Abstract
Tumor infiltrating lymphocytes (TILs) in primary melanomas are thought to represent the host anti-tumor immune response, but controversy exists over whether TILs offer independent prognostication of survival. We studied a cohort of 1,241 primary melanoma patients to assess the association of absent, non-brisk, and brisk TIL grade with survival outcomes. We tested whether quantitative TIL counts using immunohistochemical lymphocyte markers CD3, CD45, and FOXP3 add prognostic value to TIL grading compared to histology alone in 15% of the cohort. To assess for inter-group immunologic heterogeneity among TIL grades, we investigated differential expression of 594 immunoregulatory genes in 67 primary melanomas. On histologic evaluation of 1,241 primary melanomas, TILs were graded as absent (n=388, 31%), non-brisk (n=330, 27%), and brisk (n=523, 42%). Patients with brisk TILs had improved recurrence-free survival (RFS) (p=0.025) and overall survival (OS) (p=0.006) compared to patients with non-brisk and absent TILs, for which there were no differences in RFS (p=0.40) or OS (p=0.41). TIL quantitation by immunohistochemistry did not improve prognostication compared to TIL grading on hematoxylin and eosin stained sections. Melanomas with non-brisk and absent TILs share similar immunoregulatory gene expression profiles. In contrast, melanomas with brisk TILs demonstrate upregulation of T-cell activation pathways and inhibition of upstream immune checkpoint regulators. The presence of TILs in primary melanomas represents a heterogeneous group and caution in prognostic interpretation is warranted. Melanomas with brisk TILs are defined by an immunostimulatory gene expression profile and improved prognosis compared to melanomas with non-brisk or absent TILs.
Keywords: tumor infiltrating lymphocytes, melanoma, prognosis, host immune response
1. INTRODUCTION
Melanoma incidence in the United States is rising. There were 73,000 new cases of melanoma diagnosed in 2015 and over 9,000 deaths (1). The American Joint Committee on Cancer’s (AJCC) melanoma staging system is the mainstay of clinical prognostication in melanoma and is dependent upon primary tumor thickness, ulceration, mitoses, and nodal status (2-6). Despite the AJCC’s survival estimates by stage, clinical behavior of melanoma is often unpredictable. Additional prognostic markers are warranted to more precisely identify early stage, high risk melanoma patients in jeopardy of recurrence and early death (5, 7). This will aid in modification of clinical surveillance strategies and improve patient selection for adjuvant therapy administration.
Tumor-infiltrating lymphocytes (TILs) have been identified as a favorable prognostic marker across multiple cancers including breast, ovarian, and colon cancer and may serve as a surrogate indicator of the strength of the host anti-tumor immune response (8-10). While multiple studies have assessed the prognostic value of TILs in primary melanoma (11-19), pooled results from the literature remain controversial. Several studies show no association of TIL presence and improved melanoma prognosis (13, 15-17), while the studies that do show a significant correlation (11, 12, 14, 18, 19) are difficult to draw conclusions from due to heterogeneity of patient populations, research methods, and TIL grading systems used. TIL assessment also remains a semi-quantitative measure that may be challenging to consistently reproduce. To date, no cancer staging algorithms incorporate immunologic markers.
Importantly, there is also a lack of understanding of the diverse composition of the TIL infiltrate, comprised of effector and suppressor T cells, B cells, natural killer cells, macrophages, dendritic cells, and myeloid-derived suppressor cells, each of which impart competing immunostimulatory or immunosuppressive effects within the tumor microenvironment. For example, tumor-associated T cells may be immunosuppressive (i.e. T regulatory cells) or anergic and thus their presence does not ensure an effective anti-tumor immune response. Therefore, the functional impact of TILs within the tumor microenvironment, rather than their mere presence or absence, is an important consideration that has not been significantly addressed in the literature.
The primary objectives of this study are to identify patterns of immunologic heterogeneity that exist among progressing levels of TIL intensity within primary melanomas and how these profiles impact patient prognosis. We test whether quantitative immunohistochemistry (IHC) compared to TIL grading by histology alone allows for more precise prognostic predictions. We also compare immunoregulatory gene expression profiles of primary melanomas to explore whether an immunologic signature may functionally explain survival differences amongst melanomas of each TIL grade.
2. MATERIALS AND METHODS
2.1 Study Population
A cohort of patients with primary cutaneous melanoma presenting to New York University (NYU) Langone Medical Center from 2002-2013 were enrolled on the Interdisciplinary Melanoma Cooperative Group (IMCG) biospecimen database protocol. This protocol is approved by the NYU institutional review board (IRB), prospectively enrolls melanoma patients presenting to surgical and medical oncologists at the NYU Perlmutter Cancer Center, and banks patient tissue specimens for research purposes, which are linked to the clinicopathologic database. Patient demographics, and clinical, pathologic, and follow-up information are prospectively recorded and updated on a regular basis as per the standards outlined in our protocol. Informed consent for use of clinical data and tissue was obtained from all patients at the time of enrollment. Patients with multiple primary melanomas were excluded from this study.
2.2 Histopathological Review
Histopathologic features from hematoxylin and eosin (H&E) stained sections for all melanoma cases were determined by the IMCG pathologist (FD) including: thickness, ulceration, mitoses, histologic subtype, regression, vascular invasion, perineural invasion, microsatellites, and solar elastosis. TILs were graded as brisk (present throughout the vertical growth phase (VGP) or infiltrating the entire base of the VGP), non-brisk (present in one or more foci of the VGP), or absent (no lymphocytes are in contact with the VGP but may be present in perivascular or fibrotic areas), as previously defined (12). Patients who underwent sentinel lymph node (SLN) biopsy had SLN status recorded as positive or negative.
2.3 Immunohistochemical Analysis
Primary melanomas representing 15% of the entire cohort were randomly selected to undergo immunohistochemical staining for CD3, a pan T-cell marker and T-cell co-receptor, and CD45, a transmembrane glycoprotein expressed on leukocytes. Polyclonal rabbit anti-human CD3 and monoclonal mouse anti-human CD45 (Dako North America, Inc., Carpinteria, CA) were used for TIL identification. Monoclonal mouse anti-human Foxp3 clone 236A/E7 (eBioscience, San Diego, CA) was used to detect T regulatory cells (Tregs) in the same cohort.
Formalin fixed paraffin embedded (FFPE) primary melanomas were deparaffinized, rehydrated, and subjected to heat-induced epitope retrieval in citrate buffer in a microwave. Sections were quenched with hydrogen peroxidase, incubated in blocking solution (Vectastain Elite ABC Kit; rabbit IgG for CD3; mouse IgG for both CD45 and Foxp3, Vector Laboratories, Burlingame, CA), and then incubated in primary antibody diluted in blocking solution at a concentration of 1:500 for 1 hour at room temperature and at 4° C overnight. Sections were washed and incubated in a 1:200 dilution of biotinylated antibody solution (goat anti-rabbit for CD3; horse anti-mouse for both CD45 and Foxp3) for 1 hour and incubated with Vectastain ABC reagent. Avidin-biotinylated horseradish peroxidase complexes were visualized with diaminobenzidine (DAB substrate kit, Vector Laboratories, Burlingame, CA). Slides were counterstained with hematoxylin, dehydrated, and mounting medium and coverslips were applied.
The IMCG pathologist was blinded to patient clinicopathologic data. He quantitatively scored CD3 and CD45 expression as the absolute number of intratumoral TILs stained in the densest infiltrative area in one high power field (HPF) and the absolute number of peritumoral TILs stained in the dermis immediately beyond the tumor-dermis interface. Cut-off for quantitative scoring was set at TIL counts of greater than 50 cells. Foxp3 expression was scored by identifying the densest area of antibody staining and quantifying the number of Foxp3-positive cells in a single HPF within the lymphocytic infiltrate. Percentage of Foxp3-stained cells within the lymphocytic infiltrate was estimated.
2.4 Gene Expression
RNA was isolated from macrodissection of 67 FFPE primary melanoma sections using the Rneasy FFPE Kit (Qiagen, Valencia, CA) per manufacturer protocol (20). After RNA was quantified and subjected to quality control measures, gene expression analysis was conducted using Nanostring technology per manufacturer protocol (21). The selected genes consisted of a customized NCounter® GX Human Immunology Kit (Nanostring Technologies, Seattle, WA) comprised of 594 immunoregulatory genes, related to 24 immunology-related gene networks compiled from the Gene Ontology Consortium list of immunologically important genes.
2.4.1 Pathway Analysis
Gene expression data were analyzed using Ingenuity Pathway Analysis (IPA) software (Qiagen, Redwood City, CA) to identify immunologic profiles characteristic of melanomas of each TIL grade (22). Information from the core analysis function including top canonical pathways, upstream regulators, regulator effects, and gene networks with annotated disease states and functions were integrated to identify the most influential immunoregulatory pathways that defined each TIL grade.
2.5 Statistical Analysis
TIL grade and association with clinicopathologic characteristics was assessed using the Chi square test and the non-parametric test. Specifically, the p-values in Table 1 were calculated as follows: if the variable had categorical data, the chi-squared test was performed; if the variable had continuous data, the Kruskal-Wallis Rank Sum Test for median and the Analysis of Variance (ANOVA) for mean was performed. Survival curves stratified by TIL grade and quantitative IHC values were made using the Kaplan-Meier method and compared using the log-rank test. Hazard ratios and 95% confidence intervals (CIs) for recurrence-free survival (RFS), overall survival (OS), and post-recurrence survival were estimated using Cox regression models. Multivariate analyss controlling for primary tumor thickness, ulceration, and mitoseswas performed to assess the independent impact of TIL grade on survival outcomes. Sensitivity and specificity were calculated to determine the ability of quantitative CD3 and CD45 TIL counts to reflect the initial TIL grade as determined on H&E examination. Gene expression data was stratified by TIL grade using the t-test. Fold changes were calculated and a p-value<0.05 was used in IPA.
Table 1.
Characteristics of 1,241 primary cutaneous melanomas by TIL grade
| Absent (n=388) | Non-brisk (330) | Brisk (523) | p-value | ||||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| Gender | |||||||
| Female | 183 | 47.2 | 142 | 43 | 230 | 43.9 | 0.49 |
| Male | 205 | 52.8 | 188 | 57 | 293 | 56.1 | |
| Age | 0.1 | ||||||
| Median | 60.6 | 61.1 | 58.6 | ||||
| Mean | 58.8 | 60.1 | 57.4 | ||||
| Thickness | |||||||
| ≤ 1mm | 238 | 61.3 | 153 | 46.4 | 323 | 61.8 | 0.002 |
| > 1mm | 150 | 38.7 | 177 | 53.6 | 200 | 38.2 | |
| Ulceration | <0.001 | ||||||
| Absent | 338 | 87.3 | 245 | 74.2 | 437 | 83.6 | |
| Present | 49 | 12.7 | 85 | 25.8 | 86 | 16.4 | |
| Unknown | 1 | 0 | 0 | ||||
| Mitoses | <0.001 | ||||||
| Absent | 198 | 51.2 | 117 | 35.9 | 220 | 42.1 | |
| Present | 189 | 48.8 | 209 | 64.1 | 303 | 57.9 | |
| Unknown | 1 | 4 | 0 | ||||
| Histologic Subtype | <0.001 | ||||||
| Nodular | 64 | 17.1 | 85 | 27.6 | 109 | 22.8 | |
| Superficial Spreading | 243 | 65 | 171 | 56.3 | 319 | 66.7 | |
| Other | 67 | 17.9 | 48 | 16.1 | 50 | 10.5 | |
| Unclassified | 14 | 26 | 45 | ||||
| SLN biopsy | <0.001 | ||||||
| Not performed | 228 | 59.1 | 146 | 44.6 | 274 | 53 | |
| Performed | 158 | 40.9 | 181 | 55.4 | 243 | 44 | |
| SLN biopsy | 0.49 | ||||||
| Negative | 124 | 90.7 | 290 | 88.7 | 470 | 91.1 | |
| Positive | 34 | 9.3 | 37 | 11.3 | 46 | 8.9 | |
| Other | 2 | 3 | 6 | ||||
3. RESULTS
3.1 Non-brisk TIL grade was associated with the worst pathologic characteristics
Clinicopathological features for 1,241 primary melanoma patients stratified by TIL grade are shown in Table 1. TILs were graded as absent (n= 388, 31%), non-brisk (n= 330, 27%), and brisk (n= 523, 42%) on H&E. Non-brisk TIL grade had the thickest tumors (mean = 2.11 mm, p=0.002) and increased rates of ulceration (p<0.001), mitoses (p<0.001), and nodular histology (p<0.001) compared to melanomas with absent TIL grade and brisk TIL grade. Although SLN biopsy was performed most often for patients with non-brisk TIL-graded melanomas (p<0.001), TIL grade did not correlate with SLN biopsy positivity (p=0.49) (Table 1).
3.2 Brisk TIL grade is associated with the best survival outcomes
Brisk TIL grade was associated with the best RFS (p=0.025) (Figure 1a), OS (p=0.0057) (Figure 1b), and post-recurrence survival (p=0.046) compared to non-brisk and absent TIL grades. Surprisingly, there were no differences in RFS (p=0.4) or OS (p=0.41) for patients when comparing only non-brisk vs. absent TIL-graded melanomas. This demonstrates that brisk TIL grade distinctly separates out from non-brisk and absent TIL grade as a positive prognostic marker. Median follow-up time for the cohort was 3.9 years. Multivariate analysis was performed to identify whether the poor survival outcomes of patients with non-brisk TIL-graded melanomas compared to those with brisk TIL grade were driven by unfavorable pathologic features alone. However, brisk TIL grade’s association with improved OS was independent of thickness, the most important primary melanoma prognostic feature (p=0.036) (Table 2). Additionally, brisk versus non-brisk TIL grade continued to be significantly prognostic for improved OS, independent of pathologic features incorporated into current AJCC staging including thickness, ulceration, and mitoses (p=0.049). (Table 2).
Figure 1.
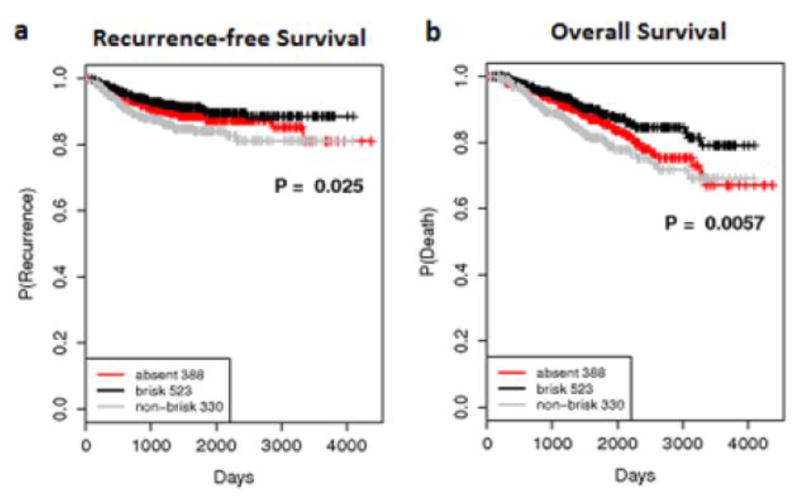
Kaplan-Meier survival curves for 1,241 primary melanoma patients with a median follow-up time of 3.9 years showing significant (a) recurrence-free survival and (b) overall survival differences comparing absent, non-brisk, and brisk TIL grade.
Table 2.
Univariate and multivariate analyses for recurrence-free and overall survival comparing brisk to non-brisk TIL grade
| Recurrence-Free Survival | |||||
| UNIVARIATE | |||||
| Baseline | HR | 95% CI | p-value | ||
| TIL GRADE | non-brisk | brisk | 0.56 | 0.37-0.85 | 0.007 |
| MULTIVARIATE | |||||
| Baseline | HR | 95% CI | p-value | ||
| TIL GRADE | non-brisk | brisk | 0.73 | 0.47-1.14 | 0.173 |
| THICKNESS | 1.14 | 1.11-1.18 | <0.001 | ||
| Baseline | HR | 95% CI | p-value | ||
| TIL GRADE | non-brisk | brisk | 0.78 | 0.50-1.21 | 0.268 |
| THICKNESS | 1.08 | 1.05-1.12 | <0.001 | ||
| ULCERATION | absent | present | 3.69 | 2.28-5.97 | <0.001 |
| MITOSES | absent | present | 4.94 | 2.10-11.65 | <0.001 |
| Overall Survival | |||||
| UNIVARIATE | |||||
| Baseline | HR | 95% CI | p-value | ||
| TIL GRADE | non-brisk | brisk | 0.54 | 0.37-0.79 | 0.002 |
| MULTIVARIATE | |||||
| Baseline | HR | 95% CI | p-value | ||
| TIL GRADE | non-brisk | brisk | 0.66 | 0.44-0.97 | 0.036 |
| THICKNESS | 1.13 | 1.10-1.16 | <0.001 | ||
| Baseline | HR | 95% CI | p-value | ||
| TIL GRADE | non-brisk | brisk | 0.67 | 95% CI | 0.049 |
| THICKNESS | 1.08 | 1.05-1.12 | <0.001 | ||
| ULCERATION | absent | present | 2.89 | 1.85-4.50 | <0.001 |
| MITOSES | absent | present | 1.75 | 1.03-2.98 | 0.037 |
3.3 CD3 and CD45 expression was highly sensitive for TIL detection, but did not add to prognostication
To identify whether a more sensitive measurement of TILs impacts prognosis, primary melanomas were stained for CD3 (n=210, 18% absent, 29% non-brisk, 53% brisk) and CD45 (n=196, 16% absent, 30% non-brisk, 54% brisk) to quantify intratumoral and peritumoral TILs. Representative staining of melanomas with absent, non-brisk, and brisk TILs and quantitative averages of total CD3 and CD45-expressing TILs (intratumoral + peritumoral) are illustrated in Figure 2a-c. Total CD3 and CD45 counts were sensitive for TIL detection (0.92 and 0.98, respectively) but not specific (0.299 and 0.146, respectively) to TIL grade as determined on H&E. For example, there were cases in which TILs graded as absent on H&E were found to be quantifiable by IHC. Despite this increased sensitivity, quantitative TIL detection by IHC did not impact survival outcomes (Figure 2d and 2e). Using a count of 50 TILs as an upper limit cut-off for defining high versus low TIL count, there were no significant survival differences for intratumoral, peritumoral, or total CD3 or CD45 TIL quantitation. When comparing only melanomas with non-brisk versus absent TIL grade, higher intratumoral CD3 and CD45 expression in melanomas with non-brisk TILs was associated with improved RFS (CD3: p=0.001; CD45: p=0.003) and OS (CD3: p=0.002; CD45: p=0.02).
Figure 2.
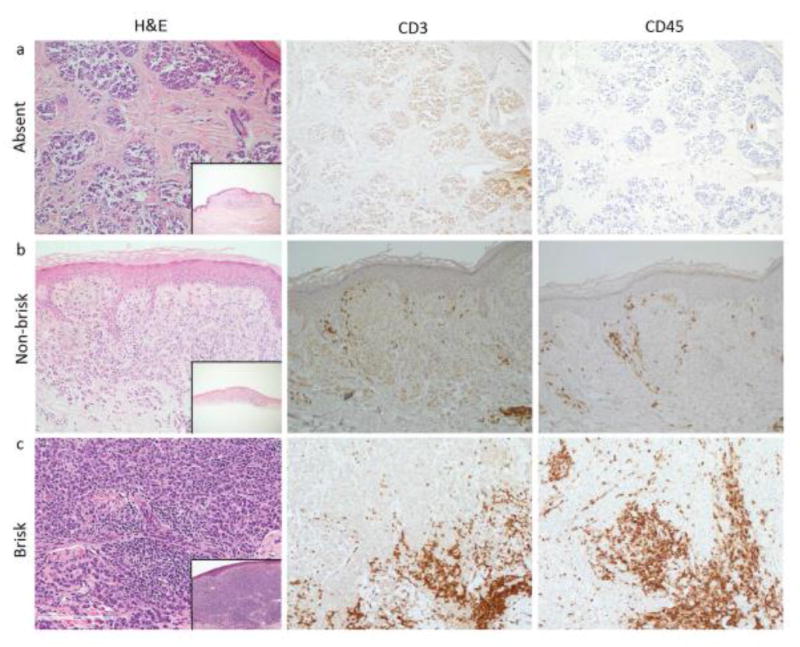
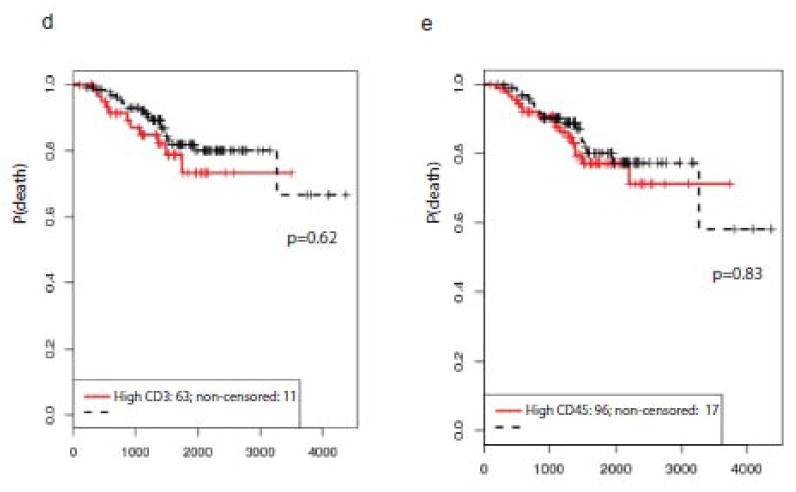
(a-c): Demonstrates primary melanomas with (a) absent, (b) non-brisk, and (c) brisk TIL grade on H&E, with corresponding CD3 and CD45 expression, all at a power of 20X. Insets in each of the H&E panels show the entire primary melanoma as viewed on H&E at a power of 4X. Of note, CD3 staining in panel (a) shows non-specific staining. (d-e): Overall survival curves for (d) quantitative intratumoral CD3 counts and (e) quantitative intratumoral CD45 counts. TIL counts greater than 50 were used as an upper limit. “High” represents counts > 50. “Low” represents counts ≤ 50.
3.4 Expression of Foxp3+ Tregs did not significantly differ by TIL grade
Foxp3 expression was measured in the same cohort stained for CD3 and CD45 (n=184, 24% absent, 30% non-brisk, and 46% brisk TIL grades) to identify whether Tregs may contribute to a more immunosuppressed tumor microenvironment in melanomas with non-brisk TILs. Although the quantity of Foxp3+ Tregs increased with melanomas of increasing TIL grade, there was no difference in the percentage of Foxp3+ Tregs (p=0.13) by TIL grade. Increased percentage of Foxp3+ cells in the lymphocytic infiltrate did not impact OS (p=0.23) (Figure 3).
Figure 3.
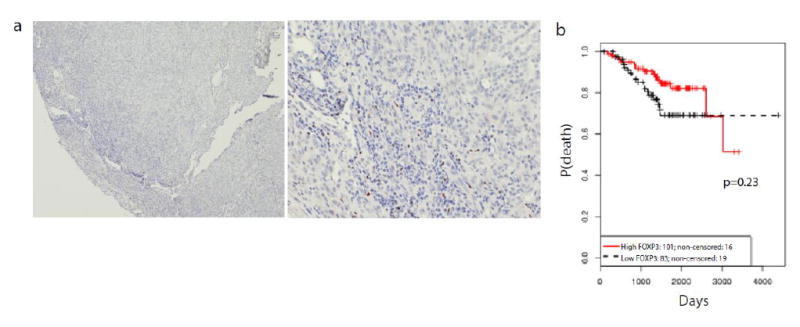
(a) H&E and corresponding FOXP3 expression at a power of 10X and 40X. (b) Overall survival curves for percentage of FOXP3 positive cells within the entire lymphocytic infiltrate. 10% was taken as the median. “High” represents > 10%. “Low” represents ≤ 10%.
3.5 Melanomas with present TILs represent an immunologically heterogeneous group
A subset of 67 primary melanomas graded as having absent (n=16), non-brisk (n=25), and brisk (n=26) TILs were evaluated for differential immunoregulatory gene expression profiles. Only 8/579 (1%) immunoregulatory genes were differentially expressed in melanomas with non-brisk versus absent TILs, suggesting these melanomas are related immunologically. In contrast, 180/579 (31%) genes were differentially expressed between the brisk and non-brisk TILs group and 297/579 (51%) genes were differentially expressed between the brisk and absent TILs group, emphasizing that melanomas with brisk TILs represent a distinct immunologic entity.
The top canonical pathways enriched in melanomas with brisk versus non-brisk TILs included pathways involved in immune cell function (T helper cell differentiation, dendritic cell maturation), T cell co-stimulation (iCOS, CD28, and CD40 signaling), cytokine signaling (IL-2), and other signaling pathways including interferon, T cell receptor, Toll-like receptor, and JAK/STAT signaling (Figure 4). Melanomas with brisk TILs also demonstrated activation of upstream regulators including cytokines (IL1B, IL2, IL21, and IFNG), co-stimulatory transmembrane receptors (CD40), and the T cell receptor complex. Interestingly, immune checkpoint regulators such as PD1 and CTLA4 were more inhibited in melanomas with brisk TILs compared to those with non-brisk TILs. Melanomas with brisk TILs had gene expression patterns involved pathways enriched for T lymphocyte activation, development, and migration. Upregulated genes common to these pathways include CD28, IFNG, CD2, ZAP70, and TNF. The immunologic profile of melanomas with brisk TILs separated out from melanomas graded as non-brisk or absent TILs. The brisk TIL group is distinct functionally and prognostically from non-brisk TILs, representing a threshold within the “present” (brisk and non-brisk) TILs category.
Figure 4.
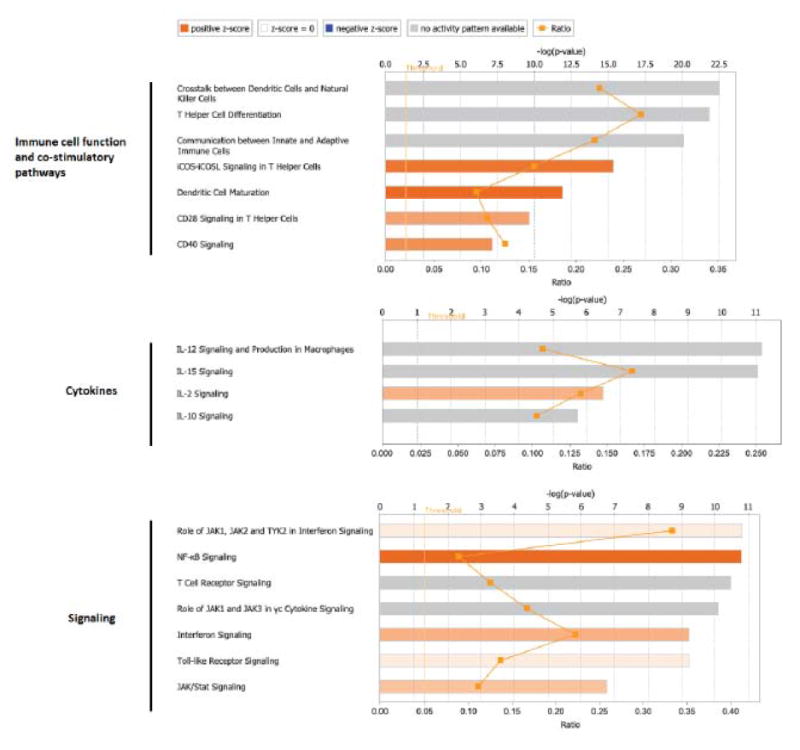
Top canonical pathways enriched in melanomas with brisk compared to non-brisk TILs using Integrative Pathway Analysis. Bar length represents statistical significance as displayed as the –log of p-value which is calculated by Fisher’s exact test. Orange squares represent the number of genes in pathway meeting the cutoff criteria in our dataset divided by the total number of genes involved in the pathway. Orange bars indicate predicted pathway activation. Gray bars indicate pathways where a prediction cannot be made.
4. DISCUSSION
There is an unresolved debate in the literature as to whether TIL grade is a robust enough prognostic feature to be included in AJCC staging. In our cohort, brisk TIL grade correlates with improved RFS and OS compared to non-brisk and absent TIL grades. Surprisingly, there are no distinct differences in survival outcomes for patients with non-brisk or absent TIL-graded melanomas. Melanomas with non-brisk TILs are associated with the most unfavorable pathologic features. These results raise the question of whether non-brisk TIL grade may be suggestive of a partially failed host immune response, particularly because patients with a non-brisk TIL grade had an equivalent prognosis to patients without any lymphocytic infiltrate. Through gene expression profiling of each TIL grade, we identified that that melanomas with brisk TIL grade harbor an immunologically activated tumor microenvironment in comparison to melanomas with non-brisk TIL grade, despite both groups having a TIL “presence.”
There are several limitations in the current literature reporting on the prognostic value of TILs that we attempt to address in our study. First, there is a lack of standardization of TIL grading methods. Although Clark’s method for TIL grading (absent, non-brisk, brisk) is used in many publications, other studies score TILs on a scale of 0 to 3, consider the presence of “slight” TILs as “absent,” or only consider TILs as “present” or “absent” (11, 12, 14). Second, heterogeneity of patient selection criteria and often small sample size may also impact the data given that TILs have a higher incidence in thin melanomas (15, 19). For example, Rao et al. studied 293 high-risk, thick (> 4 mm) primary melanomas in which TIL grade did not significantly correlate with survival outcomes (23). In contrast, Thomas et al. did find survival differences between TIL grades in a cohort of 3,330 primary invasive melanomas where the majority were thin (<1 mm) (18) and carried a higher percentage of present (brisk or non-brisk) TILs. Third, inconsistent grouping of TIL grades and loss of prognostic significance in multivariate analysis poses additional limitations in interpreting the impact of TILs. Barnhill et al. examined a majority of thin melanomas (< 1.7 mm) however no survival differences were shown between TIL grades, possibly because TIL “presence” was not stratified as brisk or non-brisk (13). Conversely, Burton et al. found 5 year OS differences between patients with brisk (95%) and non-brisk (84%) TIL grades, however statistical significance was lost in multivariate analysis and absent TIL grade was not addressed (16). Similarly, Mandala et al. found 5 year OS differences for present (94.8%) vs. absent (90.4%) TILs in assessment of 1,251 primary melanomas, but the significance of the survival difference was not confirmed in Cox regression models (17).
We addressed these limitations by conducting an analysis of TIL grade in a large prospectively enrolled cohort of primary melanoma patients representative of the general population. Interestingly, patients with non-brisk TIL grade consistently had the most unfavorable pathologic features as well as OS outcomes no better than patients who had no lymphocytic infiltrate at all. While RFS outcomes by TIL grade were dependent on primary tumor thickness, the OS outcomes for brisk TIL grade were independent of the AJCC staging parameters of primary tumor thickness, ulceration, and mitoses, suggesting that other host- and tumor-related functional factors explain the worse outcomes seen in patients with non-brisk TILs.
Immune measures, specifically TIL grade, are not standard components of melanoma pathology reports because they have not yet been shown to definitively impact survival or alter clinical management. In parallel, associations between lymphocyte marker expression and melanoma prognosis have been investigated, but results are inconsistent (24-29). In our cohort, CD3, CD45, and Foxp3 expression do not add prognostic value over examining H&E sections alone. One reason for this lack of correlation is that H&E TIL grading evaluates the entire surface area of the primary tumor, whereas our quantitative TIL grading by IHC focuses on only one high power field, designed to be an easy and quick measure for any pathologist to perform.
However, a more global analysis using gene expression profiling did identify immunologic heterogeneity among melanomas with present lymphocytic infiltrates (brisk and non-brisk). Melanomas with absent and non-brisk TIL grade had the most similar immunoregulatory gene expression patterns and were immunologically distinguishable from melanomas with brisk TIL grade. This is particularly interesting because many studies classify TILs as only “present” or “absent.” Instead, our data emphasize that presence of brisk TILs is prognostically superior and immunologically distinct from a group of lower intensity “present” (non-brisk) TILs, and certainly from absent TILs. We highlight a clear prognostic and functional distinction between melanomas with brisk and non-brisk TIL grade, which historically have been grouped together as “present” TILs.
In the pathway analysis of the primary melanoma gene expression data, melanomas with brisk TIL grade had upregulated T cell signaling, activation, and migration pathways compared to non-brisk TIL-graded melanomas. Melanomas with brisk TIL grade also demonstrated increased expression of genes involved in immune co-stimulatory signaling pathways including CD40 on antigen presenting cells and iCOS and CD28 on T cells. Zap70, also upregulated in the brisk TILs dataset, functions in T cell receptor-mediated signal transduction in combination with LCK and FYN (30). This is further independently validated in the Cancer Genome Atlas (TCGA) analysis in which melanoma patients with high lymphocyte score and elevated LCK expression in their tumors demonstrated improved survival (31). Identification of distinct immunologic gene expression profiles between melanomas with brisk and non-brisk TIL grades emphasizes that the “presence” of TILs alone is not enough to implicate them as immunologically functional.
The strengths of our study include a large sample size with comprehensive pathologic evaluation and protocol-driven follow-up data. The 4-year median follow-up time also allows ample time for detection of recurrent melanomas, many of which tend to recur within 3-5 years after initial diagnosis. Furthermore, our study population is largely generalizable to the broader population. For example, despite the fact that our institution largely represents a tertiary referral center and often attracts patients with more advanced stages of melanoma, 79% of the primary melanomas studied were less than or equal to 2 mm thick. Thin melanomas have been shown to carry a higher incidence of brisk TIL grading (15, 18) and this finding may account for the higher percentage of brisk TIL grading in our cohort, in which thin melanomas (< 2mm) have an increased incidence of brisk TILs (44%) compared to thick melanomas (> 4mm) with brisk TILs (31%). While one limitation may be that we performed immune marker expression studies and gene expression analysis on a subset of cases rather than the entire cohort, these cases were selected at random and are representative of the overall study cohort. Given the limited amount of tissue available for most primary melanomas, we included a substantial number of cases in our analyses without compromising valuable tissue in our biorepository that is in use for other investigations.
“Immunoscores” are being proposed as prognostic tools in colon cancer, melanoma, and other malignancies (32, 33). Identifying ratios and combinations of immune cell markers with or without use of gene expression profiling can highlight immunoregulatory pathways that impact survival differences. This may lead to identification of new immunologic prognostic markers that could be incorporated into melanoma staging algorithms, whereas examining each marker alone is less revealing. The immunologic heterogeneity inherent in melanomas of varying TIL grades provides clues towards factors driving the prognostic impact of TIL grade over more static measures documenting only the “presence” or “absence” of TILs.
We conclude that the presence of TILs in primary melanomas represents a heterogeneous clinical group and caution in prognostic interpretation is warranted. We challenge the current dogma that the presence of TILs, compared to an absent lymphocytic infiltrate, correlates with improved outcomes. In our cohort, melanomas with non-brisk TIL grade appear to be more closely related to melanomas with absent TIL grade, both prognostically and immunologically. Therefore, TIL presence does not uniformly translate into a heightened host anti-tumor immune response, although brisk TIL grade is a positive prognostic factor in primary melanomas and is a distinct group from non-brisk TIL grade in terms of both survival outcomes and immunoregulatory gene expression profiles. We emphasize that recognition of the underlying properties of the host immune system is crucial in accounting for differences in patient survival among TIL grades.
Acknowledgments
Funding: This research did not receive any specific grant funding from agencies in the public, commercial, or not-for-profit sectors.
ABBREVIATIONS
- AJCC
American Joint Committee on Cancer
- CI
confidence interval
- FFPE
formalin-fixed paraffin embedded
- H&E
hematoxylin and eosin
- HPF
high power field
- IHC
immunohistochemistry
- IMCG
Interdisciplinary Melanoma Cooperative Group
- IPA
Integrative Pathway Analysis
- IRB
Institutional Review Board
- NYU
New York University
- OS
overall survival
- RFS
recurrence-free survival
- SLN
sentinel lymph node
- TIL
tumor infiltrating lymphocyte
- Treg
T regulatory cell
- VGP
vertical growth phase
Footnotes
DECLARATIONS
The IMCG protocol is approved by the NYU institutional review board. Informed consent is obtained from all patients who choose to participate in the study at time of initial enrollment.
Conflicts of interest: We have no conflict of interest disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2015;65:1, 5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Balch CM, Buzaid AC, Atkins MB, Cascinelli N, Coit DG, Fleming ID, et al. A new American Joint Committee on Cancer staging system for cutaneous melanoma. Cancer. 2000;88(6):1484–91. doi: 10.1002/(sici)1097-0142(20000315)88:6<1484::aid-cncr29>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 3.Balch CM, Buzaid AC, Soong SJ, Atkins MB, Cascinelli N, Coit DG, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19(16):3635–48. doi: 10.1200/JCO.2001.19.16.3635. [DOI] [PubMed] [Google Scholar]
- 4.Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27(36):6199–206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Ding S, Byrd DR, et al. Multivariate analysis of prognostic factors among 2,313 patients with stage III melanoma: comparison of nodal micrometastases versus macrometastases. J Clin Oncol. 2010;28(14):2452–9. doi: 10.1200/JCO.2009.27.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balch CM, Soong SJ, Gershenwald JE, Thompson JF, Reintgen DS, Cascinelli N, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001;19(16):3622–34. doi: 10.1200/JCO.2001.19.16.3622. [DOI] [PubMed] [Google Scholar]
- 7.American Cancer Society. [11.11.15];What are the survival rates for melanoma skin cancer, by stage? Available at: http://www.cancer.org/cancer/skincancer-melanoma/detailedguide/melanoma-skin-cancer-survival-rates.
- 8.Ropponen KM, Eskelinen MJ, Lipponen PK, Alhava E, Kosma VM. Prognostic value of tumour-infiltrating lymphocytes (TILs) in colorectal cancer. J Pathol. 1997;182(3):318–24. doi: 10.1002/(SICI)1096-9896(199707)182:3<318::AID-PATH862>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 9.Hwang WT, Adams SF, Tahirovic E, Hagemann IS, Coukos G. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: a meta-analysis. Gynecol Oncol. 2012;124(2):192–8. doi: 10.1016/j.ygyno.2011.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adams S, Gray RJ, Demaria S, Goldstein L, Perez EA, Shulman LN, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014;32(27):2959–66. doi: 10.1200/JCO.2013.55.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Azimi F, Scolyer RA, Rumcheva P, Moncrieff M, Murali R, McCarthy SW, et al. Tumor-infiltrating lymphocyte grade is an independent predictor of sentinel lymph node status and survival in patients with cutaneous melanoma. J Clin Oncol. 2012;30(21):2678–83. doi: 10.1200/JCO.2011.37.8539. [DOI] [PubMed] [Google Scholar]
- 12.Clark WH, Jr, Elder DE, Guerry Dt, Braitman LE, Trock BJ, Schultz D, et al. Model predicting survival in stage I melanoma based on tumor progression. J Natl Cancer Inst. 1989;81(24):1893–904. doi: 10.1093/jnci/81.24.1893. [DOI] [PubMed] [Google Scholar]
- 13.Barnhill RL, Fine JA, Roush GC, Berwick M. Predicting five-year outcome for patients with cutaneous melanoma in a population-based study. Cancer. 1996;78(3):427–32. doi: 10.1002/(SICI)1097-0142(19960801)78:3<427::AID-CNCR8>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 14.Tuthill RJ, Unger JM, Liu PY, Flaherty LE, Sondak VK. Risk assessment in localized primary cutaneous melanoma: a Southwest Oncology Group study evaluating nine factors and a test of the Clark logistic regression prediction model. Am J Clin Pathol. 2002;118(4):504–11. doi: 10.1309/WBF7-N8KH-71KT-RVQ9. [DOI] [PubMed] [Google Scholar]
- 15.Taylor RC, Patel A, Panageas KS, Busam KJ, Brady MS. Tumor-infiltrating lymphocytes predict sentinel lymph node positivity in patients with cutaneous melanoma. J Clin Oncol. 2007;25(7):869–75. doi: 10.1200/JCO.2006.08.9755. [DOI] [PubMed] [Google Scholar]
- 16.Burton AL, Roach BA, Mays MP, Chen AF, Ginter BA, Vierling AM, et al. Prognostic significance of tumor infiltrating lymphocytes in melanoma. Am Surg. 2011;77(2):188–92. [PubMed] [Google Scholar]
- 17.Mandala M, Imberti GL, Piazzalunga D, Belfiglio M, Labianca R, Barberis M, et al. Clinical and histopathological risk factors to predict sentinel lymph node positivity, disease-free and overall survival in clinical stages I-II AJCC skin melanoma: outcome analysis from a single-institution prospectively collected database. Eur J Cancer. 2009;45(14):2537–45. doi: 10.1016/j.ejca.2009.05.034. [DOI] [PubMed] [Google Scholar]
- 18.Thomas NE, Busam KJ, From L, Kricker A, Armstrong BK, Anton-Culver H, et al. Tumor-infiltrating lymphocyte grade in primary melanomas is independently associated with melanoma-specific survival in the population-based genes, environment and melanoma study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31(33):4252–9. doi: 10.1200/JCO.2013.51.3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clemente CG, Mihm MC, Jr, Bufalino R, Zurrida S, Collini P, Cascinelli N. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer. 1996;77(7):1303–10. doi: 10.1002/(SICI)1097-0142(19960401)77:7<1303::AID-CNCR12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 20.Qiagen. Purification of total RNA from FFPE cores using the RNeasy FFPE kit. [11.11.15];2011 Available at: https://www.qiagen.com/us/resources/resourcedetail?id=665fc085-8691-4fcd-a458-a47c9be312db&lang=en.
- 21.Nanostring Technologies Gene Expression Analysis Protocol. [11.11.15]; Available at: www.nanostring.com.
- 22.Qiagen. Integrative Pathway Analysis. [11.11.15]; Available at: http://www.ingenuity.com/products/ipa.
- 23.Rao UN, Lee SJ, Luo W, Mihm MC, Jr, Kirkwood JM. Presence of tumor-infiltrating lymphocytes and a dominant nodule within primary melanoma are prognostic factors for relapse-free survival of patients with thick (t4) primary melanoma: pathologic analysis of the e1690 and e1694 intergroup trials. Am J Clin Pathol. 2010;133(4):646–53. doi: 10.1309/AJCPTXMEFOVYWDA6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerber AL, Munst A, Schlapbach C, Shafighi M, Kiermeir D, Husler R, et al. High expression of FOXP3 in primary melanoma is associated with tumour progression. Br J Dermatol. 2014;170(1):103–9. doi: 10.1111/bjd.12641. [DOI] [PubMed] [Google Scholar]
- 25.Ladanyi A, Mohos A, Somlai B, Liszkay G, Gilde K, Fejos Z, et al. FOXP3+ cell density in primary tumor has no prognostic impact in patients with cutaneous malignant melanoma. Pathol Oncol Res. 2010;16(3):303–9. doi: 10.1007/s12253-010-9254-x. [DOI] [PubMed] [Google Scholar]
- 26.Ladanyi A, Kiss J, Mohos A, Somlai B, Liszkay G, Gilde K, et al. Prognostic impact of B-cell density in cutaneous melanoma. Cancer Immunol Immunother. 2011;60(12):1729–38. doi: 10.1007/s00262-011-1071-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miracco C, Mourmouras V, Biagioli M, Rubegni P, Mannucci S, Monciatti I, et al. Utility of tumour-infiltrating CD25+FOXP3+ regulatory T cell evaluation in predicting local recurrence in vertical growth phase cutaneous melanoma. Oncol Rep. 2007;18(5):1115–22. [PubMed] [Google Scholar]
- 28.Piras F, Colombari R, Minerba L, Murtas D, Floris C, Maxia C, et al. The predictive value of CD8, CD4, CD68, and human leukocyte antigen-D-related cells in the prognosis of cutaneous malignant melanoma with vertical growth phase. Cancer. 2005;104(6):1246–54. doi: 10.1002/cncr.21283. [DOI] [PubMed] [Google Scholar]
- 29.Erdag G, Schaefer JT, Smolkin ME, Deacon DH, Shea SM, Dengel LT, et al. Immunotype and immunohistologic characteristics of tumor-infiltrating immune cells are associated with clinical outcome in metastatic melanoma. Cancer research. 2012;72(5):1070–80. doi: 10.1158/0008-5472.CAN-11-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang H, Kadlecek TA, Au-Yeung BB, Goodfellow HE, Hsu L, Freedman TS, et al. ZAP-70: An Essential Kinase in T-cell Signaling. Cold Spring Harb Perspect Biol. 2010;2:a002279. doi: 10.1101/cshperspect.a002279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Genomic Classification of Cutaneous Melanoma. Cell. 2015;161(7):1681–96. doi: 10.1016/j.cell.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Capone M. Immunoscore: a new possible approach for melanoma classification. Journal for ImmunoTherapy of Cancer. 2014;2(Suppl 3):193. [Google Scholar]
- 33.Galon J, Pages F, Marincola FM, Angell HK, Thurin M, Lugli A, et al. Cancer classification using the Immunoscore: a worldwide task force. J Transl Med. 2012;10:205. doi: 10.1186/1479-5876-10-205. [DOI] [PMC free article] [PubMed] [Google Scholar]


