Abstract
Consumption of kava (Piper methysticum Forst) has been linked to reduced cancer risk in the South Pacific Islands. Kavalactones are major bioactive components in kava root extracts, which have recently demonstrated anti-cancer activities. However, molecular mechanisms of kavalactones' anti-cancer action remain largely unknown. We have identified two kavalactones, yangonin and 5′ 6'-dehydrokawain, as potent inducers of autophagic cell death in bladder cancer cells. The effect of yangonin inducing autophagy is associated with increased expression of beclin and ATG5. In addition, yangonin increases the expression of LKB1 and decreases the phosphorylation of Akt, PRAS40, rpS6, p70S6K and 4E-BP1, leading to increased binding of 4E-BP1 to m7 GTP. The growth inhibitory effects of yangonin were attenuated inTSC1 or LKB1 knockout mouse embryonic fibroblasts, suggesting that TSC1 and LKB1 expression may contribute to optimal growth inhibition by yangonin. Furthermore, yangonin reduces the viability of bladder cancer cell lines derived from different stages of human bladder cancer, and acts synergistically with apoptosis-inducing agents such as docetaxel and flavokawain A. Our results support a novel anti-bladder cancer mechanism by yangonin and further studies are needed to assess the potential use of yangonin for bladder cancer prevention and treatment
Keywords: yangonin, bladder cancer, autophagy, mTOR
Introduction
Urothelial cell carcinoma of the bladder accounted for 430,000 new cases diagnosed in 2012[1]. It is the ninth most common cancer in the world accounting for 3.2% of the worldwide cancer burden, and it is the second most common malignancy of the genitourinary tract following prostate cancer[1]. Bladder cancer is subdivided into non-invasive and invasive subtypes or low and high grade. In general, low-grade non-invasive cancer has a low risk of progression to invasive disease and overall favorable prognosis, and so does muscle-confined invasive bladder cancer (pT2N 0M 0)[2–3]. The 5-year overall survival rate for muscle-confined invasive bladder cancer after cystectomy is approximately 80%. However, advanced bladder cancer (pT3, 4, any N+, any M+) is an aggressive disease with a poor survival rate despite treatment with radical cystectomy and standard adjuvant chemotherapy. Currently, cisplatin-based combination chemotherapy such as MVAC (methotrexate, vinblastine, adriamycin, cisplatin) and GC (gemcitabine, cisplatin) is considered to be the standard chemo-agent, but it results in suboptimal survival outcomes with high toxicity[4]. Although urothelial cancer is a comparatively chemosensitive tumor, it is commonly known that chemotherapy is rarely curative and relapse after first-line therapy in the case of nodal involvement or distant metastatic disease and median survival of patients with metastatic disease is 15 months[5]. Some newer chemotherapeutic regimens have been investigated and attempted, but there is not yet any compelling evidence of improved patient survival[6]. Limited advances in chemotherapy have resulted from multiple efforts across several different areas of therapy.
A number of countries in the South Pacific Islands have very low incidence of colorectal, bladder, and testicular cancer, yet have higher incidence of infection-related cancers[7–8], despite the high percentage of the population habituated to tobacco[9]. The search for dietary agents has suggested that consumption of kava, a perennial plant indigenous to the Pacific Islands, may lower the cancer incidence for that population[10], although the difference in incidence may also be affected by other dietary components and foods. Such a negative correlation between kava consumption and cancer incidence suggests that the kava plant may contain interesting anti-cancer compounds. Kavalactones and chalcones (i.e. flavokawains) are two important classes of bioactive compounds identified from kava extracts. Accumulating data from cell culture and animal studies have demonstrated that both kavalactones and chalcones have potent anti-cancer and anti-carcinogenic activity[11–16]. However, molecular mechanisms of these compounds' anti-cancer action remain largely unknown.
Autophagy has been widely studied as a cancer prevention or therapeutic target through either its pro-death or pro-survival mechanisms. Some natural compounds have been found to exhibit anti-cancer effects through the modulation of autophagy. We therefore have examined the effect of kava extracts and its active components (including kawain, yangonin, 5, 6-dehydrokawain, methysticin, flavokawain B, and flavokawain A) on autophagy in human bladder cancer cell lines. Among these compounds, yangonin and 5, 6-dehydrokawain have been identified to be autophagic death inducers. In addition, we have shown that yangonin inhibits the growth of bladder cancer cell lines and enhances the growth inhibitory effect of flavokawain A and docetaxel via inhibition of mTOR signaling.
Materials and methods
Cell lines, compounds, and reagents
The RT4, T24, UMUC3, HT1376, and HT 1197 cell lines were obtained from American Type Culture Collection (Manassas, VA). RT4 and T24 cells were maintained in McCoy's 5A medium containing 10% fetal bovine serum (FBS). UMUC3, HT1376, and HT 1197 cells were cultured in EMEM medium with 10% FBS.TSC1+/+/p53+/+, TSC1−/−/p53+/+, TSC2+/+/p53−/−, and TSC2−/−/p53−/− mouse embryonic fibroblasts (MEFs), LKB knockout and wild-type MEFs were generous gifts from David Kwiatkowski (Brigham Women's Hospital) and were maintained in DMEM supplemented with 10% FBS. Kawain, yangonin, 5, 6-dehydrokawain, methysticin, flavokawain B, and flavokawain A were isolated from kava extracts by LKT Laboratories, Inc. (St. Paul, MN, USA), dissolved in DMSO, aliquoted, and stored at −20°C. The DMSO in culture medium never exceeded 0.1% (v/v), to avoid an effect on cell proliferation. The pEGFP-LC3, PcDNA3-TSC1, and PcDNA3-TSC2 constructs were purchased from Addgene (Cambridge, MA). Antibodies for LKB1, phospho-AKT, AKT, phospho-PRAS40, PRAS40, phospho-p70S6K (Thr389), phospho-rpS6, 4EBP1, eIF4E, Beclin1 ATG7, ATG5, ATG12, and Bcl2 were purchased from Cell Signaling Technology, Inc. (Beverly, MA, USA). Tubulin antibody, protein A/G-plus agarose and protein A-plus agarose beads were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). m7 GTP Sepharose and ECL detection system were from Amersham Biosciences (Arlington Heights, IL). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and docetaxel were obtained from Sigma (St. Louis, MO, USA). RNAazol B was purchased from Tel-Test (Friendswood, TX). The Reverse Transcription System kit was purchased from Promega (Madison, WI, USA).
MTT assay
RT4, T24, UMUC3, HT1376, and HT 1197 cells, as well as TSC1+/+/p53+/+, TSC1−/−/p53+/+, TSC2+/+/p53−/−, TSC2−/−/p53−/− MEFs, LKB knockout and wild-type MEFs were plated at a density of 2 × 105 per well in 24-well culture plates in medium containing 10% FBS. After 24 hours, the medium was refreshed and then was either left untreated or was treated with yangonin, docetaxel or flavokawain A at the concentrations indicated in the figures. After treatment, MTT was added to the wells at a final concentration of 1 mg/mL and incubated at 37°C for 3 hours. The absorbance was determined at 570 nm. Cell sensitivity to drug treatment was expressed as the drug concentration that yielded 50% cell growth inhibition (IC 50). All of the experiments were performed in triplicate.
In addition, after IC 50s were determined for both docetaxel and favokawain A in UMUC-3 cells, varying concentrations of yanonin were added to treated cells. Cells were treated with the desired drug or drug combination for 72 hours. The type of interaction between drug activities was determined by the median effect principle according to the method of Chou and Talalay using CalcuSyn software (Biosoft, Cambridge, UK). The interaction among drugs was then quantified by determining a combination index (CI) at increasing levels of cell killing. A CI is lower to, equal or higher than 1 indicated synergy, additivity or antagonism, respectively. Each compound combination experiment was performed in triplicate.
Colony formation assay
UMUC-3 cells were seeded in top agar containing 0.35% agar with EMEM and 10% FBS. Bottom agar consisted of 0.8% agar, EMEM and 10% FBS. Cultures were maintained under standard culture conditions. Media with 0.1% DMSO or indicated concentrations of yangonin were added and replaced every 3 days. After 3 weeks, the number of colonies was determined with an inverted phase-contrast microscope at 100× magnification where a group of >10 cells was counted as a colony. Crystal violet (0.1%) in methanol was used to stain the 6-well plates. After washing in PBS, colonies were photographed.
Western blot analysis
Cells were treated with 0.1% DMSO and yangonin under each experimental condition. After treatment, clarified protein lysates (20 μg–80 μg) were denatured at 95°C for 5 minutes and resolved by 8%–16% SDS-PAGE. Proteins were transferred to nitrocellulose membranes, and probed with antibodies and visualized by an enhanced chemiluminescence detection system.
7-Methyl-guanosine cap binding assay
A total of 1 mg of cellular proteins in lysis buffer (20 mmol/L Tris-HCl, pH 7.5, 150 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L EGTA, 1% Triton, 2.5 mmol/L sodium pyrophosphate, 1 mmol/L β-glycerophosphate, 1 mmol/L Na 3VO 4, 1 μg/mL leupeptin) was mixed with 50 μL of 7-methyl-GTP-Sepharose-4B bead suspension (Amersham Biosciences, Pittsburgh, PA, USA) and incubated overnight. After washing the pellet, the affinity complex was recovered and boiled with SDS sample buffer. The supernatant was recovered after centrifugation and subjected to SDS-PAGE. The amount of 4E-BP1 or eIF4E in the bound fraction was detected by Western blotting using specific antibodies.
Stable transfection
T24 cells were plated at 1 × 106 per 100-mm dish. At 60% confluency, cultures were transfected with pEGFP-LC3 using FuGENE 6 (Roche, Indianapolis, IN). Transfected cells were selected with G418 (800 μg/mL) starting at 48 hours after transfection, and all of the stable transfectants were pooled to avoid cloning artifacts. Pooled stable clones of T24 cells expressing pEGFP-LC3 were maintained in RPMI containing 10% FBS and 200 μg/mL G418.
Fluorescence microscopy
T24/pEGFP-LC3 cells were cultured in chamber slides (Laboratory-Tek). After treatments with different concentrations of kavalactones, flavokawains and chloroquine, cells were fixed in 4% paraformaldehyde solution for 20 min and methanol for 2 min. Slides were mounted in Vectashield (Vector Laboratories, Inc., Burlingame, CA). Immunostaining was analyzed with a Nikon Eclipse TE2000-S fluorescent microscope (magnification, 200×) using the 488-excitation wavelength. For quantification of autophagic cells, cells with >10 GFP-LC3 punctuate dots were considered positive. Data obtained from counting triplicates of 100 cells were averaged (mean±SD).
Electron microscopy
After treatments, cell pellets were collected, fixed with 1.6% glutaraldehyde, post-fixed in 1% OsO4, dehydrated in alcohol series, and embedded in epoxy resin. Thin sections were contrasted with uranyl acetate and lead citrate. Preparations were observed either with a Philips CM12 electron microscope operating at 80 kV (FEI) or with a Jeol 1400 mounted with CCD cameras (Morada, Olympus SIS, Münster, Germany).
Statistics
Microsoft Excel software was used to calculate the mean and the standard error of the mean. Comparisons of cell viabilities, number of colonies formed in soft agar and number of positive cells with LC3-GFP puncta between treatment and control were conducted using analysis of variance (ANOVA) or Student'st-test. All statistical tests were two-sided. P values<0.05 were considered significant.
Results
Kavalactones, yangonin and 5, 6-dehydrokawain, induce autophagy
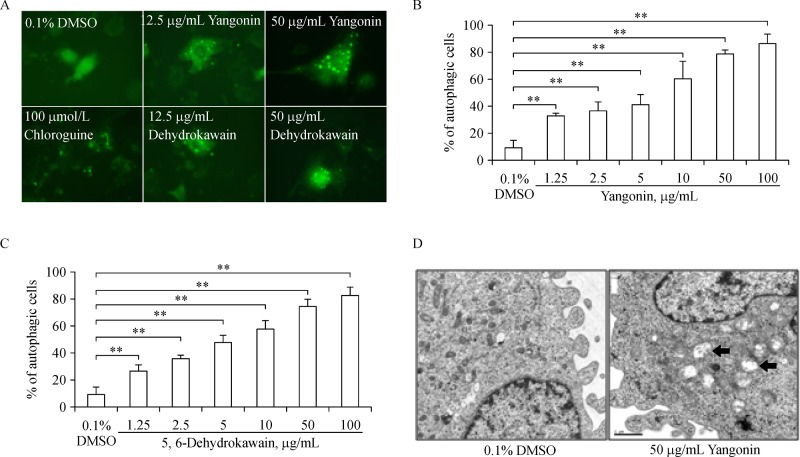
In our previously published study[11], we have determined the contents of major bioactive components of a commercial kava extract, which contains 2.7% kawain, 1.75% 5, 6-dehydrokawain, 3.08% yangonin, 1.4% methysticin, 0.33% flavokawain B, and 0.21% flavokawain A. To examine whether these compounds were capable of inducing autophagy, we have established stable T24 cell lines expressing pEGFP-LC3 as a model for autophagy detection. If the re-localization of pEGFP-LC3 in T24 cells from a diffuse cytoplasmic staining to punctate structures is visualized by fluorescence microscopy, it is indicative of autophagy as described in our previous publications[16–17] (Fig. 1A). After treatments of T24/LC3-EGFP cells with chloroquine (a positive control), kawain, 5, 6-dehydrokawain, yangonin, methysticin, flavokawain B, and flavokawain A at different concentrations for 6 hours, quantitative analysis of the percentage of T24 cells with LC3-EGFP puncta staining reveals that yangonin and 5, 6-dehydrokawain treatment at doses of 1.25 to 100 mg/mL resulted in a dose-dependent increase in percentages (27.0% to 86.5%) of cells with presence of LC3-GFP puncta, whereas vehicle control and other compounds (kawain, methysticin, flavokawain A and B)' treatments only had less than 10% of examined cells with LC3-EGFP puncta (Fig. 1B & C and data not shown, ANOVA, Ps 0.01).
Fig.1. Kavalactones (yangonin and 5, 6-dehydrokawain) induces autophagy in UMUC-3 and T24 cells.
A: T24 cells stably expressing pEGFP-LC3 were treated with vehicle control (0.1% DMSO), 100 μmol/L chloroquine, and 12.5 or 50 μg/mL yangonin or dehydrokawain for 8 hours. After treatment, cells were examined by fluorescence microscopy. Magnification: ×400. B and C: Quantitative analysis of percentages of T24 cells with LC3-punctas after yangonin and 5, 6-dehydrokawain treatments, respectively. Bar: SE, n = 3. ** P 0.01. D: UMUC-3 cells were treated with vehicle control (0.1% DMSO) or yangonin (50 μg/mL) for 8 hours. After treatment, cells were fixed and examined by electron microscopy for the presences of autophagosomes (arrows). Magnification: ×46,000. Size bar = 1 μm.
Given yangonin is the predominant kavalactone in the kava extract, we further confirmed the autophagy inducing effect of yangonin in UMUC-3 cell by electron microscopy.Fig. 1D shows that yangonin treatment resulted in a significant increase in cells with typical double-membrane autophagic vesicles compared to control. These autophagic vesicles contained extensively degraded organelles.
The effect of yangonin on expression of autophagy markers and cell death
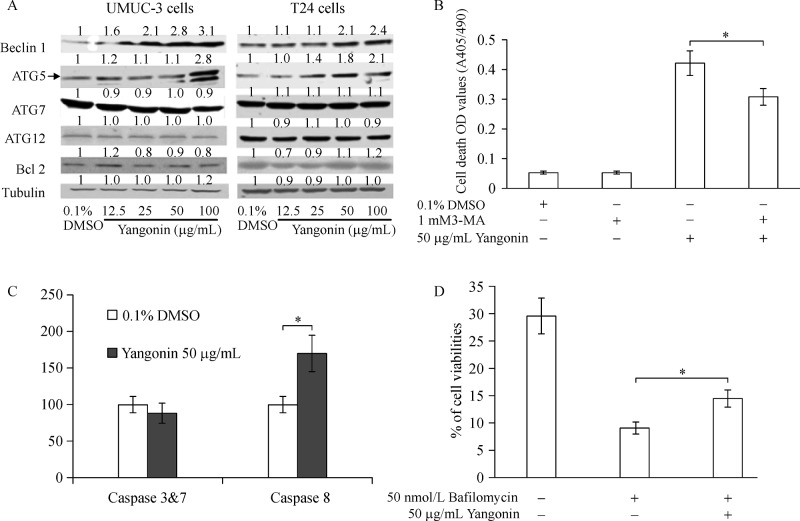
Next, Western blot analysis was performed for autophagy-associated proteins, including ATG-5, ATG-7, ATG-12, Bcl-2 and beclin-1. As shown inFig. 2A, treatment of UMUC-3 cells and T24 cells with yangonin increased the protein expression of beclin-1 and ATG-5. However, the protein levels of ATG-12 and ATG-7, as well as Bcl2 were not significantly altered by yangonin treatment. The expression level of beclin1, a key apical regulator of autophagy, coincides with autophagy activity[18]. In addition, ATG5 plays a key role in regulation of autophagosome elongation and functions as a tumor suppressor. Both beclin1 and ATG5 are also involved in apoptosis. These results further confirm the autophagic effect of yangonin and suggest a role of yangonin inducing cell death in bladder cancer cell lines.
Fig.2. The effect of yangonin on expression of autophagy markers and cell death.
A: UMUC-3 and T24 cells were treated with 0.1% DMSO or yangonin at indicated concentrations for 24 hours. The protein expression of beclin1, ATG-5, ATG-7, ATG-12, and Bcl-2 examined by Western blotting analysis shows increased levels of beclin1 and ATG5 by yangonin treatment. The density ratio (DR) for each treatment was calculated by dividing by the adjusted density of corresponding control bands. All density ratios were placed on the top of their Western blot bands. B: Quantification of mean optical density (O.D.) values measured by cell death ELISA assay after indicated treatments for 24 hours shows attenuation of yangonin-induced cell death by 3-methyladenine (3-MA; specific autophagy inhibitor). C: Quantification of caspase activities by caspase activity assays demonstrate activation of caspase-8 but not caspase-3/7 by yangonin treatment in UMUC-3 cells. D: Cell viabilities measured by MTTassay after indicated treatments for 24 hours show that bafilomycin A1 mitigates yangonin induced reduction of cell viabilities in UMUC-3 cells. Bar: SE, n= 4, *P 0.05.
Cell death ELISA analysis shows that 50 mg/mL yangonin treatment of UMUC-3 cells for 3 days increased the number of dead cells by 7.8 fold compared to vehicle control treatment (Fig. 2B, P<0.01). Treatment of 1 mmol/L 3-methyladenine (3-MA) alone, an autophagy inhibitor via inhibition of the enzyme PI3K class III[19], did not cause significant differential cell death compared to control. However, co-treatment of 3-MA and yangonin partly attenuated the cell death effect of yangonin (Fig. 2B). Further experiment shows that yangonin did not increase caspase 3 and 7 activities but significantly induced activation of caspase 8 (Fig. 2C).
Bafilomycin A1, an inhibitor of vacuolar H+-ATPase, can block late-phase autophagy[20]. Similarly, yangonin and bafilomycin acted together in an antagonist way on cell viability (Fig. 2D). Taken together, these results indicate that yangonin induces autophagic cell death.
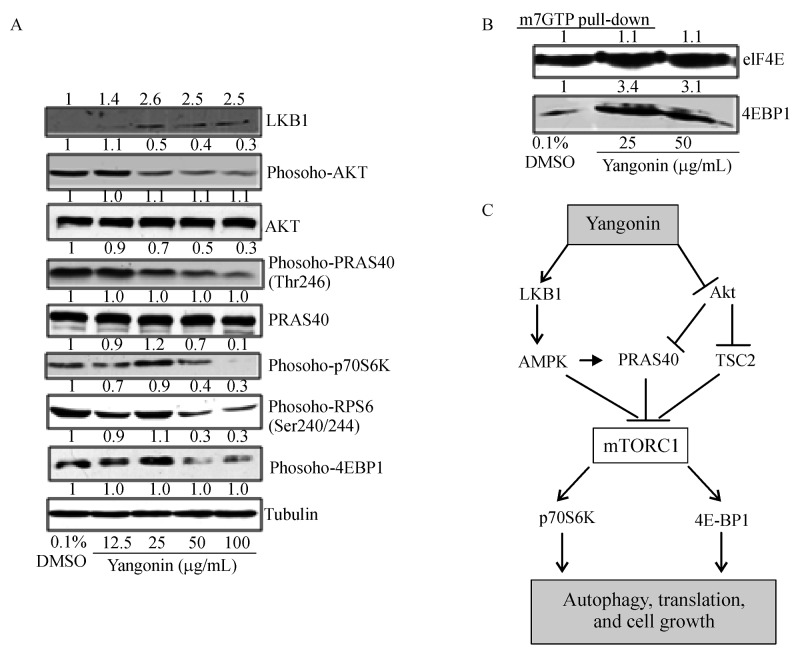
Yangonin upregulates the expression of LKB1 and downregulates the phosphorylation levels of AKT, PRAS40, rpS6, and p70S6K, and 4E-BP1 leading to increased binding of 4E-BP1 to m7 GTP in UMUC-3 cells
The mTOR pathway regulates autophagy as a cell survival mechanism under stress conditions induced by nutrient deprivation, hypoxia and drug treatments[21]. We therefore examined whether yangonin treatment affected the mTOR pathway in bladder cancer cells. Both Akt and LKB1 are up-stream events of the mTOR pathway. LKB1 inhibits the mTOR activity through activation of AMPKα, whereas AKT activates the mTOR via causing inactivating phosphorylation of TSC2 and PRAS40[20]. Dephosphorylated TSC2 and PRAS40 can bind to mTORC1 and then prevent the phosphorylation of p70S6K and 4E-BP1 by the mTORC1.Fig. 3A shows that yangonin increased the expression of LKB1 and decreased the phosphorylated levels of AKT and PRAS40 in a dose-dependent manner without a change in their total protein levels.
Fig.3. The effects yangonin on the mTOR pathway in UMUC-3 cells.
UMUC-3 cells were treated with vehicle control or the indicated doses of yangonin for 24 hours. At the end of each treatment, cell lysates were prepared as mentioned in Materials and Methods. A: Western blotting analysis of LKB1, Akt, PRAS40, rpS6, p70S6K and 4E-BP1, and membranes were stripped for reprobing with anti-tubulin antibody for protein loading correction. A representative blot from three independent experiments is shown. B: eIF4E was purified from cell extracts by m7 GTP affinity chromatography and probed with antibodies against eIF4E and 4E-BP1. A representative blot from three independent experiments is shown. C: The proposed mechanism for yangonin inducing autophagy and cell growth inhibition via inhibition of the mTOR pathway. The density ratio (DR) for each treatment was calculated by dividing by the adjusted density of corresponding control bands. All density ratios were placed on the top their Western blot bands.
In addition, phosphorylation of rpS6, p70S6K and 4E-BP1 represents major downstream targets of mTORC1. Treatment with yangonin decreased the phosphorylation of rpS6, p70S6K and 4E-BP1. These results suggest the inhibitory effect of yangonin on mTORC1 activityvia upregulation of LKB1 expression and de-phosphorylation of Akt.
Furthermore, Fig. 3B shows that yangonin treatment resulted in an increased binding of 4E-BP1 (rate-limiting translation initiation blocker) to m7 GTP without a change in eIF4E. This result suggests that yangonin may inhibit cap-dependent translation initiation.
The mechanism which yangonin inhibits the mTOR pathway can be simply proposed as Fig. 3C.
The growth inhibitory effect of yangonin is at least in part required for the expression of LKB1 and TSC1, but independent of TSC2 and p53
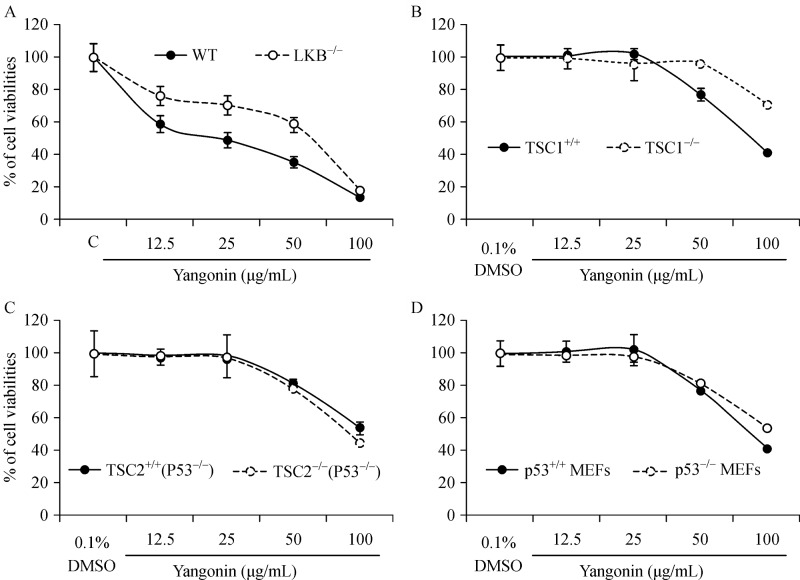
Fig. 4A shows that yangonin at doses of 12.5 to 50 mg/mL was less effective in inhibiting the growth of LKB1 knockout MEFs compared to wild-type MEFs (Ps<0.01). Knockout of TSC1 also significantly attenuated the growth inhibitory effect of yangonin at doses of 50 and 100 mg/mL in MEFs (Ps<0.01). However, there are no significant differences in the growth inhibitory effects of yangonin on wild-type MEFs andp53 or TSC2 knockout MEFs. These results suggest that the inhibitory effect of yanonin on the mTOR pathway via LKB/TSC1 is partly responsible for its growth inhibitory effect.
Fig.4. The association of p53, TSC 1/2 and LKB1 statuses with the growth inhibitory effect of yangonin in MEFs.
MEFs were treated as indicated for 72 hours. Cell viabilities were measured by MTTassay. Points, mean of four independent plates; bars, SE. The growth inhibitory effect of yangonin on MEF LKB+/+ and LKB–/–(A), MEFs TSC1+/+ (p53+/+) and TSC1–/–(p53+/+) (B), TSC2+/+(p53–/–) and TSC2–/–(p53–/–) (C), and MEF p53+/+ and p53–/–(D).
Yangonin inhibits anchorage-dependent and-independent growth of bladder cancer cell lines
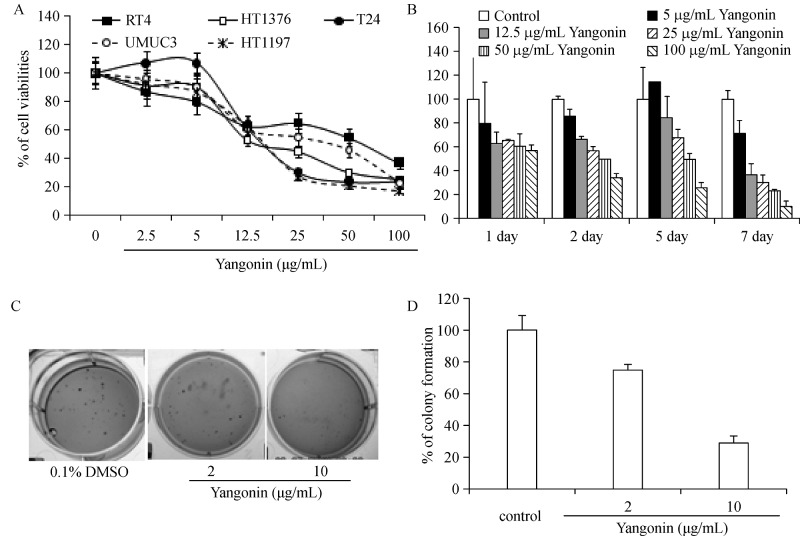
To examine the potential anti-bladder cancer effects of yangonin, human papillary (RT4) and muscle-invasive (T24, UMUC3, HT 1376 and HT 1197) bladder cancer cell lines were treated with different doses of yangonin.Fig. 5A shows that treatment with yangonin for 72 hours differentially decreased the viability of tested cell lines in a dose-dependent manner. The IC 50s in HT 1197, T24, HT 1376, UMUC3, and RT4 cell lines were estimated to be 15.1, 15.2, 13.0, 38.4, and 58.7 mg/mL, respectively. However, RT4 cells appeared to be less sensitive to treatment with high doses of yangonin compared to the other bladder cancer cell lines.Fig. 5B shows that yangonin inhibited the growth of UMUC-3 cells in both a time and dose-dependent manner. With treatment time up to 7 days, yangonin was significantly more effective in reducing cell viabilities. Since anchorage-independent growth is more predictive for in vivo tumor growth of cancer cell lines with longer treatment time, the effect of yanonin on colony formation of UMUC-3 cells in soft agar was investigated. Compared to the control treatment, treatment of UMUC-3 cells with 2 and 10 mg/mL yangonin resulted in 25% and 70% inhibition of colony formation in soft agar, respectively (Fig. 5C & D, Ps<0.01). These results are consistent with other reports that the growth inhibitory effectsvia autophagic cell death require longer treatment time.
Fig.5. The growth inhibitory effect of yangonin on anchorage-dependent growth of bladder cancer cell lines and anchorage-independent growth of UMUC-3 cells.
A: T24, UMUC3, HT 1376 and HT 1197 cells were treated with vehicle control (0.1% DMSO), or indicated doses of yangonin. After 72 hours of treatment, cell viabilities were measured by MTTassay. B: UMUC3 cell viabilities were measured by MTT assay after 1, 2, 5, and 7 days of treatment at the indicated doses, respectively. Points, mean of four independent plates; bars, SE. C and D: A soft agar colony formation assay with the indicated treatments of UMUC-3 cells was performed using 6-well plates. The data are presented by bar figures and mean±SE of four independent wells at an optimum time of 21 days post cell seeding. The pictures provide a qualitative analysis of colony formation.
The effects of yangonin in combination with docetaxel or flavokawain A on the growth of bladder cancer cells
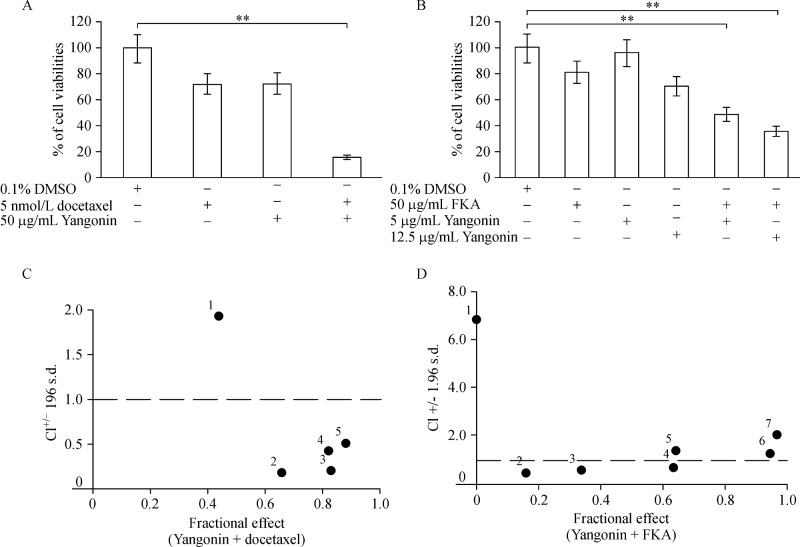
Yangonin as a single agent appears not to be a very potent agent for inhibiting the growth of bladder cancer cells. Therefore, we examined the potential of yangonin as an autophagic death inducer in combination with docetaxel or flavokawain A for inhibiting the growth of bladder cancer cells. Both docetaxel and flavokawain A are potent apoptosis inducers[12]. Fig. 6A shows that 50 mg/mL yangonin or 5 nmol/L docetaxel alone treatment of UMUC-3 cells for 48 hours inhibited the growth by 27.5% and 27.1% whereas their combination result in 83.6% of growth inhibition. The yangonin and docetaxel combined treatment increased the potency of growth inhibition by 56% compared to each alone.Fig. 6B also shows that yanonin and flavokawain A combination resulted in a significant improvement of their potency for inhibiting the growth of UMUC-3 cells by 36% to 52% compared to each treatment alone. Combination indexes (CI) of 0.183 and 0.466 for docetaxel and 0.746 and 0.560 for flavokawain A at yangonin doses of 5 and 12.5 mg/mL, respectively, were shown (Fig. 6C& D). These results suggested a synergistic effect between yangonin and docetaxel or flavokawain A.
Fig.6. Yangonin in combination with apoptosis-inducing agents (docetaxel and flavokawain A) for reduction of cell viabilities of UMUC-3 cells.
A: Quantitative analysis of the UMUC-3 cell viability after treatment with yangonin (50 βg/mL) and docetaxel (5 nmol/L) combination, in comparison with an identical concentration of yangonin or docetaxel. ** P 0.01. Points, mean of four inde pendent plates; bars, SE. B: After 72 hours of indicated treatments, MTT assay shows the combination of yangonin and flavokawain A has enhanced effect on reduction of cell viabilities compared to each agent alone. ** P 0.01. The data are presented by bar figures and mean±SE of four independent experiments. C & D: CI/fractional effect curve for UMUC-3 cells treated with a fixed yangonin:docetaxel or flavokawain A concentration ratio, as described in Materials and Methods. CIs lying below 1 indicate synergistic conditions.
Discussion
Yangonin is one of the six major kavalactones found in the Kava plant. Kava (Piper methysticum Forst) is a perennial plant indigenous to the Pacific Islands[22]. Unusually low incidences of several cancers, including lung and prostate cancers, have been reported in the Pacific Island nations despite a high proportion of smokers in these populations[23–24]. Recently, a study of the effect of kava components on prostate cancer suggested the possibility of a cancer treatment and prevention effect, necessitating further research[11].
The main findings of the present study were as follows: (1) treatment with yangonin decreased viability of bladder cancer cell lines derived from different stages of human bladder cancer andp53 knockout MEFs (p53−/−), which is partly dependent of TSC1 expression but not TSC2; (2) yangonin induced efferocytosis of bladder cancer cell lines, which is dependent on autophagy via inhibition of the mTOR pathway. These results match with molecular mechanisms demonstrating increased expression of beclin, ATG5 and LKB1 and decreased phosphorylation levels of Akt, PRAS40, rpS6, p70S6K and 4E-BP1; (3) treatment with yangonin showed the possibility of a synergistic growth inhibitory effect in combination with apoptosis-inducing agents such as docetaxel or flavokawain A. These results suggest that yangonin deserves further study as a novel agent for bladder cancer prevention and treatment.
The pathogenesis of urothelial cell carcinoma of the bladder was shown to be associated with two major molecular pathways[25–26]. The first route is characterized with gain-of-function mutations in oncogenes, e.g. H-ras; activation of the Ras pathway gives rise to superficial papillary tumors, which frequently recur, but are rarely lethal[26]. The second route entails the inactivation, usually via loss-of-major tumor suppressors genes [i.e.p53, phosphatase and tensin homolog (PTEN) and retinoblastoma (Rb1)], and leads to the formation of highly invasive and metastatic tumors[25]. The activation of the Ras pathway occurs in 70%-90% of "superficial" bladder tumors[26–27], whereas the loss of p53 function was reported in more than 50% of muscle invasive transitional cell carcinoma[28]. Importantly, these pathways (Ras, p53, and PTEN) commonly crosstalk with the mTOR pathway[20,26,28], and thus alterations in these pathways are expected to affect the mTOR pathway. The relationship between development of bladder cancer and the mTOR pathway has attracted intense scientific interest and suggests critical targets in bladder cancer prevention and treatment. Based on these findings, Seageret al.[29] performed in vivo preclinical studies using a genetically engineered PTEN and p53 double knockout mouse model and showed that intravesical delivery of rapamycin directly into the bladder lumen was highly effective for suppressing bladder tumorigenesis. Thus, these findings provide the therapeutic possibility of inhibiting mTOR signaling for treatment of bladder cancer. However, mTOR inhibitors that are clinically administered have serious side effects such as immunosuppression and pneumonitis, which can give rise to potentially life-threatening conditions[30]. Apart from expense, concern about the potential side effects remains a substantial barrier to their clinical application, especially when administered continuously. In this study, we provide results demonstrating that yangonin inhibits the mTOR pathway through the change of upstream (LKB1, AKT signaling and PRAS40) and downstream (rpS6, p70S6K and 4E-BP1) events of the mTOR pathway, which corresponds to the decreased viability of bladder cancer cell lines. Since yangonin is conveniently taken in beverage form, these results suggest the possibility for its use as a novel agent for bladder cancer prevention and treatment.
The response to yangonin treatment in bladder cancer cells differs depending on the presence of TSC1 expression status as shown inFig. 5A. T24 and UMUC-3 cells expressing high levels of TSC1/2 are more sensitive to the growth inhibitory effect of yangonin than RT4 cells without TSC 1 expression[28,31]. Consistent with this result, TSC1 knockout MEFs are also resistant to the growth inhibitory effect of yangonin than TSC1 wild type MEFs (Fig. 4B). These results suggest that TSC1 expression is at least in part required for the growth inhibitory effect of yangonin. Further studies are in progress to investigate the mechanisms by which yangonin regulates the TSC1/mTOR mediated cell growth and autophagy.
Another reason for the interest in yangonin treatment with bladder cancer cells is the possibility of synergistic effects in combination with other apoptosis-inducing agents (i.e., docetaxel or flavokawain A). In bladder cancer development, dysregulation can occur at multiple points in cell cycle control including the mTOR pathway. Until now, the medical treatment of bladder cancer has been focused on empirical approaches using cytotoxic agents. Although advances in cytotoxic therapy have suggested the possibility of overcoming biological heterogeneity, there are limits as to what a cytotoxic agent can do, and these limitations combined with the biological diversity of cancer serve as a firm obstacle and drive the discovery and validation of molecular target therapy. Currently, to better understand molecular pathways that are crucial in tumorigenesis of bladder cancer, there is a trend toward targeted treatment of malignancy. A few recent reports have provided evidence that inhibition of the mTOR pathway sensitizes and potentiates chemotherapy (i.e., docetaxel)-induced cytotoxic effects in several cancer cells such as prostate[32], lung[33] and gastric cancer[34], and also suggest underlying mechanisms such as survivin downregulation[32–34] and inhibition of thymidilate synthase[34]. Current results indicate that yangonin inhibits the mTOR pathway, which could enhance the cytotoxicity of docetaxel and flavokawain A to impact other parts of the pathway and serve as a new agent for bladder cancer prevention and treatment. However, to enhance the clinical relevance of this study, further preclinical studies are required to evaluate the combined effect of yangonin and docetaxel or flavokawain A on tumor growth in bladder cancer animal models. In addition, urine and plasma concentrations of yangonin in Kava drinking populations remain to be determined for its physiologic relevance in cancer prevention.
Acknowledgments
This work was supported in part by NIH awards 1R01CA193967-01A1, 5R01CA122558-05 and 1R21-CA152804-01A1 (to X. Zi.)
References
- 1. http://globocan.iarc.fr, accessed on 11/21/2016.
- 2. Puzio-Kuter AM, Castillo-Martin M, Kinkade CW, et al. Inactivation of p53 and Pten promotes invasive bladder cancer[J].Genes Dev, 2009, 23(6): 675–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abraham R, Pagano F, Gomella LG, et al. Chromosomal deletions in bladder cancer: shutting down pathways[J].Front Biosci, 2007, 12: 826–838. [DOI] [PubMed] [Google Scholar]
- 4. Roberts JT, von der Maase H, Sengeløv L, et al. Long-term survival results of a randomized trial comparing gemcitabine/cisplatin and methotrexate/vinblastine/doxorubicin/cisplatin in patients with locally advanced and metastatic bladder cancer[J].Ann Oncol, 2006, 17(Suppl 5): v118–v122. [DOI] [PubMed] [Google Scholar]
- 5. von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study[J].J Clin Oncol, 2000, 18(17): 3068–3077. [DOI] [PubMed] [Google Scholar]
- 6. Gallagher DJ, Milowsky MI, Bajorin DF. Advanced bladder cancer: status of first-line chemotherapy and the search for active agents in the second-line setting[J].Cancer, 2008, 113(6): 1284–1293. [DOI] [PubMed] [Google Scholar]
- 7. Fourth Symposium on Epidemiology and Cancer Registries in the Pacific Basin[J]. Proceedings of a symposium held in Kona, Hawaii, January 16-20, 1984. Natl Cancer Inst Monogr, 1985, 69: 1–282. [PubMed] [Google Scholar]
- 8. Meredith I, Sarfati D, Ikeda T, et al. Cancer in Pacific people in New Zealand[J].Cancer Causes Control, 2012, 23(7): 1173–1184. [DOI] [PubMed] [Google Scholar]
- 9. Tuomilehto J, Zimmet P, Taylor R, et al. Smoking rates in Pacific islands[J].Bull World Health Organ, 1986, 64(3): 447–456. [PMC free article] [PubMed] [Google Scholar]
- 10. Steiner GG. The correlation between cancer incidence and kava consumption[J].Hawaii Med J, 2000, 59(11): 420–422. [PubMed] [Google Scholar]
- 11. Li X, Liu Z, Xu X, et al. Kava components down-regulate expression of AR and AR splice variants and reduce growth in patient-derived prostate cancer xenografts in mice[J].PLoS One, 2012, 7(2): e31213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zi X, Simoneau AR. Flavokawain A, a novel chalcone from kava extract, induces apoptosis in bladder cancer cells by involvement of Bax protein-dependent and mitochondria-dependent apoptotic pathway and suppresses tumor growth in mice[J].Cancer Res, 2005, 65(8): 3479–3486. [DOI] [PubMed] [Google Scholar]
- 13. Narayanapillai SC, Balbo S, Leitzman P, et al. Dihydromethysticin from kava blocks tobacco carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis and differentially reduces DNA damage in A/J mice[J].Carcinogenesis, 2014, 35(10): 2365–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li X, Yokoyama NN, Zhang S, et al. Flavokawain A induces deNEDDylation and Skp2 degradation leading to inhibition of tumorigenesis and cancer progression in the TRAMP transgenic mouse model[J].Oncotarget, 2015, 6(39): 41809–41824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pinner KD, Wales CT, Gristock RA, et al. Flavokawains A and B from kava (Piper methysticum) activate heat shock and antioxidant responses and protect against hydrogen peroxide-induced cell death in HepG2 hepatocytes[J].Pharm Biol, 2016, 54(9): 1503–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu Z, Li X, Simoneau AR, et al. Rhodiola rosea extracts and salidroside decrease the growth of bladder cancer cell lines via inhibition of the mTOR pathway and induction of autophagy[J].Mol Carcinog, 2012, 51(3): 257–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu Z, Antalek M, Nguyen L, et al. The effect of gartanin, a naturally occurring xanthone in mangosteen juice, on the mTOR pathway, autophagy, apoptosis, and the growth of human urinary bladder cancer cell lines[J].Nutr Cancer, 2013, 65(Suppl 1): 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu H, He Z, Simon H. Protective role of autophagy and autophagy-related protein 5 in early tumorigenesis[J].J Mol Med (Berl), 2015, 93(2):159–164. [DOI] [PubMed] [Google Scholar]
- 19. Kallifatidis G, Hoepfner D, Jaeg T, et al. The marine natural product manzamine A targets vacuolar ATPases and inhibits autophagy in pancreatic cancer cells[J].Mar Drugs, 2013, 11(9): 3500–3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jung CH, Ro SH, Cao J, et al. mTOR regulation of autophagy[J].FEBS Lett, 2010, 584(7): 1287–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shorning BY, Griffiths D, Clarke AR. Lkb1 and Pten synergise to suppress mTOR-mediated tumorigenesis and epithelial-mesenchymal transition in the mouse bladder[J].PLoS One, 2011, 6(1): e16209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Singh YN. Kava: an overview[J].J Ethnopharmacol, 1992, 37(1): 13–45. [DOI] [PubMed] [Google Scholar]
- 23. Foliaki S, Best D, Akau'ola S, et al. Cancer incidence in four pacific countries: Tonga, Fiji Islands, Cook Islands and Niue[J].Pac Health Dialog, 2011, 17(1): 21–32. [PubMed] [Google Scholar]
- 24. Henderson BE, Kolonel LN, Dworsky R, et al. Cancer incidence in the islands of the Pacific[J].Natl Cancer Inst Monogr, 1985, 69: 73–81. [PubMed] [Google Scholar]
- 25. McConkey DJ, Lee S, Choi W, et al. Molecular genetics of bladder cancer: Emerging mechanisms of tumor initiation and progression[J].Urol Oncol, 2010, 28(4): 429–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mo L, Zheng X, Huang HY, et al. Hyperactivation of Ha-ras oncogene, but not Ink4a/Arf deficiency, triggers bladder tumorigenesis[J].J Clin Invest, 2007, 117(2): 314–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sibley K, Cuthbert-Heavens D, Knowles MA. Loss of heterozygosity at 4p16.3 and mutation of FGFR3 in transitional cell carcinoma[J].Oncogene, 2001, 20(6): 686–691. [DOI] [PubMed] [Google Scholar]
- 28. Dinney CP, McConkey DJ, Millikan RE, et al. Focus on bladder cancer[J].Cancer Cell, 2004, 6(2): 111–116. [DOI] [PubMed] [Google Scholar]
- 29. Seager CM, Puzio-Kuter AM, Patel T, et al. Intravesical delivery of rapamycin suppresses tumorigenesis in a mouse model of progressive bladder cancer[J].Cancer Prev Res (Phila), 2009, 2(12): 1008–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Motzer RJ, Escudier B, Oudard S, et al. , and the RECORD ‐ 1 Study Group. Phase 3 trial of everolimus for metastatic renal cell carcinoma: final results and analysis of prognostic factors[J].Cancer, 2010, 116(18): 4256–4265. [DOI] [PubMed] [Google Scholar]
- 31. Liu Z, Li X, Simoneau AR, et al. Rhodiola rosea extracts and salidroside decrease the growth of bladder cancer cell lines via inhibition of the mTOR pathway and induction of autophagy[J].Mol Carcinog, 2012, 51(3): 257–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Morikawa Y, Koike H, Sekine Y, et al. Rapamycin enhances docetaxel-induced cytotoxicity in a androgen-independent prostate cancer xenograft model by survivin downregulation[J].Biochem Biophys Res Commun, 2012, 419(3): 584–589. [DOI] [PubMed] [Google Scholar]
- 33. Niu H, Wang J, Li H, et al. Rapamycin potentiates cytotoxicity by docetaxel possibly through downregulation of Survivin in lung cancer cells[J].J Exp Clin Cancer Res, 2011, 30: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shigematsu H, Yoshida K, Sanada Y, et al. Rapamycin enhances chemotherapy-induced cytotoxicity by inhibiting the expressions of TS and ERK in gastric cancer cells[J].Int J Cancer, 2010, 126(11): 2716–2725. [DOI] [PubMed] [Google Scholar]