Abstract
Discovering new therapeutic interventions to treat lipid and lipoprotein disorders is of great interest and the discovery of autophagy as a regulator of lipid metabolism has opened up new avenues for targeting modulators of this pathway. Autophagy is a degradative process that targets cellular components to the lysosome and recent studies have indicated a role for autophagy in regulating hepatic lipid metabolism (known as lipophagy) as well as lipoprotein assembly. Autophagy directly targets apolipoprotein B-100 (apoB100), the structural protein component of very low-density lipoproteins (VLDLs), and further targets lipid droplets (LDs), the cellular storage for neutral lipids. Autophagy thus plays a complex and dual role in VLDL particle assembly by regulating apoB100 degradation as well as aiding the maturation of VLDL particles by hydrolyzing lipid from LDs. The purpose of this article is to review our current understanding of molecular and cellular mechanisms mediating authophagic control of hepatic lipid biogenesis and VLDL production as well as dysregulation in insulin resistance and dyslipidemia.
Keywords: autophagy, lipophagy, lipid droplets, apolipoprotein B-100, VLDL, dyslipidemia, hepatic steatosis
Introduction
Autophagy is a term derived from the Greek words "auto" meaning "self" and "phagy" meaning "to eat" and describes a catabolic process that targets cellular com-ponents to the lysosome for degradation. The formation of membrane-bound vesicle containing organelles was first reported in an experiment that exposed rat hepato-cytes to glucagon which appeared to be a stimulus for the organelle formation[1]. The process was soon understood to involve degradation and reutilization of cellular mate-rials and was given the name autophagy by de Duve in 1965[2]. Early studies characterized the regulatory role of various amino acids and hormones on hepatic autophagy, and recognized the importance of autophagy as a way to "clean out" damaged organelles and aggregated proteins that otherwise may cause cellular toxicity.
Autophagic process and cell metabolism
Three types of autophagy have been established which differ in the way cargo is targeted and carried to the lysosome. These include macroautophagy, chaperone mediated autophagy, and microautophagy. Macroautophagy is the most well-characterized and will be the main topic for this review (hereafter referred to as autophagy). Studies in yeast led to the identification of more than 32 autophagy-related genes (Atg) required for the various steps of the autophagy process. Autophagy initiates with the formation of a phagophore originating from ER or Golgi, which gradually surrounds cytoplasmic substrates and organelles in a double-membrane vesicle called the autophagosome. Vps34, a class III PI-3 kinase involved in phagophore formation in mammalian cells[3], forms a complex with Beclin-1, the mammalian ortholog of Atg6, which results in increased production of phosphatidylinosi- tol-3-phosphate (PI3P)[4] and aids in the recruitment of other autophagy related proteins to the phagophore. Following the induction of autophagy, autophagosome nucleation begins with the sequestering of cytoplasmic constituents. Sequestration is generally thought to be a non-selective process, however recent studies indicate the capability for cargo recognition. The recruitment of microtubule-associated protein light chain 3 (LC3; mammalian homolog of Atg8) into the growing phagophore by an Atg12 and Atg5 complex[5] plays an important role in selective uptake of materials by autophagy. For example, LC3 can bind p62/SQSTM1 to help clearance of polyubiquitinated protein aggregates[6], while cytosolic proteins can also act as identification tags by recognizing and binding to mitochondria that undergo structural membrane changes[7]. Following either specific or random sequestration, autophagosomes then fuse with the lysosome and cytoplasmic materials contained are degraded by lysosomal hydrolases. The final step involves recycling of the remaining amino acids for protein synthesis which is important in maintaining cellular energy balance during states of nutrient starvation. Thus, autophagy has been deemed critical for states of nutrient deprivation. However, autophagy is not limited to protein/organelle clearance and starvation adaption but are also involved in anti-degeneration, anti-aging, immunity, tumor suppression, cell death and development[8]. More recently, lipid droplets (LDs) were identifiable in double-membrane vesicles suggesting for the first time that autophagy may play a role in regulation of lipid mobilization and metabolism[6].
Lipid droplets as dynamic and multifunctional organelles
LDs are a form of cellular fat storage, which consist of lipid ester deposits surrounded by a phospholipid monolayer. A beneficial function of LDs is the ability to store free fatty acids (FFA) in the form of neutral lipids (ie/triglycerides (TG), cholesterol esters (CE)) and thereby avoid its lipotoxic effects[9]. Adipocytes are the main TG reservoir for the body and thus contain a large amount of LDs, however LDs are not specific to adipocytes and can be found in most cell types including hepatocytes. Contrary to traditional views, LDs are no longer recognized as a mere source of lipid storage but are now classified as intracellular organelles that display metabolic activity and multi-functionality. In mammals, LDs are important for states of nutrient deprivation and are conveniently located in close proximity to both mitochondria and perixosomes[10]. Therefore, when LDs undergo hydrolysis, liberated FAs can be used for β-oxidation and energy supply. On the other hand, promotion of TG production by unsaturated FAs also play a beneficial role by protect-ing against the saturated FA-induced lipotoxicity[9]. Lipid ester synthesis (and storage in the form of LDs) is also important in the defense against cellular stress and lipotoxocity[9,11-12]. Of particular importance to the liver is the role of LDs in very low density lipoprotein (VLDL) production. Previous studies have shown that VLDL-associated lipid is more commonly derived from cytosolic lipid stores as opposed to newly synthesized TG[13], suggesting a role for LDs in VLDL lipidation. More recent studies indicate Cideb, a LD associated protein, is required for VLDL lipidation. Particularly, Cideb possesses LD association domains as well as the ability to bind the structural protein component of VLDL, apolipoprotein B100 (apoB100), and plays a key role in hepatic VLDL secretion[14]. Lipidated apoB100 has also been shown to reside lumen of ER when either the proteasome or autophagy are inhib-ited[15-16]. It was proposed that the LD surface may play a functional role in the convergence of proteasomal and autophagic degradation of apoB100[15].
LD lipolysis by lipases
TG stored in hepatic LDs can be hydrolyzed in a process called lipolysis to provide lipids for β-oxidation or for the assembly of VLDL particles. Lipolysis is regulated by a wide range of enzymes called lipases, including adipose triglyceride lipase (ATGL), an important lipase highly expressed in white adipose tissue (WAT). Although ATGL expression in the liver is very low, studies in mice with liver-specific inactivation of ATGL have been shown to cause progressive hepatic steatosis[17] with a threefold increase in lipolysosomes in primary hepatocytes, suggesting a role for lysosomal TG hydrolysis in these mice. Alternatively, ATGL over-expression in hepatocytes increases hepatic β-oxidation and ameliorated hepatic steatosis[18]. Similarly, hormone sensitive lipase (HSL) has low hepatic expression levels but has been shown to increase hepatic β-oxidation indicating a role for a network of lipases that can regulate hepatic TG hydrolysis[18]. Unlike ATGL and HSL, triacylglycerol hydrolase (TGH) is a lipase that is highly expressed in the liver and is localized to the ER by an unusual retrieval sequence[19-21]. The rat hepatoma cell line McArdle, as well as the human hepatoma cell line HepG2 both lack TGH and cannot mobilize stored TG[22] and thus are mainly dependent on newly synthesized TG for VLDL assembly and secretion[26]. Various lipases that have been shown to regulate hepatic lipid metabolism have also been linked to autophagy. Chloroquine, an inhibitor of autophagy, prevents the fusion of autophagosomes with lysosomes and increases lysosomal pH, thereby inhibiting lysosomal enzymes that require an acidic pH for activation. Lysosomal acid lipase (LAL) is an essential lipase for the hydrolysis of TGs and cholesteryl esters (CE) in lysosomes and is inhibited by chloroquine treatment[23]. Chloroquine treatment has been shown to inhibit VLDL secretion from cultured rat hepatocytes[24] indicating a role of autophagy in the regulation of hepatic lipoprotein metabolism and suggesting a link between autophagy and LAL for regulation of LDs. To support this, LAL knockout mice have shown to accumulate hepatic TG and CE in their liver[25] and additional lysosomal lipases are also involved in hydrolyzing the remnants of TG-rich lipoproteins taken up by receptor-mediated endocytosis[26].
Autophagy of lipid droplets
The discovery of Atgs have uncovered the various physiological and pathophysiological roles of autophagy and the associated biological significance. Atg knockdown studies unveiled the interrelationship between autophagy and lipid metabolism, now known as lipophagy[27]. In the initial discovery of lipophagy, autophagy impairment in hepatocytes via Atg5 knockdown resulted in increased TG levels when hepatocytes were challenged with a lipid load, indicating for the first time that autophagy plays a critical role in hepatic lipid metabolism. LD and LC3 colocalization indicated a direct association between LDs and autophagosomes and this was confirmed by electron microscopy in which double membrane vesicles were shown to surround LDs[6]. This novel discovery has sparked great interest in studying the contribution of autophagy in hepatic lipid homeostasis.
Autophagy is important in the maintenance of cellular energy homeostasis and is constitutively active at basal levels in various cell types including hepatocytes. However, autophagy can also be modulated by various stimuli as demonstrated in the initial discovery with glucagon acting as an autophagy inducer[1]. Both lipid and LD associated proteins are found within autophagosomes under basal conditions, suggesting that lipophagy can occur randomly. However, upregulation of lipophagy occurs under states of nutrient deprivation and/or states of increased cellular lipid, further suggesting that lipophagy is also a selective process. While autophagy is known to be upregulated during cellular starvationvia the mTOR signalling pathway[28], the mechanism by which lipophagy is induced under cellular lipid overload is not completely understood. In other forms of selective autophagy, adaptor proteins such as p62/SQSTMl act as cargo receptors by linking ubiquitinated cargo to autophagosomal membranes via binding to LC3[29-30]. LC3 acts as an autophagosomal marker and has been shown to be associated with LDs, particularly phosphatidylethanolamine, under both fed and starved conditions[27]. The LD-LC3 association suggests that the process of LD recognition may be similar to other types of selective autophagy and may require LD-associated proteins as cargo receptors. Proteomic analysis have revealed the structure and function of an array of proteins associated with LDs which primarily include the PAT family proteins and 160 kDa capsule protein[31-33]. In mammals, the PAT family consists of perilipin (Plin1), adipose differentiation-related protein (ADRP; Plin2), tail-interacting protein of 47 kDa (TIP47; Plin3), S3-12 (Plin4) and myocardial lipid droplet protein (MLDP; Plin5). Increased expression levels of TIP47 and ADRP were observed in liver specific Atg7 knockout mice[27] suggesting that these may be cargo/LD proteins that are recognized and removed by autophagic machinery[34]. In addition to cargo receptors, ubiquitination also acts as a tag for autophagy machinery and has been found on polarized areas of LDs due to accumulation of polyubuitinated apoB100[15]. The mechanism by which apoB100 accumulation in LDs initiates autophagic/lysosomal degradation is not fully understood, but may involve ancient ubiquitous protein 1 (AUP1). AUP1 is an ER associated protein also found on the LD surface and recently shown to bind to the ubiquitination enzyme Ube2g2[35]. However, the significance of AUP1-Ube2g2 binding in LDs and the resulting degradation pathway is not fully understood.
Role of apoB100 autophagy and LD lipophagy in hepatic lipoprotein homeostasis
ApoB100, the structural component of VLDL particles, is a 550-kDa glycoprotein that is constitutively synthesized by hepatocytes[36]. ApoB100 is mainly regulated co-translationally during synthesis on ER-associated polysomes or post-translationally during transit through the secretory pathway. Synthesis of VLDL particles initiates within the ER lumen dur-ing translation of apoB100. With the aid of microsomal triglyceride transfer protein (MTP), apoB100 is lipidated to form a nascent VLDL particle[37]. These nascent particles then further mature by fusing with TG-rich particles to form secretion competent VLDL particles. In the absence of sufficient lipid supply, apoB100 translocation across the ER membrane is retarded leading to rapid targeting to proteasomal degradation by ERAD[38] . However, recent studies indicate that degradation of apoB100 can also occur through autophagy. Ohsaki et al., showed that cytoplasmic lipid droplets (CLDs) are sites where proteasomal and autophagic degradation of apoB100 converge[15]. In addition to this serendipitous finding, inhibition of autophagy by 3-MA increased the accumulation of apoB100 around CLDs in Huh7 cells while inhibition of the proteasome increased LC3 colocalization with CLDs supporting the view that CLDs may act as a site to hold misfolded apoB100 without aggregation for further degradation through autophagy[15]. Additional studies have shown that h-3 polyunsaturated fatty acids (PUFAs) such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) stimulate posttranslational degradation of apoB100 following ER exit, in a process called post-ER pre-secretory proteolytic (PERPP) pathway[39]. This degradation is due to increased generation of lipid peroxidation products that results in oxidative damage of nascent VLDL particles and formation of large aggregates by apoB100. Inhibition of autophagy has been shown to increase aggregation of apoB100 while induction of autophagy reduced apoB100 aggre-gation supporting the notion that autophagy may aid in the PERPP pathway by clearing apoB aggregates. Further studies employing DHA have shown that colocalization of LC3-GFP with apoB100 was increased in McArdle cells in the presence of DHA. Interestingly, these effects were not present after atg7 knockdown which increased apoB100 recovery from the media[40]. To confirm the effects in vivo, mice infused with DHA showed a decrease in VLDL secretion and increased hepatic lipid peroxide[39]. Interestingly, basal apoB100 degradation in primary rodent hepatocytes was shown to be regulated by lipid peroxidation as desferrioxa mine (an iron chelator) or vitamin E increased apoB100 recovery[41].
Induction of ER stress caused by glucosamine-treatment is also linked to degradation of apoB100 in an autophagy-dependent manner[42]. McArdle-7777 cells treated with glucosamine resulted in a significant increase in apoB100-GFP-LC3 puncta that was reversed by the addition of 3-MA and elevated endogenous LC3-II conversion. Interestingly, addition of PBA which facilitates protein folding, prevented ER stress-induced apoB100-autophagic degradation, however, it did not inhibit DHA-induced apoB100 autophagy in primary hepatocytes of rats[42]. This suggests the mechanism governing apoB-autophagic degradation under ER stress is likely distinct from that induced by DHA.
Autophagy is also involved in various metabolic disorders and has recently been linked to familial hypobetalipoproteinemia (FHBL); a genetic disease characterized by low levels of plasma LDL-cholesterol and apoB100. A31P was identified as a non-truncating missense ApoB mutation in heterozygous carriers of FHBL, and resulted in a significant decrease in apoB100 secretion when stably expressed in McArdle cells[43]. A31P has been shown to escape ER asso-ciated degradation and instead undergo autophagic degradation. This suggests that physiologically autophagic degradation of apoB100 may be an important regulator of VLDL secretion. In fact, apoB100 is under the regulatory control of insulin and recent studies have shown that insulin-dependent apoB100 degra-dation (IDAD) secretion is regulated by autophagy[44]. An insulin-dependent reduction in VLDL secretion was inhibited by wortmannin, a PI3-kinase inhibi-tor, in primary hepatocytes from Apobec-1-/- mice[40]. From these studies, class II PI3- kinases appeared to be the key enzymes regulating the reduction in VLDL. Interestingly, insulin treatment of primary hepatocytes from Atg5-deficient mice showed no effect on apoB100 recovery, suggesting that autophagy is required for the degradation of apoB100 in insulin-stimulated hepatocytes[40]. Conversely, Sparks et al. showed that overex-pression of phosphatase and tensin homologue (PTEN) or transfection of a kinase-deficient mutant Vps34 kinase (Class III PI3K) in McArdle cells blocked insulin suppression of apoB100 secretion thereby suggesting a role for Class I and Class III PI3K in the generation of PIP3 and PI3P in IDAD, respectively[45]. Notably, increased PIP 3 generation from ER-localized Class I PI3K led to increased interaction of apoB100 with sortilin[46] ; a sorting protein localized to the Golgi apparatus that targets various ligands to the lysosome. Increased hepatic expression of sortilin is linked to a reduction in apoB100 secretionvia lysosomal targeting[46] and has been shown to bind and facilitate the secretion of proprotein convertase subtilisin/kexin type 9 (PCSK9) in primary mouse hepatocytes[47]. PCSK9 plays an important role in the regulation of LDL-cholesterol levels by binding and targeting the LDL receptor for degradation in lysosomes[48]. Interestingly, PCSK9 overexpression can inhibit apoB100 intracel-lular degradation via the autophagosome/lysosome pathway and result in increased apoB100 secretion in various mouse models irrespective of the LDL recep-tor[49]. This highlights the complex and various roles that autophagy plays in regulating hepatic lipid home-ostasis and VLDL secretion.
Autophagy in metabolic disease and associated lipid disorders
Insulin resistant states such obesity, metabolic syndrome, and type 2 diabetes are associated with fatty liver disease, fasting dyslipidemia and hepatic insulin resistance. Despite increased understanding of the mechanisms leading to the onset and progression of these metabolic disorders, there is a lack of a well defined therapeutic strategy. Numerous therapeutic trials over the last decade have demonstrated the need for both life style/diet modifications in conjunction with pharmacological agents but have resulted in few liver-specific therapies. Targeting hepatic autophagy may have promising therapeutic implications for hepatic lipid disorders. Below we discuss the role of autophagy in the induction and progression of hepatic steatosis, dyslipidemia and insulin resistance.
Autophagy and non-alcoholic fatty liver disease
Non-alcoholic fatty liver disease (NAFLD) is characterized by excess TG accumulation in the liver (simple steatosis) and is accompanied by a continuum of histological alterations that include nonalcoholic steatohepatitis (NASH), fibrosis and hepatic cirrhosis. NAFLD affects one third of the population in developed countries[50], is associated with increased risk of cardiovascular and liver-related mortalities[51], and is considered a key manifestation of the metabolic syndrome[52]. In the initial discovery that autophagy could regulate hepatic LD turnover, it also became apparent that autophagy plays a critical role in the prevention of fatty liver disease as indicated by Atg7 liver specific knockout mice that displayed increased liver size accompanied by elevated hepatic TG and cholesterol content[27]. The mechanism of LD accumulation in these mice was proposed to involve decreased rates of FA β-oxidation. Interestingly, rates of hepatic autophagy have also been shown to be decreased in both genetic and dietary mouse models of obesity and hepatic steatosis as indicated by decreased protein levels of LC3, Beclin 1 (Atg6), Atg5 and Atg7[51]. Similar findings were observed in a recent clinical study that evaluated autophagy markers in obese non-diabetic NAFLD patients. Immunofluorescence analysis identified increased numbers of LC3-II puncta in liver biopsies of patients with either simple steatosis or NASH accompanied by increased protein levels of p62 and LC3-II/ LC3-I ratio in both patients, suggesting reduced hepatic autophagy[52]. Whether LD accumulation in obesity leads to impaired hepatic lipophagy, or vice versa is not known, however it is likely that they both occur leading to a positive feedback effect and thereby exacerbating fatty liver.
Autophagy and metabolic dyslipidemia
Fasting dyslipidemia is a central driving force for increased risk of cardiovascular disease and is directly associated with hepatic lipoprotein overproduction. Maintaining normal levels of VLDL production is important for both hepatic and circulating lipid homeostasis. VLDL overproduction can result in the formation of atherogenic small dense LDL particles that more easily penetrate the arterial wall. Alternatively, defective VLDL production prevents lipid export and results in hepatic steatosis. Autophagy plays a paradoxical role in the regulation of VLDL by mobilizing hepatic lipid stores important for apoB stability[24,27,53], but also con-tributes to apoB100 degradation (Fig. 1)[15,41]. Given that 80% of NAFLD patients display dyslipidemia[50], it may be reasonable to assume that increases in hepatic lipid accumulation due to defective autophagy would then further result in dyslipidemia. However, a study using rat hepatocytes showed increased hepatic TG secretion upon autophagy induction[54] suggesting that lipid mobilization via autophagy may be required for hepatic lipid export. Furthermore, hepatocyte-specific Atg7 knockout mice displayed decreased hepatic TG secretion[27], thereby suggesting that activation, rather than inhibition, of autophagy potentially contributes to dyslipidemia. To further support these observations, a clinical study in kidney transplant recipients treated with mTOR inhibitors sirolumus or everolimus showed increased plasma TG and LDL-cholesterol levels[55]. The activation of autophagy may induce dyslipidemia by mobilizing hepatic LDs thereby increasing lipid availability for VLDL assembly, but still causing a reduction in hepatic steatosis. Further experimental evidence is necessary to clarify the relationship between hepatic autophagy, steatosis and dyslipidemia.
Autophagy and insulin resistance
Hepatic insulin resistance is defined as the failure of insulin to adequately suppress hepatic glucose production without compromising the lipogenic actions of insulin. Due to the lack of inhibition of lipid metabolism, insulin resistance is strongly linked to the onset of hepatic steatosis and dyslipidemia[56-57], leading to increased efforts to understand the development and progression of this disease. Recently, defective autophagy has been linked to the onset of hepatic insulin resistance as shown in a study where mice fed a high fat diet (HFD) had decreased expression of hepatic Atg7 which was further associated with induction of ER stress and defective insulin signalling[51]. In the last decade, ER stress has been recognized as a con-tributor to insulin resistance due to its direct inhibitory effect on insulin signalling and indirect effect on lipid accumulation[58]. Impaired autophagy may contribute to the induction of ER stress via the accumulation of misfolded protein aggregates. Additional studies in both genetic and dietary models of obesity have shown simultaneous defects in both autophagy and insulin signalling pathways[51]. Diet-induced obese mice display increased activation of the autophagy inhibitor mTOR, associated with increased phosphorylation and inhibition of insulin receptor substrate 1 (IRS1) and impaired Akt activity[59]. In a separate study, HFD induced hyperinsulinemia was associated with decreased levels of hepatic autophagy and was reversed by streptozotocin-induced insulin deficiency[60] thereby highlighting a direct link between insulin and autophagic regulation. Recently, hepatic lipophagy has been shown to be modulated by an incretin hormone known as glucagon-like peptide-1 (GLP-1)[61]. GLP-1 is a gut-derived hormone that acts as an insulin secretagogue and has been shown to increase insulin sensitivity and effectively lower glucose levels in patients with type 2 diabetes. GLP-1 has been implicated in the regulation of lipid metabolism by decreasing diet-induced hepatic steatosis and VLDL production in rodents[62-64]. Sharma et al. implicate the involvement of autophagy in these lipid-lowering effects by highlighting an association between GLP-1 induced autophagy and the reduction of both hepatic steatosis and ER stress[65-66]. This suggests that insulin sensitizing agents may be effective in modulating hepatic autophagy and reducing hepatic steatosis.
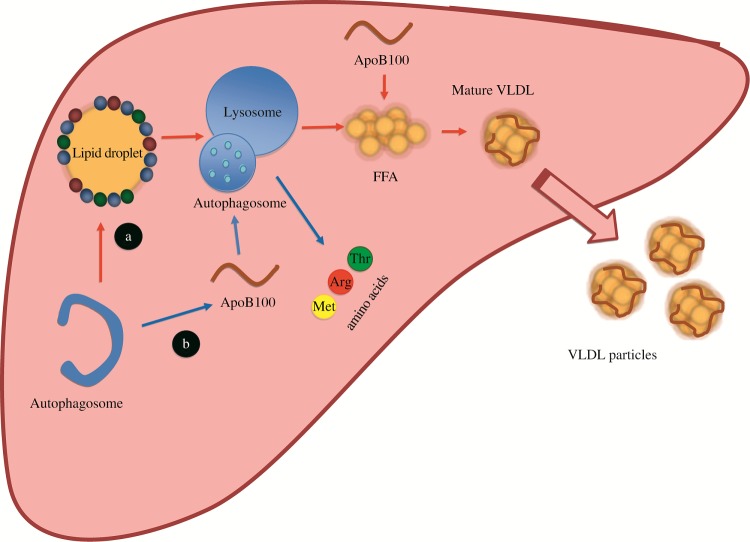
Fig.1. Dual role of autophagy in hepatic lipid and lipoprotein metabolism.
A: Autophagy directly targets LDs to mobilize FFAs which can then be incorporated with apoB100 to form mature VLDL particles for secretion (red arrows). B: Concurrently, autophagy can degrade misfolded apoB100 and pre-mature VLDLs (blue arrows). The balance between lipid mobilization and apoB degradation determines the rate of hepatic VLDL production.
Concluding remarks
Impaired autophagy has been linked to the onset and progression of various metabolic diseases there by increasing interest into determining the therapeutic potential of autophagy modulators. While pharmacological manipulation of autophagy is currently being tested in clinical trials for treatment of cancer and neuronal degeneration, regulation of lipid metabolism may prove to be more complex. Many questions remain unanswered. For example, how does autophagy selectively act on LDs? Are apoB degradation by autophagy and lipid mobilization through lipophagy under the same regulatory controls? How does insulin and other circulating hormones regulate hepatic autophagy? Answering these questions will provide a better understanding of the complex role that autophagy plays in regulating lipid metabolism and may identify potential targets for therapeutic intervention in metabolic diseases associated with hepatic steatosis and dyslipidemia.
References
- 1. Ashford TP, Porter KR. Cytoplasmic components in hepat-ic cell lysosomes[J]. J Cell Biol, 1962,12(1):198–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Klionsky DJ. Autophagy revisited: a conversation with Christian de Duve[J]. Autophagy, 2008,4(6):740–743. [DOI] [PubMed] [Google Scholar]
- 3. Jaber N, Dou Z, Chen J-S, et al. Class III PI3K Vps34 plays an essential role in autophagy and in heart and liv-er function[J]. Proc Natl Acad Sci U S A, 2012,109(6): 2003-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Funderburk SF, Wang QJ, Yue Z. The Beclin 1-VPS34 complex--at the crossroads of autophagy and beyond[J]. Trends Cell Biol, 2010,20(6):355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.[ Romanov J, Walczak M, Ibiricu I, et al. Mechanism and functions of membrane binding by the Atg5-Atg12/Atg16 complex during autophagosome formation[J]. Embo J, 2012,31(22):4304–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms[J]. J Pathol, 2010,221(1):3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Youle RJ, Narendra DP. Mechanisms of mitophagy[J]. Nat Rev Mol Cell Biol, 2011,12(1):9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mizushima N. T he pleiotropic role of autophagy: from protein metabolism to bactericide[J]. Cell Death Differ, 2005,12:1535–1541. [DOI] [PubMed] [Google Scholar]
- 9. Listenberger LL, Han X, Lewis SE, et al. Triglyceride accumulation protects against fatty acid-induced lipo-toxicity[J]. Proc Natl Acad Sci U S A, 2003,100(6): 3077–3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zehmer JK, Huang Y, Peng G, et al. A role for lipid drop-lets in inter-membrane lipid traffic[J]. Proteomics, 2009, 9(4):914–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gubern A, Barceló-Torns M, Casas J, et al. Lipid droplet biogenesis induced by stress involves triacylglycerol syn-thesis that depends on group VIA phospholipase A2[J].J Biol Chem, 2009,284(9):5697–5708. [DOI] [PubMed] [Google Scholar]
- 12. Schaffer JE. Lipotoxicity: when tissues overeat[J]. Curr Opin Lipidol, 2003,14(3):281–287. [DOI] [PubMed] [Google Scholar]
- 13. Gibbons GF, Wiggins D. Intracellular triacylglycerol lipase: its role in the assembly of hepatic very-low-density lipoprotein (VLDL)[J]. Adv Biol Regul, 1995,35:179–198. [DOI] [PubMed] [Google Scholar]
- 14. Ye J, Li JZ, Liu Y, et al. Cideb, an ER-and lipid droplet-as-sociated protein, mediates VLDL lipidation and matura-tion by interacting with apolipoprotein B[J]. Cell Metab, 2009,9(2):177–190. [DOI] [PubMed] [Google Scholar]
- 15. Ohsaki Y, Cheng J, Fujita A, et al. Cytoplasmic lipid drop-lets are sites of convergence of proteasomal and autophag-ic degradation of apolipoprotein B[J]. Mol Biol Cell, 2006, 17(6):2674–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ohsaki Y, Cheng J, Suzuki M, et al. Lipid droplets are arrested in the ER membrane by tight binding of lipidat-ed apolipoprotein B-100[J]. J Cell Sci, 2008,121(14): 2415–2422. [DOI] [PubMed] [Google Scholar]
- 17. Wu JW, Wang SP, Alvarez F, et al. Deficiency of liver adi-pose triglyceride lipase in mice causes progressive hepatic steatosis[J]. Hepatology, 2011,54(1):122–132. [DOI] [PubMed] [Google Scholar]
- 18. Reid BN, Ables GP, Otlivanchik OA, et al. Hepatic overex-pression of hormone-sensitive lipase and adipose triglycer-ide lipase promotes fatty acid oxidation, stimulates direct release of free fatty acids, and ameliorates steatosis[J]. J Biol Chem, 2008,283(19):13087–13099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lehner R, Verger R. Purification and characterization of a porcine liver microsomal triacylglycerol hydrolase[J]. Biochemistry, 1997,36(7):1861–1868. [DOI] [PubMed] [Google Scholar]
- 20. Lehner R, Vance D. Cloning and expression of a cDNA encoding a hepatic microsomal lipase that mobilizes stored triacylglycerol[J]. Biochem J, 1999,343:1–10. [PMC free article] [PubMed] [Google Scholar]
- 21. Gilham D, Alam M, Gao W, et al. Triacylglycerol hydro-lase is localized to the endoplasmic reticulum by an unu-sual retrieval sequence where it participates in VLDL assembly without utilizing VLDL lipids as substrates[J]. Mol Biol Cell, 2005,16(2):984–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lehner R, Cui Z, Vance D. Subcellullar localization, developmental expression and characterization of a liv-er triacylglycerol hydrolase[J]. Biochem J, 1999,338: 761–768. [PMC free article] [PubMed] [Google Scholar]
- 23. Rustan AC, Nossen JO, Tefre T, et al. Inhibition of very-low-density lipoprotein secretion by chloroquine, verapamil and monensin takes place in the Golgi complex-[J]. Biochim Biophys Acta, 1987,930(3):311–319. [DOI] [PubMed] [Google Scholar]
- 24. Nossen JO, Rustan AC, Barnard T, et al. Inhibition by chloroquine of the secretion of very low density lipopro-teins by cultured rat hepatocytes[J]. Biochim Biophys Acta, 1984,803(1):11–20. [DOI] [PubMed] [Google Scholar]
- 25. Du H, Heur M, Duanmu M, et al. Lysosomal acid lipase-deficient mice: depletion of white and brown fat, severe hepatosplenomegaly, and shortened life span[J]. J Lipid Res, 2001,42(4):489–500. [PubMed] [Google Scholar]
- 26. Brown MS, Goldstein JL. Receptor-mediated control of cholesterol metabolism[J]. Science, 1976,191(4223): 150–154. [DOI] [PubMed] [Google Scholar]
- 27. Singh R, Kaushik S, Wang Y, et al. Autophagy regulates lipid metabolism[J]. Nature, 2009,458(7242):1131–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Neufeld TP. TOR-dependent control of autophagy: biting the hand that feeds[J]. Curr Opin Cell Biol, 2010,22(2): 157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dikic I, Johansen T, Kirkin V. Selective autophagy in can-cer development and therapy[J]. Cancer Res, 2010,70(9): 3431–3434. [DOI] [PubMed] [Google Scholar]
- 30. Johansen T, Lamark T. Selective autophagy mediated by autophagic adapter proteins[J]. Autophagy, 2011,7(3): 279–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fujimoto Y, Itabe H, Sakai J, et al. Identification of major proteins in the lipid droplet-enriched fraction isolated from the human hepatocyte cell line HuH7[J]. Biochim Biophys Acta, 2004,1644(1):47–59. [DOI] [PubMed] [Google Scholar]
- 32. Okumura T. Role of lipid droplet proteins in liver steato-sis[J]. J Physiol Biochem, 2011,67(4):629–636. [DOI] [PubMed] [Google Scholar]
- 33. Bickel PE, Tansey JT, Welte MA. PAT proteins, an ancient family of lipid droplet proteins that regulate cellular lipid stores[J]. Biochim Biophys Acta, 2009,1791(6):419–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dong H, Czaja MJ. Regulation of lipid droplets by auto-phagy[J]. Trends Endocrinol Metab, 2011,22(6):234–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Spandl J, Lohmann D, Kuerschner L, et al. Ancient ubiqui-tous protein 1 (AUP1) localizes to lipid droplets and binds the E2 ubiquitin conjugase G2 (Ube2g2) via its G2 binding region[J]. J Biol Chem, 2011,286(7):5599–5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rutledge AC, Su Q, Adeli K. Apolipoprotein B100 biogen-esis: a complex array of intracellular mechanisms regulating folding, stability, and lipoprotein assembly[J]. Biochem Cell Biol, 2010,88(2):251–267. [DOI] [PubMed] [Google Scholar]
- 37. Hussain MM, Shi J, Dreizen P. Microsomal triglyceride transfer protein and its role in apoB-lipoprotein assembly[J]. J Lipid Res, 2003,44(1):22–32. [DOI] [PubMed] [Google Scholar]
- 38. Liao W, Chang BHJ, Mancini M, et al. Ubiquitin-dependent and-independent proteasomal degradation of apoB associated with endoplasmic reticulum and golgi apparatus, respectively, in HepG2 cells[J]. J Cell Biochem, 2003,89(5):1019–1029. [DOI] [PubMed] [Google Scholar]
- 39. Pan M, Cederbaum AI, Zhang Y-L, et al. Lipid peroxida-tion and oxidant stress regulate hepatic apolipoprotein B degradation and VLDL production[J]. J Clin Invest, 2004, 113(9):1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Andreo U, Guo L, Chirieac DV, et al. Insulin-stimulated degradation of apolipoprotein B100: roles of class II phos-phatidylinositol-3-kinase and autophagy[J]. PloS one, 2013,8(3):e57590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fisher EA, Pan M, Chen X, et al. The triple threat to nascent apolipoprotein B Evidence for multiple, distinct degradative pathways[J]. J Biol Chem, 2001,276(30): 27855–27863. [DOI] [PubMed] [Google Scholar]
- 42. Qiu W, Kohen-Avramoglu R, Mhapsekar S, et al. Glucosamine-induced endoplasmic reticulum stress promotes ApoB100 degradation evidence for Grp78-mediated targeting to proteasomal degradation[J]. Arterioscler Thromb Vasc Biol, 2005,25(3):571–577. [DOI] [PubMed] [Google Scholar]
- 43. Zhong S, Magnolo AL, Sundaram M, et al. Nonsynonymous mutations within APOB in human familial hypobetali-poproteinemia evidence for feedback inhibition of lipo-genesis and postendoplasmic reticulum degradation of apolipoprotein B[J]. J Biol Chem, 2010,285(9):6453–6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bostr�m K, Boren J, Wettesten M, et al. Studies on the assembly of apo B-100-containing lipoproteins in HepG2 cells[J].J Biol Chem, 1988,263(9):4434–4442. [PubMed] [Google Scholar]
- 45. Sparks JD, O'Dell C, Chamberlain JM, et al. Insulin-dependent apolipoprotein B degradation is mediated by autophagy and involves class I and class III phosphatidy-linositide 3-kinases[J]. Biochem Biophys Res Commun, 2013,435(4):616–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chamberlain JM, O’Dell C, Sparks CE, et al. Insulin suppres-sion of apolipoprotein B in McArdle RH7777 cells involves increased sortilin 1 interaction and lysosomal targeting[J]. Biochem Biophys Res Commun, 2013,430(1):66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gustafsen C, Kjolby M, Nyegaard M, et al. The hypercho-lesterolemia-risk gene SORT1 facilitates PCSK9 secre-tion[J]. Cell Metab, 2014,19(2):310–318. [DOI] [PubMed] [Google Scholar]
- 48. Norata GD, Tibolla G, Catapano AL. Targeting PCSK9 for hypercholesterolemia[J]. Annu Rev Pharmacol Toxicol, 2014,54:273–293. [DOI] [PubMed] [Google Scholar]
- 49. Sun H, Samarghandi A, Zhang N, et al. Proprotein conver-tase subtilisin/kexin type 9 interacts with apolipoprotein B and prevents its intracellular degradation, irrespective of the low-density lipoprotein receptor[J]. Arterioscler Thromb Vasc Biol, 2012,32(7):1585–1595. [DOI] [PubMed] [Google Scholar]
- 50. Grattagliano I, Portincasa P, Palmieri VO, et al. Managing nonalcoholic fatty liver disease Recommendations for family physicians[J]. Can Fam Physician, 2007,53(5): 857–863. [PMC free article] [PubMed] [Google Scholar]
- 51. Yang L, Li P, Fu S, et al. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resist-ance[J]. Cell Metab, 2010,11(6):467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gonzalez-Rodriguez A, Mayoral R, Agra N, et al. Impaired autophagic flux is associated with increased endoplasmic reticulum stress during the development of NAFLD[J].Cell Death Dis, 2014,5(4):e1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Francone OL, Kalopissis AD, Griffaton G. Contribution of cytoplasmic storage triacylglycerol to VLDL-triacylglycerol in isolated rat hepatocytes[J]. Biochim Biophys Acta, 1989,1002(1):28–36. [DOI] [PubMed] [Google Scholar]
- 54. Škop V, Cahová M, Papáčková Z, et al. Autophagy-lysosomal pathway is involved in lipid degradation in rat liver[J]. Physiol Res, 2012,61:287–297. [DOI] [PubMed] [Google Scholar]
- 55. Kasiske BL, De Mattos A, Flechner SM, et al. Mammalian target of rapamycin inhibitor dyslipidemia in kidney transplant recipients[J]. Am J Transplant, 2008,8(7): 1384–1392. [DOI] [PubMed] [Google Scholar]
- 56. Adeli K, Taghibiglou C, Van Iderstine SC, et al. Mechanisms of hepatic very low-density lipoprotein over-production in insulin resistance[J]. Trends Cardiovascul Med, 2001,11(5):170–176. [DOI] [PubMed] [Google Scholar]
- 57. Festi D, Colecchia A, Sacco T, et al. Hepatic steatosis in obese patients: clinical aspects and prognostic significan-ce[J]. Obes Rev, 2004,5(1):27–42. [DOI] [PubMed] [Google Scholar]
- 58. Flamment M, Hajduch E, Ferré P, et al. New insights into ER stress-induced insulin resistance[J]. Trends Endocrinol Metab, 2012,23(8):381–390. [DOI] [PubMed] [Google Scholar]
- 59. Khamzina L, Veilleux A, Bergeron S, et al. Increased acti-vation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: possible involve-ment in obesity-linked insulin resistance[J]. Endocrinology, 2005,146(3):1473–1481. [DOI] [PubMed] [Google Scholar]
- 60. Liu H-Y, Han J, Cao SY, et al. Hepatic autophagy is sup-pressed in the presence of insulin resistance and hyperinsu-linemia inhibition of FoxO1-dependent expression of key autophagy genes by insulin[J]. J Biol Chem, 2009,284(45): 31484–31492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mells JE, Fu PP, Sharma S, et al. Glp-1 analog, liraglu-tide, ameliorates hepatic steatosis and cardiac hypertrophy in C57BL/6J mice fed a Western diet[J]. Am J Physiol Gastrointest Liver Physiol, 2012,302(2):G225–G235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Taher J, Baker CL, Cuizon C, et al. GLP-1 receptor ago-nism ameliorates hepatic VLDL overproduction and de novo lipogenesis in insulin resistance[J]. Mol Metab, 2014,3(9):823–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Farr S, Taher J, Adeli K. Glucagon-like peptide-1 as a key regulator of lipid and lipoprotein metabolism in fasting and postprandial states[J]. Cardiovasc Hematol Disord Drug Targets, 2014,14(2):126–136. [DOI] [PubMed] [Google Scholar]
- 64. Parlevliet ET, Wang Y, Geerling JJ, et al. GLP-1 receptor activation inhibits VLDL production and reverses hepatic steatosis by decreasing hepatic lipogenesis in high-fat-fed APOE*3-Leiden mice[J]. PLoS One, 2012,7(11):e49152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sharma S, Mells JE, Fu PP, et al. GLP-1 analogs reduce hepatocyte steatosis and improve survival by enhancing the unfolded protein response and promoting macroauto-phagy[J]. PLoS One, 2011,6(9):e25269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Christian P, Sacco J, Adeli K. Autophagy: emerging roles in lipid homeostasis and metabolic control. Biochim Biophys Acta, 2013,1831(4):819–824. [DOI] [PubMed] [Google Scholar]