Abstract
The current review highlights the evidence supporting the use of ketogenic diets in the management of drug-resistant epilepsy and status epilepticus in adults. Ketogenic diet variants are compared and advantages and potential side effects of diet therapy are discussed.
Since 1921, ketogenic diet (KD) therapy has been a recognized effective tool in the treatment of epilepsy. Interest in diet therapy waned following the introduction of antiepileptic drugs (AEDs), until the 1990s, when studies and clinical trials emerged demonstrating its efficacy in drug-resistant patients and particular pediatric epilepsy syndromes (1–3). Indeed, there is growing evidence of diet efficacy in the management of glucose transporter type 1 deficiency syndrome, pyruvate dehydrogenase deficiency, infantile spasms, Lennox-Gastaut syndrome, Dravet syndrome, Angelman syndrome, and myoclonic-astatic epilepsy (4–9). Many patients diagnosed with these syndromes are now reaching adulthood, while others are being diagnosed de novo as adults with recent advances in genetic testing. In the management of drug-resistant epilepsy (seizures resistant to two or more AEDs), patients have a less than 5% chance of seizure freedom with additional drugs and may not be surgical candidates due to a generalized epilepsy, multifocal nature, or nonresectable seizure focus (10). Emerging evidence suggests that KDs can offer seizure reduction and seizure freedom in a subset of these patients, not only children but now adults as well.
Ketogenic Diet Variants
The classic KD is the original diet introduced into practice in the 1920s. This high-fat, low-carbohydrate diet induces ketone body production through fat metabolism with the goal of mimicking a starvation state without depriving the body of necessary calories to sustain growth and development (11,12). It is typically composed of a 4:1 ratio of fat (in grams) to protein plus carbohydrates (in grams), thus shifting the predominant caloric source from carbohydrate to fat. Lower ratios of 3:1, 2:1, or 1:1 (referred to as a modified KD) can be used depending on age, individual tolerability, levels of ketosis, and protein requirements (13). To increase flexibility and palatability, more relaxed variant forms (Figure) include the modified Atkins diet (MAD), the low glycemic index treatment (LGIT), and the KD combined with medium-chain triglyceride (MCT) oil. The MAD, introduced in 2003, typically employs a net 10 to 20 g carbohydrate per day limit, which is roughly equivalent to a ratio of 1–2:1 of fat to protein plus carbohydrates (14, 15). The MAD does not require calculating and weighing of food portions or an initial hospital stay for implementation (12, 16). The LGIT recommends 40 to 60 g of carbohydrates daily with the selection of foods with glycemic indices <50 and ~60% of dietary energy derived from fat and 20 to 30 percent from protein (17). The MCT variant KD uses medium-chain fatty acids provided in coconut and/or palm kernel oil as a diet supplement. It provides an option for individuals with carnitine deficiency, as carnitine is required for processing the long-chain fatty acids supplied by the classic KD, and allows for greater carbohydrate and protein intake than even a lower-ratio classic KD (18), which can improve compliance.
FIGURE.
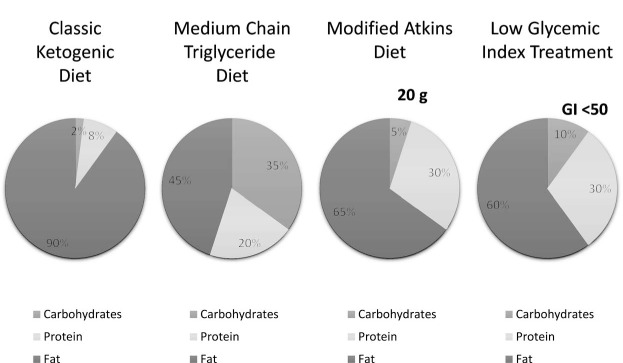
Comparison of classic (4:1 ratio) ketogenic diet and ketogenic diet variant composition used for adults. GI, glycemic index.
Ketogenic Diets in the Management of Chronic Epilepsy in Adults
The demand for KD access in adults comes from children already on KD therapy transitioning into adulthood, patients with adult-onset epilepsy refractory to medications or wishing to limit AEDs due to side-effect burden, and adults with chronic epilepsy who were not offered a KD as children and are appropriate candidates. These patients are motivated to achieve seizure control; secondary goals include improved overall quality of life and employment potential, independence in the activities of daily living, the ability to obtain driving privileges, and reduced risk of injury, sudden unexplained death in epilepsy, and AED burden. Thus, evidence supporting diet efficacy (≥50% reduction in seizure frequency) in adults is needed.
A 2011 review of dietary treatments in adolescents and adults pooled data from seven studies of the classic KD to show that 49% of 206 patients had ≥50% seizure reduction and, of these, 13% were seizure free (19). A 2015 meta-analysis reviewing ketogenic dietary treatments in adults from 12 studies of the classic KD (n = 168 patients), the MAD (n = 87 patients), and the classic KD in combination with MCT (n = 15 patients) found that the efficacy rates of KD in adult intractable epilepsy ranged from 13 to 70 percent with a combined efficacy rate of 52% for the classic KD and 34% for the MAD (20). A subsequent study of the classic KD noted that 39% of subjects had a ≥50% seizure reduction (9/23) and 8% had ≥90% seizure reduction (2/23); a subsequent MAD study noted that 31% of subjects had ≥50% seizure reduction (4/13) (21, 22). In the largest observational study of 101 adult patients naïve to diet therapy who subsequently started the MAD, 39% had ≥50% seizure reduction and 22% became seizure free following 3 months of treatment (23). Thus, the classic KD reduced seizures by ≥50% in 22 to 70 percent of patients and by ≥90% in up to 52% of patients, while the MAD reduced seizures by ≥50% in 12 to 67 percent of patients and by ≥90% in up to 67% of patients (21, 23).
Diet adherence and compliance remain significant barriers to successful implementation of ketogenic diets and an adequate assessment of efficacy. The aforementioned meta-analysis of 11 studies of KDs in adults found a combined adherence rate of 45% for all KD types, 38% for the classic KD, and 56% for the MAD (20). Similarly, in a recent observational study of 139 adult patients treated with KDs, 48% (67/139) discontinued the diet (39%) or were lost after initial follow-up (9%) with approximately half of patients citing difficulty with compliance or restrictiveness as the reason for stopping (23). These findings are in line with reports of adult dropout rates of 51% for the classic KD and 42% for the MAD in prior reviews (24). Often, providing food recipes and resources to patients and families during initial diet training and subsequent visits can emphasize the variety of food choices and ease of use rather than perceived restrictiveness. Additional methods to improve adherence and compliance, as well as access for patients who live far from a KD center, include scheduling telephone calls or electronic communication with the supervising dietitian or nutritionist, providing ketogenic supplements, and using electronic applications like the KetoDietCalculator to prevent dropout and emphasize progress and success (15). A major barrier to KD use in adults is lack of access to the treatment itself as very few epilepsy centers offer KDs to adult patients.
Dietary treatment in patients with drug-resistant epilepsy has several advantages. Dietary treatments can be rapidly initiated, and the beneficial effects can be seen almost immediately. They can be used safely and effectively for most types of epilepsy and in patients of all ages worldwide (25). Several studies in adults have also reported benefits beyond seizure control, including improvements in arousal, mood, alertness, energy, and concentration (22, 26). Other studies show that diets are effective in treating comorbid diseases, such as obesity and type 2 diabetes, which are a major health concern in the adult population (27). Furthermore, quality-of-life scores tend to increase rather than decrease with diet therapy (28).
Ketogenic Diet Use in the Management of Refractory Status Epilepticus in Adults
Status epilepticus (prolonged seizure lasting longer than 5 minutes or recurrent seizures without return to baseline between seizures) that continues despite appropriate first- and second-line AEDs is classified as refractory status epilepticus (RSE). If status epilepticus continues or recurs 24 hours or more after the initiation of treatment with anesthetic agents to induce burst or seizure suppression, patients are diagnosed with super-refractory status epilepticus (SRSE) (29). Several case reports and case series demonstrate the successful use of KD therapy for the management of RSE and SRSE. The first report of KD use for SRSE in an adult was published in France (30). Subsequently, a 4:1 ratio KD was introduced to two adult patients with SRSE at the University of Pennsylvania after 20 and 101 days of status epilepticus, respectively, and resulted in seizure cessation after 6 and 11 days, respectively (31). Similar cases of successful resolution of status epilepticus in adults within 2 weeks with the classic KD and 4 days with LGIT have also been reported (32–34). A case series of 10 adults with SRSE lasting a median of 21.5 days who were treated with a KD (nine with a 4:1 ratio KD and one with a 3:1 ratio KD) at four medical centers showed successful cessation of status epilepticus in 100% of patients who achieved ketosis (9 of 10 adults) at a median of 3 days (range = 1–31 days) (35). In the most recent and largest phase I/II clinical trial of 15 adult patients treated with 4:1 ratio KD therapy (14 of whom completed therapy) after a median of 10 days of SRSE at four medical centers, 11 (79% of patients who completed KD therapy, 73% of all patients enrolled) achieved resolution of seizures in a median of 5 days (range = 0–10 days) (36).
As both RSE and SRSE carry high rates of morbidity and mortality (37), KD therapy offers a needed adjunct strategy for management. It has the potential advantages of working rapidly and synergistically with other concurrent treatments; is relatively easy to start, monitor, and maintain in the controlled intensive care unit setting with close follow-up, does not contribute to hemodynamic instability seen with anesthetic agents; and could potentially reduce the need for prolonged use of these drugs. Moreover, there may be an additional neuroprotective benefit related to improved mitochondrial function due to increased energy reserves combined with decreased production of reactive oxygen species (38). For example, the ketogenic diet has been shown to stimulate mitochondrial biogenesis, increase cerebral ATP concentrations, and result in lower reactive oxygen species production in animal models (39, 40). There is additional growing evidence that ketone bodies exhibit protective anti-inflammatory effects (41). In preclinical studies, KD treatment reduces microglial activation, expression of proinflammatory cytokines, and pain and inflammation after thermal nociception (42–44); it also improves outcomes in models of Parkinson disease and multiple sclerosis through proposed mechanisms that include reduced brain inflammation and neurodegeneration (43, 45). These anti-inflammatory properties may explain the observed benefit of KD in treating patients with SRSE secondary to autoimmune and presumed autoimmune encephalitis, such as those with new onset refractory status epilepticus and febrile infection-related epilepsy syndrome.
Management of Adverse Effects in Adults
The most commonly reported adverse effects associated with KD use in adults are gastrointestinal effects, weight loss, and a transient increase in lipids. The gastrointestinal side effects, which include constipation, diarrhea, and occasional nausea and vomiting, are typically mild, improve with time, can often be managed with diet adjustments under the guidance of a dietitian or nutritionist, and infrequently require medical intervention. Smaller meals, increased fiber intake, exercise, and increased sodium and fluid intake can often prevent or alleviate these complaints. Weight loss may be an intended positive effect in patients who are overweight, but for those who want to maintain or gain weight, adjustments in caloric intake can be recommended. Increases in serum lipids have been shown to normalize with continued diet therapy (after 1 year) or return to normal after cessation of diet therapy (46–48). In addition, very-low-carbohydrate diets that induce ketosis have been shown to lead to reductions in serum triglycerides, low-density lipoprotein, and total cholesterol and increased levels of high-density lipoprotein cholesterol in adults (27). Whether ketogenic diets influence extended serum and vascular markers of atherogenesis in adults with epilepsy beyond standard lipid profiles warrants further investigation. Adults with persistent hyperlipidemia who are responding well to diet therapy may consider increasing fat sources of omega 3 or adhering to published lipid-lowering recommendations for the MAD, although further research is needed to demonstrate that these interventions provide benefit (48). Other potential side effects can result from vitamin and mineral deficiencies secondary to restricting carbohydrates and prolonged ketonemia, including osteopenia and osteoporosis (12, 49, 50), although the precise mechanism remains unclear. The standard practice of supplementing a recommended daily allowance of multivitamin and mineral supplements can reduce the risk of such deficiencies.
Conclusions
Clinical evidence now demonstrates the effectiveness of ketogenic diets in treating drug-resistant epilepsy in adults. Potential side effects of diet therapy are often preventable and manageable; however, additional strategies are needed to improve long-term adherence.
Highlights
Ketogenic diets are an important adjunct to AEDs for management of chronic epilepsy and RSE in adults.
Studies support the feasibility, tolerability, and efficacy of a KD in adults.
Ketogenic diets may have anti-inflammatory properties that could benefit patients with epilepsy caused by an autoimmune process.
Compliance and long-term side effects remain major considerations.
Supplementary Material
Acknowledgments
We would like to acknowledge the multidisciplinary team at the Johns Hopkins Adult Epilepsy Diet Center—Eric Kossoff, Rebecca Fisher, Joanne Barnett, Bobbie Henry-Barron, and Diane Vizthum; Elizabeth Felton at the University of Wisconsin, Madison; and our patients and their families.
Funding
None to declare.
Conflict of Interest
Dr. Cervenka receives grant support from Nutricia North America, Vitaflo, Army Research Laboratory, and BrightFocus Foundation. She receives speaking honoraria from LivaNova, Nutricia North America, and Glut1 Deficiency Foundation and she performs consulting with Nutricia North America and Sage Therapeutics.
References
- 1. Martin K, Jackson CF, Levy RG, Cooper PN.. Ketogenic diet and other dietary treatments for epilepsy. Cochrane Database Syst Rev. 2016; 2016 doi:10.1002/14651858.CD001903.pub3. [DOI] [PubMed] [Google Scholar]
- 2. Barborka CJ. Epilepsy in adults: Results of treatment by ketogenic diet in one hundred cases. Arch Neurol Psychiatry. 1930; 23: 904– 914. [Google Scholar]
- 3. Barborka CJ. Ketogenic diet treatment of epilepsy in adults. JAMA. 1928; 9: 73– 78. [Google Scholar]
- 4. Kass HR, Winesett SP, Bessone SK, Turner Z, Kossoff EH.. Use of dietary therapies amongst patients with GLUT1 deficiency syndrome. Seizure. 2016; 35: 83– 87. doi:10.1016/j.seizure.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 5. Pong AW, Geary BR, Engelstad KM, Natarajan A, Yang H, De Vivo DC.. Glucose transporter type I deficiency syndrome: Epilepsy phenotypes and outcomes. Epilepsia. 2012; 53: 1503– 1510. doi:10.1111/j.1528-1167.2012.03592.x. [DOI] [PubMed] [Google Scholar]
- 6. Nangia S, Caraballo RH, Kang HC, Nordli DR, Scheffer IE.. Is the ketogenic diet effective in specific epilepsy syndromes? Epilepsy Res. 2012; 100: 252– 257. doi:10.1016/j.eplepsyres.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 7. Thibert RL, Pfeifer HH, Larson AM, Raby AR, Reynolds AA, Morgan AK, Thiele EA.. Low glycemic index treatment for seizures in Angel-man syndrome. Epilepsia. 2012; 53: 1498– 1502. doi:10.1111/j.1528-1167.2012.03537.x. [DOI] [PubMed] [Google Scholar]
- 8. Barañano KW, Hartman AL.. The ketogenic diet: Uses in epilepsy and other neurologic illnesses. Curr Treat Options Neurol. 2008; 10: 410– 419. doi:10.1007/s11940-008-0043-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bergin AM. Ketogenic diet in established epilepsy indications. Masino SA, Ketogenic Diet and Metabolic Therapies: Expanded Roles in Health and Disease. New York: Oxford University Press; 2017: 40– 49. [Google Scholar]
- 10. Brodie MJ, Barry SJE, Bamagous GA, Norrie JD, Kwan P.. Patterns of treatment response in newly diagnosed epilepsy. Neurology. 2012; 78: 1548– 1554. doi:10.1212/WNL.0b013e3182563b19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McNally MA, Hartman AL.. Ketone bodies in epilepsy. J Neurochem. 2012; 121: 28– 35. doi:10.1111/j.1471-4159.2012.07670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cervenka MC, Kossoff EH.. Dietary treatment of intractable epilepsy. Continuum (Minneap Minn). 2013; 19: 756– 766. doi:10.1212/01.CON.0000431396.23852.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zupec-Kania BA, Spellman E.. An overview of the ketogenic diet for pediatric epilepsy. Nutr Clin Pract. 1998; 23: 589– 596. doi:10.1177/0884533608326138. [DOI] [PubMed] [Google Scholar]
- 14. Kossoff EH, Rowley H, Sinha SR, Vining EPG.. A prospective study of the modified Atkins diet for intractable epilepsy in adults. Epilepsia. 2008; 49: 316– 319. doi:10.1111/j.1528-1167.2007.01256.x. [DOI] [PubMed] [Google Scholar]
- 15. Cervenka MC, Terao NN, Bosarge JL, Henry BJ, Klees AA, Morrison PF, Kossoff EH.. E-mail management of the modified Atkins diet for adults with epilepsy is feasible and effective. Epilepsia. 2012; 53: 728– 732. doi:10.1111/j.1528-1167.2012.03406.x. [DOI] [PubMed] [Google Scholar]
- 16. Schoeler NE, Cross JH.. Ketogenic dietary therapies in adults with epilepsy: A practical guide. Pract Neurol. 2016; 16: 208– 214. doi:10.1136/practneurol-2015-001288. [DOI] [PubMed] [Google Scholar]
- 17. Muzykewicz DA, Lyczkowski DA, Memon N, Conant KD, Pfeifer HH, Thiele EA.. Efficacy, safety, and tolerability of the low glycemic index treatment in pediatric epilepsy. Epilepsia. 2009; 50: 1118– 1126. doi:10.1111/j.1528-1167.2008.01959.x. [DOI] [PubMed] [Google Scholar]
- 18. Neal EG, Cross JH.. Efficacy of dietary treatments for epilepsy. J Hum Nutr Diet. 2010; 23: 113– 9. doi:10.1111/j.1365-277X.2010.01043.x. [DOI] [PubMed] [Google Scholar]
- 19. Payne NE, Cross JH, Sander JW, Sisodiya SM.. The ketogenic and related diets in adolescents and adults—A review. Epilepsia. 2011; 52: 1941– 1948. doi:10.1111/j.1528-1167.2011.03287.x. [DOI] [PubMed] [Google Scholar]
- 20. Ye F, Li XJ, Jiang WL, Sun H Bin, Liu J.. Efficacy of and patient compliance with a ketogenic diet in adults with intractable epilepsy: A meta-analysis. J Clin Neurol. 2015; 11: 26– 31. doi:10.3988/jcn.2015.11.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kverneland M, Selmer KK, Nakken KO, Iversen PO, Taubøll E.. A prospective study of the modified Atkins diet for adults with idiopathic generalized epilepsy. Epilepsy Behav. 2015; 53: 197– 201. doi:10.1016/j.yebeh.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 22. Schoeler NE, Wood S, Aldridge V, Sander JW, Cross JH, Sisodiya SM.. Ketogenic dietary therapies for adults with epilepsy: Feasibility and classification of response. Epilepsy Behav. 2014; 37: 77– 81. doi:10.1016/j.yebeh.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 23. Cervenka MC, Henry BJ, Felton EA, Patton K, Kossoff EH.. Establishing an adult epilepsy diet center: Experience, efficacy and challenges. Epilepsy Behav. 2016; 58: 61– 68. doi:10.1016/j.yebeh.2016.02.038. [DOI] [PubMed] [Google Scholar]
- 24. Klein P, Tyrlikova I, Mathews GC.. Dietary treatment in adults with refractory epilepsy: A review. Neurology. 2014; 83: 1978– 1985. doi:10.1212/WNL.0000000000001004. [DOI] [PubMed] [Google Scholar]
- 25. Kossoff EH, Al-Macki N, Cervenka MKC, Kim HD, Liao J, Megaw K, Nathan JK, Raimann X, Rivera R, Wiemer-Kruel A, Williams E, Zupec-Kania BA.. What are the minimum requirements for ketogenic diet services in resource-limited regions? Recommendations from the International League Against Epilepsy Task Force for Dietary Therapy. Epilepsia. 2015; 56: 1337– 1342. doi:10.1111/epi.13039. [DOI] [PubMed] [Google Scholar]
- 26. Sirven J, Whedon B, Caplan D, Liporace J, Glosser D, O'Dwyer J, Sperling MR.. The ketogenic diet for intractable epilepsy in adults: Preliminary results. Epilepsia. 1999; 40: 1721– 1726. doi:10.1111/j.1528-1157.1999.tb01589.x. [DOI] [PubMed] [Google Scholar]
- 27. Paoli A, Rubini A, Volek JS, Grimaldi KA.. Beyond weight loss: A review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur J Clin Nutr. 2013; 67: 789– 796. doi:10.1038/ejcn.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lambrechts DAJE, Wielders LHP, Aldenkamp AP, Kessels FGH, de Kinderen RJA, Majoie MJM.. The ketogenic diet as a treatment option in adults with chronic refractory epilepsy: Efficacy and tolerability in clinical practice. Epilepsy Behav. 2012; 23: 310– 314. doi:10.1016/j.yebeh.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 29. Hocker SE, Britton JW, Mandrekar JN, Wijdicks EFM, Rabinstein AA.. Predictors of outcome in refractory status epilepticus. JAMA Neurol. 2013; 70: 72– 77. doi:10.1001/jamaneurol.2013.578. [DOI] [PubMed] [Google Scholar]
- 30. Bodenant M, Moreau C, Sejourné C, Auvin S, Delval A, Cuisset J-M, Derambure P, Destée A, Defebvre L.. [Interest of the ketogenic diet in a refractory status epilepticus in adults]. Rev Neurol (Paris). 2008; 164: 194– 199. doi:10.1016/j.neurol.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 31. Wusthoff CJ, Kranick SM, Morley JF, Bergqvist AGC.. The ketogenic diet in treatment of two adults with prolonged nonconvulsive status epilepticus. Epilepsia. 2010; 51: 1083– 1085. doi:10.1111/j.1528-1167.2009.02388.x. [DOI] [PubMed] [Google Scholar]
- 32. Nam SH, Lee BL, Lee CG, Yu HJ, Joo EY, Lee J, Lee M.. The role of ketogenic diet in the treatment of refractory status epilepticus. Epilepsia. 2011; 52: e181– e184. doi:10.1111/j.1528-1167.2011.03289.x. [DOI] [PubMed] [Google Scholar]
- 33. Strzelczyk A, Reif PS, Bauer S, Belke M, Oertel WH, Knake S, Rosenow F.. Intravenous initiation and maintenance of ketogenic diet: Proof of concept in super-refractory status epilepticus. Seizure. 2013; 22: 581– 583. doi:10.1016/j.seizure.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 34. Martikainen MH, Paivarinta M, Jaaskelainen S, Majamaa K.. Successful treatment of POLG-related mitochondrial epilepsy. Epileptic Disord. 2012; 14: 438– 441. [DOI] [PubMed] [Google Scholar]
- 35. Thakur KT, Probasco JC, Hocker SE, Roehl K, Henry B, Kossoff EH, Kaplan PW, Geocadin RG, Hartman AL, Venkatesan A, Cervenka MC.. Ketogenic diet for adults in super-refractory status epilepticus. Neurology. 2014; 82: 665– 670. doi:10.1212/WNL.0000000000000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cervenka MC, Hocker SE, Koenig M, Bar B, Henry-Barron B, Kossoff EH, Hartman AL, Probasco JC, Benavides DR, Venkatesan A, Hagen EC, Dittrich D, Stern T, Radzik B, Depew M, Caserta FM, Nyquist P, Kaplan PW, Geocadin RG.. A phase I/II multicenter ketogenic diet study for adult super-refractory status epilepticus. Neurology. 2017; 88: 938– 943. doi:10.1212/WNL.0000000000003690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shorvon S, Ferlisi M.. The outcome of therapies in refractory and super-refractory convulsive status epilepticus and recommendations for therapy. Brain. 2012; 135: 2314– 2328. doi:10.1093/brain/aws091. [DOI] [PubMed] [Google Scholar]
- 38. Maalouf M, Rho JM, Mattson MP.. The neuroprotective properties of calorie restriction, the ketogenic diet, and ketone bodies. Brain Res Rev. 2009; 59: 293– 315. doi:10.1016/j.brainresrev.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sullivan PG, Rippy NA, Dorenbos K, Concepcion RC, Agarwal AK, Rho JM.. The ketogenic diet increases mitochondrial uncoupling protein levels and activity. Ann Neurol. 2004; 55: 576– 580. doi:10.1002/ana.20062. [DOI] [PubMed] [Google Scholar]
- 40. Bough KJ, Wetherington J, Hassel B, Pare JF, Gawryluk JW, Greene JG, Shaw R, Smith Y, Geiger JD, Dingledine RJ.. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann Neurol. 2006; 5: 223– 35. doi:10.1002/ana.20899. [DOI] [PubMed] [Google Scholar]
- 41. Koppel SJ, Swerdlow RH.. Neuroketotherapeutics: A modern review of a century-old therapy. Neurochem Int. 2017. June 1 pii: S0197–0186(17)30227-9. doi:10.1016/j.neuint.2017.05.019. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ruskin DN, Kawamura M, Masino SA.. Reduced pain and inflammation in juvenile and adult rats fed a ketogenic diet. PLoS One. 2009; 4: 1– 6. doi:10.1371/journal.pone.0008349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yang X, Cheng B.. Neuroprotective and anti-inflammatory activities of ketogenic diet on MPTP-induced neurotoxicity. J Mol Neurosci. 2010; 42: 145– 153. doi:10.1007/s12031-010-9336-y. [DOI] [PubMed] [Google Scholar]
- 44. Youm Y, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, Agostino DD, Planavsky N, Lupfer C, Kanneganti TD, Kang S, Horvath TL, Fahmy TM, Crawford PA, Biragyn A, Alnemri E, Dixit VD.. The ketone metabolite β -hydroxybutyrate blocks NLRP3 inflammasome–mediated inflammatory disease. Nat Med. 2015; 21: 263– 269. doi:10.1038/nm.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kim DY, Hao J, Liu R, Turner G, Shi F, Rho JM.. Inflammation-mediated memory dysfunction and effects of a ketogenic diet in a murine model of multiple sclerosis. PLoS One. 2012; 7 5: e35476 doi:10.1371/journal.pone.0035476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Klein P, Janousek J, Barber A, Weissberger R.. Ketogenic diet treatment in adults with refractory epilepsy. Epilepsy Behav. 2010; 19: 575– 579. doi:10.1016/j.yebeh.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 47. Mosek A, Natour H, Neufeld MY, Shiff Y, Vaisman N.. Ketogenic diet treatment in adults with refractory epilepsy: A prospective pilot study. Seizure. 2009; 18: 30– 33. doi:10.1016/j.seizure.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 48. Cervenka MC, Patton K, Eloyan A, Henry B, Kossoff EH.. The impact of the modified Atkins diet on lipid profiles in adults with epilepsy. Nutr Neurosci. 2016; 19: 131– 137. doi:10.1179/1476830514Y.0000000162. [DOI] [PubMed] [Google Scholar]
- 49. Mackay MT, Bicknell-Royle J, Nation J, Humphrey M, Harvey AS.. The ketogenic diet in refractory childhood epilepsy. J Paediatr Child Health. 2005; 41: 353– 357. doi:10.1111/j.1440-1754.2005.00630.x. [DOI] [PubMed] [Google Scholar]
- 50. Bergqvist AGC, Schall JI, Stallings VA, Zemel BS.. Progressive bone mineral content loss in children with intractable epilepsy treated with the ketogenic diet. Am J Clin Nutr. 2008; 88: 1678– 1684. doi:10.3945/ajcn.2008.26099. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


