Abstract
Background
Recent reports have established the notion that many patients with longstanding type 1 diabetes (T1D) possess a remnant population of insulin-producing beta cells. It remains questionable, however, whether these surviving cells can physiologically sense and respond to glucose stimuli.
Methods
Frozen pancreatic sections from non-diabetic donors (n = 8), type 2 diabetic patients (n = 4), islet autoantibody-positive non-diabetic patients (n = 3), type 1 diabetic patients (n = 10) and one case of gestational diabetes were obtained via the network for Pancreatic Organ Donors. All longstanding T1D samples were selected based on the detection of insulin-producing beta cells in the pancreas by immunohistochemistry. RNA was isolated from all sections followed by cDNA preparation and quantitative real-time polymerase chain reaction for insulin, glucose transporter 1 (GLUT1), GLUT2 and GLUT3. Finally, immunofluorescent staining was performed on consecutive sections for all four of these markers and a comparison was made between the expression of GLUT2 in humans versus NOD mice.
Results
In contrast to islets from the most widely used T1D model, the NOD mouse, human islets predominantly express GLUT1 and, to a much lesser extent, GLUT3 on their surface instead of GLUT2. Relative expression levels of these receptors do not significantly change in the context of the various (pre-)diabetic conditions studied. Moreover, in both species preservation of GLUT expression was observed even under conditions of substantial leucocyte infiltration or decades of T1D duration.
Conclusions
These data suggest that despite being subjected to multiple years of physiological stress, the remaining beta-cell population in longstanding T1D patients retains a capacity to sense glucose via its GLUTs.
Keywords: glucose transporters, type 1 diabetes, beta cells, pancreas, islets of Langerhans, insulin
Introduction
The ability of the pancreatic beta cells to secrete appropriate doses of insulin depends in part on their capacity to accurately sense blood glucose levels. Like most other cell types, beta cells convert intracellular glucose to glucose-6-phosphate followed by incorporation into the glycolytic pathway. Only beta cells, however, are capable of releasing insulin granules in direct quantitative correlation with the amount of insulin they get to process [1]. A crucial preceding step in glucose processing is the actual uptake at the cellular membrane. Different cell types are known to express distinct facilitative glucose transporters (GLUTs) or combinations thereof that act by passively carrying glucose into the cytoplasm down a concentration gradient [2].
The most widely studied GLUT in the context of beta-cell glucose uptake is GLUT2. Studies in rodents have indeed consistently ascribed a pivotal role of GLUT2-mediated transport in relation to insulin secretory capacity of beta cells [3]. The physiological role of GLUT2 is equally important in humans, as evidenced by the finding that some mutations in the GLUT2 (SLC2A2) gene are responsible for the Fanconi–Bickel syndrome, an autosomal recessive disorder in carbohydrate metabolism [4]. GLUT2 polymorphisms were also found in association with T2D development [5–7], while many other reports seem to contradict this finding [8–15]. A detailed comparison by De Vos et al. between humans and rat beta cells, however, showed that GLUT2 is not detectable in isolated human pancreatic islets [16]. This study thus revealed a major interspecies discrepancy between GLUT usage by beta cells and calls into question how dominant beta-cell-specific GLUT2-mediated effects, as observed in rodent diabetes models, translate to humans. The study by De Vos et al. instead identified GLUT1 and, to a much lesser extent, GLUT3 as the dominant GLUT types expressed within pancreatic islets. GLUT1 is the most ubiquitously expressed transporter isoform, while GLUT3 is predominantly expressed in neurons [17].
Within the context of autoimmune-driven T1D, recent studies have revitalized the idea that longstanding T1D patients maintain a viable pool of functional beta cells [18,19]. It could be reasoned that beta-cell function in these individuals is likely impaired as a consequence of cellular stress caused by glucotoxicity or islet autoimmunity. Contrastingly, it was found that many longstanding T1D patients maintain the capacity to secrete small amounts of insulin in a regulated manner [18–20]. These encouraging data were even suggested to be an underestimation since the patients under investigation were often subjected to a strict insulin-replacement regimen, which could suppress endogenous insulin production by the remnant beta-cell mass [21]. From the perspective of designing beta-cell-specific regenerative drugs, this is particularly important information.
This study was therefore designed to assess the levels of GLUT expression on pancreatic beta cells from longstanding T1D organ donors in direct comparison with non-diabetic controls, islet autoantibody-positive ‘pre’-diabetic patients, T2D donors and one case of gestational diabetes. Hereto, we have selected T1D samples from the network for Pancreatic Organ Donors (nPOD) tissue collection that showed insulin staining on immunohistochemistry as performed by pathologists from the nPOD consortium (accessible to registered investigators on www.jdrfnpod.org). Our polymerase chain reaction (PCR) data demonstrate that these individuals consistently express GLUT1 and GLUT3 within their pancreatic tissue, while GLUT2 is subdominant and often undetectable. Detailed immunofluorescent analysis revealed that GLUT1 is uniformly expressed across pancreatic islets, while no GLUT2 and only limited GLUT3 expression could be demonstrated. Importantly, GLUT1 staining was preserved in all but one longstanding T1D case, a feature that was paralleled by persistent GLUT2 expression in severely inflamed islets from non-obese diabetic (NOD) mice.
Materials and methods
Sample selection
Six micrometre sections from freshly frozen pancreatic samples were obtained via the nPOD and from Dr Thomas WH Kay at the St Vincent’s Institute (case SVI021-08). Samples are from cadaveric donor pancreases and demographic information is disclosed in Table S1 (available online only). Basic histopathology is provided by the nPOD’s pathology department and we selected all insulin-positive, longstanding T1D cases together with available samples from non-diabetic subjects and donors with other diabetic conditions. Standard operating procedures for organ processing, C-peptide and autoantibody determination can be found in the Website http://www.jdrfnpod.org/sops.php. All experimental procedures were approved by the Human Subjects Committee at the La Jolla Institute for Allergy and Immunology (protocol nr. DI3-054-1209).
Mice
Female NOD mice were purchased from The Jackson Laboratories. Pancreatic tissue was harvested at 8 weeks of age and embedded in OCT for sectioning. All animal experiments were approved by the Ethics Committee at The La Jolla Institute for Allergy and Immunology.
Quantitative real-time PCR
RNA was purified from frozen sections using the RNAqueous-Micro Kit (Ambion). The protocol provided by the manufacturer was followed, with the addition of an initial step of preheating the lysis buffer to 56 °C. Subsequent to purification, 10 µL sterile-filtered ammonium acetate 7.5 M (Sigma), 30 mg oyster glycogen (20 mg/mL) (USBiological) and 75 µL 100% ethanol (Sigma-Aldrich) was added. After a minimum of 60 min at −40 °C, the pellets were centrifuged and washed with ethanol before resuspension in DEPC-treated water (Ambion). RNA yields in the samples were determined and up to 5 µg of RNA was synthesized to cDNA using SuperScript II Reverse Transcriptase® with random primers (Invitrogen). The cDNA was then diluted and used in duplicate for real-time PCR for S18, insulin, GLUT1, GLUT2 and GLUT3. All primers used in the PCR are from Quantitect Primer Assay® (Qiagen). Real-time PCR reactions were run on a LightCycler® from Roche and the results were calculated using the DeltaDeltaCt method. Specifically, the lowest sample threshold cycles (Ct) value obtained after measurement was subtracted from all quantifiable samples. Analogously, the lowest housekeeping gene (HKG) Ct was subtracted from all housekeeping gene values. For both series of values, 2−[delta] was calculated and finally normalization was performed by calculating 2−[delta]Ct,sample/2−[delta]Ct,HKG to yield the respective relative expression values for each sample.
Ct between 35 and 40 were registered as ‘detectable’ but not included in any quantitative analysis due to higher uncertainty, while Ct higher than 40 were registered as ‘undetectable’.
Immunofluorescent staining
Frozen sections were thawed, fixed in acetone and blocked with 2% goat serum. Consecutive sections were stained using rabbit antibodies for GLUT1 (Millipore), GLUT2 (Millipore) and GLUT3 (Sigma-Aldrich), all combined with subsequent staining for insulin using guinea pig anti-insulin antibodies (Dako). Goat anti-rabbit Alexa Fluor 647 (Invitrogen) and goat anti-guinea pig Alexa Fluor 488 (Invitrogen) were used as secondary antibodies for immunofluorescent detection without any possible spectral overlap. One staining contained anti-CD68 (Dako) in its primary cocktail, followed by detection with Alexa Fluor 555. Image acquisition was performed with a Leica SP5 confocal microscope equipped with an argon laser for excitation at 488 and two HeNe lasers for 543 and 633 nm and attached to a Leica DM6000 upright microscope carrying a ×40 1.3 NA oil objective.
Results
Determination of pancreatic insulin mRNA content
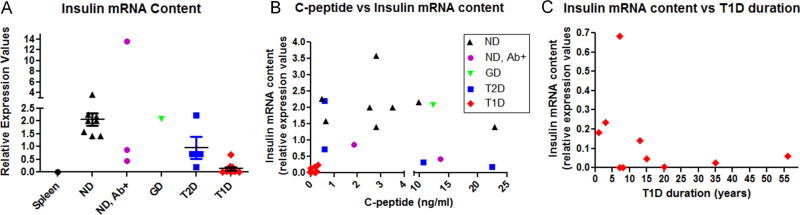
In order to confirm the successful isolation of high-quality RNA from the frozen sections provided by nPOD, we initially determined their insulin mRNA content. Using SYBR green-based quantitative real-time PCR, we were able to detect insulin message in all samples that were obtained, even from all five T1D organ donors without detectable C-peptide levels (Figure 1A). Insulin mRNA levels in the T2D patients showed a trend towards decrease as compared to control, but only the T1D group significantly differed from non-diabetic patients (p = 0.0004; Mann–Whitney). Two of the three islet autoantibody-positive donors, one of which had three autoantibodies, also showed a declining trend. When C-peptide levels are plotted against insulin mRNA content, T1D subjects cluster around the origin indicating a correlation between the relative lack of insulin mRNA and the absence or low levels of C-peptide in peripheral blood (Figure 1B). The higher ranges of C-peptide levels from controls and T2D donors did not show correlation with insulin mRNA, most likely because of temporal discordances between transcriptional events and subsequent C-peptide cleavage and release into the blood stream. The T1D subjects with the highest mRNA production status clustered within the first 5 years after diagnosis (Figure 1C).
Figure 1.
Pancreatic insulin mRNA content and correlation with peripheral C-peptide levels and type 1 diabetes (T1D) duration. RNA was prepared from frozen sections and corresponding cDNA was used in a quantitative real-time polymerase chain reaction using primers against 18S ribosomal RNA for standardization. (A) A trend towards decreased insulin gene transcription is observed in T2D samples, while a statistically significant decrease is detected only in T1D versus non-diabetic (p = 0.0004; Mann–Whitney). (B) C-peptide measurement in blood at sampling plotted against insulin mRNA content. No significant correlations were observed, although C-peptide depletion in T1D subjects correlated well with low insulin mRNA levels. (C) Distribution of T1D cases according to duration and pancreatic insulin mRNA content. Significant insulin mRNA levels cluster in the earlier time range post-diagnosis. ND, non-diabetic; Ab+, islet autoantibody-positive at sampling; GD, gestational diabetes. Relative expression values across tested samples were calculated as outlined in Materials and methods section
Taken together, these data demonstrate the reliability of our methods to isolate high-quality RNA from frozen sections and show the preservation of pancreatic insulin transcripts in longstanding T1D patients.
Detection of GLUT1, GLUT2 and GLUT3 pancreatic mRNA levels
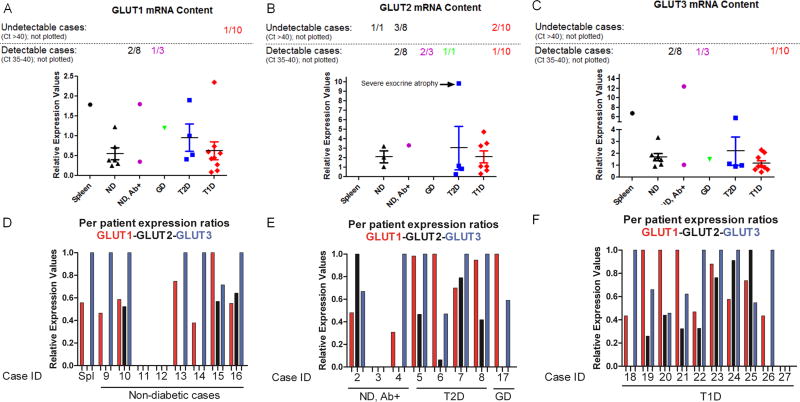
Measuring transcript levels in collagenase-digested, Ficoll gradient-purified and handpicked islets is problematic because of transcriptional artefacts introduced during the lengthy time-window after isolation and the extensive manipulations. The definitive advantage over isolated islets is that the samples studied here are obtained from freshly frozen pancreatic tissues isolated from patients on life support, which minimizes the risk of artefacts. Prior to immunofluorescent staining, we wished to get a general impression of overall pancreatic GLUT levels across healthy and diabetic samples. GLUT1 and GLUT3 could be detected in almost all pancreatic samples tested, while GLUT2 could only be quantitatively detected (definition see Materials and methods) in three of the eight non-diabetic controls (Figure 2A–C). Remarkably, no statistical differences were detected between T1D and non-diabetic patients, indicating that GLUT are still abundantly expressed within diabetic pancreata. While these data do not show islet-specific expression of GLUT isoforms, they indicate that pancreatic expression is predominantly limited to GLUT1 and GLUT3 and that detection in longstanding T1D patients is maintained. Moreover, on a per-patient basis the ratios between the three GLUT isoforms clearly show that GLUT2 is the subdominant species, even when the bulk pancreatic mRNA preparations are evaluated (Figure 2D–F).
Figure 2.
Pancreatic glucose transporter (GULT) mRNA expression across diabetic conditions. (A–C) Relative mRNA levels for GLUT1, GLUT2 and GLUT3 were compared between controls and various diabetic conditions. While GLUT1 and GLUT3 are both detectable in all but one sample, GLUT2 could not be quantitatively assessed in many samples. Note that only ‘quantifiable’ samples are plotted (threshold cycles between 1 and 35), while ‘detectable’ (threshold cycles between 35 and 40) and ‘undetectable’ (threshold cycles >40) samples are included in the numbers at the top. (D–F) Relative expression values for GLUT1, GLUT2 and GLUT3 are plotted for each individual sample. Across all samples, GLUT1 and GLUT3 ratios are dominant while GLUT2 consistently represents the minor mRNA species. ND, non-diabetic; Ab+, islet autoantibody-positive at sampling; GD, gestational diabetes. Relative expression values across tested samples were calculated as outlined in Materials and methods
GLUT2 is the dominant isoform in pancreatic islets from NOD mice and persists in the face of inflammation
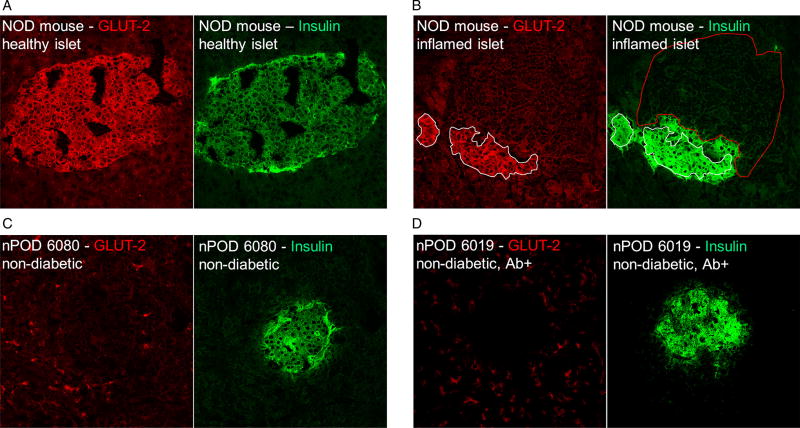
On the basis of the initial PCR data we directly compared pancreatic GLUT2 staining between human samples and sections obtained from pre-diabetic NOD mice. It is important to note that all three GLUT antibodies employed in this study were extensively validated by the Human Protein Atlas project (http://www.proteinatlas.org), a publicly available database containing application-specific validation performed for each antibody, including immunohistochemisty, Western blot analysis and, for a large fraction, a protein array assay and immunofluorescent-based confocal microscopy. We thus used the same antibodies from the same supplier and at the same concentration. For the human GLUT2-specific antibody, cross-reactivity with GLUT2 of mouse origin was reported by the supplier and confirmed in-house (data not shown).
Pancreata from three 8-week-old NOD mice were harvested and sectioned for staining. At this age, both non-inflamed and insulitic islets can be found allowing us to comparatively assess GLUT2 expression on both. Healthy islets were found to abundantly express GLUT2 (Figure 3A). Figure 3B shows a representative insulitic islet, the insulin-positive remnants of which still express GLUT2. Only a relatively small zone that is in direct contact with the inflammatory cells has lost its GLUT2 expression. These images demonstrate that mouse beta cells maintain GLUT expression even under conditions of overwhelming inflammatory stress. In contrast, none of the human pancreatic islets examined expressed any GLUT2 (Figure 3C and D), in part confirming our PCR data and further highlighting the considerable interspecies differences with regard to GLUT isoform distribution.
Figure 3.
Glucose transporter 2 (GLUT2) is the dominant GLUT isoform in the NOD mouse and remains detectable under insulitic conditions. (A) A normal islet found in an 8-week-old female NOD mouse shows normal insulin production and strong GLUT2 expression. (B) A heavily inflamed islet from the same animal shows dramatically reduced insulin staining but persistent GLUT2 expression (white outline), except for the contact zone between infiltrate (red outline) and beta-cell mass. (C, D) In sharp contrast with rodents, GLUT2 could not be detected in any of the human islets examined
GLUT1 but not GLUT3 is abundantly expressed in human pancreatic islets and persists in longstanding T1D patients that maintain functional beta-cell mass
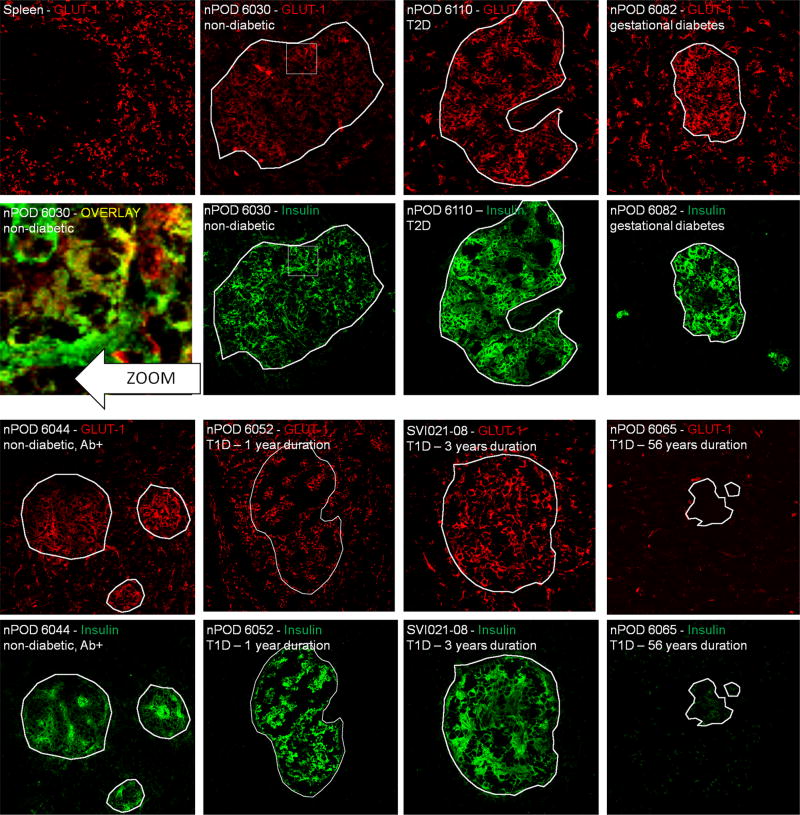
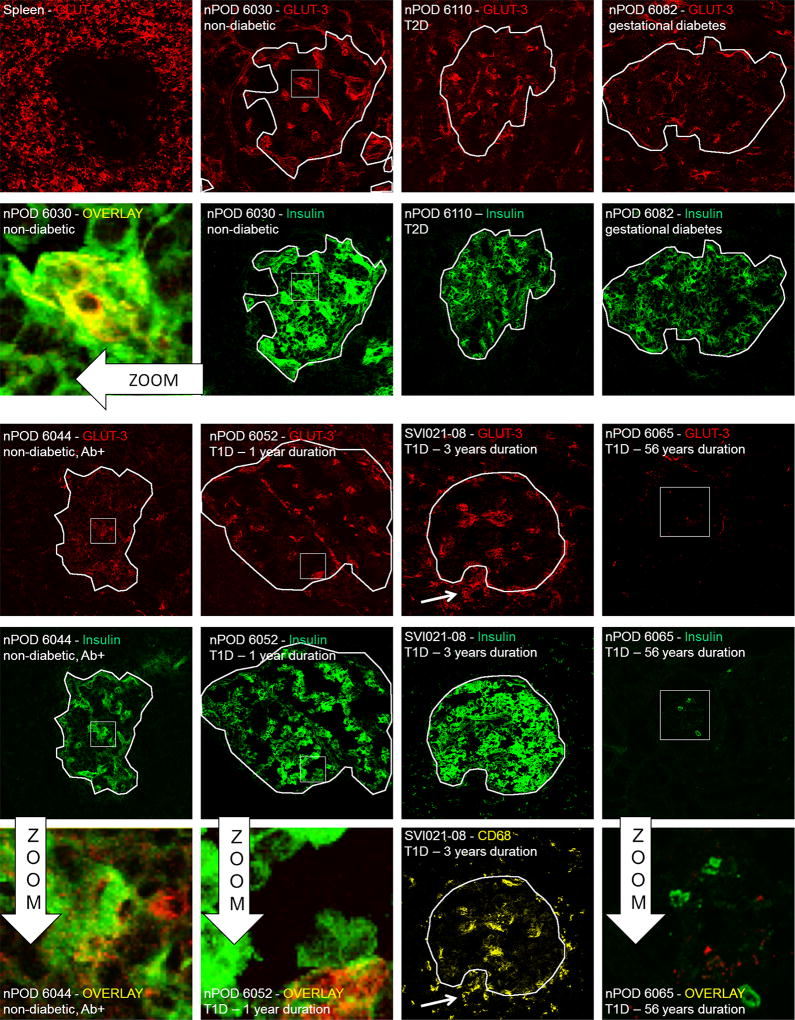
Since GLUT2 was undetectable in human islets, we focused our experiments on the detection of GLUT1 and GLUT3. GLUT1 and GLUT3 staining was verified in a healthy spleen section as GLUT1 is known to be abundantly expressed in leucocytes [22]. As a negative control we used the sample from a patient with 56 years of T1D (nPOD6065) in which none of the GLUT of interest could be detected using PCR.
GLUT1 was found to be the dominant islet isoform on consecutive sections from the same non-diabetic and diabetic human subjects (Figure 4). The observed staining pattern clearly showed that GLUT1 expression is not confined to pancreatic islets, in line with its reportedly ubiquitous distribution on, for example, endothelial cells. In non-diabetic individuals, the GLUT1 staining colocalized with the fluorescence emanating from the insulin staining. The same patterns, although with varying intensity, were found in all samples examined except for nPOD 6065. Interestingly, the results obtained on persistent GLUT expression in mice could be extended. In the case of the subject SVI021-08 where strong macrophage (and CD8+, data not shown) infiltration was demonstrated on a consecutive section (see corresponding image in Figure 5), GLUT1 staining was perfectly preserved. This data shows that even under conditions of ongoing leucocyte infiltration, beta cells are capable of maintaining expression of their principal GLUT.
Figure 4.
Immunofluorescent glucose transporter 1 (GLUT1) expression survey on pancreatic islets from donors with various diabetic conditions. All representative samples are individually labelled with sample and staining ID. Spleen section from a normal individual served as a positive control for staining, while nPOD6065 in which no GLUT1 mRNA was detected is a negative control. From this staining series of it can be concluded that GLUT1 is the dominant isoform on human pancreatic islets and persist after many years of type 1 diabetes on remnant insulin-positive islets. The zoom shows yellow colocalization between insulin and GLUT3, indicating that beta cells are among the cells that express this transporter. White outlines demarcate insulin-positive areas and were superposed onto the GLUT1 images for comparison. White squares are zoom regions
Figure 5.
Immunofluorescent glucose transporter 3 (GLUT3) expression survey on pancreatic islets from donors with various diabetic conditions. All representative samples are individually labelled with sample and staining ID. Spleen section from a normal individual served as a positive control for staining, while nPOD6065 in which no GLUT3 mRNA was detected is a negative control. In contrast to GLUT1, a more diffuse staining pattern was obtained for GLUT3, clearly showing less expression as compared to the former. The zoom shows limited areas with yellow colocalization between insulin and GLUT3, indicating that only some beta cells express this transporter. In a type 1 diabetic subject with macrophage + insulitis, these infiltrating cells can be observed as GLUT3+, in line with published data [17]. White outlines demarcate insulin-positive areas and were superposed onto the GLUT3 images for comparison. White squares are zoom regions
GLUT3 expression was observed in only limited amounts in both normal and diabetic samples (Figure 5). Colocalization with insulin staining was occasionally observed but many insulin-positive zones showed no corresponding GLUT3 expression. In the subject SVI021-08 we performed costaining with anti-CD68 and were able to demonstrate that the majority of GLUT3 staining in the islet periphery originated from infiltrating macrophages. In summary, pancreatic beta cells do not consistently and abundantly express GLUT3.
Discussion
This study confirms that GLUT1 is the dominant GLUT associated with human pancreatic islets and demonstrates that this molecule continues to be expressed in surviving beta cells from longstanding T1D subjects. These data only partially ascertain that this remnant beta-cell pool is capable of adequate insulin secretion in response to altering glucose levels. Indeed, glucose transport across the cellular membrane is just the first step in a complex cascade that eventually leads to the release of insulin secretory granules. For instance, glucokinase, an enzyme functional at proximal hydrolysis, is absolutely required for glucose sensing in both rodents and humans [16]. Mutations in the gene coding for this enzyme have been linked to gestational diabetes [23], MODY [24], T2D [25] and T1D [26]. It is thus conceivable that differences in glucokinase expression levels as a consequence of beta-cell stress caused by glucotoxicity or inflammation lead to their inability to mount insulin responses. Perhaps, the most convincing evidence that surviving beta cells from longstanding type 1 diabetic patients are still capable of responding to glucose stimuli comes from recent patient studies. In a recent report by Keenan et al., ‘Joslin medalists’ were studied, who have a documented history of over 50 years of T1D [19]. It was found that the majority of these individuals had residual endogenous insulin production. Likewise, Rother et al. demonstrated that beta cells in longstanding T1D patients are able to respond to physiological and pharmalogical stimuli and have thus maintained their capacity to secrete insulin in a regulated manner [20]. Another study performed venous sampling of stimulated C-peptide in islet transplant recipients and found that all allograft recipients produced endogenous C-peptide [18]. Collectively, these data offer in vivo support for the notion put forward by our present work, that is, surviving beta cells in longstanding T1D patients still possess the molecular machinery required for glucose sensing.
The strengths of this study lie in the direct detection of GLUTs relevant to human beta cells by immunofluorescence on well-preserved frozen sections. Protein expression and particularly mRNA levels are likely to be influenced by the drastic protocol that is used for islet isolation, a disadvantage that is avoided by our methods. We employed extensively characterized antibodies and verified their specificity on human spleen sections, which are known to contain abundant levels of GLUT1 and GLUT3. Furthermore, analysis of GLUT1 and GLUT3 mRNA expression in bulk pancreatic tissue confirmed the presence and dominance of these isoforms in contrast to the relative absence of GLUT2. In this context, it should be mentioned that the PCR in this study was performed on a mixed population of pancreatic cells and not purified islets. The readout is therefore dominated by non-endocrine cells such as endothelial cells and any decline in T1D patients remains consequentially masked in this assay. Immunfluorescent analysis, however, showed that GLUT1 was uniformly dominant over GLUT3 in terms of islet-specific expression. Most importantly and in line with the reported preservation of glucose responsiveness as described above, GLUT1 expression was maintained by the residual beta cells in longstanding type 1 diabetic patients. Although it is clearly demonstrated that GLUT2 is not detectable in human pancreatic islets, equivalent persistence is observed in the inflamed islets from pre-diabetic NOD mouse.
As it appears that these endogenous survivors carry an intact machinery to allow for insulin secretion, how may we exploit this therapeutically? Beta cells in the NOD mouse seem to have a powerful regenerative capacity, with mostly beta-cell proliferation as the driving force [27]. In humans, reports on beta-cell proliferation vary (none [28,29], limited [30] and occasionally extensive [31]) at various stages of disease. Endogenous beta-cell proliferation in longstanding T1D individuals, however, was to our knowledge never investigated. The therapeutic key potentially lies in the specific expansion of this remnant beta-cell pool using agents such as exendin-4 that promote beta-cell proliferation. Concurrent immunomodulatory treatment may well turn out to be indispensible to curb islet-specific memory cells that would recognize the replenished beta-cell mass.
Altogether, we believe our current understanding of how beta cells can rebound from inflammatory damage provides reason for cautious optimism. It is now established that the majority of patients maintain at least a fraction of their functional beta-cell population, pointing towards the existence of powerful beta-cell survival pathways.
Supplementary Material
Acknowledgments
This research was performed with the support of the network for Pancreatic Organ Donors with Diabetes, a collaborative type 1 diabetic research project sponsored by the Juvenile Diabetes Research Foundation International. Organ Procurement Organizations partnering with nPOD to providing research resources are listed at www.jdrfnpod.org/our-partners.php.
Footnotes
Conflict of interest
The authors have no conflicts of interest.
Supporting information may be found in the online version of this article.
References
- 1.Thorens B. A toggle for type 2 diabetes? N Engl J Med. 2006;354:1636–1638. doi: 10.1056/NEJMcibr060422. [DOI] [PubMed] [Google Scholar]
- 2.Mueckler M. Facilitative glucose transporters. Eur J Biochem. 1994;219:713–725. doi: 10.1111/j.1432-1033.1994.tb18550.x. [DOI] [PubMed] [Google Scholar]
- 3.Leturque A, Brot-Laroche E, Le Gall M. GLUT2 mutations, translocation, and receptor function in diet sugar managing. Am J Physiol Endocrinol Metab. 2009;296:E985–E992. doi: 10.1152/ajpendo.00004.2009. [DOI] [PubMed] [Google Scholar]
- 4.Santer R, Schneppenheim R, Dombrowski A, Gotze H, Steinmann B, Schaub J. Mutations in GLUT2, the gene for the liver-type glucose transporter, in patients with Fanconi-Bickel syndrome. Nat Genet. 1997;17:324–326. doi: 10.1038/ng1197-324. [DOI] [PubMed] [Google Scholar]
- 5.Laukkanen O, Lindstrom J, Eriksson J, et al. Polymorphisms in the SLC2A2 (GLUT2) gene are associated with the conversion from impaired glucose tolerance to type 2 diabetes: the Finnish Diabetes Prevention Study. Diabetes. 2005;54:2256–2260. doi: 10.2337/diabetes.54.7.2256. [DOI] [PubMed] [Google Scholar]
- 6.Barroso I, Luan J, Middelberg RP, et al. Candidate gene association study in type 2 diabetes indicates a role for genes involved in beta-cell function as well as insulin action. PLoS Biol. 2003;1:E20. doi: 10.1371/journal.pbio.0000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alcolado JC, Baroni MG, Li SR. Association between a restriction fragment length polymorphism at the liver/islet cell (GluT 2) glucose transporter and familial type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1991;34:734–736. doi: 10.1007/BF00401519. [DOI] [PubMed] [Google Scholar]
- 8.Matsutani A, Koranyi L, Cox N, Permutt MA. Polymorphisms of GLUT2 and GLUT4 genes. Use in evaluation of genetic susceptibility to NIDDM in blacks. Diabetes. 1990;39:1534–1542. doi: 10.2337/diab.39.12.1534. [DOI] [PubMed] [Google Scholar]
- 9.Patel P, Bell GI, Cook JT, Turner RC, Wainscoat JS. Multiple restriction fragment length polymorphisms at the GLUT2 locus: GLUT2 haplotypes for genetic analysis of type 2 (noninsulin-dependent) diabetes mellitus. Diabetologia. 1991;34:817–821. doi: 10.1007/BF00408357. [DOI] [PubMed] [Google Scholar]
- 10.Li SR, Alcolado JC, Stocks J, Baroni MG, Oelbaum RS, Galton DJ. Genetic polymorphisms at the human liver/islet glucose transporter (GLUT2) gene locus in Caucasian and West Indian subjects with type 2 (noninsulin-dependent) diabetes mellitus. Biochim Biophys Acta. 1991;1097:293–298. doi: 10.1016/0925-4439(91)90084-m. [DOI] [PubMed] [Google Scholar]
- 11.Baroni MG, Alcolado JC, Pozzilli P, Cavallo MG, Li SR, Galton DJ. Polymorphisms at the GLUT2 (beta-cell/liver) glucose transporter gene and noninsulin-dependent diabetes mellitus (NIDDM): analysis in affected pedigree members. Clin Genet. 1992;41:229–234. doi: 10.1111/j.1399-0004.1992.tb03671.x. [DOI] [PubMed] [Google Scholar]
- 12.Tanizawa Y, Riggs AC, Chiu KC, et al. Variability of the pancreatic islet beta cell/liver (GLUT 2) glucose transporter gene in NIDDM patients. Diabetologia. 1994;37:420–427. doi: 10.1007/BF00408481. [DOI] [PubMed] [Google Scholar]
- 13.Shimada F, Makino H, Iwaoka H, et al. Identification of two novel amino acid polymorphisms in beta-cell/liver (GLUT2) glucose transporter in Japanese subjects. Diabetologia. 1995;38:211–215. doi: 10.1007/BF00400096. [DOI] [PubMed] [Google Scholar]
- 14.Matsubara A, Tanizawa Y, Matsutani A, Kaneko T, Kaku K. Sequence variations of the pancreatic islet/liver glucose transporter (GLUT2) gene in Japanese subjects with noninsulin dependent diabetes mellitus. J Clin Endocrinol Metab. 1995;80:3131–3135. doi: 10.1210/jcem.80.11.7593414. [DOI] [PubMed] [Google Scholar]
- 15.Moller AM, Jensen NM, Pildal J, et al. Studies of genetic variability of the glucose transporter 2 promoter in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2001;86:2181–2186. doi: 10.1210/jcem.86.5.7499. [DOI] [PubMed] [Google Scholar]
- 16.De Vos A, Heimberg H, Quartier E, et al. Human and rat beta cells differ in glucose transporter but not in glucokinase gene expression. J Clin Invest. 1995;96:2489–2495. doi: 10.1172/JCI118308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simpson IA, Dwyer D, Malide D, Moley KH, Travis A, Vannucci SJ. The facilitative glucose transporter GLUT3: 20 years of distinction. Am J Physiol Endocrinol Metab. 2008;295:E242–E253. doi: 10.1152/ajpendo.90388.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu EH, Digon BJ, III, Hirshberg B, et al. Pancreatic beta cell function persists in many patients with chronic type 1 diabetes, but is not dramatically improved by prolonged immunosuppression and euglycaemia from a beta cell allograft. Diabetologia. 2009;52:1369–1380. doi: 10.1007/s00125-009-1342-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keenan HA, Sun JK, Levine J, et al. Residual insulin production and pancreatic ss-cell turnover after 50 years of diabetes: Joslin Medalist Study. Diabetes. 2010;59:2846–2853. doi: 10.2337/db10-0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rother KI, Spain LM, Wesley RA, et al. Effects of exenatide alone and in combination with daclizumab on beta-cell function in long-standing type 1 diabetes. Diabetes Care. 2009;32:2251–2257. doi: 10.2337/dc09-0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rother KI, Harlan DM. Comment on: Keenan, et al. (2010) residual insulin production and pancreatic ss-cell turnover after 50 years of diabetes: Joslin Medalist Study. Diabetes 2010; 59:2846–2853. Diabetes. 2010;59:e26. doi: 10.2337/db10-1207. author reply e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fu Y, Maianu L, Melbert BR, Garvey WT. Facilitative glucose transporter gene expression in human lymphocytes, monocytes, and macrophages: a role for GLUT isoforms 1, 3, and 5 in the immune response and foam cell formation. Blood Cells Mol Dis. 2004;32:182–190. doi: 10.1016/j.bcmd.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Stoffel M, Bell KL, Blackburn CL, et al. Identification of glucokinase mutations in subjects with gestational diabetes mellitus. Diabetes. 1993;42:937–940. doi: 10.2337/diab.42.6.937. [DOI] [PubMed] [Google Scholar]
- 24.Miller SP, Anand GR, Karschnia EJ, Bell GI, LaPorte DC, Lange AJ. Characterization of glucokinase mutations associated with maturity-onset diabetes of the young type 2 (MODY-2): different glucokinase defects lead to a common phenotype. Diabetes. 1999;48:1645–1651. doi: 10.2337/diabetes.48.8.1645. [DOI] [PubMed] [Google Scholar]
- 25.Gidh-Jain M, Takeda J, Xu LZ, et al. Glucokinase mutations associated with non-insulin-dependent (type 2) diabetes mellitus have decreased enzymatic activity: implications for structure/ function relationships. Proc Natl Acad Sci U S A. 1993;90:1932–1936. doi: 10.1073/pnas.90.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rowe RE, Wapelhorst B, Bell GI, Risch N, Spielman RS, Concannon P. Linkage and association between insulin-dependent diabetes mellitus (IDDM) susceptibility and markers near the glucokinase gene on chromosome 7. Nat Genet. 1995;10:240–242. doi: 10.1038/ng0695-240. [DOI] [PubMed] [Google Scholar]
- 27.Akirav E, Kushner JA, Herold KC. Beta-cell mass and type 1 diabetes: going, going, gone? Diabetes. 2008;57:2883–2888. doi: 10.2337/db07-1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Butler AE, Galasso R, Meier JJ, Basu R, Rizza RA, Butler PC. Modestly increased beta cell apoptosis but no increased beta cell replication in recent-onset type 1 diabetic patients who died of diabetic ketoacidosis. Diabetologia. 2007;50:2323–2331. doi: 10.1007/s00125-007-0794-x. [DOI] [PubMed] [Google Scholar]
- 29.Meier JJ, Bhushan A, Butler AE, Rizza RA, Butler PC. Sustained beta cell apoptosis in patients with long-standing type 1 diabetes: indirect evidence for islet regeneration? Diabetologia. 2005;48:2221–2228. doi: 10.1007/s00125-005-1949-2. [DOI] [PubMed] [Google Scholar]
- 30.In’t Veld P, Lievens D, De Grijse J, et al. Screening for insulitis in adult autoantibody-positive organ donors. Diabetes. 2007;56:2400–2404. doi: 10.2337/db07-0416. [DOI] [PubMed] [Google Scholar]
- 31.Meier JJ, Lin JC, Butler AE, Galasso R, Martinez DS, Butler PC. Direct evidence of attempted beta cell regeneration in an 89-year-old patient with recent-onset type 1 diabetes. Diabetologia. 2006;49:1838–1844. doi: 10.1007/s00125-006-0308-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.