Abstract
Aims:
The aim of the study was to check the antimicrobial activity of the 5% Sodium hypochlorite, 2% Chlorhexidine, 0.10% Octenidine (OCT), and 2% Silver Zeolite (SZ) at different time intervals against a single species biofilm of Enterococcus faecalis, Staphylococcus aureus, and Candida albicans model prepared on a nitrocellulose membrane.
Settings and Design:
In vitro nitrocellulose biofilm model was used to check antibacterial efficacy of root canal irrigants.
Materials and Methods:
The in vitro nitrocellulose biofilm model was used to check the antibacterial activity of root canal irrigants. Single species biofilms were suspended into 96-well microtiter plate and treated with root canal irrigants for 1, 5, 10, 15, 30, and 60 s, respectively. The remaining microbial load in the form of colony-forming unit/ml after antimicrobial treatment was tabulated and data were statistically analyzed.
Statistical Analysis:
SPSS version 17, Kruskal–Wallis ANOVA, Mann–Whitney U-test, and Wilcoxon matched pair test (P < 0.05) were used.
Results:
All tested microorganisms were eliminated within 30 s by all the antimicrobial substances tested except normal saline. 2% chlorhexidine and 0.10% OCT were equally effective against C. albicans at 30 s.
Conclusion:
The newly tested irrigants have shown considerable antibacterial activity against selected single species biofilm. OCT (0.10%) can be used as an alternative endodontic irrigant.
Keywords: Candida albicans, contact test, Enterococcus faecalis, root canal irrigant, Staphylococcus aureus, single species biofilm
INTRODUCTION
Biofilm-forming microorganisms such as Enterococcus faecalis, Streptococcus mutans, and Candida albicans are the primary etiological agents responsible for the development of necrotic pulp, periapical pathosis, and post treatment disease following the root canal treatment.[1] They usually produce an exopolymeric matrix which is attached to the surface and commonly known as a sessile biofilm.[2] The increasing resistance of biofilm-forming bacteria against different antimicrobial compounds is a matter of concern.[3] As these microorganisms responsible for biofilm-associated periapical infection cannot be eliminated by host defense mechanism, it is necessary to treat them by chemical and mechanical debridement procedures which include irrigation of root canal with chemical agents.[4]
Sodium hypochlorite (NaOCl, 5.25%) and chlorhexidine (CHX, 2%) are the most studied root canal irrigants in endodontics.[3] CHX in liquid form (0.2%–2%) and NaOCl (0.5%–5.25%) were found to be the most effective irrigants against Gram-positive and negative oral pathogens including yeasts.[5] Siqueira et al. and Ayhan et al. found that higher concentration of NaOCl (5.25%) was more effective than CHX (1%–2%) in irradicating E. faecalis.[6,7]
Octenidine hydrochloride (OCT 0.10%) is another bispyridine derivative mouth rinse which was found to have profound effect in removal of plaque and prevention of gingivitis development. OCT has also been suggested as an alternative endodontic irrigant due to its low cytotoxicity and better-sustained effect against the bacterial adhesive capacity in comparison to CHX.[8] An in vitro study has proved that octenidine hydrochloride was superior to NaOCl against E. faecalis, thereby revealing a good bactericidal property.[9]
Silver zeolites (SZ) are mostly microporous, crystalline solids with well-defined structures containing aluminum, silicon, and oxygen in their regular framework. SZ (2%) added to mineral trioxide aggregate (MTA) enhances the antimicrobial activity of MTA which is used as a repair material.[10] However, the antibacterial efficacy of SZ (2%) as a root canal irrigant has not been reported.
The biofilm-forming microorganisms grow slowly in their exopolymeric matrix as compared to planktonic cells; as a result, they are more resistant to antimicrobial agents.[8] Few studies have evaluated the efficacy of endodontic irrigants against microorganisms grown in biofilm.[1,3,4] The present study was undertaken to compare the antibacterial efficacy of contemporary irrigants currently used in root canal treatment on a bacterial biofilm model at different time intervals to simulate the intraoral conditions. The hypothesis of the study was that antimicrobial activity of various irrigants on biofilm-forming microorganisms depends on the contact time.
MATERIALS AND METHODS
Test solutions
The root canal irrigants used in the study were obtained from Vishal Dentocare private limited, Ahmedabad, Gujarat, India (5.25% NaOCl), Xenon Biomed, Kolkata, India (2% CHX), Mahesh pharmaceutical's, Gujarat, India (0.10% OCT), Sigma-Aldrich, St. Louis, USA (2% SZ), and Mark Biosciences, Goa, India (0.9% normal saline [NS]).
Microbial strains
The standard microbial strains of E. faecalis (ATCC 29212), Staphylococcus aureus (ATCC 25923), and C. albicans (ATCC 3736) were obtained from microbial type culture collection, Institute of Microbial Technology, Chandigarh, India.
Preparation of biofilm
The strains of E. faecalis (ATCC 29212) and S. aureus (ATCC 25923) were subcultured in brain heart infusion (BHI) agar (HiMedia Laboratories® Mumbai, India) aerobically in the incubator at 37°C for 24 h and C. albicans (ATCC 10231D-5) were subcultured in BHI agar aerobically at 37°C for 48–72 h. Fifteen microcentrifuge tubes (Eppendorf, India) were taken in three sets (each set containing five tubes). One milliliter of BHI broth was suspended into each tube. Single colony of E. faecalis was taken from the pure culture and inoculated into first five tubes containing BHI broth. Similar way S. aureus and C. albicans were also inoculated in the remaining ten tubes. The suspension was then adjusted to 0.5 McFarland standards. Five pieces of cellulose nitrate membrane (0.2 lm pore size, 13 mm diameter – Whatman International Ltd., Maidstone, UK) were then kept inside each tube. All the 15 tubes were kept in an incubator (Labotech Bacteriological Incubator, B.D Instrumentation, India) at 37°C for 3 weeks (Sena et al.). The media were discarded, and the membranes were supplemented with fresh media at least twice a week to prevent contamination. The development of single species biofilm of E. faecalis, S. aureus, and C. albicans was observed under inverted microscope, and Gram-staining was done to check microbial viability.
Contact test
Five wells in a 96-welled microtiter plate (Nest, biotech co limited) were filled with 200 μl of 5.25% NaOCl. Similarly, 200 μl of 2% CHX, 0.10% OCT, and 2% SZ and NS were added to the respective wells. The membranes containing E. faecalis biofilm were placed aseptically in 96 well microtiter plate (each membrane in 25 wells). The plate was vertically divided into five sections and incubated at five different time intervals (1 s, 5 s, 10 s, 30 s, and 60 s) to check the action of each reagent.[9]
Five sets of 25 microcentrifuge tubes were taken, in which BHI broth and neutralizers (sodium thiosulphate and Tween 80) were added. The membranes after treating with root canal irrigants were transferred to microcentrifuge tubes containing broth and neutralizer according to the different time intervals. The tubes were vortexed, 10 μl of the broth was inoculated on the BHI agar plate which is incubated at 37°C for 24 h, and colonies were counted. The turbidity was checked spectrophotometrically at 492 nm wavelength by transferring 200 μl broth in the microtiter plate. The same procedure was repeated for single species biofilm of S. aureus and C. albicans.
RESULTS
Effect of each test reagent on biofilm was evaluated by calculating the percentage kill of viable bacteria. The action of irrigants at different time interval was analyzed by Kruskal–Wallis ANOVA, keeping time period as fixed factor while percentage kill as a dependent variable. Intergroup pair-wise comparison was done using Mann–Whitney U-test and Wilcoxon matched pair test when necessary at significance level of P < 0.05.
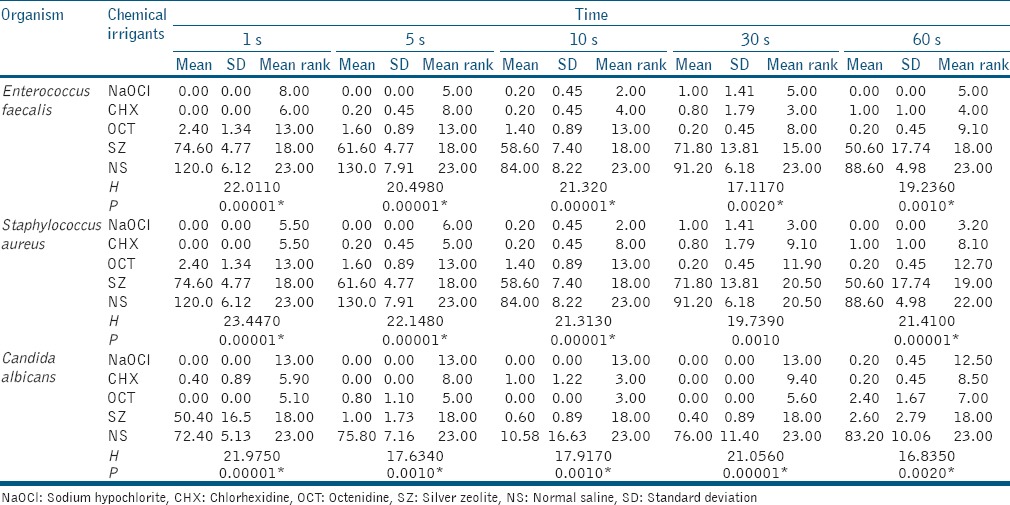
The above table shows antibacterial activity of test reagents against viable bacteria and fungi at different time intervals. Kruskal–Wallis ANOVA results revealed a significant relationship between test agent and percentage of bactericidal and fungicidal activity (P < 0.05). The results demonstrated that there was a significant difference in the percentage of bactericidal activity of E. faecalis, S. aureus, and C. albicans among the tested solutions [Table 1] at different intervals. All tested microorganisms were eliminated within 30 s by all the antimicrobial substances tested except NS. NaOCl (5.25%) was found to eliminate 100% of E. faecalis and S. aureus microorganisms in 10 s, but it has shown action against C. albicans at 60 s (100% kill of microorganism). CHX (2%) has shown 100% bactericidal activity against E. faecalis, S. aureus, and C. albicans at 1 s. No statistically significant difference existed between CHX (2%) and NaOCl (5.25%) to eliminate E. faecalis and S. aureus. CHX (2%) was found to be more effective against C. albicans at all the given intervals of time as compared to NaOCl (5.25%), OCT (0.10%), and SZ (2%). OCT (0.10%) eliminated 93.8% E. faecalis biofilm and 91.7% of S. aureus biofilm at 30 s interval whereas 100% C. albicans biofilm was eliminated at 10 s. CHX (2%) has killed more number of E. faecalis as compared to OCT (0.10%), but statistically significant difference exists at 5 s and 60 s interval. 2% CHX and 0.10% OCT are equally effective at 30 s interval against C. albicans. SZ (2%) showed least antibacterial and antifungal activity. Statistically significant difference existed between SZ (2%) and NaOCl (5.25%), CHX (2%), and OCT (0.10%) at all given interval of the time. However, it was statistically different from control group saline (P < 0.05).
Table 1.
Comparison of mean, standard deviation and mean rank of five test reagents with respect to colony-forming unit of Enterococcus faecalis, Staphylococcus aureus, and Candida albicans at different time intervals by Kruskal-Wallis ANOVA
DISCUSSION
Infections in the root canals are of polymicrobial nature and microorganisms such as E. faecalis, Streptococcus mutans, and C. albicans were generally detected in such cases.[9] Bacterial elimination from the root canal is usually achieved by means of the mechanical action of the instruments along with flushing and antibacterial activity of irrigants.
The use of planktonic culture to study the antimicrobial efficacy of endodontic irrigants was observed in various studies.[10,11] The cells grown in the biofilm culture show uneven development among all species of microorganisms as compared to cells grown in planktonic cultures. Bacterial cells in biofilm adhere to dentinal wall with their exopolymeric matrix which is difficult to remove by root canal irrigants.[12] Therefore, to mimic in vivo conditions as closely as possible, the biofilm model was used in the present study.[5]
The microorganisms selected in our study were found to be the most common pathogens in the oral cavity that forms single species biofilms. The basic methodologies applied in the present study were similar to the work of Sena et al. with little modification. The liquid cultures used to inoculate nitrocellulose membrane were adjusted to standard optical density and resuspended in fresh medium.[1] The contact time between biofilm and irrigant in our experiment was typically 1 min (as opposed to 15 or 60 min) to allow a comparison to be made to in vivo conditions. Although the pore sizes of filter membranes (0.2–0.45 μm) used in the study were unlikely to affect the results, as these pore diameters will neither limit the diffusion of nutrients and penetration of irrigant nor movement of bacteria from the agar to biofilm.[3]
The results of the present study indicated that the data were nonparametric due to high standard deviation in the contact test and number of colony-forming unit. Thus, rank transformation was indicated in present tables. NaOCl (5.25%) showed bactericidal effect against E. faecalis, S. aureus, and C. albicans biofilm with an antibacterial efficacy against E. faecalis at 10 s and C. albicans at 60 s. It was earlier reported that 5.25% NaOCl exerts antimicrobial action within 30 s on E. faecalis grown in planktonic suspension and biofilm model.[13] NaOCl (5.25%) with or without mechanical agitation eliminated E. faecalis in 30 s.[1] According to Yap et al., NaOCl (0.9%) is highly effective against E. faecalis grown alone and as a part of multispecies biofilm consisting of Fusobacterium nucleatum and Streptococcus sanguinis.[14]
The bactericidal effect of CHX (2%) on E. faecalis, S. aureus, and C. albicans biofilm was found in 1 s. No significant difference was observed between antibacterial activity of CHX (2%) and NaOCl (5.25%) on all the species of biofilm-forming organisms. According to Sena et al., CHX (2%) in liquid formulation with mechanical agitation has eliminated all the microorganisms in 30 s and without mechanical agitation in 60 min.[1] Vianna et al. evaluated the contact time required for CHX and NaOCl to produce a negative culture where CHX (2%) eliminated all anaerobic microorganisms in 15 s. The action of CHX (2%) in biofilm dissolution by direct contact test is lower than NaOCl on the dentin block model. The reason for different result could be attributed to different study designs.[15] A recent study stated that atmospheric pressure of nonequilibrium plasma treatment (5 min) and CHX (2%) were found to be equally effective in inactivating E. faecalis grown in biofilm.[16]
OCT (0.10%) exerted its antimicrobial activity against E. faecalis and S. aureus at 30 s and C. albicans at 10 s. OCT (0.10%) showed equally good results as CHX (2%) against C. albicans but its bactericidal activity against E. faecalis and S. aureus was less as compared to NaOCl (5.25%). The results of the present study were not in accordance with Tirali et al. where it was found that OCT dihydrochloride was as effective as NaOCl on E. faecalis and C. albicans. This variable results could be due to the agar diffusion test model which had been used in the study.[8] The antibacterial activity of OCT (0.10%) is attributed to its action on binding to cell wall and membranes causing cell lysis.[17]
The antimicrobial activity of SZ (2%) was found to be less effective in comparison to NaOCl, CHX, and OCT against all the tested microorganisms. The bactericidal action of SZ (2% SZ) could be attributed to either the release of silver from zeolite or the reactivation of oxygen generated from silver in the matrix.[18] SZ is an aluminum silicate crystalline structure present in the void spaces within the frame work of 3–10 angstroms that are capable of hosting cations, water, or organic molecules. According to Odabas et al., mineral trioxide aggregate (MTA) with 2% and 0.2% SZ showed inhibitory effects on some microorganisms at all time periods, whereas no antimicrobial activity was observed for Prevotella intermedia and Actinomyces israelii. MTA without SZ was found to be inhibiting C. albicans, Escherichia coli, and P. intermedia.[10] The reason for reduced antibacterial activity of SZ could be due to the presence of certain organic substances in the biofilm model.[18] In the present study, SZ is used as an irrigant which disrupts the frame of set matrix which is dissolved in aqueous solution reducing its antibacterial efficacy.
Within the limitation of this in vitro study, OCT (0.10%) has shown antimicrobial activity equivalent to 2% CHX against biofilm of C. albicans at 10 s. SZ (2%) used in the study against biofilm of E. faecalis, S. aureus, and C. albicans was proved to be least effective. Taking in an account of different methodological procedures, different forms of antimicrobial agents, use of mechanical agitation, and different presentation of microorganisms were the modulating factors that could affect the accurate comparison of inherent antibacterial activity. Future research using other species and multispecies biofilm grown in complex root canal system under strictly controlled clinical conditions should be carried out to support these findings.
CONCLUSION
The results of the present study demonstrated that NaOCl (5.25%) is the most effective endodontic irrigant against biofilm forming microorganisms. CHX (2%) and OCT (0.10%) are equally effective against C. albicans at 30 seconds time interval. However, SZ (2%) showed least antibacterial activity against all tested microorganisms. Results of the present study indicated that OCT (0.10%) solution can also be used as an endodontic irrigant.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Sena NT, Gomes BP, Vianna ME, Berber VB, Zaia AA, Ferraz CC, et al. In vitro antimicrobial activity of sodium hypochlorite and chlorhexidine against selected single-species biofilms. Int Endod J. 2006;39:878–85. doi: 10.1111/j.1365-2591.2006.01161.x. [DOI] [PubMed] [Google Scholar]
- 2.Giardino L, Ambu E, Savoldi E, Rimondini R, Cassanelli C, Debbia EA. Comparative evaluation of antimicrobial efficacy of sodium hypochlorite, MTAD, and Tetraclean against Enterococcus faecalis biofilm. J Endod. 2007;33:852–5. doi: 10.1016/j.joen.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 3.Hope CK, Garton SG, Wang Q, Burnside G, Farrelly PJ. A direct comparison between extracted tooth and filter-membrane biofilm models of endodontic irrigation using Enterococcus faecalis. Arch Microbiol. 2010;192:775–81. doi: 10.1007/s00203-010-0604-6. [DOI] [PubMed] [Google Scholar]
- 4.Chai WL, Hamimah H, Abdullha M. Evaluation of antibacterial efficacy of antibiotics and calcium hydroxide against Enterococcus faecalis biofilm in dentin. Sains Malasiana. 2013;42:73–80. [Google Scholar]
- 5.Luddin N, Ahmed HM. The antibacterial activity of sodium hypochlorite and chlorhexidine against Enterococcus faecalis: A review on agar diffusion and direct contact methods. J Conserv Dent. 2013;16:9–16. doi: 10.4103/0972-0707.105291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siqueira JF, Jr, Batista MM, Fraga RC, de Uzeda M. Antibacterial effects of endodontic irrigants on black-pigmented gram-negative anaerobes and facultative bacteria. J Endod. 1998;24:414–6. doi: 10.1016/S0099-2399(98)80023-X. [DOI] [PubMed] [Google Scholar]
- 7.Ayhan H, Sultan N, Cirak M, Ruhi MZ, Bodur H. Antimicrobial effects of various endodontic irrigants on selected microorganisms. Int Endod J. 1999;32:99–102. doi: 10.1046/j.1365-2591.1999.00196.x. [DOI] [PubMed] [Google Scholar]
- 8.Tirali RE, Bodur H, Sipahi B, Sungurtekin E. Evaluation of the antimicrobial activities of chlorhexidine gluconate, sodium hypochlorite and octenidine hydrochloride in vitro. Aust Endod J. 2013;39:15–8. doi: 10.1111/j.1747-4477.2010.00266.x. [DOI] [PubMed] [Google Scholar]
- 9.Anuradha B, Rajamoni I, Lalitha MK, Sriram T. A new irrigant against E. faecalis in the root canal disinfection. Biosci Biotech Res Asia. 2014;11:121–7. [Google Scholar]
- 10.Odabas ME, Cinar C, Akça G, Araz I, Ulusu T, Yücel H. Short-term antimicrobial properties of mineral trioxide aggregate with incorporated silver-zeolite. Dent Traumatol. 2011;27:189–94. doi: 10.1111/j.1600-9657.2011.00986.x. [DOI] [PubMed] [Google Scholar]
- 11.Zehnder M. Root canal irrigants. J Endod. 2006;32:389–98. doi: 10.1016/j.joen.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 12.Dunavant TR, Regan JD, Glickman GN, Solomon ES, Honeyman AL. Comparative evaluation of endodontic irrigants against Enterococcus faecalis biofilms. J Endod. 2006;32:527–31. doi: 10.1016/j.joen.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Gomes BP, Ferraz CC, Vianna ME, Berber VB, Teixeira FB, Souza-Filho FJ. In vitro antimicrobial activity of several concentrations of sodium hypochlorite and chlorhexidine gluconate in the elimination of Enterococcus faecalis. Int Endod J. 2001;34:424–8. doi: 10.1046/j.1365-2591.2001.00410.x. [DOI] [PubMed] [Google Scholar]
- 14.Yap B, Zilm PS, Briggs N, Rogers AH, Cathro PC. The effect of sodium hypochlorite on Enterococcus faecalis when grown on dentine as a single- and multi-species biofilm. Aust Endod J. 2014;40:101–10. doi: 10.1111/aej.12073. [DOI] [PubMed] [Google Scholar]
- 15.Del Carpio-Perochena AE, Bramante CM, Duarte MA, Cavenago BC, Villas-Boas MH, Graeff MS, et al. Biofilm dissolution and cleaning ability of different irrigant solutions on intraorally infected dentin. J Endod. 2011;37:1134–8. doi: 10.1016/j.joen.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 16.Du T, Ma J, Yang P, Xiong Z, Lu X, Cao Y. Evaluation of antibacterial effects by atmospheric pressure nonequilibrium plasmas against Enterococcus faecalis biofilms in vitro. J Endod. 2012;38:545–9. doi: 10.1016/j.joen.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 17.Tandjung L, Waltimo T, Hauser I, Heide P, Decker EM, Weiger R. Octenidine in root canal and dentine disinfection ex vivo. Int Endod J. 2007;40:845–51. doi: 10.1111/j.1365-2591.2007.01279.x. [DOI] [PubMed] [Google Scholar]
- 18.Matsumura Y, Yoshikata K, Kunisaki S, Tsuchido T. Mode of bactericidal action of silver zeolite and its comparison with that of silver nitrate. Appl Environ Microbiol. 2003;69:4278–81. doi: 10.1128/AEM.69.7.4278-4281.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


