cancer mortality.
This study has several important limitations. First, our model framework is a simplified representation of a complex set of considerations related to low-dose CT lung cancer screening and its implementation in Medicare. Accordingly, we focused on population-level factors that are expected to have the greatest influence on the clinical, resource use, and fiscal impacts of low-dose CT screening in Medicare enrollees age 55 to 77 years. Additionally, our outcomes compared the impacts of low-dose CT screening with those of no screening. In reality, a small proportion of the Medicare population might already receive lung cancer screening, and this could lead to small changes in the clinical, resource use, and fiscal impacts of implementing large-scale low-dose CT screening. Second, we limited the time horizon of our analysis to 5 years, although lung cancer screening will have important impacts beyond that period. We chose a short-term time horizon because extending the analysis beyond 5 years would have required many more assumptions, and screening test performance and cancer care costs may change as new technologies and clinical strategies are introduced in the long term. However, a short-term time horizon also introduces lead-time bias, where low-dose CT screening detects some lung cancers that would be clinically detected beyond the analysis time horizon. This is the primary driver of the high 5-year screen-detected patient case overdiagnosis rate, and it increases low-dose CT screening incremental diagnostic and cancer care expenditures relative to a lifetime horizon. We also did not model the clinical impact of smoking cessation counseling provided alongside screening because this is expected to be small over a 5-year time horizon. Finally, we estimated the clinical and economic impacts of implementing screening, but we did not assess the value of screening, as is done in a cost-effectiveness analysis.21 A recent economic analysis of NLST estimated the cost effectiveness of low-dose CT lung cancer screening at $52,000 per life-year gained and $81,000 per quality-adjusted life-year gained.22 These findings suggest that low-dose CT screening has good value relative to implied willingness-to-pay thresholds in cancer in the United States, but these are not necessarily applicable to Medicare enrollees, who are older and generally less healthy than the NLST participants.2,23 Future research should assess the value of low-dose CT lung cancer screening specifically in Medicare.
In conclusion, our analyses suggest that coverage of low-dose CT screening in Medicare enrollees age 55 to 77 years with ≥ 30–pack-year smoking history who are current smokers or quit in the last 15 years will result in more lung cancer diagnoses, a greater proportion of patient cases diagnosed at an early stage, and increased Medicare expenditure. Although the degree of expenditure impact is uncertain, it is clear that the increased cost of conducting millions of screening examinations will greatly outweigh any potential cancer care savings from a stage shift. These results can inform planning efforts by Medicare administrators, contracted health care providers, and other stakeholders.
Acknowledgment
Supported by Genentech and by Grant No. K12HS022982 from the Agency for Healthcare Research and Quality (J.A.R.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality. The funding sources had no role in the design or conduct of the study or the collection, management, analysis, or interpretation of the data; however, Genentech was involved in the preparation, review, and approval of the manuscript and agreed to submission of the manuscript for publication. J.A.R. had full access to all data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis. Presented in part at the 50th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 30-June 3, 2014, and 19th Annual International Meeting of the International Society for Pharmacoeconomic and Outcomes Research, Montreal, Quebec, Canada, May 31-June 4, 2014.
Appendix
Table A1.
Model Input Point Estimates, Uncertainty Ranges, Distributions, and Data Sources
| Input | Point Estimate | Low | High | Distribution | Reference |
|---|---|---|---|---|---|
| Lung cancer stage distribution in no-screening scenario | |||||
| Proportion of incident patient cases with | |||||
| Stage I | 20.2% | 18.2% | 22.2% | Beta | Dinan et al9 |
| Stage II | 2.4% | 2.2% | 2.6% | Beta | Dinan et al9 |
| Stage III | 33.9% | 27.3% | 40.4% | Beta | Dinan et al9 |
| Stage IV | 43.5% | 39.2% | 47.9% | Beta | Dinan et al9 |
| Risk classification | |||||
| Proportion of population age 65 to 77 years classified as high risk by NLST criteria | 20.3% | 16.2% | 24.4% | Beta | Oken et al6 |
| Screen-detected lung cancer overdiagnosis rate versus no screening (all years) | 53.0% | 48.0% | 56.0% | Beta | Patz et al10 |
| Lung cancer annualized incidence in NLST high-risk patients who are not screened | 0.005 | 0.004 | 0.006 | Beta | Dinan et al9 |
| Screening diffusion rate | |||||
| Proportion of high-risk individuals offered low-dose CT screening in | |||||
| Year 1 | 30.0% | NA | Assumption | ||
| Year 2 | 45.0% | NA | Assumption | ||
| Year 3 | 60.0% | NA | Assumption | ||
| Year 4 | 75.0% | NA | Assumption | ||
| Year 5 | 90.0% | NA | Assumption | ||
| Screening use among high-risk individuals by NLST criteria in | |||||
| Year 1 | 50.0% | 20.0% | 80.0% | Beta | Assumption |
| Year 2 | 55.0% | 25.0% | 75.0% | Beta | Assumption |
| Year 3 | 60.0% | 30.0% | 70.0% | Beta | Assumption |
| Year 4 | 65.0% | 35.0% | 65.0% | Beta | Assumption |
| Year 5 | 70.0% | 35.0% | 65.0% | Beta | Assumption |
| Screening | |||||
| Proportion screening positive | 28.7% | 25.8% | 31.6% | Beta | Pinsky et al4 |
| Proportion screening positive and have lung cancer | |||||
| Any stage | 4.9% | 4.4% | 5.4% | Beta | Pinsky et al4 |
| Stage I | 61.1% | NA | Pinsky et al4 | ||
| Stage II | 8.2% | NA | Pinsky et al4 | ||
| Stage III | 17.5% | NA | Pinsky et al4 | ||
| Stage IV | 13.2% | NA | Pinsky et al4 | ||
| Proportion screening negative | |||||
| Proportion screening negative and have lung cancer | |||||
| Any stage | 0.1% | 0.1% | 0.1% | Beta | Pinsky et al4 |
| Stage I | 15.9% | NA | Aberle et al2 | ||
| Stage II | 11.4% | NA | Aberle et al2 | ||
| Stage III | 40.9% | NA | Aberle et al2 | ||
| Stage IV | 31.8% | NA | Aberle et al2 | ||
| Screening and diagnostic cost, 2014 $US | |||||
| Low-dose CT scan cost | $220 | $176 | $264 | Normal | CPT code 71250 |
| Low-complexity office visit cost | $80 | $72 | $88 | Normal | CPT code 99213 |
| Smoking-cessation counseling cost | $30 | $24 | $36 | Normal | CPT code 99407 |
| Smoking-cessation nicotine replacement intervention cost | $200 | $160 | $220 | Normal | Villanti et al12 |
| Low-dose CT scan incidental finding cost | $13 | $7 | $20 | Normal | Priola et al14 |
| Bronchoscopy with biopsy cost | $1,270 | $1,016 | $1,524 | Normal | Goulart et al13 |
| Lung cancer treatment cost, 2014 $US | |||||
| Stage I | |||||
| Initial year | $41,694 | $33,355 | $50,033 | Normal | Yabroff et al15 |
| Continuing | $5,357 | $4,286 | $6,429 | Normal | Yabroff et al15 |
| End of life | $53,810 | $43,048 | $64,572 | Normal | Yabroff et al15 |
| Stage II | |||||
| Initial year | $41,694 | $33,355 | $50,033 | Normal | Yabroff et al15 |
| Continuing | $5,357 | $4,286 | $6,429 | Normal | Yabroff et al15 |
| End of life | $53,810 | $43,048 | $64,572 | Normal | Yabroff et al15 |
| Stage III | |||||
| Initial year | $53,106 | $42,485 | $63,727 | Normal | Yabroff et al15 |
| Continuing | $5,357 | $4,286 | $6,429 | Normal | Yabroff et al15 |
| End of life | $73,316 | $58,653 | $87,979 | Normal | Yabroff et al15 |
| Stage IV | |||||
| Initial year | $58,450 | $46,760 | $70,140 | Normal | Yabroff et al15 |
| Continuing | $5,357 | $4,286 | $6,429 | Normal | Yabroff et al15 |
| End of life | $91,386 | $73,109 | $109,663 | Normal | Yabroff et al15 |
| Lung cancer mortality, survival | |||||
| Stage I or II | |||||
| 1 year | 78.4% | 74.5% | 82.3% | Beta | Howlader et al1 |
| 2 years | 66.1% | 62.8% | 69.4% | Beta | Howlader et al1 |
| 3 years | 58.4% | 55.5% | 61.3% | Beta | Howlader et al1 |
| 4 years | 52.9% | 50.3% | 55.5% | Beta | Howlader et al1 |
| 5 years | 48.4% | 46.0% | 50.8% | Beta | Howlader et al1 |
| Stage III | |||||
| 1 year | 58.0% | 55.1% | 60.9% | Beta | Howlader et al1 |
| 2 years | 40.1% | 38.1% | 42.1% | Beta | Howlader et al1 |
| 3 years | 31.2% | 29.6% | 32.8% | Beta | Howlader et al1 |
| 4 years | 26.5% | 25.2% | 27.8% | Beta | Howlader et al1 |
| 5 years | 22.8% | 21.7% | 23.94 | Beta | Howlader et al1 |
| Stage IV | |||||
| 1 year | 22.0% | 20.9% | 23.1% | Beta | Howlader et al1 |
| 2 years | 10.0% | 9.5% | 10.5% | Beta | Howlader et al1 |
| 3 years | 6.1% | 5.8% | 6.4% | Beta | Howlader et al1 |
| 4 years | 4.2% | 4.0% | 4.4% | Beta | Howlader et al1 |
| 5 years | 3.1% | 2.9% | 3.3% | Beta | Howlader et al1 |
| Other-cause mortality | |||||
| Annual mortality rate | 2.2% | 1.8% | 2.7% | Beta | Rosenberg et al16 |
Abbreviation: CT, computed tomography; NLST, National Lung Screening Trial.
Authors' Disclosures of Potential Conflicts of Interest
Disclosures provided by the authors are available with this article at jop.ascopubs.org.
Author Contributions
Conception and design: Joshua A. Roth, Sean D. Sullivan, Arliene Ravelo, Joanna C. Sanderson, Scott D. Ramsey
Collection and assembly of data: Joshua A. Roth, Joanna C. Sanderson
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Projected Clinical, Resource Use, and Fiscal Impacts of Implementing Low-Dose Computed Tomography Lung Cancer Screening in Medicare
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jop.ascopubs.org/site/misc/ifc.xhtml.
Joshua A. Roth
Consulting or Advisory Role: Genentech
Travel, Accommodations, Expenses: Life Technologies
Sean D. Sullivan
Consulting or Advisory Role: Genentech
Bernardo H.L. Goulart
No relationship to disclose
Arliene Ravelo
Employment: Genentech
Joanna C. Sanderson
Consulting or Advisory Role: Genentech, Allergan, Ipsen, Avanir, AstraZeneca/MedImmune
Scott D. Ramsey
Consulting or Advisory Role: Genentech
References
- 1.Howlader N Noone AM Krapcho M, etal: SEER Cancer Statistics Review, 1975-2009 2012Bethesda, MD: National Cancer Institute [Google Scholar]
- 2.Aberle DR Adams AM Berg CD, etal: Reduced lung-cancer mortality with low-dose computed tomographic screening N Engl J Med 365:395–409,2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moyer VA: Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement Ann Intern Med 160:330–338,2014 [DOI] [PubMed] [Google Scholar]
- 4.Pinsky PF Gierada DS Hocking W, etal: National Lung Screening Trial findings by age: Medicare-eligible versus under-65 population Ann Intern Med 161:627–633,2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Congressional Budget Office. Medicare: Baseline Projections, April 2014. http://www.cbo.gov/publication/44205.
- 6.Oken MM Hocking WG Kvale PA, etal: Screening by chest radiograph and lung cancer mortality: The Prostate, Lung, Colorectal, and Ovarian (PLCO) randomized trial JAMA 306:1865–1873,2011 [DOI] [PubMed] [Google Scholar]
- 7.Cronin KA Yu B Krapcho M, etal: Modeling the dissemination of mammography in the United States Cancer Causes Control 16:701–712,2005 [DOI] [PubMed] [Google Scholar]
- 8.Church TR Black WC Aberle DR, etal: Results of initial low-dose computed tomographic screening for lung cancer N Engl J Med 368:1980–1991,2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dinan MA Curtis LH Carpenter WR, etal: Stage migration, selection bias, and survival associated with the adoption of positron emission tomography among medicare beneficiaries with non–small-cell lung cancer, 1998-2003 J Clin Oncol 30:2725–2730,2012 [DOI] [PubMed] [Google Scholar]
- 10.Patz EF Jr Pinsky P Gatsonis C, etal: Overdiagnosis in low-dose computed tomography screening for lung cancer JAMA Intern Med 174:269–274,2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Lung Association. Providing Guidance on Lung Cancer Screening to Patients and Physicians. http://www.lung.org/lung-disease/lung-cancer/lung-cancer-screening-guidelines/lung-cancer-screening.pdf.
- 12.Villanti AC Jiang Y Abrams DB, etal: A cost-utility analysis of lung cancer screening and the additional benefits of incorporating smoking cessation interventions PLoS One 8:e71379,2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goulart BH Bensink ME Mummy DG, etal: Lung cancer screening with low-dose computed tomography: Costs, national expenditures, and cost-effectiveness J Natl Compr Canc Netw 10:267–275,2012 [DOI] [PubMed] [Google Scholar]
- 14.Priola AM Priola SM Giaj-Levra M, etal: Clinical implications and added costs of incidental findings in an early detection study of lung cancer by using low-dose spiral computed tomography Clin Lung Cancer 14:139–148,2013 [DOI] [PubMed] [Google Scholar]
- 15.Yabroff KR Lamont EB Mariotto A, etal: Cost of care for elderly cancer patients in the United States J Natl Cancer Inst 100:630–641,2008 [DOI] [PubMed] [Google Scholar]
- 16.Rosenberg MA Feuer EJ Yu B, etal: Chapter 3: Cohort life tables by smoking status, removing lung cancer as a cause of death Risk Anal 32:S25–S38,2012suppl 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Briggs AH, Ades AE, Price MJ: Probabilistic sensitivity analysis for decision trees with multiple branches: Use of the Dirichlet distribution in a Bayesian framework Med Decis Making 23:341–350,2003 [DOI] [PubMed] [Google Scholar]
- 18.O'Hagan A McCabe C Akehurst R, etal: Incorporation of uncertainty in health economic modelling studies Pharmacoeconomics 23:529–536,2005 [DOI] [PubMed] [Google Scholar]
- 19.Pyenson BS Sander MS Jiang Y, etal: An actuarial analysis shows that offering lung cancer screening as an insurance benefit would save lives at relatively low cost Health Aff (Millwood) 31:770–779,2012 [DOI] [PubMed] [Google Scholar]
- 20.Pyenson BS Henschke CI Yankelevitz DF, etal: Offering lung cancer screening to high-risk Medicare beneficiaries saves lives and is cost-effective: An actuarial analysis Am Health Drug Benefits 7:272–282,2014 [PMC free article] [PubMed] [Google Scholar]
- 21.Gold MR Siegel JE Russell LB, etal: Cost-Effectiveness in Health and Medicine 1996New York, NY: Oxford University Press USA [Google Scholar]
- 22.Black WC Gareen IF Soneji SS, etal: Cost-effectiveness of CT screening in the National Lung Screening Trial N Engl J Med 371:1793–1802,2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neumann PJ, Cohen JT, Weinstein MC: Updating cost-effectiveness: The curious resilience of the $50,000-per-QALY threshold N Engl J Med 371:796–797,2014 [DOI] [PubMed] [Google Scholar]