Summary
The safety of induced pluripotent stem cells (iPSCs) in autologous recipients has been questioned after iPSCs, but not embryonic stem cells (ESCs), were reported to be rejected in syngeneic mice. This important topic has remained controversial because there has not been a mechanistic explanation for this phenomenon. Here, we hypothesize that iPSCs, but not ESCs, readily differentiate into gamete‐forming cells that express meiotic antigens normally found in immune‐privileged gonads. Because peripheral blood T cells are not tolerized to these antigens in the thymus, gamete‐associated‐proteins (GAPs) sensitize T cells leading to rejection. Here, we provide evidence that GAPs expressed in iPSC teratomas, but not in ESC teratomas, are responsible for the immunological rejection of iPSCs. Furthermore, silencing the expression of Stra8, ‘the master regulator of meiosis’, in iPSCs, using short hairpin RNA led to significant abrogation of the rejection of iPSCs, supporting our central hypothesis that GAPs expressed after initiation of meiosis in iPSCs were responsible for rejection. In contrast to iPSCs, iPSC‐derivatives, such as haematopoietic progenitor cells, are able to engraft long‐term into syngeneic recipients because they no longer express GAPs. Our findings, for the first time, provide a unifying explanation of why iPSCs, but not ESCs, are rejected in syngeneic recipients, ending the current controversy on the safety of iPSCs and their derivatives.
Keywords: CD4+ T cells, gamete‐associated proteins, rejection of induced pluripotent stem cells
Abbreviation
- EB
embryoid body
- ESCs
embryonic stem cells
- ES‐EB
ES cell‐derived embryoid body
- GAPs
gamete‐associated proteins
- HPC
haematopoietic progenitor cells
- iPSC‐EB
iPS cell‐derived embryoid body
- iPSCs
induced pluripotent stem cells
- RA
retinoic acid
- TT
testosterone
Introduction
Induced pluripotent stem cells (iPSCs) are derived from somatic cells through ectopic expression of reprogramming factors, and appear to be very similar to embryonic stem cells (ESCs), which holds great promise for regenerative medicine.1, 2, 3 Recent data on the immunogenicity of iPSCs have deepened the controversy regarding the utility of iPSCs and failed to unequivocally answer the question of why iPSCs, but not ESCs, are rejected in syngeneic mice. The original article by Zhao et al. identified meiotic and cancer genes in iPSC teratomas but not in ESC teratomas.4, 5 The Zhao group reported that two genes (Hormad1 and Zg16) were abnormally expressed in iPS teratomas and resulted in rejection of iPSCs in syngeneic mice through T‐cell activation.4 In contrast, two other groups found negligible or complete lack of immunogenicity in iPSC derivatives.6, 7 Since iPSCs are derived from syngeneic mice and are transplanted back to the donor of the somatic cells from which the iPSCs are derived, allogeneic immunological rejection should not be of concern. However, preliminary studies in our laboratory indicated that iPSCs were capable of forming teratomas in immunodeficient mice but not in syngeneic immunocompetent mice. To explain these findings, we reasoned that iPSCs highly expressed gamete‐associated proteins (GAPs) to which peripheral T cells are not tolerized. Physiologically, GAPs are sequestered in immune‐privileged sites and are not exposed to peripheral T cells. Further, GAPs do not circulate through the thymus, avoiding thymic tolerization of T cells. Here, we hypothesized that by subcutaneously injecting iPSCs in syngeneic mice and allowing them to form teratomas that express GAPs, peripheral T cells are sensitized to GAPs leading to rejection of the iPSCs. Here, we showed that GAPs are highly expressed in iPSCs during differentiation in vitro (embryoid bodies; EBs) and in vivo (teratoma). In particular, we identified that the ‘stimulated by retinoic acid 8’ gene (Stra8), which is one of the GAPs, is highly expressed in only iPS‐EBs, but not ES‐EBs. In addition, rejection of Stra8 gene‐silenced iPSCs was delayed compared with control iPSCs in syngeneic mice. Hence, our findings suggest that reprogrammed iPSCs highly express GAPs during the differentiation into three germ layers, which sensitize T cells and initiate immune responses that lead to the rejection of iPSCs.
Materials and methods
Pluripotent stem cell lines
The 129x1/SvJ iPSC lines were kindly provided by Dr Budd Tucker, University of Iowa. The 129SvJ HM‐1 ESC line was purchased from Open Biosystems (Huntsville, AL). All cell lines were transduced with pLU‐Tet‐EF1a‐FFluc‐mCherry lentivirus (The WISTAR Institute, Philadelphia, PA). The mCherry+ cells were sorted using the BD FACS Aria II and were plated onto irradiated mouse embryonic fibroblasts (GlobalStem, Rockville, MD) and cultivated in ES medium.
Other methods are described in the Supplementary material (Data S1).
Statistical analysis
Evaluation of experimental data for significant differences was performed through the Student's t‐test, which was conducted using the prism software package (graphpad Software, San Diego, CA).
A value of P < 0·05 was considered significant for these studies.
Results
iPSCs, but not ESCs, form teratomas in syngeneic immunocompetent mice
To investigate whether iPSCs induce immune responses to syngeneic recipient mice, luciferase‐expressing 129x1/SvJ iPSCs or ESCs were injected subcutaneously into 129x1/SvJ recipient mice, respectively. Interestingly, iPSCs were rejected after a mean of 14 days (n = 6 per each group), Fig. 1(a) and Fig. S1 (see Supplementary material). To explore whether T cells cause this rejection of iPSCs, we performed a second transplantation into mice already sensitized with iPSCs or into naive mice subcutaneously. Figure 1(b) shows that rejection of iPSCs was quicker upon secondary challenge. Indeed, as opposed to the primary rejection kinetics of 7–14 days, we observed that mice challenged with iPSCs a second time rejected iPSCs in 5–6 days. In contrast, ESCs remained detectable for more than 40 days. To confirm that both ESCs and iPSCs were pluripotent, both cell types were subcutaneously transplanted in NOD‐SCID mice. They successfully formed teratomas (Fig. 1c), confirming that both cell types were indeed pluripotent.
Figure 1.
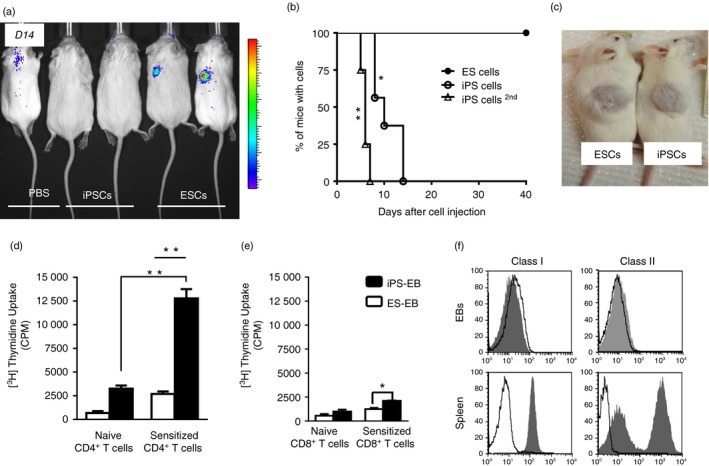
Induced pluripotent stem cells (iPSCs) are rejected by CD4+ T cells. (a) To determine whether iPSCs are rejected in syngeneic mice, luciferase‐expressing 129x1/SvJ iPS or embryonic stem cells (ESCs) were injected into 129x1/SvJ mice, n = 6. Mice were imaged regularly to determine the engraftment of the cells. iPSCs could not be detected after 14 days. (b) ESCs ( ) were not rejected in syngeneic mice over the 40 days of observation. In contrast iPSCs (
) were not rejected in syngeneic mice over the 40 days of observation. In contrast iPSCs ( ) were rejected after a mean of 12 days. Furthermore, mice challenged for a second time with iPSCs (
) were rejected after a mean of 12 days. Furthermore, mice challenged for a second time with iPSCs ( ) rejected those iPSCs within 5–6 days. For statistical analysis, the Log rank test was used. *P < 0·05, **P < 0·01. (c) To prove that both iPSCs and ESCs were pluripotent, the teratoma assay was performed in NOD‐SCID mice. In both cases, large teratomas developed. This is a representative result for the 129SvJ cells. (d) To determine the mechanism of iPSC rejection, splenocytes of mice that had rejected iPSCs were collected and CD4+ and CD8+ cells were sorted. The cells were exposed to iPS‐embryoid body (EB) cells in a proliferation assay. iPSCs, but not ESCs, stimulated CD4+ T cells derived from animals that had rejected iPSCs. In contrast, CD8+ T cells minimally proliferated to stimulation by iPS‐EB cells (e). Bothe CD4+ and CD8+ T cells from naive animals proliferated minimally. iPS‐EB cells induce T‐cell stimulation much more than ES‐EBs. These experiments were performed in triplicates in three mice and repeated twice. **P < 0·01 and *P < 0·05. (f) iPSCs are pluripotent and iPS‐EB cells poorly express MHC I and MHC II molecules. iPS‐EB cells do not express MHC antigens. EBs were harvested on day 7 and the cells were used to measure MHC class I and class II expression. 129x1/SvJ splenocytes were used as controls. EB cells hardly express any class I or class II antigens. Open histograms indicate class I or class II positive population and filled histograms indicate isotype control staining. [Colour figure can be viewed at wileyonlinelibrary.com]
) rejected those iPSCs within 5–6 days. For statistical analysis, the Log rank test was used. *P < 0·05, **P < 0·01. (c) To prove that both iPSCs and ESCs were pluripotent, the teratoma assay was performed in NOD‐SCID mice. In both cases, large teratomas developed. This is a representative result for the 129SvJ cells. (d) To determine the mechanism of iPSC rejection, splenocytes of mice that had rejected iPSCs were collected and CD4+ and CD8+ cells were sorted. The cells were exposed to iPS‐embryoid body (EB) cells in a proliferation assay. iPSCs, but not ESCs, stimulated CD4+ T cells derived from animals that had rejected iPSCs. In contrast, CD8+ T cells minimally proliferated to stimulation by iPS‐EB cells (e). Bothe CD4+ and CD8+ T cells from naive animals proliferated minimally. iPS‐EB cells induce T‐cell stimulation much more than ES‐EBs. These experiments were performed in triplicates in three mice and repeated twice. **P < 0·01 and *P < 0·05. (f) iPSCs are pluripotent and iPS‐EB cells poorly express MHC I and MHC II molecules. iPS‐EB cells do not express MHC antigens. EBs were harvested on day 7 and the cells were used to measure MHC class I and class II expression. 129x1/SvJ splenocytes were used as controls. EB cells hardly express any class I or class II antigens. Open histograms indicate class I or class II positive population and filled histograms indicate isotype control staining. [Colour figure can be viewed at wileyonlinelibrary.com]
To understand the mechanism by which iPSCs were rejected, mice that had rejected iPSCs were killed and their spleens were harvested (n = 3). CD8+ and CD4+ T cells were isolated by MACS. These T cells were used as responders to dissociated iPS‐EB or ES‐EB cells that were used as stimulator cells in a mixed lymphocyte reaction assay. Sensitized CD4+ T cells strongly responded to the iPS‐EB cells (Fig. 1d), but the response was minimal in the CD8+ T‐cell cultures (Fig. 1e). In contrast, ES‐EB cells stimulated neither CD4+ nor CD8+ T cells. These data clearly suggest that CD4+ T cells cause iPSC rejection in syngeneic mice. Furthermore, these iPS‐EB cells hardly express any MHC antigens compared with splenocytes (Fig. 1f), suggesting that the antigen on the iPS‐derived teratomas was picked up by the dendritic cells of the host and indirectly presented to T cells,8 which rejected the EBs. Furthermore, the site where SSEA‐1+ iPSCs were injected into syngeneic mice showed a higher number of infiltrating CD4+ CD3+ T cells in the area compared with the sites of syngeneic mice where SSEA‐1+ ES cells were injected (see Supplementary material, Fig. S2).
iPSC‐derived haematopoietic progenitor cells engraft long‐term in syngeneic mice
To further rule out that MHC molecules expressed by the teratomas were involved in the rejection process, we derived haematopoietic progenitor cells (HPCs) from the iPSCs (iPS‐HPCs), which were transduced with FMEV‐eGFP2A‐HA‐HOXB4 retrovirus, as previously reported.9, 10, 11 As shown in Fig. 2(a), these cells expressed Sca‐1+/c‐kit+, which are markers of HPCs. The goal was to transplant these GFP+ iPS‐HPCs into recipient mice, thereby generating ‘chimeric’ mice with syngeneic iPS‐HPCs. These ‘chimeric’ mice would tolerize the syngeneic mice to the antigens that cause rejection, so preventing rejection.12, 13, 14 To determine whether the iPS‐HPCs are not rejected in syngeneic recipients, recipient mice (n = 10) were sub‐lethally irradiated and injected with 2 × 106–3 × 106 iPS‐HPCs. Engraftment of GFP+ iPS‐HPCs was monitored by FACS in peripheral blood. These ‘chimeric’ mice were then subcutaneously injected with ESCs or iPSCs, or with both on contralateral sides, and were imaged after 21 days by bioluminescence imaging. Teratomas were visible in mice that were injected with ESCs (red circle), but not in mice injected with iPSCs (blue circle) (Fig. 2b). Interestingly, the mice injected with both iPSCs and ESCs rejected iPSCs, but not ESCs. These results demonstrated that the antigens that incited the rejection of iPSCs were not expressed by iPSC derivatives, since ‘chimerism’ (4–5%) was maintained over 9 weeks (Fig. 2c and see Supplementary material, Fig. S4). In addition, the results showed that those immunogens were not expressed on ESC teratomas, otherwise mice that received both ESCs and iPSCs would have additionally rejected the ESCs.
Figure 2.
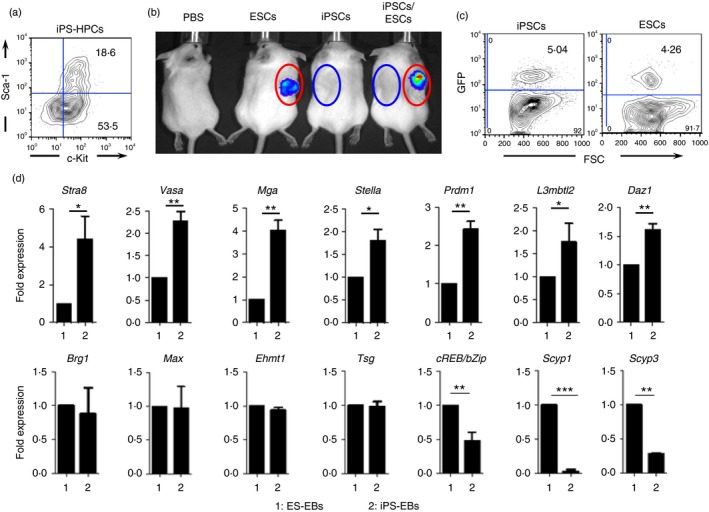
Meiotic and spermatogenetic genes expressing induced pluripotent stem cells (iPSC) embryoid bodies (EBs) are rejected in syngeneic mice. (a) To determine, whether iPSC derivatives are rejected in syngeneic mice like iPSCs, we differentiated the 129x1/SvJ iPSCs into haematopoietic progenitor cells (HPCs). These iPSC‐derived HPCs express sca‐1+/c‐kit+, which are haematopoietic progenitor markers, indicating that those iPSCs are successfully differentiated into HPCs. (b) To determine whether iPSC‐derived HPCs engraft in syngeneic mice, 129x1/SvJ mice were sublethally irradiated and transplanted with the 129x1/SvJ iPS‐HPCs. After that, these mice were subsequently injected with 129x1/SvJ iPSCs, embryonic stem cells (ESCs) or with both, subcutaneously. The luminescence emitted by the teratomas (21 days post transplantation) are shown if they are not rejected (circles denote original injection sites). Mice transplanted with luciferase‐expressing iPSCs either singly or together with luciferase‐expressing ESCs rejected iPSCs, but not ESCs (Each group of mice comprised 10 mice, and these experiments were repeated four times). However, GFP‐expressing iPS‐HPCs were detected in chimeric mice long term in the peripheral blood system (6 weeks after iPSC transplantations) (c). To identify the genes that are differently expressed, we performed quantitative PCR analysis expression between iPSCs and ESCs. (d) iPS‐EBs but not ES‐EBs strongly expresses genes involved in meiosis and spermatogenesis (top row). In particular, Stra8, Vasa and Mga were strongly expressed in iPS‐EBs compared with in ES‐EBs. The genes displayed in the lower row either remained unchanged or were down‐regulated. These quantitative PCR experiments were repeated at least four times in triplicates. ***P < 0·001, **P < 0·01 and *P < 0·05. [Colour figure can be viewed at wileyonlinelibrary.com]
iPSC teratomas highly express meiotic and spermatogenesis‐associated genes
Next, to determine the antigens that cause rejection, we ran the cell lysates of iPSC teratomas and those of ESC teratomas on two‐dimensional gels separately and performed protein analysis (see Supplementary material, Fig. S3). Interestingly, we found that the proteins associated with germ cells were up‐regulated in the gel containing iPSC teratoma lysates, suggesting that iPSCs readily form GAPs to which peripheral T cells of the host are not tolerized (see Supplementary material, Fig. S3, Table S1). To further confirm the expression of GAPs by iPS‐EBs, quantitative PCR was performed on both ES‐EBs and iPS‐EBs using primers of GAPs. The results were normalized using the results of the ES‐EBs. We found that Stra8, Vasa, Mga, Stella, Prdm1, L3mbtl2 and Daz1,15, 16 were known to regulate spermatogenesis, highly expressed in iPS‐EBs compared with ES‐EBs. Stra8 has been shown to be the critical gene for early meiosis. For example, gamete formation was completely abrogated in mice that were Stra8 −/−,17 confirming the importance of Stra8 in gamete formation.
Silencing of Stra8 significantly delays the rejection of iPSCs
Having established the expression of GAPs in iPS‐EBs, we wondered whether silencing Stra8, ‘the gatekeeper of meiosis’, abrogates the rejection of iPSCs. Although Stra8 is a major regulator of meiosis,18 its absence does not completely shut down meiosis, because it has been recently reported that meiosis may initiate in the absence of Stra8. However, spermatogenesis does not complete due to chromosome condensation in spermatocytes.17, 18 It has also been reported that ESCs can undergo meiosis under the influence of retinoic acid (RA) and testosterone (TT).19 Here, to confirm this finding, we treated ES‐EBs or iPS‐EBs with both RA and TT. Only ES‐EBs treated with RA and TT expressed Stra8. In contrast, iPS‐EBs expressed Stra8 spontaneously in EBs, as expected (Fig. 3a).
Figure 3.
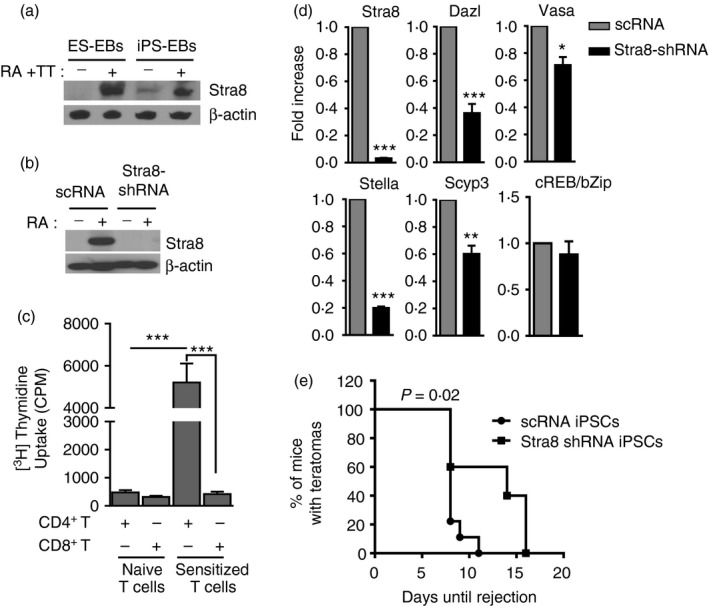
Silencing of Stra8 abrogates rejection of induced pluripotent stem cells (iPSCs). (a) Embryonic stem cells (ESCs) express Stra8 under treatment with retinoic acid (RA) and testosterone (TT). To demonstrate that ESCs are capable of undergoing meiosis, ESCs were allowed to form embryoid bodies (EBs) with or without treatment with both RA and TT. The iPSCs were treated the same way. Indeed ES‐EBs only expressed Stra8 under treatment with TT and RA but not when left untreated. In contrast iPS‐EBs expressed Stra8 without further treatment with RA and TT. However, Stra8 expression was stronger in the treated iPSCs. (b) Stra8 protein expression is silenced in Stra8‐shRNA‐iPSCs. The control iPSCs (scRNA‐iPSCs) and Stra8‐silenced iPSCs (Stra8‐shRNA‐iPSCs) were either left alone or treated with RA. scRNA‐iPSCs responded to RA treatment by expression of Stra8, whereas Stra8‐shRNA‐iPSCs do not express Stra8 as expected. (c) Sensitized CD4+ T cells with iPSCs highly proliferated to dendritic cells pulsed with Stra8 overexpressed cell lysates. In contrast, sensitized CD8+ T cells showed similar level of naive T‐cell responses (***P < 0·001). (d) Silencing of Stra8 leads to down‐regulation of the expression of several critical genes in meiosis. To determine whether meiotic gene expression is regulated by Stra8, Stra8‐shRNA‐iPS cell‐EBs and scRNA‐iPS cell‐EBs were formed and used to extract RNA for quantitative PCR. As expected, Stra8 gene expression was abrogated in the Stra8‐shRNA‐iPS cell‐EBs but not in the scRNA‐iPS cell‐EBs. In addition, Dazl, Stella, Vasa and Scyp3 were significantly down‐regulated. Ct values were first normalized within the sample to the housekeeping gene GAPDH before comparison across samples. ***P < 0·001, **P < 0·01 and *P < 0·05. (e) Rejection of iPSCs is after silencing Stra8. To determine the impact of the down‐regulation of the meiotic programme on iPS rejection, 129x1/SvJ mice were transplanted subcutaneously with either shRNA‐iPSCs (n = 10) or Stra8‐shRNA‐iPSCs (n = 10). ESCs were used as controls (n = 10). scRNA‐iPSCs were rejected in normal fashion, the mean of the rejection day was day 12. As expected, ESCs were not rejected. However, Stra8‐shRNA‐iPS cell showed delayed rejection suggesting that abrogation of meiosis delays rejection of iPSCs. The difference in the rejection mean times was highly significant, For statistical analysis, Log rank test was used (P = 0·02).
To test the impact of Stra8 on the rejection of iPSCs, we silenced Stra8 gene expression, using lentiviral‐Stra8‐shRNA. Lentiviral‐scrambled RNA (scRNA) was used as a control. Stra8 silenced iPSCs (Stra8‐shRNA‐iPSCs) and scRNA‐iPSCs were treated with RA to induce meiosis. Figure 3(b) shows that control cells highly express Stra8; however, Stra8‐shRNA‐iPSCs remained negative even after treatment of the cells with RA. These data indicate that Stra8 was successfully silenced in these cells. To investigate whether Stra8 is one of the antigens in the teratomas that can cause the rejection of iPSCs, we pulsed bone marrow‐derived dendritic cells with Stra8 overexpressing cell lysates (Stra8‐lysates) (see Supplementary material, Fig. S5) and those dendritic cells were used as stimulators for CD4+ or CD8+ T cells from sensitized mice with iPSCs. Sensitized CD4+ T cells responded strongly to dendritic cells pulsed with Stra8‐lyates, but not naive CD4+ T cells. In comparison, sensitized CD8+ T cells hardly responded, like naive CD4+ or CD8+ T cells (Fig. 3c). These data suggest that Stra8 might be one of the antigens that indirectly induce a CD4+ T‐cell response, leading to rejection of iPSCs.
To determine whether the lack of Stra8 expression interferes with the regulation of genes that are involved in meiosis and spermatogenesis, quantitative PCR was performed. Stra8 was not detected in Stra8‐shRNA‐iPS‐EBs as expected (Fig. 3d). In addition, the expression of other genes involved in meiosis and spermatogenesis, which include Dazl, Stella, Vasa and Scyp3, were significantly abrogated in Stra8‐shRNA‐iPS‐EBs (Fig. 3d). Next, we subcutaneously transplanted the Stra8‐shRNA‐iPSCs in 129x1/SvJ mice to determine whether the kinetics of iPSC rejection was altered in mice where Stra8 had been silenced. As an appropriate control, scRNA‐iPSCs were used. Mice were imaged on a regular basis using bioluminescence imaging. scRNA‐iPSCs were rejected after a mean of 12 days (n = 10), whereas Stra8‐shRNA‐iPSCs were rejected after 15 days. From 10 mice that received Stra8‐shRNA‐iPSCs, only three mice were rejected between days 5 and 10, and most mice showed delayed rejection suggesting that abrogation of meiosis delays the rejection of iPSCs (Fig. 3e). This showed that although Stra8 silencing prevented early rejection of iPSCs, it failed to completely prevent their rejection. Hence, we suggest that in the mice that rejected iPSCs, meiosis was not completely shut down despite the Stra8 silencing. In summary, we conclude that the ability of iPS‐EBs to express GAPs and not their expression of cancer‐associated proteins is the reason why iPSCs, but not ESCs, are rejected in syngeneic mice.
Discussion
Recently, there has been some controversy in the field regarding the immunogenicity of self‐derived iPSCs and what impact this may have on the immunogenicity of their derivatives, which significantly influences the application of iPSCs in clinical therapy. Zhao et al. showed that iPSCs were immunogenic in syngeneic hosts owing to the expression of meiotic and cancer genes in iPSC‐derived teratomas but not in ESC‐derived teratomas.4, 5 This was followed up with studies that suggested that iPSC‐derivatives were not immunogenic and that the unique immunogenicity of iPSCs should be of no consequence to the immunological properties of their derivatives.6, 7 However, the mechanism underlying the immunogenicity of iPSCs as opposed to ESCs, as well as an explanation of how iPSC‐derivatives lose the potential to induce immunological rejection, have not been unequivocally elucidated.
Here, we conclusively demonstrate the mechanism by which iPSCs, but not ESCs, are rejected in immunocompetent syngeneic mice. Our data demonstrate that iPSCs express high levels of GAPs after initiating meiosis, and that these GAPs sensitize CD4+ T cells, which initiate immune responses that lead to the rejection of iPSCs. In contrast, meiosis is impaired in ESCs, as has been previously demonstrated.15 GAPs are expressed in the gonads and not exposed to peripheral blood lymphocytes. Gonads are immune privileged sites, where inflammation hardly occurs because the immune system is shut out. As such, T cells are not tolerized to these antigens, because gametes do not circulate through the thymus. Stra8, which is one of the GAPs, regulates meiotic initiation in both spermatogenesis and oogenesis. Our finding that iPSCs are rejected in syngeneic mice between 7 and 14 days is consistent with the timing of primary immune responses. Importantly, our demonstration that these iPSCs form teratomas in immunodeficient mice unequivocally shows that these cells are pluripotent, eliminating concern that the iPSCs themselves are inherently incapable of forming teratomas.
Most significantly, we demonstrate here that iPSCs lose the potential to express GAPs upon their differentiation into HPCs. Indeed, mice that are ‘chimeric’ with iPS‐HPCs still reject the iPSCs, demonstrating that they do not confer tolerance toward GAPs expressed by iPSC teratomas. Overall, our data suggest that iPSCs express GAPs after initiating meiosis whereas ESCs do not. However, we also show that iPS‐HPCs do not express GAPs (see Supplementary material, Fig. S6) and engraft long‐term in syngeneic immunocompetent hosts, which reassures us that iPSC‐derived HPCs are safe when given back to the donor of the iPSCs.
Other groups have reported that iPSC derivatives showed different degrees of immunogenicity.7, 20, 21 In particular, those groups showed that iPSC‐derived cardiomyocytes and endothelial cells can induce more prominent immune responses in syngeneic mice compared with hepatocytes, neuronal cells and bone marrow cells.6 During the reprogramming processes in iPS cells from somatic cells, epigenetic changes can induce the expression of minor antigens, which can possibly directly or indirectly lead to the rejection of iPSC‐derivatives in syngeneic mice.7 We should be concerned as to whether those minor antigens can lead to the immune rejection of all iPSC derivatives. To probe this question further, genetic and epigenetic analysis of various iPSC derivatives is needed to find specific minor antigens that can cause the rejection of iPSC derivatives themselves. However, our studies elucidate that with regard to GAPs, which induce rejection of iPSCs, these antigens are not expressed by iPSC derivatives, such as HPCs, and should pose no concern for rejection as was originally identified for iPSCs.
Here, we clearly show that iPSCs express GAPs during their differentiation into three germ layers and that these GAPs sensitize CD4+ T cells, resulting in rejection of the iPSCs. Our data suggest that the immune system may serve as a natural fortification against iPSC‐derived teratomas. Collectively, these findings for the first time provide the mechanism by which iPSCs, but not ESCs, are rejected in syngeneic mice. In the broader picture, our studies demonstrate that somatic cells derived from self iPSCs are safe and do not cause teratomas.
Disclosure
The authors declare no conflict of interest.
Supporting information
Table S1. Selected profiles of up‐regulated proteins identified in induced pluripotent stem cell teratomas by two‐dimensional gel analysis and mass spectrometry.
Table S2. Quantitative PCR primers used for experiments.
Figure S1. Induced pluripotent stem cells are rejected in syngeneic mice.
Figure S2. CD4+ T cells infiltrate into induced pluripotent stem cell‐injected sites of syngeneic mice.
Figure S3. Induced pluripotent stem cell teratomas express meiotic and spermatogenetic proteins and transcription factors.
Figure S4. Engraftment of induced pluripotent stem (iPS) cell‐derived haematopoietic progenitor cells (HPCs) in 129x1/SvJ iPS cells rejected syngeneic mice.
Figure S5. Generated Stra8 overexpressing cell line.
Figure S6. Induced pluripotent stem cell‐derived haematopoietic progenitor cells do not express gamete‐associated genes.
Data S1. Material and methods.
Acknowledgements
EMK designed the research, performed all experiments, analysed data and edited the manuscript. GSM analysed the two‐dimensional gel data and assisted with experiments. NZ designed the research, analysed data, and wrote the manuscript. This study was made possible by grant number NIH/NHLBI #R01 HLO73015 to NZ, a VA Merit Review Award #1I01BX001125‐01A1 to NZ and by an American Heart Association Career Development Award to EMK, Award No 14DG18690008.
References
- 1. Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K et al Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007; 131:861–72. [DOI] [PubMed] [Google Scholar]
- 2. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006; 126:663–76. [DOI] [PubMed] [Google Scholar]
- 3. Yu J, Vodyanik MA, Smuga‐Otto K, ntosiewicz‐Bourget J, Frane JL, Tian S et al Induced pluripotent stem cell lines derived from human somatic cells. Science 2007; 318:1917–20. [DOI] [PubMed] [Google Scholar]
- 4. Zhao T, Zhang Z‐N, Rong Z, Xu Y. Immunogenicity of induced pluripotent stem cells. Nature 2011; 474:212–5. [DOI] [PubMed] [Google Scholar]
- 5. Cao J, Li X, Lu X, Zhang C, Yu H, Zhao T. Cells derived from iPSC can be immunogenic – yes or no? Protein Cell 2014; 5:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guha P, Morgan JW, Mostoslavsky G, Rodrigues NP, Boyd AS. Lack of immune response to differentiated cells derived from syngeneic induced pluripotent stem cells. Cell Stem Cell 2013; 12:407–12. [DOI] [PubMed] [Google Scholar]
- 7. Araki R, Uda M, Hoki Y, Sunayama M, Nakamura M, Ando S et al Negligible immunogenicity of terminally differentiated cells derived from induced pluripotent or embryonic stem cells. Nature 2013; 494:100–4. [DOI] [PubMed] [Google Scholar]
- 8. Nijagal A, Derderian C, Le T, Jarvis E, Nguyen L, Tang Q et al Direct and indirect antigen presentation lead to deletion of donor‐specific T cells after in utero hematopoietic cell transplantation in mice. Blood 2013; 121:4595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bonde S, Chan KM, Zavazava N. ES‐cell derived hematopoietic cells induce transplantation tolerance. PLoS One 2008; 3:e3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bonde S, Dowden AM, Chan KM, Tabayoyong WB, Zavazava N. HOXB4 but not BMP4 confers self‐renewal properties to ES‐derived hematopoietic progenitor cells. Transplantation 2008; 86:1803–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chan KM, Bonde S, Klump H, Zavazava N. Hematopoiesis and immunity of HOXB4‐transduced embryonic stem cell‐derived hematopoietic progenitor cells. Blood 2008; 111:2953–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sykes M. Mixed chimerism and transplant tolerance. Immunity 2001; 14:417–24. [DOI] [PubMed] [Google Scholar]
- 13. Yamada K, Shimizu A, Utsugi R, Ierino FL, Gargollo P, Haller GW et al Thymic transplantation in miniature swine. II. Induction of tolerance by transplantation of composite thymokidneys to thymectomized recipients. J Immunol 2000; 164:3079–86. [DOI] [PubMed] [Google Scholar]
- 14. Leventhal J, Abecassis M, Miller J, Gallon L, Ravindra K, Tollerud DJ et al Chimerism and tolerance without GVHD or engraftment syndrome in HLA‐mismatched combined kidney and hematopoietic stem cell transplantation. Sci Transl Med 2012; 4:124ra128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maeda I, Okamura D, Tokitake Y, Ikeda M, Kawaguchi H, Mise N et al Max is a repressor of germ cell‐related gene expression in mouse embryonic stem cells. Nat Commun 2013; 4:1754. [DOI] [PubMed] [Google Scholar]
- 16. Lesch BJ, Page DC. Genetics of germ cell development. Nat Rev Genet 2012; 13:781–94. [DOI] [PubMed] [Google Scholar]
- 17. Baltus AE, Menke DB, Hu Y‐C, Goodheart ML, Carpenter AE, de Rooij DG et al In germ cells of mouse embryonic ovaries, the decision to enter meiosis precedes premeiotic DNA replication. Nat Genet 2006; 38:1430–4. [DOI] [PubMed] [Google Scholar]
- 18. Mark M, Jacobs H, Oulad‐Abdelghani M, Dennefeld C, Feret B, Vernet N et al STRA8‐deficient spermatocytes initiate, but fail to complete, meiosis and undergo premature chromosome condensation. J Cell Sci 2008; 121:3233–42. [DOI] [PubMed] [Google Scholar]
- 19. Silva C, Wood JR, Salvador L, Zhang Z, Kostetskii I, Williams CJ et al Expression profile of male germ cell‐associated genes in mouse embryonic stem cell cultures treated with all‐trans retinoic acid and testosterone. Mol Reprod Dev 2009; 76:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang X, Qin J, Zhao RC, Zenke M. Reduced immunogenicity of induced pluripotent stem cells derived from Sertoli cells. PLoS One 2014; 9:e106110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Koch CA, Geraldes P, Platt JL. Immunosuppression by embryonic stem cells. Stem Cells 2008; 26:89–98. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Selected profiles of up‐regulated proteins identified in induced pluripotent stem cell teratomas by two‐dimensional gel analysis and mass spectrometry.
Table S2. Quantitative PCR primers used for experiments.
Figure S1. Induced pluripotent stem cells are rejected in syngeneic mice.
Figure S2. CD4+ T cells infiltrate into induced pluripotent stem cell‐injected sites of syngeneic mice.
Figure S3. Induced pluripotent stem cell teratomas express meiotic and spermatogenetic proteins and transcription factors.
Figure S4. Engraftment of induced pluripotent stem (iPS) cell‐derived haematopoietic progenitor cells (HPCs) in 129x1/SvJ iPS cells rejected syngeneic mice.
Figure S5. Generated Stra8 overexpressing cell line.
Figure S6. Induced pluripotent stem cell‐derived haematopoietic progenitor cells do not express gamete‐associated genes.
Data S1. Material and methods.


