Abstract
Background
Gastric cancer (GC) with cisplatin resistance is one of the leading causes of limitations to therapy. Inhibition of apoptosis-stimulating protein of p53 (iASPP) plays a key role in GC. However, the role of iASPP in GC with cisplatin resistance remains unclear. The aim of this study was to investigate iASPP expression in GC, and the functions of iASPP in cisplatin-resistant cell lines.
Material/Methods
In this study, the expression of iASPP was investigated in normal GC patients and patients with cisplatin resistance, along with GC cell lines and cell lines with cisplatin resistance. Furthermore, knockdown of iASPP was conducted in cell lines; and cell proliferation, apoptosis rate, cell cycle distribution, and cell migration and invasion were determined through CCK8, flow cytometry, Scratch test and Transwell assay, respectively.
Results
The expression of iASPP in GC patients with cisplatin resistance was significant higher than in the health control group. Higher expression of iASPP was detected in cisplatin-resistant cancer cell lines. Cell proliferation of SGC-7901 and MGC-803 was inhibited by transfection with siRNA, along with evaluated apoptosis rate and G1 phase retardant. Furthermore, cells viability, including migration and invasion, was suppressed post-transfection with siRNA.
Conclusions
iASPP induced cisplatin resistance in GC patients. Thus, knockdown of iASPP might be a novel therapeutic strategy for the treatment of GC cisplatin-resistant patients.
MeSH Keywords: Cell Cycle, Cell Proliferation, Cisplatin, Drug Resistance, Stomach Neoplasms
Background
Despite advancements in new diagnosis and therapy strategies, gastric cancer (GC) is associated with high mortality rate owing to its resistance to anticancer drugs, such as cisplatin [1]. Cisplatin is one of the most widely used traditional chemotherapeutic agents for GC therapy [2]. While acquired resistance is found to be a major obstacle in treatment, the mechanism of chemo-resistance remains unclear. Therefore, exploring the molecular mechanism to chemo-resistance will offer basic information and new therapeutic strategy to effectively manage GC.
Studies have shown that inhibition of apoptosis-stimulating protein of p53 (iASPP) is the only homologue of the ASPP family, and its abnormal expression is the leading cause of various diseases. Studies have shown that iASPP presents with a higher expression level in several types of solid tumors, such as hepatocellular carcinoma (HCC) [3], GC [4], lung cancer [5], and ovarian cancer [6]. These findings suggest that iASPP may play an important role in tumorigenesis in humans. Also, iASPP can downregulate the expression of the tumor suppressor gene p53, by binding to its DNA domain [6].
Overexpression of iASPP can inhibit cells apoptosis, which can be induced by radiation or cisplatin therapy. Previous research demonstrated that increased expression of iASPP increased its resistance to radiation and cisplatin-induced apoptosis [7]. Jiang et al. demonstrated an association between iASPP overexpression and gene amplification in ovarian cancer and suggested a role of iASPP in poor patient outcomes and chemo-resistance to paclitaxel by blocking mitotic catastrophe [6].
In this study, the expression level of iASPP, coded by PPP1R13L in GC tissues and cell lines, was determined. This study is the first to demonstrate the role of iASPP in GC. This study provided key information on new therapeutic strategy for GC.
Material and Methods
Tissues collection and cell lines
We collected ten pairs of tissue specimens (tumor tissues and adjacent non-tumor tissues) collected from patients with GC in Shandong Cancer Hospital Affiliated to Shandong University (Table 1). This study was approved by the Ethics Committee of Shandong Institute of Cancer Research. Collected tissues were immediately cooled using liquid nitrogen and then transfer into an ultra-low temperature freezer (–80°C).
Table 1.
Details of involved patients’ features.
| Patients features | N | iASPP expression | ||
|---|---|---|---|---|
| Positive | Negative | |||
| Age | <50 | 2 | 1 | 1 |
| ≥50 | 8 | 6 | 2 | |
| Gender | Male | 4 | 2 | 2 |
| Female | 6 | 4 | 2 | |
| Tumor size | <3 cm | 3 | 2 | 1 |
| ≥3 cm | 7 | 3 | 4 | |
| TNM stage | I and II | 4 | 2 | 2 |
| III and IV | 6 | 5 | 1 | |
The cell lines SGC-7901 and MGC-803, and their cisplatin-resistant cell lines, SGC-7901/DDP and MGC-803/DDP, were purchased from Bioleaf Biotech (Shanghai, China). The GC cell lines were cultured in RPMI 1640 medium supplemented with penicillin (100 U/mL), streptomycin (100 μg/mL) and 10% (v/v) fetal bovine serum (Gibco Life Technologies, Carlsbad, CA, USA) in a humidified atmosphere of 5% CO2 at 37°C.
RNA isolation and real-time PCR
Total RNA was extracted from the cell lines using the RNAiso reagent provided by Takara (Takara Bio, Inc., Otsu, Japan). cDNA was synthesized using myoblastosis virus reverse transcriptase (Invitrogen; Thermo Fisher Scientific, Inc.) with 1 μg of total RNA. The qPCR assay was performed using DNA Engine Opticon 2 Continuous Fluorescence Detection System (Bio-Rad Laboratories, Inc., Hercules, CA, USA) with the SYBR Premix ExTaq kit (Takara Bio, Inc.). Primers were designed using primer 5.0 version (Peimier Company, Canada) and synthesized by Sangon (Shanghai, China). Primers sequences used are as follow:
iASPP forward: 5′-GCGGTGAAGGAGATGAACGA-3′,
iASPP reverse: 5′-TGATGAGGAAATCCACGATAGAGTAG-3′;
GAPDH forward: 5′-TGTTCGTCATGGGTGTGAAC-3′,
GAPDH reverse: 5′-ATGGCATGGACTGTGGTCAT-3′.
Primers iASPP was used as described by Wang et al. [8]. All experiments were carried out in triplicates.
Western blot
Total protein of cell lines was isolated using a total protein extraction kit (Boster Biological Technology, Wuhan, China); concentration of protein was determined using a bicinchoninic acid kit (Pierce; Thermo Fisher Scientific, Inc.) at 570 nm absorption value. Western blotting was conducted as described by Li et al. [9]. Briefly, lysate protein was separated using SDS-PAGE, using 10% gel, and was transferred to nitrocellulose membrane. The expression signal was detected using an enhanced chemiluminescence system (EMD Millipore, Billerica, MA, USA). Primary antibodies used in this assay were as follow: iASPP primary antibody (ab115605, Abcam, UK), GAPDH primary antibody (ab9385, Abcam, UK), and anti-rabbit secondary antibody (ab191866, Abcam, UK).
Immunofluorescence staining
Cells were pre-plated on slides and then fixed in 1 mL of 4% paraformaldehyde for 20 minutes. The samples were permeabilized with 0.2% Triton-X100 for 90 seconds. The cells were then incubated with primary antibody (ab115605; Abcam, UK); then with a secondary antibody conjugated with fluorescein isothiocyanate for one hour. Primary antibody of iASPP was used to detect the expression of protein at a dilution of 1: 200.
Cell viability and colony formation assays
To study the effect of iASPP on GC cell proliferation, siRNA of iASPP was selected to transfect cisplatin-resistant cells. Briefly, the target cells were plated in six-well plates at a density of 1,000 cells/plate. Cell proliferation was detected using a CCK8 kit (Lianke Biotech, Zhejiang, China) according to manufacturer’s instructions. Briefly, after transfection with siRNA or NC (negative control), the cells were harvested and washed with PBS and incubated with CCK8 working reagent for two hours. The cells were washed with PBS three times and the absorbance was measured at 450 nm using a microplate reader.
Detection of apoptosis
Flow cytometry was used to study the association between iASPP, cisplatin-resistant GC cells and the level of cell apoptosis. Briefly, the cells were plated in six-well plate at density of 5×105/mL before transfection. Afterwards, the cells were collected and washed with precooled PBS buffer two times. The level of cell apoptosis in each group was detected using an Annexin V-FITC apoptosis detection kit (eBioscience, Thermo Fisher Scientific) and PI (propidium iodide) (Sigma-Aldrich) according to the manufacturer’s instructions. The cell apoptosis level was analyzed using the BD flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA). Finally, the collected data was equipped with CellQuest software (BD Biosciences).
Hoechst staining assay
To further explore the association between iASPP expression and cell DNA damage, Hoechst staining was used. Briefly, the cells were treated with siRNA-iASPP or NC and harvested and collected for further washing with PBS, and resuspended in binding buffer. Afterwards, the cells were stained with Hoechst 33342 (Sigma, St Louis, MO, USA) for 25 minutes, and fluorescence microscopy (Olympus IX71; Olympus Corporation, Tokyo, Japan) with a filter for Hoechst 33342 (365 nm) was used to detect the nuclear morphology.
Statistical analysis
All the data presented in this research is represented as mean ±SD. The results from treated and untreated control cells were analyzed using student’s t-test or one-way ANOVA by GraphPad Prism 6.0 program. A value of p<0.05 was considered as a statistically significant difference.
Results
Expression of iASPP in GC tissues and adjacent non-tumor tissues
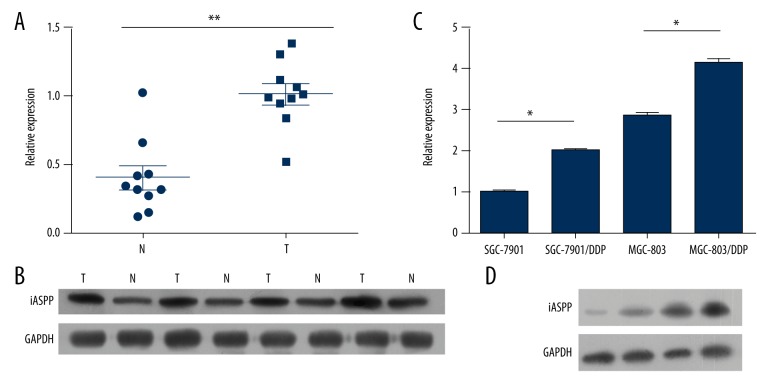
To investigate the difference in expression level of iASPP in GC tissues and adjacent non-tumor tissues, 10 pairs of clinical samples were collected, and expression was determined using qPCR and western blotting (Figure 1A). As shown in Figure 1A and 1B, expression of iASPP was significantly higher in GC tissue. To further confirm the potential oncogenic role of iASPP in cisplatin-resistant cell lines, expression of iASPP was determined using qPCR and western blotting. As shown in Figure 1C and 1D, higher expression of iASPP was observed in SGC-7901/DDP and MGC-803/DDP compared to SGC-7901 and MGC-803.
Figure 1.
iASPP expression in clinical tissues (A, B) and cisplatin resistant cell lines (C, D).
Effect of iASPP on cells proliferation, apoptosis and cell cycle
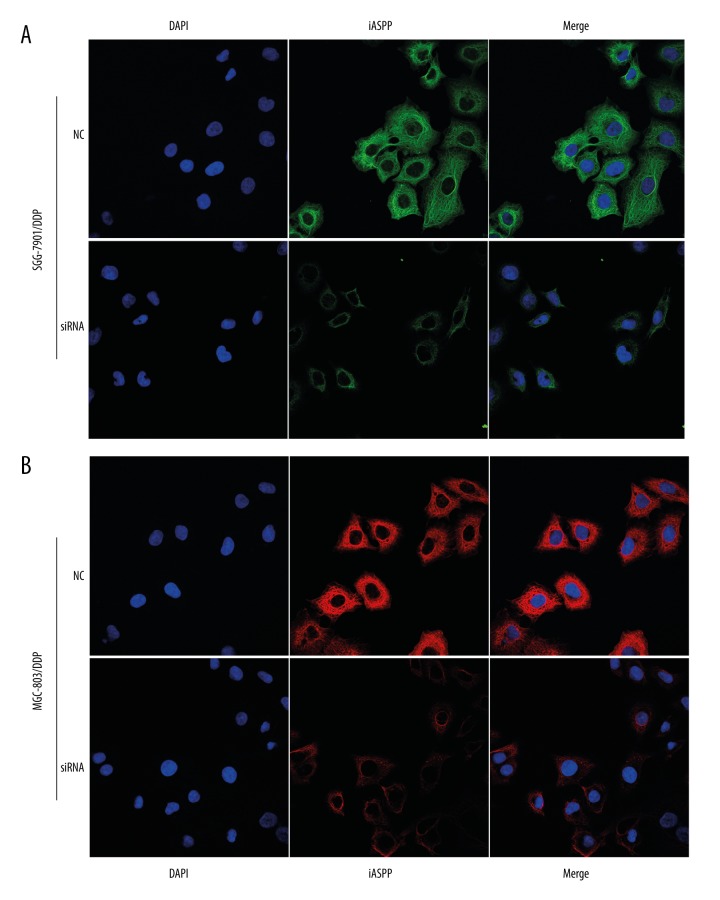
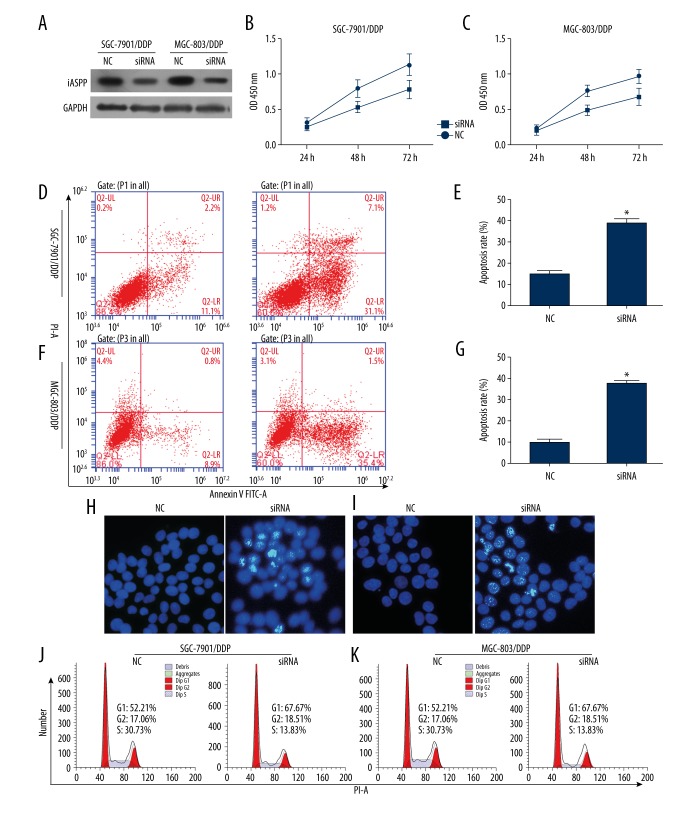
The cells were transfected with siRNA to knockdown iASPP for determining the role of iASPP in resistant cells. Following the treatment of cells with siRNA, iASPP expression was determined using western blotting and immunofluorescence staining (Figures 2, 3A). It was observed that cell proliferation was significantly inhibited 48 hours and 72 hours post-transfection compared to the control (Figure 3B, 3C). As shown in Figure 3D, decrease in iASPP expression significantly increased cell apoptosis in SGC-7901/DDP compared to cells transfected with negative control (Figure 3E–3G). As shown in Figure 3H and 3I, drug resistant cells exhibited enhanced DNA damage induced by transfected with siRNA or NC. To further explore the effect of iASPP on cell cycle distribution, flow cytometric analysis was conducted. Results showed that knockdown of iASPP increased blocking of G1 phase compared to cells treated with negative control in SGC-7901/DDP and MGC-803/DDP (Figure 3J, 3K).
Figure 2.
Determination of iASPP expression by immunofluorescence and in SGC-7901/DDP (A) and MGC-803/DDP (B).
Figure 3.
Downregulated expression of iASPP inhibited cell proliferation, enhanced cell apoptosis and induced G1-phase block in SGC-7901/DDP and MGC-803/DDP. (A) Knockdown of iASPP was determined by western blotting. (B) Inhibition of cell proliferation after transfection with siRNA for 48 hours in cell lines. (D–G) Cell apoptosis was determined using flow cytometry, and the apoptosis level was enhanced after transfection with siRNA for 48 hours in cell lines. (H, I) Nuclear DNA damage was determined using Hoechst staining; damage was induced by transfection of siRNA in cell lines. (J, K) Cisplatin-resistant cell line inhibited G1 phase after transfection with siRNA for 48 hours; * indicates p<0.05.
Effect of iASPP on cell viability
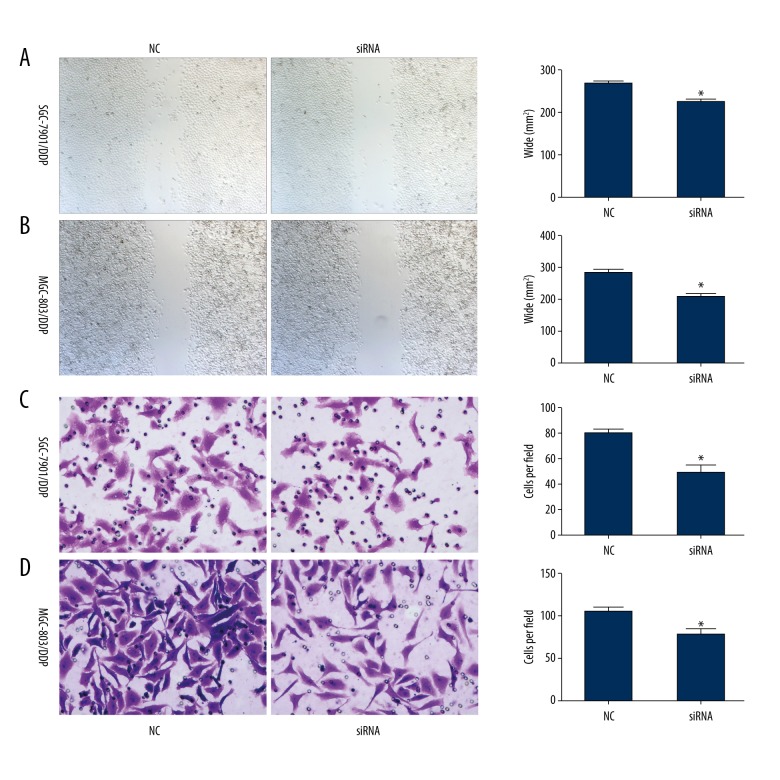
To further investigate the effect of iASPP on cell viability, scratch test and Transwell assay were conducted. SGC-7901/DDP was transfected with siRNA. Results showed that cell migration was significantly inhibited when the cells were treated with siRNA compared to NC (Figure 4A). Inhibition of cell migration in MGC-803/DDP is presented in Figure 4B. As shown in Figure 4C and D, cell invasion was significantly inhibited in SGC-7901/DDP and MGC-803/DDP (Figure 4C, 4D).
Figure 4.
Cell mobility was inhibited after transfection with iASPP siRNA in SGC-7901/DDP and MGC-803/DDP cell lines. (A, B) Cell migration was determined using scratch assay. Cells with knockdown iASPP exhibited significantly lower migration after transfection with siRNA in SGC-7901/DDP and MGC-803/DDP cell lines. (C, D) Cell invasion was determined using Transwell assay. Cell invasion was significantly inhibited after transfection with siRNA; * indicates p<0.05.
Discussion
GC is the major cause of death worldwide, and is regarded as the fourth most common cancer in the world. GC is the second most common cancer in China [10]. Despite advancement in drug discovery and surgical techniques, the five-year survival rate for GC patients remains below 30%. Chemotherapy remains one of the most effective treatment methods for GC patients [11]. However, drug resistance limits the anticancer effect in the majority of GC patients [12]. The present study examined the expression of iASPP in cell lines with cisplatin resistance. Cisplatin is one of the most common first-line anticancer drugs, and it has been used widely in the treatment of GC. Inhibition of DNA synthesis is induced by DDP by forming several types of DNA adducts [13]. The mechanism of DDP-resistance is due to DNA damage repair via nucleotide excision repair pathway, however, the exact mechanism remains unknown [14]. Growing evidence suggests that the efflux of anticancer drugs is associated with the accumulation of various ATP-binding cassette transporters (ABCT), such as ABCB1 and ABCG2 [15].
iASPP plays an important role in regulating metastasis owing to its ability to bind DNA-binding region of p53 [16]. iASPP acts as an oncogene in different types of cancers. Various studies have shown that iASPP can enhance cell proliferation in the glioblastoma cell line U251 [17]. Downregulation of iASPP expression significantly inhibits cell proliferation in GC cell lines. Also, cell apoptosis is also increased by transfection of iASPP siRNA [8]. In this study, the expression of iASPP was upregulated in cisplatin-resistant GC cell lines, and the knockdown of iASPP inhibited cell proliferation and induced cell apoptosis. A recent study has showed that iASPP increases the expression of miR-20a, which targets anticancer genes FBXL5 and BTG3 and enhances epithelial-mesenchymal transition (EMT) and cisplatin-resistance in cervical cancer cells [18]. Previous research has indicated that mitotic catastrophes can be induced by cisplatin, which in turns induces cell apoptosis [19]. However, overexpression of iASPP could abrogate paclitaxel-induced apoptosis [6].
Conclusions
iASPP expression was upregulated in GC cell lines with cisplatin-resistance. Further, knockdown of iASPP induced by siRNA resulted in abrogation of cell proliferation, enhancement of cell apoptosis, and inhibition of cell mobility. Our results highlight the great potential of iASPP as a chemo-sensitivity target for clinical prognosis in GC.
Footnotes
Conflict of interest
None.
Source of support: This research was supported by grants from the Natural Sciences Foundation of Shandong (ZR2013HL043)
References
- 1.Yoo BC, Ku JL, Hong SH, et al. Decreased pyruvate kinase M2 activity linked to cisplatin resistance in human gastric carcinoma cell lines. Int J Cancer. 2004;108(4):532–39. doi: 10.1002/ijc.11604. [DOI] [PubMed] [Google Scholar]
- 2.Pasini F, Fraccon AP, DE Manzoni G. The role of chemotherapy in metastatic gastric cancer. Anticancer Res. 2011;31(10):3543–54. [PubMed] [Google Scholar]
- 3.Liu HM, Liu MG, Tang C, et al. Effect of iASPP RNAi vector transfection on apoptosis of liver cancer cell. Journal of Hainan Medical College. 2009;15(9):1014–17. [Google Scholar]
- 4.Chun-Zhi Z, Lei H, An-Ling Z, et al. MicroRNA-221 and microRNA-222 regulate gastric carcinoma cell proliferation and radioresistance by targeting PTEN. BMC Cancer. 2010;10:367. doi: 10.1186/1471-2407-10-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deng Q, Sheng L, Su D, et al. Genetic polymorphisms in ATM, ERCC1, APE1 and iASPP genes and lung cancer risk in a population of southeast China. Med Oncol. 2011;28(3):667–72. doi: 10.1007/s12032-010-9507-2. [DOI] [PubMed] [Google Scholar]
- 6.Jiang L, Siu MK, Wong OG, et al. iASPP and chemoresistance in ovarian cancers: Effects on paclitaxel-mediated mitotic catastrophe. Clin Cancer Res. 2011;17(21):6924–33. doi: 10.1158/1078-0432.CCR-11-0588. [DOI] [PubMed] [Google Scholar]
- 7.Bergamaschi D, Samuels Y, O’Neil NJ, et al. iASPP oncoprotein is a key inhibitor of p53 conserved from worm to human. Nat Genet. 2003;33(2):162–67. doi: 10.1038/ng1070. [DOI] [PubMed] [Google Scholar]
- 8.Wang LL, Xu Z, Peng Y, et al. Downregulation of inhibitor of apoptosisstimulating protein of p53 inhibits proliferation and promotes apoptosis of gastric cancer cells. Mol Med Rep. 2015;12(2):1653–58. doi: 10.3892/mmr.2015.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li M, Zhang M, Zhang ZL, et al. Induction of apoptosis by berberine in hepatocellular carcinoma HepG2 cells via downregulation of NF-κB. Oncol Res. 2017;25(2):233–39. doi: 10.3727/096504016X14742891049073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao P, Dai M, Chen W, Li N, et al. Cancer trends in China. Jpn J Clin Oncol. 2010;40(4):281–85. doi: 10.1093/jjco/hyp187. [DOI] [PubMed] [Google Scholar]
- 11.Shah MA, Kelsen DP. Gastric cancer: A primer on the epidemiology and biology of the disease and an overview of the medical management of advanced disease. J Natl Compr Canc Netw. 2010;8(4):437–47. doi: 10.6004/jnccn.2010.0033. [DOI] [PubMed] [Google Scholar]
- 12.Li W, Jie Z, Li Z, et al. ERCC1 siRNA ameliorates drug resistance to cisplatin in gastric carcinoma cell lines. Mol Med Rep. 2014;9(6):2423–28. doi: 10.3892/mmr.2014.2112. [DOI] [PubMed] [Google Scholar]
- 13.Chijiwa S, Masutani C, Hanaoka F, et al. Polymerization by DNA polymerase eta is blocked by cis-diamminedichloroplatinum(II) 1,3-d(GpTpG) cross-link: Implications for cytotoxic effects in nucleotide excision repair-negative tumor cells. Carcinogenesis. 2010;31(3):388–93. doi: 10.1093/carcin/bgp316. [DOI] [PubMed] [Google Scholar]
- 14.Kweekel DM, Gelderblom H, Guchelaar HJ. Pharmacology of oxaliplatin and the use of pharmacogenomics to individualize therapy. Cancer Treat Rev. 2005;31(2):90–105. doi: 10.1016/j.ctrv.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 15.Gottesman MM, Ling V. The molecular basis of multidrug resistance in cancer: The early years of P-glycoprotein research. Febs Letters. 2006;580(4):998–1009. doi: 10.1016/j.febslet.2005.12.060. [DOI] [PubMed] [Google Scholar]
- 16.Samuels-Lev Y, O’Connor DJ, Bergamaschi D, et al. ASPP proteins specifically stimulate the apoptotic function of p53. Mol Cell. 2001;8(4):781–94. doi: 10.1016/s1097-2765(01)00367-7. [DOI] [PubMed] [Google Scholar]
- 17.Li G, Wang R, Gao J, et al. RNA interference-mediated silencing of iASPP induces cell proliferation inhibition and G0/G1 cell cycle arrest in U251 human glioblastoma cells. Mol Cell Biochem. 2011;350(1):193–200. doi: 10.1007/s11010-010-0698-9. [DOI] [PubMed] [Google Scholar]
- 18.Xiong Y, Sun F, Dong P, et al. iASPP induces EMT and cisplatin resistance in human cervical cancer through miR-20a-FBXL5/BTG3 signaling. J Exp Clin Cancer Res. 2017;36(1):48. doi: 10.1186/s13046-017-0520-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobayashi D, Oike T, Shibata A, et al. Mitotic catastrophe is a putative mechanism underlying the weak correlation between sensitivity to carbon ions and cisplatin. Sci Rep. 2017;7:40588. doi: 10.1038/srep40588. [DOI] [PMC free article] [PubMed] [Google Scholar]