Abstract
Purpose of review
We will describe recently discovered smart aptamers with tumor specificity, with an emphasis on targeted delivery of novel therapeutic molecules, cancer-specific biomarkers, and immunotherapy.
Recent findings
The development of cancer-specific aptamers has facilitated targeted delivery of potent therapeutic molecules to cancer cells without harming non-tumoral cells. This specificity also makes it possible to discover novel cancer biomarkers. Furthermore, alternative immune-checkpoint blockade aptamers have been developed for combinational immunotherapy.
Summary
Aptamers selected against cancer cells show cancer specificity, which has great potential for targeting. First, functionalizing targeted aptamers with therapeutic molecule payloads (e.g., small activating RNAs (saRNAs), anti-mitotic drugs, therapeutic antibodies, and peptides) facilitates successful delivery into cancer cells. This approach greatly improves the therapeutic index by minimizing side effects in non-tumoral cells. Second, cancer-specific proteins have been identified as cancer biomarkers through in vitro and in vivo selection, aptamer pull-down assays, and mass spectrometry. These newly discovered biomarkers improve therapeutic intervention and diagnostic specificity. In addition, the development of alternative immune-checkpoint blockade aptamers are suggested for use in combinational immunotherapeutic with current immune blockade regimens, to reduce the resistance and exhaustion of T cells in clinical trials.
Keywords: cancer specificity, targeted delivery, biomarker discovery, alternative immune-checkpoint blockade
INTRODUCTION
Aptamers, structured nucleic acid ligands, are selected to recognize a wide variety of targets from proteins, cultured cells, and ex vivo organ cultures (1–6) and have great advantages for therapeutics; high specificity and affinity to targets, structural stability, lower toxicity, and lower immunogenicity (7). Therapeutic aptamer discovery has traditionally focused on occupancy of a binding site that directly affects target protein function. Hence, selection of an appropriate target determines the biological function of a therapeutic aptamer and their outcomes in the clinic. In recent decades, aptamers have been isolated against cell surface markers for targeted delivery of cargos. This class of therapeutic aptamers has shown great success for targeted therapeutics (8), active targeting agents (8), and theranostics (9). The recent expiration of patents for the aptamer selection process known as “Systematic Evolution of Ligands by EXponential enrichment (SELEX)” (10), is expected to promote the development of aptamers for human therapeutics. In this review, promising new discoveries of cancer-specific therapeutic aptamers will be discussed in terms of functionalizing aptamers to cancer-specific antigens for targeted delivery with novel therapeutic molecules, novel biomarker discovery, and non-immunogenic immunomodulatory aptamers for cancer therapy. This well-designed approach allows delivery of therapeutics to cancer cells specifically without harming non-tumoral cells.
FUNCTIONALIZING APTAMERS FOR TARGETED DELIVERY
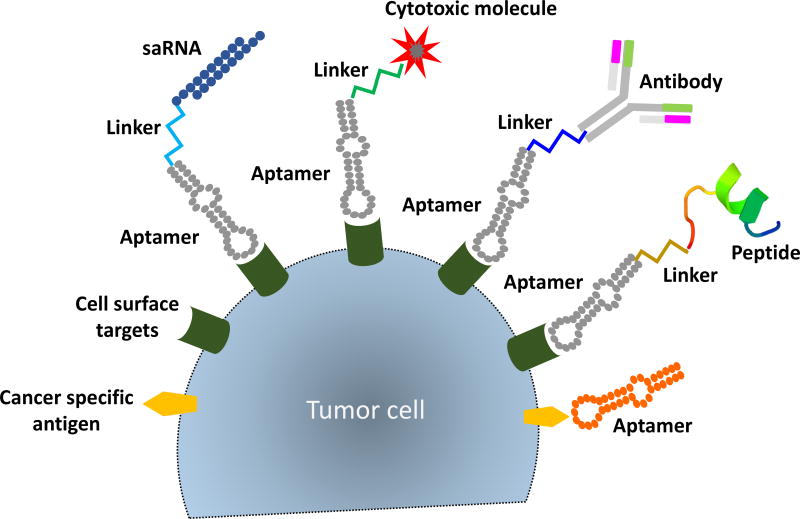
Aptamers isolated against cancer cells have been functionalized with therapeutic payloads for various targeting modalities (Fig. 1). Aptamer-mediated targeted delivery of therapeutics has improved therapeutic index while minimizing side effects in normal cells.
Figure 1.
Functionalizing aptamers for targeted delivery of therapeutic modalities. Selected RNA aptamers against the cancer cell surface are functionalized as delivery cargos. Aptamers conjugated with therapeutic payloads including small activating RNAs (saRNAs), cytotoxic molecules, antibodies, or peptides allow targeted delivery of therapeutic molecules to cancer cells, which improves the therapeutic intervention. Aptamers against cancer-specific antigens also confer cancer specificity and inhibit the function of the target for anti-tumor effects.
Cancer-specific targeted delivery of small activating RNAs (saRNAs)
Upregulating target gene expression by promoter-targeted saRNA is a recently developed new concept in nucleic acid therapeutics. Likewise, functionalizing aptamers for targeted delivery of saRNA has emerged as a new field in cancer therapeutics. Pancreatic cancer, one of the most aggressive types of cancers, has a 5-year survival rate of only 5% (11). In contrast to other cancer types, the mortality rate for pancreatic ductal adenocarcinoma (PDAC) is increasing, and it has been predicted that PDAC will become the second leading cause of cancer-related mortality by 2020 (12). However, currently, there is no effective treatment in pancreatic cancer. In previous studies in liver cancer, saRNA targeting C/EBPα in vivo induced a potent antitumor effect through positive regulation of C/EBPα and its downstream targets, including cyclin-dependent kinase inhibitor 1 (p21) (13). Loss of the histone demethylase KDB6B enhances the aggressiveness of pancreatic cancer via downregulation of C/EBPα (14). Thus, C/EBPα-saRNA shows potential for therapeutic targeting in pancreatic cancer. For targeted delivery of C/EBPα-saRNA, pancreatic cancer-specific RNA aptamers have been isolated against naïve pancreatic cancer using cell SELEX and conjugated with C/EBPα-saRNA via sticky-bridge sequences (15). Aptamer-mediated C/EBPα-saRNA has shown increased target gene and protein expression, and a strong anti-tumor effect in in vivo studies, without toxicity (15).
Although prostate cancer has a high overall survival rate, metastasis remains the major cause of mortality: 10-year survival is 79%, but drops to only 12% for patients with lymph node metastasis. In previous studies, dihydropyrimidinase-related protein 3 (DPYSL3), also called collapsing response mediator protein 4 (CRMP4), is identified as a tumor metastasis suppressor in prostate cancer (16). Thus, to suppress prostate cancer cell invasion and metastasis, saRNA targeting the DPYSL3 promotor region has been developed to upregulate target gene expression at the transcriptional level; it is conjugated with prostate-specific membrane antigen aptamers for targeted delivery (17). The aptamer-conjugated DPYSL3-saRNA induces target gene expression without any transfection agents and completely suppresses distal metastasis in orthotopic xenografted nude mice with no side effects.
Together, these two studies indicate that the conjugation of a newly identified cancer-specific aptamer with saRNA will be a potent future cancer therapeutic that reduces side effects on non-targeted cells.
Cancer-specific targeted delivery of anti-mitotic drugs
Cytotoxic drugs targeting microtubules have been developed as potent anti-tumor therapeutics. Although tubulin-targeting drugs are clinically efficacious, because of their high toxicity, lack of specificity, and poor biostability, they have limited use in patients. To improve the target specificity of cytotoxic anti-mitotic drugs, aptamers against the glycoprotein mucin 1 cell surface associated (MUC1) have been conjugated with liposomes containing the tubulin-targeted dodecapeptide (TTP) nanoassembly, which encapsulate the anti-mitotic drug docetaxel (DX, a microtubule stabilizing taxane) (18). Targeted delivery of this combination (TTP and DX) by MUC1 aptamer shows selective cytotoxicity in breast cancer cells (MCF7), with significantly reduced effects in normal breast epithelial cells (MCF 10A) and non-MUC1 expressing liver carcinoma cells (HepG2).
The highly potent anti-mitotic drugs, monomethyl auristatin E (MMAE) and maytansinoid (DM1), have both been investigated in several pre-clinical studies (19, 20). However, because of their high toxicity, neither MMAE nor DM1 can be used as cytotoxic drugs in their own right in clinic. As an targeted delivery of both MMAE and DM1, truncated pancreatic cancer-targeted aptamers have been chemically conjugated to MMAE and DM1 to construct aptamer-drug conjugates (21). Aptamer-MMAE and -DM1 show significant pancreatic cancer cell-specific cytotoxicity, but non-toxic effects in non-targeted breast cancer cells (MCF7) or normal skin fibroblast cells (BJ).
Together, these two studies clearly demonstrate that functionalized aptamers are effective for delivering cargos of anti-mitotic drugs without affecting normal cells.
Cancer-specific targeted delivery of macromolecules and peptides
Insufficient penetration of therapeutic macromolecules into tumor tissues induces inadequate therapeutic efficacy. For instance, although antibodies have been commonly used for targeted delivery in recent years, they are ~150 kDa macromolecules, which limits tumor tissue penetration. In contrast, aptamers show superior performance for both tumor penetration and homogenous biodistribution compared to antibodies (22). An aptamer-antibody complex (oligobody, oligomer + antibody) is constructed as a novel delivery platform to overcome these limitations (23). An oligobody is a chimeric construct of RNA (e.g., anti-VEGF aptamer) with protein (e.g., anti-cotinine antibody). In in vivo assays, it retains the original biological function and binding affinity of aptamers, but shows improved pharmacokinetics, increased tumor penetration, and effective inhibition of lung cancer growth (23).
T regulatory cells (Tregs) are major culprits for induction of tumor immunosuppression. Foxp3, an intracellular transcription factor, is a specific marker for Tregs and its disruption entails the loss of Treg immunosuppressive function. A 15-mer synthetic peptide (P60) inhibiting Foxp3 has been developed by phase displayed peptide screen (24). However, the anti-Foxp3 peptides penetrate into cells in non-specific ways and require a large dose for therapeutic efficacy, which hampers clinical feasibility. To reduce the activity of Tregs and improve the therapeutic index for T cells specifically, a CD26 aptamer (CD26Apt) is chemically conjugated with the anti-Foxp3 P60 peptide via an oligo sequence linker to make chimeric aptamer-peptide (CD26Apt-P60) (25). The CD26Apt-P60 binds to CD28-expressing cells and reduces the immunosuppressive activity of Tregs. Notably, the chimeric aptamer-peptide conjugate (CD26Apt-P60) shows a remarkable improvement in vaccine efficacy at low concentration with ovalbumin and poly I:C and improves anti-tumor immunity in an in vivo model of colon carcinoma cells (CT26) (25).
Together, these two studies prove that aptamers are efficient carriers of macromolecules into tissues and efficient for delivery of cargos, even to immune cells.
Targeting cancer-specific antigens or mutations
Targeting tumor-specific antigens improves therapeutic intervention. The shared tumor-specific antigens, often called cancer/testis antigens (CTAs), are important targets for cancer therapy (26). The National Cancer Institute pilot project has prioritized the identification of such CTAs to develop a well-vetted and priority-ranked list of cancer vaccine and translational research targets (27). Among 75 cancer targets, the epidermal growth factor receptor variant III (EGFRvIII) and the melanoma associated antigen A3 (MAGE-A3) are ranked in the top 10. These cancer-specific antigens are thus ideal target for therapeutic aptamer development, as the aptamers show great specificity against the targets, and can discriminate against even single amino acid mutations (28).
Treatment of glioblastoma multiforme (GBM) has seen little improvement in the last 20 years despite targeted therapy and progress in surgery, radiation delivery, and chemotherapy. Therefore, new therapeutic approaches are needed for this highly lethal cancer. Elevated EGFR expression is a hallmark for enhanced tumorigenicity of GBM. An aptamer binding a mutant form of EGFRvIII has been isolated and its function inhibits the formation of phosphorylated disulfide-bonded EGFRvIII complexes in GBM (29). It also reduces the phosphorylation of downstream effectors, including signal transducer and activator of transcription (STAT3) and extracellular signal-regulated kinase (ERK). The biological function of EGFRvIII aptamer reduces GBM cell motility and growth (29).
MAGE-A3 is one of the most frequently and highly expressed CTAs in tumors, including in 74% of metastatic melanomas, 57% of esophageal carcinomas, 39% of non-small cell lung cancers, and 38% of gastric carcinomas (30). As the expression of MAGE-A3 has been associated with malignancy and poor prognosis, it is considered a significant target for therapeutic development. For the potential use in therapeutics, a DNA aptamer is isolated against a MAGE-A3111–125 peptide using peptide-SELEX. The isolated MAGE-A3 aptamer shows cancer-specific binding to various cancer cells such as MCF-7 (breast cancer), SK-MEL-28 (melanoma), Cal-27 (oral cancer), DLD-1 (colorectal cancer), HepG2 (liver cancer), A-549 (lung cancer), and AsPC-1 (pancreatic cancer), but does not bind in non-tumoral cells (30). Although the authors does not perform any functional assays of MAGE-A3 aptamer activity, it has great potential for as a targeting agent to deliver therapeutics.
Together, these two studies indicate that aptamers distinguish the caner-specific isoforms from normal proteins.
ANTI-METASTATIC APTAMERS TARGETING THE CYTOSKELETON
The major cause of cancer mortality can be attributed to metastasis, rather than the primary tumors from which these malignant lesions arise. Cancer metastasis involves dissemination of cancer cells to anatomically distant organ sites and their subsequent adaptation to tissue microenvironments. To promote metastatic signaling, cancer cells use cytoskeleton remodeling. Thus, aptamers targeting the cytoskeleton have great potential as anti-metastatic drugs.
Integrins are αβ heterodimeric cell surface receptors that mediate stable adhesion of cancer cells in the microenvironment (31). Among 18 α-subunits and 8 β-subunits, the laminin-binding α6β4 integrin is associated with poor prognosis (32) and the pre-metastatic niche (33). To block laminin binding to the α6β4 integrin, DNA aptamers is isolated via combined protein- and cell-SELEX (34). The α6β4 integrin aptamers show prostate cancer specificity and inhibit the interaction of α6β4 integrin and laminin-332. Unfortunately, no further in vitro or in vivo functional assay of prostate cancer migration have been performed. Truncated anti-integrin aptamers lose the functionality to block integrin-laminin binding in the study.
Truncation of aptamers is required to enable efficient and large-scale chemical synthesis for subsequent evaluation using in vivo assays or for clinical trials. However, their functionality and binding affinity are often compromised during the process of truncation. To avoid these unwanted effects, careful truncation is necessary.
NOVEL BIOMARKER DISCOVERY
The genomic revolution has allowed novel protein targets to be linked to diseases (35). Identification of novel biomarkers associated with cancer is fundamental to improving therapeutic intervention and diagnostic specificity. In the majority of biomarker discovery platforms, mass spectrometry is used as the analytical method to identify target proteins. However, as biomarkers frequently show very low abundance, capturing biomarkers with antibodies has limitations, due to interference with the detection of the biomarker peptide sequence, particularly after digestion of the antigen/antibody complex. As with non-protein-based reagents, an aptamer-facilitated biomarker discovery (AptaBiD) platform (36) is highly desired for detecting biomarkers differentially expressed on the cell surface. Figure 2 shows a flow chart of the aptamer biomarker discovery platform in vitro and in vivo.
Figure 2.
Schematic flow chart of aptamer-based biomarker discovery. Identifying novel targets associated with cancer is a critical issue for biomarker discovery to improve therapeutic intervention. Aptamers are enriched using in vitro or in vivo SELEX. Target ligands are retrieved using affinity pull-down assay. Peptide fingerprinting is achieved using LC-MS/MS. The binding of aptamer with the target ligand is validated by surface plasmon resonance (SPR) or flow cytometry.
Biomarker discovery in vitro
Pancreatic cancer is a leading high mortality cancer, and ranks as the fourth cause of cancer-related death in both Europe and the United States (37, 38). The survival rate remains poor, as most patients present with metastatic disease at first diagnosis (39), and there is currently no effective way to inhibit metastasis. The liver is known the most common site of metastasis (40, 41). To identify novel pancreatic cancer-specific biomarkers, untargeted SELEX (“blind-SELEX”) has been performed against pancreatic cancer cells (PANC-1), with liver cancer cells (Huh7) used for counter-SELEX to remove non-specific binding (42). After 14 rounds of SELEX, a pancreatic cancer-specific RNA aptamer is selected. To identify the aptamer-bound plasma membrane protein, liquid chromatography tandem mass spectrometry (LC-MS/MS) is used. The proteomic mass spectrometry approach identifies the target of the aptamer as the intermediate filament vimentin, a biomarker for epithelial mesenchymal transition (EMT). The binding of aptamer to vimentin is validated by flow cytometry and biosensor assays. Aptamer binding to the vimentin shows internalization into pancreatic cancer cells. As EMT plays a pivotal role to transform cancer cells into invasive cells, tumor cell metastasis assays is performed and shows that anti-vimentin aptamer-treated pancreatic cancer cells inhibit significantly pancreatic tumor metastasis in vitro (42). Thus, the anti-vimentin aptamer has potential as an anti-metastatic therapy in pancreatic cancer.
Nasopharyngeal carcinoma (NPC) is a squamous cell carcinoma that occurs in the epithelial lining of the nasopharynx. Due to the lack of early detection, most NPC patients are diagnosed at an advanced stage, resulting in poor prognosis and a low survival rate. As dysregulation and abnormal expression of membrane proteins usually takes place in cancer cells, membrane proteins provide potential disease biomarkers for diagnosis, therapy, and prognosis. To discover potential biomarkers for NPC, untargeted naïve cell-SELEX is employed, and the aptamer-binding ligands is retrieved by pull-down assay and identified by LC-MS/MS. The NPC aptamers bind the cell surface biomarker CD109 (43). The CD109 aptamers show specificity for CD109 on the cell surface of NPC cell lines and clinical NPC tissue specimens, but not for normal nasopharyngeal cell line and clinic normal nasopharyngeal tissues that expressed little to no CD109.
For the advanced application of biomarker discovery using aptamers, previously isolated DNA aptamers have been used in immunohistochemistry assays (aptahistochemistry) in postoperative human lung adenocarcinoma tissues (44). The relatively new concept of aptahistochemisty uses cancer-specific aptamers instead of antibodies. In this study, the histological structure of lung adenocarcinoma is characterized in human specimens using three aptamers labeled with fluorescence. The fluorescently labeled aptamers show an overlapping pattern with antibodies to lamins, tubulin, and actin, respectively, by histopathological screen, confirming their targets. Importantly, cancer and normal cells could be easily distinguished by aptamers. Interestingly, throughout this study, the authors confirm that anti-actin aptamers could distinguish a specific post-translational modification (PTM): methylation of histidine at position 73. This clearly demonstrates that aptamers are effective molecular probes for pathology imaging, biomarker identification, and discrimination of PTMs.
These studies suggest that AptaBiD is a promising tool for discovering new attractive targets for cancer therapeutics and diagnostics.
Biomarker discovery in vivo
Typically, the SELEX process is performed against purified proteins or overexpressed stable cell lines. However, aptamers selected by in vitro SELEX fail to bind to the targets and are not efficient in vivo. One possible explanation for this difference is that the conformation of target proteins might vary between in vitro and in vivo conditions. To circumvent this obstacle, in vivo SELEX has been developed as a more clinically relevant strategy. In one example of in vivo SELEX, intrahepatic xenografts are established in immunodeficient mice using colorectal cancer cells isolated from human patients (45). To remove the non-specific distribution of aptamers, the aptamer library is systemically injected via tail vein and the SELEX is repeated for 12 rounds. The binding ligand of the selected aptamer, ATP-dependent RNA helicase A (DHX9), is identified using affinity pull-down assay in the in vivo SELEX. DHX9 is predominantly a nuclear protein and shuttles to the cytoplasm to carry out some of its functions in translational regulation and miRNA processing (46). Its functions include regulation of DNA replication, transcription, translation, microRNA biogenesis, RNA processing and transport, and maintenance of genomic stability (46). As the inhibition of DHX9 expression is lethal to human cancer cell lines (47), a DHX9 aptamer will be promising therapeutic molecule for colorectal cancer.
This study provides additional evidence that aptamers can capture translocated intracellular proteins on the cancer membrane.
COMBINATIONAL IMMUNOTHERAPEUTIC APTAMERS
Anti-CD aptamers have been isolated to treat cancers and for use as immune therapies. These anti-CD aptamers demonstrate the unique feature of acting simultaneously as an agonist and antagonist for CD receptors, depending on the degree of aptamer oligomerization (48). Recently, disruption of the programmed cell death protein 1 (PD-1) with immune checkpoint antibodies has emerged as an immunotherapeutic breakthrough in multiple cancers. However, blocking PD-1 with antibodies shows severe immune cytotoxicity, and leads to the resistance and exhaustion of T cells in clinical trials. Thus, synthetic and non-immunogenic immunotherapeutic molecules, including aptamers against PD-1 (49) and the programmed cell death protein 1 ligand (PD-L1) (50), have been developed and show inhibition of tumor growth in vivo. Additionally, for use in combinational immunotherapeutics, alternative immune-checkpoint blockade aptamers, T-cell immunoglobulin and mucin-domain containing-3 (TIM-3) (51) and complement component 5a (C5a) (52), have been isolated and their efficacy has been determined in vivo. The immune-checkpoint blockade aptamers have been summarized in Table 1.
Table 1.
Non-immunogenic immune-checkpoint blockade aptamers.
Alternative immune-checkpoint blockade aptamers for combinational immunotherapeutics
Immune therapies against the immune checkpoint molecules CTLA-4 and PD-1 have achieved good responses in some type of cancers: melanoma, renal cancer, and lung cancer (53). However, a large number of cancer patients are not benefiting from these therapies. It is now widely accepted that tumors have co-stimulatory immune checkpoint pathways for the immune suppression. It has catalyzed interest in developing novel immune checkpoint blockades for combinational immunotherapeutics. TIM-3 is expressed on IFN-γ producing CD4+ T helper 1 (Th1) and CD8+ T cytotoxic 1 (Tc1) cells (54). As TIM-3 functions specifically to limit the duration and magnitude of Th1 and Tc1 T cell responses, in this regard, the immune checkpoint receptor TIM-3 exhibits an intriguing candidate for the next generation of immune checkpoints in cancer. In pre-clinical studies, the blockade of the PD-1:PDL-1 axis together with TIM3 exhibits synergistic antitumor effects in colon carcinoma (53). To aim the evaluation of combinational immunotherapeutic aptamers of TIM3 with immune checkpoint antibodies, antagonistic TIM3 aptamers have been selected (51). The anti-TIM3 aptamers revert T cell exhaustion in vitro and significantly reduce colon carcinoma burden in combination with PD-L1 antibody blockade (51).
Anaphylatoxin complement component 5a (C5a), a protein fragment released from cleavage of complement component C5, contributes to lung cancer progression by promoting an immunosuppressive microenvironment. To reverse the tumor immunosuppressive microenvironment, a C5a aptamer has been selected (52). For plasma-stability, the anti-C5a aptamer is synthesized with Spiegelmer® L-aptamer, an artificial oligonucleotide that is a mirror image of natural oligonucleotides (RNA-like molecules built from L-ribose units). The anti-C5a aptamers show the increased survival rate and substantial improvement of anti-PD-1 blockades against lung cancer growth and metastasis. This synergistic effect is accompanied by a negative regulation of immune suppressive activity of myeloid-derived suppressor cells (MDSCs) and increased mRNA expression of IL2.
These studies prove that aptamers have great potential in immune therapy and have synergistic effects with current immune checkpoint antibodies.
IN SILICO APPROACH TO DESIGNING APTAMERS
The SELEX (10, 55) is conceptually simple and practical. However, it requires tedious work, through multiple steps of amplification, and 9 to 14 rounds of enrichment for selection of aptamer clones. To reduce the uncertainty and risk of SELEX, designing aptamer-based computational structure analysis of targets has recently been initiated.
Improving aptamer selection: In silico design of aptamers
The conceptually simple SELEX procedure (10, 55) is based on enrichment of unidentified nucleic acid ligands. Because of the inherent uncertainty of the SELEX method, the initial randomized library required to achieve an even distribution of aptamers against a specific target remains unknown. To reduce the tremendous risk and improve the chance of successful selection outcomes, mathematical modeling and computational algorithms have been developed to analyze the combined impact of important evolutional success factors including competition, randomness, and target concentration, with a systemic analysis of the role the initial library plays in affinity distribution (56). Based on stochastic models, the initial library KD distribution has been identified as having a huge impact on selection efficacy and playing a significant role in experimental parameters (56). Therefore, a well-randomized aptamer library pool determines the success of smart aptamer selection.
The classical SELEX method cannot avoid repetitive selection for enrichment. To facilitate the process of aptamer selection using a non-SELEX method, an in silico approach has been developed for targeted aptamer selection (57). An estrogen receptor α (ERα) aptamer developed using an in silico method combining in vivo structural chemistry and interaction with computational remodeling and molecular docking showed the high target specificity (58). As the non-SELEX in silico method is more cost-effective and requires less effort, it is likely to be applicable for selection of other nuclear receptor aptamers. Furthermore, the in silico-selected ERα aptamer will be used as a therapeutic molecule in ER-positive malignant cancers. In silico-based aptamer design has potential for some targets, but requires that target protein structures be identified in advance.
CONCLUSION
Cancer-specific aptamers that can be exploited for therapeutic modalities are attracting considerable attention in the aptamer field, owing to the advantage of high target specificity. These advantages include the potential to reduce systemic exposure, and the ability to reduce undesirable off-target effects. Furthermore, aptamer biomarker discovery platform technologies continue to evolve and are certain to facilitate discovery of novel biomarkers for therapeutics and diagnostics.
The remaining underdeveloped area in the field is identification of the aptamer target-binding motif. Identifying the binding motif is crucial to determining the binding affinity and the biological function of aptamers. For instance, there are two known thrombin aptamers. A 15-base thrombin aptamer (G15D) binds the fibrinogen-recognition exosite with a KD of 100 nM, and shows competitive enzymatic inhibitory function; in contrast, a 29-base thrombin aptamer (DNA 60-18) binds the heparin-binding exosite with KD of 0.5 nM, and shows non-competitive inhibition of clot formation (59). Thus, there is a clear advantage to identifying ligands that bind to specific sites or epitopes on a target, in order to define the working mechanism of aptamers. In addition, identification of the binding motif helps to design improved variants of aptamers in silico. As aptamers are nucleotide-based molecules, challenges to improve nuclease resistance and to increase binding affinity remain. The use of a phosphorodithioate (PS2) substitution on a single nucleotide as an alternative method to increase both of these properties shows dramatic improvement of binding affinity (~1000-fold) and serum stability (60).
It is very clear that aptamers are great therapeutic modalities to reach their targets. Therefore, to take full advantage of the compelling features of aptamers, the smart selection of a specific target of interest is a critically important component of therapeutic aptamer development. The selection of aptamers against predefined sophisticated epitopes on targets, tumor-specific neoantigens, and incorporation with newly developed chemically modified nucleotides (61) promote therapeutic aptamers from bench to bedside for cancer treatment.
Supplementary Material
KEY POINTS.
Identification of cancer-specific aptamers and their functionalization with novel therapeutic payloads greatly improves targeted therapeutic intervention.
Untargeted in vitro- or in vivo-SELEX, or blind-SELEX, provides enormous opportunities for novel biomarker discovery and application in cancer diagnostics.
Synthetic and non-immunogenic alternative immunomodulatory aptamers induce synergetic effects when combined with current immune-checkpoint blockade regimens for combinational immunotherapy that reduces the resistance and exhaustion of T cells.
Computational remodeling and molecular docking opens the possibility of in silico-based design of aptamers, but is limited to the known structure of target proteins.
Acknowledgments
Research reported in this publication also included work performed in shared resource facilities supported by the National Cancer Institute of the National Institutes of Health under award number P30CA033572. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We also thank Dr. Sarah Wilkinson, scientific writer, for language editing.
Financial support and sponsorship
This work is partly funded by Apterna Ltd and NIH grant AI029329 to J.J.R.
Footnotes
Conflicts of interest
J.J.R. is a co-founder of Apterna Ltd, a privately held pre-clinical stage company specializing in aptamer-based therapeutic development. S.Y. holds stock in Apterna Ltd.
REFERENCES AND RECOMMENDED READING
■ of special interest
■■ of outstanding interest
- 1.Ulrich H, Magdesian MH, Alves MJ, Colli W. In vitro selection of RNA aptamers that bind to cell adhesion receptors of Trypanosoma cruzi and inhibit cell invasion. J Biol Chem. 2002;277(23):20756–62. doi: 10.1074/jbc.M111859200. [DOI] [PubMed] [Google Scholar]
- 2.Wang J, Jiang H, Liu F. In vitro selection of novel RNA ligands that bind human cytomegalovirus and block viral infection. RNA. 2000;6(4):571–83. doi: 10.1017/s1355838200992215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blank M, Weinschenk T, Priemer M, Schluesener H. Systematic evolution of a DNA aptamer binding to rat brain tumor microvessels. selective targeting of endothelial regulatory protein pigpen. J Biol Chem. 2001;276(19):16464–8. doi: 10.1074/jbc.M100347200. [DOI] [PubMed] [Google Scholar]
- 4.Daniels DA, Chen H, Hicke BJ, Swiderek KM, Gold L. A tenascin-C aptamer identified by tumor cell SELEX: systematic evolution of ligands by exponential enrichment. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(26):15416–21. doi: 10.1073/pnas.2136683100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hicke BJ, Marion C, Chang YF, Gould T, Lynott CK, Parma D, et al. Tenascin-C aptamers are generated using tumor cells and purified protein. J Biol Chem. 2001;276(52):48644–54. doi: 10.1074/jbc.M104651200. [DOI] [PubMed] [Google Scholar]
- 6.Wilson DS, Szostak JW. In vitro selection of functional nucleic acids. Annu Rev Biochem. 1999;68:611–47. doi: 10.1146/annurev.biochem.68.1.611. [DOI] [PubMed] [Google Scholar]
- 7.Que-Gewirth NS, Sullenger BA. Gene therapy progress and prospects: RNA aptamers. Gene therapy. 2007;14(4):283–91. doi: 10.1038/sj.gt.3302900. [DOI] [PubMed] [Google Scholar]
- 8■■.Zhou J, Rossi J. Aptamers as targeted therapeutics: current potential and challenges. Nature reviews Drug discovery. 2017;16:181–202. doi: 10.1038/nrd.2016.199. This review paper summarizes all about aptamers from overall SELEX procedure to therapeutic applications. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiang D, Shigdar S, Qiao G, Wang T, Kouzani AZ, Zhou SF, et al. Nucleic acid aptamer-guided cancer therapeutics and diagnostics: the next generation of cancer medicine. Theranostics. 2015;5(1):23–42. doi: 10.7150/thno.10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tuerk C, Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science. 1990;249(4968):505–10. doi: 10.1126/science.2200121. [DOI] [PubMed] [Google Scholar]
- 11.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, et al. Cancer statistics, 2006. CA: a cancer journal for clinicians. 2006;56(2):106–30. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 12.Stathis A, Moore MJ. Advanced pancreatic carcinoma: current treatment and future challenges. Nature reviews Clinical oncology. 2010;7(3):163–72. doi: 10.1038/nrclinonc.2009.236. [DOI] [PubMed] [Google Scholar]
- 13.Reebye V, Saetrom P, Mintz PJ, Huang KW, Swiderski P, Peng L, et al. Novel RNA oligonucleotide improves liver function and inhibits liver carcinogenesis in vivo. Hepatology. 2014;59(1):216–27. doi: 10.1002/hep.26669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamamoto K, Tateishi K, Kudo Y, Sato T, Yamamoto S, Miyabayashi K, et al. Loss of histone demethylase KDM6B enhances aggressiveness of pancreatic cancer through downregulation of C/EBPalpha. Carcinogenesis. 2014;35(11):2404–14. doi: 10.1093/carcin/bgu136. [DOI] [PubMed] [Google Scholar]
- 15.Yoon S, Huang KW, Reebye V, Mintz P, Tien YW, Lai HS, et al. Targeted Delivery of C/EBPalpha -saRNA by Pancreatic Ductal Adenocarcinoma-specific RNA Aptamers Inhibits Tumor Growth In Vivo. Molecular therapy : the journal of the American Society of Gene Therapy. 2016;24(6):1106–16. doi: 10.1038/mt.2016.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao X, Pang J, Li LY, Liu WP, Di JM, Sun QP, et al. Expression profiling identifies new function of collapsin response mediator protein 4 as a metastasis-suppressor in prostate cancer. Oncogene. 2010;29(32):4555–66. doi: 10.1038/onc.2010.213. [DOI] [PubMed] [Google Scholar]
- 17.Li C, Jiang W, Hu Q, Li LC, Dong L, Chen R, et al. Enhancing DPYSL3 gene expression via a promoter-targeted small activating RNA approach suppresses cancer cell motility and metastasis. Oncotarget. 2016;7(16):22893–910. doi: 10.18632/oncotarget.8290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohapatra S, Saha A, Mondal P, Jana B, Ghosh S, Biswas A, et al. Synergistic Anticancer Effect of Peptide-Docetaxel Nanoassembly Targeted to Tubulin: Toward Development of Dual Warhead Containing Nanomedicine. Advanced healthcare materials. 2017;6(2) doi: 10.1002/adhm.201600718. [DOI] [PubMed] [Google Scholar]
- 19.Koga Y, Manabe S, Aihara Y, Sato R, Tsumura R, Iwafuji H, et al. Antitumor effect of antitissue factor antibody-MMAE conjugate in human pancreatic tumor xenografts. International journal of cancer Journal international du cancer. 2015;137(6):1457–66. doi: 10.1002/ijc.29492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yao HP, Feng L, Zhou JW, Zhang RW, Wang MH. Therapeutic evaluation of monoclonal antibody-maytansinoid conjugate as a model of RON-targeted drug delivery for pancreatic cancer treatment. Am J Cancer Res. 2016;6(5):937–56. [PMC free article] [PubMed] [Google Scholar]
- 21.Yoon S, Huang KW, Reebye V, Spalding D, Przytycka TM, Wang Y, et al. Aptamer-Drug Conjugates of Active Metabolites of Nucleoside Analogs and Cytotoxic Agents Inhibit Pancreatic Tumor Cell Growth. Molecular therapy Nucleic acids. 2017;6:80–8. doi: 10.1016/j.omtn.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiang D, Zheng C, Zhou SF, Qiao S, Tran PH, Pu C, et al. Superior Performance of Aptamer in Tumor Penetration over Antibody: Implication of Aptamer-Based Theranostics in Solid Tumors. Theranostics. 2015;5(10):1083–97. doi: 10.7150/thno.11711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heo K, Min SW, Sung HJ, Kim HG, Kim HJ, Kim YH, et al. An aptamer-antibody complex (oligobody) as a novel delivery platform for targeted cancer therapies. Journal of controlled release : official journal of the Controlled Release Society. 2016;229:1–9. doi: 10.1016/j.jconrel.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 24.Casares N, Rudilla F, Arribillaga L, Llopiz D, Riezu-Boj JI, Lozano T, et al. A peptide inhibitor of FOXP3 impairs regulatory T cell activity and improves vaccine efficacy in mice. Journal of immunology. 2010;185(9):5150–9. doi: 10.4049/jimmunol.1001114. [DOI] [PubMed] [Google Scholar]
- 25■.Lozano T, Soldevilla MM, Casares N, Villanueva H, et al. Targeting inhibition of Foxp3 by a CD28 2'-Fluro oligonucleotide aptamer conjugated to P60-peptide enhances active cancer immunotherapy. Biomaterials. 2016;91:73–80. doi: 10.1016/j.biomaterials.2016.03.007. This study suggests the aptamer-mediated inhibition of Treg function in cancer vaccine context. [DOI] [PubMed] [Google Scholar]
- 26.Van Der Bruggen P, Zhang Y, Chaux P, Stroobant V, Panichelli C, Schultz ES, et al. Tumor-specific shared antigenic peptides recognized by human T cells. Immunological reviews. 2002;188:51–64. doi: 10.1034/j.1600-065x.2002.18806.x. [DOI] [PubMed] [Google Scholar]
- 27.Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, et al. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15(17):5323–37. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen L, Rashid F, Shah A, Awan HM, Wu M, Liu A, et al. The isolation of an RNA aptamer targeting to p53 protein with single amino acid mutation. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(32):10002–7. doi: 10.1073/pnas.1502159112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Camorani S, Crescenzi E, Colecchia D, Carpentieri A, Amoresano A, Fedele M, et al. Aptamer targeting EGFRvIII mutant hampers its constitutive autophosphorylation and affects migration, invasion and proliferation of glioblastoma cells. Oncotarget. 2015;6(35):37570–87. doi: 10.18632/oncotarget.6066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang CY, Lin BL, Chen CH. An aptamer targeting shared tumor-specific peptide antigen of MAGE-A3 in multiple cancers. International journal of cancer. 2016;138(4):918–26. doi: 10.1002/ijc.29826. [DOI] [PubMed] [Google Scholar]
- 31.Stewart RL, O'Connor KL. Clinical significance of the integrin alpha6beta4 in human malignancies. Laboratory investigation; a journal of technical methods and pathology. 2015;95(9):976–86. doi: 10.1038/labinvest.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cruz-Monserrate Z, Qiu S, Evers BM, O'Connor KL. Upregulation and redistribution of integrin alpha6beta4 expression occurs at an early stage in pancreatic adenocarcinoma progression. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2007;20(6):656–67. doi: 10.1038/modpathol.3800782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329–35. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berg K, Lange T, Mittelberger F, Schumacher U, Hahn U. Selection and Characterization of an alpha6beta4 Integrin blocking DNA Aptamer. Molecular therapy Nucleic acids. 2016;5:e294. doi: 10.1038/mtna.2016.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang T, Birsoy K, Hughes NW, Krupczak KM, Post Y, Wei JJ, et al. Identification and characterization of essential genes in the human genome. Science. 2015;350(6264):1096–101. doi: 10.1126/science.aac7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berezovski MV, Lechmann M, Musheev MU, Mak TW, Krylov SN. Aptamer-facilitated biomarker discovery (AptaBiD) Journal of the American Chemical Society. 2008;130(28):9137–43. doi: 10.1021/ja801951p. [DOI] [PubMed] [Google Scholar]
- 37.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: a cancer journal for clinicians. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 38.Malvezzi M, Bertuccio P, Levi F, La Vecchia C, Negri E. European cancer mortality predictions for the year 2013. Annals of oncology : official journal of the European Society for Medical Oncology. 2013;24(3):792–800. doi: 10.1093/annonc/mdt010. [DOI] [PubMed] [Google Scholar]
- 39.Mayo SC, Nathan H, Cameron JL, Olino K, Edil BH, Herman JM, et al. Conditional survival in patients with pancreatic ductal adenocarcinoma resected with curative intent. Cancer. 2012;118(10):2674–81. doi: 10.1002/cncr.26553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kamisawa T, Isawa T, Koike M, Tsuruta K, Okamoto A. Hematogenous metastases of pancreatic ductal carcinoma. Pancreas. 1995;11(4):345–9. doi: 10.1097/00006676-199511000-00005. [DOI] [PubMed] [Google Scholar]
- 41.Disibio G, French SW. Metastatic patterns of cancers: results from a large autopsy study. Archives of pathology & laboratory medicine. 2008;132(6):931–9. doi: 10.5858/2008-132-931-MPOCRF. [DOI] [PubMed] [Google Scholar]
- 42.Yoon S, Armstrong B, Habib N, Rossi JJ. Blind SELEX Approach Identifies RNA Aptamers that Regulate EMT and Inhibit Metastasis. Molecular cancer research : MCR. 2017 doi: 10.1158/5141-7786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jia W, Ren C, Wang L, Zhu B, Jia W, Gao M, et al. CD109 is identified as a potential nasopharyngeal carcinoma biomarker using aptamer selected by cell-SELEX. Oncotarget. 2016;7(34):55328–42. doi: 10.18632/oncotarget.10530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zamay GIT, Zamay T, Grigorieva V, Glazyrin Y, Kolovskaya O, Garanzha I, Barinov A, Krat A, Mironov G, Gargaun A, Veprintsev D, Bekuzarov S, Kirichenko A, Zukov R, Petrova M, Modestov A, Berezovski M, Zamay A. DNA Aptamers for the Characterization of Histological Structure of Lung Adenocarcinoma. Molecular therapy Nucleic acids. 2017;6:150–62. doi: 10.1016/j.omtn.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mi J, Ray P, Liu J, Kuan CT, Xu J, Hsu D, et al. In Vivo Selection Against Human Colorectal Cancer Xenografts Identifies an Aptamer That Targets RNA Helicase Protein DHX9. Molecular therapy Nucleic acids. 2016;5:e315. doi: 10.1038/mtna.2016.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee T, Pelletier J. The biology of DHX9 and its potential as a therapeutic target. Oncotarget. 2016;7(27):42716–39. doi: 10.18632/oncotarget.8446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee T, Paquet M, Larsson O, Pelletier J. Tumor cell survival dependence on the DHX9 DExH-box helicase. Oncogene. 2016;35(39):5093–105. doi: 10.1038/onc.2016.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nozari A, Berezovski MV. Aptamers for CD Antigens: From Cell Profiling to Activity Modulation. Molecular therapy Nucleic acids. 2017;6:29–44. doi: 10.1016/j.omtn.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prodeus A, Abdul-Wahid A, Fischer NW, Huang EH, Cydzik M, Gariepy J. Targeting the PD-1/PD-L1 Immune Evasion Axis With DNA Aptamers as a Novel Therapeutic Strategy for the Treatment of Disseminated Cancers. Molecular therapy Nucleic acids. 2015;4:e237. doi: 10.1038/mtna.2015.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lai WY, Huang BT, Wang JW, Lin PY, Yang PC. A Novel PD-L1-targeting Antagonistic DNA Aptamer With Antitumor Effects. Molecular therapy Nucleic acids. 2016;5:e397. doi: 10.1038/mtna.2016.102. [DOI] [PubMed] [Google Scholar]
- 51.Hervas-Stubbs S, Soldevilla MM, Villanueva H, Mancheno U, Bendandi M, Pastor F. Identification of TIM3 2'-fluoro oligonucleotide aptamer by HT-SELEX for cancer immunotherapy. Oncotarget. 2016;7(4):4522–30. doi: 10.18632/oncotarget.6608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52■■.Ajona D, Ortiz-Espinosa S, Moreno H, Lozano T, et al. A Combined PD-1/C5a Blockade Synergistically Protects Against Lung Cancer Growth and Metastasis. Cancer discovery. 2017 doi: 10.1158/2159-8290. This study provides a novel combination immune therapeutic strategy with antibodies and aptamers for lung cancer in clinical relevant way. [DOI] [PubMed] [Google Scholar]
- 53.Anderson AC. Tim-3: an emerging target in the cancer immunotherapy landscape. Cancer immunology research. 2014;2(5):393–8. doi: 10.1158/2326-6066.CIR-14-0039. [DOI] [PubMed] [Google Scholar]
- 54.Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T, et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415(6871):536–41. doi: 10.1038/415536a. [DOI] [PubMed] [Google Scholar]
- 55.Ellington AD, Szostak JW. In vitro selection of RNA molecules that bind specific ligands. Nature. 1990;346(6287):818–22. doi: 10.1038/346818a0. [DOI] [PubMed] [Google Scholar]
- 56■.Spill F, Weinstein ZB, Irani Shemirani A, Ho N, et al. Controlling uncertainty in aptamer selection. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:12076–81. doi: 10.1073/pnas.1605086113. This study describes the important factors to reduce the uncertainty of SELEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shcherbinin DS, Gnedenko OV, Khmeleva SA, Usanov SA, Gilep AA, Yantsevich AV, et al. Computer-aided design of aptamers for cytochrome p450. Journal of structural biology. 2015;191(2):112–9. doi: 10.1016/j.jsb.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 58.Ahirwar R, Nahar S, Aggarwal S, Ramachandran S, Maiti S, Nahar P. In silico selection of an aptamer to estrogen receptor alpha using computational docking employing estrogen response elements as aptamer-alike molecules. Scientific reports. 2016;6:21285. doi: 10.1038/srep21285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tasset DM, Kubik MF, Steiner W. Oligonucleotide inhibitors of human thrombin that bind distinct epitopes. Journal of molecular biology. 1997;272(5):688–98. doi: 10.1006/jmbi.1997.1275. [DOI] [PubMed] [Google Scholar]
- 60.Abeydeera ND, Egli M, Cox N, Mercier K, Conde JN, Pallan PS, et al. Evoking picomolar binding in RNA by a single phosphorodithioate linkage. Nucleic acids research. 2016;44(17):8052–64. doi: 10.1093/nar/gkw725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61■.Yoon S, Rossi JJ. Future strategies for the discovery of therapeutic aptamers. Expert Opin Drug Discov. 2017:1–3. doi: 10.1080/17460441.2017.1290077. This paper suggests the future strategies for developing therapeutic aptamers. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.