Abstract
Background
(Over-)expression of arginase may limit local availability of arginine for nitric oxide synthesis. We investigated the significance of arginase1 (ARG1) for the development of airway hyperresponsiveness (AHR) and lung inflammation in female mice with ovalbumin (OVA)-induced allergic asthma.
Methods
Arg1 was ablated in the lung by crossing Arg1 fl/fl and Tie2Cre tg/− mice. OVA sensitization and challenge were conducted, and AHR to methacholine was determined using the Flexivent system. Changes in gene expression, chemokine and cytokine secretion, plasma IgE, and lung histology were quantified using RT-qPCR, ELISA, and immunohistochemistry, respectively.
Results
Arg1 ablation had no influence on the development of OVA-induced AHR, but attenuated OVA-induced increases in expression of Arg2 and Nos2, Slc7a1, Slc7a2, and Slc7a7 (arginine transporters), Il4, Il5 and Il13 (TH2-type cytokines), Ccl2 and Ccl11 (chemokines), Ifng (TH1-type cytokine), Clca3 and Muc5ac (goblet cell markers), and OVA-specific IgE. Pulmonary IL-10 protein content increased, but IL-4, IL-5, IL-13, TNFα and IFNγ content, and lung histopathology, were not affected. Arg1 elimination also decreased number and tightness of correlations between adaptive changes in lung function and inflammatory parameters in OVA/OVA-treated female mice. OVA/OVA-treated female mice mounted a higher OVA-IgE response than males, but the correlation between lung function and inflammation was lower. Arg1-deficient OVA/OVA-treated females differed from males in a more pronounced decline of arginine-metabolizing and -transporting genes, higher plasma arginine levels, a smaller OVA-specific IgE response, and no improvement of peripheral lung function.
Conclusion
Complete ablation of Arg1 in the lung affects mRNA abundance of arginine-transporting and -metabolizing genes, and pro-inflammatory genes, but not methacholine responsiveness or accumulation of inflammatory cells.
Electronic supplementary material
The online version of this article (10.1186/s12890-017-0490-7) contains supplementary material, which is available to authorized users.
Keywords: Arginase1, Airway hyperresponsiveness, Inflammation
Background
Allergic asthma is a prevalent disease characterized by airway inflammation, hyperresponsiveness, and eventually remodeling. Development of allergic asthma starts upon binding of inhaled allergens to their complementary IgEs, which activates IgE receptor-bearing mast cells and basophils. These cells, in turn, activate an inflammatory cascade that causes lung infiltration with eosinophils, neutrophils, alternatively activated (M2) macrophages and (TH2) lymphocytes. The inflammatory response is typically accompanied by mucus production, mucosal edema, and smooth-muscle hyperresponsiveness, which all contribute to the development of airway hyperresponsiveness (AHR) upon exposure to e.g. histamine and methacholine [1, 2]. Repeated episodes of allergic inflammation eventually lead to fibrosis and wall thickening in large and small airways (“airway remodeling”).
Adult women suffer more often and more severely from asthma than men do (for a recent review, see: [3]). Female (BALB/c) mice develop a more severe airway inflammation with more myeloid dendritic cells, effector T cells, alternatively activated macrophages, eosinophils, and higher concentrations of IgE and cytokines than male mice in ovalbumin (OVA)-induced allergic asthma models [4–6]. Male (C57BL/6) [7, 8] and progesterone-treated female mice [9], on the other hand, develop stronger AHR due to the relaxing effect of estrogens on airway smooth muscle [8, 10, 11]. Taken together, these data reveal a sex difference in mice with respect to the respiratory and immune responses to allergens (for a recent review, see [12]).
Depending on the pro-inflammatory agent, alveolar macrophages can develop into classically activated (M1) macrophages, alternatively activated M2) macrophages, and intermediate forms [13]. Alternatively activated lung macrophages appear to be major players in the development of airway inflammation of OVA-sensitized and -challenged (OVA/OVA) mice, and are more prevalent in female than in male mice [14]. The arginase1 (Arg1) gene is highly expressed in the cytoplasm of M2 macrophages [13, 15]. Accordingly, OVA-induced Arg1 expression is present in cells with the morphological characteristics of macrophages [16, 17]. The prominence of ARG1-containing macrophages underlies the hypothesis that arginine availability plays a key role in the development and presentation of asthma [18–20].
Arginase and nitric oxide synthase (NOS) share, and compete for the substrate L-arginine. Since NO relaxes the smooth-muscle cells of the bronchi and blood vessels, insufficient NO production may underlie AHR [4]. A high concentration of NO, however, promotes inflammation, mucosal swelling, and mucus secretion in the lung [21, 22]. Although the NOS enzymes have a ~500-fold higher affinity for arginine than arginase, the Vmax of arginase is ~1000-fold higher than that of NOS, which makes regional substrate depletion of arginine possible [21, 23]. Inhibition of arginine uptake into macrophages by ablation of the arginine transporter Cat2 [24] and excess cationic proteins, such as eosinophil-derived major basic protein (MBP) [25], also inhibits NO production, whereas pharmacological inhibition of arginase activity attenuates AHR, Arg1 expression, cell number in bronchoalveolar lavage fluid, and expression of the inflammatory markers IL-4, IL-5, IL-13, and NOS2 [26–29], and induces NO-mediated smooth-muscle relaxation [30]. Furthermore, supplementation of arginine mitigates the inflammatory airway response, increases arginase expression and activity, and elevates NOx levels [31, 32]. These findings suggest that arginase-mediated differences in substrate availability for NO synthesis may determine the clinical presentation of allergic asthma.
In an earlier study, we reported that Arg1 ablation in macrophages improved peripheral lung function and decreased mRNA expression of arginine-metabolizing and -transporting genes in OVA/OVA-treated male C57Bl/6 mice, but had no effect on airway inflammation [33]. In a similar study with Arg1 fl/fl /Tie2Cre tg/− mice, in which Arg1 exons 7 and 8 instead of exon 4 were flanked by loxP sites, Barron et al. also reported the lack of an effect of Arg1 elimination in myeloid cells on allergic lung inflammation, but found no effect on peripheral lung mechanics [34]. However, these authors did not differentiate between male and female mice. In the present study, we examined the role of ARG1 on lung function and inflammation in OVA/OVA-treated female Arg1 fl/fl /Tie2Cre tg/− mice. We report that Arg1 ablation in the lung of female C57Bl/6 mice did not protect peripheral lung mechanics, as in male mice, but did cause a smaller increase in the expression of L-arginine-metabolizing enzymes and transporters, and of inflammatory cytokines and chemokines.
Methods
Generation of transgenic mice and husbandry
All animal experiments were approved by the Committee for Animal Care and Use of Maastricht University (DEC2005-146). The generation of the transgenic mice was described before [33]. In brief: exon 4 of the mouse Arg1 gene was flanked with loxP sites [33] and offspring were genotyped with primers Arg1-F1 and Arg1-R1 (Additional file 1: Table S1). To ablate the floxed Arg1 allele (Arg1 fl) specifically in macrophages, Arg1 fl/fl mice were crossed with either LysM-Cre [35] or Tie2-Cre mice [36]. The resulting Arg fl/fl /LysM-Cre tg/− (Arg1-KOLysM) mice and their Arg fl/fl /LysM-Cre −/− littermates (Arg1-Con) or Arg fl/fl /Tie2-Cre tg/− (Arg1- KOTie2) mice and Arg fl/fl /Tie2-Cre −/− littermates (Arg1-Con) were analyzed for the presence of the LysM-Cre or Tie2-Cre transgene by PCR using primer pairs LysM-F and LysM-R or Tie2-F and Tie2-R, respectively (Additional file 1: Table S1). The Cre-excised Arg1 allele (298 bp) was detected with the primers Arg1-F2 (Additional file 1: Table S1) and Arg1-R1. Mice were kept with 2-3 animals per filter-top cage with wood shavings as bedding on a 12-h light/dark cycle, with standard chow and water ad libitum. Mice were checked daily for their well being by dedicated personnel of the animal facility.
Antigen sensitization and challenge
Antigen sensitization and challenge have been described before [33]. In brief, ten-week old female mice were injected intraperitoneally on days 0 and 14 with 10 μg of ovalbumin (OVA), grade V (Sigma-Aldrich, Zwijndrecht, The Netherlands) in the presence of 1 mg/mL of alum adjuvant (Imject Alum®, Thermo Scientific, Rockford, IL, USA). On days 21-27, mice were exposed daily for 30 min to 1% (w/v) aerosolized OVA in PBS. Lung function was assessed 12 h after the last challenge. Groups, each containing 2-3 Arg1-Con or Arg1-KO mice pretreated with PBS or OVA (in total 7-8 mice per genotype and treatment), were tested at 3-4 different occasions to correct for any “session” effects [37].
Airway hyperresponsiveness
Assessment of methacholine responsiveness was carried out as previously described [33]. Briefly, 7-8 mice per genotype and treatment were anesthetized with 80 mg/kg sodium pentobarbital and an additional 40 mg/kg 30 min later. An 18-gauge blunt needle was inserted into the trachea and connected to a mechanical small-animal ventilator (FlexiVent, Scireq, Montreal, Canada). Mice were ventilated at 200 breaths/min with a delivered tidal volume of 0.25 mL against a positive end-expiratory pressure (PEEP) of 3 cm H2O applied by a water trap. Lungs were challenged by delivering successively 0, 3.1, 12.5 and 50 mg/mL aerosolized methacholine (Sigma) in PBS through the tracheal cannula using an ultrasonic nebulizer during 10 deep inhalations with a tidal volume of 0.8 mL. Following each methacholine challenge, the input impedance (Zrs) of the respiratory system was measured. Parameters measured were: RN, the Newtonian airflow resistance, mostly in the conducting pulmonary airways; H, “tissue elastance”, or the elastic energy stored in tissues; G, “tissue resistance”, or viscous dissipation of energy in respiratory tissues and airflow heterogeneity.
Plasma collection and analysis
After each experiment, blood was collected from the inferior caval vein of 7-8 mice per group in heparin-coated tubes, centrifuged 3 min at 5000*g, snap-frozen and stored at −80 °C as described previously [33]. Plasma OVA-specific IgE levels were determined by ELISA (MD Biosciences, M036005, Zürich, Switzerland). For the determination of plasma amino acids, 50 μL of plasma was added to 4 mg sulfosalicylic acid, vortexed, snap-frozen in liquid nitrogen and stored at −80 °C until use. Plasma amino-acid concentrations were measured as described [33].
Tissue isolation
Following euthanasia, the left lung was filled with 10% buffered formalin (Klinipath, Deventer, The Netherlands) for 10 min at a pressure of 20 cm H2O and submersed overnight in 4% formaldehyde at room temperature prior to paraffin embedding. The right lung was snap frozen in liquid nitrogen, pulverized in a liquid-nitrogen-chilled mortar, and stored at −80 °C as described previously [33].
Immunostaining
Immunostaining was performed as described previously [33]. In brief, paraffin-embedded tissue was cut into 4 μm sections and stained with hematoxylin & eosin (H&E), reticulin, or Sirius red. Epitope retrieval was carried out by heating the slides for 5 min in 10 mM sodium citrate (pH 6 at room temperature (RT)) at 95 °C and cooling to RT for 30 min. Endogenous peroxidase was blocked with peroxidase block (DAKO, S2001, Enschede, the Netherlands) for 10 min at RT. Non-specific antibody binding was blocked with 10% normal goat serum for 30 min. After washing in PBS, slides were incubated with anti-ARG1 (Amsterdam Liver Center, AMS40.11.13), anti-myeloperoxidase (MPO; DAKO), or anti-major basic protein (MBP; kindly provided by Dr. James Lee, MT14.3.7, Mayo Clinic Scottsdale, AZ, USA) as described [33]. After washing, sections were incubated with a 1:200 diluted biotinylated rabbit anti-rat secondary antibody (DAKO) for 45 min at RT, washed, incubated with streptavidin/HRP (Vector) for 30 min at RT, and developed with 3,3′-diaminobenzidine (Sigma) for 10 min. Sections stained for ARG1 were incubated with an AP-labeled secondary antibody (DAKO) for 45 min, developed with nitroblue-tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate (Roche, Almere, The Netherlands) dissolved in 50 mM MgSO4, 100 mM Tris·HCl (pH 9.5) for 30 min, and cover-slipped with an aqueous mounting medium (DAKO). To facilitate quantification, the immunostained slides were not counterstained.
Histological assessment
Lung inflammation was assessed on H&E-stained sections. MBP and MPO images were digitized with the Pannoramic slide scanner 250 (3DHistech, Budapest, Hungary), and counted using the Pannoramic viewer software application. Color deconvolution was adjusted to detect diaminobenzidine-stained cells. Only cells with a diameter between 10 and 15 μm were included. Arginase-positive cells were counted manually in three different locations (peribronchiolar, perivenous and in the intervening parenchyma). The density of the counted cells was expressed per mm2 tissue.
Milliplex assay
Tissue powder was homogenized in PBS, pH 7.6, in the presence of a proteinase inhibitor cocktail (Complete, Roche). Cytokines (CCL11 (eotaxin-1), IL-4, IL-5, IL-10, IL-13, TNFα, and IFNγ) were quantified using a Luminex ® xMAP® multiplex platform, combined with a customized Milliplex™ mouse chemokine/cytokine panel from Millipore™, as described previously [33].
RNA isolation and quantification
RNA purification and quantification was performed as described previously [33]. In brief, tissue powder was homogenized in Tri reagent (Sigma) with the Mini Bead-Beater (Biospec products, Bartlesville, OK, USA). To remove genomic DNA, RNA was precipitated with 2 M LiCl for at least 30 min at −20 °C. RNA integrity was checked by denaturing gel electrophoresis. RNA concentration was determined with a NanoDrop-ND-1000 spectrophotometer at 260 nm (Isogen Life Sciences, Wilmington, DE, USA). 400 ng of total RNA was transcribed using a first-strand synthesis kit (Roche). Quantitative PCR was performed in a Lightcycler 480 (Roche), using Lightcycler 480® SYBRgreen mastermix (Roche) and the following settings: denaturation: 30 s at 95 °C; annealing 30 s at 60 °C; elongation 30 s at 72 °C; 45 cycles; and a final elongation step for 5 min at 72 °C. If reverse transcriptase was omitted, no product was formed. Primary fluorescence data were quantified as described [33] and expressed relative to that of 18S rRNA. Primer sequences are given in Additional file 1: Table S1.
Western blot
Western blot analysis was performed as described previously [33]. In brief, tissue powder was homogenized in RIPA buffer (25 mM Tris·HCl (pH 7.6), 150 mM NaCl, 1% NP-40, 1% Na-deoxycholate, 0.1% SDS, containing Complete® cocktail (Roche). Protein concentration was measured with the bicinchoninic-acid assay (Pierce, Rockford, IL, USA). Twenty-five μg protein was separated by SDS-PAGE, transferred onto 0.45 μm nitrocellulose membranes, using a wet transfer system (Biorad, Hercules, CA, USA). Equal loading of lanes was confirmed by Ponceau S staining, followed by a wash-step with TBS (50 mM Tris, 150 mM NaCl, pH 7.6) and blocking of non-specific binding with 5% skimmed milk in TBS/0.5% Tween-2. Rabbit anti-ARG1 antibody (1:200), followed by an HRP-conjugated swine anti-rabbit secondary antibody (DAKO) was used to visualize ARG1. Signal was developed with the Super Signal West Pico Substrate (Pierce) and quantified in a Fuji systems darkbox (Fuji Film Life Sciences, Tokyo, Japan).
Statistical analyses
Groups were compared using the Kruskal-Wallis test for PBS/OVA- versus OVA/OVA-treated, and Arg1-Con versus Arg1-KOTie2 mice, as described previously [33]. If this nonparametric test indicated a difference, a multiple comparison of the groups was carried out. Values were considered as statistically significant if P < 0.05, and as indicating a trend if P < 0.10.
The bivariate two-tailed Pearson correlation coefficients between each of the lung-function parameters, mRNA and protein concentrations, histology quantification data and plasma amino-acid concentrations were determined after combining the data from the comparable PBS/OVA and OVA/OVA groups. In the Tables, P-values of the resulting correlation coefficients were color-coded, with red indicating a P < 0.001, orange 0.01 > P > 0.001, and yellow 0.05 > P > 0.01.
Results
Arg1 expression in the lungs of OVA-sensitized and -challenged mice with Arg1-deficient macrophages
Arg1-KOLysM mice, Arg1- KOTie2 mice, and their Arg1-Con littermates were either sensitized-and-challenged with ovalbumin (OVA/OVA), or mock-sensitized and then challenged with ovalbumin (PBS/OVA). As expected, we found no ARG1-positive cells in the lungs of PBS/OVA-treated mice, whereas the lungs of OVA/OVA-treated Arg1-Con mice contained many ARG1-positive cells (Fig. 1a). ARG1-positive cells were completely absent from the lungs of OVA/OVA-treated Arg1-KOTie2, whereas the number of ARG1-positive cells was reduced, but not eliminated, in the lungs of similarly treated Arg1-KOLysM mice. Of note, we observed no appreciable ARG1 in the endothelial cells in PBS/OVA (Fig. 1a) or OVA/OVA-treated lung blood vessels (Fig. 1d). Furthermore, we did not observe any ARG1 protein or mRNA expression in the lungs of mice undergoing the PBS/OVA protocol, whereas a robust induction of ARG1 protein and mRNA expression was found in the lungs of Arg1-Con mice exposed to the OVA/OVA protocol (black columns in Figs. 1b and 2). Pulmonary Arg1 mRNA and protein concentration in OVA/OVA-treated Arg1-KOTie2 mice were reduced to 5 ± 2% and 2 ± 0.2%, respectively, and in OVA/OVA-treated Arg1-KOLysM mice to 20 ± 8% and 19 ± 10%, respectively, of that found in similarly treated Arg1-Con littermates (Figs. 1b and 2, white columns). We also counted ARG1-positive cells surrounding bronchioles and arteries, and in parenchyma of OVA/OVA-treated Arg1-Con and Arg1-KO Tie2 mice, and found no ARG1-positive cells in lung tissue of Arg1-KO Tie2 mice (Fig. 1c). From this finding, we conclude that the floxed 4th exon of Arg1 was accessible to the Cre enzyme and that the reduction in Arg1 expression was near complete in female Arg1-KOTie2 mice (reduction to ~2%), whereas residual Arg1 expression was present in female Arg1-KOLysM mice (reduction to ~20%). Since Arg1-KOLysM mice showed an incomplete elimination of Arg1 expression and an intermediate phenotype, we limit the description of our study to Arg1-KOTie2 mice and their Arg1-Con littermates.
Fig. 1.
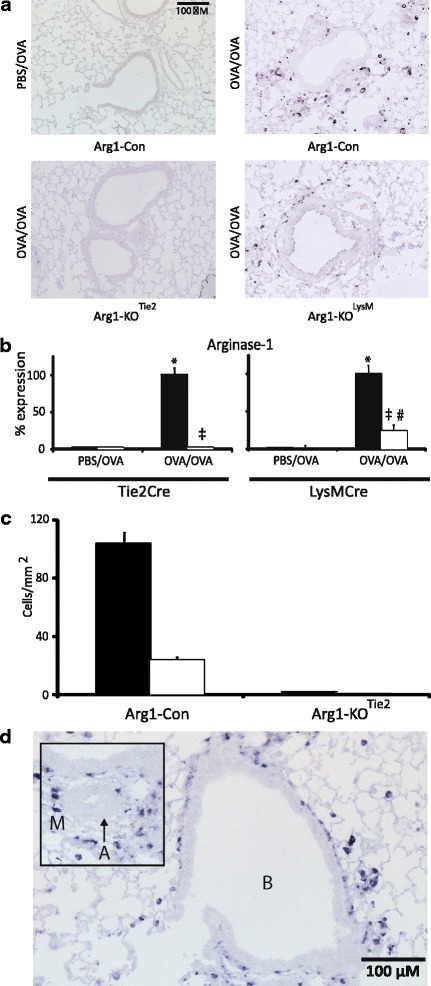
Validation of Arg1 ablation in lung of female Arg1 fl/fl mice. a Lungs of PBS/OVA- and OVA/OVA-treated female control mice, respectively (top row), and of OVA/OVA-treated female Arg1-KOTie2 and Arg1-KOLysM mice, respectively (bottom row). Note the complete absence of ARG1-positive macrophages in Arg1-KOTie2 mice and the reduction in Arg1-KOLysM mice. b Reduction of ARG1 protein content in the lungs of OVA/OVA-treated Arg1-KOTie2 and Arg1-KOLysM mice (white bars) compared to the corresponding Arg1-ConLysM or Arg1-ConTie2 mice (black bars). Means ± SEM (n = 8 mice per group). c Number of ARG1-positive cells per mm2 lung tissue. Cells were counted near bronchioles and arteries (black bars) and in the lung parenchyma (white bars). Means ± SEM are shown (n = 8 mice per group). d Detail of ARG1 protein content in OVA/OVA-treated control lung. The absence of ARG1 in arterial endothelium is noteworthy. Abbreviations: A = artery, and M = ARG1-positive macrophages. Significance symbols: *: P < 0.001 OVA/OVA vs. PBS/OVA Arg1-Con; #: P < 0.001 OVA/OVA vs. PBS/OVA Arg1-KOLysm; ‡: P < 0.001 OVA/OVA Arg1-KOLysm or Arg1-KOTie2 vs. OVA/OVA Arg1-Con
Fig. 2.
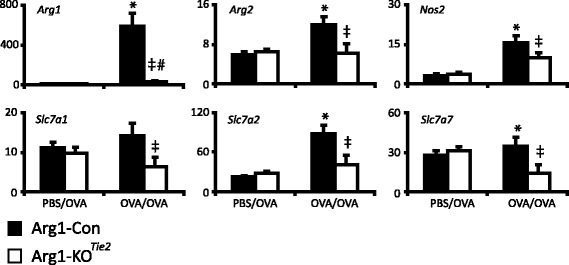
Effect of Arg1 ablation in the lung on the expression of arginine-metabolizing enzymes and arginine transporters. Black bars represent Arg1-Con mice and white bars Arg1-KOTie2 mice. The specific treatment (PBS/OVA or OVA/OVA) is indicated below the columns. mRNA abundance (AU = number of mRNA copies normalized to 18S rRNA expression and multiplied by 104) of the arginine-metabolizing enzymes Arg1, Arg2, and Nos2 and the arginine transporters Slc7a1, Slc7a2 and Slc7a7 is shown as mean ± SEM of 7-8 mice per group. Significance symbols: *: P < 0.05 OVA/OVA vs. PBS/OVA Arg1-Con; #: P < 0.05 OVA/OVA vs. PBS/OVA Arg1-KOTie2; ‡: P < 0.05 OVA/OVA Arg1-Con vs. Arg1-KOTie2
Effect of Arg1 deletion on arginine-metabolizing and -transporting genes in the lung
We investigated to what extent ablation of Arg1 in the lung caused changes in the expression of arginine-metabolizing enzymes and arginine transporters. Figure 2 shows that the pulmonary mRNA abundance of Arg1, Arg2, Nos2, Slc7a1, Slc7a2 and Slc7a7 was induced by the OVA/OVA protocol compared to the PBS/OVA protocol. More importantly, the induction was consistently lower in Arg1-KOTie2 than in Arg1-Con female mice subjected to the OVA/OVA protocol. As a result, OVA/OVA treatment no longer induced the expression of Arg2, Nos2, Slc7a1, Slc7a2, and Slc7a7 in Arg1-KOTie2 mice. These data clearly show that effective ablation of Arg1 expression in macrophages suppressed the induction of arginine-metabolizing enzymes and arginine transporters in the lungs of OVA/OVA-treated female mice.
The effect of Arg1 ablation on circulating amino acids
OVA/OVA treatment of female Arg1-Con mice caused a significant drop in the concentration of circulating arginine levels relative to their PBS/OVA-treated littermates (Additional file 1: Table S2). The decline was not seen in OVA/OVA-treated Arg1-KOTie2 mice, implying an effect of ARG1. In both Arg1-KOTie2 and Arg1-Con mice, OVA/OVA treatment also caused an increase in the plasma concentration of ornithine and a decline in that of glycine relative to the corresponding PBS/OVA-treated mice (Additional file 1: Table S2). As a result, the arginine bioavailability index (the ratio of arginine and ornithine plus lysine concentrations) decreased non-significantly to ~71% (P = 0.369) in OVA/OVA-treated Arg-Con, but remained unchanged in Arg1-KOTie2 mice.
Ablation of Arg1 in the lung has no effect on allergen-induced airway hyperresponsiveness
To address the question whether Arg1 ablation in the lung affects respiratory mechanics, we measured methacholine responsiveness in PBS/OVA- and OVA/OVA-treated Arg1-KOTie2 mice and their Arg1-Con littermates (Fig. 3). Compared to PBS/OVA treatment, OVA/OVA treatment increased airway resistance (RN), tissue elastance (H), and tissue resistance (G) in female Arg1-Con and Arg1-KOTie2 mice, without an effect of Arg1 depletion. Collectively, these findings show that Arg1 ablation in the lung did not affect AHR in female mice.
Fig. 3.
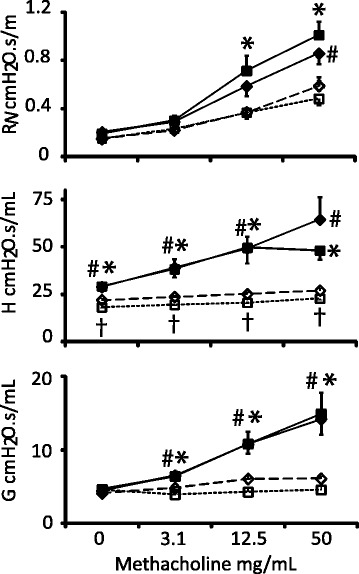
Effect of Arg1 ablation in the lung on lung function as measured with the FlexiVent. PBS/OVA-treated Arg1-Con mice are represented by open diamonds connected by dashed lines and PBS/OVA-treated Arg1-KOTie2 mice by open squares connected by dotted lines. OVA/OVA-treated Arg1-Con mice are represented by filled diamonds and OVA/OVA treated Arg1-KOTie2 mice by filled squares both connected by continuous lines. Airway resistance RN, tissue elastance H, and tissue resistance G are shown in panels A, B, and C, respectively. Values on the X-axis indicate the concentrations of aerosolized methacholine. Means ± SEM (n = 7-8 mice per group). Significance symbols: #: P < 0.01 (OVA/OVA vs. PBS/OVA Arg1-Con); *: P < 0.01 (OVA/OVA Arg1-KOTie2 vs. PBS/OVA Arg1-KOTie2); †: P < 0.01 (PBS/OVA Arg1-KOTie2 vs. PBS/OVA Arg1-Con). Arg1-KOTie2 and Arg1-Con mice did not differ in their response to OVA/OVA treatment
Ablation of Arg1 in the lung affects mRNA abundance of inflammatory genes
We investigated whether Arg1 ablation in the lung affected the expression of asthma-associated cytokines (Fig. 4). OVA/OVA treatment increased the abundance of Il13, Il4, Il5, Ccl2, Ccl11 (Eotaxin-1), and Il10 mRNAs in Arg1-Con mice as expected, while the expression of Tnfa and Ifng remained unchanged. Furthermore, OVA/OVA treatment of Arg1-Con mice increased gene expression of respiratory epithelium-specific Muc5ac and Clca3. OVA/OVA-treated female Arg1-KOTie2 mice differed from their OVA/OVA-treated Arg1-Con littermates by showing a significantly lower expression of the Il13, Il4, Ccl2, Ccl11, Ifng, Muc5ac, and Clca3 mRNAs. These findings imply a direct relation between Arg1 elimination in the lung and the decreased expression of inflammatory genes in the lungs of OVA/OVA-treated mice.
Fig. 4.
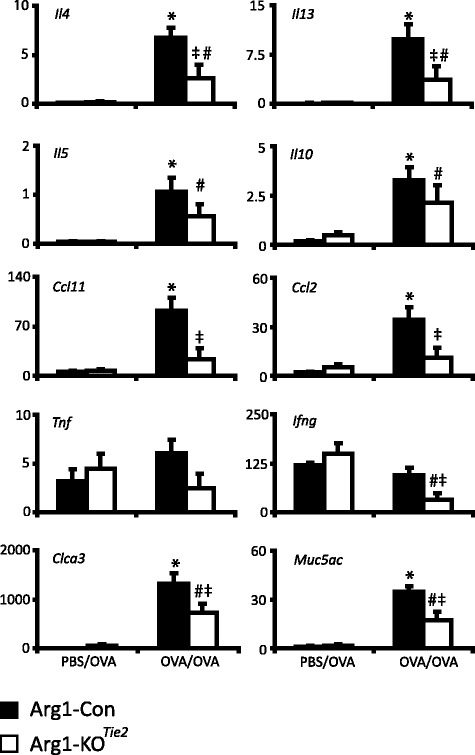
Effect of Arg1 ablation in the lung on the expression of inflammatory genes. Black bars represent Arg1-Con mice and white bars Arg1-KOTie2 mice. mRNA abundance (AU = number of mRNA copies normalized to 18S rRNA expression and multiplied by 104) of the TH2-related inflammatory genes Il4, Il13, Il5 and Ccl11, the macrophage-chemotactic protein Ccl2, the anti-inflammatory gene Il10, the TH1-related inflammatory genes Tnfa and Ifng, and the marker genes for the activation of bronchiolar epithelium Muc5ac and Clca3 are shown as means ± SEM (n = 7-8 mice per group). Significance symbols: *: P < 0.05 (OVA/OVA vs. PBS/OVA Arg1-Con); #: P < 0.05 (OVA/OVA vs. PBS/OVA Arg1-KOTie2); ‡: P < 0.05 (OVA/OVA Arg1-KOTie2 vs OVA/OVA Arg1-Con)
Arg1 ablation in the lung does not affect protein levels of cytokines and chemokines
To determine whether Arg1 ablation in the lung had an effect on the protein concentration of pulmonary cytokines and chemokines, we measured IL-4, IL-5, IL-10, IL-13, CCL11, TNFα and IFNγ protein in extracts of whole-lung homogenates (Fig. 5). Compared to PBS/OVA-treated female mice, OVA/OVA-treated Arg1-Con and Arg1-KOTie2 mice had increased concentrations of IL-5, CCL11 and TNFα in their lungs, whereas the concentration of IL-4, IL-13, and IFNγ was unchanged. IL-10 was lower in OVA/OVA- than in PBS/OVA-treated Arg1-Con mice and higher in OVA/OVA- treated Arg1-KOTie2 mice than in similarly treated Arg1-Con mice. Apart from IL-10, no differences in cytokine and chemokine protein concentration were found between OVA/OVA-treated Arg1-Con and Arg1-KOTie2 mice.
Fig. 5.
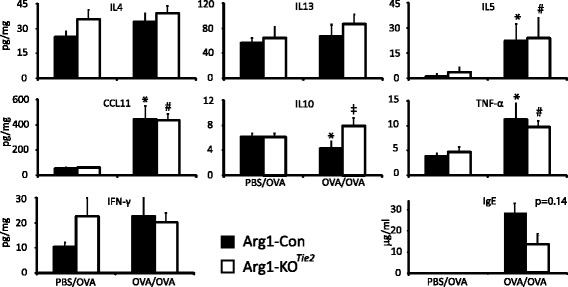
Effect of Arg1 ablation in the lung on the pulmonary concentration of inflammatory cytokines, chemokines, and OVA-specific IgE. Black bars represent Arg1-Con mice and white bars Arg1-KOTie2 mice. Treatment of the mice (PBS/OVA or OVA/OVA) is indicated. The pulmonary concentrations of IL-4, IL-13, IL-5, CCL11, IL-10, TNFα and IFNγ (pg/mg total protein), and OVA-specific IgE (ng/mL) in plasma are shown as means ± SEM (n = 7-8 mice per group). Significance symbols: *: P < 0.05 (OVA/OVA vs. PBS/OVA Arg1-Con); #: P < 0.05 (OVA/OVA vs. PBS/OVA Arg1-KOTie2); ‡: P < 0.05 (OVA/OVA Arg1-KOTie2 vs OVA/OVA Arg1-Con)
Effect of Arg1 deletion on the immune response to OVA
We investigated whether the production of ovalbumin-specific IgE antibodies was affected by the ablation of Arg1. OVA-specific IgE was not detectable in plasma of PBS/OVA-treated mice. OVA-specific IgE levels in OVA/OVA-treated female Arg1-KOTie2 mice were reduced to ~50% of that in OVA/OVA-treated Arg1-Con mice, but due to the high variance in plasma IgE concentrations this difference did not reach statistical significance (P = 0.14).
Effects of Arg1 deletion in the lung on pulmonary inflammation
We investigated the effect of Arg1 ablation on the OVA/OVA-induced inflammatory response in lung tissue (Fig. 6). H&E-stained sections of lung tissue did not reveal any inflammatory cells in PBS/OVA-treated mice (Fig. 6A). This finding was confirmed by staining the same lungs for the presence of eosinophils and neutrophils using MBP and MPO as markers, respectively (Fig. 6A). As expected, many inflammatory cells were present in the lungs of OVA/OVA-treated mice, but their number was not different between female Arg1-KOTie2 mice and their Arg1-Con littermates (Fig. 6B). To investigate whether there was a difference in lung remodeling between female Arg1-KOTie2 mice and their Arg1-Con littermates, we stained slides for reticulin and collagen (Sirius red), but did not detect differences either (Fig. 7).
Fig. 6.
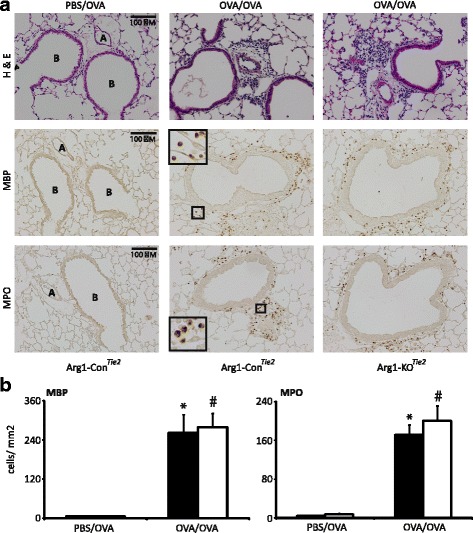
Effect of Arg1 ablation in the lung on the prevalence of inflammatory cells in the lungs. A: The left two columns show lungs of PBS/OVA- and OVA/OVA-treated Arg1-Con mice, while the right column shows lungs of OVA/OVA-treated Arg1-KOTie2 mice. Top row: hematoxylin and eosin; middle row: MBP staining for eosinophils; bottom row: MPO staining for neutrophils. Bar: 100 μm. B: Quantification of the inflammatory cells in OVA/OVA-treated Arg1-Con (black bars) and Arg1-KOTie2 (white bars) mice. All sections were stained simultaneously. Means and SEM of 7-8 mice per group are shown. *: P < 0.05 (OVA/OVA vs. PBS/OVA Arg1-Con); #: P < 0.05 (OVA/OVA vs. PBS/OVA Arg1-KOTie2)
Fig. 7.
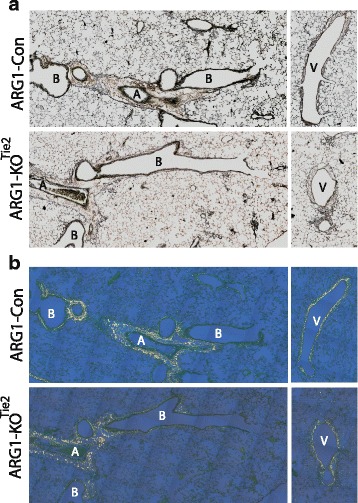
Effect of Arg1 ablation on lung remodeling. a: Bright-field images of reticulin-stained sections of OVA/OVA-treated female Arg1-Con and Arg1-KOTie2 mice. b: Polarized-light images of Sirius red-stained sections of OVA/OVA-treated female Arg1-Con and Arg1-KOTie2 mice to assess collagen content (serial sections of panel A). The left-sided panels show sections of periarterial areas and the right-handed panels sections of perivenous areas. Abbreviations: B = bronchiole, A = artery, and V = vein
Comparison of responses to allergic asthma in female Arg1-Con and Arg1-KOTie2 mice
To investigate whether the presence or absence of ARG1 activity affected the adaptive responses to OVA-induced asthma in a quantitatively similar way, we also investigated to what extent changes in lung-function parameters, mRNA and protein concentrations, histology quantitation and plasma amino-acid concentrations correlated in either Arg1-Con or Arg1-KOTie2 mice (Fig. 8). Strikingly, lung-function parameters and mRNA concentrations in Arg1-Con females strongly corresponded within, but hardly between both categories. Noticeable exceptions were a correlation between the response of lung-function parameters H and G and the expression of Il4 mRNA, and the near absence of a correlation between the response of the pro-inflammatory cytokines Tnfa and especially Ifng, and all other mRNAs measured. Female Arg1-KOTie2 differed from Arg1-Con mice (Fig. 8) by a pronounced decrease in the tightness of the correspondence between the adaptive responses that were mounted. Striking examples were the loss of correspondence between the response of H and G, and Il4 mRNA expression, and the loss of correspondence of the expression of the mRNAs of arginine transporters Slc7a7 and Slc7a1, and that of all other mRNAs.
Fig. 8.
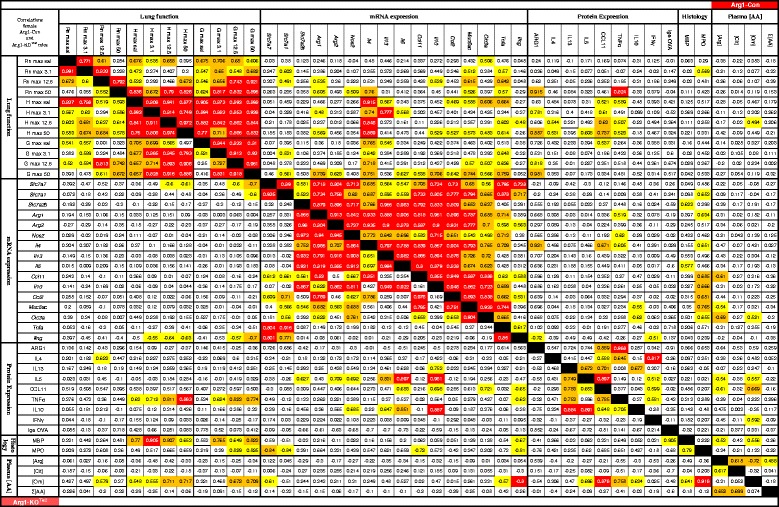
Effect of Arg1 ablation in the lung on the correlation of lung function, abundance of pulmonary mRNAs and proteins, pulmonary histopathology, and concentrations of plasma amino acids in female mice. The triangle on the upper right side refers to data from Arg1-Con mice and the triangle on the lower left to data from Arg1-KOTie2 mice. The correlation coefficients between the parameters named above the columns and left to the rows, respectively, as measured in all 15 or 16 PBS/OVA- and OVA/OVA-treated mice of the Arg1-Con or Arg1-KOTie2 groups are shown. The significance of the correlations is color-coded: yellow: 0.05 > P > 0.01, orange: 0.01 > P > 0.001, and red: P < 0.001. The near-absence of cross-correlations of parameters between the categories named in the title and the decline of the number or degree of significant correlations in Arg1-KOTie2 compared to Arg1-Con mice is noteworthy
Discussion
Tie2Cre-dependent deletion of Arg1 expression in myeloid cells prevented the expression of ARG1 in lung inflammatory cells of OVA/OVA-treated Arg1-KOTie2 mice. Compared to female OVA/OVA-treated Arg1-Con mice, Arg1-KOTie2 mice had lower OVA-specific IgE levels and a lower expression of arginine-metabolizing or -transporting genes (Arg2, Nos2, Slc7a1, Slc7a2, and Slc7a7), of cyto- and chemokine genes (Il4, Il13, Il5, Ccl11, Ccl2, and Ifng), and of epithelial marker genes (Muc5ac and Clc3a), but Arg1 deficiency did not affect airway resistance (RN), tissue elastance (H), or tissue resistance (G). Furthermore, the tightness of the correspondence between the adaptive responses that were mounted in OVA/OVA-treated female mice were strongly reduced if these mice were unable to express Arg1 in their alveolar macrophages.
Efficacy of Tie2Cre-mediated Arg1 ablation in allergically inflamed lungs
Tie2Cre mice excise loxP-flanked DNA in both endothelial and early hematopoietic progenitor cells [36, 38]. As in male mice [33] and in other studies [34, 36, 39, 40], Tie2Cre-mediated excision of Arg1 resulted in a near-complete deletion of Arg1 mRNA and protein expression in macrophages. A near-complete Arg1 excision was also observed in peritoneal macrophages of Arg1-KOTie2 mice [41]. Importantly, we did not observe ARG1 protein in the endothelium of the pulmonary blood vessels of PBS/OVA- or OVA/OVA-treated Arg1-Con mice (Fig. 1a,d), even after prolonged incubation with the alkaline-phosphatase substrate (up to 90 min). In support of this finding, most other studies found no or only minimal arginase1 protein in lung epithelium and smooth muscle [42–44], also after inducing allergic inflammation [45–47]. Based on these findings, we conclude that Arg1-KOTie2 mice can be used for the ablation of Arg1 in macrophages of allergically inflamed lungs.
The near 100% efficacy of Tie2Cre-mediated excision of loxP-flanked Arg1 fl sequences contrasts with the 70-80% efficacy LysMCre-mediated Arg1 fl excision found in the present study and in our earlier study in male Arg1-KO mice [33]. Other studies [35, 48–50] also report LysMCre-mediated target excision in only 50-80% of the target cells. Since the 20% remaining expression of Arg1 in the lungs of OVA/OVA-treated Arg1-KOLysM mediated an intermediate phenotype between OVA/OVA-treated Arg1-Con and Arg1-KOTie2Cre mice, we limited our description of the effects of pulmonary Arg1 deficiency on allergic asthma to Arg1-KOTie2Cre mice.
Arg1 ablation in macrophages and arginine availability do not affect lung biomechanics in allergically inflamed mouse lungs
Lung mechanics were measured using the forced-oscillation technique, which yields data on airway resistance (RN) in the large airways, and on tissue elastance (H) and resistance (G) that reflect the function of the peripheral parts of the lung [51, 52]. Arg1-KOTie2 and Arg1-Con mice did not differ in any of these parameters with or without OVA/OVA treatment. This finding in female mice contrasts with our earlier observation in male mice, in which Arg1 deficiency in macrophages improved the peripheral lung mechanics parameters H and G [33]. Since the experiments in male and female mice were carried out concurrently with mice from the same litters, the difference cannot be attributed to a session effect. However, the present finding does agree with studies in which deficiency of Arg1 in macrophages was mediated by bone-marrow transfer of constitutively Arg1-deficient stem cells [53] or Tie2Cre-mediated elimination of Arg1 exons 7 and 8 [34]. Both studies tested, in addition to the OVA/OVA protocol, allergic inflammation in response to Aspergillus fumigatus and Schistosoma mansoni eggs in both C57BL/6 and BALB/c mice, but did not state whether the lung function tests were carried out in male and/or female mice.
The lack of an effect of Arg1 deficiency on lung mechanics is in line with a smaller increase in the expression of arginine-metabolizing and -transporting genes upon OVA/OVA treatment (Fig. 2). Administration of pharmacological arginase inhibitors reduced AHR of allergen-induced asthma in both male and female mice. Whereas arginase inhibitors reduced lung inflammation in male mice [28, 29], they enhanced inflammation in female mice [26]. Because we observed no effect on AHR and a reduced inflammatory response in Arg1-deficient lungs, the effects of systemic inhibition of arginase-1 and -2 activities apparently differ from those of a deletion of Arg1 from macrophages. Arginine analogues may exert additional effects, as NOS2 depletion or inhibition did not affect AHR, but that of NOS1- and −3 did [30, 54, 55].
The circulating concentration of L-arginine (Additional file 1: Table S2) was ~17% lower in OVA/OVA-treated Arg1-Con mice than in similarly treated Arg1-KOTie2 mice, showing that Arg1 expression in hematopoietic tissue had an impact on plasma arginine. Because the concentration of plasma ornithine increased and that of citrulline was unchanged, the plasma [Arg]/([Orn] + [Cit]) index decreased, in agreement with a recent report in female BALB/c mice [56]. Scott et al. [56] attributed an important role to lung arginase in the increase of plasma ornithine, but we observed a similar increase in plasma ornithine in mice with and without arginase1 in their lungs. Furthermore, we did not find an increase in plasma ornithine or a decrease in the arginine availability index in OVA/OVA-treated male Arg1-Con and Arg1-KO mice [33], even though methacholine responsiveness was decreased by Arg1 deletion in male mice. The plasma [Arg]/([Orn] + [Lys]) index, which may reflect the availability of basic amino acids, only declined in OVA/OVA-treated female Arg1-KO mice. The question, therefore, is whether plasma arginine concentration or any plasma arginine availability index is important for respiratory function. Barron et al. [34] suggested that the enormous perfusion of the lung, which equals the cardiac output, assures that the supply of arginine from plasma to tissue cells is hardly impaired by local arginase activity. In addition, we have recently shown that citrulline rather than arginine is the more efficient extracellular source for intracellular arginine [57].
Arg1 ablation does not affect pro-inflammatory protein content in allergically inflamed lungs
OVA/OVA treatment of Arg1-KO mice resulted in a similar degree of lung infiltration with inflammatory cells as seen in Arg1-Con littermates, but Il13, Il4, Il5, Ccl2, Ccl11, Ifng, Muc5ac, and Clca3 were expressed to a lesser extent in Arg1-KO Tie2 than in Arg1-Con mice. Such a coordinated transcriptional response of genes probably reflects the activity of one or more common regulatory genes or proteins. However, as far as measured, changes in mRNA abundance of the chemo- and cytokines were not reflected in the corresponding protein concentrations in lung tissue (Fig. 8). This finding can be explained by the fact that cyto- and chemokines are secreted proteins, but inefficient translation of the mRNAs in Arg1-KO mice can also contribute, because posttranscriptional regulation of expression is a well-known feature of cyto- and chemokines [58]. In OVA/OVA-treated Arg1-Con males, we did observe a reasonable correspondence between mRNA and protein levels in 4 out of 8 combinations measured, but this correspondence disappeared in OVA/OVA-treated Arg1-KO male mice. The decline in circulating arginine concentration in OVA/OVA-treated males [33] was similar to that observed in females. Low circulating arginine concentrations are known to affect translation by inducing endoplasmic reticulum (ER) stress [59].
Comparison of the adaptive responses to OVA sensitization and challenge in male and female Arg1-Con mice
In a parallel study, we investigated the effects of Arg1 ablation in the lung of genetically identical, similarly treated male mice [33]. Since the biology of female mice is underreported and since females are more often affected by allergic asthma than males [4–6, 60], we compared the adaptive responses of Arg1-Con mice in both sexes (Additional file 1: Table S3). The OVA-specific IgE concentration was ~3-fold higher in plasma of OVA/OVA-treated female than male mice. The sex difference in IgE accumulation is well-established [4]. Arg1 deficiency did not reduce the induction of plasma IgE concentration in males, but tended to reduce it by ~2-fold in females. However, this reduction in plasma IgE did not modify the inflammatory response in OVA/OVA-treated Arg1-deficient lungs. OVA/OVA-treated BALB/c female mice further have higher lung concentrations of IL-4, IL-5, IL-10, IL-13, IFN-γ, and TNF-α protein [4, 6, 61] than male BALB/c mice, but we observed similar IL-4, IL-13, IL10, CCL11, TNFα, and IFNγ concentrations in OVA/OVA-treated male and female C57BL/6 mice. It is well-recognized that C57BL/6 and BALB/C mice differ in their response to OVA/OVA treatment [62, 63].
OVA/OVA-treated Arg1-Con females accumulated ~3-fold more Arg1 mRNA than similarly treated males, without showing different numbers of arginase1-positive macrophages in the lung. As far as we are aware, a sex difference in macrophage Arg1 mRNA and protein expression in OVA/OVA-treated mice has not yet been reported. Furthermore, the expression of Il4, Il13 and, to a lesser extent, Il5 was affected by Arg1 deletion in female mice only, suggesting a sex difference in the expression of M2 cytokines in OVA/OVA-treated mice. In Coxsackie virus-induced myocarditis, the ~4-fold higher Arg1 expression in macrophages of female over male mice was attributed to skewing of the macrophage polarization towards an M2 phenotype in females, and towards an M1 phenotype in males [64]. Depending on the inducing agent, alveolar macrophages can also develop an M1, M2, or even an intermediate phenotype [13]. These sex difference may arise because female steroid hormones promote alternative activation of macrophages, whereas testosterone inhibits the M2 phenotype [65, 66].
We also studied whether there were sex differences in the coordination of the responses to OVA/OVA treatment (Additional file 2: Figure S1). Adaptive changes in methacholine responsiveness and mRNA expression (except the M1-macrophage markers Ifng and, to a lesser extent, Tnfa) correlated more strongly within their category in females than in males, whereas, as already discussed, IL-4, IL-13, IL-5, CCL11, and TNFα protein concentrations correlated better with the corresponding mRNA concentrations and with lung function parameters H and G in males. These data imply that the respiratory responses to OVA/OVA treatment were less integrated with the inflammatory responses in female than in male mice.
Conclusion
Complete ablation of Arg1 in the lung does not affect methacholine responsiveness or the invasion of inflammatory cells, but does change the gene-expression profile of these cells in OVA/OVA-treated female mice. Since the asthmatic responses in mice and humans show similar sex-related differences, and since ablation of Arg1 did improve peripheral lung function in male mice only, we hypothesize that treatment of asthma by modulating arginase activity in the lung will primarily benefit male patients.
Additional files
Table S1. Primers pairs used in genotyping and quantitative PCR. Table S2. Amino-acid concentrations in venous plasma (μM ± SEM) of Arg1-Con and Arg1-KOTie2Cre mice *: P < 0.05 OVA/OVA vs. PBS/OVA Arg1-Con; #: P < 0.05 OVA/OVA vs. PBS/OVA Arg1-KOTie2. Table S3. Differences between male and female Arg1-Con mice after treatment with the OVA/OVA protocol. (DOCX 24 kb)
Correlation of lung-function, abundance of pulmonary mRNAs and proteins, pulmonary histopathology, and concentrations of plasma amino acids in wild-type male and female mice. The lower-left triangle refers to data obtained female and the upper-right triangle to data from male Arg1-Con mice. The numbers show the correlation coefficients between parameters indicated above and to the left of the columns and rows, respectively, as measured in all 15 or 16 male and female Arg1-Con mice studied, that is, both the PBS/OVA- and the OVA/OVA-treated groups. The significance of the correlations is color-coded according to the P-value of the correlation coefficient: yellow: 0.05 > P > 0.01, orange: 0.01 > P > 0.001, and red: P < 0.001. Note the difference in the degree of cross-correlations of parameters between the categories named in the title in males and females. (PDF 219 kb)
Acknowledgements
Not applicable
Funding
This study was supported by a grant from the Transnational University Limburg, the Netherlands, 31,962,140 T. The funding body had no role in the design of the study, data collection, analysis, interpretation, or writing of the manuscript.
Availability of data and materials
All data generated or analysed during this study are included in this published article [and its Additional files].
Abbreviations
- AHR
airway hyperresponsiveness
- ARG1, Arg1
arginase 1 protein or gene
- Arg1-Con
Arg fl/fl /LysM-Cre −/− or Arg fl/fl /Tie2-Cre −/− control littermates
- Arg1-KOLysM or Arg1-KOTie2
Arg fl/fl /LysM-Cre tg/− or Arg fl/fl /Tie2-Cre tg/− tissue-specific knockout mice
- Arg2
arginase 2 gene
- Cat2
cationic amino acid transporter aka Slc7a2
- Ccl11
C-C motif chemokine 11, aka eotaxin-1
- Ccl2
C-C-motif chemokine ligand 2
- Clca3
chloride channel accessory 3
- G
tissue resistance
- H
tissue elastance
- H&E
hematoxylin & eosin
- IFNγ, Ifng
interferon gamma
- IgE
immunoglobulin E
- IL10
interleukin 10
- IL13
interleukin 13
- IL4
interleukin 4
- IL5
interleukin 5
- MBP
major basic protein
- MPO
myeloperoxidase
- Muc5ac
mucin 5 ac
- NOS2
nitric oxide synthase
- OVA
ovalbumin
- OVA/OVA
ovalbumin-sensitized and challenged
- PBS/OVA
mock-sensitized and ovalbumin-challenged
- RN
Newtonian airflow resistance
- RT
room temperature
- Slc7a1
solute carrier family 7 member 1, aka as Cat1
- Slc7a2
solute carrier family 7 member 2, aka as Cat2
- Slc7a7
solute carrier family 7 member 7
- TNFα, Tfna
tumour necrosis factor alpha
Authors’ contributions
RC and SS participated in the design of the study, data acquisition, statistical analysis, and drafting of the manuscript. MP participated in acquisition of the data and reviewing the drafted manuscript. ET and PvD participated in acquisition of the data and technical support. WL and EK conceived of the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Ethics approval
All animal experiments were approved by the Committee for Animal Care and Use of Maastricht University (DEC 2055-146).
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12890-017-0490-7) contains supplementary material, which is available to authorized users.
Contributor Information
Roy H. E. Cloots, Email: roy.cloots@maastrichtuniversity.nl
Selvakumari Sankaranarayanan, Email: s4selvakumari@gmail.com.
Matthew E. Poynter, Email: matthew.poynter@med.uvm.edu
Els Terwindt, Email: e.terwindt@maastrichtuniversity.nl.
Paul van Dijk, Email: p.vandijk@maastrichtuniversity.nl.
Wouter H. Lamers, Email: wh.lamers@maastrichtuniversity.nl
S. Eleonore Köhler, Phone: +31 43 3881191, Email: leo.koehler@maastrichtuniversity.nl
References
- 1.Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma. From bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med. 2000;161(5):1720–1745. doi: 10.1164/ajrccm.161.5.9903102. [DOI] [PubMed] [Google Scholar]
- 2.Busse WW, Lemanske RF., Jr Asthma. N Engl J Med. 2001;344(5):350–362. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- 3.Sathish V, Martin YN, Prakash YS. Sex steroid signaling: implications for lung diseases. Pharmacol Ther. 2015;150:94–108. doi: 10.1016/j.pharmthera.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Melgert BN, Postma DS, Kuipers I, Geerlings M, Luinge MA, van der Strate BW, Kerstjens HA, Timens W, Hylkema MN. Female mice are more susceptible to the development of allergic airway inflammation than male mice. Clin Exp Allergy. 2005;35(11):1496–1503. doi: 10.1111/j.1365-2222.2005.02362.x. [DOI] [PubMed] [Google Scholar]
- 5.Riffo-Vasquez Y, Ligeiro de Oliveira AP, Page CP, Spina D, Tavares-de-Lima W. Role of sex hormones in allergic inflammation in mice. Clin Exp Allergy. 2007;37(3):459–470. doi: 10.1111/j.1365-2222.2007.02670.x. [DOI] [PubMed] [Google Scholar]
- 6.Blacquiere MJ, Hylkema MN, Postma DS, Geerlings M, Timens W, Melgert BN. Airway inflammation and remodeling in two mouse models of asthma: comparison of males and females. Int Arch Allergy Immunol. 2010;153(2):173–181. doi: 10.1159/000312635. [DOI] [PubMed] [Google Scholar]
- 7.Card JW, Carey MA, Bradbury JA, DeGraff LM, Morgan DL, Moorman MP, Flake GP, Zeldin DC. Gender differences in murine airway responsiveness and lipopolysaccharide-induced inflammation. J Immunol. 2006;177(1):621–630. doi: 10.4049/jimmunol.177.1.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsubara S, Swasey CH, Loader JE, Dakhama A, Joetham A, Ohnishi H, Balhorn A, Miyahara N, Takeda K, Gelfand EW. Estrogen determines sex differences in airway responsiveness after allergen exposure. Am J Respir Cell Mol Biol. 2008;38(5):501–508. doi: 10.1165/rcmb.2007-0298OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hellings PW, Vandekerckhove P, Claeys R, Billen J, Kasran A, Ceuppens JL. Progesterone increases airway eosinophilia and hyper-responsiveness in a murine model of allergic asthma. Clin Exp Allergy. 2003;33(10):1457–1463. doi: 10.1046/j.1365-2222.2003.01743.x. [DOI] [PubMed] [Google Scholar]
- 10.Carey MA, Card JW, Bradbury JA, Moorman MP, Haykal-Coates N, Gavett SH, Graves JP, Walker VR, Flake GP, Voltz JW, et al. Spontaneous airway hyperresponsiveness in estrogen receptor-alpha-deficient mice. Am J Respir Crit Care Med. 2007;175(2):126–135. doi: 10.1164/rccm.200509-1493OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dimitropoulou C, Drakopanagiotakis F, Chatterjee A, Snead C, Catravas JD. Estrogen replacement therapy prevents airway dysfunction in a murine model of allergen-induced asthma. Lung. 2009;187(2):116–127. doi: 10.1007/s00408-008-9129-z. [DOI] [PubMed] [Google Scholar]
- 12.Bosse Y. Endocrine regulation of airway contractility is overlooked. J Endocrinol. 2014;222(2):R61–R73. doi: 10.1530/JOE-14-0220. [DOI] [PubMed] [Google Scholar]
- 13.Katsura Y, Harada N, Harada S, Ishimori A, Makino F, Ito J, Kamachi F, Okumura K, Akiba H, Atsuta R, et al. Characteristics of alveolar macrophages from murine models of OVA-induced allergic airway inflammation and LPS-induced acute airway inflammation. Exp Lung Res. 2015;41(7):370–382. doi: 10.3109/01902148.2015.1044137. [DOI] [PubMed] [Google Scholar]
- 14.Melgert BN, Oriss TB, Qi Z, Dixon-McCarthy B, Geerlings M, Hylkema MN, Ray A. Macrophages: regulators of sex differences in asthma? Am J Respir Cell Mol Biol. 2010;42(5):595–603. doi: 10.1165/rcmb.2009-0016OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Munder M. Arginase: an emerging key player in the mammalian immune system. Br J Pharmacol. 2009;158(3):638–651. doi: 10.1111/j.1476-5381.2009.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zimmermann N, King NE, Laporte J, Yang M, Mishra A, Pope SM, Muntel EE, Witte DP, Pegg AA, Foster PS, et al. Dissection of experimental asthma with DNA microarray analysis identifies arginase in asthma pathogenesis. J Clin Invest. 2003;111(12):1863–1874. doi: 10.1172/JCI17912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takemoto K, Ogino K, Shibamori M, Gondo T, Hitomi Y, Takigawa T, Wang DH, Takaki J, Ichimura H, Fujikura Y, et al. Transiently, paralleled upregulation of arginase and nitric oxide synthase and the effect of both enzymes on the pathology of asthma. Am J Physiol Lung Cell Mol Physiol. 2007;293(6):L1419–L1426. doi: 10.1152/ajplung.00418.2006. [DOI] [PubMed] [Google Scholar]
- 18.Morris CR, Poljakovic M, Lavrisha L, Machado L, Kuypers FA, Morris SM., Jr Decreased arginine bioavailability and increased serum arginase activity in asthma. Am J Respir Crit Care Med. 2004;170(2):148–153. doi: 10.1164/rccm.200309-1304OC. [DOI] [PubMed] [Google Scholar]
- 19.Mabalirajan U, Ahmad T, Leishangthem GD, Joseph DA, Dinda AK, Agrawal A, Ghosh B. Beneficial effects of high dose of L-arginine on airway hyperresponsiveness and airway inflammation in a murine model of asthma. J Allergy Clin Immunol. 2010;125(3):626–635. doi: 10.1016/j.jaci.2009.10.065. [DOI] [PubMed] [Google Scholar]
- 20.Benson RC, Hardy KA, Morris CR. Arginase and arginine dysregulation in asthma. J Allergy (Cairo) 2011;2011:736319. doi: 10.1155/2011/736319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akata K, Yatera K, Wang KY, Naito K, Ogoshi T, Noguchi S, Kido T, Toyohira Y, Shimokawa H, Yanagihara N, et al. Decreased bronchial Eosinophilic inflammation and mucus Hypersecretion in asthmatic mice lacking all nitric oxide Synthase Isoforms. Lung. 2016;194(1):121–124. doi: 10.1007/s00408-015-9833-4. [DOI] [PubMed] [Google Scholar]
- 22.Naura AS, Zerfaoui M, Kim H, Abd Elmageed ZY, Rodriguez PC, Hans CP, Ju J, Errami Y, Park J, Ochoa AC, et al. Requirement for inducible nitric oxide synthase in chronic allergen exposure-induced pulmonary fibrosis but not inflammation. J Immunol. 2010;185(5):3076–3085. doi: 10.4049/jimmunol.0904214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bratt JM, Franzi LM, Linderholm AL, O'Roark EM, Kenyon NJ, Last JA. Arginase inhibition in airways from normal and nitric oxide synthase 2-knockout mice exposed to ovalbumin. Toxicol Appl Pharmacol. 2010;242(1):1–8. doi: 10.1016/j.taap.2009.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rothenberg ME, Doepker MP, Lewkowich IP, Chiaramonte MG, Stringer KF, Finkelman FD, MacLeod CL, Ellies LG, Zimmermann N. Cationic amino acid transporter 2 regulates inflammatory homeostasis in the lung. Proc Natl Acad Sci U S A. 2006;103(40):14895–14900. doi: 10.1073/pnas.0605478103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hammermann R, Hirschmann J, Hey C, Mossner J, Folkerts G, Nijkamp FP, Wessler I, Racke K. Cationic proteins inhibit L-arginine uptake in rat alveolar macrophages and tracheal epithelial cells. Implications for nitric oxide synthesis. Am J Respir Cell Mol Biol. 1999;21(2):155–162. doi: 10.1165/ajrcmb.21.2.3574. [DOI] [PubMed] [Google Scholar]
- 26.Ckless K, Lampert A, Reiss J, Kasahara D, Poynter ME, Irvin CG, Lundblad LK, Norton R, van der Vliet A, Janssen-Heininger YM. Inhibition of arginase activity enhances inflammation in mice with allergic airway disease, in association with increases in protein S-nitrosylation and tyrosine nitration. J Immunol. 2008;181(6):4255–4264. doi: 10.4049/jimmunol.181.6.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.North ML, Khanna N, Marsden PA, Grasemann H, Scott JA. Functionally important role for arginase 1 in the airway hyperresponsiveness of asthma. Am J Physiol Lung Cell Mol Physiol. 2009;296(6):L911–L920. doi: 10.1152/ajplung.00025.2009. [DOI] [PubMed] [Google Scholar]
- 28.Ogino K, Kubo M, Takahashi H, Zhang R, Zou Y, Fujikura Y. Anti-inflammatory effect of arginase inhibitor and corticosteroid on airway allergic reactions in a Dermatophogoides farinae-induced NC/Nga mouse model. Inflammation. 2013;36(1):141–151. doi: 10.1007/s10753-012-9529-3. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi N, Ogino K, Takemoto K, Hamanishi S, Wang DH, Takigawa T, Shibamori M, Ishiyama H, Fujikura Y. Direct inhibition of arginase attenuated airway allergic reactions and inflammation in a Dermatophagoides farinae-induced NC/Nga mouse model. Am J Physiol Lung Cell Mol Physiol. 2010; 299(1):L17–24. [DOI] [PubMed]
- 30.Meurs H, McKay S, Maarsingh H, Hamer MA, Macic L, Molendijk N, Zaagsma J. Increased arginase activity underlies allergen-induced deficiency of cNOS-derived nitric oxide and airway hyperresponsiveness. Br J Pharmacol. 2002;136(3):391–398. doi: 10.1038/sj.bjp.0704725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arikan-Ayyildiz Z, Karaman M, Tuncel T, Kiray M, Bagriyanik A, Yilmaz O, Uzuner N, Karaman O. Beneficial effects of arginase inhibition and inhaled L-arginine administration on airway histology in a murine model of chronic asthma. Allergol Immunopathol (Madr) 2014;42(4):316–323. doi: 10.1016/j.aller.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 32.Zhang R, Kubo M, Murakami I, Setiawan H, Takemoto K, Inoue K, Fujikura Y, Ogino K. L-Arginine administration attenuates airway inflammation by altering l-arginine metabolism in an NC/Nga mouse model of asthma. J Clin Biochem Nutr. 2015;56(3):201–207. doi: 10.3164/jcbn.14-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cloots RH, Sankaranarayanan S, de Theije CC, Poynter ME, Terwindt E, van Dijk P, Hakvoort TB, Lamers WH, Kohler SE. Ablation of Arg1 in hematopoietic cells improves respiratory function of lung parenchyma, but not that of larger airways or inflammation in asthmatic mice. Am J Physiol Lung Cell Mol Physiol. 2013;305(5):L364–L376. doi: 10.1152/ajplung.00341.2012. [DOI] [PubMed] [Google Scholar]
- 34.Barron L, Smith AM, El Kasmi KC, Qualls JE, Huang X, Cheever A, Borthwick LA, Wilson MS, Murray PJ, Wynn TA. Role of arginase 1 from myeloid cells in th2-dominated lung inflammation. PLoS One. 2013;8(4):e61961. doi: 10.1371/journal.pone.0061961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8(4):265–277. doi: 10.1023/A:1008942828960. [DOI] [PubMed] [Google Scholar]
- 36.Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev Biol. 2001;230(2):230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- 37.Ruijter JM, Thygesen HH, Schoneveld OJ, Das AT, Berkhout B, Lamers WH. Factor correction as a tool to eliminate between-session variation in replicate experiments: application to molecular biology and retrovirology. Retrovirology. 2006;3:2. doi: 10.1186/1742-4690-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schlaeger TM, Mikkola HK, Gekas C, Helgadottir HB, Orkin SH. Tie2Cre-mediated gene ablation defines the stem-cell leukemia gene (SCL/tal1)-dependent window during hematopoietic stem-cell development. Blood. 2005;105(10):3871–3874. doi: 10.1182/blood-2004-11-4467. [DOI] [PubMed] [Google Scholar]
- 39.Koni PA, Joshi SK, Temann UA, Olson D, Burkly L, Flavell RA. Conditional vascular cell adhesion molecule 1 deletion in mice: impaired lymphocyte migration to bone marrow. J Exp Med. 2001;193(6):741–754. doi: 10.1084/jem.193.6.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamada K, Sasaki T, Koni PA, Natsui M, Kishimoto H, Sasaki J, Yajima N, Horie Y, Hasegawa G, Naito M, et al. The PTEN/PI3K pathway governs normal vascular development and tumor angiogenesis. Genes Dev. 2005;19(17):2054–2065. doi: 10.1101/gad.1308805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van den Bossche J, Lamers WH, Koehler ES, Geuns JM, Alhonen L, Uimari A, Pirnes-Karhu S, Van Overmeire E, Morias Y, Brys L, et al. Pivotal advance: Arginase-1-independent polyamine production stimulates the expression of IL-4-induced alternatively activated macrophage markers while inhibiting LPS-induced expression of inflammatory genes. J Leukoc Biol. 2012;91(5):685–699. doi: 10.1189/jlb.0911453. [DOI] [PubMed] [Google Scholar]
- 42.Lee J, Ryu H, Ferrante RJ, Morris SM, Jr, Ratan RR. Translational control of inducible nitric oxide synthase expression by arginine can explain the arginine paradox. Proc Natl Acad Sci U S A. 2003;100(8):4843–4848. doi: 10.1073/pnas.0735876100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choi S, Park C, Ahn M, Lee JH, Shin T. Immunohistochemical study of arginase 1 and 2 in various tissues of rats. Acta Histochem. 2012;114(5):487–494. doi: 10.1016/j.acthis.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 44.Hochstedler CM, Leidinger MR, Maher-Sturm MT, Gibson-Corley KN, Meyerholz DK. Immunohistochemical detection of arginase-I expression in formalin-fixed lung and other tissues. J Histotechnol. 2013;36(4):128–134. doi: 10.1179/2046023613Y.0000000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zeki AA, Bratt JM, Rabowsky M, Last JA, Kenyon NJ. Simvastatin inhibits goblet cell hyperplasia and lung arginase in a mouse model of allergic asthma: a novel treatment for airway remodeling? Transl Res. 2010;156(6):335–349. doi: 10.1016/j.trsl.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.North ML, Amatullah H, Khanna N, Urch B, Grasemann H, Silverman F, Scott JA. Augmentation of arginase 1 expression by exposure to air pollution exacerbates the airways hyperresponsiveness in murine models of asthma. Respir Res. 2011;12:19. doi: 10.1186/1465-9921-12-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu W, Ghosh S, Comhair SA, Asosingh K, Janocha AJ, Mavrakis DA, Bennett CD, Gruca LL, Graham BB, Queisser KA, et al. Increased mitochondrial arginine metabolism supports bioenergetics in asthma. J Clin Invest. 2016;126(7):2465–2481. doi: 10.1172/JCI82925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gordy C, Pua H, Sempowski GD, He YW. Regulation of steady-state neutrophil homeostasis by macrophages. Blood. 2011;117(2):618–629. doi: 10.1182/blood-2010-01-265959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jakubzick C, Bogunovic M, Bonito AJ, Kuan EL, Merad M, Randolph GJ. Lymph-migrating, tissue-derived dendritic cells are minor constituents within steady-state lymph nodes. J Exp Med. 2008;205(12):2839–2850. doi: 10.1084/jem.20081430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Z, Zhang F, An P, Guo X, Shen Y, Tao Y, Wu Q, Zhang Y, Yu Y, Ning B, et al. Ferroportin1 deficiency in mouse macrophages impairs iron homeostasis and inflammatory responses. Blood. 2011;118(7):1912–1922. doi: 10.1182/blood-2011-01-330324. [DOI] [PubMed] [Google Scholar]
- 51.Bates JH, Rincon M, Irvin CG. Animal models of asthma. Am J Physiol Lung Cell Mol Physiol. 2009;297(3):L401–L410. doi: 10.1152/ajplung.00027.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lundblad LK, Rinaldi LM, Poynter ME, Riesenfeld EP, Wu M, Aimi S, Barone LM, Bates JH, Irvin CG. Detrimental effects of albuterol on airway responsiveness requires airway inflammation and is independent of beta-receptor affinity in murine models of asthma. Respir Res. 2011;12:27. doi: 10.1186/1465-9921-12-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Niese KA, Collier AR, Hajek AR, Cederbaum SD, O'Brien WE, Wills-Karp M, Rothenberg ME, Zimmermann N. Bone marrow cell derived arginase I is the major source of allergen-induced lung arginase but is not required for airway hyperresponsiveness, remodeling and lung inflammatory responses in mice. BMC Immunol. 2009;10:33. doi: 10.1186/1471-2172-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xiong Y, Karupiah G, Hogan SP, Foster PS, Ramsay AJ. Inhibition of allergic airway inflammation in mice lacking nitric oxide synthase 2. J Immunol. 1999;162(1):445–452. [PubMed] [Google Scholar]
- 55.Boer J, Duyvendak M, Schuurman FE, Pouw FM, Zaagsma J, Meurs H. Role of L-arginine in the deficiency of nitric oxide and airway hyperreactivity after the allergen-induced early asthmatic reaction in guinea-pigs. Br J Pharmacol. 1999;128(5):1114–1120. doi: 10.1038/sj.bjp.0702882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scott JA, North ML, Rafii M, Huang H, Pencharz P, Grasemann H. Plasma arginine metabolites reflect airway dysfunction in a murine model of allergic airway inflammation. J Appl Physiol (1985) 2015;118(10):1229–1233. doi: 10.1152/japplphysiol.00865.2014. [DOI] [PubMed] [Google Scholar]
- 57.Wijnands KA, Vink H, Briede JJ, van Faassen EE, Lamers WH, Buurman WA, Poeze M. Citrulline a more suitable substrate than arginine to restore NO production and the microcirculation during endotoxemia. PLoS One. 2012;7(5):e37439. doi: 10.1371/journal.pone.0037439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rattenbacher B, Bohjanen PR. Evaluating posttranscriptional regulation of cytokine genes. Methods Mol Biol. 2012;820:71–89. doi: 10.1007/978-1-61779-439-1_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marion V, Sankaranarayanan S, de Theije C, van Dijk P, Lindsey P, Lamers MC, Harding HP, Ron D, Lamers WH, Kohler SE. Arginine deficiency causes runting in the suckling period by selectively activating the stress kinase GCN2. J Biol Chem. 2011;286(11):8866–8874. doi: 10.1074/jbc.M110.216119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Postma DS. Gender differences in asthma development and progression. Gend Med. 2007;4 Suppl B:S133–S146. doi: 10.1016/S1550-8579(07)80054-4. [DOI] [PubMed] [Google Scholar]
- 61.Hayashi T, Adachi Y, Hasegawa K, Morimoto M. Less sensitivity for late airway inflammation in males than females in BALB/c mice. Scand J Immunol. 2003;57(6):562–567. doi: 10.1046/j.1365-3083.2003.01269.x. [DOI] [PubMed] [Google Scholar]
- 62.Takeda K, Haczku A, Lee JJ, Irvin CG, Gelfand EW. Strain dependence of airway hyperresponsiveness reflects differences in eosinophil localization in the lung. Am J Physiol Lung Cell Mol Physiol. 2001;281(2):L394–L402. doi: 10.1152/ajplung.2001.281.2.L394. [DOI] [PubMed] [Google Scholar]
- 63.Gueders MM, Paulissen G, Crahay C, Quesada-Calvo F, Hacha J, Van Hove C, Tournoy K, Louis R, Foidart JM, Noel A, et al. Mouse models of asthma: a comparison between C57BL/6 and BALB/c strains regarding bronchial responsiveness, inflammation, and cytokine production. Inflamm Res. 2009;58(12):845–854. doi: 10.1007/s00011-009-0054-2. [DOI] [PubMed] [Google Scholar]
- 64.Ahmad T, Mabalirajan U, Ghosh B, Agrawal A. Altered asymmetric dimethyl arginine metabolism in allergically inflamed mouse lungs. Am J Respir Cell Mol Biol. 2010;42(1):3–8. doi: 10.1165/rcmb.2009-0137RC. [DOI] [PubMed] [Google Scholar]
- 65.Routley CE, Ashcroft GS. Effect of estrogen and progesterone on macrophage activation during wound healing. Wound Repair Regen. 2009;17(1):42–50. doi: 10.1111/j.1524-475X.2008.00440.x. [DOI] [PubMed] [Google Scholar]
- 66.Frisancho-Kiss S, Coronado MJ, Frisancho JA, Lau VM, Rose NR, Klein SL, Fairweather D. Gonadectomy of male BALB/c mice increases Tim-3(+) alternatively activated M2 macrophages, Tim-3(+) T cells, Th2 cells and Treg in the heart during acute coxsackievirus-induced myocarditis. Brain Behav Immun. 2009;23(5):649–657. doi: 10.1016/j.bbi.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Primers pairs used in genotyping and quantitative PCR. Table S2. Amino-acid concentrations in venous plasma (μM ± SEM) of Arg1-Con and Arg1-KOTie2Cre mice *: P < 0.05 OVA/OVA vs. PBS/OVA Arg1-Con; #: P < 0.05 OVA/OVA vs. PBS/OVA Arg1-KOTie2. Table S3. Differences between male and female Arg1-Con mice after treatment with the OVA/OVA protocol. (DOCX 24 kb)
Correlation of lung-function, abundance of pulmonary mRNAs and proteins, pulmonary histopathology, and concentrations of plasma amino acids in wild-type male and female mice. The lower-left triangle refers to data obtained female and the upper-right triangle to data from male Arg1-Con mice. The numbers show the correlation coefficients between parameters indicated above and to the left of the columns and rows, respectively, as measured in all 15 or 16 male and female Arg1-Con mice studied, that is, both the PBS/OVA- and the OVA/OVA-treated groups. The significance of the correlations is color-coded according to the P-value of the correlation coefficient: yellow: 0.05 > P > 0.01, orange: 0.01 > P > 0.001, and red: P < 0.001. Note the difference in the degree of cross-correlations of parameters between the categories named in the title in males and females. (PDF 219 kb)
Data Availability Statement
All data generated or analysed during this study are included in this published article [and its Additional files].


