Abstract
Liver injury represents a continuum of pathophysiological processes involving a complex interplay between hepatocytes, macrophages, and hepatic stellate cells. The mechanism whereby these intercellular interactions contribute to liver injury and fibrosis is not completely understood. We report here that angiogenic factor with G patch and FHA domains 1 (Aggf1) was downregulated in the livers of cirrhotic patients compared to healthy controls and in primary hepatocytes in response to carbon tetrachloride (CCl4) stimulation. Overexpression of Aggf1 attenuated macrophage chemotaxis. Aggf1 interacted with NF-κB to block its binding to theCcl2 gene promoter and repressed Ccl2 transcription in hepatocytes. Macrophages cultured in the conditioned media collected from Aggf1-overexpressing hepatocytes antagonized HSC activation. Taken together, our data illustrate a novel role for Aggf1 in regulating hepatic inflammation and provide insights on the development of interventional strategies against cirrhosis.
Keywords: Aggf1, liver fibrosis, hepatocyte, hepatic stellate cell, macrophage
Introduction
The liver is home to a number of different cell types including hepatocytes, hepatic macrophages (Kupffer cells), and hepatic stellate cells (HSCs). Under normal conditions, these cells form dynamic communications and collectively maintain the hepatic homeostasis. In response to various injurious signals, however, hepatic cells undergo morphological and functional alterations heralding a transition of the liver from a physiologic state to a pathological state[1]. Hepatocytes can upregulate the expression of pro-inflammatory cytokines and chemokines when exposed to toxins or excessive nutrients[2-3]. Macrophages can be educated to differentiate into an alternatively activated, pro-fibrogenic (M2) phenotype by hepatitis B virus in patients with acute liver failure[4]. HSCs, confronted with a host of stimuli ranging from nutrients to pathogens, can switch from a fat-storing phenotype to a contractile phenotype known as myofibroblast specialized in extracellular matrix (ECM) deposition[5]. The cell-specific changes associated with the pathophysiological processes are typically not cell-autonomous. Instead, phenotypic changes of one cell type may influence those of the other. For instance, damage-associated molecular pattern (DAMP) derived from injured or necrotic hepatocytes recruits/activates macrophages and contributes to hepatic inflammation[6]. Meanwhile, macrophages can produce large quantities of pro-fibrogenic growth factors such as TGF-β and TNF-α to promote HSC activation and consequently liver fibrosis[7]. This complex interplay of different hepatic cells constitutes the basis of liver injury and fibrosis.
Angiogenic factor with G patch and FHA domains 1, or Aggf1, was initially identified as a vascular endothelium-derived protein with the ability to promote endothelial cell proliferation[8]. Overexpression of Aggf1 is associated with enhanced angiogenesis and predicts poor prognosis of hepatocellular carcinoma in humans[9]. On the other hand, Aggf1 gain-of-function has been shown to protect the mice from ischemic injuries[10-11]. Here, we report that Aggf1 is downregulated in cirrhotic livers in humans and in primary hepatocytes exposed to carbon tetrachloride (CCl4). We also show that Aggf1 is able to broker the communication between hepatocytes, macrophages, and HSCs by repressing Ccl2 transcription. Therefore, screening for small-molecule compounds that can boost Aggf1 expression/activity may provide novel therapeutic solutions to treat liver injury and fibrosis.
Materials and methods
Cell culture
Immortalized mouse monocytic/macrophage-like cells (RAW264.7, ATCC) were maintained in DMEM supplemented with 10% fetal bovine serum (FBS). Primary murine hepatic stellate cells and primary hepatocytes were isolated as described previously[12-13].
Plasmids, transfection, and reporter assay
FLAG-tagged Aggf1, Ccl2 promoter-luciferase construct, and NF-κB/p65 expression construct have been described previously[14–18]. Transient transfections were performed with Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). Luciferase activities were assayed using a luciferase reporter assay system (Promega, Madison, WI, USA).
Protein extraction, immunoprecipitation, and Western blotting assay
Whole cell lysates and nuclear proteins were obtained as previously described[19]. Specific antibodies or pre-immune IgGs (P.I.I.) were added to and incubated with cell lysates overnight before being absorbed by Protein A/G-plus Agarose beads (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Precipitated immune complex was released by boiling with 1X SDS electrophoresis sample buffer. Western blot analyses were performed with anti-collagene type I (Rockland), anti-α-SMA, anti-β-actin (Sigma), anti-Aggf1, or anti-p65 (Santa Cruz Biotechnology) antibodies.
Chromatin immunoprecipitation (ChIP)
ChIP assays were performed essentially as described before[20] with anti-p65 (Santa Cruz Biotechnology). Precipitated genomic DNA was amplified by real-time PCR with primers as previously described[20].
Animals
All animal protocols were approved by the NJMU Intramural Ethics Committee on Animal Studies and adhere to the criteria outlined in the "Guide for the Care and Use of Laboratory Animals." To induce liver injury, 6-8 week-old male C57/BL6 mice were given four i.p. administrations of CCl4 dissolved in olive oil (1.0 mL/kg bodyweight as 50%, vol/vol) within a week with a one-day interval between each injection. For selected groups, mice were injected weeklyvia the tail vein adenovirus (1×1012 MOI) carrying FLAG-tagged AGGF1 (Ad-AGGF1) or an empty vector (Ad-V).
RNA isolation and Real-time PCR
RNA was extracted with the RNeasy RNA isolation kit (Qiagen). Reverse transcriptase reactions were performed as previously described using a SuperScript First-strand Synthesis System (Invitrogen)[21]. Real-time quantitative PCR reactions were performed using previously described primers[3,22].
Histology
Human studies were reviewed and approved by the Committee on Ethical Conduct of Human Studies of Nanjing Medical University. Human liver tissue microarray chips contain 30 normal human liver tissues and 40 cirrhotic human liver tissues. Cirrhotic tissues were obtained from patients with cirrhosis receiving treatment at Xi-Jing Hospital, Xi'an, China. Normal liver tissues, adjacent to cancerous tissues, were obtained from patients with hepatocellular carcinoma (HCC) undergoing biopsy. All the samples were collected under informed consent. Histological analyses were performed essentially as described before[3,23].
In vitro migration assay
The migration/chemotaxis of macrophages was evaluated using a Boyden chamber (BD Biosciences) system. Briefly, macrophages were seeded into the upper chamber of the cell culture inserts while hepatocytes were seeded into the lower chamber for co-culture. Following 24 hours of co-culture, the inserts were washed by PBS, fixed by 4% paraformaldehyde, and the unmigrated cells were removed by cotton swabs. The migrated cells were stained with crystal violet for microscopic analysis. Quantification was performed with Image J.
Statistical analysis
One-way ANOVA with post-hoc Scheffe analyses was performed using an SPSS package. Unless otherwise specified, P values smaller than 0.05 were considered statistically significant (*).
Results
Aggf1 expression is downregulated during liver injury
To probe the role of Aggf1 in liver injury, we first examined liver Aggf1 expression in humans by immunohistochemical staining. As shown inFig. 1, Aggf1 protein levels were downregulated in cirrhotic livers compared to healthy livers. Aggf1 expression was also decreased in primary mouse hepatocytes exposed to CCl4, a common hepatotoxic substance, at both mRNA and protein levels (Fig. 2A). Together, these data demonstrate that Aggf1 expression levels may be correlated with liver injury both in vivo and in vitro.
Fig.1. Aggf1 is downregulated in the liver during fibrogenesis in humans.
Immunohistochemical stainings were performed with anti-Aggf1 antibodies using liver specimens from cirrhotic patients or healthy controls. Shown here are two representative pictures from each group. Scale bar, 100 μm.
Fig.2. Aggf1 overexpression attenuates hepatic inflammation.
A: Primary hepatocytes were treated with corn oil or CCl4 (10 μmol/L) for 12 hours. Aggf1 expression was measured by qPCR and Western blotting. B-D: Liver injury was induced in C57/BL6 mice by CCl4 injection. Aggf1 overexpression was mediated by adenovirus. Western blotting was performed to evaluate Aggf1 protein levels in the liver (B). Immunochemical stainings were performed with anti-F4/80 using paraffin embedded liver sections (C). Scale bar, 25 μm; N = 4 mice for each group. Paraffin embedded liver sections were stained with anti-CD31 antibodies (D). Quantifications were performed with Image J. N = 4 mice for each group. E: Transwell assay was performed as described under Materials and methods.
Aggf1 overexpression attenuates hepatic inflammation
Next, we examined the effect of Aggf1 overexpression on liver injury induced by carbon tetrachloride (CCl4) in mice. Consistent with the data shown inFig. 1 and Fig. 2A, endogenous Aggf1 was downregulated in the livers of mice receiving CCl4 injection; infection of adenovirus carrying an Aggf1 expression vector led to a robust increase in Agg1 levels while at the same time dampened liver fibrosis (Fig. 2B). Aggf1 overexpression in mice attenuated hepatic inflammation as measured by immunohistochemical staining showing fewer F4/80+ macrophages compared to the mice infected by adenovirus carrying an empty vector (Fig. 2C). Unexpectedly, we did not find significant alterations in angiogenesis in the CCl4-injected livers following Aggf1 overexpression (Fig. 2D), suggesting that Aggf1 might play angiogenesis-independent roles to regulate liver injury. To further support a role for hepatocyte Aggf1 in antagonizing macrophage recruitment, we performed the Boyden chamber transwell assay. As shown inFig. 2E, primary mouse hepatocytes exposed CCl4 was able to attract more macrophages to migrate to the lower chamber when cultured together, a process blocked by Aggf1 overexpression in hepatocytes. Collectively, these data suggest that Aggf1 may attenuate hepatic inflammation possibly by preventing hepatocyte-derived chemoattraction of macrophages.
Aggf1 interacts with NF-κB to regulate Ccl2 transcription in hepatocytes
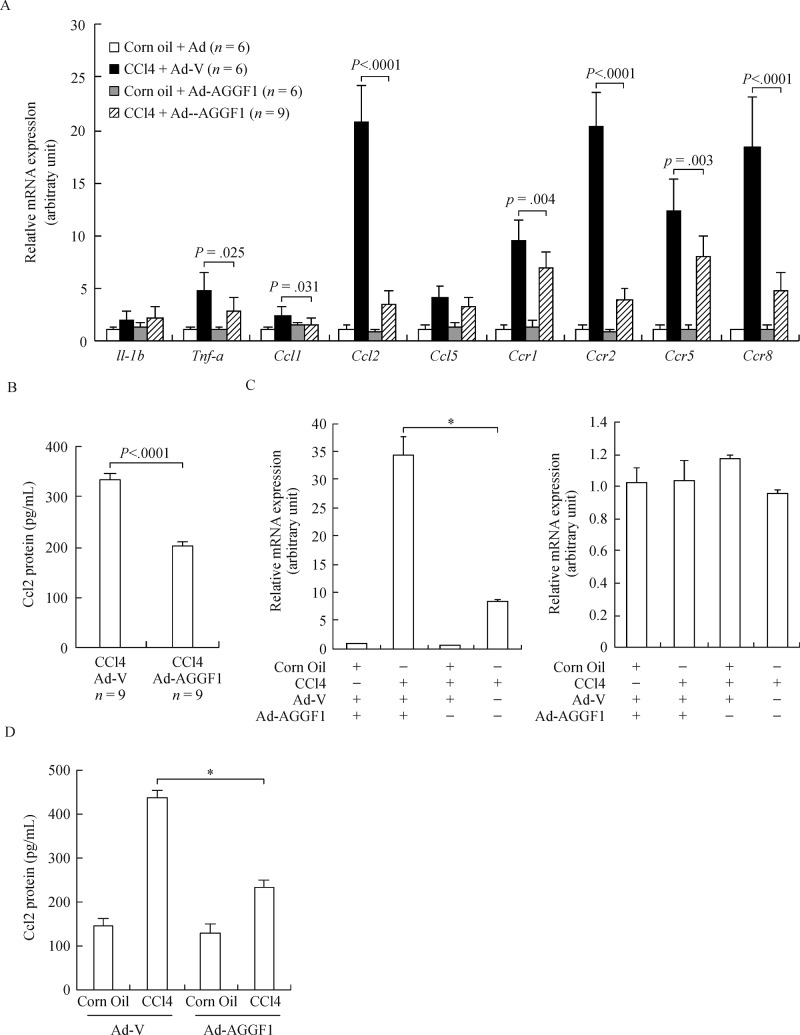
Macrophages are actively recruited to the injured liver to promote fibrogenesis; hepatocytes contributes to this process by emitting chemoattractive signals[24-25]. Therefore, we investigated the role of Aggf1 in hepatocyte-mediated chemotaxis. QPCR analyses found that Aggf1 overexpression altered the expression of several chemokines and their receptors in the liver, among which Ccl2 stood out as the one being most significantly stimulated by CCl4 injury and repressed by Aggf1 (Fig. 3A, 3B). Treatment with CCl4 directly stimulated the synthesis and secretion of Ccl2, but not Ccl1, in hepatocytes, which could be blocked by Aggf1 (Fig. 3C, 3D).
Fig.3. Aggf1 represses Ccl2 transcription in hepatocytes.
A, B: Liver injury was induced in C57/BL6 mice by CCl4 injection. Aggf1 overexpression was mediated by adenovirus. Expression levels of chemokines and chemokine receptors in liver tissues were evaluated with qPCR (A) and ELISA (B). C, D: Primary hepatocytes were infected with with Ad-V or Ad-AGGF1 followed by treatment with corn oil or CCl4 (10μM) for 12 hours. Ccl2 expression was measured by qPCR (C) and ELISA (D).
NF-κB is the master regulator of cellular inflammation responsible for Ccl2 transactivation[26]. We postulated that Aggf1 might suppress NF-κB activity in hepatocyte. Co-immunoprecipitation assay indicates that endogenous Aggf1 formed a complex with NF-κB/p65 (Fig. 4A). Consistently, Aggf1 was able to antagonize p65-induced Ccl2 transcription in a dose-dependent manner in reporter assay (Fig. 4B). Concomitantly, ChIP assays show that the binding of p65 to the proximal Ccl2 promoter was significantly weakened by Aggf1 in both liver tissues and hepatocytes following CCl4 stimulation (Fig. 4C). Together, these data suggest that Aggf1 might play a role in regulating the production of hepatocyte-derived chemoattractant.
Fig.4. Aggf1 interacts with NF-κB and suppresses its binding to the Ccl2 promoter.
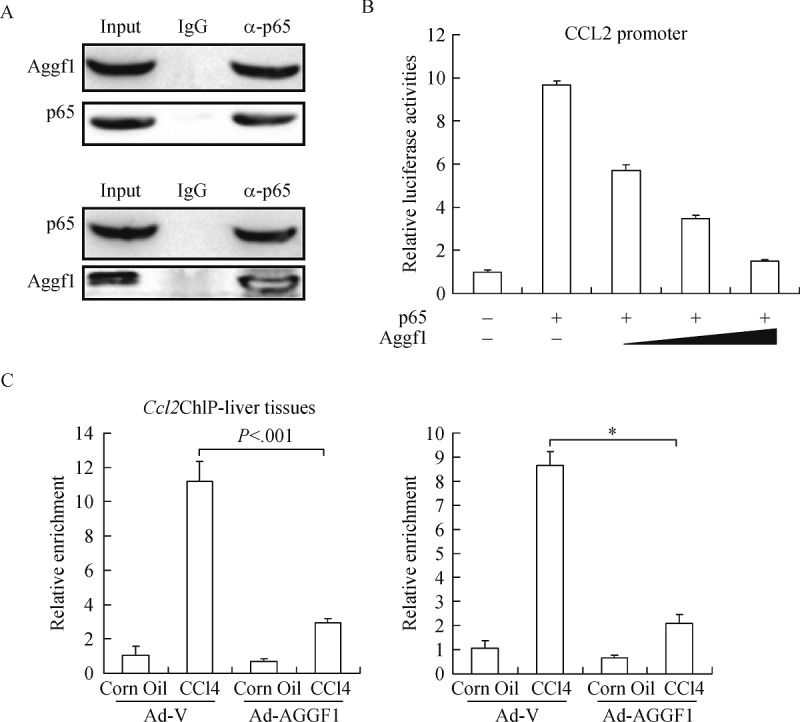
A: Lysates extracted from primary hepatocytes were immunoprecipitated with anti-NF-κB or anti-AGGF1 antibodies. B: A Ccl2 promoter-luciferase construct was transfected into mouse primary hepatocytes along with indicated expression constructs. Luciferase activities were expressed as relative luciferase activity compared to the control group. C: ChIP assays were performed with anti-NF-κB using lysates extracted from liver tissues (left panel) or primary hepatocytes (right panel).
Aggf1 blocks HSC activation by antagonizing hepatocyte-derived chemotaxis
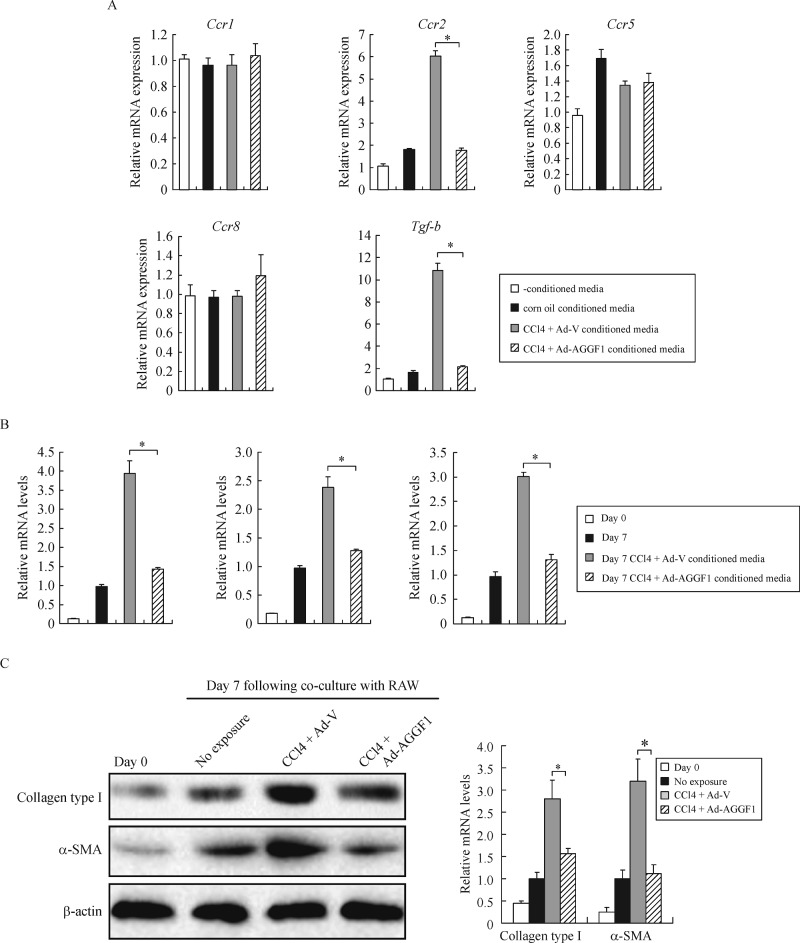
The observation that Aggf1 could curb Ccl2 transactivation prompted us to hypothesize that Aggf1 could indirectly affect HSC activation by moderating the crosstalk between hepatocyte and macrophages. Indeed, conditioned media from CCl4-treated hepatocytes increased the expression of Ccr2, a specific receptor for Ccl2 and Tgf-b, in macrophages; both were dampened by Aggf1 overexpression (Fig. 5A). We further evaluated the (indirect) effect of Aggf1 on communication between macrophages and HSCs by using a co-culture system: there was augmented activation of HSCs when macrophages were pre-exposed to hepatocyte-derived, CCl4-stimulated conditioned media, but not when Aggf1 was overexpressed in hepatocytes (Fig. 5B, 5C). Taken together, these data support a role for hepatocyte-Aggf1 in restricting the release of a pro-fibrogenic signal that could promote HSC-macrophage dialog and HSC activation.
Fig.5. Aggf1 blocks HSC activation by antagonizing hepatocyte-derived chemotaxis.
A: Conditioned media collected from primary hepatocytes were used to treat macrophages (RAW264.7). Gene expression levels were examined by qPCR. B, C: Primary mouse HSCs were cocultured with macrophages (RAW264.7). Expression of fibrogenic genes was measured by qPCR (B) and Western (C).
Discussion
It has been well documented that a dynamic dialog between hepatic cells, including hepatocytes, hepatic stellate cells, and macrophages, contribute to the maintenance of liver homeostasis; disruption of this intricate balance leads to a host of liver pathologies such as cirrhosis[1]. In the present study we investigated the role of Aggf1 in mediating the crosstalk between different cells and our data indicate that Aggf1, by regulating Ccl2 transcription in hepatocytes, is essential in antagonizing macrophage recruitment and consequently HSC activation.
Several independent investigations have shown that hepatocyte-derived Ccl2 plays a key role in macrophage infiltration and HSC activation in both model animals and in humans[25,27–29]. Moreover, mice depleted of Ccr2, the receptor of Ccl2, exhibit decreased fibrosis when challenged with CCl4[30]. In response to liver injury, Ccl2 levels are upregulated primarily at the transcriptional level by the master regulator of inflammation, NF-κB. Indeed, inhibition of NF-κB activity by a small-molecule compound is capable of preventing/reversing fibrosis in mice[31]. In contrast, there is enhanced hepatic inflammation and fibrogenesis in mice with a deficiency of p50, the inhibitory subunit of NF-κB[32]. Our data indicate that Aggf1 selectively suppresses Ccl2 transcription by interacting with and blocking the recruitment of NF-κB/p65 to the Ccl2 thus functioning as a check to curb macrophage infiltration and hepatic inflammation. This is consistent with a previous report showing that Aggf1 attenuates TNF-α induced, NF-κB mediated endothelial activation by inhibiting ERK phosphorylation[10]. At this point, it is unclear how Aggf1 specifically impacts the transcription of Ccl2 among a panel of pro-inflammatory mediators in hepatocytes, all of which are NF-κB target genes. One possible explanation would be that Aggf1 is preferentially recruited by a Ccl2 promoter-specific transcription factor/co-factor. Alternatively, different NF-κB target genes may require differential stringency in terms of NF-κB binding affinity and therefore differential sensitivity to Aggf1 inhibition[33].
We show here that in addition to blocking Ccl2 synthesis in hepatocytes and hence macrophage attraction, Aggf1 overexpression also skews macrophage phenotype as evidenced by a decrease in both the capacity to synthesize Tgf-b and the ability to promote HSC activation. This line of data suggests that Aggf1 may contribute indirectly to the regulation of HSC activation and fibrogenesis by mediating the dialog between hepatocytes and macrophages. These results, however, should be interpreted with caution. Previous studies have established a vascular endothelium-derived “angiocrine” signal in maintaining liver homeostasis and modulating liver fibrosis[34-35]. Although our data suggest that Aggf1 exerted little influence over angiogenesis in the context of liver fibrosis (Fig. 2C), the possibility that Aggf1 may fine-tune this “angiocrine” signal cannot be excluded since Aggf1 is abundantly expression in endothelial cells. Future investigations employing cell-restricted Aggf1 deletion mice should shed additional light on the precise role of Aggf1 in liver fibrosis.
In summary, we show here that Aggf1 modulates hepatic inflammation and fibrosis by brokering the communications between hepatocytes, macrophages, and HSCs. Further investigations are warranted to define a more precise and holistic role for Aggf1 in this process in order to devise novel therapeutic strategies to combat liver failure.
Acknowledgements
This work was supported, in part, by grants from the Natural Science Foundation of China (81402550), the Natural Science Foundation of Jiangsu Province (BK20140906), the Natural Science Foundation of Jiangsu Higher Education Institutions (14KJB310007), and the Science & Technology Development Foundation of Nanjing Medical University (2013NJMU015). Wenping Xu received funding from Jiangsu Jiankang Vocational University (JK201405).
References
- 1. Seki E, Schwabe RF. Hepatic inflammation and fibrosis: functional links and key pathways[J].Hepatology, 2015, 61(3): 1066–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mandrekar P, Ambade A, Lim A, et al. An essential role for monocyte chemoattractant protein-1 in alcoholic liver injury: regulation of proinflammatory cytokines and hepatic steatosis in mice[J].Hepatology, 2011, 54(6): 2185–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tian W, Xu H, Fang F, et al. Brahma-related gene 1 bridges epigenetic regulation of proinflammatory cytokine production to steatohepatitis in mice[J].Hepatology, 2013, 58(2): 576–588. [DOI] [PubMed] [Google Scholar]
- 4. Bility MT, Cheng L, Zhang Z, et al. Hepatitis B virus infection and immunopathogenesis in a humanized mouse model: induction of human-specific liver fibrosis and M2-like macrophages[J].PLoS Pathog, 2014, 10(3): e1004032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Trautwein C, Friedman SL, Schuppan D, et al. Hepatic fibrosis: Concept to treatment[J].J Hepatol, 2015, 62(1 Suppl): S15–S24. [DOI] [PubMed] [Google Scholar]
- 6. Kubes P, Mehal WZ. Sterile inflammation in the liver[J].Gastroenterology, 2012, 143(5): 1158–1172. [DOI] [PubMed] [Google Scholar]
- 7. Chu PS, Nakamoto N, Ebinuma H, et al. C-C motif chemokine receptor 9 positive macrophages activate hepatic stellate cells and promote liver fibrosis in mice[J].Hepatology, 2013, 58(1): 337–350. [DOI] [PubMed] [Google Scholar]
- 8. Tian XL, Kadaba R, You SA, et al. Identification of an angiogenic factor that when mutated causes susceptibility to Klippel-Trenaunay syndrome[J].Nature, 2004, 427(6975): 640–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang W, Li GY, Zhu JY, et al. Overexpression of AGGF1 is correlated with angiogenesis and poor prognosis of hepatocellular carcinoma[J].Med Oncol, 2015, 32(4): 131. [DOI] [PubMed] [Google Scholar]
- 10. Hu FY, Wu C, Li Y, et al. AGGF1 is a novel anti-inflammatory factor associated with TNF-α-induced endothelial activation[J].Cell Signal, 2013, 25(8): 1645–1653. [DOI] [PubMed] [Google Scholar]
- 11. Liu Y, Yang H, Song L, et al. AGGF1 protects from myocardial ischemia/reperfusion injury by regulating myocardial apoptosis and angiogenesis. Apoptosis: an international journal on programmed cell death[J]. 2014, 19(8):1254–1268. Epub 2014/06/05. [DOI] [PubMed] [Google Scholar]
- 12. Schneiderhan W, Schmid-Kotsas A, Zhao J, et al. Oxidized low-density lipoproteins bind to the scavenger receptor, CD36, of hepatic stellate cells and stimulate extracellular matrix synthesis[J].Hepatology, 2001, 34(4 Pt 1): 729–737. [DOI] [PubMed] [Google Scholar]
- 13. Shen H, Sheng L, Chen Z, et al. Mouse hepatocyte overexpression of NF-κB-inducing kinase (NIK) triggers fatal macrophage-dependent liver injury and fibrosis[J].Hepatology, 2014, 60(6): 2065–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fan C, Ouyang P, Timur AA, et al. Novel roles of GATA1 in regulation of angiogenic factor AGGF1 and endothelial cell function[J].J Biol Chem, 2009, 284(35): 23331–23343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li H, Rauch T, Chen ZX, et al. The histone methyltransferase SETDB1 and the DNA methyltransferase DNMT3A interact directly and localize to promoters silenced in cancer cells[J].J Biol Chem, 2006, 281(28): 19489–19500. [DOI] [PubMed] [Google Scholar]
- 16. Major MB, Roberts BS, Berndt JD, et al. New regulators of Wnt/beta-catenin signaling revealed by integrative molecular screening[J].Sci Signal, 2008, 1(45): ra12. [DOI] [PubMed] [Google Scholar]
- 17. Yu L, Weng X, Liang P, et al. MRTF-A mediates LPS-induced pro-inflammatory transcription by interacting with the COMPASS complex[J].J Cell Sci, 2014, 127(Pt 21): 4645–4657. [DOI] [PubMed] [Google Scholar]
- 18. Yang Y, Cheng X, Tian W, et al. MRTF-A steers an epigenetic complex to activate endothelin-induced pro-inflammatory transcription in vascular smooth muscle cells[J].Nucleic Acids Res, 2014, 42(16): 10460–10472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fang F, Yang Y, Yuan Z, et al. Myocardin-related transcription factor A mediates OxLDL-induced endothelial injury[J].Circ Res, 2011, 108(7): 797–807. [DOI] [PubMed] [Google Scholar]
- 20. Fang F, Chen D, Yu L, et al. Proinflammatory stimuli engage Brahma related gene 1 and Brahma in endothelial injury[J].Circ Res, 2013, 113(8): 986–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sun L, Li H, Chen J, et al. PIASy mediates hypoxia-induced SIRT1 transcriptional repression and epithelial-to-mesenchymal transition in ovarian cancer cells[J].J Cell Sci, 2013, 126(Pt 17): 3939–3947. [DOI] [PubMed] [Google Scholar]
- 22. Tian W, Hao C, Fan Z, et al. Myocardin related transcription factor A programs epigenetic activation of hepatic stellate cells[J].J Hepatol, 2015, 62(1): 165–174. [DOI] [PubMed] [Google Scholar]
- 23. Sun L, Li H, Chen J, et al. A SUMOylation-dependent pathway regulates SIRT1 transcription and lung cancer metastasis[J].J Natl Cancer Inst, 2013, 105(12): 887–898. [DOI] [PubMed] [Google Scholar]
- 24. Saiman Y, Friedman SL. The role of chemokines in acute liver injury[J].Front Physiol, 2012, 3: 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ramm GA, Shepherd RW, Hoskins AC, et al. Fibrogenesis in pediatric cholestatic liver disease: role of taurocholate and hepatocyte-derived monocyte chemotaxis protein-1 in hepatic stellate cell recruitment[J].Hepatology, 2009, 49(2): 533–544. [DOI] [PubMed] [Google Scholar]
- 26. Ping D, Boekhoudt GH, Rogers EM, et al. Nuclear factor-kappa B p65 mediates the assembly and activation of the TNF-responsive element of the murine monocyte chemoattractant-1 gene[J].J Immunol, 1999, 162(2): 727–734. [PubMed] [Google Scholar]
- 27. Marra F, DeFranco R, Grappone C, et al. Increased expression of monocyte chemotactic protein-1 during active hepatic fibrogenesis: correlation with monocyte infiltration[J].Am J Pathol, 1998, 152(2): 423–430. [PMC free article] [PubMed] [Google Scholar]
- 28. Kamada Y, Kiso S, Yoshida Y, et al. Estrogen deficiency worsens steatohepatitis in mice fed high-fat and high-cholesterol diet[J].Am J Physiol Gastrointest Liver Physiol, 2011, 301(6): G1031–G1043. [DOI] [PubMed] [Google Scholar]
- 29. Borkham-Kamphorst E, van de Leur E, Zimmermann HW, et al. Protective effects of lipocalin-2 (LCN2) in acute liver injury suggest a novel function in liver homeostasis[J].Biochim Biophys Acta, 2013, 1832(5): 660–673. [DOI] [PubMed] [Google Scholar]
- 30. Mitchell C, Couton D, Couty JP, et al. Dual role of CCR2 in the constitution and the resolution of liver fibrosis in mice[J].Am J Pathol, 2009, 174(5): 1766–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lv P, Luo HS, Zhou XP, et al. Reversal effect of thalidomide on established hepatic cirrhosis in rats via inhibition of nuclear factor-kappaB/inhibitor of nuclear factor-kappaB pathway[J].Arch Med Res, 2007, 38(1): 15–27. [DOI] [PubMed] [Google Scholar]
- 32. Oakley F, Mann J, Nailard S, et al. Nuclear factor-kappaB1 (p50) limits the inflammatory and fibrogenic responses to chronic injury[J].Am J Pathol, 2005, 166(3): 695–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wan F, Lenardo MJ. Specification of DNA binding activity of NF-kappaB proteins[J].Cold Spring Harb Perspect Biol, 2009, 1(4): a000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ding BS, Cao Z, Lis R, et al. Divergent angiocrine signals from vascular niche balance liver regeneration and fibrosis[J].Nature, 2014, 505(7481): 97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ding BS, Nolan DJ, Butler JM, et al. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration[J].Nature, 2010, 468(7321): 310–315. [DOI] [PMC free article] [PubMed] [Google Scholar]