Abstract
The importance of extracellular vesicles (EVs) in cell-cell communication has long been recognized due to their ability to transfer important cellular cargoes such as DNA, mRNA, miRNAs, and proteins to target cells. Compelling evidence supports the role of EVs in the horizontal transfer of cellular material which has the potential to influence normal cellular physiology and promote various disease states. Of the different types of EVs, exosomes have garnered much attention in the past decade due to their abundance in various biological fluids and ability to affect multiple organ systems. The main focus of this review will be on cancer and how cancer-derived exosomes are important mediators of metastasis, angiogenesis, immune modulation, and the tumor macro-/microenvironment. We will also discuss exosomes as potential biomarkers for cancers due to their abundance in biological fluids, ease of uptake, and cellular content. Exosome use in diagnosis, prognosis, and in establishing treatment regimens has enormous potential to revolutionize patient care.
Keywords: tumor macro-environment, microenvironment, exosomes, cancer, biomarker, therapeutics, metastasis
Introduction
Intercellular communication via membrane-derived vesicles has been studied for over three decades with new advances related to its synthesis as well as functions in several biological systems[1-7]. The initial discovery of membrane-derived vesicles occurred in 1946 when Chargaff and West hypothesized that human plasma contained a coagulation factor that promoted plasma clotting after finding that high-speed centrifugation of plasma and removal of the fractionated pellet led to the suppression of clot formation[1]. Twenty years later British physician Peter Wolf termed this pelleted plasma fraction as "platelet dust"[8] and found that these small platelet-derived vesicles were 20-50 nm in diameter with a density of 1.020-1.025 g/mL in the plasma[8]. In 1987, vesicles approximately 50 nm in diameter were found in the culture medium of sheep reticulocytes after high-speed centrifugation[2]. These vesicles were highly active and included ace-tylcholinesterase, ctyochalasin B binding (glucose transporter), nucleoside binding, and Na-amino acid transport. In addition, components of the reticulocyte plasma membrane (eg. transferrin receptor) but no other cellular components of the blood were present[2].
The identification of any membrane-derived vesicles would soon be classified in a general sense as "extracellular vesicles" (EVs). Three main classes of EVs emerged: microvesicles (100-1,000 nm), exosomes (30-150 nm), and apoptotic blebs (1-5 μm)[9-10]. All three classes of EVs differ not only in size, but also in morphology, content, mode of generation, and mechanism of release, for example. Both microvesicles and exosomes play critical roles in intracellular communication through the horizontal transfer of cellular cargoes such as DNA, mRNA, miRNAs, peptides, and proteins[10-11]. Although both types of EVs have been shown to have significant impacts on various disease states, this review will focus on providing a comprehensive overview of exosomes and their overall impact in various disease processes with the primary focus being on cancer and the tumor micro-/macroenvironment.
Biogenesis of exosomes
Understanding the mechanisms of exosome biogenesis could shed light on important biological processes and aid in the development of novel extracellular vesicle-based therapies for early detection and treatment of various diseases. It is well known that almost all cell types in the human body secrete exosomes. The "classical pathway" for exosomal generation has been extensively studied and is believed to originate in multivesicular bodies (MVBs) as intraluminal vesicles (ILVs). The sorting and eventual formation of ILVs requires endosomal-sorting complex required for transport (ESCRT) machinery[12-13]. A report from Colombo and colleagues identified that silencing a large panel of ESCRT components revealed ESCRT-0 (HRS, STAM1) and ESCRT-I (TSG101) altered exosome secretion of exosomal CD63 and MHC II, but each component had varying effects on the size and protein composition of these exosomes[14]. In addition, tetraspanin proteins CD9 and CD63 are believed to be involved in sorting transmembrane proteins into ILVs for secretion from the cell[15,16]. However, there are other ESCRT-independent processes that have been shown to contribute to exosome formation. Trajkovicet al.revealed that sphingolipid ceramide-containing exosomes isolated from an oligodendrocyte cell line does not require ESCRT machinery for formation and release[17].
Once ILVs are formed, the MVB can either fuse to the lysosome to degrade its contents or fuse to the plasma membrane and release its contentsvia newly-formed exosomes. Fusion of exosomes with the plasma membrane has been shown to require specific Rab proteins for docking and fusion of the MVB to the plasma membrane, however, this is dependent on the cell type[18-22]. Although Rab GTPases are necessary for exosome release, the exact Rab GTPases is used and how cell type uses them is not fully understood. For example, Rab 27a and Rab 27b were both shown to have differing roles in the exosomal pathway, but both were necessary for the docking of the MVB to the plasma membrane[22]. Additionally, Rab 11 was found to be required for Ca2+-induced exosome release in K562 cells (leukemia cell line), but the specific mechanism is unknown[19].
Content of exosomes
Exosomes are 30-150 nm sized membranous vesicles that are endogenously produced by almost all cell types. Additional characteristics of exosomes include a buoyant density of 1.10-1.21 g/mL, a lipid composition that includes cholesterols, ceramides, lipid rafts, and sphingomyelin, and surface protein markers such as Alix, TSG101 (ESCRT machinery), CD63, CD9, CD81 (tetraspanins) and HSP70. Exosomes can be isolated from a variety of biological fluids including blood, urine, breast milk, and saliva. The content of exosomes has been shown to vary based on the cell type secreting it, however in general, exosomes have been shown to contain DNA, RNA, miRNA, cytoskeletal and heatshock proteins, MHC Class I and II molecules, and peptides[7]. Understanding the content of exosomes can impact the study of various diseases. A recent report by Thakur et al. demonstrated that double-stranded DNA (dsDNA) was contained within human myelog-enous leukemia cell line exosomes. They showed that this "exosomal DNA" was a reflection of the entirety of the genome, and that this information could be used to identify the mutational status of cancerous cells[5]. The diverse content and shuttling of exosomal content is particularly relevant in the context of tumorigenesis due to local and distal tissues having been shown to acquire the characteristics of the primary tumor. The remainder of this review will focus primarily on this topic, specifically on how exosomes affect the tumor macro/microenvironment.
Exosomes in pathophysiology
Immunology: Exosomes have been implicated in the immunological mechanisms of disease and through both promotion and suppression of immune responses. As a consequence, various immunological processes are affected, such as immune surveillance, immuno-suppression, and intercellular communication. Quah and colleagues demonstrated a method that could modulate immune responses by using exosomes that were produced by immature dendritic cells (DCs) in spleen long-term culture (LTC)[24]. In another study, DC exosomes were found to contain MHC class II, CD40, CD83, and TNF receptors[25]. These DC-derived
Fig.1.
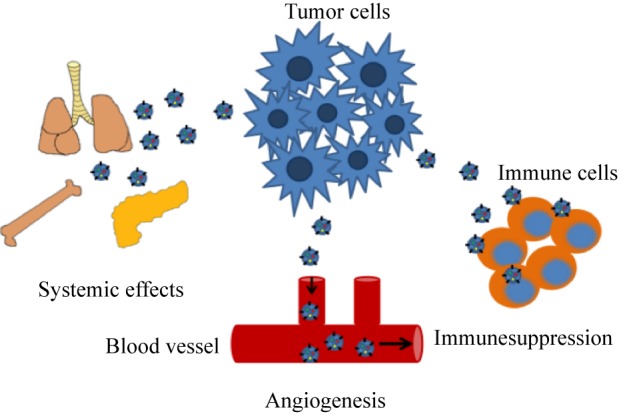
Effects of tumor-derived exosomes on the macroenvironment
exosomes could readily enter target epithelial cells, stimulate cytokine and chemokine release, and elicit both innate and adaptive immunity responses[25]. In the context of cancer, tumor-derived exosomes have also been shown to carry immunological factors that can either promote or suppress immune responses. For example, exosomes isolated from human and murine B lymphocytes induced antigen-specific MHC class II-restricted T cell responses, which suggests a pivotal role for tumor-derived exosomes in antigen presentation[26]. Interestingly, MHC class I and II complexes have been shown on the surface of exosomes. In a study by Montecalvoet al., exosomes from splenic DCs were shown to transport alloantigens, which consequently led to the activation of anti-donor CD4+ T cells[27]. It has also been reported that tumor-derived exosomes converted myeloid cells into myeloid-derived suppressor cell (MDSCs) (CD11b+Gr1+) which promotes tumor growth[28]. MDSC populations have been found to be expanded in a variety of cancers, leading to fur-ther tumor progression and immune evasion[28-30]. This topic will be revisited with the focus being on the role of exosomes in immune-mediated responses to the tumor microenvironment.
Cardiovascular diseases: The study of the exosomes in cardiovascular diseases is an emerging field with many unanswered questions. Exosomes have been implicated in the pathology of cardiac remodeling including cardiac hypertrophy, diabetic cardiomyopathy, and sepsis-induced cardiovascularity[31]. Bang et al. showed that miRNA-enriched exosomes were secreted by car-diac fibroblasts and interacted with cardiac myocytes, leading to cellular hypertrophy[32]. They identified miR-21* in cardiac fibroblast-derived exosomes, which induced cardiomyocyte hypertrophy, while pharmacological inhibition of miR-21* in a mouse model of Ang II-induced cardiomyocyte hypertrophy significantly decreased this pathology[32].
In addition to cardiac remodeling, exosomes have been shown to have protective effects in the myocardium. Recently, the release of paracrine and autocrine factors such as proteins, miRNAs, cytokines/growth factors, and now exosomes and microvesicles from stem cells has been the subject of much interest in cardiac repair[33]. In one study, mice underwent ischemia followed by reperfusion[34]. Exosomes isolated from mesenchymal stem cells were administered prior to reperfusion, which reduced infarct size by 45% compared to saline alone[34]. In addition, cardiac function was preserved up to 28 days after exosome treatment, which also decreased oxidative stress and increased ATP, and NADH levels[34]. In total, this collective evi-dence supports their hypothesis that mesenchymal-cell derived exosomes have the potential for use as an adjuvant to reperfusion injury.
Exosomes and cancer
The development of cancer is a complex process that involves the coordination of events such as a chronic inflammatory response, environmental insults, and genetic factors all of which can lead to tumor development[35]. The tumor microenvironment, which consists of the tumor stroma, surrounding blood vessels, and the tumor cells themselves, is pivotal for the growth and eventual dissemination of the tumor, consequently leading to drug resistance and metastasis. Deregulation of these cellular interactions can lead to a disruption in normal cell-cell communication, thereby promoting tumorigenesis[35]. In addition, genetic instability of the cancerous cells, stress stimuli such as hypoxia and oxidative stress, as well as an inflammatory response can collectively promote tumorigenesis[35]. Emerging evidence has shown that exosomes play a critical role in causing deregulated local and systemic cellular communication in the tumor microenvironment.
Metastasis: The sequence of events leading up to the development of tumor cell invasion and eventual metastasis is a key factor in the prognosis of cancer patients. Tumor metastasis requires the intricate coordination of events that lead to the formation of secondary tumors at distant locations including invasion of escaped tumor cells. Invasion requires malignant cells to lose their adhesion capacity, allowing cells to disseminate from the primary tumor and invade local stroma. The mechanisms of invasion are complex and involve cytoskeletal alterations, which lead to degradation of the basement membrane and either upregulation or suppression of the genes involved in migration and motility[36]. Exosomes may provide a mechanistic link to tumor metastasis based on their cellular content and ability to transport content to target cells. Ramteke and colleagues showed that exosomes secreted by hypoxic prostate cancer cells can influence distant and neighboring tumor cells and the surrounding microenvironment[37]. These tumor-derived exosomes in turn enhanced prostate cancer cell invasiveness and metastasis[37]. Proteomic profiling of exosomes isolated from epithelial Madin-Darby canine kidney (MDCK) cells transformed with oncogenic H-Ras revealed the presence of several fac-tors associated with altering the tumor microenvironment[38]. These factors included proteases, annexins, secreted extra cellular matrix (ECM) components, and integrins[38]. Jeppesen and colleagues studied the content of exosomes isolated from a human bladder carcinoma cell line (T24) without metastatic capacity and two isogenic derivative cell lines that caused either lung or liver metastases in mice[39]. They reported an increase in tumor-derived exosomes, which show increased proteins related to epithelial to mesenchymal transition (EMT) including vimentin, hepatoma-derived growth factor (HDGF), casein kinase II, and annexin A2[39].
The formation of a pre-metastatic niche in distal sites is integral to the formation of metastatic disease. A growing body of evidence has shown that tumor-derived exosomes are necessary for pre-metastatic niche formation. A premetastatic niche was first hypothesized by Pagetet al. in their "seed in soil" model which reported that critical oncogenic events such as the recruitment of pro-tumorigenic factors and extracellular matrix remodeling were necessary for metastasis[40]. In addition to the metastatic niche, it is now widely accepted that a premetastatic niche can form by the release of various cytokines and growth factors from the primary tumor. Release of these factors consequently mobilizes bone-marrow-derived cells which have the capacity to be recruited to distal organs for future metastasis. Seminal work by Peinadoet al. demonstrated the importance of exosomes in the "seed and soil" hypothesis[41]. Exosomes isolated from highly-metastatic melanomas increased the metastatic potential of the primary tumors by ‘educating’ bone marrow progenitor cells through the receptor tyrosine kinase MET[41]. Vascular leakiness was also induced by melanoma-derived exosomes, which aided in reprogramming bone marrow progenitor cells towards a pro-vasculogenic phenotype[41]. A report by Zhou and colleagues investigated the role of exosome-mediated transfer of miR-105 in metastatic breast cancer cells[42]. miR-105 is found to be expressed and secreted by met-astatic breast cancer cells and targets the tight junction protein ZO-1[42]. Exosome-mediated transfer from breast cancer exosomes was shown to destroy the integrity of the tight junctions by release of miR-105 which targets ZO-1. As a consequence, this interaction was shown to enhance vascular permeability[42].
Recent evidence of exosomes in the formation of the pre-metastatic niche was demonstrated by Costa-Silva et al. in which pancreatic ductal adenocarcinoma (PDAC)-derived exosomes initiated a pre-metastatic niche in the liver[43]. The uptake of these exosomes by Kupffer cells was shown to activate and release trans-forming growth factor β (TGFβ) via exosomal migration inhibitory factor (MIF), thus promoting a proinflammatory environment suitable for metastasis[43].
Angiogenesis: Angiogenesis is imperative during cancer progression and may be initiated by hypoxic, nutrient-depleted conditions, and inflammatory responses evident in the tumor microenvironment[44]. Tumors can induce angiogenesis by secreting growth factors such as VEGF, TGF-β, PDGF, and bFGF that promote capillary growth to the tumor. Such growth factors are known to supply necessary nutrients for its survival[45]. Specifically, these factors aid in the promotion of angiogenesis by regulating quiescence, migration, and proliferation of endothelial cells[45,46].
In a recent study, exosomes isolated from hypoxic-resistant multiple myeloma cells were shown to contain an abundance of oncogenic miR-135b, which directly suppressed its target, factor-inhibiting hypoxia-inducible factor 1 (FIH-1) in endothelial cells[47]. Interestingly, exosomal miR-135b was found to promote endothelial tube formation under hypoxic conditions through HIF-FIH signaling[47]. In a study conducted by Kucharzewska and colleagues exosomes isolated from glioblastoma multiforme (GBM) cells grown in hypoxic conditions promoted angiogenesis bothex vivo and in vitro in endothelial cells[48]. Hypoxic GBM cell-derived exosomes were found to program endothelial cells to secrete growth factors and cytokines in addition to activating pericyte PI3K/AKT signaling and migration[48].
Immune-mediated responses to tumor microenvi-ronment: Immune responses in cancer consist of both innate and adaptive immunity, with the tumor microenvironment containing cells from each spectrum. Innate immune cells, such as macrophages, dendritic cells (DCs), natural killer cells, neutrophils, and myeloid derived suppressor cells are present in the tumor microenvironment. Adaptive immune cells, which consist of T and B lymphocytes, can also be found in the tumor microenvironment. However, the most predominant cells present in the tumor are T cells and tumorassociated macrophages (TAMs)[49]. These cells form a complex network in which they communicate through direct contact or secretion of cytokines/chemokines in an autocrine or paracrine manner in order to promote tumorigenesis. Chronically produced proinflammatory mediators are thought to be the key factor in tumor promotion and progression[49-51]. A delicate balance between the expression of these key immune mediators with the abundance and activation of specific cell types in the tumor microenvironment can influence whether inflammation will promote tumor growth or an anti-tumor response will occur[49,52].
Exosomes have been found to play an important role in cancer immune surveillance and tumor escape through communication between immune cells and cancer cells. In addition, exosomes have been shown to modulate the tumor microenvironmentvia transfer of immune mediators, such as cytokines and chemokines[53]. A growing body of evidence has shown that DCs and B cells release an abundance of exosomes after interacting with T cells[16,54,55]. For example, Zitvogel and colleagues demonstrated that exosomes derived from DCs express functional MHC Class I and II and T-cell costimulatory molecules[4]. These DC-derived exosomes were capable of inducing CD8+ T-lymphocyte-dependent antitumor responses in vivo[4,15]. Tumor-derived exosomes produce several detrimental effects on immune cell populations such as apoptosis of activated T cells and suppression of DC maturation[56,57]. In addition, tumor-derived exosomes have suppressive effects in the generation and expansion of regulatory T cells (Tregs)[58-59]. For example, molecules contained in tumor-derived exosomes such as FasL, TRAIL, and TGF-β have been shown to modulate immune responses[60-61]. This collective weight of evidence shows the importance of tumor-derived exosomes in the suppression of immune responses.
Tumor Macro-environment: In addition to local effects, tumors can mediate a systemic response through the release of soluble factors into circulation, lymph vessels, orvia immune cell interactions with the microenvironment[62]. There are several complications mediated by tumors which can lead to cancerinduced cachexia, Cushing’s syndrome, hypercalcemia, and Trousseau’s syndrome[63]. Collectively, these complications are termed "paraneoplastic syndromes" as they are a consequence of the presence of the tumor but are not caused by its local effects. To date, the impact of tumor-derived exosomes on the tumor macroenvironment has yet to be explored. Work done by our group has shown a novel mechanism for the development of paraneoplastic diabetes seen in pancreatic cancer patients[64]. We found that pancreatic cancer secretes exosomes that enter systemic circulation to cause β-cell dysfunction by the transfer of an exosomal polypeptide known to inhibit insulin secretion[64]. This study demonstrates the importance of the tumor macroenvironment and how exosomes play a pivotal role in the transfer of critical cargo that can promote paraneoplastic effects.
Exosomes and cancer therapeutics
The potential role of exosomes as vehicles for targeted drug delivery is a topic that has received much attention. The endogenous role of exosomes as carriers of cellular cargo between donor and recipient cells and in eliciting biological responses makes these vesicles prime candidates for targeted drug delivery. Exosomes are abundantly secreted in various biological fluids, are readily taken up by cells, are membrane permeable, and are targetable to different tissues. All of these factors make exosomes ideal candidates for drug therapies, delivery of proteins, peptides, miRNAs, and other important mole-cules that would normally be degraded by the cell. There are several means in which exosomes could be used for therapeutics. Several strategies have used exosomes for 1) drug delivery of antitumor agents; 2) immune-modulation; 3) removal of tumor-derived exosomes from bodily fluids; or 4) modulation of exosome content for the prevention of tumorigenesis/ metastasis.
Although exosomes are released by almost all cell types, tumors produce and secrete them in excess quantities in which they contain altered content (e.g., oncoproteins and immunosuppressive molecules that promote metastasis)[65]. It has been suggested that preventing the production of tumor-derived exosomes could block tumorigenesis. In one study, the authors found Rab27a to berequired for exosome secretion and knockdown of Rab27a resulted in decreased primary tumor growth[66]. To prevent exosome production, other studies have specifically targeted microtubule dynamics, key players in endosomal sorting, and modulators of pH conditions[66-68]. New methods such as the complete removal of tumor-derived exosomes from the circulation have been proposed. One study used a hemofiltration system to selectively capture targeted exosomes from the entire circulatory system[65].
Worked by Zitvogel and colleagues first described the role of exosomes in immunity and suggested an alternative to DC-based therapies resulting in a delay in tumor growth[4]. Their results demonstrated that tumor-peptide loaded DC-derived exosomes could elicit a potent immune response in vivo, resulting in stunted tumor growth or the complete eradication of the tumor[4]. This work led to three phase I clinical trials utilizing DC- and ascite-derived exosomes, but they showed only modest improvements in patients[69-71]. A phase II cancer vaccine trial was conducted in 2009 using DC-derived exosomes (Dex) loaded with NKg2D and Il-15Rα[72].
Exosomes as cancer biomarkers
Emerging evidence strongly suggests that exosomes can be used as biomarkers for cancers and a variety of other diseases. The need for novel biomarkers for early detection, diagnosis, and prognosis of cancer is evident. With their diverse content, bioavailability, and ease of uptake, exosomes may be prime candidates for monitoring disease progression, prognosis, and for establishing treatment regimes[67]. The varying content of exosomes often reflects the tissue from which they were secreted and the particular disease state. For example, tumor-derived exosomes have been shown to contain a variety of cellular cargoes that can promote tumorigenesis[73]. Studies have also looked at the proteomic profiles of tumor-derived exosomes to determine suitable biomarkers[74,75]. For example, Ji and colleagues used exosomes isolated from both primary and metastatic human isogenic colorectal cell lines to compare proteomic profiles[75]. An enrichment of several metastatic factors, signal transduction molecules, and lipid raft components were found in the exosomes from the metastatic cell lines[75]. Further analysis of these exosomes showed the presence and colocalization of EPCAM-CLDN7 and TNIK-RAP2A, which suggests crosstalk between the tumor and stroma in the tumor microenviron-ment[75] . Recent work by Melo et al. detected a cell surface proteoglycan, glypican-1 (GPC1) specifi-cally enriched in tumor-derived exosomes[76]. GPC1+ exosomes were detected in the serum of pancreatic cancer patients with levels correlating to tumor bur-den and overall survival of these patients[76].Therefore, GPC1+ circulating exosomes (crExos) could be used as a potential biomarker for diagnostics and screening of the disease[76].
In addition to proteins, exosomes have also been shown to contain peptides. Recent work by our group demonstrated that a 52-amino-acid polypeptide, adrenomedullin, was present in cell line-derived and patient-derived pancreatic cancer exosomes (PC-Exo). Interestingly, these PC-Exo were shown to cause β-cell dysfunctionin vitro[64]. This work demonstrated the importance of tumor-derived exosomes in paraneoplas-tic syndrome for pancreatic cancer patients as a sub-set of patients had been shown to develop new-onset diabetes (<36 months in duration), which was characterized by insulin resistance and β-cell dysfunction[64]. Therefore, PC-Exo have the potential to be early detec-tion markers for pancreatic cancer on the basis of the presence and functionality of their content.
The discovery of both mRNAs and miRNAs in exosomes suggests their ability to harbor important genetic material. miRNAs have been found in a variety of tumor-derived exosomes from both cancerous cell lines and patient plasma/sera[77-79]. It has been shown that exosomal miRNAs are protected from RNase degradation and can be detected in the plasma/sera of patients[6,80,81]. One study using exosomes isolated from ovarian cancer patient sera found 8 diagnostic miR-NAs, which were also consistently found in ovarian cancer[6]. These findings suggest that profiling of exosomal miRNAs could be a potential diagnostic tool for ovarian cancer. This in turn could extend to screening of asymptomatic individuals for early detection of the disease[6]. In addition to miRNA and mRNAs present in exosomes, an interesting finding by Thakur and colleagues demonstrated the presence of double-stranded DNA in tumor-derived exosomes[5]. They showed that this exosomal DNA (exoDNA) reflects the entirety of the genome and thereby the mutational status of parental tumor cells[5].
Conclusion
Interest in exosomes over the past decade has been exponentially growing due to increasing knowledge of the function of these vesicles. In this review, the role of exosomes in a variety of pathologies was explored with the primary focus being on exosomes in the tumor micro-/macro-environment. Due to the ability of exosomes to bidirectionally communicate with distal tissues/cells, several reports have indicated the importance of these vesicles in tumorigenesis, particularly in the development and progression of the disease. Further understanding of the role of exosomes in intercellular communication during both normal and pathological conditions has yet to be elucidated. Exosomes have the potential to provide critical insight into the development of novel non-invasive therapeutic approaches to treat cancer due to their bioavailability, ease of col-lection, and resemblance to parental cells. Strategies utilizing exosomes as methods for nucleic acid and/or drug delivery to treat cancer are currently underway. Methods such as the delivery of small interfering RNA (siRNA) and packaging of chemotherapeutic drugs in exosomes to target cancer cells offer novel treatment alternatives for cancer. New exosome therapeutic strategies such as the use of dendritic cell-derived exosomes (dexosomes) as anti-cancer agents and hemofiltration of exosomes from advanced cancer patients show much promise although further evaluation of the efficacy and safety of these methods will need to be assessed. The development of novel treatment strategies and the further refinement of current strategies will benefit from the identification of the content of exosomes, more efficient isolation of exosomes, and optimization of targeted delivery of exosomes to tissues/cells of interest. Understanding the fundamentals of exosomes and their roles in disease pathology will ultimately lead to better strategies for cancer diagnosis, prognosis, and therapeutics.
Acknowledgments
This work was partly supported by Florida Department of Health Cancer Research Chair Funding, NIH fund-ing (CA150190, HL 70567 and CA78383, and Mayo Clinic Pancreatic Cancer SPORE P50 CA102701 Pilot Project) to DM and NCI training grant 1T32 CA148073 (to DM) Pre-doctoral fellowship to NJ.
References
- 1. Chargaff E, West R. The biological significance of the thromboplastic protein of blood[J]. J Biol Chem, 1946, 166(1): 189–197. [PubMed] [Google Scholar]
- 2. Johnstone RM, Adam M, Hammond JR, et al. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes)[J]. J Biol Chem, 1987, 262(19): 9412–9420. [PubMed] [Google Scholar]
- 3. Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses[J]. Nat Rev Immunol, 2009, 9(8): 581–593. [DOI] [PubMed] [Google Scholar]
- 4. Zitvogel L, Regnault A, Lozier A, et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes[J]. Nat Med, 1998, 4(5): 594–600. [DOI] [PubMed] [Google Scholar]
- 5. Thakur BK, Zhang H, Becker A, et al. Double-stranded DNA in exosomes: a novel biomarker in cancer detection[J]. Cell Res, 2014, 24(6): 766–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovar-ian cancer[J]. Gynecol Oncol, 2008, 110(1): 13–21. [DOI] [PubMed] [Google Scholar]
- 7. Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication[J]. J Proteomics, 2010, 73(10): 1907–1920. [DOI] [PubMed] [Google Scholar]
- 8. Wolf P. The nature and significance of platelet products in human plasma[J]. Br J Haematol, 1967, 13(3): 269–288. [DOI] [PubMed] [Google Scholar]
- 9. Meckes DG Jr, Raab-Traub N. Microvesicles and viral infection[J]. J Virol, 2011, 85(24): 12844–12854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. György B, Szabó TG, Pásztói M, et al. Membrane vesicles, current state-of-the-art: emerging role of extracellular ves-icles[J]. Cell Mol Life Sci, 2011, 68(16): 2667–2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication[J]. J Proteomics, 2010, 73(10): 1907–1920. [DOI] [PubMed] [Google Scholar]
- 12. Morvan J, Rinaldi B, Friant S. Pkh1/2-dependent phospho-rylation of Vps27 regulates ESCRT-I recruitment to endosomes[J]. Mol Biol Cell, 2012, 23(20): 4054–4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Adell MA, Vogel GF, Pakdel M, et al. Coordinated binding of Vps4 to ESCRT-III drives membrane neck constriction during MVB vesicle formation[J]. J Cell Biol, 2014, 205(1): 33–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Colombo M, Moita C, van Niel G, et al. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles[J]. J Cell Sci, 2013, 126(Pt 24): 5553–5565. [DOI] [PubMed] [Google Scholar]
- 15. Bobrie A, Colombo M, Raposo G, et al. Exosome secretion: molecular mechanisms and roles in immune responses[J]. Traffic, 2011, 12(12): 1659–1668. [DOI] [PubMed] [Google Scholar]
- 16. Buschow SI, Nolte-’t Hoen EN, van Niel G, et al. MHC II in dendritic cells is targeted to lysosomes or T cell-induced exosomes via distinct multivesicular body pathways[J]. Traffic, 2009, 10(10): 1528–1542. [DOI] [PubMed] [Google Scholar]
- 17. Trajkovic K, Hsu C, Chiantia S, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes[J]. Science, 2008, 319(5867): 1244–1247. [DOI] [PubMed] [Google Scholar]
- 18. Baietti MF, Zhang Z, Mortier E, et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes[J]. Nat Cell Biol, 2012, 14(7): 677–685. [DOI] [PubMed] [Google Scholar]
- 19. Savina A, Vidal M, Colombo MI. The exosome pathway in K562 cells is regulated by Rab11[J]. J Cell Sci, 2002, 115(Pt 12): 2505–2515. [DOI] [PubMed] [Google Scholar]
- 20. Savina A, Fader CM, Damiani MT, et al. Rab11 promotes docking and fusion of multivesicular bodies in a calci-um-dependent manner[J]. Traffic, 2005, 6(2): 131–143. [DOI] [PubMed] [Google Scholar]
- 21. Hsu C, Morohashi Y, Yoshimura S, et al. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C[J]. J Cell Biol, 2010, 189(2): 223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ostrowski M, Carmo NB, Krumeich S, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway[J]. Nat Cell Biol, 2010, 12(1): 19–30., 1–13. [DOI] [PubMed] [Google Scholar]
- 23. Théry C. Exosomes: secreted vesicles and intercellular communications[J]. F1000 Biol Rep, 2011, 3(15): 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Quah BJ, O’Neill HC. The immunogenicity of dendritic cell-derived exosomes[J]. Blood Cells Mol Dis, 2005, 35(2): 94–110. [DOI] [PubMed] [Google Scholar]
- 25. Obregon C, Rothen-Rutishauser B, Gerber P, et al. Active uptake of dendritic cell-derived exovesicles by epithe-lial cells induces the release of inflammatory mediators through a TNF-alpha-mediated pathway[J]. Am J Pathol, 2009, 175(2): 696–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Raposo G, Nijman HW, Stoorvogel W, et al. B lympho-cytes secrete antigen-presenting vesicles[J]. J Exp Med, 1996, 183(3): 1161–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Montecalvo A, Larregina AT, Shufesky WJ, et al. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes[J]. Blood, 2012, 119(3): 756–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xiang X, Poliakov A, Liu C, et al. Induction of mye-loid-derived suppressor cells by tumor exosomes[J]. Int J Cancer, 2009, 124(11): 2621–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Danilin S, Merkel AR, Johnson JR, et al. Myeloid-derived suppressor cells expand during breast cancer progres-sion and promote tumor-induced bone destruction[J]. Oncoimmunology, 2012, 1(9): 1484–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li H, Han Y, Guo Q, et al. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-beta 1[J]. J Immunol, 2009, 182(1): 240–249. [DOI] [PubMed] [Google Scholar]
- 31. Ailawadi S, Wang X, Gu H, et al. Pathologic function and therapeutic potential of exosomes in cardiovascular dis-ease[J]. Biochim Biophys Acta, 2015, 1852(1): 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bang C, Batkai S, Dangwal S, et al. Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy[J]. J Clin Invest, 2014, 124(5): 2136–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Makridakis M, Roubelakis MG, Vlahou A. Stem cells: insights into the secretome[J]. Biochim Biophys Acta, 2013, 1834(11): 2380–2384. [DOI] [PubMed] [Google Scholar]
- 34. Arslan F, Lai RC, Smeets MB, et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury[J]. Stem Cell Res, 2013, 10(3): 301–312. [DOI] [PubMed] [Google Scholar]
- 35. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation[J]. Cell, 2011, 144(5): 646–674. [DOI] [PubMed] [Google Scholar]
- 36. Wittekind C, Neid M. Cancer invasion and metastasis[J]. Oncology, 2005, 69(Suppl 1): 14–16. [DOI] [PubMed] [Google Scholar]
- 37. Ramteke A, Ting H, Agarwal C, et al. Exosomes secreted under hypoxia enhance invasiveness and stemness of prostate cancer cells by targeting adherens junction mol-ecules[J]. Mol Carcinog, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tauro BJ, Mathias RA, Greening DW, et al. Oncogenic H-ras reprograms Madin-Darby canine kidney (MDCK) cell-derived exosomal proteins following epithelial-mesenchymal transition[J]. Mol Cell Proteomics, 2013, 12(8): 2148–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jeppesen DK, Hvam ML, Primdahl-Bengtson B, et al. Comparative analysis of discrete exosome fractions obtained by differential centrifugation[J]. J Extracell Vesicles, 2014, 3(25011): 25011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited[J]. Nat Rev Cancer, 2003, 3(6): 453–458. [DOI] [PubMed] [Google Scholar]
- 41. Peinado H, Alečković M, Lavotshkin S, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET[J]. Nat Med, 2012, 18(6): 883–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhou W, Fong MY, Min Y, et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis[J]. Cancer Cell, 2014, 25(4): 501–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Costa-Silva B, Aiello NM, Ocean AJ, et al. Pancreatic can-cer exosomes initiate pre-metastatic niche formation in the liver[J]. Nat Cell Biol, 2015, 17(6): 816–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing[J]. N Engl J Med, 1986, 315(26): 1650–1659. [DOI] [PubMed] [Google Scholar]
- 45. Katoh M. Therapeutics targeting angiogenesis: genetics and epigenetics, extracellular miRNAs and signaling networks (Review).[Review] [J].Int J Mol Med, 2013, 32(4): 763–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Roma-Rodrigues C, Fernandes AR, Baptista PV. Exosome in tumour microenvironment: overview of the crosstalk between normal and cancer cells[J]. Biomed Res Int, 2014, 2014(179486): 179486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Umezu T, Tadokoro H, Azuma K, et al. Exosomal miR-135b shed from hypoxic multiple myeloma cells enhances angiogenesis by targeting factor-inhibiting HIF-1[J]. Blood, 2014, 124(25): 3748–3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kucharzewska P, Christianson HC, Welch JE, et al. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development[J]. Proc Natl Acad Sci U S A, 2013, 110(18): 7312–7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer[J]. J Clin Invest, 2007, 117(5): 1175–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Coussens LM, Werb Z. Inflammation and cancer[J]. Nature, 2002, 420(6917): 860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease[J]. Cancer Cell, 2005, 7(3): 211–217. [DOI] [PubMed] [Google Scholar]
- 52. Grivennikov SI, Greten FR, Karin M. Immunity, inflam-mation, and cancer[J]. Cell, 2010, 140(6): 883–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Akers JC, Gonda D, Kim R, et al. Biogenesis of extracellu-lar vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies[J]. J Neurooncol, 2013, 113(1): 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nolte-’t Hoen EN, Buschow SI, Anderton SM, et al. Activated T cells recruit exosomes secreted by dendritic cells via LFA-1[J]. Blood, 2009, 113(9): 1977–1981. [DOI] [PubMed] [Google Scholar]
- 55. Muntasell A, Berger AC, Roche PA. T cell-induced secretion of MHC class II-peptide complexes on B cell exosomes[J]. EMBO J, 2007, 26(19): 4263–4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Iero M, Valenti R, Huber V, et al. Tumour-released exosomes and their implications in cancer immunity[J]. Cell Death Differ, 2008, 15(1): 80–88. [DOI] [PubMed] [Google Scholar]
- 57. Szczepanski MJ, Szajnik M, Welsh A, et al. Blast-derived microvesicles in sera from patients with acute myeloid leukemia suppress natural killer cell function via mem-brane-associated transforming growth factor-beta1[J]. Haematologica, 2011, 96(9): 1302–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wieckowski EU, Visus C, Szajnik M, et al. Tumor-derived microvesicles promote regulatory T cell expansion and induce apoptosis in tumor-reactive activated CD8+ T lym-phocytes[J]. J Immunol, 2009, 183(6): 3720–3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Szajnik M, Czystowska M, Szczepanski MJ, et al. Tumor-derived microvesicles induce, expand and up-regulate biological activities of human regulatory T cells (Treg)[J]. PLoS One, 2010, 5(7): e11469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Abusamra AJ, Zhong Z, Zheng X, et al. Tumor exosomes expressing Fas ligand mediate CD8+ T-cell apoptosis[J]. Blood Cells Mol Dis, 2005, 35(2): 169–173. [DOI] [PubMed] [Google Scholar]
- 61. Théry C, Regnault A, Garin J, et al. Molecular characteri-zation of dendritic cell-derived exosomes. Selective accu-mulation of the heat shock protein hsc73[J]. J Cell Biol, 1999, 147(3): 599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Al-Zoughbi W, Huang J, Paramasivan GS, et al. Tumor macroenvironment and metabolism[J]. Semin Oncol, 2014, 41(2): 281–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Darnell RB, Posner JB. Paraneoplastic syndromes involv-ing the nervous system[J]. N Engl J Med, 2003, 349(16): 1543–1554. [DOI] [PubMed] [Google Scholar]
- 64. Javeed N, Sagar G, Dutta SK, et al. Pancreatic Cancer-Derived Exosomes Cause Paraneoplastic β-cell Dysfunction[J]. Clin Cancer Res, 2015, 21(7): 1722–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Marleau AM, Chen CS, Joyce JA, et al. Exosome removal as a therapeutic adjuvant in cancer[J]. J Transl Med, 2012, 10(134): 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bobrie A, Krumeich S, Reyal F, et al. Rab27a supports exosome-dependent and-independent mechanisms that modify the tumor microenvironment and can pro-mote tumor progression[J]. Cancer Res, 2012, 72(19): 4920–4930. [DOI] [PubMed] [Google Scholar]
- 67. Tickner JA, Urquhart AJ, Stephenson SA, et al. Functions and therapeutic roles of exosomes in cancer[J]. Front Oncol, 2014, 4(127): 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Parolini I, Federici C, Raggi C, et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells[J]. J Biol Chem, 2009, 284(49): 34211–34222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Escudier B, Dorval T, Chaput N, et al. Vaccination of met-astatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of thefirst phase I clinical trial[J]. J Transl Med, 2005, 3(1): 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Morse MA, Garst J, Osada T, et al. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer[J].J Transl Med, 2005, 3(1): 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Dai S, Wei D, Wu Z, et al. Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer[J]. Mol Ther, 2008, 16(4): 782–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Viaud S, Terme M, Flament C, et al. Dendritic cell-derived exosomes promote natural killer cell activation and pro-liferation: a role for NKG2D ligands and IL-15Ralpha[J]. PLoS One, 2009, 4(3): e4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Henderson MC, Azorsa DO. The genomic and proteomic content of cancer cell-derived exosomes[J].Front Oncol, 2012, 2(38): 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Duijvesz D, Burnum-Johnson KE, Gritsenko MA, et al. Proteomic profiling of exosomes leads to the identification of novel biomarkers for prostate cancer[J]. PLoS One, 2013, 8(12): e82589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ji H, Greening DW, Barnes TW, et al. Proteome profiling of exosomes derived from human primary and metastatic colorectal cancer cells reveal differential expression of key metastatic factors and signal transduction components[J]. Proteomics, 2013, 13(10-11): 1672–1686. [DOI] [PubMed] [Google Scholar]
- 76. Melo SA, Luecke LB, Kahlert C, et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer[J]. Nature, 2015, 523(7559): 177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Rabinowits G, Gerçel-Taylor C, Day JM, et al. Exosomal microRNA: a diagnostic marker for lung cancer[J]. Clin Lung Cancer, 2009, 10(1): 42–46. [DOI] [PubMed] [Google Scholar]
- 78. Que R, Ding G, Chen J, et al. Analysis of serum exosomal microRNAs and clinicopathologic features of patients with pancreatic adenocarcinoma[J]. World J Surg Oncol, 2013, 11(219): 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Takeshita N, Hoshino I, Mori M, et al. Serum microRNA expression profile: miR-1246 as a novel diagnostic and prognostic biomarker for oesophageal squamous cell car-cinoma[J]. Br J Cancer, 2013, 108(3): 644–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Mitchell PS, Parkin RK, Kroh EM, et al. Circulating microRNAs as stable blood-based markers for cancer detection[J]. Proc Natl Acad Sci U S A, 2008, 105(30): 10513–10518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lin J, Li J, Huang B, et al. Exosomes: novel biomarkers for clinical diagnosis[J]. Scientific World Journal, 2015, 2015(657086): 657086. [DOI] [PMC free article] [PubMed] [Google Scholar]


